Abstract
Background
Survivors of childhood cancer treated with platinum-based chemotherapy and/or cranial radiation are at risk of treatment-induced hearing loss; however, the effects of such hearing loss on adult social attainment have not been well elucidated.
Methods
Adult survivors of pediatric central nervous system (CNS; n=180) and non-CNS solid tumors (n=226) treated with potentially ototoxic cancer therapy completed audiologic evaluations and questionnaires assessing perception of social functioning and social attainment (i.e. independent living, marriage, employment). Audiograms were graded with the Chang Ototoxicity Grading Scale. Analyses were stratified by tumor type (i.e. CNS vs. non-CNS). Multivariable logistic regression models were conducted with adjustment for age, sex, chronic health conditions, and for the CNS group, IQ. Adjusted odds ratios (OR) and 95% confidence intervals (CI) are reported.
Results
Serious hearing loss (requiring a hearing aid or deafness) was detected in 36% of CNS and 39% of non-CNS tumor survivors. Serious hearing loss was associated with increased risk for perceived negative impact in one or more areas of social functioning (non-CNS: OR=1.83, 95% CI, 1.00-3.34). Among non-CNS tumor survivors, serious hearing loss was associated with 2-fold increased risk of non-independent living (OR=2.19, 95% CI, 1.19-4.04) and unemployment or not graduating from high school (OR=1.85, 95% CI, 1.00-3.34).
Conclusions
A substantial proportion of adult survivors of childhood cancer treated with potentially ototoxic therapy have serious hearing loss. Treatment-induced hearing loss was associated with reduced social attainment, both perceived and actual, in this study sample.
Introduction
Advances in treatment regimens have led to substantial increases in survival rates for many pediatric central nervous system (CNS) and non-CNS solid tumors over the past several decades.1 Platinum-based chemotherapy, primarily cisplatin and carboplatin, are therapeutic agents routinely used for the treatment of pediatric extracranial solid tumors (i.e., neuroblastoma, osteosarcoma, nasopharyngeal carcinoma, hepatoblastoma, germ cell tumors) while cranial irradiation, with or without adjuvant chemotherapy, is often used to treat tumors located within the CNS. Curative treatments for childhood cancer often result in early and late-onset adverse organ toxicities,2, 3 with up to 90% of children estimated to acquire sensorineural hearing loss secondary to cisplatin treatment.4-9
The use of cisplatin, carboplatin, and cranial irradiation is associated with hearing loss resulting from damage to the organ of Corti, specifically degeneration of the cochlear inner and outer hair cell stereocilia, the stria vascularis, and the spiral ganglion.10-14 Damage to the outer hair cells occurs prior to inner hair cell damage and is more pronounced at the basal end of the cochlea,15 typically resulting in bilateral sensorineural hearing loss beginning in the high frequency range and extending to the lower frequencies with continued exposure.7 Young age at exposure (<5 years) and high cumulative dose (≥400 mg/m2) further increase risk of developing cisplatin-induced serious hearing loss.7 In addition, platinum- and cranial radiation-induced hearing loss may worsen over time or have a latency period prior to manifestation.5, 6, 16-18
The significant impact of severe to profound hearing loss on speech and language acquisition, educational performance, and psychosocial functioning in child and adolescent survivors of cancer has been documented;4, 5, 19 however, the long-term effects of childhood hearing loss on adult functional outcomes are less well appreciated. Among older adults in the general population, hearing loss has been associated with social isolation, depressive symptoms, and reduced quality of life.20-22 Despite these concerns, the effects of treatment-induced hearing loss on social functioning and attainment in adult survivors of childhood cancer have not been well elucidated.
The aims of the current study were to characterize the prevalence and degree of hearing loss in a large cohort of adult survivors of childhood cancer considered at-risk for hearing loss secondary to treatment with ototoxic therapies, and compare indicators of perceived and attained social outcomes in adult survivors with and without serious treatment-induced hearing loss.
Methods
St. Jude Lifetime Cohort Study (SJLIFE)
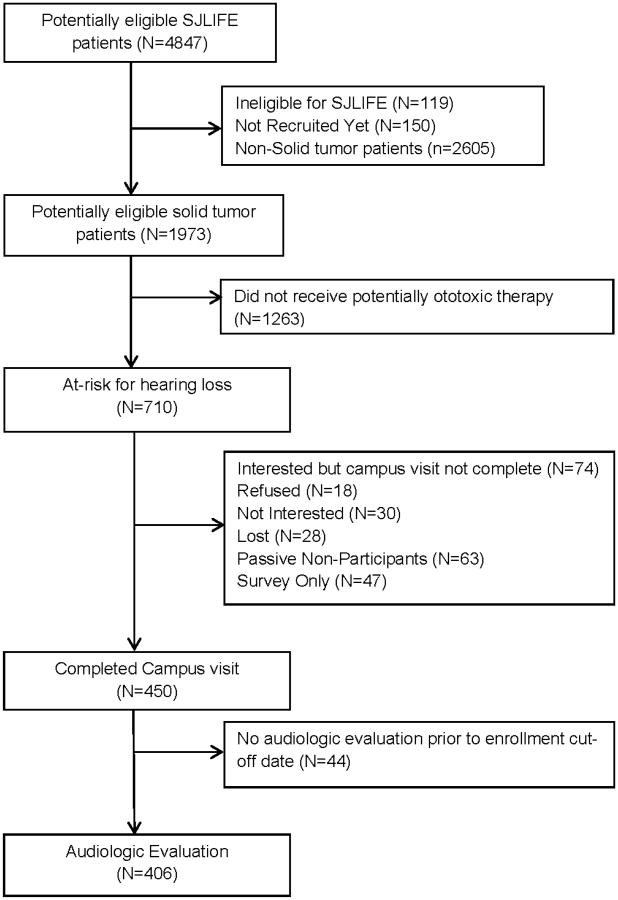
The study design and cohort characteristics of the St. Jude Lifetime Cohort Study (SJLFE) have previously been described.23, 24 Briefly, SJLIFE is a continuous enrollment, IRB-approved institutional follow-up study designed to better understand the multi-factorial etiology and severity of long-term adverse effects of treatments for adult childhood cancer survivors. Survivors eligible for study participation were treated at St. Jude Children's Research Hospital (SJCRH) for childhood cancer, currently ≥18 years of age, and survived ≥10 years since original diagnosis. SJLIFE participants receive a comprehensive risk-based clinical and laboratory assessment consistent with the Children's Oncology Group Long-term Follow-up Guidelines (COG LTFU).25 In addition, survivors complete comprehensive health questionnaires to assess social and demographic factors, health behaviors, and psychosocial functioning. Our study sample was comprised of SJLIFE CNS and non-CNS solid tumor survivors considered to be at high risk for treatment-induced hearing loss secondary to treatment with cisplatin, carboplatin, and/or cranial radiation. As of June 30, 2014, the cutoff date for this analysis, 710 potentially eligible participants were identified, 450 (63%) completed a SJLIFE clinic visit and 406 (90%) of these completed on-site audiologic evaluations (Figure 1; see Supplemental Figures 1 and 2 for participation by disease group).
Figure 1. Consort diagram of study participation.
Outcomes and Exposures
The primary outcome of interest was serious hearing loss. For audiologic evaluations, pure tone air conduction thresholds were assessed at frequencies .25, .5, 1, 2, 3, 4, 6, and 8 kHz in decibel (dB) hearing level (HL). Bone conduction thresholds were measured at .25, .5, 1, 2, 3, and 4 kHz in dBHL as needed to determine the type of hearing loss. Tympanometry was reviewed to assess the integrity of the conductive mechanism at the time of testing. Each audiogram was assigned a grade based on the Chang Ototoxicity Grading Scale26 (Table 1) and serious hearing loss was defined as a Chang grade of ≥2b. For survivors with asymmetrical hearing loss, the grade for the better ear was used in analyses.
Table 1. Chang Ototoxicity Grading Scale.
| Chang Grade | Sensorineural Hearing Threshold (dB HL) bone conduction or air conduction with normal tympanogram |
|---|---|
|
| |
| 0 | ≤ 20 dB at 1, 2, and 4 kHz |
|
| |
| 1a | ≥ 40 dB at any freq 6 to 12 kHz |
| 1b | > 20 and < 40 dB at 4kHz |
|
| |
| 2a | ≥ 40 dB at 4 kHz and above |
| 2b | > 20 and < 40 dB at any freq below 4kHz |
|
| |
| 3 | ≥ 40 dB at 2 or 3 kHz and above |
|
| |
| 4 | ≥ 40 dB at 1 kHz and above |
Response variables of interest were indicators of adult social functioning, including those related to perceived and social attainment. Perceived social outcomes were assessed using select items from the Brief Cancer Impact Assessment.27, 28 Items included perceived impact of cancer on educational plans, work life or career, and social life using a five-point Likert scale anchored by ‘very negative impact’ and ‘very positive impact’. A response of ‘somewhat negative impact’ or ‘very negative impact’ was coded as negative impact. To assess the extent to which, and amount of time, perceived health problems interfere with social function,29 two items from the Medical Outcomes Survery-36 Short Form Health Survey (SF-36) social function scale were used. Negative impact on social life was considered present if survivors reported their health interfered with normal social activities ‘moderately’, ‘quite a bit’ or ‘extremely’ or if their health interfered with social activities ‘some of the time’, ‘most of the time’, or ‘all of the time’. Social attainment was measured by survivor report of marital status, current employment, and independent living.
Covariates included chronic health conditions and intellectual functioning. Chronic health conditions were graded for endocrine and visual categories in accordance with the Common Terminology Criteria Adverse Events (CTCAE) version 4.0. Consistent with COG LTFU guidelines, CNS tumor survivors underwent neurocognitive testing as part of their standard evaluation, including assessment of intelligence (Wechsler Abbreviated Scale of Intelligence).30 Scores more than two standard deviations below the normative mean were indicative of IQ impairment.
Data analysis
Because of expected differences in social attainment, all analyses were stratified by solid tumor type (i.e. CNS vs. non-CNS). Descriptive statistics, including means, standard deviations and percentages were calculated for all exposures and outcomes. Unadjusted analysis of outcomes by hearing loss status was examined using the Chi-Square test. Negative impact on education/vocation was coded as present if survivors reported perceived negative impact on education plans or work life or career. Negative impact on social functioning was coded as present if survivors reported perceived negative impact on social life or on either social item from the SF-36. For attainment analyses, educational/vocational attainment was considered below developmental expectations if survivors completed less than high school or were currently unemployed. Independent living was coded as present if survivors reported living alone or with a spouse. Marriage was coded as present if survivors reported a history of marriage or living with a partner as married. Logistic regression analysis, adjusted for age at the audiologic evaluation, sex, and any CTCAE Grade 3 or 4 endocrine or visual health condition were conducted for each perceived social outcome and each social attainment outcome. Models for the CNS tumor group were adjusted for IQ impairment. Adjusted odds ratios (OR) and 95% confidence intervals (CIs) are reported.
Results
Study participants and non-participants were similar with respect to demographic, diagnostic, and treatment characteristics (Supplemental Table 1).
Non-CNS Solid Tumor Sample
Non-CNS solid tumor survivors (N=226; 51% male) were on average 31 years (range: 19.1-53.4) of age and 22.4 years (range: 11.1- 45.5) from original cancer diagnosis. The most common cancer diagnoses included osteosarcoma (19.9%), germ cell tumor (15.0%), neuroblastoma (16.8%), rhabdomyosarcoma (13.7%), and nasopharyngeal carcinoma (9.3%). Thirty seven percent were exposed to CNS radiation. Sixty nine percent were treated with cisplatin and 14% with carboplatin. Characteristics of non-CNS tumor survivors with and without serious hearing loss are provided in Supplemental Table 2.
CNS Tumor Sample
CNS tumor survivors (N=180; 62% male) were on average 27 years (range: 19.1-53.1) of age and 18.2 years (range: 11.1-41.8) from original cancer diagnosis at the time of their audiologic evaluation (Table 2). Thirty-eight percent were diagnosed with medulloblastoma, 24.4% with astrocytic tumors, 16.7% with ependymoma, and 21.1% with other brain tumors. Nearly all CNS tumor survivors were treated with cranial irradiation and 32.8% were treated with platinum-based chemotherapy (cisplatin n=55; carboplatin n=8). Characteristics of CNS tumor survivors with and without serious hearing loss are provided in Supplemental Table 3.
Table 2. Participant characteristics.
| CNS tumors (N=180) | Non-CNS solid tumors (N=226) | ||
|---|---|---|---|
|
| |||
| M (SD) | M (SD) | P-value | |
|
|
|||
| Age at follow-up (years) | 26.60 (5.49) | 31.02 (7.86) | |
| Age at diagnosis (years) | 8.41 (4.56) | 8.67 (6.27) | |
|
|
|||
| N (%) | N (%) | ||
|
|
|||
| Years since diagnosis | <0.0001 | ||
| 10-19 | 122 (67.78) | 86 (38.05) | |
| 20-29 | 53 (29.44) | 115 (50.88) | |
| 30-39 | 3 (1.67) | 21 (9.29) | |
| 40+ | 2 (1.11) | 4 (1.77) | |
| Sex | 0.03 | ||
| Male | 111 (61.67) | 115 (50.88) | |
| Female | 69 (38.33) | 111 (49.12) | |
| Cranial Radiation | <0.0001 | ||
| Yes | 179 (99.44) | 84 (37.17) | |
| No | 1 (0.56) | 142 (62.83) | |
| Carboplatin | 0.002 | ||
| Yes | 8 (4.44) | 31 (13.72) | |
| No | 172 (95.56) | 195 (86.28) | |
| Cisplatin | <0.0001 | ||
| Yes | 55 (30.56) | 156 (69.03) | |
| No | 125 (69.44) | 70 (30.97) | |
| Relapse/Recurrent | 0.74 | ||
| Yes | 21 (11.7) | 24 (10.6) | |
| No | 159 (88.3) | 202 (89.4) | |
| Endocrine Condition ≥ Grade 3 | 0.01 | ||
| Yes | 69 (38.33) | 59 (26.11) | |
| No | 111 (61.67) | 167 (73.89) | |
| Visual Condition ≥ Grade 3 | 0.71 | ||
| Yes | 27 (15.00) | 31 (13.72) | |
| No | 153 (85.00) | 195 (86.28) | |
| Neurocognitive Impairment | - | ||
| Yes | 42 (23.33) | n/a | |
| No | 135 (75.00) | n/a | |
| Missing | 3 (1.67) | n/a | |
Note. n/a = not available. SMN = subsequent malignant neoplasm.
Hearing Loss
Non-CNS Solid Tumors
The prevalence of serious hearing loss in non-CNS solid tumor survivors treated with potentially ototoxic therapy was 39% (95% CI, 33%-45%). Table 3 provides the distribution of hearing loss for better and worse ears stratified by solid tumor group. Survivors with serious hearing loss, relative to those without serious hearing loss, were significantly more likely to be male (P=0.01), younger at time of diagnosis (P=0.003), and diagnosed with neuroblastoma or osteosarcoma (P<0.001). Among the 89 non-CNS tumor survivors with serious hearing loss, 20% reported use of a hearing intervention (i.e., hearing aid, cochlear implant).
Table 3. Chang Grade Distribution for Better and Worse Ear.
| Better Ear | Worse Ear | |||
|---|---|---|---|---|
| Non-CNS N=226 | CNS N=180 | Non-CNS N=226 | CNS N=180 | |
| Chang Grade | N (%) | N (%) | N (%) | N (%) |
| 0 | 92 (40.7) | 88 (48.9) | 76 (33.6) | 73 (40.6) |
| 1a | 23 (10.2) | 12 (6.7) | 20 (8.9) | 6 (3.3) |
| 1b | 11 (4.9) | 7 (3.9) | 13 (5.8) | 6 (3.3) |
| 2a | 11 (4.9) | 8 (4.4) | 7 (3.1) | 1 (0.6) |
| 2b | 21 (9.3) | 10 (5.6) | 21 (9.3) | 10 (5.6) |
| 3 | 55 (24.3) | 27 (15.0) | 69 (30.5) | 27 (15.0) |
| 4 | 13 (5.8) | 28 (15.6) | 20 (8.9) | 57 (31.7) |
Note. Serious hearing loss defined as Grade ≥2b in better ear.
CNS Tumors
The prevalence of serious hearing loss in CNS tumor survivors was 36% (95% CI, 29%-43%). Age at diagnosis was not significantly associated with serious hearing loss among the CNS tumor group, although survivors of medulloblastoma were more likely to have hearing loss than were those with other histologic subtypes (P<0.001). Among the 64 CNS tumor survivors with serious hearing loss, 48% reported use of a hearing intervention.
Perceived Impact on Social Functioning
Non-CNS Solid Tumors
Among non-CNS tumor survivors with serious hearing loss, 41% (95% CI, 30%-52%) reported perceived negative impact of cancer on their education/vocation compared to 29% (95% CI, 21%-38%) of survivors without serious hearing loss. After adjustment for current age, sex, and health status, survivors with serious hearing loss were twice as likely to report perceived negative impact on social functioning (OR=2.28, 95% CI, 1.09-4.75) compared to survivors without serious hearing loss. Although not statistically significant, survivors with serious hearing loss also reported perceived negative impact on educational/vocational plans (OR=1.74, 95% CI, 0.93-3.26) (Table 4).
Table 4. Serious hearing loss and perceived negative impact on social functioning.
| 4a. Educational plans OR work life or career | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| CNS survivors | Non-CNS survivors | |||||||
|
| ||||||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | |||
| Age at evaluation | 1.04 | 0.97-1.12 | 0.26 | 1.05 | 1.00-1.10 | 0.05 | ||
| Age at diagnosis | 0.90 | 0.82-0.99 | 0.03 | 1.01 | 0.95-1.07 | 0.75 | ||
| Sex | ||||||||
| Male | 1.00 | 1.00 | ||||||
| Female | 0.58 | 0.29-1.15 | 0.12 | 0.86 | 0.46-1.59 | 0.63 | ||
| Hearing Loss* | ||||||||
| No | 1.00 | 1.00 | ||||||
| Yes | 1.53 | 0.72-3.29 | 0.27 | 1.74 | 0.93-3.26 | 0.08 | ||
| IQ Impairment | ||||||||
| No | 1.00 | - | ||||||
| Yes | 2.46 | 0.88-6.85 | 0.09 | - | - | - | ||
| ≥Grade 3 condition† | ||||||||
| No | 1.00 | 1.00 | ||||||
| Yes | 1.15 | 0.58-2.28 | 0.68 | 0.81 | 0.42-1.54 | 0.52 | ||
|
| ||||||||
| 4b. Social functioning | ||||||||
|
| ||||||||
| CNS survivors | Non-CNS survivors | |||||||
|
| ||||||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | |||
|
| ||||||||
| Age at evaluation | 0.99 | 0.91-1.07 | 0.80 | 1.02 | 0.96-1.08 | 0.47 | ||
| Age at diagnosis | 1.05 | 0.95-1.17 | 0.34 | 1.02 | 0.95-1.10 | 0.58 | ||
| Sex | ||||||||
| Male | 1.00 | 1.00 | ||||||
| Female | 1.70 | 0.77-3.79 | 0.19 | 1.77 | 0.85-3.69 | 0.13 | ||
| Hearing Loss* | ||||||||
| No | 1.00 | 1.00 | ||||||
| Yes | 1.60 | 0.69-3.69 | 0.27 | 2.28 | 1.09-4.75 | 0.03 | ||
| IQ Impairment | ||||||||
| No | 1.00 | - | ||||||
| Yes | 5.74 | 2.19-15.01 | 0.0004 | - | - | - | ||
| ≥Grade 3 condition† | ||||||||
| No | 1.00 | 1.00 | ||||||
| Yes | 1.52 | 0.68-3.39 | 0.30 | 1.36 | 0.65-2.81 | 0.41 | ||
|
| ||||||||
| 4c. Education/vocation or social functioning | ||||||||
|
| ||||||||
| CNS survivors | Non-CNS survivors | |||||||
|
| ||||||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | |||
|
| ||||||||
| Age at evaluation | 1.04 | 0.97-1.13 | 0.28 | 1.04 | 0.99-1.09 | 0.08 | ||
| Age at diagnosis | 0.94 | 0.85-1.03 | 0.17 | 1.01 | 0.95-1.07 | 0.80 | ||
| Sex | ||||||||
| Male | 1.00 | 1.00 | ||||||
| Female | 0.72 | 0.35-1.47 | 0.37 | 0.93 | 0.52-1.67 | 0.81 | ||
| Hearing Loss* | ||||||||
| No | 1.00 | 1.00 | ||||||
| Yes | 1.78 | 0.79-4.01 | 0.17 | 1.83 | 1.00-3.34 | 0.05 | ||
| IQ Impairment | ||||||||
| No | 1.00 | - | ||||||
| Yes | 3.31 | 1.02-10.68 | 0.05 | - | - | - | ||
| ≥Grade 3 condition† | ||||||||
| No | 1.00 | 1.00 | ||||||
| Yes | 1.28 | 0.63-2.59 | 0.49 | 0.93 | 0.50-1.72 | 0.82 | ||
Chang grade ≥2b hearing level versus ≤2a hearing level
Endocrine and visual health conditions
CNS Tumors
More than 60% of CNS tumor survivors reported perceived negative impact of cancer on their educational/vocational plans, irrespective of hearing status. After adjustment for current age, sex, health status, and IQ impairment, serious hearing loss was not significantly associated with perceived impact on social functioning, although the estimate of effect was elevated (OR=1.60, 95% CI, 0.69-3.69). Similarly, CNS tumor survivors with serious hearing loss were 1.5 times more likely to report perceived negative impact on their education/vocation (OR=1.53, 95% CI, 0.72-3.29) compared to survivors without serious hearing loss.
Social Attainment
Non-CNS Tumors
Among non-CNS tumor survivors, 39% (95% CI, 32%-46%) were not living independently, 45% (95% CI, 38%-52%) never married, and 34% (95% CI, 28%-41%) had not graduated high school or were unemployed. Survivors with serious hearing loss were at increased risk of not living independently (OR=2.19, 95% CI, 1.19-4.04) and never being married (OR=1.61, 95% CI, 0.81-3.20) compared to non-CNS tumor survivors without hearing loss. Serious hearing loss was associated with a nearly 2-fold increased odds of not graduating from high school or being unemployed (OR=1.85, 95% CI 1.02-3.35) (Table 5).
Table 5. Hearing loss and social attainment.
| 5a. Non-independent living | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| CNS survivors | Non-CNS survivors | |||||
|
| ||||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age at evaluation | 0.88 | 0.81-0.95 | 0.003 | 0.95 | 0.91-1.00 | 0.06 |
| Age at diagnosis | 0.99 | 0.90-1.09 | 0.88 | 0.98 | 0.93-1.04 | 0.60 |
| Sex | ||||||
| Male | 1.00 | 1.00 | ||||
| Female | 1.32 | 0.61-2.81 | 0.48 | 0.92 | 0.50-1.68 | 0.77 |
| Hearing Loss* | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.57 | 0.69-3.57 | 0.28 | 2.19 | 1.19-4.04 | 0.01 |
| IQ Impairment | ||||||
| No | 1.00 | - | ||||
| Yes | 12.15 | 2.38-62.08 | 0.003 | - | - | - |
| ≥Grade 3 condition† | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.91 | 0.44-1.90 | 0.80 | 1.56 | 0.85-2.89 | 0.15 |
|
| ||||||
| 5b. Never married | ||||||
|
| ||||||
| CNS survivors | Non-CNS survivors | |||||
|
| ||||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
|
| ||||||
| Age at evaluation | 0.83 | 0.75-0.91 | <0.0001 | 0.88 | 0.83-0.93 | <0.0001 |
| Age at diagnosis | 0.96 | 0.86-1.07 | 0.43 | 0.94 | 0.88-1.00 | 0.05 |
| Sex | ||||||
| Male | 1.00 | 1.00 | ||||
| Female | 0.77 | 0.32-1.86 | 0.56 | 0.69 | 0.35-1.35 | 0.28 |
| Hearing Loss* | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.02 | 0.40-2.62 | 0.96 | 1.61 | 0.81-3.20 | 0.17 |
| IQ Impairment | ||||||
| No | 1.00 | - | ||||
| Yes | 3.25 | 0.70-15.04 | 0.13 | - | - | - |
| ≥Grade 3 condition† | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.50 | 0.62-3.66 | 0.37 | 0.71 | 0.36-1.42 | 0.33 |
|
| ||||||
| 5c. Unemployed and/or did not graduate from high school | ||||||
|
| ||||||
| CNS survivors | Non-CNS survivors | |||||
|
| ||||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
|
| ||||||
| Age at evaluation | 1.01 | 0.94-1.08 | 0.79 | 1.01 | 0.97-1.06 | 0.60 |
| Age at diagnosis | 0.96 | 0.88-1.05 | 0.36 | 0.98 | 0.92-1.04 | 0.43 |
| Sex | ||||||
| Male | 1.00 | 1.00 | ||||
| Female | 1.48 | 0.74-2.96 | 0.27 | 0.87 | 0.48-1.57 | 0.64 |
| Hearing Loss* | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.83 | 0.87-3.89 | 0.11 | 1.85 | 1.02-3.35 | 0.04 |
| IQ Impairment | ||||||
| No | 1.00 | - | ||||
| Yes | 6.85 | 2.16-21.70 | 0.001 | - | - | - |
| ≥Grade 3 condition† | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.06 | 0.54-2.09 | 0.86 | 1.26 | 0.69-2.28 | 0.46 |
|
| ||||||
| 5d. Any adverse social attainment outcome | ||||||
|
| ||||||
| CNS survivors | Non-CNS survivors | |||||
|
| ||||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
|
| ||||||
| Age at evaluation | 0.88 | 0.80-0.96 | 0.003 | 0.93 | 0.89-0.98 | 0.005 |
| Age at diagnosis | 0.95 | 0.84-1.09 | 0.48 | 0.95 | 0.89-1.01 | 0.09 |
| Sex | ||||||
| Male | 1.00 | 1.00 | ||||
| Female | 2.03 | 0.61-6.76 | 0.25 | 1.04 | 0.54-1.98 | 0.92 |
| Hearing Loss* | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.19 | 0.37-3.88 | 0.77 | 2.27 | 1.15-4.48 | 0.02 |
| IQ Impairment | ||||||
| No | 1.00 | - | ||||
| Yes | 4.68 | 0.51-43.25 | 0.17 | - | - | - |
| ≥Grade 3 condition† | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.52 | 0.17-1.56 | 0.24 | 1.09 | 0.55-2.14 | 0.80 |
Chang grade ≥2b hearing level versus ≤2a hearing level
Endocrine and visual health conditions
CNS Tumors
Overall, 69% (95% CI, 62%-76%) of CNS tumor survivors were not living independently, 79% (95% CI, 72%-85%) never married, and 61% (95% CI, 54%-68%) had not graduated high school or were unemployed, much higher frequencies than those observed in the non-CNS tumor survivor group. Impaired IQ conferred the greatest risk for reduced social attainment in survivors of CNS tumors. However, beyond IQ, survivors with serious hearing loss were at increased risk of not living independently (OR=1.57, 95% CI, 0.69-3.57) and not graduating from high school or being unemployed (OR=1.83, 95% CI, 0.87-3.89). Although estimates did not meet statistical significance, risk estimates suggest a deleterious effect of hearing loss.
Conclusions
Approximately 38 percent of adult survivors of childhood non-CNS solid tumors and CNS tumors who received ototoxic cancer-directed therapies during childhood had serious hearing loss, i.e. deafness or loss that equates to need for a hearing aid. However, only one-third of survivors used some type of hearing intervention. Our results generally indicate that both non-CNS and CNS tumor survivors with serious hearing loss report poorer perception of social functioning and reduced social attainment compared to survivors without serious hearing loss. Although CNS-tumor survivors had substantially poorer social attainment than did non-CNS tumor survivors, the effect of serious hearing loss was more pronounced among the non-CNS tumor survivors.
Past studies have documented increased risk of hearing loss in pediatric patients treated with platinum compounds and cranial radiation therapy, however limited data on adult survivors are available. One notable exception is a report on auditory complications from the Childhood Cancer Survivor Study (CCSS). Whelan and colleagues31 reported that exposure to platinum compounds was associated with 4.1-fold increased risk of hearing loss requiring the use of a hearing aid and that temporal lobe and posterior fossa radiation was associated with adverse hearing outcomes in a dose-dependent fashion. This study, however, was limited to self-report of hearing outcomes and did not include survivors of malignancies such as germ cell tumors and nasopharyngeal carcinomas.
Non-CNS solid tumor survivors with serious hearing loss had almost twice the odds of not graduating from high school and/or unemployment compared to those without serious hearing loss. Similar to these findings, in a study of 137 children (age 8 to 17 years) who were treated on Children's Oncology Group protocols, survivors of neuroblastoma with hearing loss, as reported by their parents, had at least twice the risk of an identified academic problem, and a similarly higher risk of a general learning disability and/or special educational needs, than did neuroblastoma survivors without hearing loss.19 Our results substantially extend this past work by considering direct audiologic assessment and by demonstrating that hearing loss has potentially adverse effects on educational and occupational attainment well into adulthood.
Non-CNS solid tumor survivors with serious hearing loss also perceived greater negative impact of cancer on their social functioning compared to non-CNS solid tumors survivors without serious hearing loss. Consistent with this perception, hearing loss was associated with reduced likelihood of independent living and marriage in non-CNS tumor survivors. Independent living and marriage, albeit relatively crude indicators, are often conceptualized as important social developmental milestones that provide opportunities for socialization and community integration. Although we did not directly measure social isolation, we did find that survivors with serious hearing loss reported spending significantly more time outside of work watching television or using the computer, suggesting increased engagement in leisure activities associated with isolation.
Overall, nearly two-thirds of CNS tumor survivors, with and without serious hearing impairment, reported negative impact on education plans and work life or career. This is not surprising given that survivors of CNS malignancies are at high risk for cognitive morbidities, which may adversely impact educational and occupational attainment. Studies from CCSS indicate that adult survivors of CNS tumors are twice as likely to be unemployed compared to survivors of other malignancies32 and less likely than sibling comparisons to attend college.33 Our data suggest that hearing loss may confer additional risk for poor educational and occupational attainment, such that 75% of survivors with serious hearing loss did not graduate from high school and/or were unemployed compared to 54% of CNS tumor survivors without serious hearing loss. With respect to social integration, CNS tumor survivors with serious hearing loss were at 60% increased risk of not living independently compared to those without serious hearing loss.
Among older adults (aged >60 years) in the general population, age-related hearing loss has been associated with impaired activities of daily living, social isolation and depressive symptoms, particularly among those with moderate to severe hearing loss.20-22 As our data suggest that survivors with serious hearing loss are vulnerable to adverse social outcomes in young and middle adulthood, it will be important to continue to monitor their social functioning and integration as they age. Of note, we generally observed that older age at examination was associated with reduced relative odds of adverse social outcomes. We speculate that this may be due to additional time to attain outcomes such as employment and marriage with increasing age.
The strengths of this study include cohorts of both non-CNS solid tumor and CNS tumor survivors with standardized audiometric testing and concurrent reporting of social attainment. However, certain limitations need to be considered when interpreting the findings. This analysis was restricted to survivors who were alive at least 10 years post-diagnosis. Therefore, hearing loss and social attainment data for patients who did not survive to participate in the study are unavailable. It also is possible that non-participants had a different distribution of hearing loss or social outcomes than did participants. Because only a small proportion of survivors reported use of a hearing intervention we could not compare social outcomes for those with and without aided hearing. Presumably, timely intervention could offset deleterious social effects of hearing loss. However, our data are consistent with data from the general population suggesting that the majority of adults who could benefit from hearing aids do not use them.34, 35 Participants in SJLIFE received risk-based medical screenings; therefore, not all participants underwent systematic evaluations of organ function (e.g. echocardiography, pulmonary function testing, bone mineral density testing). As a result, conditions detected through these assessments could not be considered in our analyses. However, it is likely that most social outcomes were attained prior to the development of chronic health conditions whereas many auditory complications, including cisplatin induced hearing loss,36 occur within the first five years of diagnosis31 and likely precede adult social attainment. Because this analysis was cross-sectional in nature a clear understanding of specific causal relations is limited. Lastly, there is a degree of uncertainty in the findings, for example with perceived negative impact in social functioning from hearing loss among CNS patients (OR 1.78; 95% CI 0.79-4.01), as reflected by the confidence intervals that include the null value of 1.0.
In summary, treatment-induced hearing loss in childhood cancer survivors was associated with reduced perception of and actual adult social attainment. Nearly 38% of adult survivors in our sample had serious hearing loss and a sizeable proportion of these survivors reported restrictions in their social functioning. Continued follow-up of adult survivors of childhood cancer is necessary to evaluate future onset or worsening of auditory complications as well as associated impact on social participation and attainment.
Supplementary Material
Acknowledgments
This work was supported by St. Jude Children's Research Hospital Cancer Center Support CORE Grant CA21765 from the National Cancer Institute and by ALSAC.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Howlader N, N A, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review. 1975-2011 Available from URL: http://seer.cancer.gov/csr/1975_2011/based on November 2013 SEER data submission, posted to the SEER web site, April 2014.
- 2.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14:61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grewal S, Merchant T, Reymond R, McInerney M, Hodge C, Shearer P. Auditory late effects of childhood cancer therapy: a report from the Children's Oncology Group. Pediatrics. 2010;125:e938–950. doi: 10.1542/peds.2009-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23:8588–8596. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 6.Brock PR, Bellman SC, Yeomans EC, Pinkerton CR, Pritchard J. Cisplatin ototoxicity in children: a practical grading system. Med Pediatr Oncol. 1991;19:295–300. doi: 10.1002/mpo.2950190415. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur J Cancer. 2004;40:2445–2451. doi: 10.1016/j.ejca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Einar-Jon E, Trausti O, Asgeir H, et al. Hearing impairment after platinum-based chemotherapy in childhood. Pediatr Blood Cancer. 2011;56:631–637. doi: 10.1002/pbc.22876. [DOI] [PubMed] [Google Scholar]
- 9.Landier W, Knight K, Wong FL, et al. Ototoxicity in children with high-risk neuroblastoma: prevalence, risk factors, and concordance of grading scales--a report from the Children's Oncology Group. J Clin Oncol. 2014;32:527–534. doi: 10.1200/JCO.2013.51.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brock PR, Knight KR, Freyer DR, et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol. 2012;30:2408–2417. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright CG, Schaefer SD. Inner ear histopathology in patients treated with cis-platinum. Laryngoscope. 1982;92:1408–1413. doi: 10.1288/00005537-198212000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Meech RP, Campbell KC, Hughes LP, Rybak LP. A semiquantitative analysis of the effects of cisplatin on the rat stria vascularis. Hear Res. 1998;124:44–59. doi: 10.1016/s0378-5955(98)00116-6. [DOI] [PubMed] [Google Scholar]
- 13.Comis SD, Rhys-Evans PH, Osborne MP, Pickles JO, Jeffries DJ, Pearse HA. Early morphological and chemical changes induced by cisplatin in the guinea pig organ of Corti. J Laryngol Otol. 1986;100:1375–1383. doi: 10.1017/s0022215100101161. [DOI] [PubMed] [Google Scholar]
- 14.Hinojosa R, Riggs LC, Strauss M, Matz GJ. Temporal bone histopathology of cisplatin ototoxicity. Am J Otol. 1995;16:731–740. [PubMed] [Google Scholar]
- 15.Scheitzer V, Hawkins J, Lilly D, Litterst C, Abrams G, Davis J. Ototoxic and nephrotoxic effects of combined treatment with cisdiamminedichloroplatinum and kanamycin in the guinea pig. Otolaryngol Head Neck Surg. 1984;92:38–19. doi: 10.1177/019459988409200109. [DOI] [PubMed] [Google Scholar]
- 16.Bertolini P, Lassalle M, Mercier G, et al. Platinum compound-related ototoxicity in children: long-term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol. 2004;26:649–655. doi: 10.1097/01.mph.0000141348.62532.73. [DOI] [PubMed] [Google Scholar]
- 17.Jehanne M, Lumbroso-Le Rouic L, Savignoni A, et al. Analysis of ototoxicity in young children receiving carboplatin in the context of conservative management of unilateral or bilateral retinoblastoma. Pediatr Blood Cancer. 2009;52:637–643. doi: 10.1002/pbc.21898. [DOI] [PubMed] [Google Scholar]
- 18.Hua C, Bass JK, Khan R, Kun LE, Merchant TE. Hearing loss after radiotherapy for pediatric brain tumors: effect of cochlear dose. Int J Radiat Oncol Biol Phys. 2008;72:892–899. doi: 10.1016/j.ijrobp.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 19.Gurney JG, Tersak JM, Ness KK, et al. Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: a report from the Children's Oncology Group. Pediatrics. 2007;120:e1229–1236. doi: 10.1542/peds.2007-0178. [DOI] [PubMed] [Google Scholar]
- 20.Karpa MJ, Gopinath B, Beath K, et al. Associations between hearing impairment and mortality risk in older persons: the Blue Mountains Hearing Study. Ann Epidemiol. 2010;20:452–459. doi: 10.1016/j.annepidem.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Gopinath B, Schneider J, McMahon CM, Teber E, Leeder SR, Mitchell P. Severity of age-related hearing loss is associated with impaired activities of daily living. Age Ageing. 2012;41:195–200. doi: 10.1093/ageing/afr155. [DOI] [PubMed] [Google Scholar]
- 22.Gopinath B, Wang JJ, Schneider J, et al. Depressive symptoms in older adults with hearing impairments: the Blue Mountains Study. J Am Geriatr Soc. 2009;57:1306–1308. doi: 10.1111/j.1532-5415.2009.02317.x. [DOI] [PubMed] [Google Scholar]
- 23.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56:825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ojha RP, Oancea SC, Ness KK, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: Results from the St. Jude lifetime cohort study. Pediatric Blood & Cancer. 2013;60:856–864. doi: 10.1002/pbc.24348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. Journal of Clinical Oncology. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 26.Chang KW, Chinosornvatana N. Practical grading system for evaluating cisplatin ototoxicity in children. J Clin Oncol. 2010;28:1788–1795. doi: 10.1200/JCO.2009.24.4228. [DOI] [PubMed] [Google Scholar]
- 27.Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. Journal of the National Cancer Institute. 2002;94:39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 28.Alfano CM, McGregor BA, Kuniyuki A, et al. Psychometric evaluation of the Brief Cancer Impact Assessment among breast cancer survivors. Oncology. 2006;70:190–202. doi: 10.1159/000094320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 30.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 31.Whelan K, Stratton K, Kawashima T, et al. Auditory complications in childhood cancer survivors: a report from the childhood cancer survivor study. Pediatr Blood Cancer. 2011;57:126–134. doi: 10.1002/pbc.23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirchhoff AC, Leisenring W, Krull KR, et al. Unemployment among adult survivors of childhood cancer: a report from the childhood cancer survivor study. Med Care. 2010;48:1015–1025. doi: 10.1097/MLR.0b013e3181eaf880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurney JG, Krull KR, Kadan-Lottick N, et al. Social outcomes in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2390–2395. doi: 10.1200/JCO.2008.21.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meister H, Walger M, Brehmer D, von Wedel UC, von Wedel H. The relationship between pre-fitting expectations and willingness to use hearing aids. Int J Audiol. 2008;47:153–159. doi: 10.1080/14992020701843111. [DOI] [PubMed] [Google Scholar]
- 35.McCormack A, Fortnum H. Why do people fitted with hearing aids not wear them? Int J Audiol. 2013;52:360–368. doi: 10.3109/14992027.2013.769066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurney JG, Bass JK, Onar-Thomas A, et al. Evaluation of amifostine for protection against cisplatin-induced serious hearing loss in children treated for average-risk or high-risk medulloblastoma. Neuro Oncol. 2014;16:848–855. doi: 10.1093/neuonc/not241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.