Abstract
Hot flashes (HFs) are a common side effect of cancer treatment. The purpose of this systematic review was to evaluate evidence related to the use of acupuncture for HFs in cancer patients. EMBASE, MEDLINE, Cochrane (all databases), PubMed, the Cumulative Index to Nursing and Allied Health Literature, and Scopus were searched from their inception through December 2014. Included studies had to be randomized controlled trials with a usual-care and/or placebo comparison group that investigated acupuncture to treat HFs in cancer patients. No language limits were applied. The risk of bias (ROB) was rated as low, high, or unclear according to Cochrane criteria. Both within-group and between-group changes were evaluated. Four hundred two items were identified, and 192 duplicates were omitted; this left 210 publications to be screened. Eight studies met the inclusion criteria, and all involved women with breast cancer. All studies showed significant within-group improvement from the baseline for true acupuncture (TA). One study showed significant improvement in favor of TA over sham acupuncture (SA; P < .001), 1 study found in favor of TA over SA for nighttime HFs only (P = .03), and 1 study found in favor of TA over SA or untreated controls (P < .01 and P < .001, respectively). Between-group (TA vs SA) effect size (ES) estimates for daytime and nighttime HFs were calculated (ES range, 0.04–0.9) whenever possible. No studies were rated with a low ROB. In conclusion, the current level of evidence is insufficient to either support or refute the benefits of acupuncture for the management of HFs in cancer patients. Future studies should provide within-group and between-group ES estimates in addition to P values.
Keywords: acupuncture, cancer, hot flashes, hot flushes, integrative medicine, systematic review
INTRODUCTION
Hot flashes (HFs), a sensation of heat flushing over the skin,1,2 are a common complaint among cancer patients undergoing treatment as well as cancer survivors, particularly those who have undergone oophorectomy, orchiectomy, chemotherapy, and/or hormone therapy.3 HFs can be severe and are often accompanied by sweating, palpitations, dizziness, nausea, chills, fatigue, poor sleep, or mood disturbances. These symptoms may persist for years and can affect work, social activities, concentration, energy levels, and overall quality of life.4 In a cross-sectional survey of women treated for breast cancer,5 the severity of HFs and associated sweats was significantly correlated with poorer patient-reported overall quality of life (rs = 0.47). Up to 80% of women taking tamoxifen reported experiencing HFs, with 30% rating them as severe. Only 21% of women experiencing HFs were receiving treatment for them, and most participants described no knowledge or poor knowledge of HF treatment options.5 Patients taking anastrozole or other aromatase inhibitors also experience a higher incidence and severity of HFs than the general population.3,6,7
Effective treatment options for HFs are particularly limited for women with estrogen receptor–positive disease, and some treatments may not provide complete relief or may have unpleasant side effects. For example, gabapentin or antidepressants such as selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors may cause nausea, drowsiness, dizziness, dry mouth, or headache, and many women decline selective serotonin reuptake inhibitors/serotonin-norepinephrine reuptake inhibitors because of the associated stigma of taking an antidepressant.8 if HF symptoms are severe and cannot be managed effectively, some patients may choose to discontinue their cancer treatments.3,8–10
Because of a lack of treatment options, cancer patients often turn to integrative therapies to help manage HF symptoms, with many adding acupuncture to their treatment plan.11 According to the theory of traditional Chinese medicine, the body’s Qi (pronounced “chee”) or vital energy can be stimulated by the insertion of small needles into specific body locations. Acupuncture is defined as the placement of very small, sterile, solid, stainless steel needles into points on the body that are believed to be more bioelectrically conductive and less resistant than surrounding tissues.12 Although the specific mechanisms of how acupuncture may reduce HFs in cancer patients are unknown, acupuncture has been shown to alter the concentration of β-endorphins.13 It has been suggested that HFs due to hormonal changes may be related to a reduced concentration of β-endorphin in the hypothalamus, which leads to a drop in the set point of the thermoregulation center14–16 and increased release of calcitonin gene–related peptide, a potent vasodilator that may mediate vasomotor symptoms.17,18
Although previous systematic reviews of acupuncture for HFs in cancer patients have been published,19–21 only 1 study21 was conducted within the past 5 years. The authors of that study concluded that acupuncture improved HF symptoms in cancer patients, with benefits lasting at least 3 months after the end of acupuncture treatment, although other reviews have reported equivocal findings.19 To assess the quality of included studies, the review21 used a Jadad score22 rather than the more comprehensive Cochrane risk of bias (ROB) assessment23 and did not report effect size (ES) estimates. Therefore, to assist with informed clinical decision making, the purpose of the current review was to update findings from previous studies by 1) identifying randomized controlled trials (RCTs) evaluating acupuncture for HFs in cancer patients, 2) evaluating the ROB for included studies on the basis of Cochrane criteria,23 and 3) calculating treatment ES estimates where appropriate.
MATERIALS AND METHODS
PubMed, Scopus, EMBASE, MEDLINE, the Cumulative Index to Nursing and Allied Health Literature, and Cochrane (all databases) were searched from the time of their inception through December 2014 by a professional medical research librarian for articles reporting the results of RCTs related to the use of acupuncture for HFs in cancer patients. Key search terms included randomized controlled trial, clinical trial, hot flash, hot flush, acupuncture, electroacupuncture, cancer, carcinoma, oncology, and neoplasm. No language limitations were applied.
Study Selection
Two authors (M.K.G. and L.G.-G.) independently screened articles for inclusion. The studies were included if they were prospective RCTs that evaluated acupuncture to treat HFs in patients with cancer and involved needle insertion at acupuncture points. Likewise, studies that did not include an acupuncture-only treatment group, evaluated therapies that were similar to acupuncture but did not involve needle insertion (acupressure, laser acupuncture, and electrostimulation without needles), compared only different types of active acupuncture, or did not include a usual-care and/or placebo comparison group and for which a final data analysis was not available were also excluded. When data were unclear, the corresponding author was contacted via e-mail for further clarification.
Data Abstraction and ROB Rating
All articles that met the inclusion criteria were independently rated with the Cochrane ROB criteria (Table 1).23 The articles were reviewed by 2 authors (M.K.G. and L.G.-G.) for sequence generation/randomization, allocation concealment, selective outcome reporting, missing data, and any other potential source of bias. A study was rated as having a low ROB if all 5 criteria were met. If any single criterion was not met, the study was rated as having a high ROB. If it was unclear whether 1 or more of the criteria were met, the study was rated as having an unclear ROB. A lack of agreement between the authors rating the studies was resolved by discussion. After the ROB was determined, each study was evaluated for both within-group and between-group differences in HF symptoms. The magnitude of the treatment effect was estimated for trials that had at least 25 patients randomized per group and presented adequate summary statistics for calculating ES estimates between true acupuncture (TA) and sham acupuncture (SA).
TABLE 1.
Risk-of-Bias Criteria
| Domain | Criteria | Examples |
|---|---|---|
| Sequence generation | Allocation sequence was adequately generated. |
Used random number table Used computer random number generator Used coin tossing Used card or envelope shuffling Drew lots |
| Allocation concealment | Allocation of group assignment could not be foreseen before randomization. |
Used central allocation such as telephone or Web-based randomization Used sequentially numbered, opaque, sealed envelopes |
| Blinding of participants, personnel, and outcome assessors |
Knowledge of the allocated intervention was adequately prevented during the study. |
Blinding ensured for participants and key study personnel and unlikely to have been broken No blinding but unlikely that the outcome was influenced |
| Incomplete outcome data | Incomplete outcome data were adequately addressed. |
No missing outcome data Missing outcome data unlikely related to true outcome Missing outcome data balanced across groups with similar reasons for missing data across groups |
| Selective outcome reporting | The study was free of apparent selective outcome reporting. |
Study protocol available and all prespecified outcomes of interest reported Study protocol not available but all expected prespecified outcomes reported |
| Other sources of bias | The study was free of other problems that could introduce bias. |
Small sample size (<30/group randomized) All treatments provided by 1 acupuncturist to active and sham groups Baseline group differences Inconsistent or unclear recruitment strategies or treatment methods Study stopped early Vague/unclear outcome measures Short washout period in a crossover design |
Complete criteria for the judgment of a high, low, or unclear risk of bias can be found in Table 8.5.d of Cochrane Handbook for Systematic Reviews of Interventions23 (accessible at http://handbook.cochrane.org/chapter_8/table_8_5_d_criteria_for_judging_risk_of_bias_in_the_risk_of.htm).
RESULTS
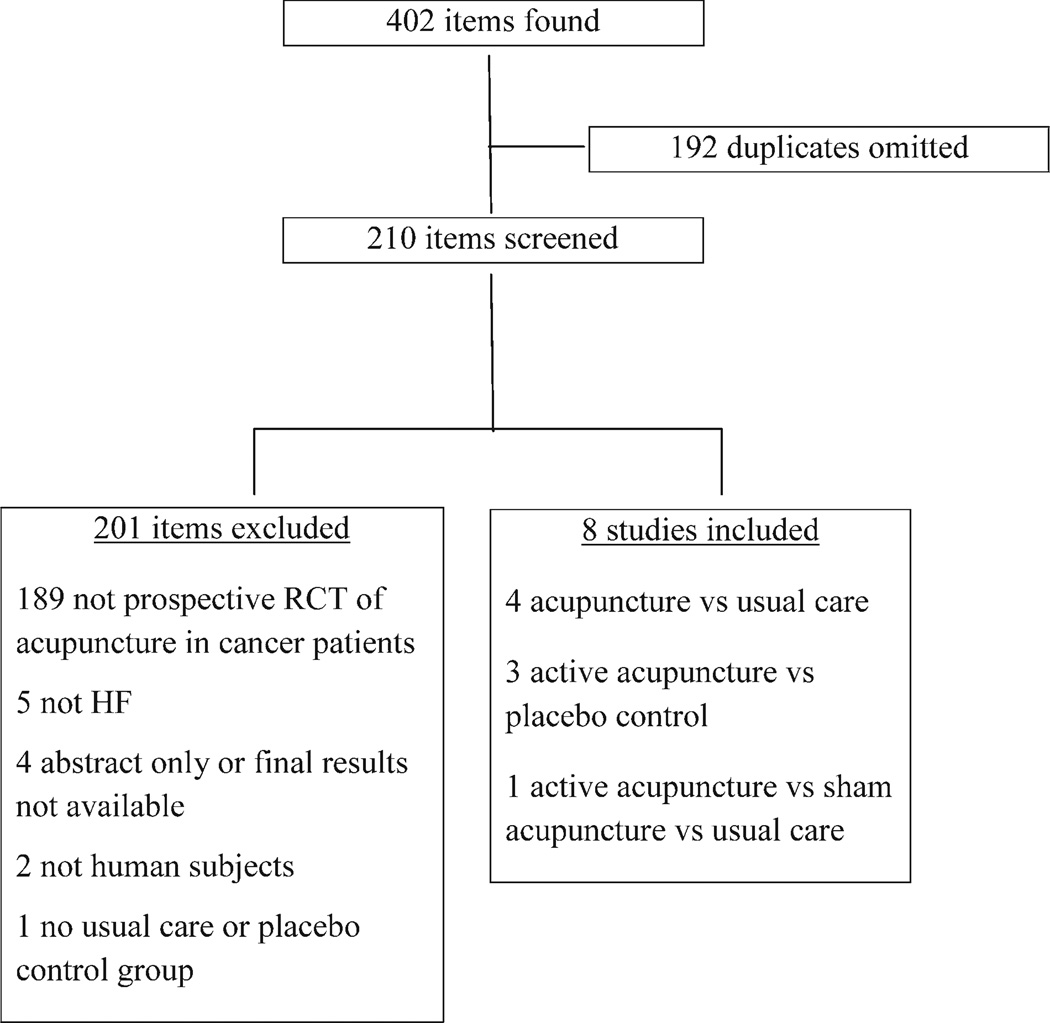
A total of 402 articles were identified with the search terms. One hundred ninety-two duplicates were omitted, and this left 210 publications that were screened. The screened studies were excluded for the following reasons: 189 were not prospective RCTs of acupuncture in cancer patients, 5 did not assess HFs, 4 were abstracts only or did not include final results, 2 did not include human subjects, and 1 did not include a usual-care or placebo comparison group. No studies evaluating the use of acupuncture to treat HFs in men with prostate cancer met our inclusion criteria. Therefore, 8 studies (n = 474 patients), all of which involved women with breast cancer, were included.7,10,24–29 The mean follow-up after the end of treatment for the 8 studies was 7.5 months (range, 1 week to 24 months).
One study compared TA to applied relaxation, 2 studies compared TA to pharmacological therapy, 4 studies compared TA to SA, and 1 study compared TA to both SA and no acupuncture. All studies showed significant within-group improvement from the baseline to the end of treatment for TA, and 4 studies showed significant within-group improvement from the baseline for SA. For significant between-group findings, 1 study showed significantly greater improvement for TA over SA (P < .001),27 1 study found in favor of TA over SA for nighttime HFs only (P = .03),28 and 1 study found in favor of TA over SA (P < .01) or untreated controls (P < .001).29 Importantly, in the 2 studies comparing TA to pharmacotherapy, TA was as effective in controlling HFs as venlafaxine7 and hormone replacement therapy.26 At 12 weeks, the median number of HFs per 24 hours decreased from 9.6 (interquartile range [IQR], 6.6–9.9) to 4.3 (IQR, 1.0– 7.1; P < .001) for electroacupuncture (EA; n = 19) and from 6.6 (IQR, 4.0–8.9) to 0.0 (IQR, 0.0–1.6; P = .001) for hormone therapy (n = 18).26 One study provided information that allowed between-group (TA vs SA) ES estimates for daytime HFs (≥0.9 ES),27 and 2 studies provided information or summary statistics for calculating between-group ES estimates for nighttime HFs (≥0.8 and 0.04 , respectively).27,28
No studies were rated as having a low ROB (Table 2). Four studies had a high ROB because of problems with blinding. 7,24,26,28 Three studies had an unclear ROB because of small sample sizes or an unclear blinding assessment, and 1 study with a crossover design was rated as having an unclear ROB because, although there were no significant between-group differences, additional improvement was seen in the SA group after participants crossed over to TA.25 The authors stated that short treatment and washout periods might have contributed to these findings.
TABLE 2.
Risk of Bias for Included Studies
| Study | Sequence Generation |
Allocation Concealment |
Blinding | Incomplete Outcome Data |
Selective Outcome Reporting |
Other Sources of Bias |
Level of Risk |
|---|---|---|---|---|---|---|---|
| Nedstrand 200624 | + | + | − | + | + | − | H |
| Deng 200725 | + | + | + | + | + | 0 | U |
| Frisk 200826 and Frisk 201231 (same data) | + | + | − | + | + | − | H |
| Hervik & Mjaland 200927 | + | + | + | + | + | 0 | U |
| Liljegren 201228 | + | + | − | + | + | 0 | H |
| Walker 20107 | + | 0 | − | + | − | − | H |
| Bao 201410 | + | + | + | + | + | 0 | U |
| Bokmand & Flyger 201329 | + | + | + | + | + | 0 | U |
Abbreviations: −, high risk of bias; +, low risk of bias; 0, unclear risk of bias; H, high risk of bias for 1 or more domains; U, unclear risk of bias for 1 or more domains.
The acupuncture treatment parameters of the studies are provided in Table 3. Among all the studies, a total of 29 points involving 11 channels (all major channels except Small Intestine) as well as the Governing Vessel and the Conception Vessel30 were used. The most common points used were Spleen 6, Liver 3, Kidney 3, and Stomach 36. Six studies used manual stimulation only,7,10,25,27–29 and 3 studies used electrostimulation.24,26,31 Among all trials, the average number of treatments given was 12 (range, 5–16; median, 14). The most common frequency of treatment sessions was twice weekly, with follow-up ranging from 0 to 24 months. No studies reported adverse events.
TABLE 3.
Treatment Parameters for Included Studies
| Study | Study Design/ Participants |
Acupuncture Points |
Type of Stimulation/ Retention |
No. of Acupuncture Treatments |
Treatment Frequency/ Follow-Up |
Primary Outcome(s) | Results/Conclusions | Limitations |
|---|---|---|---|---|---|---|---|---|
| Nedstrand 200624 |
RCT of women with breast cancer; EA (n = 19) vs applied relaxation (n = 19) |
EA: bilaterally from B23 to B32 + GV20 and bilateral B15, H7, Sp6, Sp9, Lv3, and P6 |
Electrostimulation; retained 30 min |
14 | Twice per wk for 2 wk, then once per wk for 10 wk Weekly 60-min applied-relaxation sessions for 12 wk Follow-up for 6 mo |
No. of HFs/24 h (per daily logbook) KI |
No sig differences were seen between groups, but a sig decrease was seen in No. of HFs/24 h from the baseline in both groups. No. of HFs decreased from 8.4 (95% CI, 6.6– 10.2) to 4.1 (95% CI, 3.0– 5.2) after 12 wk and to 3.5 (95% CI, 1.7–5.3) after 6 mo in the EA group (n = 17; P <.0001) and from 9.2 (95% CI, 6.6–11.9) to 4.5 (95% CI, 3.2–5.8) after 12 wk and to 3.9 (95% CI, 1.8–6.0) after 6 mo in the applied-relaxation group (n = 14; P < .001). |
Small sample size/ low statistical power; no patient blinding; no usual-care comparison group |
| Similarly for KI, a sig within- group improvement (P < .001 for both) was seen, but no sig between-group differences were found. |
||||||||
| Conclusion: There were within-group improve- ments from the baseline only. |
||||||||
| Deng 200725 | Crossover RCT of women with breast cancer and 3 or more HFs/d; TA (n = 39 at 6 wk; n = 33 at 6 mo) vs SA (n = 28 at 6 wk; n = 17 at 6 mo); after 6 wk, 17 SA patients crossed over to TA. |
TA: GV14, bilateral Gb20, B13, P7, H6, K7, St36, and Sp6 + ear Shenmen and ear Sympathetic SA: nonpenetrating needles applied a few centimeters from TA points |
Manual; retained 20 min |
8 | Twice per wk for 4 wk Follow-up for 6 mo |
No. of HFs/24 h (per daily diary |
Mean No. of HFs/24 h was reduced from 8.7 (SD, 3.9) to 6.2 (SD, 4.2) in the TA group and from 10.0 (SD, 6.1) to 7.6 (SD, 5.7) in the SA group. TA was associ- ated with 0.8 fewer HFs/d than SA at 6 wk, but no sig between-group differ- ences were found (95% CI, −0.7 to 2.4; P = .3). When participants in the SA group crossed over to TA, a further reduction in HFs was seen, and it per- sisted for up to 6 mo after the completion of treatment. |
Crossover design with small sam- ple; short treat- ment and washout period |
| Conclusion: No sig between- group differences were seen, but additional improvement was seen af- ter SA patients crossed over to TA. |
||||||||
| Frisk 200826 and Frisk 201231 (same data) |
RCT of women with breast cancer; EA (n = 27) for 12 wk vs HT (n = 18) for 24 mo |
EA bilaterally from B23 to B32 + GV20 and bilateral B15, H7, Sp6, Sp9, Lv3, and P6 |
Electrostimulation; retained 30 min |
14 | Twice per wk for 2 wk followed by 10 weekly sessions HT given for 2 y and then stopped Follow-up for 24 mo |
No. of HFs/d and No. of HFs/night for 24 h (per daily logbook); HF distress KI |
At 12 wk, median No. of HFs/24 h decreased from 9.6 (IQR, 6.6–9.9) to 4.3 (IQR, 1.0–7.1; P < .001) for EA (n = 19) vs 6.6 (IQR, 4.0–8.9) to 0.0 (IQR, 0.0– 1.6; P = .001) for HT (n = 18). |
Small sample size; no patient blinding |
| For KI, a sig improvement was found over time within groups and in favor of HT for between-group com- parisons (at 12 mo, P = .002; at 24 mo, P = .039). |
||||||||
| Conclusion: Both EA and HT had a persistent sig effect over time, with differences between groups in favor of HT (P < .001) 12 mo after the start of treatment. EA is a possible treatment for HFs in this population. |
||||||||
| Hervik & Mjaland 200927 |
Women with breast cancer; TA (n = 30) vs SA (n = 29) |
TA: Lv3, Gb20, Lu7, K3, Sp6, CV4, P7, and Lv8 SA: superficial nee- dling at 4 bilateral points located 5, 10, and 15 cm proximal to the upper border of the patella and 1 point over the highest point of the trape- zius muscle |
Manual; retained 30 min |
15 | Twice per wk for 5 wk followed by 5 weekly sessions Follow-up for 12 wk |
No. of HFs/d and No. of HFs/night (recorded on the same day each wk for 4 wk before treat- ment, during 10-wk treatment period, and 12 wk after treatment) KI |
A sig between-group differ- ence was found for day- time HFs in favor of TA during treatment and at 12 wk (P < .001 for both), with similar findings for nighttime HFs (P = .009 during treatment; P < .001 at 12 wk). For KI, a sig difference was found in favor of TA after treatment (P = .004) and at 12 wk (P = .001). |
Small sample size; both groups treated by 1 acupuncturist (unblinded) |
| Conclusion: A sig decrease in daytime and nighttime HFs in favor of TA over SA was seen. |
||||||||
| Liljegren 201228 | Women with breast cancer; TA (n = 38) vs SA (n = 36) |
TA: LI4, H6, Lv3, and St36 unilaterally and Sp6 and K7 bilaterally SA: nonpenetrating needle on same meridian 1 cm from TA points |
Manual; retained 20 min |
10 | Twice per wk for 5 wk Follow-up for 1 wk |
No. of HFs/d and No. of HFs/ night (recorded at the baseline and 1, 3, and 18 wk after EOT) |
Both groups reported improvement from the baseline in the severity and frequency of HFs (42% [16/38] at 6 wk [ie, 1 wk after EOT] for TA vs 47% [17/36] for SA [95% CI, −28% to 18%]). A sig improvement was also found in favor of TA for nighttime HF severity (P = .03), but no other sig between-group differences were found. |
Both TA and SA participants able to accu- rately guess group assign- ment at EOS (69.4% and 63.9%, respectively); almost all treatments given by same (unblinded) acupuncturist |
| CONCLUSION: Sig between- group differences in favor of TA were observed for nighttime HFs only |
||||||||
| Walker 20107 | Women with breast cancer; TA (n = 25) vs venlafaxine (n = 25) |
Bilateral core points: B23, K3, and Sp6 Secondary points based on TCM di- agnosis: GV14, GV20, Gb20, Lu9, Lv3, St36, CV6, P7, and H7 |
Manual; retained 30 min |
16 | Twice per wk for 4 wk, then 8 weekly sessions Venlafaxine at 37.5 mg PO HS for 1 wk followed by 75 mg HS for 11 wk Follow-up for 1 y |
No. of HFs/24 h (per HFD) |
Sig decreases (50%) in HFs were observed between the baseline and EOT for both groups with no sig between-group differen- ces; this indicated that TA was as effective as venla- faxine. By 2 wk after treat- ment, the venlafaxine group experienced sig increases in HFs, whereas HFs in the TA group remained low. The venla- faxine group experienced 18 occurrences of adverse effects (eg, nausea, dry mouth, dizziness, and anx- iety), whereas there were no adverse effects in the TA group. |
Small sample size; no blinding |
| CONCLUSION: TA was as effective as drug therapy with fewer adverse events. |
||||||||
| Bao 201410 | Women with stage 0- III breast cancer; TA (n = 23) vs SA (n = 24) |
TA: CV4, CV6, CV12, bilateral LI4, SJ6, Gb34, St36, K3, and B65 SA: nonpenetrating device in 14 nona- cupuncture points |
Manual; retained 20 min |
8 | Weekly Follow-up for 24 wk |
Severity; No. of HFs/24 h (per HFD) HFRDI |
For TA, sig improvements at 8 wk were found for HF severity (P = .006), HF fre- quency (P = .011), and HFRDI (P = .014), whereas for SA, improvement was found only for HFRDI (P = .043). African American patients (n = 9) benefited more from TA than SA in comparison with non-Afri- can American patients (n = 38) in reducing HF se- verity (P <.001) and fre- quency (P <.001). |
Small sample size; study powered to detect improvements in AIMSS, not sig differences between TA and SA |
| CONCLUSION: Within-group improvements were found for both TA and SA with no sig between-group differences. |
||||||||
| Bokmand & Flyger 201329 |
Women with breast cancer; TA (n = 31) vs SA (n = 29) vs NA (n = 34) |
TA: bilateral H6, K3, Sp6, and Lv3 SA: bilateral nonacu- puncture points (4) with superficial insertion |
Manual; retained 15–20 min |
5 | Weekly Follow-up for 12 wk |
HF distress (0–10 on VAS per logbook) |
Sig less distress was found in favor of TA vs SA (P < 0.01) or NA (P < .001), with effects occurring after the second acupuncture session and lasting at least 12 wk. CONCLUSION: TA was more effective than either SA or NA for HF distress in this population. |
Unclear how patient-reported HF distress per VAS compares with measures used in other studies (eg, No. of HFs/24 h) |
Abbreviations: AIMSS, aromatase inhibitor musculoskeletal symptoms; B, bladder; CI, confidence interval; GV, governing vessel; EA, electroacupuncture; EOS, end of study; EOT, end of treatment; Gb, gallbladder; H, heart; HF, hot flash; HFD, hot flash diary; HFRDI, Hot Flash-Related Daily Interference Scale; HS, at bedtime; HT, hormone replacement therapy; IQR, interquartile range; K, kidney; KI, Kupperman index; LI, large intestine; Lu, lung; Lv, liver; NA. no acupuncture; R pericardium; PO, by mouth; RCT, randomized controlled trial; CV, conception vessel; SA, sham acupuncture; SD, standard deviation; sig, statistically significant; SJ, san jiao (triple burner); Sp, spleen; St, stomach; TA, true acupuncture; TCM, traditional Chinese medicine; VAS, visual analogue scale.
DISCUSSION
This systematic review was conducted to provide an update to prior reviews of the use of acupuncture for the treatment of HFs in cancer patients. On the basis of our analysis of 8 studies (n = 474), we found that the current level of evidence is insufficient to either support or refute the benefits of acupuncture for treating HFs in cancer patients. First, the heterogeneity of comparators (applied relaxation, pharmacological therapy, SA, and no acupuncture) among the 8 trials makes the interpretation of findings difficult. Significant between-group differences in favor of TA were reported in 3 studies,27–29 and 1 study reported that TA was as effective as venlafaxine in controlling HFs with fewer side effects.7 Another trial that compared acupuncture with electrical stimulation (ie, EA) to hormone therapy concluded that both EA and hormone therapy had a persistent, significant effect over time.26 Although symptoms in the EA group tended to increase over the 24-month follow-up period, the authors stated that acupuncture is a viable treatment option for HFs, especially for women with estrogen receptor–positive disease who cannot take hormone therapy. The authors conceded a remaining question: whether or not symptoms returned because putative mechanisms were related to nonspecific placebo effects.
Three studies found within-group improvements only; however, none of these trials were rated as having a low ROB.10,24,25 Because of methodological problems such as a lack of blinding or small sample sizes, findings must be viewed with caution. Although adequately powered 3- or 4-armed RCTs comparing TA, SA, and pharmacologic therapy and/or no-treatment controls would be the most meaningful in terms of making informed clinical decisions, such trials are time-consuming and expensive and have not yet been conducted.
In pharmaceutical research, an ideal sham comparison must be indistinguishable from the active treatment and physiologically inert. Which methods best achieve this same standard in acupuncture research remains highly debated; thus, the various methods used to deliver SA treatment (a placebo needle at a nonacupuncture point, a placebo needle located on the same meridian as acupuncture points, and superficial needling) in the included studies complicated the interpretation. Because of the many difficulties associated with SA, the National Center for Complementary and Integrative Health at the National Institutes of Health currently states that the inclusion of an SA treatment arm in RCTs is of low programmatic priority.32 Some investigators, however, feel that parsing out specific effects related to needling from nonspecific placebo effects provides important information that is helpful for optimizing treatment methods. Because the patient-provider relationship is a robust component of a placebo effect,33 it is also important to note that in many acupuncture studies, a single acupuncturist who is not blinded to group assignment provides all treatments. Even inadvertently, an increased ROB can be introduced when 1 individual provides all treatments to both active and sham groups.
Another common problem in acupuncture research is the lack of understanding of putative mechanisms. Without clear biological measures, separating the specific effects of needling from nonspecific effects is challenging. However, we also know that even placebo effects have a biological component.34 Although functional magnetic resonance imaging studies have demonstrated differences between active acupuncture and SA, the central nervous system effects of acupuncture remain unknown. Some researchers35 have suggested that acupuncture produces unique nonspecific effects that are different from those of other interventions (medications, physical therapy, or psychotherapy). Central nervous system changes due to acupuncture may initiate patient engagement in a way unique to this intervention. Functional magnetic resonance imaging studies have shown that TA can induce changes in brain activity that are different from those induced by SA.11 The extent to which patients are actively (vs passively) engaged in a therapeutic encounter may be different for acupuncture than for other interventions, such as taking a pill or participating in physical therapy. Further exploration of these differences through central nervous system imaging studies during acupuncture could provide much needed information regarding putative mechanisms.
Finally, studies with small sample sizes and low statistical power, as found with the 8 studies included in this review, can lead to inaccurate conclusions. Even though estimating the magnitude of the treatment effect can be challenging in acupuncture trials, it is equally important to evaluate both clinical and statistical significance. Researchers have used a variety of approaches (Cohen’s d, number needed to treat, and success rate difference)36 for determining a clinically interpretable ES, but the best method remains an open question. In this review, as in our previous reviews,12,37 few publications provided the information necessary to calculate the treatment ES, yet this information is imperative for synthesizing available evidence for informed clinical decision making. The Consolidated Standards of Reporting Trials guidelines38 recommend reporting ES estimates in addition to P values because P values alone are not enough to determine whether or not an effect is meaningful,35 and conclusions can be misleading if the picture is incomplete.
ES estimates allow researchers and clinicians to have a standardized value that allows comparisons across different interventions and/or populations. Ideally, future publications should include an ES estimate for within-group and between-group changes with a description of the method used and its rationale. At the very least, publications should present data in such a way that ES estimates can be performed by others as part of systematic reviews and meta-analyses. Specifically, summary statistics for change scores, or the correlation between scores across time points in addition to the summary statistics at each time point, should be included for the primary outcome measure. The existing literature includes guidance on ES measures, interpretability, and even ways to convert one to another when appropriate. Chinn,39 for example, recommends converting odds ratios to Cohen’s d by dividing by 1.81. Ensuring adequate statistical power and establishing ES estimates for various types of controls are also key to informing the design of future studies. To date, few acupuncture clinical trials have been adequately powered to detect significant differences between TA and SA.
Despite the many issues related to the design and implementation of acupuncture trials, some patients clearly benefit from the addition of acupuncture to their treatment plans. A secondary analysis of an individual patient data meta-analysis for chronic musculoskeletal pain (20 studies with sham controls [n = 5230] and 18 studies with nonsham controls [n = 14,597]) found that TA was significantly superior to controls, regardless of the subtype of control.35 On the basis of this finding, exploring the use of acupuncture for other symptom management in a population of cancer patients with few treatment options seems to be an appropriate and important endeavor. However, this work should be undertaken with unbiased diligence, complete transparency, and a strong scientific rationale.
Limitations
As with any systematic review, several limitations are important to consider. First, although we attempted to include databases that the study team thought would be most inclusive of relevant literature, other databases may have produced a different listing of RCTs, and there is also the possibility that related studies were inadvertently overlooked. Second, heterogeneity in study design, outcome measures, and treatment parameters makes meaningful comparisons across studies difficult. It is also unclear whether weaknesses in specific trials were due to reporting deficits or methodology. Third, for all included studies, HF determination was by patient report, and according to some studies,40–42 HFs tend to be underreported when subjective reports are compared to objective measurements such as sternal skin conductance. This is especially true for nighttime HFs. Finally, the lack of clear biologic mechanisms makes it difficult to determine whether outcomes were due to nonspecific patient-reported responses or the specific effects of needling at acupuncture points. Publication bias is also of concern. Despite these many limitations, all included studies showed significant within-group improvements over the baseline for TA; this is an important finding because 50% of women with breast cancer report HFs lasting 10 years after menopause,42 and spontaneous improvement is not likely.
In conclusion, according to this review, the current level of evidence is insufficient to either support or refute the benefits of acupuncture for treating HFs in cancer patients. Future RCTs should follow Consolidated Standards of Reporting Trials/Standards for Reporting Interventions in Clinical Trials of Acupuncture guidelines38 for reporting; focus on standardizing blinding methods, comparison groups, and treatment approaches; evaluate biologic mechanisms; have adequate statistical power; involve multiple acupuncturists; and diligently report data that allow both P value and ES estimates.
Figure 1.
Flowchart of study selection. HF indicates hot flash; RCT, randomized controlled trial.
Acknowledgments
We thank Greg Pratt and Yimin Geng for library support and database searches as well as the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center for assistance with this article.
FUNDING SUPPORT
Support was provided in part by the National Institutes of Health/National Cancer Institute (grants CA160880 and CA148707) and The University of Texas MD Anderson Cancer Center.
Footnotes
All authors were involved in study planning, development, design, and final article approval. M. Kay Garcia, Leslie Graham-Getty, Yisheng Li, and Lorenzo Cohen were also involved in data collection, management, analysis, and interpretation.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Otte JL, Carpenter JS, Zhong X, Johnstone PA. Feasibility study of acupuncture for reducing sleep disturbances and hot flashes in post-menopausal breast cancer survivors. Clin Nurse Spec. 2011;25:228–236. doi: 10.1097/NUR.0b013e318229950b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filshie J, Bolton T, Browne D, Ashley S. Acupuncture and self acupuncture for long-term treatment of vasomotor symptoms in cancer patients—audit and treatment algorithm. Acupunct Med. 2005;23:171–180. doi: 10.1136/aim.23.4.171. [DOI] [PubMed] [Google Scholar]
- 3.Moraska AR, Moraska JM, Sideras K, Loprinzi CL. Management of hot flashes in breast cancer patients. Eur J Clin Med Oncol. 2012;4:1–9. [Google Scholar]
- 4.Azizi H, Liu YF, Du L, et al. Menopause-related symptoms: traditional Chinese medicine vs hormone therapy. Altern Ther Health Med. 2011;17:48–53. [PubMed] [Google Scholar]
- 5.Gupta P, Sturdee DW, Palin SL, et al. Menopausal symptoms in women treated for breast cancer: the prevalence and severity of symptoms and their perceived effects on quality of life. Climacteric. 2006;9:49–58. doi: 10.1080/13697130500487224. [DOI] [PubMed] [Google Scholar]
- 6.Filshie J, Rubens CNJ. Complementary and alternative medicine. Anesthesiol Clin. 2006;24:81–111. doi: 10.1016/j.atc.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Walker EM, Rodriguez AI, Kohn B, et al. Acupuncture versus venlafaxine for the management of vasomotor symptoms in patients with hormone receptor-positive breast cancer: a randomized controlled trial. J Clin Oncol. 2010;28:634–640. doi: 10.1200/JCO.2009.23.5150. [DOI] [PubMed] [Google Scholar]
- 8.Hickey M, Saunders C, Partridge A, Santoro N, Joffe H, Stearns V. Practical clinical guidelines for assessing and managing menopausal symptoms after breast cancer. Ann Oncol. 2008;19:1669–1680. doi: 10.1093/annonc/mdn353. [DOI] [PubMed] [Google Scholar]
- 9.Crew KD, Capodice JL, Greenlee H, et al. Randomized, blinded, sham-controlled trial of acupuncture for the management of aromatase inhibitor-associated joint symptoms in women with early-stage breast cancer. J Clin Oncol. 2010;28:1154–1160. doi: 10.1200/JCO.2009.23.4708. [DOI] [PubMed] [Google Scholar]
- 10.Bao T, Cai L, Snyder C, et al. Patient-reported outcomes in women with breast cancer enrolled in a dual-center, double-blind, randomized controlled trial assessing the effect of acupuncture in reducing aromatase inhibitor-induced musculoskeletal symptoms. Cancer. 2014;120:381–389. doi: 10.1002/cncr.28352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Regan D, Filshie J. Acupuncture and cancer. Auton Neurosci. 2010;157:96–100. doi: 10.1016/j.autneu.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Garcia MK, McQuade J, Haddad R, et al. Systematic review of acupuncture in cancer care: a synthesis of the evidence. J Clin Oncol. 2013;31:952–960. doi: 10.1200/JCO.2012.43.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Cancer Institute. [Accessed July 19, 2015];Acupuncture (PDQ®) http://www.cancer.gov/about-cancer/treatment/cam/patient/acupuncture-pdq#section/_1.
- 14.Chiu HY, Pan CH, Shyu YK, Han BC, Tsai PS. Effects of acupuncture on menopause-related symptoms and quality of life in women in natural menopause: a meta-analysis of randomized controlled trials. Menopause. 2015;22:234–244. doi: 10.1097/GME.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 15.Borud E, Grimsgaard S, White A. Menopausal problems and acupuncture. Auton Neurosci. 2010;157:57–62. doi: 10.1016/j.autneu.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Sturdee DW. The menopausal hot flush—anything new? Maturitas. 2008;60:42–49. doi: 10.1016/j.maturitas.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Collin E, Frechilla D, Pohl M, et al. Opioid control of the release of calcitonin gene-related peptide-like material from the rat spinal cord in vivo. Brain Res. 1993;609:211–222. doi: 10.1016/0006-8993(93)90875-n. [DOI] [PubMed] [Google Scholar]
- 18.Wyon Y, Frisk J, Lundeberg T, Theodorsson E, Hammar M. Post-menopausal women with vasomotor symptoms have increased urinary excretion of calcitonin gene-related peptide. Maturitas. 1998;30:289–294. doi: 10.1016/s0378-5122(98)00047-4. [DOI] [PubMed] [Google Scholar]
- 19.Lee MS, Kim KH, Choi SM, Ernst E. Acupuncture for treating hot flashes in breast cancer patients: a systematic review. Breast Cancer Res Treat. 2009;115:497–503. doi: 10.1007/s10549-008-0230-z. [DOI] [PubMed] [Google Scholar]
- 20.Lee MS, Shin BC, Ernst E. Acupuncture for treating menopausal hot flushes: a systematic review. Climacteric. 2009;12:16–25. doi: 10.1080/13697130802566980. [DOI] [PubMed] [Google Scholar]
- 21.Frisk JW, Hammar ML, Ingvar M, Spetz Holm AC. How long do the effects of acupuncture on hot flashes persist in cancer patients? Support Care Cancer. 2014;22:1409–1415. doi: 10.1007/s00520-014-2126-2. [DOI] [PubMed] [Google Scholar]
- 22.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Altman DG, Sterne JAC. Assessing risk of bias in included studies. chap 8. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Oxford, England: Cochrane Collaboration; 2011. [Google Scholar]
- 24.Nedstrand E, Wyon Y, Hammar M, Wijma K. Psychological well-being improves in women with breast cancer after treatment with applied relaxation or electro-acupuncture for vasomotor symptom. J Psychosom Obstet Gynaecol. 2006;27:193–199. doi: 10.1080/01674820600724797. [DOI] [PubMed] [Google Scholar]
- 25.Deng G, Vickers A, Yeung S, et al. Randomized, controlled trial of acupuncture for the treatment of hot flashes in breast cancer patients. J Clin Oncol. 2007;25:5584–5590. doi: 10.1200/JCO.2007.12.0774. [DOI] [PubMed] [Google Scholar]
- 26.Frisk J, Carlhall S, Kallstrom AC, Lindh-Astrand L, Malmstrom A, Hammar M. Long-term follow-up of acupuncture and hormone therapy on hot flushes in women with breast cancer: a prospective, randomized, controlled multicenter trial. Climacteric. 2008;11:166–174. doi: 10.1080/13697130801958709. [DOI] [PubMed] [Google Scholar]
- 27.Hervik J, Mjaland O. Acupuncture for the treatment of hot flashes in breast cancer patients, a randomized, controlled trial. Breast Cancer Res Treat. 2009;116:311–316. doi: 10.1007/s10549-008-0210-3. [DOI] [PubMed] [Google Scholar]
- 28.Liljegren A, Gunnarsson P, Landgren BM, Robeus N, Johansson H, Rotstein S. Reducing vasomotor symptoms with acupuncture in breast cancer patients treated with adjuvant tamoxifen: a randomized controlled trial. Breast Cancer Res Treat. 2012;135:791–798. doi: 10.1007/s10549-010-1283-3. [DOI] [PubMed] [Google Scholar]
- 29.Bokmand S, Flyger H. Acupuncture relieves menopausal discomfort in breast cancer patients: a prospective, double blinded, randomized study. Breast. 2013;22:320–323. doi: 10.1016/j.breast.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Deng LY. Chinese Acupuncture and Moxibustion. Beijing, China: Foreign Languages Press; 1997. [Google Scholar]
- 31.Frisk J, Kallstrom AC, Wall N, Fredrikson M, Hammar M. Acupuncture improves health-related quality-of-life (HRQoL) and sleep in women with breast cancer and hot flushes. Support Care Cancer. 2012;20:715–724. doi: 10.1007/s00520-011-1134-8. [DOI] [PubMed] [Google Scholar]
- 32.National Center for Complementary and Integrative Health. [Accessed July 19, 2015];Acupuncture research—areas of high and low programmatic priorities. https://nccih.nih.gov/grants/acupuncture/priorities.
- 33.Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336:999–1003. doi: 10.1136/bmj.39524.439618.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375:686–695. doi: 10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacPherson H, Vertosick E, Lewith G, et al. Influence of control group on effect size in trials of acupuncture for chronic pain: a secondary analysis of an individual patient data meta-analysis. PLoS One. 2014;9:e93739. doi: 10.1371/journal.pone.0093739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraemer HC, Frank E, Kupfer DJ. How to assess the clinical impact of treatments on patients, rather than the statistical impact of treatments on measures. Int J Methods Psychiatr Res. 2011;20:63–72. doi: 10.1002/mpr.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia MK, McQuade J, Lee R, Haddad R, Spano M, Cohen L. Acupuncture for symptom management in cancer care: an update. Curr Oncol Rep. 2014;16:418. doi: 10.1007/s11912-014-0418-9. [DOI] [PubMed] [Google Scholar]
- 38.MacPherson H, Altman DG, Hammerschlag R, et al. Revised Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. J Evid Based Med. 2010;3:140–155. doi: 10.1111/j.1756-5391.2010.01086.x. [DOI] [PubMed] [Google Scholar]
- 39.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19:3127–3131. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 40.Savard J, Davidson JR, Ivers H, et al. The association between nocturnal hot flashes and sleep in breast cancer survivors. J Pain Symptom Manage. 2004;27:513–522. doi: 10.1016/j.jpainsymman.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Carpenter JS. State of the science: hot flashes and cancer Part 1: definition, scope, impact, physiology, and measurement. Oncol Nurs Forum. 2005;32:959–968. doi: 10.1188/05.ONF.959-968. [DOI] [PubMed] [Google Scholar]
- 42.Carpenter JS, Andrykowski MA, Cordova M, et al. Hot flashes in postmenopausal women treated for breast carcinoma: prevalence, severity, correlates, management, and relation to quality of life. Cancer. 1998;82:1682–1691. [PubMed] [Google Scholar]