Abstract
AIMS
Serum chemerin concentrations are elevated in obese individuals and may play a role in type 2 diabetes. Exercise improves insulin sensitivity, which may be related to changes in chemerin. This study explored how an acute bout of aerobic exercise affected chemerin levels in non-diabetic obese adults.
METHODS
Blood samples from 11 obese adults were obtained during two separate conditions: sedentary (SED) and exercise (EX; 60-65% VO2peak). Samples were drawn at baseline, immediately following exercise and hourly for an additional 2 hours. ANOVA was used to test for differences in chemerin between conditions.
RESULTS
Unadjusted analysis showed no difference in overall change (baseline to 2 hrs post) in chemerin between conditions. During the 2-hr post-exercise period, chemerin decreased to 12% below baseline, compared to a 2.5% increase above baseline during that time period on the sedentary day (p=0.06, difference in post-to-2hr change between conditions). Controlling for homeostatic model assessment of insulin resistance (HOMA-IR), a significant difference existed between EX and SED in the change in chemerin from baseline to 2-hr post (p=0.02). Stratified analyses showed a consistent exercise-induced decrease in chemerin among non-insulin resistant subjects, while chemerin increased during exercise among insulin resistant subjects, and then decreased post-exercise.
CONCLUSION
An acute bout of exercise in obese individuals may elicit a drop in chemerin levels during the post-exercise period, and this response may vary based on insulin resistance.
Keywords: chemerin, insulin resistance, exercise
Introduction
Chemerin is an adipokine that has been shown to be elevated in obese individuals and associated with the metabolic impairments of type 2 diabetes mellitus (T2DM). The precise role of chemerin in the pathophysiology of diabetes remains unclear, although its positive association with BMI and obesity (Bozaoglu et al. 2007, Bozaoglu et al. 2009, Blüher et al. 2012) and its regulatory function in adipocyte differentiation in vitro (Goralski et al. 2007) have been demonstrated.
Chemerin acts as a ligand for the G-protein coupled receptor CMKLR1, which is highly expressed in adipose tissue (Bozaoglu et al. 2007, Goralski et al. 2007, Takahashi et al. 2008, Sell et al. 2009). Chemerin was recently shown to regulate glucose uptake in both adipose tissue and skeletal muscle, which suggests its potential role in the development of T2DM (Takahashi et al. 2008, Kralisch et al. 2009, Lehrke et al. 2009, Weigert et al. 2010). It was found that Stimulation of cultured 3T3-L1 cells with the pro-inflammatory cytokine interleukin 1-Beta (IL1-β) enhanced chemerin expression and decrease insulin-stimulated glucose uptake (Kralisch et al. 2009). Chemerin treatment in obese mice worsened glucose intolerance by decreasing serum insulin concentrations and glucose uptake in the liver and adipose tissue (Ernst et al. 2010). Further, Goralski et al (2007) supported chemerin's necessary contribution to adipocyte development by showing that knocking down chemerin by adenoviral siRNA led to impaired 3T3-L1 pre-adipocyte differentiation, as well as reduced expression of adipocyte genes involved in glucose and lipid homeostasis. These findings suggest that adipocytes are both releasing and utilizing chemerin in an autocrine manner. Takahashi et al (2008), on the other hand, found that chemerin incubation restored insulin sensitivity by 41% in cultured 3T3-L1 adipocytes via improved insulin-stimulated glucose uptake and insulin receptor substrate (IRS)-1 tyrosine phosphorylation. The implications of those findings, however, are debatable given that glucose uptake into adipocytes could be interpreted as both preventing hyperglycemia and facilitating lipogenesis.
Both acute and chronic exercise enhance glucose uptake and increase insulin action (Bradley et al. 2008, Bordenave et al. 2008). Several studies demonstrated an association between chronic exercise and both diminished chemerin levels and enhanced insulin action (Chakaroun et al. 2012, Saremi et al. 2010, Lee et al. 2013, Kim et al. 2013, Veojarvi et al. 2013). Acute bouts of exercise were also associated with immediate enhancements in insulin action, as evidenced by improvements in whole body glucose disposal (Goodyear et al. 1996), insulin-stimulated skeletal muscle glucose transport (Oakes et al. 1997), and GLUT-4 translocation (King et al. 1993) following a single exercise session. We theorize that the acute effects of exercise on insulin sensitivity may be related to acute decreases in circulating chemerin following a single bout of exercise. Thus, the purpose of this study was to determine whether serum chemerin levels would decrease following an acute exercise bout in obese individuals.
Materials and Methods
Subjects
This study was a secondary analysis of data and biological samples from a completed crossover study of the effects of exercise on satiety and circulating hormone levels (Holmstrup et al. 2013). The original study and the current analysis were approved by the Syracuse University Institutional Review Board and all subjects (n=11) signed an informed consent document prior to participation in the study. Inclusion criteria were: age between 18-35 years (mean=25.3 ± 4.3) and BMI >30 kg/m2 (mean=33.8 ± 4.0). Exclusion criteria included weight loss or gain of ≥ 5 lbs. three months prior to the study, diagnosed gastrointestinal problems, or diagnosed orthopedic limitations to normal walking activity. Subjects could not be using any glucose-lowering medications or other medications that may affect glucose metabolism (e.g. antidepressants, hormonal contraceptives, steroid hormones). Indices of resting blood pressure (Omron HEM automatic blood pressure monitor, Omron, Kyoto, Japan), cardiovascular disease (medical questionnaire), and lipid profile (Cholestech LDX, Cholestech Corporation, Hayward, CA) were evaluated in order to ensure a healthy subject cohort. Specifically, resting blood pressure of 140/90 mm/Hg, total cholesterol of 200 mg/dL, and low density lipoprotein cholesterol of 160 mg/dL were used as cut-offs for exclusion. Female subjects (n=3) were tested within the first eight days of their menstrual cycle to minimize possible hormonal effects on glucose and chemerin levels.
VO2peak assessment
Aerobic capacity was assessed as previously described (Kanaley et al. 2009). Briefly, VO2peak was measured during a gradual treadmill exercise stress test using breath by breath analysis performed with a metabolic cart (True One 2400, ParvoMedics, Sandy, UT). Expired gases and heart rate were analyzed during the test and VO2peak was calculated. Subjects initially walked at a pace of 2.5 miles per hour (mph) and a 0% grade, with speed increased by increments of 0.5 mph per stage until a speed of 3.5 mph was achieved at minute six. Starting at minute eight, the workload increased 2% per stage until the subject reached volitional fatigue. Criteria for a successful test were determined in accordance with ACSM guidelines (2009).
During the initial visit, each subject's habitual dietary intake as well as general health and physical activity were recorded using Institutional Review Board (IRB) approved questionnaires. Individuals were measured for height and weight and body composition was assessed using air-displacement plethysmography (BODPOD system, Life Measurement, Inc., Concorde, CA) according to manufacturer's specifications.
Study Protocol
This study was a crossover design in which each subject served as their own control. For the original study, each subject reported to the Syracuse University Human Performance Lab on two separate occasions for twelve hours of testing, beginning at 0700 h. (Holmstrup et al. 2013). Subjects participated in the sedentary (SED) and exercise (EX) protocols in random order with a minimum 7-day period between conditions.
Subjects were fasted and had abstained from caffeine consumption for 12 hours, and abstained from alcohol consumption and structured exercise for at least 24 hours prior to testing. Upon arrival to the lab on testing days the subjects had a Teflon catheter inserted in their antecubital vein. On the exercise day, blood samples were drawn immediately prior to exercise for baseline reference, immediately following the cessation of exercise (post), one hour post-exercise (1hr), and two hours post–exercise (2hr). The exercise protocol consisted of one hour of walking at an intensity corresponding to 60-65% of VO2peak. Blood samples were drawn at the same one-hour intervals on the sedentary day.
As part of the original study, liquid meals were administered immediately after the first and third blood draws during both conditions. All meals were matched for energy content (15% protein, 65% carbohydrate, 20% fat; Wegmans Nutritional Beverage, Wegmans, Rochester, NY), with the majority of carbohydrate coming from sucrose and corn syrup, and the majority of protein from soy and whey. All of the subjects were required to remain in the laboratory and participated in quiet, sedentary activities including reading, studying, playing board games, and watching movies, with extremely limited physical activity (e.g. walking to restroom) outside of the designated exercise period.
Blood analysis
Blood samples were obtained and transferred to serum separator tubes (BD Vacutainer, Franklin Lakes, NJ), separated by centrifugation, divided into two sets of polypropylene tubes, and stored at −80°C for subsequent analysis. Samples were assayed in duplicate for serum glucose using a commercially available glucose oxidase assay (Sigma-Aldrich Corp., St. Louis, MO). A second set of samples were briefly centrifuged (3,000g, 5 min, 4°C) and assayed for serum insulin concentrations using Luminex xMap Technology (Linco Research, St. Charles, MO) on a Luminex 100/200 platform (Luminex Corporation, Austin, TX). All procedures followed manufacturer's instructions (Millipore, Billerica, MA) with quality controls assayed within expected ranges. Inter-assay and intra-assay coefficients for insulin were 4.9% and 8.5% respectively. The lowest limits of detection in this assay were 137 pg/ml.
Blood plasma collected from all subjects was analyzed in duplicate to assess circulating chemerin levels. The Enzyme-linked Immunosorbent Assay (ELISA) was performed according to the manufacturer's instructions (BioVendor LLC, Candler, NC). Briefly, unbound sights were blocked to prevent false positive results with BSA. The chemerin antibody was added to the appropriate wells, followed by a secondary IgG conjugated antibody. The colorimetric measurements were obtained using a plate reader (BioTek Powerwave HT, BioTek Instruments Inc, Winooski, VT) and software (Gen5 software, BioTek, Instruments Inc, Winooski, VT)
Statistical analyses
Means (±SD) were calculated for all baseline anthropometric and serum biomarker measurements. Pearson correlation coefficients were utilized to examine the relationships between chemerin and all baseline anthropometric (BMI, body fat percentage) and metabolic (HOMA-IR, HOMA-β, QUICKI) parameters. A multivariate approach using the PROC GLM procedure in SAS (SAS Institute, Cary, NC) was used to test for the interaction of condition (EX vs. SED) and time (pre, post, 1hr, 2hrs), which would indicate an effect of exercise on changes in chemerin. This model was also run with the separate inclusion of three covariates; 1) the subjects’ baseline scores for the homeostatic model assessment of insulin resistance (HOMA-IR), 2) beta cell function (HOMA-β), and 3) the quantitative insulin sensitivity check index (QUICKI) values to determine whether the association between exercise and chemerin concentrations changed after controlling for subjects’ insulin sensitivity and/or beta cell function. Paired-samples t-tests were used to test for differences between conditions in chemerin changes between specific time points (pre to post, post to 2 hrs, etc.). All statistical analyses were conducted using SAS version 9.2. An alpha level of 0.05 was used to determine statistical significance.
Results
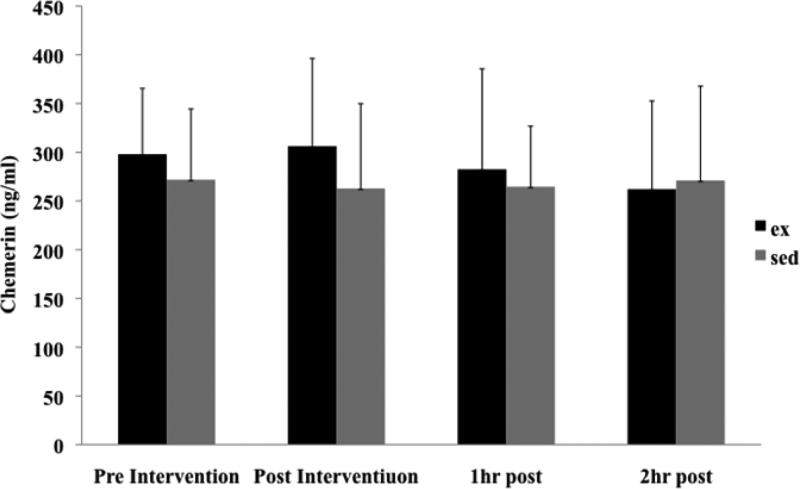
Descriptive characteristics for the 11 subjects (8 males, 3 females) are shown in Table 1. At baseline, females had a greater mean body fat percentage than males, as well as higher mean circulating chemerin concentrations (p<0.05). Baseline chemerin levels were positively correlated with percent body fat (r= 0.69; Table 2), HOMA-IR (r= 0.53) and HOMA-β (r= 0.84), and negatively correlated with QUICKI (r= −0.70). Mean chemerin levels did not differ significantly at baseline or for any of the subsequent time points between the SED and EX conditions (Figure 1).
Table 1.
Mean anthropometric and metabolic measurements, overall and by gender.
| Total (n=11) | Males (n=8) | Females (n=3) | |
|---|---|---|---|
| Age (yrs) | 25.2±4.3 | 29.5±4.3 | 25.0±5.29 |
| Height (cm) | 174.4±7.0 | 175.5±7.6 | 170.0±0.87 |
| Weight (kg) | 104.3±13.6 | 115.2±13.2 | 103.6±17.6 |
| BMI (kg/m2) | 34.3±4.4 | 37.7±4.0 | 35.8±5.8 |
| Body Fat % | 32.5±9.2 | 31.2±7.7 | 42.0±5.8* |
| HOMA-IR | 7.6±7.3 | 7.0±7.5 | 9.1±8.2 |
| HOMA-β | 16.1±4.7 | 15.0±3.0 | 19.3±7.6 |
| QUICKI | 3.0±0.3 | 3.0±0.3 | 2.8±0.2 |
p<0.05
Table 2.
Pearson correlation among anthropometric values and metabolic values.
| BMI | Body fat (%) | HOMA-β | HOMA-IR | QUICKI | |
|---|---|---|---|---|---|
| Chemerin (ng/ml) | 0.48 | 0.69* | 0.84† | 0.53 | −0.70 |
| BMI | – | 0.64* | 0.59 | 0.85† | −0.68* |
| Body fat (%) | – | – | 0.62* | 0.45 | −0.83† |
| HOMA-β | – | – | – | 0.45 | −0.66† |
| HOMA-IR | – | – | – | – | −.60† |
p < 0.05
p < 0.01
Figure 1.
Pre- and post-intervention circulating chemerin levels, by condition. Values are presented as Mean ± SD.
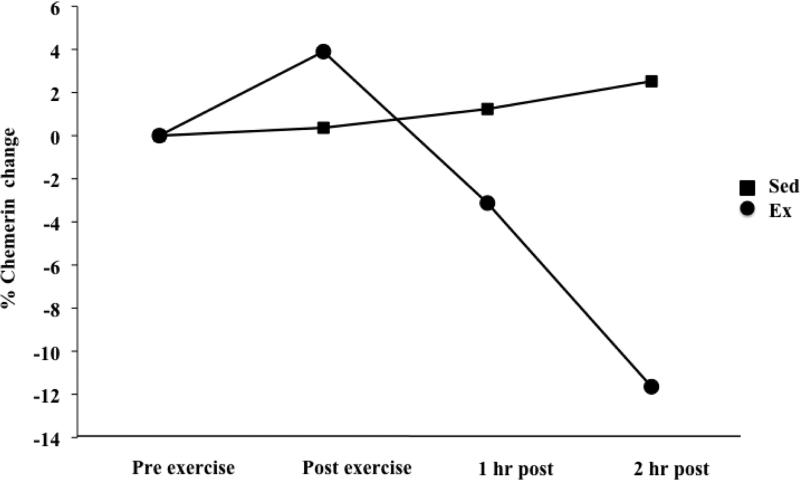
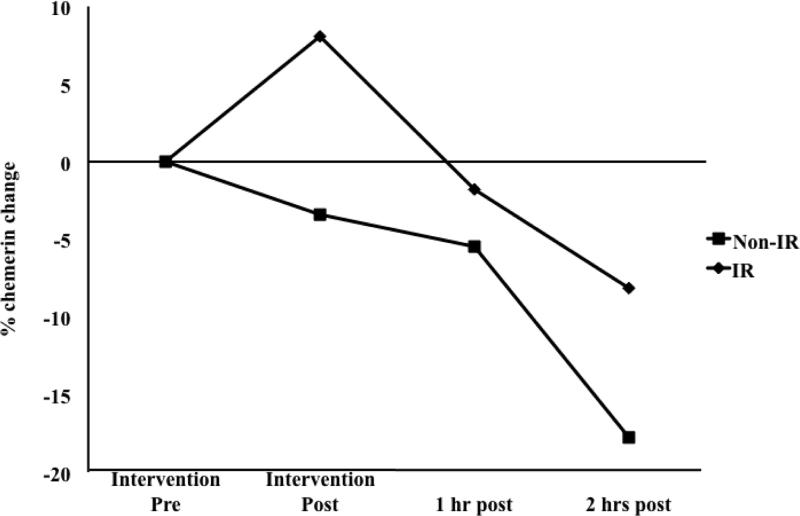
The general linear model showed no significant time by condition interaction; indicating that the exercise condition did not result in changes in chemerin concentrations over time that were significantly different from the changes that occurred during the sedentary condition when considering all time points. Paired-samples t-tests did, however, show a 12.9% (44.0 ± 70.6 ng/mL) mean decrease in chemerin concentration from the immediate post-exercise time point to two hours post-exercise (p=0.07), with a small mean increase (2.8%; 8.1 ±57.9 ng/mL; p=0.65) observed during that time period in the sedentary condition (p=0.06 for difference in post-to-2hr chemerin change between conditions; Figure 2). After controlling for subjects’ HOMA-IR scores, the change in chemerin levels from baseline to two hours post-exercise was significantly different than the change observed during the same time period in the sedentary condition (p=0.02). No significant effect of exercise was observed in separate models adjusting for subjects’ HOMA-β or QUICKI values. Post hoc stratified analyses showed differences in exercise-induced changes in chemerin between insulin resistant (HOMA-IR ≥ 3.5) and non-insulin resistant subjects (HOMA-IR < 3.5; Figure 3). A mean increase (8.1%; 15.6 ±72.7 ng/mL) in chemerin concentrations immediately following exercise occurred only in insulin resistant individuals (n=7), while chemerin concentrations decreased consistently over time during the exercise condition in non-insulin resistant subjects (n=4). At 2h post-exercise, chemerin concentrations in insulin resistant subjects were an average of 8.1% (29.2 ±71.1 ng/mL) below pre-exercise levels, compared to a mean of 17.8% (47.5 ±70.1 ng/mL) below pre-exercise levels in non-insulin resistant subjects. However, the differences in chemerin changes between insulin resistant and non-insulin resistant subjects were not statistically significant.
Figure 2.
Percentage change in chemerin from pre- to post-intervention time points.
Figure 3.
Percentage change in circulating chemerin in the exercise condition, by insulin resistance status (non-IR: n=4; IR: n=7).
Discussion
This study aimed to determine the effect of an acute bout of moderate intensity walking exercise on chemerin levels in obese individuals. We used a within-subjects crossover protocol to compare changes in chemerin concentration between acute exercise and sedentary conditions. We chose to assess the effect of an acute bout of exercise on chemerin levels because: 1) to our knowledge, the effect of a single exercise bout on chemerin has not been explored previously; and 2) this protocol allowed us to isolate the physiological effect of exercise without the accompanying weight loss typically seen in obese individuals undergoing chronic activity. Although our hypothesis that an hour of treadmill exercise would decrease circulating chemerin levels was not supported by our unadjusted analysis, we did uncover a significant effect of exercise on chemerin concentrations after controlling for subjects’ HOMA-IR scores; a measure of insulin resistance. We also observed a difference in chemerin dynamics following exercise between obese subjects with and without insulin resistance, although this study was not powered to detect a statistical difference between these two groups.
Our findings of exercise-induced decreases in chemerin are consistent with previous research showing decreases in chemerin concentrations following chronic exercise interventions (Chakaroun et al. 2012, Saremi et al. 2010, Lee et al. 2013, Kim et al. 2013, Veojarvi et al. 2013). However, to our knowledge, this is the first study to observe decreases in chemerin concentrations elicited by a single bout of exercise, as well as the first to suggest a difference in the effect of exercise between individuals who are insulin resistant and those who are non-insulin resistant. Given that the association between exercise and chemerin changes observed in this study cannot be attributed to changes in body weight and composition that can result from chronic exercise, our findings support the notion that exercise-induced changes in insulin sensitivity may be facilitated by changes in chemerin. Preliminary findings from an animal study in our lab indicate that decreases in circulating chemerin following an exercise intervention are associated with decreases in HOMA-IR. Additionally, two recent studies observed associations between changes in chemerin and improvements in insulin sensitivity following chronic exercise interventions. Stefanov et al. (2014) found decreases in serum chemerin following a 6-month endurance and resistance training regimen to be associated with concomitant decreases in HOMA-IR. Likewise, Kim et al. (2013) observed a correlation between decreases in serum chemerin and improvements in the insulin sensitivity index following a 12-week lifestyle modification program which also included both aerobic and resistance exercise.
Our results also suggest that chemerin concentrations may increase during exercise in insulin resistant individuals, thereby resulting in an attenuation of the post-exercise decrease relative to baseline levels. This is in contrast to the consistent decrease observed during and following exercise in non-insulin resistant subjects. The differences in chemerin dynamics between these two groups, however, were not significant, possibly due to the very small sample sizes. Future studies designed specifically to compare insulin resistant to non-insulin resistant adults should be done to further examine the potential differences in exercise-induced chemerin changes between these groups. Further work may also be necessary to determine whether a higher intensity or longer duration of exercise is necessary to acutely lower circulating chemerin levels in insulin resistant individuals, similar to the chemerin changes observed in subjects without insulin resistance.
Some studies have shown a positive relationship between circulating chemerin levels and concentrations of the pro-inflammatory cytokine interleukin (IL)-6. (Lehrke et al. 2009, Weigert et al. 2010). A study by Kern et al (2001) demonstrated that obese subjects have significantly greater plasma levels of IL-6, compared to normal-weight controls, and these IL-6 levels are significantly inversely related to insulin sensitivity. Circulating IL-6 concentrations, already elevated in an insulin resistant state, are further elevated during exercise because muscular contraction promotes the release of IL-6 and other cytokines into the systemic circulation (Pedersen et al. 2004). Though IL-6 is known to be elevated during exercise and peak immediately after, concentrations subsequently drop for several hours following exercise (Pedersen et al. 2000). This trend in exercise-induced IL-6 dynamics is similar to that observed for circulating chemerin in our study, which may warrant further investigation into chemerin's relationship with IL-6. The combined effects of exercise and insulin resistance on serum IL-6 may provide reasoning for our results of an increase in chemerin concentrations during exercise in insulin resistant subjects.
The single bout of aerobic exercise in this study was performed at a moderate intensity (60-65% of VO2peak) and it may not have been sufficient to acutely impact chemerin levels in some individuals. However, some of our subjects were fatigued at the end of the session and it would have been difficult for them to sustain a more intense level of exercise. We did, however, observe a near-significant (p<0.06) 12% mean drop in chemerin two hours after exercise, with no indication of a plateau within this time period. Additionally, it is possible that chemerin levels continued to decrease beyond the two hour post-exercise time point as the metabolic effect of an acute exercise bout can last up to 24 hours, but we could not verify this phenomenon with the data available for this analysis. Future research should analyze time points extrapolated out further than two hours.
Per the protocol of the original study of feeding, exercise, and satiety (Holmstrup et al. 2013), subjects ingested liquid meals at two different time points on both the sedentary and exercise intervention days. Though it may be argued that acute meal ingestion could affect circulating chemerin levels, studies by Chamberland et al. (2013) and van Herpen et al. (2013) observed only extreme fasting to significantly alter circulating chemerin levels, with no differences observed following non-fasted feeding trials.
In this study, we observed not only great between-subject variability in exercise-induced changes in chemerin levels from pre-exercise to two hours post-exercise (range: −43% to +22%), but also great within-subject variability in chemerin levels across time points during the sedentary condition (range: −40% to +81% change from pre to 2 hrs post). Given the apparent fluctuations in chemerin concentrations even during times of relative inactivity, a larger sample may be necessary to more precisely characterize the changes in chemerin elicited by an acute bout of exercise.
Our findings show that baseline chemerin levels correlated with percentage of body fat but not with BMI. This result suggests that chemerin systemic levels are linked more to adipocyte number than body mass, as body fat percentage is a more accurate assessment of adipocyte contribution to overall body weight. We also observed positive relationships between baseline circulating chemerin levels and HOMA-IR, QUICKI, and HOMA-β. HOMA-β is a measure of pancreatic β-cell function, which is responsible for insulin secretion. In a study by Takahashi et al (2011), chemerin and its receptor Chem23 were shown to be present in β-cells in mice, suggesting that the pancreatic insulin-producing cells may be a target for chemerin action. In our study, however, the correlation observed between circulating chemerin and HOMA-β was positive, indicating a relationship between the two in which higher chemerin concentrations are associated with greater insulin secretion from the pancreatic beta cells. Though this appears counterintuitive given the observed association of elevated chemerin levels with impairments in insulin action, it may be that the beta cell function of these non-diabetic subjects has yet to be negatively affected by chemerin and the other inflammatory cytokines that contribute to insulin resistance. The beta cells, then, may continue to compensate for the systemic impairments in insulin-stimulated glucose uptake resulting in the positive correlation between circulating chemerin and HOMA-β. However, further research is needed to fully characterize the relationship between chemerin and beta cell function.
A recent report suggested that circulating chemerin levels may be a robust biomarker of early insulin disturbance, as chemerin levels were shown to be different between subjects presenting glucose impairment and glucose tolerance (Ouwens et al. 2012). Our data point in the same direction as we showed that the magnitude of exercise-induced changes in circulating chemerin varied depending on HOMA-IR scores and were less favorable for subjects with scores that depicted insulin resistance. In other words, these findings suggest that individuals who exhibit normal insulin utilization may display a greater decrease in circulating chemerin levels following an acute bout of exercise, compared to those with HOMA-IR scores indicative of impaired insulin action.
We also examined other variables that we believed could potentially be associated with chemerin levels, including gender. Although Weigert et al (2010) found that chemerin levels were similar between females and males, we observed significantly greater resting chemerin levels among females (346.2 ±44.5 vs. males: 261.9 ±50.2), as well as substantial differences in correlations of chemerin with BMI (r=0.27 vs. r=0.97), body fat percentage (r=0.36 vs. r=0.99), HOMA-IR (r=0.53 vs. r=0.79), and QUICKI (r=−0.65 vs. r=−0.99) between males and females, respectively. However, this discrepancy between our observed gender differences and previous research could be explained by the low number of females (n=3) recruited into the original study from which our data were drawn, as well as the fact that Weigert et al. recruited subjects that suffered from type 2 diabetes (2010). Future investigations using gender-stratified analyses would aid our understanding of obesity-induced chemerin kinetics in response to exercise and the potential for sexually dimorphic effects.
In conclusion, this study suggests a potential effect of acute exercise in decreasing circulating chemerin levels in obese adults, with a greater decrease observed among non-insulin resistant subjects. These data allude to a trend of decreasing chemerin levels in the post-exercise period, which should be investigated beyond the 2 hour window observed in this study. Our observation of a greater change in chemerin with our exercise protocol among subjects without insulin resistance suggests that insulin resistant individuals may require a more intense or prolonged exercise bout to elicit reductions in circulating chemerin. These findings contribute to the growing body of knowledge of how exercise can affect circulating chemerin levels in obese individuals and provides the basis for future investigations.
Acknowledgments
We thank all of the research subjects for their participation in this study. This study was supported the Syracuse University School of Education Graduate Student Creative Writing grant Joan L Berstein (JL), and NIH grant R21DK063179.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bozaoglu K, Bolton K, McMillian J, et al. Chemerin is a novel adipokine associated with the obesity and metabolic syndrome. Endocrinology. 2007;148:4687–4694. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 2.Bozaoglu K, Segal D, Shields K, et al. Chemerin Is Associated with Metabolic Syndrome Phenotypes in a Mexican-American Population. J. Clin. Endocrinol. Metab. 2009;94:3085–3088. doi: 10.1210/jc.2008-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blüher M, Rudich A, Klôting N, et al. Two patterns of adipokine and other biomarker dynamics in a long-term weight loss intervention. Diabetes Care. 2012;35:342–349. doi: 10.2337/dc11-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goralski K, McCarthy T, Hannimann E, et al. Chemerin: A novel sadipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007;282:28175–28188. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi M, Takahashi Y, Takahashi K, et al. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. FEBS Lett. 2008;582:573–8. doi: 10.1016/j.febslet.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Sell H, Laurencikiene J, Taube A, et al. Chemerin is a Novel Adipokine-Derived Factor Inducing Insulin Resistance in Primary Human Skeletal Muscle Cells. Diabetes. 2009;58(12):2731–2740. doi: 10.2337/db09-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kralisch S, Weise S, Sommer G, et al. Interleukin-1beta induces the novel adipokine chemerin in adipocytes in vitro. Regul. Pept. 2009;154:102–106. doi: 10.1016/j.regpep.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Lehrke M, Becker A, Greif M, et al. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary heart atherosclerosis. Eur. J. Endocriniol. 2009;161:339–344. doi: 10.1530/EJE-09-0380. [DOI] [PubMed] [Google Scholar]
- 9.Weigert J, Neumeier M, Wanninger J, et al. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin. Endocrinol. 2010;72:342–348. doi: 10.1111/j.1365-2265.2009.03664.x. [DOI] [PubMed] [Google Scholar]
- 10.Ernst M, Issa M, Goralski K, et al. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology. 2010;154:1998–2007. doi: 10.1210/en.2009-1098. [DOI] [PubMed] [Google Scholar]
- 11.Bradley RL, Jeon JY, Liu F, et al. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2008;295:586–594. doi: 10.1152/ajpendo.00309.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bordenave S, Brandou F, Manetta J, et al. Effects of acute exercise on insulin sensitivity, glucose effectiveness and disposition index in type 2 diabetic patients. Diabetes & Metabolism. 2008;34:250–257. doi: 10.1016/j.diabet.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Chakaroun R, Raschpichler M, Kloting N, et al. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism Clin. And Experimental. 2012;61:706–714. doi: 10.1016/j.metabol.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Saremi A, Shavandi N, Parastesh M, et al. Twelve-week aerobic training decreases chemerin level and improves cardiometabolic risk factors in overweight and obese men. Asian Jour. Of Sports Med. 2010;3:151–158. doi: 10.5812/asjsm.34860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee MK, Chu SH, Lee DC, et al. The association between chemerin and homeostasis assessment of insulin resistance at baseline and after weight reduction via lifestyle modifications in young obese adults. Clin Chim Acta. 2013;5(421):109–115. doi: 10.1016/j.cca.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH,, Lee SH, Ahn KY, et al. Effects of lifestyle modifications on serum chemerin concentration and its association with insulin sensitivity in overweight and obese adults with type 2 diabetes. Clin Endocrinol. 2014;80(6):825–33. doi: 10.1111/cen.12249. [DOI] [PubMed] [Google Scholar]
- 17.Veojarvi M, Wasenius N, Manderoos S, et al. Nordic walking decreased circulating chemerin and leptin concentrations in middle-aged men with impaired glucose regulation. 2013;45:162–170. doi: 10.3109/07853890.2012.727020. [DOI] [PubMed] [Google Scholar]
- 18.Goodyear LJ, Chang PY, Sherwood DJ, et al. Effects of exercise and insulin on mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Am J Physiol Endocrinol Metab. 1996;271:E403–E408. doi: 10.1152/ajpendo.1996.271.2.E403. [DOI] [PubMed] [Google Scholar]
- 19.Oakes ND, Bell KS, Furler SM, et al. Diet-induced muscle insulin resistance in rats is ameliorated by acute dietary lipid withdrawal or a single bout of exercise: parallel relationship between insulin stimulation of glucose uptake and suppression of long-chain fatty acyl-CoA. Diabetes. 1997;46:2022–2028. doi: 10.2337/diab.46.12.2022. [DOI] [PubMed] [Google Scholar]
- 20.King PA, Betts JJ, Horton ED, et al. Exercise, unlike insulin, promotes glucose transporter translocation in obese Zucker rat muscle. Am J Physiol Regulatory Integrative Comp Physiol. 1993;265:R447–R452. doi: 10.1152/ajpregu.1993.265.2.R447. [DOI] [PubMed] [Google Scholar]
- 21.Holmstrup ME, Fairchild TJ, Keslacy S, et al. Satiety, but not total PYY, is increased with continuous and intermittent exercise. Obesity. 2013;21(10):2014–2020. doi: 10.1002/oby.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanaley JA, Goulopoulou S, Franklin RM, et al. Plasticity of heart rate signaling and complexity with exercise training in obese individuals with and without type 2 diabetes. Int J Obesity. 2009;33(10):1198–1206. doi: 10.1038/ijo.2009.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American College of Sports Medicine . ACSM's Guidelines for Exercise Testing and Prescription. 8th ed. Lippincott, Williams, & Wilkins; Philadelphia: 2009. [Google Scholar]
- 24.Stefanov T, Blüher M, Vekova A, et al. Circulating chemerin decreases in response to a combined strength and endurance training. Endocrine. 2014;45:382–391. doi: 10.1007/s12020-013-0003-2. [DOI] [PubMed] [Google Scholar]
- 25.Kern P, Ranganathan S, Li C, et al. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen B, Steensberg A, Fischer C, et al. The metabolic role of IL-6 produced during exercise: is IL-6 an exercise factor? Proceedings of the Nutrition Society. 2004;63:263–267. doi: 10.1079/PNS2004338. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen BK, Toft AD. Effects of exercise on lymphocytes and cytokines. Br. J. Sports Med. 2000;34:246–251. doi: 10.1136/bjsm.34.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chamberland JP, Berman RL, Aronis KN, et al. Chemerin is expressed mainly in pancreas and liver, is regulated by energy deprivation and lacks day/night variation in humans. Eur J Endocrinol. 2013;169:453–462. doi: 10.1530/EJE-13-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Herpen NA, Sell H, Eckel J, et al. Prolonged fastging and the effects on biomarkers of inflammation and on adipokines in healthy lean men. Horm Metab Res. 2013;45:378–382. doi: 10.1055/s-0032-1330015. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi M, Okimura Y, Iguchi G, et al. Chemerin regulates β-cell function in mice. Sci Rep. 2011;1:123. doi: 10.1038/srep00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ouwens DM, Bekaert M, Lapauw B, et al. Chemerin as biomarker for insulin sensitivity in males without typical characteristics of metabolic syndrome. Arch Physiol Biochem. 2012;118(3):135–8. doi: 10.3109/13813455.2012.654800. [DOI] [PubMed] [Google Scholar]