Summary
The influenza nonstructural protein 1 (NS1) plays a critical role in antagonizing the innate immune response to infection. One interaction that facilitates this function is between NS1 and RIG-I, one of the main sensors of influenza virus infection. While NS1 and RIG-I are known to interact, it is currently unclear whether this interaction is direct or if it is mediated by other biomolecules. In the present study, we demonstrate a direct, strain dependent interaction between the NS1 RNA binding domain (NS1RBD) of the influenza A/Brevig Mission/1918 H1N1 (1918H1N1) virus and the second CARD domain of RIG-I. Solving the solution structure of the 1918H1N1 NS1RBD revealed features in a functionally novel region that may facilitate the observed interaction. The biophysical and structural data herein suggest a possible mechanism by which strain specific differences in NS1 modulate influenza virulence.
Graphical Abstract
Introduction
The influenza virus is a serious public health concern causing seasonal epidemics and sporadic pandemics that result in significant morbidity and mortality worldwide. Every year, unique strains of the influenza virus cause seasonal outbreaks that result in over 200,000 hospitalizations (Thompson et al., 2006) and approximately 35,000 deaths in the United States (Molinari et al., 2007). The annual impact on public health is largely determined by each strain’s ability to be spread from one host to another, termed transmissibility, and its relative ability to cause disease, or virulence. The sporadic pandemics caused by the influenza virus provide clear illustrations of how these factors can affect the severity of a particular strain. For instance, the H1N1 virus that caused the 1918 “Spanish” influenza pandemic (1918H1N1) infected one-third of the world’s population and resulted in approximately 50–100 million deaths worldwide (Taubenberger and Morens, 2006), illustrating both high transmissibility and virulence. This is in contrast to the 2009 H1N1 virus that caused the “Swine Flu” influenza epidemic that displayed high transmissibility and low virulence. While the interactions between viral and host proteins that underlie transmissibility have been extensively studied (Neumann and Kawaoka, 2015), our understanding of the strain specific interactions that underlie virulence is limited. Influenza viruses encode multiple proteins that contribute to virulence, including the proteins associated with the viral RNA dependent RNA polymerase (Graef et al., 2010) and the multifunctional non-structural protein 1 (Ayllon and Garcia-Sastre, 2014).
The non-structural protein 1 (NS1) is a 217–237 amino acid protein encoded by segment 8 of the influenza virus with an approximate weight of 26 kDa. NS1 is composed of two independently folding functional domains, the N-terminal RNA binding domain (NS1RBD) and the C-terminal effector domain (NS1ED). NS1 plays a critical role in modulating virulence by facilitating viral evasion of the host innate immune response (Ayllon and Garcia-Sastre, 2014). The importance of this function has been demonstrated by numerous studies showing that certain NS1 variants are capable of dramatically influencing the virulence of a particular strain (Ayllon et al., 2014; Dankar et al., 2013; Jackson et al., 2008; Spesock et al., 2011). Each domain contributes to this function by antagonizing the type I interferon response to viral infection via interactions with multiple host proteins. The NS1RBD, encoded by residues 1–73 of NS1, is an approximately 17 kDa obligate homodimer that adopts a novel six-helical RNA binding fold (Chien et al., 1997). Critical for its RNA binding function are the conserved arginine and lysine residues at positions 38 and 41 respectively. (Wang et al., 1999) Mutation of these residues abrogates the ability of the NS1RBD to bind RNA and greatly impedes the ability of the influenza virus to block interferon production upon infection (Pichlmair et al., 2006). One explanation for this observation is that a critical function of the NS1RBD is to sequester dsRNA from cellular machinery that induces the type I interferon expression upon detection of dsRNA in the cell. However, the relative low affinity of NS1RBD for dsRNA (Chien et al., 2004) makes this possibility less likely due to the high affinity of other cellular dsRNA binding proteins such as RIG-I, the main sensor of influenza virus infection in the host cell (Yoneyama et al., 2004). In addition to facilitating dsRNA binding to the NS1RBD, Arg 38 and Lys 41 have been shown to be involved in the interactions of NS1 with TRIM25 (Gack et al., 2009), RIPLET (Oshiumi et al., 2009), and RIG-I (Guo et al., 2007; Mibayashi et al., 2007; Opitz et al., 2007; Pichlmair et al., 2006). These interactions are vital to inhibiting the induction of the type I interferon response, an essential component of the innate immune response to influenza infection. This inhibition of the innate immune response occurs at the origin of the RIG-I signaling pathway. It has been previously shown that the NS1:RIG-I complex is detec by co-immunoprecipitation (Guo et al., 2007; Mibayashi et al., 2007; Opitz et al., 2007; Pichlmair et al., 2006); however, there is no clear evidence distinguishing the nature of the complex (i.e. whether the association is direct or mediated by other interactions). A more thorough understanding of this interaction is of particular importance due to RIG-I’s role as an innate sensor of RNA virus infection and activator of the type I interferon response (Yoneyama et al., 2004).
RIG-I is composed of two N-terminal caspase activation and recruitment domains (CARDs), three helicase domains (Hel 1, Hel 2, and Hel 2i), and a regulatory C-terminal domain (CTD). It functions by binding to viral RNAs possessing a 5′ triphosphate group (Schlee and Hartmann, 2010) which, in turn, induces a structural change in RIG-I that exposes the two N-terminal caspase activation and recruitment domains (CARD1 and CARD2) (Myong et al., 2009). This conformational change allows CARD2 of RIG-I to be ubiquitinated at Lys 172 by TRIM25 (Gack et al., 2009) and RIPLET (Oshiumi et al., 2009). The ubiquitinated CARDs of RIG-I then bind to the CARDs of the mitochondrial adaptor MAVS (mitochondrial antiviral signaling). This ultimately leads to the nuclear translocation of the transcription factors IRF3, AP-1(c-Jun/ATF-2) and NFκB which drive transcription of the type I IFN genes, thereby stimulating the innate anti-viral response to influenza infection (Jiang et al., 2011; Kowalinski et al., 2011). Furthermore, expression of the CARDs alone is adequate for inducing IFN-β production in vitro, underscoring their involvement in the host innate immune response. (Yoneyama et al., 2004) The interaction between RIG-I and the influenza NS1RBD suppresses proper RIG-I function, thereby dramatically decreasing IFN-β production (Guo et al., 2007; Mibayashi et al., 2007; Opitz et al., 2007; Pichlmair et al., 2006). This inhibition of the innate immune response allows the virus to replicate more efficiently and ultimately leads to a more severe disease state (Yoneyama et al., 2004).
We demonstrate in this study that the 1918H1N1 NS1RBD interacts directly with the second CARD domain of RIG-I (CARD2) using NMR chemical shift perturbation (CSP) analysis. Currently, the interface at which this interaction was observed on the 1918H1N1 NS1RBD has no function attributed to it. The identification of a functionally novel region of the NS1RBD underscores the importance of our observations. Strikingly, no interaction was observed when the same conditions were tested for the NS1RBD derived from the influenza A/Udorn/1972 (H3N2) virus (Udorn). This observation suggests that the interaction between the NS1RBD and CARD2 is dependent on the strain from which the NS1RBD is derived. Given the lack of sequence identity between the two NS1RBDs in the regions shown to interact with CARD2 and the number of chemical shifts observed, the solution structure of the 1918H1N1 NS1RBD was solved to identify structural differences that may facilitate the strain dependent interaction. Comparing the newly solved 1918H1N1 NS1RBD solution structure to the previously solved Udorn NS1RBD solution structure revealed several structural differences that may underlie the observed differences in the binding of RIG-I. These data suggest a direct, strain dependent interaction between RIG-I and the NS1RBD, highlighting a potential structural explanation for the observed differences in virulence between strains of influenza.
Results
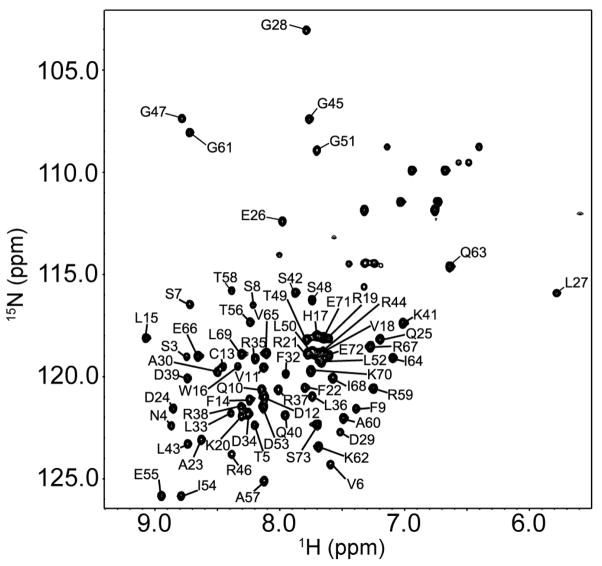
The N-terminal domain of the NS1 protein (residues 1–73) encodes the independently folding NS1RBD, an obligate homodimer that exhibits a wide range of RNA-binding activities (Ayllon and Garcia-Sastre, 2014). To investigate a possible direct interaction between RIG-I and the 1918H1N1 NS1RBD, it was first necessary to determine if the 1918H1N1 NS1RBD was amenable to NMR. The 1H-15N heteronuclear single quantum coherence (HSQC) spectrum shows a dispersed single set of peaks which is indicative of a well-folded protein (Figure 1). Because the NS1RBD is a symmetric homodimer, only 72 amide resonances (excluding prolines) were expected and ultimately observed, in addition to amides from the side chains of glutamine and asparagine. These data indicate that the 1918H1N1 NS1RBD is amenable to NMR analysis.
Figure 1.
1H-15N HSQC spectra of the 1918H1N1 NS1RBD with residue-specific backbone assignments indicated.
The second CARD domain of RIG-I binds directly to the 1918H1N1 NS1RBD
RIG-I, one of the main sensors of influenza virus infection (Yoneyama et al., 2004), plays an essential role in activating the type I interferon response to infection (Kato et al., 2006). CSP analysis was used to probe for a direct interaction between the 1918H1N1 NS1RBD and the second caspase activation and recruitment domain (CARD2) of RIG-I. NMR chemical shifts are exquisitely sensitive to atomic chemical environments, making it possible to determine protein-ligand interaction sites by identifying residues whose chemical shifts are perturbed upon the addition of a ligand (Foster et al., 1998). For this analysis, 1H-15N HSQC spectra for the 1918H1N1 NS1RBD alone and in the presence of unlabeled CARD2 were collected. The first step in the analysis was to obtain resonance assignments for the 1918H1N1 NS1RBD in the unbound state. High quality triple-resonance spectra allowed backbone resonance assignments to be obtained for 70 of the 72 (97%) possible assigned residues. Analysis of 13Cα chemical shifts (Wishart and Sykes, 1994) indicates that each monomer of the 1918H1N1 NS1RBD is composed of an α1-turn-α2-turn-α3 structure with the homodimer exhibiting the six-helical dimeric structure that is consistent with the previously solved Udorn NS1RBD solution structure (Chien et al., 1997).
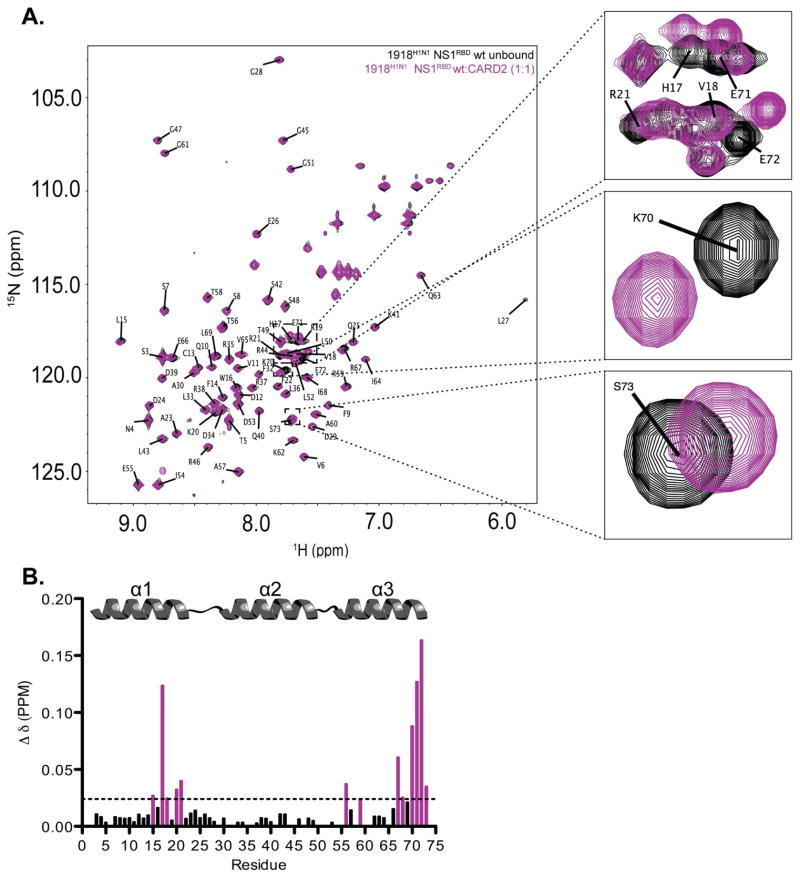
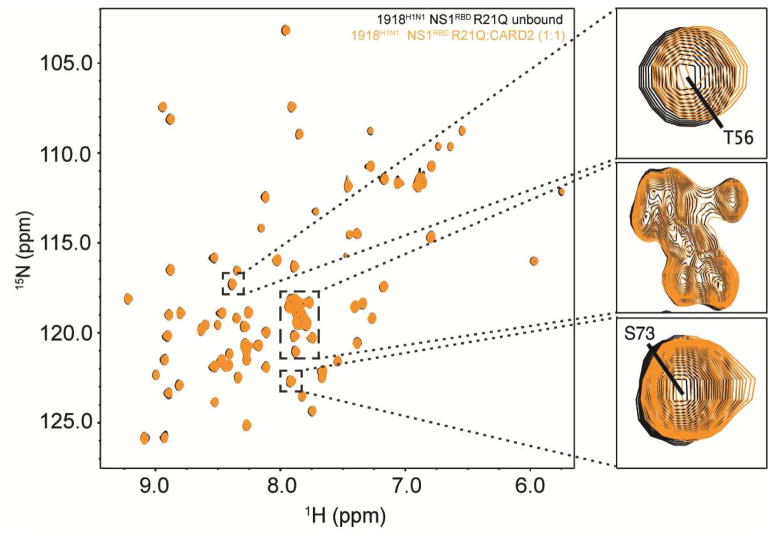
Upon addition of unlabeled CARD2, CSPs were observed for multiple residues when compared to the reference 1H-15N HSQC spectra of the 1918H1N1 NS1RBD alone (Figure 2A). Molar ratios above 1:1 resulted in precipitation of the protein sample. Therefore, a ratio of 1:1 was used in all CSP experiments as it gave the most robust chemical shifts. Normalized weighted average 1H and 15N shift differences were plotted as a function of residue position (Figure 2B). Statistical analysis of the weighted shift differences determined that several residues that exhibited significant perturbations were primarily localized in two regions: the α1 helix (residues 14 – 21) and the C-terminal α3 helix (residues 66 – 73) as determined by analysis of the 13Cα chemical shifts (Wishart and Sykes, 1994). The cutoff value used to determine statistically significant chemical shift differences ( ) was calculated using a previously published method that was determined to result in less than 4.5% false positives when discriminating between interacting and non-interacting residues (Schumann et al., 2007). Remarkably, the residues of the NS1RBD for which CSPs were observed have no previous function attributed to them. To validate chemical shift perturbation analysis, 2D 15N HSQC NOESY spectra were collected to further characterize the interaction between the 1918H1N1 NS1RBD and CARD2. We observed a significant decrease in the intensity of several cross-peaks in the 1918H1N1 NS1RBD:CARD2 spectra when compared to the spectra of the free 1918H1N1 NS1RBD (Figure S1). This decrease in peak intensity or, in some cases, disappearance all together is most likely due to increased relaxation caused by spin-diffusion contact with protons on CARD2. Using assignments obtained from our structure calculation, we determined that the weakened cross-peaks correlated with residues identified using chemical shift perturbation analysis of the 1918H1N1 NS1RBD in the presence of CARD2.
Figure 2. NMR chemical shift perturbations reveal an interaction between the 1918H1N1 NS1RBD and the second CARD domain of RIG-I (CARD2).
(A) Overlay of 1H-15N HSQC spectra of 1918 H1N1 NS1RBD (black) and 1918 H1N1 NS1RBD after adding unlabeled CARD2 (purple) at a 1:1 molar ratio. (B) Chemical shift perturbations were quantified and plotted on a per-residue basis. The dotted line represents the calculated cutoff (.024 ppm) with those resides experiencing shifts greater than the cutoff highlighted in purple (see Materials and Methods).
In addition, we were able to obtain an estimate of the binding affinity using non-denaturing gel electrophoresis. An estimated Kd of 42.82 ± 11.47 μM and 97.31 ± 26.26 μM were obtained using the relative differences in density and mobility of the 1918H1N1 NS1RBD band observed upon the addition of increasing concentrations of CARD2, respectively (Figure S2). Although Kd is not necessarily a reliable determinant of biological relevance, our measured Kd value is consistent with other interactions between viral and cellular proteins that are known to be biologically relevant (Briknarova et al., 2011; Garrus et al., 2001; Yoo et al., 1997). Based on these results, we conclude that there is a direct interaction between the NS1RBD derived from the 1918H1N1 influenza virus and the CARD2 of RIG-I at a functionally novel interface. Interfering with the proper function of CARD2 through this interaction could effectively abrogate the INF-β response, thereby facilitating influenza’s circumvention of the host cell’s innate immune response (Yoneyama et al., 2004). Further studies will be needed in order to fully understand the functional consequences of this interaction.
The second CARD domain of RIG-I does not bind to the Udorn NS1RBD
It is becoming increasingly clear that many of the properties attributed to the NS1 protein are strain dependent. These properties include but are not limited to intracellular localization, virulence, post-translational modification, and evasion of the innate immune response (Ayllon and Garcia-Sastre, 2014). This quality of NS1 is reflected by its remarkable genetic plasticity when compared to other influenza viral proteins (Heaton et al., 2013). We therefore sought to determine if the observed interaction between the 1918H1N1 NS1RBD and CARD2 is dependent on the strain of influenza from which the NS1RBD is derived. The Udorn strain was chosen due to the availability of a high-resolution solution structure (Chien et al., 1997), making comparison of any differences more efficient, as well as the use of this strain in numerous studies of influenza infection. As with the 1918H1N1 NS1RBD, it was first necessary to express and purify the NS1RBD derived from the Udorn strain of influenza. Once a high quality 1H-15N HSQC spectra was recorded (data not shown), high quality three-dimensional spectra allowed us to obtain resonances assignments for 71 of the 72 (99%) of the possible residues. It was determined, using 13Cα chemical shift analysis (Wishart and Sykes, 1994), that the expressed and purified Udorn NS1RBD possessed the same secondary structure observed in the previously solved solution structure.
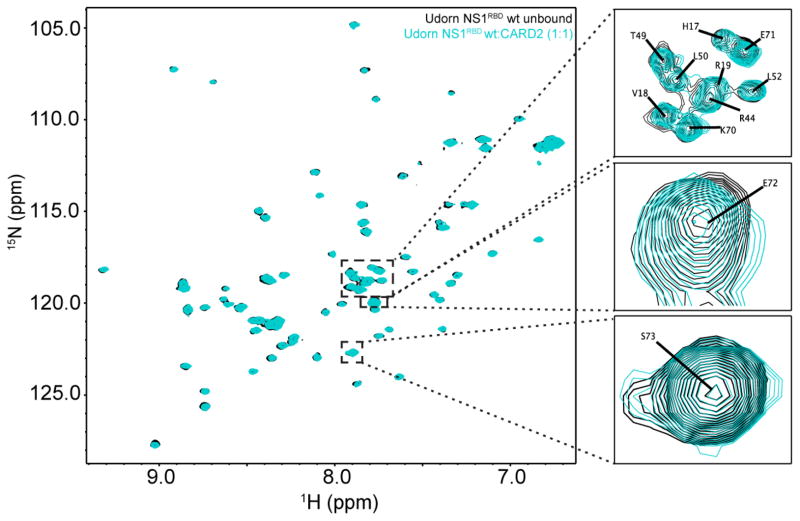
To determine if the Udorn NS1RBD interacts directly with CARD2, we performed similar 1H-15N HSQC based CSP experiments. For these experiments, 1H-15N HSQC spectra were collected for the 1972 NS1RBD in the unbound state and in the presence of unlabeled CARD2. An overlay of the unbound 1972 NS1RBD spectra and the 1972 NS1RBD:CARD2 spectra revealed that upon addition of CARD2, no residues in the Udorn NS1RBD appeared to be perturbed (Figure 3). To ensure the absence of significant CSPs, normalized weighted average 1H and 15N shift differences were plotted on a per residue basis. No significant perturbations were observed that were greater than the resolution of the 1H-15N HSQC spectra recorded (data not shown). The lack of significant perturbations demonstrates that there is no discernible interaction between the Udorn NS1RBD and CARD2, confirming that the interaction between the NS1RBD and CARD2 is strain dependent.
Figure 3. NMR chemical shift perturbation demonstrates no discernable interaction between the Udorn NS1RBD and the second CARD domain of RIG-I.
Overlay of 1H-15N HSQC spectra of Udorn NS1RBD (black) and in the presence of CARD2 (cyan) indicate no chemical shift perturbations upon the addition of unlabeled CARD2 at a 1:1 molar ratio.
Solution structure of the 1918H1N1 NS1RBD
Sequence alignment of the 1918H1N1 and Udorn NS1RBD identified changes in the α3 helix (Figure 4) as well as residues observed to be involved in the interaction with CARD2 (R21Q and R67K). To elucidate a possible structural explanation for the strain specific interaction observed, the solution structure of the 1918H1N1 NS1RBD was solved and compared to the previously solved structure of the Udorn NS1RBD. An assortment of 2D and 3D NMR experiments, including measurements of residual dipolar couplings (RDCs), were used to solve the solution structure. The agreement of the structures with experimental RDCs is shown in Table S1 and the structure statistical quality indicators are found in Table S2. The similar values of the R factors between measured and calculated 1DNH and 1DCαHα RDCs of the monomeric subunits and the dimeric state in both alignment media confirmed that the two mainly rigid monomeric backbone structures are accurately oriented relative to one another in the homodimer (data not shown). The magnitude and rhombicity of the alignment tensors and the RDC agreement with the NMR structures are included in Table S1.
Figure 4. Alignment of the 1918H1N1 and Udorn NS1RBD.
Residues that are not conserved between the two strains are outlined in grey.
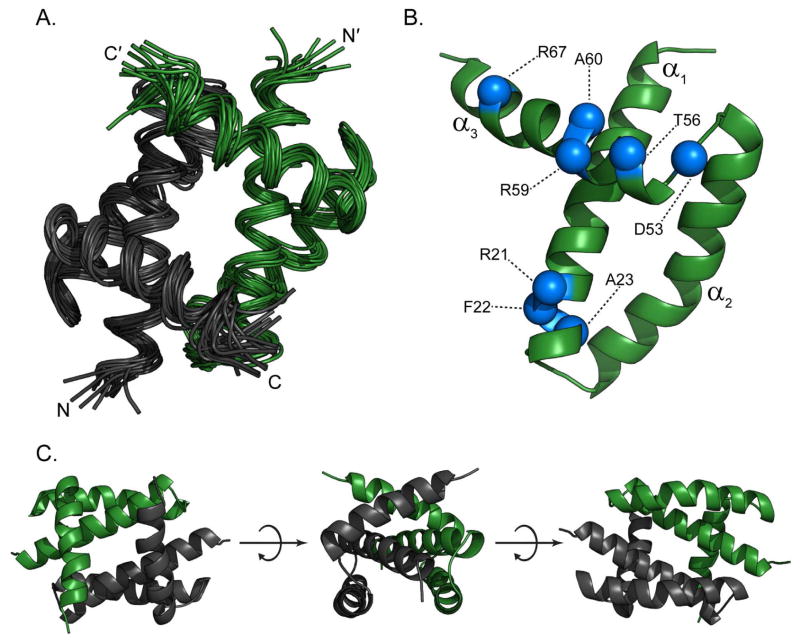
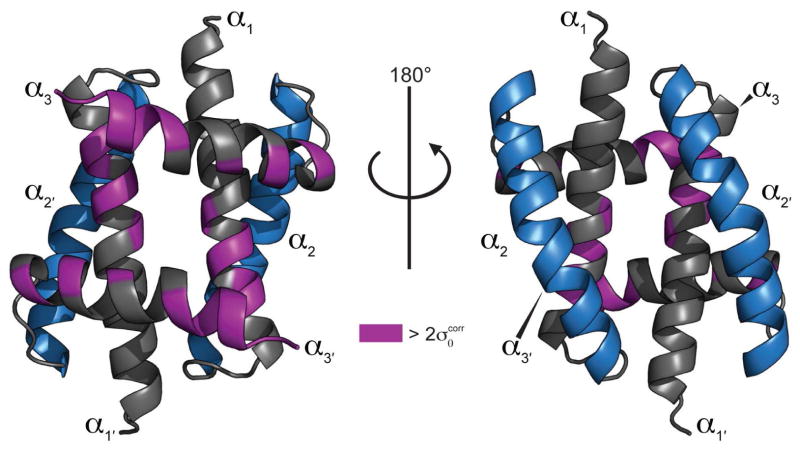
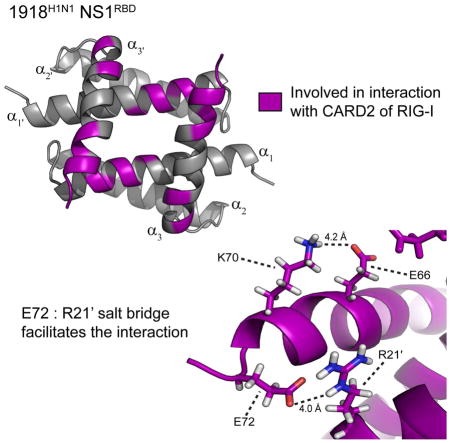
Consistent with the Udorn NS1RBD structure and the 13Cα analysis above, the structure of the 1918H1N1 NS1RBD is a homodimer that adopts a six-helix antiparallel bundle (Figure 5A). As previously noted, there are several differences in amino acid sequence when comparing the 1918H1N1 and Udorn NS1RBDs (Figure 5B). Mapping the observed CSPs on to the solved 1918H1N1 NS1RBD structure revealed that the residues involved in the interaction were localized to a small portion of the α1 helix and a much larger portion of the α3 helix (Figure 6). This structure map illustrates that the 1918H1N1 NS1RBD:CARD2 interaction occurs via a novel binding interface that is localized to the surface opposite to that of the RNA binding interface.
Figure 5. Solution NMR structural studies of 1918H1N1 NS1RBD.
(A) Ribbon diagram of the 16 energy-minimized conformers that represent the NMR structure of the 1918H1N1 NS1RBD (1–73) homodimer. The two monomers are shown in black and green respectively with the N- and C-termini labeled for each. (B) Monomeric structure with residues that are mutated when comparing the 1918H1N1 NS1RBD to the Udorn NS1RBD indicated in blue. (C) Multiple views of the 1918 H1N1 NS1RBD indicating that it retains the canonical six-helical fold demonstrated by previously solved solution structure of influenza Udorn NS1RBD.
Figure 6. Chemical shift perturbations induced by the addition of CARD2 reveal a functionally novel region of NS1RBD.
Perturbations are mapped onto the lowest energy structure of the 1918 H1N1 NS1RBD with shifts greater than in purple. Helices known to be involved in RNA binding (blue) exist opposite to the identified CARD2 binding interface.
Insight into the structural basis for the strain specific interaction between 1918H1N1 NS1RBD and RIG-I
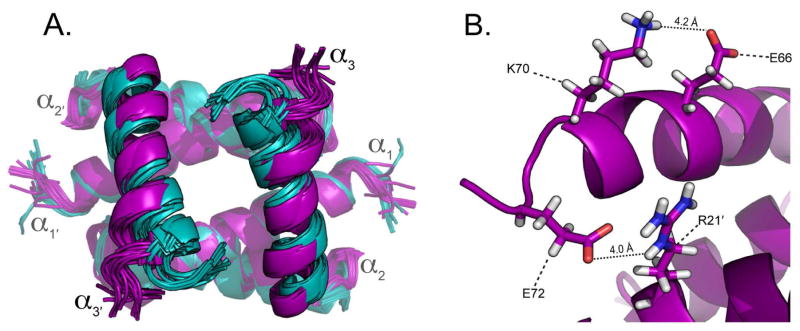
Given our data regarding CARD2 binding, we sought to determine what structural features may account for the observed strain specificity of this interaction. To accomplish this, the α3 and α3′ helices of the 1918H1N1 NS1RBDs were analyzed to compare their relative backbone and side-chain spatial orientations. Examination of the individual structures revealed that the orientation of 1918H1N1 NS1RBD α3 and α3′ helices is altered when compared to that of the Udorn NS1RBD. Alignment of the structural model ensembles reveals significant variation in the spatial arrangement of the α3 and α3′ helices when comparing the two strains (Figure 7A). Further analysis of the α3 and α3′ helices revealed two potential salt bridges that are present in the 1918H1N1 NS1RBD but absent in the Udorn NS1RBD. The potential salt bridges are between Glu 66 : Lys 70 and Arg 21′ : Glu 72 of the 1918H1N1 NS1RBD (Figure 7B). The absence of these potential salt bridges in the Udorn NS1RBD appears to be due to a single residue change from Arg 21 in the 1918H1N1 strain compared to Gln 21 in the Udorn strain. It is therefore likely that the Arg 21′ : Glu 72 salt bridge is necessary for the formation of the salt bridge between Glu 66 and Lys 70. The potential salt bridges alter the relative positions of the α3 and α3′ helices in the 1918H1N1 NS1RBD that are involved in the interaction with CARD2, providing a potential structural explanation for the strain specific nature of this interaction. Analysis of the 13C NOESY spectra offers two lines of evidence that support the presence of the Arg 21′ : Glu 72 salt bridge. First, an NOE peak is observed from the perspective of Arg 21 Hδ3 to Glu 72 Hβ2 and from the perspective of Glu 72 Hβ2 to Arg 21 Hδ3 (data not shown) indicating that these two atoms are in close proximity. Second, the overall position of Glu 72 in the lowest energy structure parallels Leu 69. NOEs are present from the perspective of Glu 72 Hα, Hβ2, Hβ3, and Hγ2 to the Leu Hα (data not shown). If the orientation were different (e.g. Glu 72 pointing away from Arg 21), these observed NOEs between Leu 69 and Glu 72 would not be visible. Taken together, the presence of the NOE peaks are highly suggestive of the presence of a salt bridge between Arg 21′ and Glu 72.
Figure 7. Prospective salt bridge alters the orientation of α3 and α 3′ and may facilitate strain specificity.
(A) Alignment of the ensemble Udorn NS1RBD (cyan) and 1918H1N1 NS1RBD (purple) structures. (B) Ribbon diagram of the NS1RBD with residues involved in the potential salt bridges labeled and the distances between them indicated.
To validate the hypothesized functional role of Arg 21, we expressed and purified a mutant 1918H1N1 NS1RBD that incorporated a Gln at position 21 as is found in the 1972H3N2 Udorn strain. CSP analysis was then performed using the R21Q mutant protein upon addition of CARD2. As seen in Figure 8, there are no significant perturbations observed when comparing the 1H-15N HSQC spectra of the mutant in the presence of CARD2 to the reference spectra. However, there are minute perturbations observed for residues previously shown to be involved in the interaction between the NS1RBD and CARD2. This indicates that the interaction between the NS1RBD and CARD2 may not be completely abrogated by the R21Q mutation but severely inhibited. Furthermore, we expressed and purified a mutant Udorn NS1RBD that incorporated an Arg at position 21 to determine if CARD2 binding could be rescued by mutating Gln 21 to Arg in the NS1RBD derived from the Udorn strain of influenza. CSP analysis indicated that there is no discernable interaction between the Udorn Q21R mutant and CARD2 (Figure S3). In conclusion, the 1918H1N1 R21Q and Udorn Q21R mutant data indicate that the presence of an Arg at position 21 is necessary but not sufficient to facilitate the interaction between the NS1RBD and CARD.
Figure 8. NMR chemical shift perturbation demonstrates no discernable interaction between the 1918H1N1 NS1RBD R21Q and the second CARD domain of RIG-I.
Overlay of 1H-15N HSQC spectra of 1918H1N1 NS1RBD R21Q (black) and in the presence of CARD2 (green) indicate no chemical shift perturbations upon the addition of unlabeled CARD2 at a 1:1 molar ratio.
Quantitative analysis reveals statistically significant differences in the relative positions of the α3 and α3′ helices
Although the differences in the surface models are apparent, we sought to quantitatively analyze the variation of the spatial arrangements between the two strains. To accomplish this, the atomic coordinate information was extracted for each atom of the lowest energy structure for the 1918H1N1 and Udorn NS1RBDs. Using these data, the intramolecular distances were determined between homologous atoms in the 1918H1N1 and Udorn α3 and α3′ helices (Ile 54 – Ser 73). Measurements were only made between residues that are conserved between both strains (Figure 9A) to ensure that these data were not biased by differences in side chain lengths between the strains. Several different measurements were made to ensure that none of them were biased to any particular set of atoms. These measurements include Cα atoms only, backbone atoms, side chain atoms, and all atoms. Each measurement revealed that the average intramolecular distance between the α3 and α3′ helices were significantly greater for the 1918H1N1 when compared to the Udorn strain. As illustrated in Figure 9, the average differences were calculated to be 2.1 Å for Cα atoms (p = 0.0483), 1.9 Å for backbone atoms (p < 0.0001), 1.6 Å for side chain atoms (p < 0.0001), and 1.8 Å for all atoms in the α3 and α3′ helices (p < 0.0001). It should be noted that because the measurements took into account the entire α3 and α3′ helices and not the subset of residues on the helices that undergo CSP, the average distances calculated are a conservative estimate. For example, if only the residues that undergo CSP are used in the calculation of intramolecular Cα distances, the average distance increases from 2.1 Å to 3.8 Å. These data support the view that the potential salt bridge in the 1918H1N1 strain may facilitate the interaction with CARD2 by altering the relative positions of the α3 and α3′ helices when compared to the Udorn strain.
Figure 9. Quantitative structural analysis reveals differences in the relative spatial arrangements of the α3 and α3′ helices from the 1918 H1N1 and Udorn NS1RBDs.
(A) Clustal alignment of the 1918H1N1 and Udorn NS1RBDs with residue differences indicated in grey. (B–D) Comparison of the distances between the 1918H1N1 α3 and α3′ Cα atoms (B), atoms composing the peptidyl backbone (C), atoms composing the side chains (D), and all atoms (E) demonstrate a significant difference in the average distance between the α3 and α3′ helices from the in the 1918H1N1 NS1RBD compared to the Udorn NS1RBD.
To accompany these data, RDC analysis was used to further confirm structural differences between the two strains. RDC measurements provide information about the orientation of the bond vector of interest relative to the external magnetic field. By comparing observed RDC measurements for the 1918H1N1 NS1RBD to back-calculated RDCs using the previously solved Udorn NS1RBD solution structure, it can be determined if there are any bond vector orientations that significantly deviate between the two structures (data not shown). When fitting the 1DNH/1DCαHα and 1DCαHα RDC measurements made using 5% C12E5 polyethylene glycol/hexanol mixture (0.96 surfactant/alcohol molar ratio) (Ruckert and Otting, 2000) and negatively charged acrylamide gel stretched from 4.2 to 5.4 mm (Ulmer et al., 2003) as alignment media, the root mean square differences (RMSDs) were calculated to be 4.0 Hz and 2.5 Hz, respectively. To ensure a robust comparison, only differences between experimental and structure derived RDCs of twice the RMSD (95% confidence interval) were used to identify differences between the backbone structures. The RDCs back calculated from the Udorn strain that are significantly different (using this criterion) from those measured for 1918H1N1 NS1RBD solution structure correspond to Asp 2, Ser 3, His 17, Gln 25, Leu 69, Lys 70, Glu 71, and Glu 72. This observation supports the hypothesis that the potential salt bridge between Arg 21′ and Glu 72 in the 1918H1N1 NS1RBD alters the orientation of the α3 and α3′ helices when compared to the Udorn strain and that this deviation may play a role in the strain specificity of the CARD2 interaction. Furthermore, the structural difference observed in His 17 between the two structures may indicate a role in the interaction. This idea is supported by the magnitude of the observed CSP of His 17 upon addition of CARD2 (Figure 2B) and the prominent role histidine residues are known to play in protein-protein interactions (Liao et al., 2013). Mutating His 17 to an Ala resulted in abrogation of the interaction with CARD2, although this mutation may have destabilized the 1918H1N1 NS1RBD resulting in a partially unfolded protein (Figure S4) making it difficult to draw any robust conclusions from the data.
Discussion
Here we report a novel binding surface that may facilitate a strain dependent interaction between the NS1RBD and the CARD2 of RIG-I. Although we observed a direct interaction between the 1918H1N1 NS1RBD and CARD2 using NMR CSP analysis, no discernable interaction was observed when assessing the NS1RBD derived from the Udorn strain of influenza. Analysis of the solution structure revealed the presence of two potential salt bridges between Glu 66 : Lys 70 and Arg 21′ : Glu 72 of the 1918H1N1 NS1RBD. The previously solved structure of the Udorn NS1RBD did not possess either of these potential salt bridges possibly due to a single point mutation at position 21 (Arg 21 for 1918H1N1 and Gln 21 for Udorn). Additional analysis of the variation in the intramolecular distances between the α3 and α3′ helices indicated that the deviation between the two strains was statistically significant. RDC analysis using the previously solved Udorn NS1RBD solution structure confirmed structural deviations in the α3 and α3′ helices between the two strains for residues involved in and adjacent to the potential salt bridge (Leu 69 – Glu 72) in the 1918H1N1 NS1RBD. Taken together, these data suggest that the interaction between the NS1RBD and CARD2 is strain specific and may be facilitated by the novel potential salt bridge observed in the 1918H1N1 NS1RBD structure. Indeed, mutating the Arg at position 21 in the 1918H1N1 NS1RBD to a Gln resulted in abrogation of the interaction between the NS1RBD and CARD2, further illustrating the selectivity of this interaction.
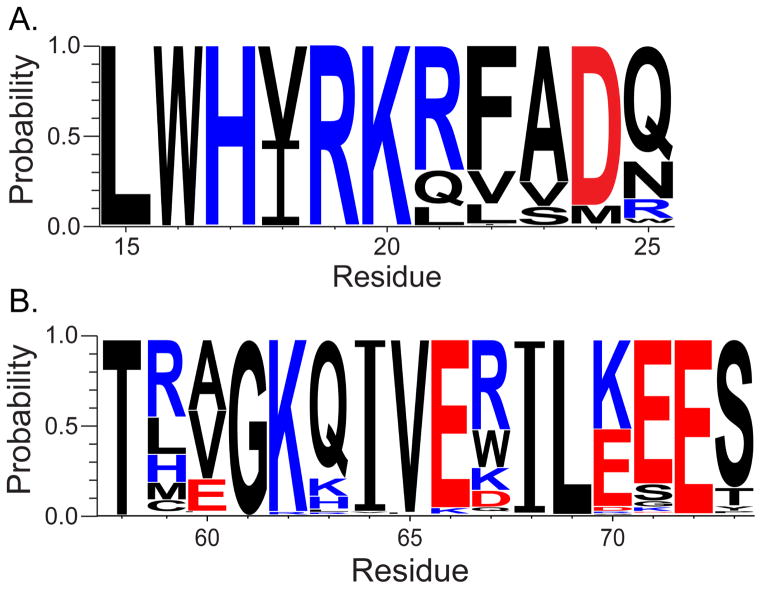
Genetic analysis of the region shown to interact with CARD2 reveals evolutionary conserved residues that may play a role in suppression of the innate immune response. Of particular interest to our study are positions that may form the salt bridge between Arg 21′ : Glu 72 of the 1918H1N1 NS1RBD that stabilize the α3 and α3′ helicies. While Glu 72 is conserved among all influenza viruses, it was determined that ~69% of influenza viruses encode an Arg at position 21 with Gln and Leu being found at position 21 in ~20% and ~11% of all influenza viruses, respectively (Figure 10). This conservation may have biological implications in the form of suppression of the IFN-β response. Indeed, a study in 2010 looked at which strains blocked IFN-β and IRF3 induction (Kuo et al., 2010). Of the strains that were unable to block IFN-β and IRF3 induction, only 10% contained an Arg at position 21. In contrast, 60% of strains containing an Arg at position at 21 were able to block IFN-β and IRF3 induction (Kuo et al., 2010). These data suggest that while an Arg at position 21 is not required for NS1 to inhibit IFN-β induction, the presence of Arg 21 may augment this function of NS1. One of our hypotheses presented in the paper is that other domains of RIG-I interact with NS1 and that the interaction between the NS1RBD and CARD2 further stabilizes the NS1:RIG-I complex. This hypothesis is supported by the data suggesting that Arg 21 enhances the inhibition of IFN-β induction but is not required. However, the lack of data regarding specific interactions between NS1 and RIG-I prevent us from drawing any robust conclusions regarding prediction of the interaction from amino acid sequence. An additional caveat that may prevent predictions from amino acid sequences alone is the presence of compensatory mutations that modulate suppression of IFN-β induction. It has been shown that although NS1 inhibits the innate immune response, the precise mechanism by which it achieves this suppression is strain specific and involves various pathways.
Figure 10. Sequence logo displaying the conservation of residues in the regions of the NS1RBD shown that interact with CARD2.
Sequence alignment was performed on all NS1 sequences (8,828) found in the influenza database (www.fludb.org). This figure was generated using WebLogo (Crooks et al., 2004).
As indicated previously, studies have shown that RIG-I exhibits an interaction with NS1 that is critical for abrogating the innate immune response and may augment influenza virulence. However, no study conducted to date has ruled out or confirmed a direct interaction between the influenza NS1 and RIG-I. Our observation of a direct interaction between two domains of these proteins allows for a more thorough understanding of the molecular interactions that may modulate virulence. Our data supports and expands on previous work that has demonstrated the importance and presence of the interaction for the PR8 strain of influenza (A/Puerto Rico/1934) by revealing a molecular interaction that may be critical for the interaction between the NS1 and RIG-I. This interaction may abrogate RIG-I function in a number of ways. One possible scenario is that the NS1RBD masks Lys 172 of CARD2 and prevents its ubiquitination by TRIM25 (Gack et al., 2009) and RIPLET (Oshiumi et al., 2009) thereby inhibiting the signal cascade that leads to expression of type I IFN, a necessary component of the innate immune response. Another possibility is that interaction between NS1RBD and CARD2 may prevent the structural rearrangement of RIG-I necessary for activating the downstream elements that induce the innate immune response. While the scope of the current study pertains exclusively to the interaction between CARD2 and the NS1RBD, the presence of direct interactions between other domains of RIG-I and either the NS1RBD or NS1ED derived from additional strains of influenza cannot be ruled out. For example, the interaction between CARD2 and NS1RBD may stabilize a larger complex involving NS1, RIG-I, and other biomolecules which, in turn, allows efficient abrogation of the innate immune response. Furthermore, the presence or absence of this stabilization may modulate each strain’s capability to abrogate the type I interferon response, which may ultimately provide a more thorough understanding of the virulence potential of a particular strain. Ongoing studies are currently underway to further characterize and understand the interaction between NS1 and RIG-I at the molecular level.
Strain dependent functions of NS1 and their potential impact on virulence is pertinent to interpreting and understanding the data presented. The concept of modulating influenza virulence via strain dependent interactions involving NS1 and host cellular proteins has been previously documented. Specifically, the interaction between the NS1ED and the 30 kD subunit of the cleavage and polyadenylation specificity factor (CPSF30) (Das et al., 2008; Nemeroff et al., 1998) has been studied in a number of strains of influenza. By sequestering CPSF30, a key component of the mammalian 3′-end mRNA processing machinery (Li et al., 2001), NS1 down-regulates global host gene expression and antagonizes the type I interferon response (Dankar et al., 2013; Kochs et al., 2007; Twu et al., 2007). This interaction was first discovered and structurally characterized using the Udorn strain of influenza (Das et al., 2008). However, subsequent studies have determined that other strains such as the pandemic 2009 H1N1 and the highly pathogenic 2013 H7N9 strains do not interact with CPSF30 (Ayllon et al., 2014; Hale et al., 2010). Additionally, highly pathogenic H5N1 strains that have circulated in China since 1996 have shown variable CPSF30 binding capability (Dankar et al., 2013; Spesock et al., 2011). The NS1ED:CPSF30 interaction has been established as a virulence determinant for multiple strains of influenza. Thus, the NS1ED:CPSF30 interaction marks an example of NS1 function that is not only strain dependent but is also capable of modulating virulence. Our results provide further support for the presence of strain specific interactions with host proteins that have the potential to modulate influenza virulence.
Experimental Procedures
Cloning, Protein Expression, and Purification
The RNA binding domains of the 1918H1N1 and Udorn influenza non-structural protein 1 (NS1) gene were subcloned into a 6X His-SMT3 fusion T7 expression vector (pE-SUMO; Life Sensors) using primers designed to amplify residues 1–73 of the full-length plasmids of 1918H1N1 and Udorn influenza NS1 (Biomatik). For the second caspase activation and recruitment domain (CARD2) of the retinoic acid inducible gene I (RIG-I), primers designed to amplify to residues 90–188 of the full-length plasmid of the RIG-I protein (Biomatik) were used to subclone CARD2 into the 6X His-SMT3 fusion T7 expression vector (pE-SUMO; Life Sensors). For the 1918H1N1 and Udorn NS1RBD samples, expression plasmids were transformed into DE3 Star BL21 Escherichia coli cells and grown in minimal media supplemented with 15NH4Cl and/or D–glucose (U–13C6–99%) depending on the desired labeling scheme. The NS1RBDs were induced at an OD600 of 0.6 with 1mM IPTG for 24 hrs at 25°C while CARD2 was induced at an OD600 of 0.6 with 0.1 mM IPTG for 18hrs at 18°C. Typical yields were 18 mg/L and 20 mg/L with >95% purity, as verified by SDS-PAGE (data not shown), for the NS1RBDs and CARD2 respectively.
NMR Spectroscopy
NMR experiments were carried out at 25°C using Bruker Avance III spectrometers equipped with TCI cryoprobes operating at 600 Mhz and 850 Mhz 1H frequencies. All NMR data were processed using NMRPipe (Delaglio et al., 1995) and analyzed using NMRView (Johnson and Blevins, 1994) complied on Linux workstations. Backbone 1H, 13C, and 15N resonances were assigned using standard triple-resonance assignment experiments. Side chain resonance assignments were obtained by manual analysis of 3D hCCH-TOCSY, hCCH-COSY, HNHA, and HCC(CO)NH experiments. Aromatic resonances were assigned using the 2D [1H-1H]-COSY, 2D [1H-1H] TOCSY, and 2D [1H-1H]-NOESY experiments with samples solvated in 100% D2O.
Residual Dipolar Coupling
All isotropic NMR data were acquired at 25°C on a 300 μl sample at pH 6.0 containing 1.0 mM [U-15N; U-13C]-RBD, 300 mM ammonium acetate, 2.2 mM EDTA, 0.15% NaN3 in 93% H2O and 7% D2O. Similar samples of 0.3 or 0.6 mM [U-15N; U-13C]-RBD, supplemented with 5% C12E5 polyethylene glycol (Fluka)/hexanol mixture (0.96 surfactant/alcohol molar ratio)(Ruckert and Otting, 2000) or aligned in a negatively charged acrylamide gel stretched from 5.4 to 4.2 mm (Ulmer et al., 2003), respectively, were used to record 1DNH and 1DCαHα residual dipolar couplings (RDCs). HN and CαHα couplings were measured from 2D ARTSY (Fitzkee and Bax, 2010) and 3D HCA(CO)N antiphase 1H-coupled in the 13Cα dimension spectra, respectively.
Structure calculation
The Cyana program with automatic NOE assignments was used to produce initial folds. Subsequently these were refined in Xplor-NIH with radius of gyration and RDC constraints. The final deposited structures were additionally refined in the new implicit solvation potential (EEFx) of Xplor-NIH. (Tian et al., 2014) Distance restraints of 1.9 Å and 2.9 Å per involved pair of residues were used to represent hydrogen bonds for HN-O and N-O, respectively (Wüthrich, 1986). NOE peak intensities in 3D NOESY spectra were automatically assigned using Cyana and converted for Xplor-NIH into a continuous distribution of 1133 approximate interproton distance restraints, with a uniform 40% distance error applied to take into account spin diffusion. All monomeric constraints were symmetrically duplicated for dimer refinement. The Cyana upper limit distance restraints were scaled (by a 0.82 factor) so that the converted Xplor-NIH NOE distance restraints reach a 6.3 Å maximum (including a 40% error to account for spin diffusion). The Cyana dimeric model was obtained using approximately 20 inter-subunit manual NOE assignments. We noted a major structural distortion in the Cyana generated caboxyl terminal helix (α3/α3′), which was bent at the middle by about 60 degrees. The Xplor-NIH RDC refined structures show a straight carboxyl terminal helix.
Structure calculations and refinement made use of the torsion angle molecular dynamics and the internal variable dynamics modules of Xplor-NIH (Schwieters et al., 2003) to ensure preservation of the correct peptide geometry when applying RDC and distance constraints simultaneously. The X-PLOR non-crystallographic symmetry potential term was included to maintain identical structure of the monomeric subunits. PyMol (Delano Scientific, LLC) and VMD-XPLOR (Schwieters and Clore, 2001) were used to analyze the structures. Neither the final set of calculated structures nor the subset of lowest energy structures (i.e. 20 out of 100) that were selected for further solvation refinement showed any consistent (i.e. in more than 17% of the calculated structures) NOE violations larger than 0.5 Å.
Chemical Shift Perturbation
Interactions between the NS1RBD (1918H1N1 and Udorn) and CARD2 of RIG-I were assessed using 1H-15N HSQC based CSP experiments. For these experiments, we collected 1H-15N HSQC spectra from the NS1RBD of interest in the unbound state and in the presence of CARD2. When comparing the two spectra the changes in chemical shift resonances were calculated using the weighted combination of chemical shifts given by:
The cutoff value used to determine statistically significant chemical shift differences ( ) was calculated using a previously published method. This method was determined to result in less than 4.5% false positives when discriminating between interacting and non-interacting residues (Schumann et al., 2007). These experiments were performed in CSP buffer [150 mM ammonium acetate, 2.2 mM EDTA, and .15% sodium azide (pH 6.0)].
Quantitative analysis of structural deviation
To assess structural variation, we used the lowest energy structures for the 1918H1N1 and the Udorn (PDB: 1NS1) NS1RBD solution structures. Coordinates for each atom were extracted from each solution structure and used for obtaining distance information. Atomic intramolecular distances between the α3 and α′3 helices (Ile 54 – Ser 73) were measured by using the following equation:
The mean average intramolecular for each strain was calculated. When assessing intramolecular differences between the α3 and α′3 helices, only residues conserved between the two strains were considered to avoid biasing any data by varying lengths of side chains. The resultant data were analyzed using the Student’s T-Test and all data are represented as the mean ± SEM.
Supplementary Material
Highlights.
Observation of a direct interaction between the influenza NS1 protein and RIG-I.
The interaction is dependent on the strain of influenza from which NS1 is derived.
Solved the structure of the NS1RBD from the 1918 “Spanish Influenza” virus.
Analysis of the solution structure reveals a functionally novel region of NS1.
Acknowledgments
We thank Ronald Shin (UAB) and Charles Schwieters (Division of Computational BioScience of the Center for Information Technology, NIH) for their technical assistance. We also thank Anthony B. Law and Paul J. Sapienza for helpful discussions. The Bruker 850 MHz and 600 MHz magnets used herein were funded by NCI Grant 1P30 CA-13148, NCRR Grant 1S10 RR022994-01A1, and NCI Grant 1P30 CA-13148. This work was funded by American Cancer Society Grant IRG-60-001-53 (C.M.P.) and NIH P41GM103399 (G.C. and C.C.C).
Footnotes
Author Contributions: A.S.J. and C.M.P. designed research; A.S.J., G.C., and C.M.P. performed research; A.S.J., A.B.K., G.C., C.C.C., and C.M.P. analyzed data; and A.S.J., A.B.K., and C.M.P. wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ayllon J, Domingues P, Rajsbaum R, Miorin L, Schmolke M, Hale BG, Garcia-Sastre A. A Single Amino Acid Substitution in the Novel H7N9 Influenza A Virus NS1 Protein Increases CPSF30 Binding and Virulence. Journal of virology. 2014 doi: 10.1128/JVI.01567-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayllon J, Garcia-Sastre A. The NS1 Protein: A Multitasking Virulence Factor. Current topics in microbiology and immunology. 2014 doi: 10.1007/82_2014_400. [DOI] [PubMed] [Google Scholar]
- Briknarova K, Thomas CJ, York J, Nunberg JH. Structure of a zinc-binding domain in the Junin virus envelope glycoprotein. The Journal of biological chemistry. 2011;286:1528–1536. doi: 10.1074/jbc.M110.166025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien CY, Tejero R, Huang Y, Zimmerman DE, Rios CB, Krug RM, Montelione GT. A novel RNA-binding motif in influenza A virus non-structural protein 1. Nature structural biology. 1997;4:891–895. doi: 10.1038/nsb1197-891. [DOI] [PubMed] [Google Scholar]
- Chien CY, Xu Y, Xiao R, Aramini JM, Sahasrabudhe PV, Krug RM, Montelione GT. Biophysical characterization of the complex between double-stranded RNA and the N-terminal domain of the NS1 protein from influenza A virus: evidence for a novel RNA-binding mode. Biochemistry. 2004;43:1950–1962. doi: 10.1021/bi030176o. [DOI] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome research. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankar SK, Miranda E, Forbes NE, Pelchat M, Tavassoli A, Selman M, Ping J, Jia J, Brown EG. Influenza A/Hong Kong/156/1997(H5N1) virus NS1 gene mutations F103L and M106I both increase IFN antagonism, virulence and cytoplasmic localization but differ in binding to RIG-I and CPSF30. Virology journal. 2013;10:243. doi: 10.1186/1743-422X-10-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K, Ma LC, Xiao R, Radvansky B, Aramini J, Zhao L, Marklund J, Kuo RL, Twu KY, Arnold E, et al. Structural basis for suppression of a host antiviral response by influenza A virus. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13093–13098. doi: 10.1073/pnas.0805213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. Nmrpipe - a Multidimensional Spectral Processing System Based on Unix Pipes. Journal of biomolecular NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Fitzkee NC, Bax A. Facile measurement of (1)H-(1)5N residual dipolar couplings in larger perdeuterated proteins. Journal of biomolecular NMR. 2010;48:65–70. doi: 10.1007/s10858-010-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster MP, Wuttke DS, Clemens KR, Jahnke W, Radhakrishnan I, Tennant L, Reymond M, Chung J, Wright PE. Chemical shift as a probe of molecular interfaces: NMR studies of DNA binding by the three amino-terminal zinc finger domains from transcription factor IIIA. Journal of biomolecular NMR. 1998;12:51–71. doi: 10.1023/a:1008290631575. [DOI] [PubMed] [Google Scholar]
- Gack MU, Albrecht RA, Urano T, Inn KS, Huang IC, Carnero E, Farzan M, Inoue S, Jung JU, Garcia-Sastre A. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell host & microbe. 2009;5:439–449. doi: 10.1016/j.chom.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrus JE, von Schwedler UK, Pornillos OW, Morham SG, Zavitz KH, Wang HE, Wettstein DA, Stray KM, Cote M, Rich RL, et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Graef KM, Vreede FT, Lau YF, McCall AW, Carr SM, Subbarao K, Fodor E. The PB2 subunit of the influenza virus RNA polymerase affects virulence by interacting with the mitochondrial antiviral signaling protein and inhibiting expression of beta interferon. Journal of virology. 2010;84:8433–8445. doi: 10.1128/JVI.00879-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Chen LM, Zeng H, Gomez JA, Plowden J, Fujita T, Katz JM, Donis RO, Sambhara S. NS1 protein of influenza A virus inhibits the function of intracytoplasmic pathogen sensor, RIG-I. American journal of respiratory cell and molecular biology. 2007;36:263–269. doi: 10.1165/rcmb.2006-0283RC. [DOI] [PubMed] [Google Scholar]
- Hale BG, Steel J, Medina RA, Manicassamy B, Ye J, Hickman D, Hai R, Schmolke M, Lowen AC, Perez DR, et al. Inefficient control of host gene expression by the 2009 pandemic H1N1 influenza A virus NS1 protein. Journal of virology. 2010;84:6909–6922. doi: 10.1128/JVI.00081-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton NS, Sachs D, Chen CJ, Hai R, Palese P. Genome-wide mutagenesis of influenza virus reveals unique plasticity of the hemagglutinin and NS1 proteins. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20248–20253. doi: 10.1073/pnas.1320524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D, Hossain MJ, Hickman D, Perez DR, Lamb RA. A new influenza virus virulence determinant: the NS1 protein four C-terminal residues modulate pathogenicity. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4381–4386. doi: 10.1073/pnas.0800482105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Ramanathan A, Miller MT, Tang GQ, Gale M, Jr, Patel SS, Marcotrigiano J. Structural basis of RNA recognition and activation by innate immune receptor RIG-I. Nature. 2011;479:423–427. doi: 10.1038/nature10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Blevins RA. NMR View: A computer program for the visualization and analysis of NMR data. Journal of biomolecular NMR. 1994;4:603–614. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kochs G, Garcia-Sastre A, Martinez-Sobrido L. Multiple anti-interferon actions of the influenza A virus NS1 protein. Journal of virology. 2007;81:7011–7021. doi: 10.1128/JVI.02581-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalinski E, Lunardi T, McCarthy AA, Louber J, Brunel J, Grigorov B, Gerlier D, Cusack S. Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell. 2011;147:423–435. doi: 10.1016/j.cell.2011.09.039. [DOI] [PubMed] [Google Scholar]
- Kuo RL, Zhao C, Malur M, Krug RM. Influenza A virus strains that circulate in humans differ in the ability of their NS1 proteins to block the activation of IRF3 and interferon-beta transcription. Virology. 2010;408:146–158. doi: 10.1016/j.virol.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen ZY, Wang W, Baker CC, Krug RM. The 3′-end-processing factor CPSF is required for the splicing of single-intron pre-mRNAs in vivo. Rna. 2001;7:920–931. doi: 10.1017/s1355838201010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SM, Du QS, Meng JZ, Pang ZW, Huang RB. The multiple roles of histidine in protein interactions. Chemistry Central journal. 2013;7:44. doi: 10.1186/1752-153X-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mibayashi M, Martinez-Sobrido L, Loo YM, Cardenas WB, Gale M, Jr, Garcia-Sastre A. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. Journal of virology. 2007;81:514–524. doi: 10.1128/JVI.01265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- Myong S, Cui S, Cornish PV, Kirchhofer A, Gack MU, Jung JU, Hopfner KP, Ha T. Cytosolic viral sensor RIG-I is a 5′-triphosphate-dependent translocase on double-stranded RNA. Science. 2009;323:1070–1074. doi: 10.1126/science.1168352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff ME, Barabino SM, Li Y, Keller W, Krug RM. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3′end formation of cellular pre-mRNAs. Molecular cell. 1998;1:991–1000. doi: 10.1016/s1097-2765(00)80099-4. [DOI] [PubMed] [Google Scholar]
- Neumann G, Kawaoka Y. Transmission of influenza A viruses. Virology. 2015 doi: 10.1016/j.virol.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz B, Rejaibi A, Dauber B, Eckhard J, Vinzing M, Schmeck B, Hippenstiel S, Suttorp N, Wolff T. IFNbeta induction by influenza A virus is mediated by RIG-I which is regulated by the viral NS1 protein. Cellular microbiology. 2007;9:930–938. doi: 10.1111/j.1462-5822.2006.00841.x. [DOI] [PubMed] [Google Scholar]
- Oshiumi H, Matsumoto M, Hatakeyama S, Seya T. Riplet/RNF135, a RING finger protein, ubiquitinates RIG-I to promote interferon-beta induction during the early phase of viral infection. The Journal of biological chemistry. 2009;284:807–817. doi: 10.1074/jbc.M804259200. [DOI] [PubMed] [Google Scholar]
- Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- Ruckert M, Otting G. Alignment of biological macromolecules in novel nonionic liquid crystalline media for NMR experiments. Journal of the American Chemical Society. 2000;122:7793–7797. [Google Scholar]
- Schlee M, Hartmann G. The chase for the RIG-I ligand--recent advances. Molecular therapy: the journal of the American Society of Gene Therapy. 2010;18:1254–1262. doi: 10.1038/mt.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann FH, Riepl H, Maurer T, Gronwald W, Neidig KP, Kalbitzer HR. Combined chemical shift changes and amino acid specific chemical shift mapping of protein-protein interactions. Journal of biomolecular NMR. 2007;39:275–289. doi: 10.1007/s10858-007-9197-z. [DOI] [PubMed] [Google Scholar]
- Schwieters CD, Clore GM. The VMD-XPLOR visualization package for NMR structure refinement. J Magn Reson. 2001;149:239–244. doi: 10.1006/jmre.2001.2300. [DOI] [PubMed] [Google Scholar]
- Schwieters CD, Kuszewski JJ, Tjandra N, Clore GM. The XPLOR-NIH NMR molecular structure determination package. J Magn Reson. 2003;160:65–73. doi: 10.1016/s1090-7807(02)00014-9. [DOI] [PubMed] [Google Scholar]
- Spesock A, Malur M, Hossain MJ, Chen LM, Njaa BL, Davis CT, Lipatov AS, York IA, Krug RM, Donis RO. The virulence of 1997 H5N1 influenza viruses in the mouse model is increased by correcting a defect in their NS1 proteins. Journal of virology. 2011;85:7048–7058. doi: 10.1128/JVI.00417-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerging infectious diseases. 2006;12:15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WW, Comanor L, Shay DK. Epidemiology of seasonal influenza: use of surveillance data and statistical models to estimate the burden of disease. The Journal of infectious diseases. 2006;194(Suppl 2):S82–91. doi: 10.1086/507558. [DOI] [PubMed] [Google Scholar]
- Tian Y, Schwieters CD, Opella SJ, Marassi FM. A practical implicit solvent potential for NMR structure calculation. Journal of magnetic resonance. 2014;243:54–64. doi: 10.1016/j.jmr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu KY, Kuo RL, Marklund J, Krug RM. The H5N1 influenza virus NS genes selected after 1998 enhance virus replication in mammalian cells. Journal of virology. 2007;81:8112–8121. doi: 10.1128/JVI.00006-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer TS, Ramirez BE, Delaglio F, Bax A. Evaluation of backbone proton positions and dynamics in a small protein by liquid crystal NMR spectroscopy. J Am Chem Soc. 2003;125:9179–9191. doi: 10.1021/ja0350684. [DOI] [PubMed] [Google Scholar]
- Wang W, Riedel K, Lynch P, Chien CY, Montelione GT, Krug RM. RNA binding by the novel helical domain of the influenza virus NS1 protein requires its dimer structure and a small number of specific basic amino acids. Rna. 1999;5:195–205. doi: 10.1017/s1355838299981621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, Sykes BD. The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. Journal of biomolecular NMR. 1994;4:171–180. doi: 10.1007/BF00175245. [DOI] [PubMed] [Google Scholar]
- Wüthrich K. NMR of Proteins and Nucleic Acids. New York, NY: Wiley Interscience; 1986. [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature immunology. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Yoo S, Myszka DG, Yeh C, McMurray M, Hill CP, Sundquist WI. Molecular recognition in the HIV-1 capsid/cyclophilin A complex. Journal of molecular biology. 1997;269:780–795. doi: 10.1006/jmbi.1997.1051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.