Summary
A diurnal rhythm of eating-fasting promotes health, but humans’ eating pattern is rarely assessed. Using a mobile app, we monitored ingestion events in healthy adults with no shift-work for several days. Most subjects ate frequently and erratically throughout wakeful hours and overnight fasting duration paralleled time in bed. There was a bias toward eating late, with estimated <25% calories being consumed before noon and >35% after 6pm. “Metabolic jetlag” resulting from weekday/weekend variation in eating pattern akin to travel across time-zones was prevalent. The daily intake duration (95% interval) exceeded 14.75 h for half the cohort. When overweight individuals with >14 h eating duration ate for only 10–11 h daily for 16 weeks assisted by a data visualization (raster plot of dietary intake pattern, “feedogram”) that we developed, they reduced body weight, reported being energetic, and improved sleep. Benefits persisted for a year.
Graphical Abstract

INTRODUCTION
Life on earth has evolved in the context of a 24-hour periodicity in environmental conditions and a dependent daily rhythm in food availability and predator avoidance. Consequently, organisms have evolved endogenous circadian oscillators that allow them to anticipate and prepare for activity, sleep, and food intake at a specific time of the day. Both food ingestion and fasting can alter the metabolic state. Therefore, molecular responses to feeding and fasting exhibit temporal dynamics with a 24 h period. The circadian oscillator and time-of-feeding act together to drive daily rhythms in gene expression and protein function such that the anticipation of and responses to feeding events are properly timed (Adamovich et al., 2014; Vollmers et al., 2009) every day. Genetic disruption of circadian rhythms in experimental animals and behavioral disruption of circadian rhythm among shift workers likely perturbs such temporal regulation and predisposes to metabolic diseases (Asher and Schibler, 2011). Frequent caloric intake in animal models of diet induced obesity also dampens molecular circadian rhythms (Kohsaka et al., 2007). Conversely, recent studies in both nocturnal (mice) and diurnal (Drosophila melanogaster) model organisms have demonstrated that restricting the time of caloric intake to a window of 8–12 hours without altering of the quantity or quality of diet can impart pleiotropic physiological benefits (Chaix et al., 2014; Gill et al., 2015; Hatori et al., 2012; Sherman et al., 2012; Zarrinpar et al., 2014). Such ‘time-restricted feeding’ (TRF) supports a robust circadian rhythm and is associated with reduced adiposity, elevated lean mass, longer sleep duration, increased endurance, reduced systemic inflammation, decelerated cardiac aging, gut homeostasis, and improvement in other clinically-relevant biomarkers.
Despite these observed benefits in model organisms, the applicability of TRF for human health has remained unknown because the temporal aspect of human eating pattern is rarely measured. A lack of methods and parameters to describe the daily eating pattern in humans makes it difficult to ascertain whether eating events in humans are spread over a long enough segment of the 24 h day such that there is an opportunity to reduce this duration. Furthermore, differences between metabolic properties of humans and those of small model organisms make it difficult to predict whether an 8–12 h eating window will impart physiological benefits in humans. Nevertheless, accumulating data indicate that the temporal aspect of food intake, in addition to total caloric intake, can be important determinant of predisposition to chronic diseases (Mattson et al., 2014), which now are the predominant cause of morbidity and mortality in developed nations (Bauer et al., 2014). Therefore, the objective longitudinal assessment of the content and the daily temporal pattern of human nutrition could have a major public health impact in terms of finding at-risk individuals for chronic disease and subsequent corrective action through lifestyle interventions.
Current methods to monitor human nutrition are subjective (such as questionnaires), remove subjects from their usual spatiotemporal niche (e.g., in-lab videography with limited food choices) or provide negative feedback that interferes with subjects’ behavior (food diaries). Moreover, these methods gather information on the quantity and quality of nutrition and generally do not seek information regarding the time at which the food or beverage was consumed. The availability of smartphones presents an opportunity to objectively monitor human nutrition along with the advantage of complete control over feedback. The wide-ranging adoption of smartphones across age, gender, and socioeconomic segments can be leveraged to study behaviors of free-living individuals at scale in heterogeneous populations.
In this study, we developed and used a smartphone-based monitoring method with minimal feedback to collect the natural daily eating pattern of free-living healthy adults. In contrast to the conventional wisdom that modern humans eat 3 meals a day within a 12 h interval, eating pattern was found to be largely erratic and differing between weekdays and weekends. In a pilot feasibility study, we tested whether reducing the eating interval to 10–12 h without an overt attempt to change nutrition could lead to weight loss in healthy overweight individuals. Overall, the results presented here show that a large segment of the human adult population displays an erratic daily rhythm of eating-fasting, which can be manipulated to obtain desirable health benefits.
Results
A smartphone app to monitor daily eating patterns
We built a smartphone software application (“app”) to longitudinally monitor the daily temporal pattern of caloric intake in free-living humans. To record an ingestive event, the participants used the camera function of the smartphone to take a picture of the food or beverage. Food pictures taken in the JPEG format were downscaled to 1/10 their original size on the device itself to reduce network data usage. Participants also had the option for textual entries to substitute for food/drink pictures, when picture taking was difficult or when subjects forgot to log their picture entries. Immediately after data logging, the food picture, and text entries along with time-stamp and geolocation were immediately transferred to a server. Upon confirmation of successful data transfer, the data and the associated metadata (i.e., the time-stamp and geolocation) were erased from the subject’s device, eliminating the possibility for the ‘feedback effect’ of prior-recorded information upon present behavior.
We made use of push notifications as an orthogonal measurement technique for assessing the time of diet intake. These push notifications were manually triggered at a random time of the day, during the stated wakeful period of the subject and numbered 1–2 per day. The specific push notification query presented to the subject was dependent on the time when he/she responds to it, not when it was originally dispatched from the server. The push notification presented a query on the user’s device inquiring whether they ate/drank anything in the past 30 minutes. Subjects had to push a “Yes” or “No” button displayed on their screen, and their responses were recorded on the server. From such responses to push notifications sent at a random time during wakeful hours, we estimated the false negative rate (i.e., when the subject consumed food/beverage/water, but forgot to log the event) for our methodology to be 10.34 %.
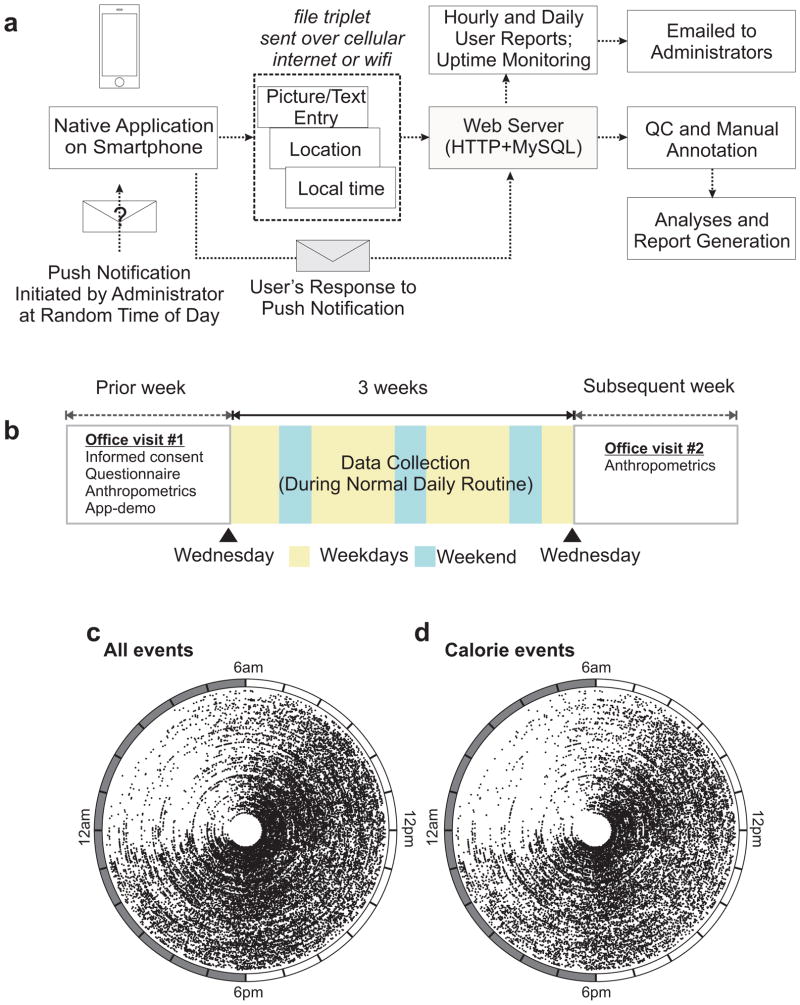
We monitored healthy, non-shift-worker adult males and females (Fig. 1a and b, Table S1) for 3 weeks. After meeting the inclusion and exclusion criteria (Table S2) and signing an informed consent document during an office visit, subjects used the custom mobile application (“Salk Metabolic App”) installed on their smartphone to take pictures of every food, beverage or water item they consumed, irrespective of volume or calorie just prior to consumption (Fig. S1, S2). Appending a textual annotation describing the amount and the item(s) consumed to the pictures was optional.
Figure 1.
A scalable method to monitor daily patterns of dietary intake in humans. (a) Schematic of the smartphone-based approach to monitor human eating pattern used to monitor all ingestion events for (b) three week period in healthy adults. Polar plot of (c) all or (d) calorie containing (≥5 kcal) ingestion events of each individual plotted against time of the day (radial axis) in each concentric circle. Data from 156 individuals are shown.
The food pictures or occasional text entries (2.1% of all events) were further annotated by looking up the reference nutrition values from Calorie King or FNDDS (Six et al., 2011). Out of the 26676 events recorded, 22% (5846) were water, 28% (7420) consisted of pre-packaged items with readily accessible nutrition information and 50% (13410) were mixed meals with multiple items. We hypothesized that the reported caloric intake should at least meet the resting energy expenditure or maintenance caloric (MC) intake (Roza and Shizgal, 1984). The average daily estimated caloric intake for the group (mean 1947 Kcal; 95% CI: 1917–1977) was more than their respective maintenance caloric intake (mean 1.233 fold over MC; 95% CI: 1.214–1.251). From push notifications, we had measured a false negative rate or underreporting of food/beverage/water to be 10.34%. Therefore, the actual caloric intake was likely little higher. The extra caloric intake likely accounts for activity above resting metabolism. There was no significant change in body weight during the 3 weeks reporting period (Table S1) indicating any potential ‘feedback effect’ on weight-loss due to recording food intake was absent.
Daily eating pattern in humans
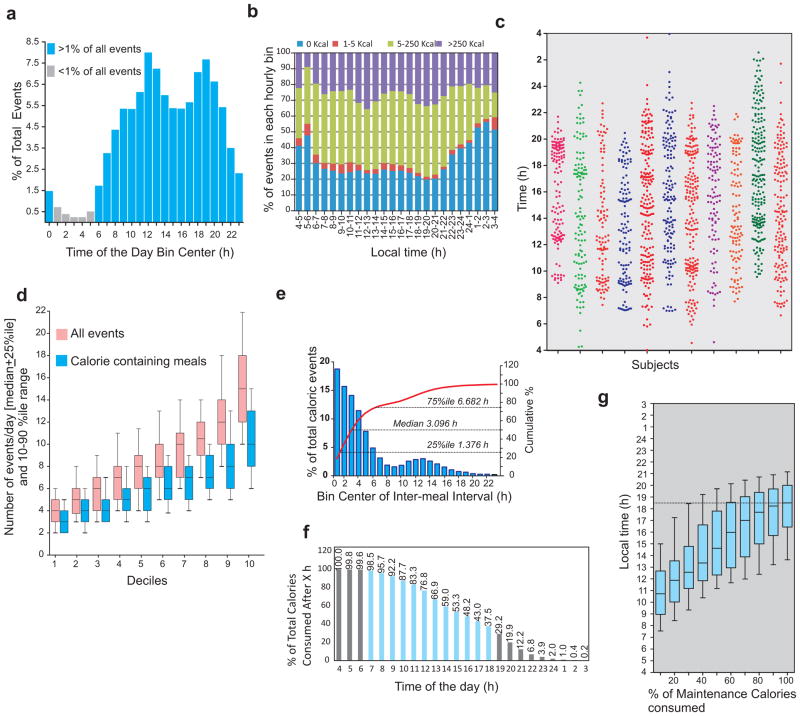
The time-stamp of every ingestion event allowed analyses of the temporal aspect of eating. Aggregate data from three weeks of monitoring was used to assess eating pattern of the cohort. Caloric (i.e., >5kcal) events populated a large segment of the 24 h day (Fig. 1c,d) and there were only 5 h between 1–6 am when the number of events/h were <1% of total events (Fig 2a). The fraction of events with estimated energy content >5kcal also reached its nadir (Fig 2b) in that interval. Because at the population level, human digital activity reaches the trough between 2–4 am (Golder and Macy, 2011), none of the subjects was a self-reported shift-worker, and the reporting trough was close to 4 am (Fig 2a,b), we considered 4 am as the onset of “metabolic day” (i.e. events between 00:00:00 and 03:59:59 h were included in the previous calendar day).
Figure 2.
Human eating lacks 3-meals-a-day structure. (a) Percentage of all ingestion events in 1 h bin shows the nadir at 4 am. (b) The fraction of events with <5kcal also reaches its peak at 4am. Therefore we considered 4 am (instead of the midnight) as the beginning of the metabolic day. (c) Representative scatter plot of ingestion events of 11 subjects during the observation period shows the lack of clustering of events into three principal bins for most individuals and a large variation in the total number of events. (d) Number of ingestion events/day in all subjects binned into 10 deciles show a wide distribution of number of total and calorie containing events every day. (e) Frequency distribution and cumulative frequency of inter-meal-interval for the entire cohort. (f) Percentage of calories remaining to be consumed in each hourly bin shows >75% of all calories are consumed after mid-day. (g) Time (median + 25%–50% range and 10–90% interval) at which percentage of maintenance calories consumed in 10% increments are shown.
Surprisingly, in contrast to the self-reported 3 meals/day structure of meals from most of the participants, a breakfast-lunch-dinner temporal pattern was largely absent (Fig 2c). At the individual level, the number of events/day showed wide variation ranging from 4.22±0.1 (mean+s.e.m) for the bottom decile to 15.52±0.34 for the top decile of which 3.33±0.07 and 10.55±0.24 respectively were caloric events (Fig. 2d).
From a subset of events that marked the beginning and end of an eating report (i.e., pictures of food at the start and of leftovers at the end of the meal), we calculated the average meal duration to be 14 min 36 sec. Therefore, “events” from an individual with <15 minutes inter-event interval were combined into one “meal”. At the group level, 25% of all meals were within 1 h 25 min of another meal and the median inter-meal interval was 3 h 6 min. Only 25% of the meals occurred after > 6 h 41 min of fasting (Fig 2e).
The fraction of total calories consumed (starting at 4 am) showed that less than 25% of caloric intake occurs before noon (Fig 2f). The percentage of total calories consumed after 6 pm, 9 pm and 11 pm (and before 4 am of the next day) were 37.5%, 12.2% and 3.9% respectively (Fig 2f). After adjusting for Maintenance Calories (MC) for each individual (Roza and Shizgal, 1984), the average cumulative percentage of MC ingested over diurnal time showed the average time by which 50%, 70%, 90% and 100% of MC were consumed to be 3:32 pm, 5:04 pm, 6:11 pm, and 6:36 pm respectively (see Fig 2g for median values). In summary, there is a systematic bias towards consuming a larger portion of the daily caloric intake towards the late afternoon and evening hours. At the cohort level, in general, food consumed after 6:36 pm exceeded the maintenance calories requirement.
Eating pattern relative to wakeful hours
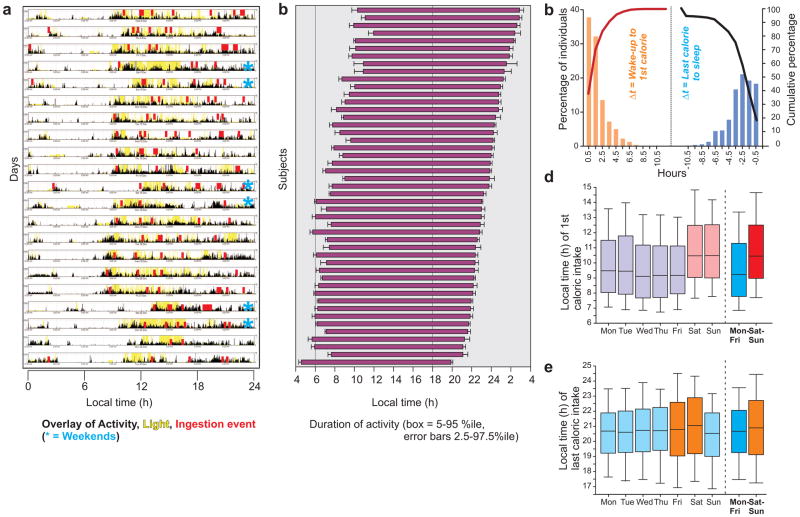
To compare the eating pattern with diurnal activity period, wrist actigraphy (Blood et al., 1997) data was collected from 47 randomly selected participants using a CamNTech Motionwatch 8, a device that measured both activity and light. A nighttime drop in activity and an absence of light was scored as time in bed. Integration of activity, light, and ingestion events allowed analyses of eating time relative to activity period (Fig. 3a). The average time of activity onset and of time to bed showed large variation even among this non-shift worker cohort (Fig. 3b). The median time interval between daily activity onset and first caloric intake was 1 h 18 min, while the median time between the last caloric intake and going to bed was 2 h 22 min (Fig. 3c). Therefore, the total overnight fasting duration paralleled the time of inactivity (sleep) at night.
Figure 3.
Activity and eating duration in adult humans. (a) Representative actogram and light exposure pattern (from a wrist-worn device) of a subject for 3 weeks overlaid with ingestion events (from smartphone app) shows that the latter occur erratically throughout the active period. (b) Wakeful activity duration in a subset of the subjects is shown. Each horizontal bar shows the interval between average wake up and bedtime (+ s.e.m., up to 21 days of monitoring). (c) Time interval between waking up and the first caloric ingestion or the last caloric ingestion and going to bed. Bars (orange and blue, y-axis) indicate the percent of the individuals for whom actigraphy was performed with the indicated number of hours (x-axis, 1 h bins) from waking up to the first caloric intake or from the last caloric intake to sleep. Cumulative percentages (secondary y-axis) are shown in color-matched lines. Median time of (d) first and (e) last caloric event of all individuals on different days of the week. Median (25–75% interval in box, 10–90% interval in lines) local time is shown.
The day-to-day variation in the time of first or last caloric intake was spread over a few hours (Fig S3). Feeding after several hours of fasting is known to affect neuroendocrine-, metabolic- pathways and adjust the phase of the circadian clock in peripheral organs (Vollmers et al., 2009) so that physiological state transitions from the fasting to the fed state. Changes in the sleep-wake cycle between social/work days and free/weekend days is similar to the circadian desynchrony arising from jet travel between time zones and is called social jetlag (Roenneberg et al., 2012). By analogy, we postulated that the variation in breakfast time between working/week days and free/weekend days likely affects the peripheral clocks in metabolic organs causing metabolic desynchrony or “metabolic jetlag”. The median breakfast (i.e., the first caloric intake event) time for the entire population changed on Saturday and Sunday (Fig. 3d, Fig. S3), so we considered those two days as the “metabolic weekend”. The time of last caloric intake did not significantly change in any of the days, but the variability was relatively large on Friday and Saturday (Fig. 3e, Fig S3). At the population level, the mean times of first caloric intake during weekdays and weekend were 9:21 am (95% CI: 9:15–9:27 am) and 10:26 am (95% CI:10:15–10:37 am) respectively. Delaying breakfast was more common than advancing it, with 40% of the subjects delaying breakfast by 1 h or longer and 25% by 2.18h, while only 7% advanced their breakfast time by >1h. The time of last caloric intake was more variable than breakfast. The average last caloric intake time was advanced by >1 h in 17%, while only 15% delayed the time of last caloric intake by >1 h in the weekend.
Eating duration and eating pattern
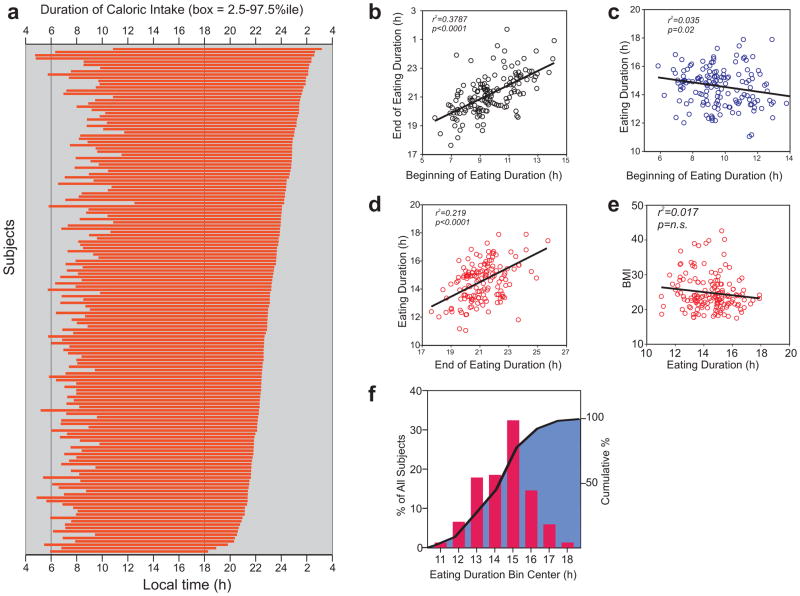
Having observed a large variance in the first and last caloric intake (Fig. S3) and the absence of a clear 3 meals/day eating pattern (Fig. 2c,d ) for most subjects, a potentially better description of an individual’s eating pattern could be the daily duration of caloric intake. Because food intake triggers post-prandial changes in neuroendocrine state that can take minutes to hours to return to resting or fasting state, eating too often could clamp the physiological state between frequent meals to the post-prandial state. Therefore, we defined the daily eating duration as the time interval (4 am onwards) that contained 95% (2.5%ile–97.5%ile) of all intake events during the monitoring period (Fig. 4a). This approach for arriving at the eating duration from aggregate data over several days is tolerant of occasional non-reporting of some random eating events. Breakfast time weakly positively correlated with the last caloric intake (r2= 0.379), so that individuals with earlier breakfast also had their last caloric intake earlier in the evening (Fig. 4b). The eating duration better correlated with the time of last caloric intake (r2= 0.215) than the time of breakfast (r2= 0.035) (Fig. 4c,d), or with BMI (r2=0.017) (Fig. 4e). The median daily eating duration was 14 h 45 min, and only 9.7% of the subjects had a daily eating duration <12 h (Fig. 4f) long. The weak correlation (r2= 0.017) between the eating duration and BMI could be due to the limited sample size, the heterogeneity of the participants in terms of gender and age, and the fact that the eating pattern recorded in the monitoring period is a short-term snapshot of a person’s long term diet related behaviors.
Figure 4.
Daily duration of caloric intake.
(a) Eating duration of all individuals is shown in the order of late (top) to early (bottom) nighttime fasting onset time. (b) The time of last caloric intake weakly positively correlates with the time of first caloric intake. (c) The daily duration of eating does not correlate with the time of first caloric intake (d) but weakly positively correlates with the time of the last caloric intake. The subjects’ daily eating duration does not correlate with their (e) body mass index (BMI, kg/m2) (f) Frequency distribution (red bars) and cumulative percentage (black line and blue filled area) of eating duration (hours).
Restricting eating duration reduces body weight
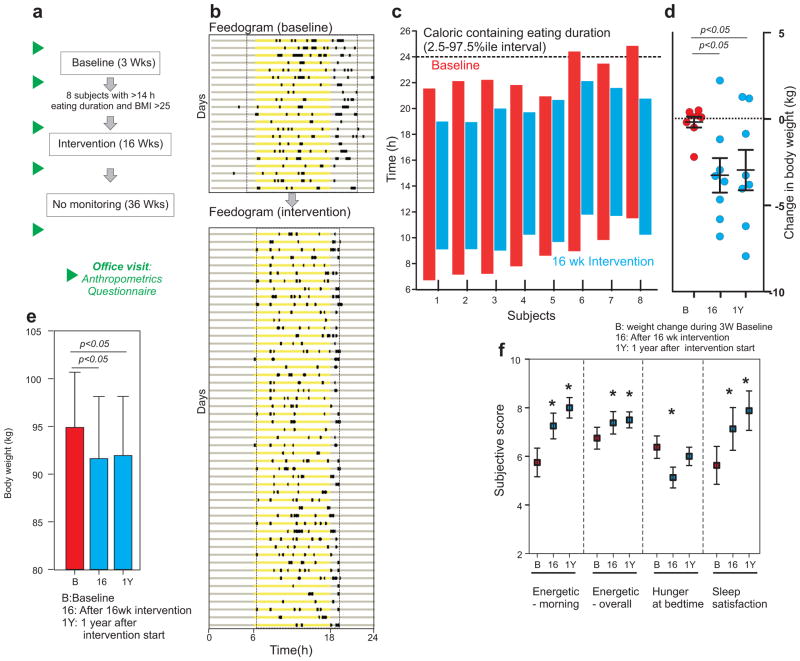
Many factors including nutrition quality, quantity, physical activity and genetics contribute to obesity. Although we did not find a simple correlation between BMI and eating duration, we wanted to test whether longer eating duration and erratic eating pattern are contributing factors in subjects with co-occurrence of >25 BMI and >14 h eating duration. We tested if reducing the eating duration and metabolic jetlag associated with weekday/weekend differences in a subset of individuals would lead to reduction in body weight. We recruited 8 individuals with >14h eating duration for a 16 weeks pilot intervention study, such that each individual’s own baseline data served as the control (Fig. 5a) condition. Individualized “feedogram” graphics representing a temporal raster plot of ingestion events in successive days were constructed (Fig. 5b). After obtaining informed consent for intervention, the participants were provided data regarding their eating pattern accrued in the 3 weeks baseline period (95% eating duration, variance in first and last caloric intake and weekday-weekend metabolic jetlag) and were shown their own baseline “feedogram” prior to the intervention. Because in rodents, a daily eating period of up to 12 h improves metabolic fitness (Chaix et al., 2014), the participants were requested to reduce their caloric containing eating duration to a self-selected window of 10–12 h, and to consistently follow this duration both during weekdays and weekends so that the metabolic jetlag could be minimized. No overt suggestion concerning nutrition quality, quantity or caloric content was provided. The individuals continued logging their food pictures using the same app as used in the baseline period for the next 16 weeks and also received a weekly summary of their feedograms and daily eating duration.
Figure 5.
Improved eating pattern reduces body weight in healthy overweight individuals. (a) Schematic of the study design to test the effect of eating pattern on body weight. (b) Representative “feedogram” of a participant during baseline and during intervention. The times of ingestion events are denoted as prominent black rectangles along the 24 h day represented in each horizontal line (x-axis). Yellow represents the time between 6 am and 6 pm. Eating duration during baseline and intervention are shown as broken lines. (c) The daily eating duration of each individual during baseline (red) and intervention (blue) plotted against the local time (y-axis). (d) Scatter plot and average (± s.e.m.) change in body weight in 8 participants during 3 weeks of baseline monitoring, after 16 weeks of intervention and after 1 year. (e) Average (+ s.e.m.) body weight at the end of 3 weeks baseline, after 16 weeks of monitored intervention and at 1 y. (f) Average (+ s.e.m.) of subjective measures of energy level, hunger and sleep in subjects. These subjective measures were assessed on a scale of 1 to 10, with 10 being the preferred (healthier) end of the range. Higher numbers thus indicated healthier values for the quantity, i.e., more energetic, less hunger at bedtime and more sleep satisfaction. *: p<0.05, T-test.
All subjects reduced their eating duration (average reduction: 4 h 35 min; 95% CI: 3 h 30 min–5 h 40 min; p<0.001) and their weekday/weekend metabolic jetlag was also reduced to <1h (Fig. 5b,c). The participants showed a reduction in total body weight (average loss 3.27 kg; 95% CI: 0.9081–5.624 kg) and accordingly, excess body weight (Fig. 5d–e, Table S3), and BMI (average reduction 1.15 kg/m2; 95% CI: 0.3247–1.980 kg/m2). In a subjective self-assessment of sleep satisfaction, hunger at bedtime and energy level (in the mornings, and overall over the past few days), statistically significant improvement was observed (Fig. 5f). All participants voluntarily expressed an interest in continuing unsupervised with the 10–11 time-restricted eating regimen after the conclusion of the 16 weeks supervised intervention. After 36 weeks (1 year since the intervention began), the participants maintained weight loss, sleep improvement, and felt more energetic (Fig. 5d–f, Table S3).
Although the participants were not overtly asked to change nutrition quality or quantity, reducing the eating duration led to reduced estimated caloric intake. Unlike mice where reducing the eating duration to ~10 h does not alter total caloric intake (Hatori et al., 2012), our human intervention cohort reduced the estimated daily caloric intake (average reduction 20.26%, 95% CI 4.92%–35.6%; paired t-test p<0.05). Humans consume heterogeneous food types in a time-of-the-day dependent manner (Fig. S4), e.g., coffee is almost always consumed in mornings, while alcohol at night. As a result, during the intervention, it was not the case that items that would have otherwise (in the baseline period) been consumed in the designated 14 h nighttime fasting hours had been moved to the self-selected 10 h feeding period during the intervention. Instead, the person would simply not consume such an item rather than consume it at the wrong time of day. This could be one potential explanation for the reduction in caloric intake.
Discussion
Collecting human nutrition information in the free-living condition has been a persistent challenge. Recording dietary intake using text entries, selecting from a large library of food items, and specifying the portion size is a ubiquitous feature found in most nutrition apps. Although such apps improve adherence relative to the traditional diary log (Carter et al., 2013), data logging can be cumbersome for mixed meals, and consequently, users may not bother to log small snacks. Furthermore, portion size reporting can be subjective. By adopting an approach centered on food pictures with an optional user-side annotation together with infrequent but randomly timed push notifications, we reduced the barrier to data recording. Server side annotation of the picture metadata ensured uniformity across the cohort. Supervised crowd-sourced annotation can make this approach scalable to large cohorts.
By overlaying the daily patterns of food and beverage intake, activity-rest, and light exposure, we could uncover relationships among them (Fig 3a–c). Data integration from multiple such longitudinal data-streams has immense promise for disease prognosis. Although the subjects did not have any chronic medical conditions, they also logged their consumption of vitamin, supplement and occasional over-the-counter medications for minor ailments, thus offering a temporal pattern of drug and supplement use (Fig. S4). More than 50% of the mammalian transcriptome exhibits diurnal rhythms in a tissue-specific manner (Zhang et al., 2014), the gut microbiome shows daily rhythms (Thaiss et al., 2014), the timing of food affects these rhythms in peripheral organs (Vollmers et al., 2009), and the targets of a large number of FDA approved drugs show circadian expression (Zhang et al., 2014). Therefore, by monitoring the timing of drug intake relative to the sleep-wake or feeding-fasting cycle can have significant impact on disease prognosis and unraveling interactions among food, sleep and drugs in free-living individuals.
Formally, our work introduces a method and the critical defining parameters for describing the diurnal and longer-term temporal characteristics of nutrition in humans. By creating a scalable method to longitudinally monitor human nutrition in an evidence-based manner, we discovered that the daily eating pattern even among healthy non-shift young workers is highly variable from day to day. For more than a half of the participants in the baseline monitoring study, the eating pattern is erratic, energy intake events span over a large fraction of a 24 h day, with a relatively short fasting period (Fig. 4). Although the first caloric intake after leaving the bed happened within 1h 18m (median value) (Fig. 3c), less than 25% of the daily caloric intake occurred before noon while 37.5% was consumed after 6pm (Fig. 2f). This suggests that breakfast is relatively small in terms of energetic input and that major caloric intake is delayed until later in the afternoon or evening, in this set of relatively young (Table S1) non-shift-worker subjects. To address the universality of our observations concerning eating patterns, this method may be extended to a larger population spread over different geographical regions, work schedules (e.g. shift-workers, retired individuals, nurses, pilots), agegroups, and/or cultures. It would also be useful to describe the diurnal patterns of caloric intake in humans that do not have a modern lifestyle influenced by electricity, such as hunter-gather societies.
Individuals in our study largely ate throughout the wakeful hours (Fig. 3). Consequently, sleep duration and quality largely dictated the eating pattern. Furthermore, since the sleep pattern changes between weekdays (workdays) and weekends leading to social jetlag (Roenneberg et al., 2012; Wittmann et al., 2006), the breakfast time also changes between weekdays and weekends. These changes in breakfast time are analogous to a person traveling across time zones every weekend and can be described as metabolic jetlag. The intricate connection we observed between sleep and overnight fasting duration suggests that the observed relationship between a short sleep duration and predisposition to metabolic diseases (Cappuccio et al., 2011; Copinschi et al., 2014) may be partly explained by the reduced duration of overnight fasting. Similarly, the reported correlation between social jetlag and BMI may also involve metabolic jetlag. The increased daily eating duration likely contributes to increased caloric intake. A change in eating pattern between days (e.g. weekday vs. weekend) can affect time-of-day/night specific changes in food intake from specific food groups (Fig. S4). Therefore, one mode by which reduced sleep duration contributes to the increased risk for metabolic diseases could be the increased daily eating duration and associated changes in caloric intake and nutrition quality.
We did not find a simple positive correlation between the daily eating duration and BMI in our cohort. This may be for several reasons including a limited sample size, heterogeneity of the cohort and a likely scenario that individuals with long eating duration may also have more physical activity. Nevertheless, reducing the temporal eating period in a feasibility study imparted measurable benefits of clinically relevant magnitude in terms of body weight reduction and sleep improvement without increasing the subjective sense of hunger. This relatively large effect on body weight reduction even in the small intervention cohort implies that the benefits might result from multiple changes: restoration of the diurnal rhythm of feeding/fasting, reduction of the weekday/weekend metabolic jetlag and a reduction in the daily caloric intake. Some benefits of TRF might arise from caloric reduction (CR). At the same time we cannot rule out the possibility that some benefits of CR in vertebrates including humans might be from TRF, as most CR studies involve caloric intake within a defined time-frame. Nevertheless, if time restriction under free-living condition inadvertently leads to caloric reduction, TRF as a method to reduce caloric intake is more attractive option as the individuals, caregiver, case managers, physicians, and scientists do not have to adapt expensive and laborious methods to accurately track caloric count. Hence, irrespective of mechanism, time restriction offers an effective approach to improve health.
While the relative contribution of daily eating pattern, calories, and nutrition quality to multifaceted health improvement in humans should be examined in detail in future studies, our results highlight that suitable manipulation of the diurnal temporal pattern of caloric intake is a feasible therapeutic approach for improving human health in the free-living condition, in spite of the vast variety of food and beverage types consumed by the average person from day to day. This opens up the possibility for utilizing this strategy by itself or in combination with existing approaches for health improvement.
Materials and Methods
This study was approved by the IRB of the Salk Institute. Participants were recruited during 2012–2013 through a newspaper advertisement, paper flyers, and online advertisements. Inclusion and exclusion criteria were determined by an online questionnaire and an in-person interview. All subjects provided written informed consent during the first office visit and were asked to record all of their food, beverage and water intake using the smartphone app. Subjects’ height and weight were measured using a calibrated scale and tape measure at the beginning and end of the 3 weeks baseline period. Participants were nominally compensated for their time and effort.
For the baseline study aimed at observing eating patterns in free living adults, data were collected from a Tuesday/Wednesday midnight to another Tuesday/Wednesday midnight 21 days later. Subjects received instructions to record every item consumed (food, drink, water) regardless of its size using the app on their smartphone. The leftovers from items that were not completely consumed were to be recorded again, but described as such in the annotation. Days with less than 3 total events (including non-caloric content items) were flagged and verified with the participants for any observed fasting day or whether they forgot to log data.
Subjects from the baseline study with BMI >25 kg.m−2 and whose intake interval exceeding 14h were offered the opportunity to participate in the intervention study. After a detailed presentation on the known benefits of time-restricted feeding in rodents, data on their own eating pattern and what the intervention study would entail, subjects chose a 10-h eating interval of their choice, and were to limit all non-water intake (including coffee and tea) to that 10-h interval. Eight subjects entered the study; 5 males (age 34.4 ± 2.9 years, weight 96.7 ± 4.8 kg, BMI 31.77 ± 2.05 kg/m2), 3 females (age 36.3 ± 4.3 years, weight 91.8 ± 15 kg, BMI 34.91 ± 3.84 kg/m2; average ± s.e.m.). Ethnicity: 2 Hispanics, 6 Non Hispanic or Latino. Race: 3 Asian, 4 White, 1 More than one race. To identify undiagnosed fasting hypoglycemia, a fasting blood metabolic panel was performed at a certified clinic prior to the intervention study for every subject. During the 16 weeks long intervention study, the participants were instructed to log in to a personalized website every week to check their eating duration. Anthropometrics were performed prior to the start of the intervention, just after the completion of the 16-week long intervention and 1 year after the start of the intervention. As the eligibility criteria for this feasibility pilot study was to test TRF in healthy overweight subjects with no recent history of fasting blood sugar, cholesterol, and triglycerides outside the reference range, we did not thoroughly follow clinical biomarkers of metabolic diseases.
Software
The smartphone application was coded in Objective-C. When the application is run for the first time, it generates a unique 20 character alphanumeric code to identify the device, as well as records the user’s Push Notification Service identifier in a database. Access to the application was restricted to study participants by a one-time username-based validation procedure. Food pictures taken in JPEG format were downscaled to 1/10 their original size on the device itself to reduce data usage. The functionality of each app tab is shown in Fig S1. Push notifications were manually triggered at a random time of the day during the stated wakeful period of the subject and numbered 1–2 per day. The specific push notification based query presented to the subject is dependent on the time when he/she sees it, not when it was originally initiated from the server. The presented query and the response were recorded on the server.
The “Salk Metabolic Study” app was available from the Apple Appstore during the study period. To limit usage to subjects who provided a written informed consent, the app activation required a randomly generated unique activation key for each participant. Recently, the app was revised, upgraded, and re-written to run on the latest operating systems in iOS and Android devices under the name “myCircadianClock”. Under Salk IRB approved study the app is available to adults living in the US. Potential users can visit the website http://mycircadianclock.mycircadianclock.org/ to review the ongoing study objectives and consent document. If they consent to participate through electronic-consenting, they are sent an activation key to use the app.
Data annotation and analysis
Duplicate pictures were first automatically removed by comparing MD5 checksums, which serve as a fingerprint of a file. A second round of de-duplication was performed by manually inspecting the contents of sequentially received food/drink pictures. De-duplicated data were then independently annotated by two researchers, for multiple characteristics that describe each item. The items and estimated portion size were also looked up in FNDDS or CalorieKing website for estimated caloric content. A third researcher tallied the annotations and where discrepancies existed, all three individuals conferred and arrived at a consensus description. In recognition of the fact that lifestyles and work schedules are controlled more by conventional time than solar time, the time data shown herein referred to as ‘local time’ incorporate daylight savings time when applicable. In other words, according as daylight savings time was in effect (March–November) or not (November–March), a local time of 9.5 would refer to 9:30 am PDT or 9:30 am PST for an event recorded in California. Polar plots were generated using Mathematica 9 (Wolfram Inc.). All other plots and statistical analysis were prepared using Prism 5 (GraphPad Software).
Feedogram
To generate a feedogram raster plot, events were binned into 15-minute intervals starting at midnight. Corresponding to each such bin, if an event was recorded in it, the segment in the raster plot was colored black. If no event was present, the segment was colored gray if it is between 6 pm and 6 am, and yellow otherwise.
Supplementary Material
Research Highlights.
The daily eating pattern in healthy adults is highly variable from day to day
More than a half of the adults eat for 15 h or longer everyday
Sleep duration parallels the fasting duration
Reducing the daily eating duration can contribute to weight loss
Acknowledgments
We thank our research assistants Sami Michishita and Toriana Dabkowski for invaluable help with the data collection and annotation. This work was partially supported by NIH grants DK091618, EY016807, DK063491, AFAR grant #M14322, Leona M. and Harry B. Helmsley Charitable Trust’s grant #2012-PG-MED002, and Joe W. and Dorothy Dorsett Brown Foundation. S.G. was supported by H.A. and Mary K. Chapman Trust and Aginsky Research Scholar Award. Additional support came from the Glenn Center for Aging.
Footnotes
Author contribution: Both S.G. and S.P. conceived the idea, designed the experiments, analyzed data, and wrote the manuscript. S.G. wrote the smartphone application and server-side software, recruited subjects and collected data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamovich Y, Rousso-Noori L, Zwighaft Z, Neufeld-Cohen A, Golik M, Kraut-Cohen J, Wang M, Han X, Asher G. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell metabolism. 2014;19:319–330. doi: 10.1016/j.cmet.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell metabolism. 2011;13:125–137. doi: 10.1016/j.cmet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Bauer UE, Briss PA, Goodman RA, Bowman BA. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384:45–52. doi: 10.1016/S0140-6736(14)60648-6. [DOI] [PubMed] [Google Scholar]
- Blood ML, Sack RL, Percy DC, Pen JC. A comparison of sleep detection by wrist actigraphy, behavioral response, and polysomnography. Sleep. 1997;20:388–395. [PubMed] [Google Scholar]
- Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. European heart journal. 2011;32:1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- Carter MC, Burley VJ, Nykjaer C, Cade JE. Adherence to a smartphone application for weight loss compared to website and paper diary: pilot randomized controlled trial. Journal of medical Internet research. 2013;15:e32. doi: 10.2196/jmir.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P, Panda S. Time-Restricted Feeding Is a Preventative and Therapeutic Intervention against Diverse Nutritional Challenges. Cell metabolism. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copinschi G, Leproult R, Spiegel K. The important role of sleep in metabolism. Frontiers of hormone research. 2014;42:59–72. doi: 10.1159/000358858. [DOI] [PubMed] [Google Scholar]
- Gill S, Le HD, Melkani GC, Panda S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science. 2015;347:1265–1269. doi: 10.1126/science.1256682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder SA, Macy MW. Diurnal and seasonal mood vary with work, sleep, and daylength across diverse cultures. Science. 2011;333:1878–1881. doi: 10.1126/science.1202775. [DOI] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, Ditacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell metabolism. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell metabolism. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer FA, Seyfried TN, Varady KA, Panda S. Meal frequency and timing in health and disease. Proc Natl Acad Sci U S A. 2014;111:16647–16653. doi: 10.1073/pnas.1413965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22:939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- Roza AM, Shizgal HM. The Harris Benedict equation reevaluated: resting energy requirements and the body cell mass. The American journal of clinical nutrition. 1984;40:168–182. doi: 10.1093/ajcn/40.1.168. [DOI] [PubMed] [Google Scholar]
- Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012;26:3493–3502. doi: 10.1096/fj.12-208868. [DOI] [PubMed] [Google Scholar]
- Six BL, Schap TE, Kerr DA, Boushey CJ. Evaluation of the Food and Nutrient Database for Dietary Studies for use with a mobile telephone food record. Journal of food composition and analysis: an official publication of the United Nations University, International Network of Food Data Systems. 2011;24:1160–1167. doi: 10.1016/j.jfca.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora N, Kuperman Y, Biton I, Gilad S, Harmelin A, Shapiro H, Halpern Z, Segal E, Elinav E. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159:514–529. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci U S A. 2009;106:21453–21458. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell metabolism. 2014;20:1006–1017. doi: 10.1016/j.cmet.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.