Summary
Mortality in ducks and geese caused by highly pathogenic avian influenza A (H5N1) infection had not been previously identified in Bangladesh. In June–July 2011, we investigated mortality in ducks, geese and chickens with suspected H5N1 infection in a north-eastern district of the country to identify the aetiologic agent and extent of the outbreak and identify possible associated human infections. We surveyed households and farms with affected poultry flocks in six villages in Netrokona district and collected cloacal and oropharyngeal swabs from sick birds and tissue samples from dead poultry. We conducted a survey in three of these villages to identify suspected human influenza-like illness cases and collected nasopharyngeal and throat swabs. We tested all swabs by real-time RT-PCR, sequenced cultured viruses, and examined tissue samples by histopathology and immunohistochemistry to detect and characterize influenza virus infection. In the six villages, among the 240 surveyed households and 11 small-scale farms, 61% (1789/2930) of chickens, 47% (4816/10 184) of ducks and 73% (358/493) of geese died within 14 days preceding the investigation. Of 70 sick poultry swabbed, 80% (56/70) had detectable RNA for influenza A/H5, including 89% (49/55) of ducks, 40% (2/5) of geese and 50% (5/10) of chickens. We isolated virus from six of 25 samples; sequence analysis of the hemagglutinin and neuraminidase gene of these six isolates indicated clade 2.3.2.1a of H5N1 virus. Histopathological changes and immunohistochemistry staining of avian influenza viral antigens were recognized in the brain, pancreas and intestines of ducks and chickens. We identified ten human cases showing signs compatible with influenza-like illness; four were positive for influenza A/H3; however, none were positive for influenza A/H5. The recently introduced H5N1 clade 2.3.2.1a virus caused unusually high mortality in ducks and geese. Heightened surveillance in poultry is warranted to guide appropriate diagnostic testing and detect novel influenza strains.
Keywords: outbreaks, influenza A virus, H5N1 subtype, clade 2.3.2.1a, duck, goose, histopathology, immunohistochemistry, Bangladesh
Introduction
Highly pathogenic avian influenza (HPAI) A(H5N1) virus is a major concern worldwide because of its ability to cause significant morbidity and mortality in birds, its ability to infect humans with high mortality and its pandemic potential (Swayne and Suarez, 2000). The first recorded cases of infection of H5N1 viruses in domestic ducks in Asia were in 1997 from live bird markets in Hong Kong (Shortridge et al., 1998). The first outbreak with mortality of ducks and geese caused by H5N1 viruses was reported in 2002–2003 in Hong Kong. Although mortality caused by H5N1 in domestic chickens is high (75–100%) (Li et al., 2011), the virus does not typically cause clinical disease or death in ducks or wild aquatic birds (Alexander, 1987; Ellis et al., 2004; Sturm-Ramirez et al., 2004; Songserm et al., 2006). However, recent outbreaks caused by H5N1 with a high mortality in ducks and geese were reported in India in 2011 and Indonesia in 2012 (Nagarajan et al., 2012; Dharmayanti et al., 2014).
Bangladesh has an estimated 270 million poultry including 221 million chickens and 41 million ducks (Ministry of Finance, 2009). Rearing ducks and geese is an important source of protein and self-employment for rural households in Bangladesh (Sultana et al., 2012), where poultry are reared in backyards (flock size: 1–99) and small-scale (flock size: 101–500) and commercial farms (flock size >500) (icddrb, 2013a). Since 2007, there have been >545 HPAI H5N1 outbreaks in chickens reported in Bangladesh (World Organization for Animal Health, 2012), causing an estimated US$746 million in financial loss to the poultry industry (FAO-Bangladesh, 2008). Although surveillance conducted on presumably healthy ducks and geese in live bird markets has identified H5N1 virus in Bangladesh since 2007, mortality in ducks and geese as a result of H5N1 infection had not been previously identified in the country (Khan et al., 2010).
As of March 2013, there have been seven reported human cases of H5N1 infection identified in Bangladesh, including one fatal case (icddrb, 2011a; Institute of Epidemiology Disease Control and Research, 2013). All cases had a history of close exposure to sick poultry (Brooks et al., 2009; icddrb, 2011a; Institute of Epidemiology Disease Control and Research, 2013; Rahman et al., 2013). A recent study of backyard poultry raisers in Bangladesh found that 95% of backyard poultry raisers reared ducks, geese and chickens together in free range conditions: 84% handled sick or dead poultry at least once a day, 52% slaughtered sick poultry, and 55% cared for sick poultry inside their houses (Shanta et al., 2012). The sporadic detection of avian influenza viruses in humans between 2008 and 2013 indicates the ongoing risk of human infection with novel influenza strains.
In Asia, there have been several reports of H5N1-related mortality in ducks and geese. In 2002, Sturm-Ramirez investigated outbreaks where 43.7% (80/105) of ducks and 37.5% (9/24) of geese died, and avian influenza A/H5N1 was recovered from 95% of these birds (Sturm-Ramirez et al., 2004). In China, during 2005, outbreaks caused by H5N1 were associated with an 80% mortality rate in ducks and geese (Zhou et al., 2006). In Korea, during 2010/2011, up to 31% of mortality associated with clade 2.3.2.1 H5N1 was recorded in ducks under five weeks old (Choi et al., 2013). In India, a 61% mortality rate was observed in a government duck breeding farm where clade 2.3.2.1a H5N1 was isolated (Nagarajan et al., 2012).
In South Asia, H5N1 virus was first detected in domestic poultry in India and Pakistan during February 2006 and subsequently detected in Bangladesh, Nepal and Bhutan in March 2007 (Zhou et al., 2006). Until 2010, all H5N1 viruses isolated from poultry in South Asia belonged to clade 2.2 (Wasilenko et al., 2011). The first detection of clade 2.3.2.1 H5N1 virus in South Asia was reported in Nepal in February 2010 (Food and Agriculture Organization, 2010). This clade was recently classified as 2.3.2.1a by WHO/OIE/FAO H5N1 Evolution Working Group (WHO/OIE & FAO H5N1 Evolution Working Group, 2014). The virus was subsequently detected in poultry in India and crows in Bangladesh in 2011 (Islam et al., 2012; Nagarajan et al., 2012; Khan et al., 2014). A recent study published in 2014 indicates that the clade 2.3.2.1a is one of the most common clades circulating in poultry of Bangladesh (Marinova-Petkova et al., 2014).
Since 2009, the Bangladesh Ministry of Health and Family Welfare's Institute of Epidemiology, Disease Control and Research (IEDCR) has monitored media reports of unusual clinical manifestations and health-related outcomes in humans and animals. IEDCR contracts with a local company to scan 10 daily newspapers and eight television stations for health-related news items. A daily report is emailed to the National Rapid Response Team at IEDCR for review, verification, risk assessment and, if necessary, rapid response to a reported outbreak (Rahman et al., 2012; icddrb, 2013b). On 29 June 2011, this media-based surveillance identified a newspaper report of unusual illness and death among ducks and geese in backyards and on small-scale farms in Netrokona, a north-eastern district of Bangladesh. The report described neurological signs in birds including twisting of the head and neck (torticollis), lack of coordination in movements, head tilting and death of more than 25 000 ducks and geese in a week. These neurological signs are infrequently observed among sick poultry in Bangladesh (Dr. Aminul Haque, Veterinary Surgeon, Netrokona, Personal Communication: 2013).
Upon identification of the report, IEDCR, icddr,b and the Government of Bangladesh's Department of Livestock Services (DLS) formed a collaborative team of epidemiologists, veterinarians, physicians and microbiologists to conduct an outbreak investigation. The objectives of this investigation were to identify the aetiologic agent and the extent of poultry mortality on backyard and small-scale farms among ducks, geese and chickens and to identify any possible associated human infections and illnesses associated with this outbreak.
Methods
Poultry investigation
In collaboration with the DLS, the investigative team prepared a line list of villages experiencing unusually high mortality among ducks and geese and visited the outbreak sites (Fig. 1) on 1 July 2011. From 1 July to 23 July 2011, we identified the index households/farms (Fig. 1) where the poultry mortality was first reported and then searched the surrounding households to look for poultry mortality. We conducted door-to-door visits to households in the affected villages to identify any morbidity or mortality in poultry. We defined a case of poultry illness as a chicken, duck or goose that had become sick, showed torticollis and/or died in the 14 days preceding the interview We considered a 14-day period because this is double the upper limit of the incubation period of avian influenza A/H5 virus in chickens (3–7 days) (FAO/OIE guidelines for field investigation) (FAO and OIE, 2006) so that we do not miss any case. Another reason for using this 14-day period was that onset of illness occurred on different dates in different villages, and we only became aware of the outbreaks after they were reported in local media and/or DLS office. In three villages, the team collected data from households rearing backyard poultry until they came to a natural barrier (e.g. a river or canal) that separated the village from other villages. In two villages, the team visited households and stopped collecting data when they did not find any reports of dead poultry at six adjacent households from the last known household with poultry morbidity and mortality of chickens, ducks and geese in the 14 days before the date of the interview. Our assumption was that the chances of other dead poultry being found in a household were low if six adjacent households were surveyed and no poultry had recently died there. While surveying households with backyard flocks, the team asked owners of small-scale duck and/or geese farms in an approximately 1 km radius if they observed increased mortality with torticollis on their farm in the three days prior to interview. The team enrolled any of these eligible small-scale duck and/or goose farms. The team used a questionnaire to collect data about flock sizes, dates of illness onset and clinical signs seen in poultry by the primary raisers or heads of households or, in the absence of household heads, any adult present at surveyed households or farms (Appendix S1. Questionnaire for HPAI outbreak investigation in ducks and geese in Netrokona).
Fig. 1.
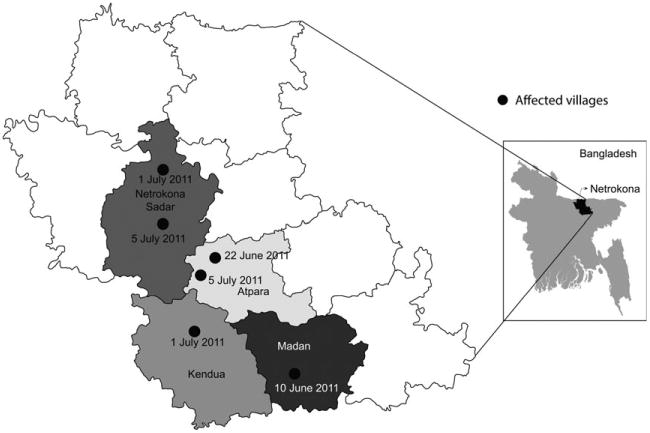
Location of villages affected with highly pathogenic avian influenza A(H5N1) virus and dates of onset of illness in ducks and geese in subdistricts of Netrokona District, Bangladesh, June–July 2011. Different colours within the district map indicate boundaries of subdistricts.
Specimen collection
Veterinarians on the outbreak investigation team collected individual oropharyngeal and cloacal swab samples from sick poultry flocks, preferentially from ducks or geese with torticollis on farms in which more than 10% of the poultry had reportedly died (slightly modified from the Food and Agriculture Organization of the United Nations trigger for investigation of farms potentially infected with avian influenza) (FAO and OIE, 2006) in the past three days of our visit. The swabs were collected and placed in viral transport media (VTM) for subsequent diagnosis and possible virus isolation (Appendix S2 – Viral Transport Media for animal samples). In three flocks of geese, we collected pooled oropharyngeal swabs from 10 geese. Swab samples were pooled to reduce the cost of testing. All the swabs were stored and transported from Netrokona to icddr,b using a cold box maintaining a temperature of 4–8°C. For post-mortem examinations, we purposively selected birds that had died recently. We performed examinations on three ducks and one chicken within 2 h of death. We also performed a post-mortem examination on a goose within 18 h of death and whose owner reported head tilting before dying. We collected various tissue samples from these birds including brain, pancreas, duodenum, heart, liver, lung, trachea, kidney and spleen and preserved them in 10% formalin. Swabs were tested for influenza A(H5N1) virus in icddr,b's animal virology laboratory by real-time reverse transcription polymerase chain reaction (real-time RT-PCR). One aliquot of each sample was sent to the laboratories of the Influenza Division of the U.S. Centers for Disease Control and Prevention (CDC; Atlanta, GA, USA), where virus isolation was performed for further characterization by sequence analysis. Tissue samples were sent for conducting histopathology and immunohistochemistry to the University of California Davis Veterinary Medicine Teaching & Research Center (Tulare, CA, USA).
Human illness investigation
In the households where unusual poultry sickness was identified, field investigators inquired about any influenza-like illness among the household members. Any person who developed fever (subjective or measured) and either cough or sore throat in the seven days prior to the interview was classified as having a case of influenza-like illness (ILI) (Vascellari et al., 2007). Among the six villages with reported unusually high waterfowl mortality, we searched for human ILI cases in three villages. In each of the three villages, field investigators surveyed ILI case-patients who were identified. Using a structured questionnaire, physicians interviewed ILI case-patients, recorded age, sex, clinical signs and exposure to poultry and collected nasopharyngeal and throat swabs in VTM. All swab specimens were stored and transported in a cold box from Netrokona to IEDCR, where they were tested for influenza A and B viruses and subtypes of influenza A/H1pdm, A/H1, A/H3 and A/H5 by real-time RT-PCR.
Laboratory testing
Influenza A virus detection, subtyping and characterization of the Hemagglutinin (HA) surface protein by phylogenetic analysis
We performed real-time RT-PCR to identify influenza A virus by targeting the matrix (M) and hemagglutinin (HA) genes for typing and subtyping and clade-specific real-time RT-PCR as previously described (Kis et al., 2013). The human influenza A positive samples were then subtyped with H1, H3 and H5 primers provided by the Influenza Division of CDC (CDC, 2009). We considered a ct value of 37 as a cut-off point for positivity in real-time RT-PCR.
At CDC, virus culture, isolation, RNA extraction and sequencing were performed as previously described (Szretter et al., 2006). The HA gene sequences of six isolates [Global Initiative on Sharing All Influenza Data (GISAID) accession numbers: EPI448207, EPI353365, EPI353370, EPI448215, EPI448223, EPI448191] were compared to sequences deposited in GenBank (http://www.ncbi.nlm.nih.gov/GenBank) using the basic local alignment search tool (BLAST) to identify close phylogenetic relatives. The sequence alignment was performed with other H5N1 virus HA sequences retrieved from GISAID (http://platform.gisaid.org/epi3/frontendb) using BioEdit version 7.0.9.0 and the ClustatW multiple alignment option. Multiple sequence alignment was used to assess the phylogenetic relatedness of isolates by the neighbour-joining method (Saitou and Nei, 1987). The bootstrap test was set at 1000 replications (Felsenstein, 1985). The phylogenetic tree was drawn to scale with branch lengths in the same units as those of the evolutionary distances. The evolutionary distances were computed using the Kimura 2-parameter (Kimura, 1980). All positions containing gaps and missing data were eliminated. Neuraminidase gene were characterized from the isolates, and the methods are described elsewhere (Gerloff et al., 2014).
Histopathology and immunohistochemistry
We performed histopathology and immunohistochemistry (IHC) on tissue samples collected from three ducks, one chicken and one goose. The tissues were processed, sectioned and stained for light microscopy as previously described (Khan et al., 2014). Immunohistochemistry was performed utilizing rabbit anti-nucleoprotein antibody directed towards influenza A(H5N1) virus and counterstained according to the methods described by Klopfleisch et al. (2006).
Data analysis
We conducted descriptive statistics to calculate the mean and frequencies of poultry reared and number of poultry deaths in the community using statistical software STATA 13 (StataCorp LP, College Station, TX, USA). We also compared the differences in influenza A/H5 positivity rates from oropharyngeal versus cloacal swabs tested by real-time RT-PCR using the chi-square test.
Ethics
We obtained informed verbal consent from the owners of the poultry that were surveyed. We also obtained verbal consent from adult respondents with suspected with ILI. For participating children aged 7–17 years, we obtained informed verbal consent from their guardians and assent from the children. Because this study was an outbreak investigation, a study protocol was not reviewed by a human subjects committee, but the investigation plan was approved by the Government of Bangladesh.
Results
Poultry illness and mortality in affected villages
We identified six villages in four subdistricts of Netrokona district with unusually high waterfowl mortality and surveyed 240 households with backyard poultry, 11 small-scale duck farms and two small-scale goose farms within those villages. The mean distance between two adjacent households was 24.3 m (SD: 18.6 m), and cumulative distance between six adjacent households in a row in the surveyed communities was 121.3 m (SD: 92.9 m). A total of 7124 birds were reared in the 240 surveyed backyard flocks, 6255 ducks were reared on the 11 surveyed duck farms, and 228 geese were reared on the two surveyed goose farms (Table 1). The median number of poultry in backyard flocks was 16 [inter-quartile range (IQR): 9–26] and in commercial duck farms was 395 (IQR: 209–400). Among the 240 investigated backyard flocks, 93% (222/240) of households reported that they observed at least one bird in their flocks become sick and 88% (212/240) of households had experienced at least one poultry death in the 14 days prior to the investigation. All surveyed households from the first (26/26) and second villages (35/35) had at least one poultry death in their poultry flocks. The third village had at least one poultry death in 31% (4/13) of households; the fourth village had at least one poultry death in 67% (18/27) of households; the fifth village had at least one poultry death in 97% (98/101) of households; and the sixth village had at least one poultry death in 82% (31/38) of households. Altogether, 51% (6963/13 607) of all poultry died within 14 days preceding the date of investigation: 61% (1789/2930) of chickens, 47% (4816/10 184) of ducks and 73% (358/493) of geese (Table 1).
Table 1. Mortality observed during a waterfowl outbreak in Netrokona, Bangladesh, June–July 2011.
| Poultry surveyed* | Died n (%) | |
|---|---|---|
| Backyard poultry | ||
| Chickens | 2930 | 1789 (61) |
| Ducks | 3929 | 1425 (36) |
| Geese | 265 | 220 (83) |
| Commercial poultry | ||
| Chickens | – | – |
| Ducks | 6255 | 3391 (54) |
| Geese | 228 | 138 (61) |
| Total poultry | 13 607 | 6963 (51) |
Number of poultry were enumerated by door-to-door visits of the field research assistant by interviewing the head of the households/adults person of the family present during survey (in backyard flocks) and farm owners/farm workers (in small-scale commercial farm).
Clinical and post-mortem presentations in ducks and geese
The common signs in observed sick duck flocks were torticollis (Fig. 2), whitish watery diarrhoea, lack of coordination, loss of appetite and sudden death. Most of the observed ill geese exhibited similar characteristics: torticollis, lack of coordination, leg paralysis and sudden death. Most of the ill chickens were observed to have cyanosis and oedema involving their combs and wattles, leg haemorrhage, drowsiness and sudden death. The internal organs of the examined ducks, geese and chickens appeared normal except for mild congestion and pinpoint haemorrhages noted in the liver and pancreas, and evidence of air sac infections in post-mortem examination.
Fig. 2.

Photographs of a duck with severe twisting of the head and neck (torticollis), Netrokona, Bangladesh, June–July 2011.
Laboratory findings
Detection of HPAI H5N1 virus in poultry samples
We tested swab specimens from 70 individual birds and three pooled samples in 15 flocks for the presence of influenza A/H5 RNA by real-time RT-PCR. Most of the samples were from ducks (n = 55), and a few were from chickens (n = 10) and geese (n = 8). Most of the samples were collected from ducks because when we visited the villages, most of the chickens were dead. Moreover, as these neurological signs and increased rate of death in ducks and geese are relatively uncommon, and the aetiologic agent of the illness was unknown, we were primarily interested in sampling ducks and geese rather than chickens. Of the 15 flocks sampled, 67% (10/15) had at least one bird with detectable RNA for influenza A/H5. Of the 70 poultry sampled, 80% (56/70) were positive for influenza A/H5, including 89% (49/55) of ducks, 40% (2/5) of geese and 50% (5/10) of chickens. Of the three pooled samples from geese flocks, influenza A/H5 virus RNA was detected from two flocks. Overall, compared with cloacal swabs, a greater proportion of oropharyngeal swab samples had detectable influenza A/H5 RNA (79% versus 52%, P < 0.001) (Table 2).
Table 2. Oropharyngeal and cloacal swab samples collected from different poultry species and tested for highly pathogenic avian influenza A(H5N1) virus during an unusually high waterfowl mortality outbreak investigation in Netrokona, Bangladesh, June–July 2011.
| Species | Samples (N) | Oropharyngeal swabs tested positive n (%) | Cloacal swabs tested positive n (%) |
|---|---|---|---|
| Ducks (individual) | 55 | 49 (89) | 31 (56) |
| Chickens (individual) | 10 | 5 (50) | 4 (40) |
| Geese (individual) | 5 | 2 (40) | 1 (20) |
| Total (individual)* | 70 | 56 (80) | 36 (51) |
| Geese (pooled)† | 3 | 2 (67) | 2 (67) |
Differences are significant (P < 0.001).
Samples pooled from 10 geese.
Phylogeny of the HA gene of H5N1 virus in ducks, geese and chickens
Of the 73 samples sent to CDC, virus isolation was attempted from 25 specimens and influenza A/H5 virus was successfully isolated from six of the samples (Gerloff et al., 2014). Phylogenetic analysis identified a close relationship with the HA gene of virus strains from H5N1 clade 2.3.2.1a (WHO/OIE & FAO H5N1 Evolution Working Group, 2014) (Fig. 3). Full-length HA genes of H5N1 virus of a duck in this outbreak showed 97.5–99.7% similarity with the crow H5N1 isolates that caused widespread mortality in crows in Bangladesh in January–February in 2011 (Khan et al., 2014). The Indian isolates from the February 2011 outbreaks shared 97.3–99.6% similarity, while the Nepal isolates from 2010 shared 97.1–99.3% similarity with the isolates obtained in this outbreak (Fig. 3). Although viruses were not successfully isolated from 19 samples, phylogenetic analysis of HA sequence fragments obtained from direct sequencing showed that these viruses were closely related and also belonged to clade 2.3.2.1a. Neuraminidase analysis identified Neuraminidase 1 (N1) from these six isolates, and the findings have been previously described (Gerloff et al., 2014).
Fig. 3.
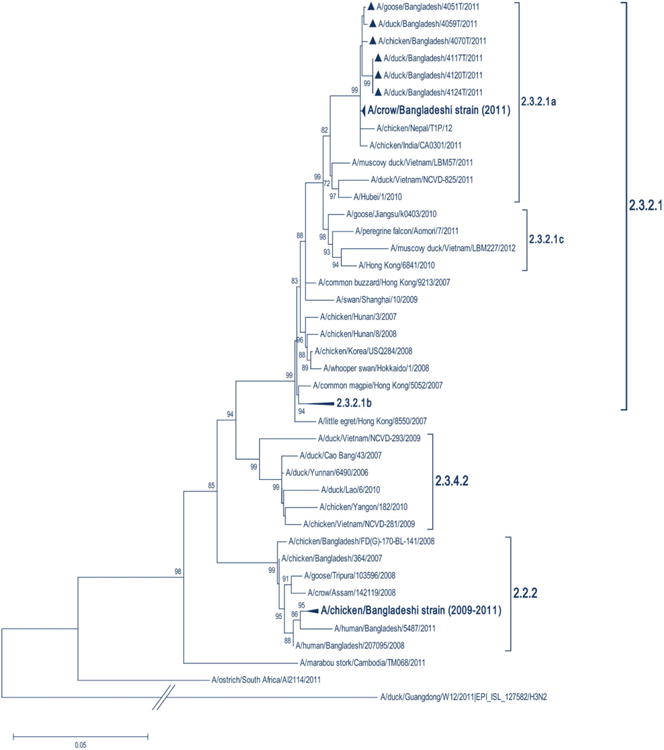
Phylogeny of hemagglutinin (HA) gene sequences of HPAI H5N1 viruses collected from waterfowl experiencing unusually high mortality in Netrokona, Bangladesh during June–July 2011. Viruses detected in Bangladesh during this outbreak investigation are denoted by a triangle in clade 2.3.2.1a. Collapsed branches indicate other large clades of H5N1 virus with strain names not shown. HA clades are indicated by brackets. The tree was calculated using the neighbour-joining method with the Kimura 2-parameter distance model.
Histopathology of duck, goose and chicken tissues
Histopathological lesions were noted in various organs of chickens and ducks. Chicken tissues including lung, spleen, pancreas, brain, kidney, liver and intestine revealed evidence of systemic lesions caused by the influenza virus infection (Swayne and Suarez, 2000; Nakatani et al., 2005). These included mononuclear cell infiltration of the lung interstitium, increased numbers of reticuloendothelial cells and hemosiderosis of the spleen, individual acinar cell necrosis with inflammatory cells within the pancreas, and gliosis and occasional focal necrosis of the neuropil of the brain, dilatation of tubules and eosino-philic debris in the lumens of kidneys, increased number of Kupffer cells in the sinusoids of liver and increased cellularity of the lamina propria in the villi of intestine. In duck tissues, perivascular cuffing with gliosis of the brain was noted (Fig. 4a); severe, multifocal coagulative necrosis of pancreatic acinar cells (Fig. 4b), focal necrotizing enteritis of the intestine (Fig. 4c), and an acute and extensive coagulative necrosis of hepatocytes were identified. These findings were common in all the three ducks' organs.
Fig. 4.
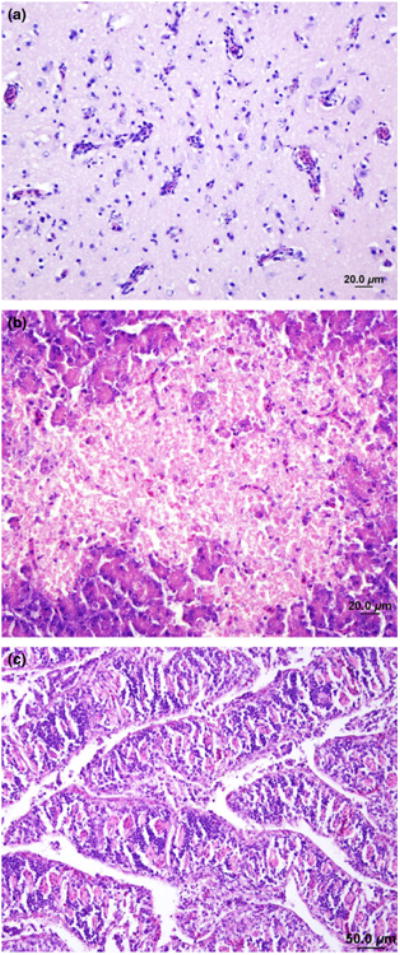
Haematoxylin- and eosin-stained sections of the brain and pancreas of ducks and of the intestine of chickens infected with avian influenza A (H5N1) virus in Netrokona, 2011. (a) Brain – mild perivascular cuffing and increased glial cells. (b) Pancreas – acinar cell necrosis (c) Intestine – increased cellularity of the lamina propria in the villi.
Immunohistochemistry (IHC) of duck, goose and chicken tissues
IHC staining directed against the nucleoprotein (NP) of avian influenza virus was evident in various chicken and duck organs. In chickens, there was evidence of virus antigen within the cytoplasm and nuclei of myocytes of the heart. There was massive accumulation of NP within the nucleus and cytoplasm of submucosal ganglia (myenteric plexus) as well as in the endothelial cells of vessels and mononuclear cells within the lamina propria of the intestine. We also noted multifocal infection of the mononuclear cells lining the sinusoids as well as in the endothelial cells of the vessels in the liver, in the nucleus and cytoplasm of acinar cells and in the mononuclear cells of the ducts in the pancreas. There was higher accumulation of NP in the nucleus, glial cells and dendrites scattered through the brain. In ducks, there was huge accumulation of NP within the nucleus and cytoplasm of the neurons, in the glial and ependymal cells of the brain (Fig. 5a) in the acinar cells of the pancreas (Fig. 5b), within the submucosal ganglion (myenteric plexus) of the intestine (Fig. 5c), and mild staining of cytoplasm and nuclei of cardiac myocytes. The liver tissue samples that we collected from a goose had evidence of infection in the hepatic sinusoidal lining cells with avian influenza virus.
Fig. 5.
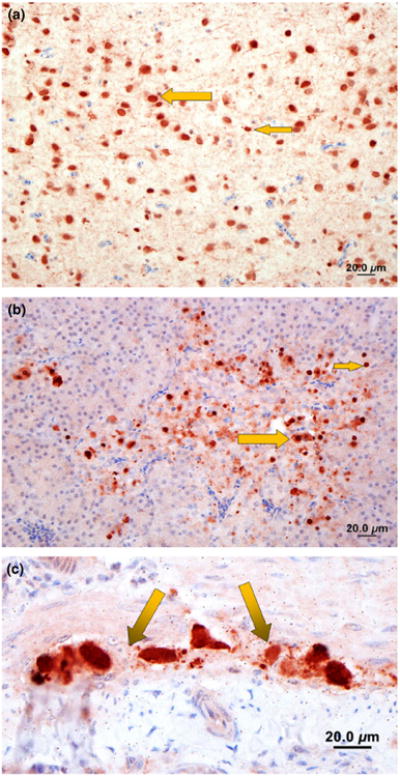
Immunohistochemistry staining for viral nucleoprotein (brown) of brain, pancreas and intestine of ducks infected with avian influenza A (H5N1) virus in Netrokona, Bangladesh, 2011. (a) Brain: moderate amount of nucleoprotein of avian influenza (AI) in the nucleus and cytoplasm of neurons (large arrow) and glial cells (small arrow). (b) Pancreas: large amount of AI antigen in the nucleus and cytoplasm of acinar cells (small arrow) and a few mononuclear inflammatory cells (large arrow) (c) Intestine: large amount of nucleoprotein of AI antigen in the nucleus and cytoplasm of subserosal and submucosal ganglia (arrows, myenteric plexus).
Human illness investigation
We have surveyed 139 households in three villages and identified ten human cases-patients with ILI. Among the ten identified ILI cases, six were male and four were female. The median age of ILI case-patients was 12.5 (range: 7–75) years. None of the cases had symptoms or signs of severe illness. In the 14 days preceding the onset of illness, nine had poultry in their houses, eight observed dead poultry in their household compounds, six had held poultry with their bare hands, four had assisted in poultry slaughtering, and two had visited a live bird market. Of the 10 ILI case-patients, nine had cough, eight fevers, seven rhinorrhea, five sore throat, four myalgia, and three headache in the 14 days preceding the onset of illness. Four (40%) of 10 nasopharyngeal swab specimens had detectable influenza A virus M gene RNA and were further subtyped as influenza A/H3. None of the case-patients had specimens that were positive for influenza A/H5 or influenza B.
Discussion
Clinical manifestations, laboratory tests, and epidemiological data from this investigation indicated that the morbidity and mortality observed among ducks, geese and chickens were caused by highly pathogenic avian influenza A(H5N1) clade 2.3.2.1a virus. H5N1 viruses are usually highly pathogenic among chickens but not typically among ducks or wild aquatic birds, although some exceptions have been reported (Alexander, 1987; Ellis et al., 2004; Sturm-Ramirez et al., 2004; Songserm et al., 2006). H5N1 viruses isolated in Hong Kong from chickens in 1997, geese in 1999 and 2000, and chickens in 2001 were not pathogenic in ducks and were only mildly to moderately pathogenic in geese (Shortridge et al., 1998; Lee et al., 2005; Sturm-Ramirez et al., 2005; Brown et al., 2008). However, since 2002, a number of H5N1 viruses have been shown to cause clinical signs and mortality in ducks either by natural infection or in experimental studies (Ellis et al., 2004; Sturm-Ramirez et al., 2005; Songserm et al., 2006). Recently, there have been several outbreaks of H5N1 clade 2.3.2.1a in domestic ducks and/or geese with significant mortality reported from India, Egypt, China and Indonesia (Food and Agriculture Organization, 2010; Nagarajan et al., 2012; Dharmayanti et al., 2014).
In January–February 2011, 5 months prior to this investigation, multiple outbreaks in crows caused by H5N1 clade 2.3.2.1a were reported from a central and a southern district of Bangladesh, most probably the introduction of the virus in the country (Khan et al., 2014; WHO/OIE & FAO H5N1 Evolution Working Group, 2014). In this outbreak, we observed a high mortality rate in ducks (47%) and geese (73%). These mortality rates were unusually high for these species. However, in India, 61% mortality in ducks caused by clade 2.3.2.1a virus was reported on one duck breeding farm during January–February 2011 (Nagarajan et al., 2012; WHO/OIE & FAO H5N1 Evolution Working Group, 2014). This new clade was apparently introduced into South Asia early in 2011 and since that time has caused massive mortality in poultry and wild birds (Islam et al., 2012; Nagarajan et al., 2012; Khan et al., 2014). In 2013, clade 2.3.2.1a virus was detected from apparently healthy ducks in live bird markets in Bangladesh (Personal communication, Sukanta Chowdhury, icddr,b, Bangladesh), and a recent publication indicates that H5N1 clade 2.3.2.1a has replaced the older clade 2.2.2 in poultry in Bangladesh (Marinova-Petkova et al., 2014).
The spread of H5N1 within waterfowl in north-east Bangladesh could result in severe economic losses for backyard poultry raisers throughout the country due to widespread geographical distribution and potential for causing morbidity and mortality in both backyard chickens and quails. Furthermore, further exposure to the virulent strain H5N1 clade 2.2.3.1a virus could increase the potential for human infection.
Since the first detection of H5N1 virus in Bangladesh in 2007, different clades have been introduced in the country including 2.2, 2.3.2.1a and 2.3.2.4. All the H5N1 viruses from Bangladesh reported from 2007 to 2010 clustered within clade 2.2 (WHO/OIE/FAO H5N1 Evolution Working Group, 2008; Gerloff et al., 2014). Since its introduction in 2009 in Vietnam, clade 2.3.2.1 has been shown to be rapidly replacing established clades of H5N1, including clade 2.3.4 (WHO/OIE/FAO H5N1 Evolution Working Group, 2008). Two years after the outbreak reported in this study, it appears that clade 2.3.2.1a is now the predominant H5N1 virus clade circulating in Bangladesh (Gerloff et al., 2014; Marinova-Petkova et al., 2014). This clade was associated with the first reported fatal human H5N1 infection in Bangladesh in February 2013, in which there were clinical signs and laboratory data indicating central nervous system (CNS) involvement (Rahman et al., 2013).
Pathological findings and avian influenza virus NP accumulation in brain, pancreas and intestine of the ducks and chickens were caused by H5N1 virus (Ellis et al., 2004; Nakatani et al., 2005). We found severe lesions in the brain, pancreas, liver and intestine and moderate lesions in the kidney and lung. These lesions and the presence of IHC staining in the brain, liver, lungs and pancreas would explain the higher mortality in ducks and chickens. Both the histopathological lesions and IHC seen in various organs of the poultry in this study are consistent with the findings of Vascellari et al. (Vascellari et al., 2007) and others (Sturm-Ramirez et al., 2004; Nakatani et al., 2005; Brown et al., 2006; Songserm et al., 2006). Although we did not find any microscopic changes in the tissue of the goose that was examined, there was IHC evidence of avian influenza virus in the liver of this bird. Moreover, swabs collected from the liver of this goose had detectable RNA for influenza A/H5 in real-time RT-PCR.
Most of the observed ducks and geese showed signs of CNS dysfunction including torticollis, lack of coordination and leg paralysis. These types of neurological signs were observed in both natural and experimental H5N1 infections in waterfowl during an outbreak in Hong Kong in 2002 (Sturm-Ramirez et al., 2004) and in other places (Swayne and Suarez, 2000; Brown et al., 2006). H5N1 virus has been reported to infect the CNS and cause changes in brains and other organs in domestic ducks and geese (Perkins and Swayne, 2002). The clinical signs observed in chickens in this outbreak were consistent with H5N1 outbreaks in chickens reported elsewhere (Biswas et al., 2011). However, the neurological signs exhibited in ducks and geese in this outbreak were uncommon and alarming for poultry raisers in the study area. This created great interest and drew the attention of local media.
In this outbreak, H5N1 was not isolated from humans, indicating that there was no detectable transmission of the virus from poultry to humans. The detection of influenza A/H3 in the poultry raisers is congruent with findings from hospital-based influenza surveillance indicating circulation of seasonal influenza in humans in Bangladesh at the time of the outbreak (icddrb, 2011b). However, the simultaneous circulation of seasonal influenza in humans and H5N1 in birds in the locality is a concern as this could increase the chances of re-assortment between human-adapted influenza strains and H5N1 virus.
Limitations
We investigated this outbreak in one district in Bangladesh. However, the outbreak subsequently spread to other districts and subdistricts in the surrounding areas. In this study, we only investigated a small area in which the outbreak occurred, during a short time interval. We did not determine the total number of households with poultry deaths in the six villages we visited, and we restricted our investigation to only ducks, geese and chickens – deaths of other poultry types were not investigated. The total number of birds in the three surveyed villages was not known – hence, we could not estimate the proportion of birds that died during the outbreak among all birds kept in the villages. Likewise, we did not survey the owners of commercial duck farms if they did not report having sick or dead birds and neurological illness in poultry. Although we collected few specimens from chickens and geese, the interpretation of this outbreak investigation (H5N1 clade 2.3.2.1a as a cause of unusually high mortality in ducks and goose) would not have changed if collected more samples because of the virus detection, characterization, histopathology and immunohistochemistry identified among available samples. We collected and tested a small number of samples (n = 73) from ducks, geese, chickens and HA and N1 gene were characterized from six isolates, as described in a previous report (Gerloff et al., 2014). There may have been selection bias resulting from the small number of samples that yielded isolates. Had we been able to collect more samples, the findings might have varied. However, as all the samples were collected from the same outbreak and during the same time period from the birds with similar clinical manifestation, it is unlikely that additional sampling would have added to this data set. The results presented showing that among the six viruses sequenced, there remained only minor genetic changes underscore this assumption. Furthermore, partial HA sequencing of the 19 isolation-negative samples yields highly related viruses indicating a shared source of infection resulting in this outbreak. Based on this epidemiologic link, we assume that the proportion of isolates positive for H5N1 would be similar had they been characterized.
Virus isolation was attempted on only the samples that had ct values <30 due to limitations on the availability of embryonated chicken eggs and other resources. Previous experience working with avian swabs that have ct values >30 and have been through more than one freeze–thaw cycle demonstrates a low isolation efficiency. In addition, because the samples were collected from birds at the same location and within a short period of time, we feel the viruses were genetically closely related and that additional isolation/sequencing (sampling) would not add to the data set.
Conclusions
The recently introduced HPAI A(H5N1) clade 2.3.2.1a virus caused neurological signs and unusually high mortality in ducks and geese. Heightened surveillance for clinical signs in waterfowl in Bangladesh is warranted so that appropriate diagnostic testing can be performed to detect novel influenza strains.
Supplementary Material
Appendix S1. Unusual waterfowl mortality investigation questionnaire, Netrokona.
Appendix S2. Viral Transport Media (VTM).
Acknowledgments
This research was funded by the U.S. Centers for Disease Control and Prevention under cooperative agreement U01 CI000298. icddr,b is grateful for the commitment of the CDC to its research efforts and outbreak investigations in Bangladesh. We acknowledge the Bangladesh Ministry of Health and Family Welfare's Institute of Epidemiology, Disease Control and Research and Department of Livestock Services for their cooperation in conducting this investigation. We are grateful to the veterinary surgeons and district livestock officers of Netrokona district for their assistance in the field investigation. We gratefully acknowledge the authors and the originating and submitting laboratories of the sequences from GISAID's EpiFlu™ Database, which were used in this analysis. We are grateful to Dr. Arach Wilson and Nadine Beckwith for their collaboration and initial testing of poultry tissues. We would like to thank Dr. Jens Teifke of the Friedrich-Loeffler-Institut, Germany, for performing immunohistochemistry. We acknowledge Nancy Gerloff and MISMS (Multinational Influenza Seasonal Mortality Study) team for their assistance in sequencing of virus isolates and phylogenetic analysis. Finally, we are grateful to Ms. Diana DiazGranados and Meghan L. Scott for their review and insightful comments regarding this manuscript. icddr,b is thankful to the Governments of Australia, Bangladesh, Canada, Sweden and the UK for providing core/unrestricted support.
Footnotes
Disclosure: The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, the Department of Defense or the Association of Schools and Programs of Public Health.
Supporting Information: Additional Supporting Information may be found in the online version of this article:
References
- Alexander DJ. The Second International Symposium on Avian Influenza, 1986. University of Wisconsin; Madison: 1987. Avian influenza — historical aspects; pp. 4–13. [Google Scholar]
- Biswas PK, Christensen JP, Ahmed SSU, Barua H, Das A, Rahman MH, Giasuddin M, Habib MA, Hannan ASMA, Debnath NC. Mortality rate and clinical features of highly pathogenic avian influenza in naturally infected chickens in Bangladesh. Rev Sci Tech. 2011;30:871–878. doi: 10.20506/rst.30.3.2080. [DOI] [PubMed] [Google Scholar]
- Brooks WA, Alamgir AS, Sultana R, Islam MS, Rahman M, Fry AM, Shu B, Lindstrom S, Nahar K, Goswami D, Haider MS, Nahar S, Butler E, Hancock K, Donis RO, Davis CT, Zaman RU, Luby SP, Uyeki TM, Rahman M. Avian influenza virus A (H5N1), detected through routine surveillance, in child, Bangladesh. Emerg Infect Dis. 2009;15:1311–1313. doi: 10.3201/eid1508.090283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JD, Stallknecht DE, Beck JR, Suarez DL, Swayne DE. Susceptibility of North American ducks and gulls to H5N1 highly pathogenic avian influenza viruses. Emerg Infect Dis. 2006;12:1663–1670. doi: 10.3201/eid1211.060652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JD, Stallknecht DE, Swayne DE. Experimental infection of swans and geese with highly pathogenic avian influenza virus (H5N1) of Asian lineage. Emerg Infect Dis. 2008;14:136–142. doi: 10.3201/eid1401.070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. CDC protocol of realtime RTPCR for influenza A (H1N1) 2009 [Google Scholar]
- Choi JG, Kang HM, Jeon WJ, Choi KS, Kim KI, Song BM, Lee HS, Kim JH, Lee YJ. Characterization of clade 2.3.2.1 H5N1 highly pathogenic avian influenza viruses isolated from wild birds (mandarin duck and Eurasian eagle owl) in 2010 in Korea. Viruses. 2013;5:1153–1174. doi: 10.3390/v5041153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmayanti NL, Hartawan R, Pudjiatmoko, Wibawa H, Balish A, Donis R, Davis CT, Samaan G. Genetic characterization of clade 2.3.2.1 avian influenza A(H5N1) viruses, Indonesia, 2012. Emerg Infect Dis. 2014;20:671–674. doi: 10.3201/eid2004.130517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis TM, Bousfield RB, Bissett LA, Dyrting KC, Luk GS, Tsim ST, Sturm-Ramirez K, Webster RG, Guan Y, Malik Peiris JS. Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathol. 2004;33:492–505. doi: 10.1080/03079450400003601. [DOI] [PubMed] [Google Scholar]
- FAO and OIE. Animal Production and Health Manual: Preparing for highly pathogenic avian influenza virus. FAO and OIE; Rome, Italy: 2006. [Google Scholar]
- FAO-Bangladesh. Rapid assessment on socio economic impact due to Highly Pathogenic Avian Influenza in Bangladesh. Dhaka, Bangladesh: 2008. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization. H5N1 HPAI global overview- July and August 2010. EMPRESS/GLEW, Issues No 24 2010 [Google Scholar]
- Gerloff NA, Khan SU, Simpson N, Shanta IS, Amanda B, Berman L, Haider N, Poh MK, Islam A, Gurley ES, Hasnat MA, Dey TK, Lindstrom S, Haque A, Klimov A, Villanueva J, Azziz-Baumgartner E, Luby SP, Zeidner N, Donis RO, Sturm-Ramirez K, Davis CT. Multiple reassortment events among highly pathogenic avian influenza A(H5N1) viruses detected in Bangladesh from 2008–2012. Virology. 2014:450–451. 297–307. doi: 10.1016/j.virol.2013.12.023. [DOI] [PubMed] [Google Scholar]
- icddrb. Outbreak of mild respiratory disease caused by H5N1 and H9N2 infections among young children in Dhaka, Bangladesh, 2011. Health Sci Bull. 2011a;9:5–10. [Google Scholar]
- icddrb. Proportion of laboratory confirmed influenza among hospitalized severe acute respiratory illness, and outpatient influenza like illness cases between July 2008 and August 2011. Health Sci Bull. 2011b;9:24. [Google Scholar]
- icddrb. Avian Infleunza virus surveillance at live bird markets in Bangladesh, 2007–2012. Health Sci Bull. 2013a;11:8–16. [Google Scholar]
- icddrb. Unusual waterfowl mortality due to highly pathogenic avian influenza A (H5N1) virus in Netrokona, Bangladesh, 2011. Health Sci Bull. 2013b;11:15–20. [Google Scholar]
- Institute of Epidemiology Disease Control and Research. Fourth case of AI, fifth and sixth cases of H5N1. [accessed February 4 2014];2013 Available at http://www.iedcr.org/pdf/files/influenza/Fourth-H5N1-human-case-in-Bangladesh.pdf and http://www.iedcr.org/pdf/files/influenza/Fifth_and_Sixth_H5N1.pdf.
- Islam MR, Haque ME, Giasuddin M, Chowdhury EH, Samad MA, Parvin R, Nooruzzaman M, Rahman MM, Monoura P. New Introduction of Clade 2.3.2.1 Avian Influenza Virus (H5N1) into Bangladesh. Transbound Emerg Dis. 2012;59:460–463. doi: 10.1111/j.1865-1682.2011.01297.x. [DOI] [PubMed] [Google Scholar]
- Khan MSU, Gurley ES, Rahman M, Hossain MJ, Nasreen S, Azim T, Mikolon AB, Azziz-Baumgartner E, Luby SP. Live bird market surveillance for avian influenza in Bangladesh, 2007-2009. International Conference on Emerging Infectious Diseases, ICEID 2012, Abstract book 2010 [Google Scholar]
- Khan SU, Berman L, Haider N, Gerloff N, Rahman MZ, Shu B, Rahman M, Dey TK, Davis TC, Das BC, Balish A, Islam A, Teifke JP, Zeidner N, Lindstrom S, Klimov A, Donis RO, Luby SP, Shivaprasad HL, Mikolon AB. Investigating a crow die-off in January-February 2011 during the introduction of a new clade of highly pathogenic avian influenza virus H5N1 into Bangladesh. Arch Virol. 2014;159:509–518. doi: 10.1007/s00705-013-1842-0. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kis Z, Jones J, Creanga A, Ferdinand K, Inui K, Gerloff N, Davis C, Nguyen T, Donis R. Real-time RT-PCR assay to differentiate clades of H5N1 Avian influenza viruses circulating in Vietnam. J Virol Methods. 2013;193:452–458. doi: 10.1016/j.jviromet.2013.06.023. [DOI] [PubMed] [Google Scholar]
- Klopfleisch R, Werner O, Mundt E, Harder T, Teifke JP. Neurotropism of highly pathogenic avian influenza virus A/chicken/Indonesia/2003 (H5N1) in experimentally infected pigeons (Columbia livia f. domestica) Vet Pathol. 2006;43:463–470. doi: 10.1354/vp.43-4-463. [DOI] [PubMed] [Google Scholar]
- Lee CW, Suarez DL, Tumpey TM, Sung HW, Kwon YK, Lee YJ, Choi JG, Joh SJ, Kim MC, Lee EK, Park JM, Lu X, Katz JM, Spackman E, Swayne DE, Kim JH. Characterization of highly pathogenic H5N1 avian influenza A viruses isolated from South Korea. J Virol. 2005;79:3692–3702. doi: 10.1128/JVI.79.6.3692-3702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu L, Zhang Y, Duan Z, Tian G, Zeng X, Shi J, Zhang L, Chen H. New avian influenza virus (H5N1) in wild birds, Qinghai, China. Emerg Infect Dis. 2011;17:265–267. doi: 10.3201/eid1702.100732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinova-Petkova A, Feeroz MM, Alam SR, Hasan MK, Akhtar S, Jones-Engel L, Walker D, McClenaghan L, Rubrum A, Franks J, Seiler P, Jeevan T, McKenzie P, Krauss S, Webby RJ, Webster RG. Multiple introductions of highly pathogenic avian influenza (H5N1) viruses into Bangladesh. Emerg Microbes Infect. 2014;3:e11. doi: 10.1038/emi.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Finance. Bangladesh Economic Review-2009. Government of The People's Republic of Bangladesh; 2009. [Google Scholar]
- Nagarajan S, Tosh C, Smith DK, Peiris JS, Murugkar HV, Sridevi R, Kumar M, Katare M, Jain R, Syed Z, Behera P, Cheung CL, Khandia R, Tripathi S, Guan Y, Dubey SC. Avian influenza H5N1 virus of clade 2.3.2 in domestic poultry in India. PLoS One. 2012;7:e31844. doi: 10.1371/journal.pone.0031844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani H, Nakamura K, Yamamoto Y, Yamada M, Yamamoto Y. Epidemiology, pathology, and immunohistochemistry of layer hens naturally affected with H5N1 highly pathogenic avian influenza in Japan. Avian Dis. 2005;49:436–441. doi: 10.1637/7304-110504R1.1. [DOI] [PubMed] [Google Scholar]
- Perkins LE, Swayne DE. Pathogenicity of a Hong Kong-origin H5N1 highly pathogenic avian influenza virus for emus, geese, ducks, and pigeons. Avian Dis. 2002;46:53–63. doi: 10.1637/0005-2086(2002)046[0053:POAHKO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Rahman M, Ao T, Alamgir ASM, Haider MS, Haque F, Chakraborty A, Hossain J, Sobel J, Luby SP, Gurley ES. Outbreak detection in resource-limited settings: a national media-based surveillance system for public health events in Bangladesh; International Conference on Emerging Infectious Diseases; Atlanta, GA, USA. 2012. pp. 165–166. [Google Scholar]
- Rahman MW, Gurley ES, Islam MS, Aleem MA, Sali-muzzaman M, Sharif AR, Bhuiyan MU, Chowdhury S, Rahman MZ, Azim T, Balish A, Gerloff N, Simpson N, Berman L, Davis W, Alamgir A, Davis CT, Lindstrom S, Heffelfinger JD, Zeidner NS, Uyeki TM, Rahman M, Sturm-Ramirez K. Option for the Control of Influenza. Cape Town, South Africa: 2013. The first reported fatal pediatric case of H5N1 with atypical symptoms, Bangladesh. [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Shanta IS, Hasnat M, Mikolon M, Khan M, Haider N, Bhuyan AM, Hossain MA, Gurley ES, Azziz-Baumgartner E, Luby SP. Backyard Poultry Rearing Practices in Bangladesh: Implications for Risk of Avian Influenza; Abstract book: 2012 International Conference on Emerging Infectious Diseases; Hyatt Regency Atlanta, Georgia. 2012. [Google Scholar]
- Shortridge KF, Zhou NN, Guan Y, Gao P, Ito T, Kawaoka Y, Kodihalli S, Krauss S, Markwell D, Murti KG, Norwood M, Senne D, Sims L, Takada A, Webster RG. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology. 1998;252:331–342. doi: 10.1006/viro.1998.9488. [DOI] [PubMed] [Google Scholar]
- Songserm TR, Jam-on N, Sae-Heng N, Meemak N, Hulse-Post DJ, Sturm-Ramirez KM, Webster RG. Domestic ducks and H5N1 influenza epidemic, Thailand. Emerg Infect Dis. 2006;12:575–581. doi: 10.3201/eid1204.051614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm-Ramirez KM, Ellis T, Bousfield B, Bissett L, Dyrting K, Rehg JE, Poon L, Guan Y, Peiris M, Webster RG. Reemerging H5N1 influenza viruses in Hong Kong in 2002 are highly pathogenic to ducks. J Virol. 2004;78:4892–4901. doi: 10.1128/JVI.78.9.4892-4901.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm-Ramirez KM, Hulse-Post DJ, Govorkova EA, Humberd J, Seiler P, Puthavathana P, Buranathai C, Nguyen TD, Chaisingh A, Long HT, Naipospos TS, Chen H, Ellis TM, Guan Y, Peiris JS, Webster RG. Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia? J Virol. 2005;79:11269–11279. doi: 10.1128/JVI.79.17.11269-11279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Nahar N, Rimi NA, Azad S, Islam MS, Gurley ES, Luby SP. Backyard poultry raising in Bangladesh: a valued resource for the villagers and a setting for zoonotic transmission of avian influenza. A qualitative study Rural Remote Health. 2012;12:1927. [PubMed] [Google Scholar]
- Swayne DE, Suarez DL. Highly pathogenic avian influenza. Rev Sci Tech. 2000;19:463–482. doi: 10.20506/rst.19.2.1230. [DOI] [PubMed] [Google Scholar]
- Szretter KJ, Balish AL, Katz JM. Influenza: propagation, quantification, and storage. Curr Protoc Microbiol. 2006;Chapter 15 doi: 10.1002/0471729256.mc15g01s3. Unit 15G 11. [DOI] [PubMed] [Google Scholar]
- Vascellari M, Granato A, Trevisan L, Basilicata L, Toffan A, Milani A, Mutinelli F. Pathologic findings of highly pathogenic avian influenza virus A/Duck/Vietnam/12/05 (H5N1) in experimentally infected pekin ducks, based on immunohistochemistry and in situ hybridization. Vet Pathol. 2007;44:635–642. doi: 10.1354/vp.44-5-635. [DOI] [PubMed] [Google Scholar]
- Wasilenko JL, Arafa AM, Selim AA, Hassan MK, Aly MM, Ali A, Nassif S, Elebiary E, Balish A, Klimov A, Suarez DL, Swayne DE, Pantin-Jackwood MJ. Pathogenicity of two Egyptian H5N1 highly pathogenic avian influenza viruses in domestic ducks. Arch Virol. 2011;156:37–51. doi: 10.1007/s00705-010-0813-y. [DOI] [PubMed] [Google Scholar]
- WHO/OIE/FAO H5N1 Evolution Working Group. Toward a unified nomenclature system for highly pathogenic avian influenza virus (H5N1) Emerg Infect Dis. 2008;14:e1. doi: 10.3201/eid1407.071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO/OIE & FAO H5N1 Evolution Working Group. Revised and updated nomenclature for highly pathogenic avian influenza A (H5N1) viruses. Influenza Other Respir Viruses. 2014;8:384–388. doi: 10.1111/irv.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Organization for Animal Health. Follow-up report No.: 39. Report reference: OIE Ref: 11881, Report Date: 22/04/2012, Country: Bangladesh. [Accessed on 28 August 2012];Update on Highly Pathogenic avian influenza in Animals (Type H5 and H7) 2012 [Google Scholar]
- Zhou JY, Shen HG, Chen HX, Tong GZ, Liao M, Yang HC, Liu JX. Characterization of a highly pathogenic H5N1 influenza virus derived from bar-headed geese in China. J Gen Virol. 2006;87:1823–1833. doi: 10.1099/vir.0.81800-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Unusual waterfowl mortality investigation questionnaire, Netrokona.
Appendix S2. Viral Transport Media (VTM).


