Abstract
Background
Migraine headache is a neurological disorder affecting millions worldwide. However, little is known about the mechanisms contributing to migraine. Recent genome-wide association studies have found single nucleotide polymorphisms in the gene encoding transient receptor potential channel M8. Transient receptor potential channel M8 is generally known as a cold receptor but it has been implicated in pain signaling and may play a role in migraine pain.
Methods
In order to investigate whether transient receptor potential channel M8 may contribute to the pain of migraine, the transient receptor potential channel M8 activator icilin was applied to the dura mater using a rat behavioral model of headache. Cutaneous allodynia was measured for 5 hours using Von Frey filaments.
Results
Dural application of icilin produced cutaneous facial and hind paw allodynia that was attenuated by systemic pretreatment with the transient receptor potential channel M8-selective antagonist AMG1161 (10 mg/kg p.o.). Further, the anti-migraine agent sumatriptan (0.6 mg/kg s.c.) or the non-selective NOS inhibitor L-NAME (20 mg/kg i.p.) also attenuated allodynia when given as a pretreatment.
Conclusions
These data indicate that transient receptor potential channel M8 activation in the meninges produces behaviors in rats that are consistent with migraine and that are sensitive to pharmacological mechanisms known to have efficacy for migraine in humans. The findings suggest that activation of meningeal transient receptor potential channel M8 may contribute to the pain of migraine.
Keywords: Transient receptor potential channel M8, headache, dura, migraine, allodynia, cold, sumatriptan
Background
Migraine is a common, often debilitating, neurological disorder that can last from hours to several days. The recent Global Burden of Disease Study found migraine headache to be the third most prevalent disease on the planet, following dental caries and tension type headache (1). Despite the magnitude and impact of migraine, the mechanisms that lead to this disorder remain unclear. Trigeminal nociceptors innervate the meninges and are sensitive to both chemical and mechanical stimulation (1,2). These nociceptors may play a role in the development of migraine pain, but the mechanisms by which they are activated remain to be fully elucidated.
The transient receptor potential (TRP) family of non-selective cation channels is involved in several physiological and pathological processes (3). TRP channels are sensitive to both thermal and chemical stimuli and have been proposed to act in mammals as thermosensors as well as detectors of endogenous inflammatory states and external irritants (4,5). In support of this hypothesis, pharmacological as well as genetic evidence has clearly implicated certain TRP channels in the detection or transduction of sensory stimuli. There are three super-families of TRP channels, TRPV, TRPC and TRPM (6), as well as several other smaller TRP families, some with only a single member (e.g. TRPA). The TRPM subfamily has eight members: TRPM1–M8. The TRPM8 channel was formerly known as the cold and menthol receptor (CMR1) due to its responsiveness to cold temperature as well as to cooling agents such as menthol (7–9). TRPM8 is activated by noxious and non-noxious cold ranging from ~28°C, down to 8°C (10). TRPM8 can also be activated by cooling agents such as icilin (as well as menthol mentioned above), behaving like many ligand-gated channels in response to these agents (11). Transcripts of TRPM8 are found in a subset (<15%) of small diameter sensory neurons (7,9). As alluded to above, TRPM8 expression in the trigeminal and dorsal root ganglia is thought to confer innocuous cold sensitivity to the somatosensory system (12,13), particularly in sensory neurons innervating cutaneous tissues. However, TRPM8 is also expressed in neurons innervating deep tissues, such as the bladder and colon (14,15). As these neurons are not exposed to cold temperatures, cold may not be an activating stimulus in these tissues and the channel may respond to other endogenous activators (16,17). Possible endogenous activators/sensitizers in deep-tissue afferents include stimuli such as lysophospholipids, cyclopentenone prostaglandins and phosphatidylinositol biphosphate among others (18). Thus, TRPM8 may be a sensor of a variety of internal and external stimuli.
One of the most consistent genetic findings in migraine patients comes from several recent genome-wide association studies (GWAS) that revealed single nucleotide polymorphisms (SNPs) in and around the TRPM8 locus (19–21). The mutations can lie in coding regions of the gene, but are often within the 5′ untranslated region. It remains unclear how these mutations influence TRPM8, e.g. by altering channel expression or function, but these mutations have been verified across several populations of migraineurs (22–25) and suggest that TRPM8 may play a role in migraine. The purpose of this study was to investigate whether activation of TRPM8 in the meninges produces behaviors in rodents consistent with migraine pain using a preclinical model of headache.
Methods
Animals
Adult male Sprague–Dawley rats (250–300 g, Harlan) were maintained in a temperature-controlled room on a 12-hour light/dark cycle with food and water ad libitum. All procedures were performed in accordance with the policies of the IASP as well as the NIH guidelines for use of laboratory animals. All procedures were approved by the IACUC of the University of Arizona.
Surgeries
Dura cannulation surgeries were performed on rats (250–300 g) as previously described (26,27). Briefly, animals were anesthetized and an incision exposing the skull was made to the top of the skull. Once the skull was exposed, a 1-mm hole was made in the skull to expose the dura (1 mm left of midline, 1 mm anterior to bregma). A 1 mm guide cannula (Plastics One) was then inserted into the hole and secured with Vetbond™ (3M™). Two screws (Small Parts) were placed rostral to the cannula on either side of the skull. Dental acrylic was used to adhere the cannula and screws to the skull. A dummy cannula (Plastics One) was placed into the cannula to ensure patency. Postoperatively, animals received gentamicin (8 mg/kg) to minimize infection. Rats were housed individually and given 6–8 days for recovery prior to behavioral testing.
Testing
The animals were allowed to habituate in the testing chambers for one hour prior to baseline. Animal weights were recorded and oral gavage was given post baseline. At 30 min post oral gavage, dura injections were given. Testing of both facial and hind paw allodynia was conducted every hour for five hours using calibrated Von Frey filaments, thresholds were determined by the ‘up-down’ method (28). The Von Frey filaments were applied to the peri-orbital region of the face or to the plantar surface of the hind paw perpendicularly until the entire force was applied and held for approximately 5 seconds or until animals withdrew. Maximum filaments used were 8 g for the peri-orbital region and 15 g for the hindpaw. Upon completion of allodynia testing, all animals’ cannulas patency was verified by ink injection into the cannula.
Solution preparation
The TRPM8 antagonist AMG1161, previously published as Compound 4529,(10 mg/mL) was dissolved in 2.5% methylcellulose diluted from 5% stock and was kept at room temperature prior to oral gavage. Icilin (Cayman Chemical) was prepared at a concentration of 1 nmol in polyethylene glycol-300 (PEG 300). Then 10 μL of the 1 nmol solution was injected at approximately 2 μL per second. Sumatriptan succinate (Amgen) was dissolved into saline and a dose of 0.6 mg/kg was administered via sub-cutaneous (s.c.) injection, as previously described (27,30). The non-selective nitric oxide synthase (NOS) inhibitor L-NG-nitroarginine methyl ester (L-NAME) (Cayman Chemical) was given at 20 mg/kg via intraperitoneal injection.
In vitro TRPM8 functional assay
Recombinant rat TRPM8 plasmid DNA was stably transfected into Chinese hamster ovary (CHO) cell lines using a tetracycline-inducible T-REx™ expression plasmid from Invitrogen, Inc. (Carlsbad, CA). To enable a luminescence readout based on intracellular calcium increase (31), the cell lines were co-transfected with a pcDNA3.1 plasmid containing jellyfish aequorin cDNA. The cells were maintained in Ham’s F-12 nutrient media containing tetracycline-free fetal bovine serum, glutamine-penicillin-streptomycin, genetecin, blasticidin-S-HCl and zeocin. Twenty four hours before assay, the cells were induced with 0.5 μg/mL tetracycline in Ham’s F-12 for TRPM8 expression and plated at a density of 3.0 × 104 per well, in 96-well black plates with clear bottoms and grown at 37°C in a humidified atmosphere of 5% CO2. On the day of assay, culture media was removed and cells were incubated for two hours at 37°C with assay buffer (Ham’s F-12 containing 30 mM HEPES) containing 15 μM coelenterazine (stock prepared in ethanol). Stock solution of AMG1161 was prepared in 100% DMSO and diluted to required final concentrations (0.2 nM to 20 μM) in assay buffer, limiting final concentration of DMSO to <0.5%. TRPM8 antagonist AMG1161 or a positive control (AMG0762) was added 2.5 min prior to the addition of agonist (1 μM icilin) or 1 min prior to the addition of cold buffer (10°C) (32,33). Luminescence was measured on a charge-coupled device camera-based FLASH-luminometer built by Amgen, Inc. A cooling device attached to the FLASH luminometer was used for cold (10°C) activation of TRPM8. A TRPM8 antagonist control (AMG0762) at a final concentration of 1 μM was considered zero percent control for cold activation. Compound activity was calculated using GraphPad Prism, version 5.03 (GraphPad Software Inc, San Diego, CA) or Genedata Screener (San Francisco, CA).
Data analysis
All data are graphed as means ±SEM. Allodynia studies were analyzed among groups and across time by two-factor analysis of variance (ANOVA) for treatment and time. Data were also converted to area over the time-effect curve to allow for analysis of multiple treatment groups and analyzed with a one-factor ANOVA and Bonferroni’s post test. Statistics were calculated using GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA). Significance was set at P < .05 for all data analysis.
Results
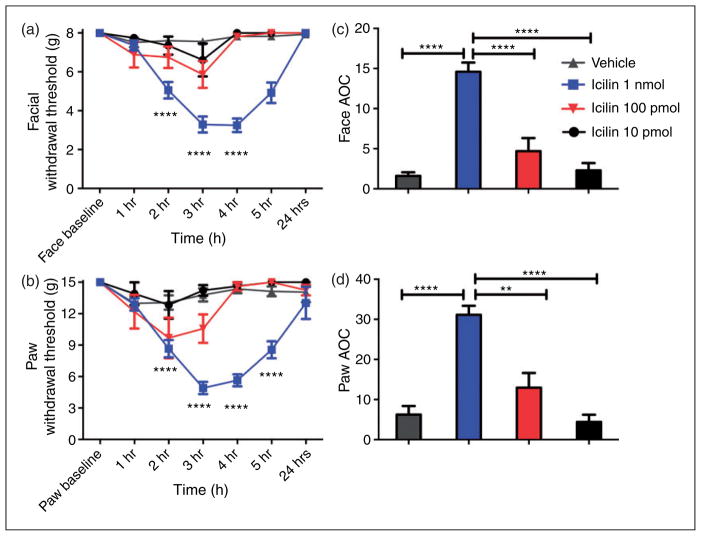
Dural application of 1 nmol icilin produced robust facial (Figure 1(a)) and hindpaw (Figure 1(b)) allodynia that peaked 3–4 hours later. This allodynia was dose-dependent as shown in the time courses and by area-over-curve plots in Figure 1(c,d). Allodynia was not observed in response to dural application of vehicle (PEG 300). Animals treated with 1 nmol icilin displayed facial withdrawal thresholds significantly different from controls from 2–5 hours, thresholds were trending towards baseline at five hours and had completely returned to baseline by 24 hours. Animals treated with 100 pmol or 10 pmol icilin did not produce facial or hindpaw responses significantly different than controls.
Figure 1.
Activation of meningeal TRPM8 produces headache-related behaviors. Dural application of 1 nmol icilin induced cutaneous facial and hindpaw allodynia. Withdrawal thresholds to tactile stimuli applied to the face (a) and the hind paws (b) were measured in rats prior to and after dural application of 1 nmol icilin (N = 39 at time points 1–5 hours, N = 8 at 24 hours), 100 pmol icilin (N = 8 at all timepoints), 10 pmol icilin (N = 8 at all timepoints) vehicle (PEG300) (N = 29 at time points 1–5 hour, N = 8 at 24 hours) For both facial and hind-paw responses, two-factor ANOVA indicated a significant effect of both treatment and time of both the face and hind paws. This figure comprises all data run in this manuscript with these stimuli (icilin or vehicle). Withdrawal thresholds to tactile stimuli measured for five hours and data were converted to area over the time-effect curve (AOC) for face (c) and hind paw (d). A one-factor ANOVA with Bonferroni’s post test revealed significantly more allodynia with 1 nmol icilin injection compared to both vehicle, 100 pmol icilin and 10 pmol icilin in both the face and hind paws. Facial: treatment F(3, 508) = 26.38, P < 0.0001, time F(6, 508) = 9.764, P < 0.0001; Hind paw: time F(6, 508) = 9.543, P < 0.0001, treatment F(3, 508) = 31.13, P < 0.0001.
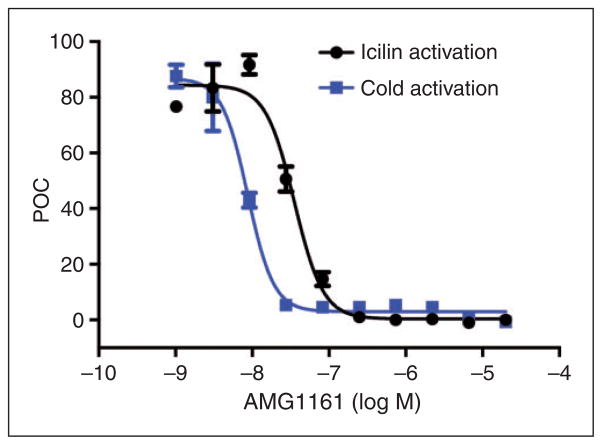
Although icilin is commonly used as an activator of TRPM8, blockade of this channel with a selective antagonist would further support the conclusion that headache-like responses following dural icilin are mediated by TRPM8. First, an in vitro calcium influx assay was used to examine blockade of TRPM8 by the antagonist AMG1161. In cultured CHO cells stably transfected with the rat TRPM8 channel, AMG 1161 (Figure 2) displayed concentration-dependent inhibition of TRPM8 activation by icilin and cold with IC50 values of 23 ± 0.9 nM (n = 6 independent experiments with three replicates for each concentration) and 11 ± 0.4 nM (n = 2 independent experiments with three replicates for each concentration), respectively. In order to address selectivity, similar studies were performed against related TRP channels including TRPV1 (activated with capsaicin), TRPV3 (activated with 2-APB), TRPV4 (activated with 4-alpha PDD), TRPC5 (activated with cold temperature) and TRPA1 (activated with allyl isothipcyanate; AITC). Corresponding IC50 values were >10 μm, >10 μm, >20 μm, >40 μm and >40 μm, respectively (data not shown). These data indicate that AMG1161 has functional selectivity over similarly related TRP channels.
Figure 2.
AMG1161 is a TRPM8 antagonist. AMG 1161 showed a concentration-dependent inhibition of TRPM8 activation by icilin and cold in cultured CHO cells stably transfected with rat TRPM8 channel. IC50 values were 23 ± 0.9 nM (n = 6 independent experiments with three replicates for each concentration) and 11 ± 0.4 nM (n = 2 independent experiments with three replicates for each concentration) against icilin and cold, respectively and Y-axis is percent of control (POC).
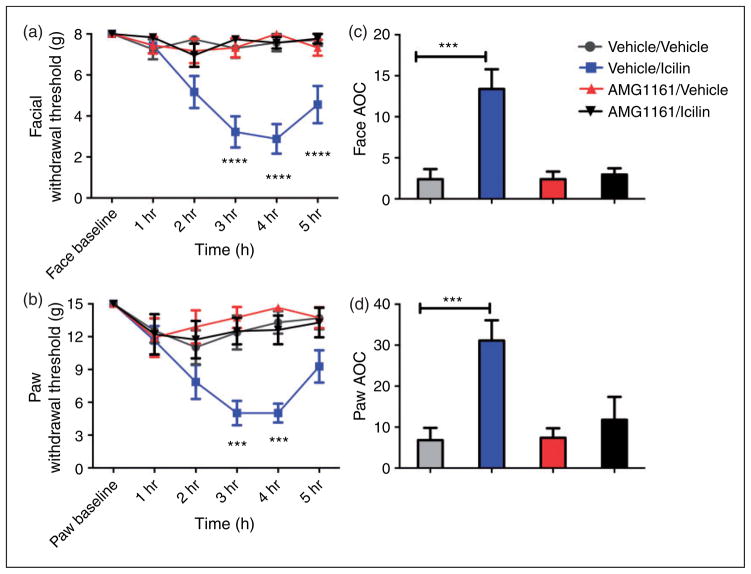
In order to determine whether icilin produces its behavioral effects via activation of TRPM8, AMG1161 was given to rats prior to dural stimulation. Oral pretreatment with AMG1161 (10 mg/kg) 30 min prior to application of 1 nmol icilin on the dura prevented the reduction in facial and paw withdrawal thresholds due to icilin administration. There was a significant decrease in facial and hindpaw allodynia at the three hour and four hour time points compared to icilin alone and also for facial allodynia at the five hour time point (hindpaw allodynia was not significant at five hours) (Figure 3(a,b)). Oral pretreatment with vehicle or AMG1161 had no effect on animals given vehicle on the dura (Figure 3(a,b)) indicating that AMG1161 alone had no effect on withdrawal thresholds.
Figure 3.
Headache-like responses due to dural icilin are prevented by a systemic TRPM8 antagonist. Pretreatment with AMG1161 (10 mg/kg) 30 min prior to application of 1 nmol icilin attenuated cutaneous allodynia. Withdrawal thresholds to tactile stimuli applied to the face (a) and the hind paws (b) were measured in rats prior with AMG1161 or vehicle then given dural application of 1 nmol icilin. AMG1161/vehicle (N = 9), AMG1161/icilin (N = 9), vehicle/vehicle (N = 11) or vehicle/icilin (PEG300) (N = 14) (for both facial and hind-paw responses, two-factor ANOVA indicated a significant effect of both treatment and time of both the face and hind paws). Facial: treatment F(3, 234) = 31.07, P < 0.0001, time F(5, 234) = 5.621, P < 0.0001; Hind paw: time F(5, 138) = 8.023, F(5, 234) = 6.168, P < 0.0001, treatment F(3, 234) = 17.63, P < 0.0001.
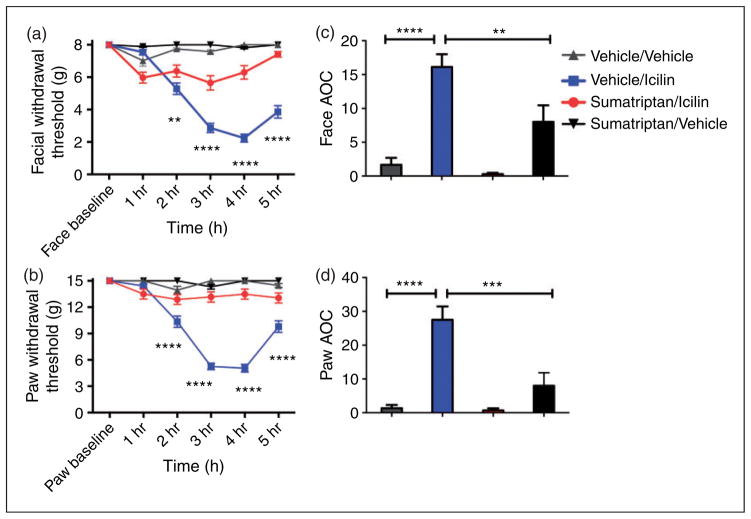
Sumatriptan (as well as other triptans) is considered to be a standard first-line abortive agent in the treatment of human migraine patients but is not given for other forms of pain (34). Prior animal studies have used sumatriptan as a probe to determine whether behavioral responses are headache-like (27,30). Thus, efficacy of sumatriptan against icilin-induced behavioral responses was examined to determine whether these behaviors are also consistent with headache. Simultaneous treatment of rats with sumatriptan (0.6 mg/kg, s.c.) and dural icilin led to partial attenuation of the decrease in both facial and paw withdrawal thresholds observed with icilin alone (Figure 4). Sumatriptan treatment did not cause any changes in withdrawal thresholds in animals given vehicle (PEG-300) onto the dura (Figure 4).
Figure 4.
Headache-like responses following dural icilin are sensitive to the migraine abortive agent sumatriptan. Dural application of 1 nmol icilin induced cutaneous allodynia that is prevented by simultaneous treatment of rats with sumatriptan (0.6 mg/kg, s.c.). Withdrawal thresholds to tactile stimuli applied to the face (a) and the hind paws (b) were measured in rats prior to and after dural application of sumatriptan/vehicle (N = 7), sumatriptan/icilin (N = 7), vehicle/vehicle (N = 6) or vehicle/icilin (PEG300) (N = 8) Withdrawal thresholds to tactile stimuli measured for 5 hours and data were converted to area over the time-effect curve (AOC) for face (c) and hind paw (d). A one-factor ANOVA with Bonferroni’s post test revealed significantly more allodynia with vehicle injection followed by icilin compared to sumatriptan followed by icilin in both the face and hind paws. Facial: treatment F(15, 144) = 27.38, P < 0.0001, time F(5, 144) = 37.30, P < 0.0001; Hind paw: time F(15, 144) = 29.90, P < 0.0001, treatment F(5, 144) = 41.71, P < 0.0001.
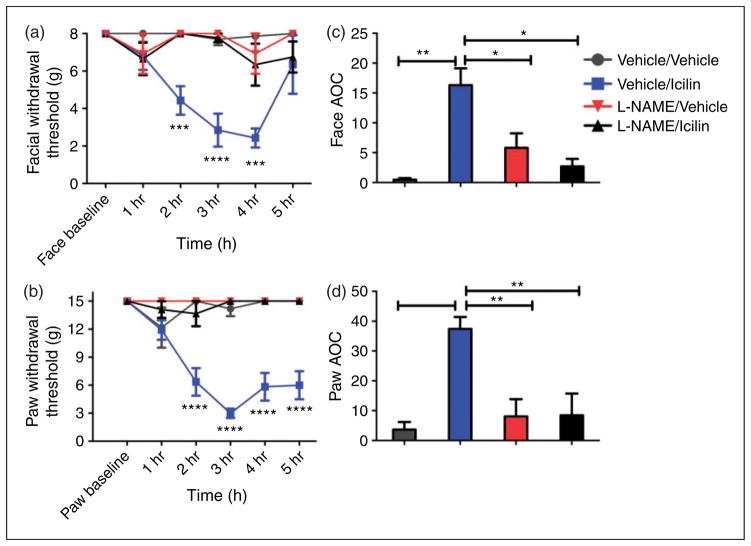
NOS inhibitors have also been found to be efficacious in humans with migraine as pharmacological blockade of NOS with the non-selective inhibitor L-NG-Monomethylarginine (L-NMMA) significantly reduced migraine pain in a small clinical trial (35,36). Similar to the experiments described above with sumatriptan, we also assessed whether the icilin-induced behaviors in this preclinical model were sensitive to NOS inhibitors. Animals were pretreated with another non-selective NOS inhibitor L-NAME (20 mg/kg, s.c.) or vehicle 15 min prior to icilin administration (Figure 5(a,b)). Animals that received L-NAME/icilin displayed significantly higher thresholds (i.e. reduced allodynia) both in the face and hindpaw compared to those of the vehicle/icilin group. No significant differences were found between the L-NAME/icilin group and vehicle groups. Together with the efficacy of sumatriptan, these data indicate that the behavioral response in rats due to dural TRPM8 activation is consistent with headache.
Figure 5.
Headache-like responses following dural icilin are sensitive to NOS inhibition. Administration of L-NAME (20 mg/kg) i.p.15 min prior to dural icilin (1 nmol) prevented cutaneous allodynia. Withdrawal thresholds to tactile stimuli applied to the face (a) and the hind paws (b) were measured in rats L-NAME/vehicle (N = 7), L-NAME/icilin (N = 9), vehicle/vehicle (N = 4) or vehicle/icilin (PEG300) (N = 4). Withdrawal thresholds to tactile stimuli measured for five hours and data were converted to area over the time-effect curve (AOC) for face (c) and hind paw (d). A one-factor ANOVA with Bonferroni’s post test revealed significantly more allodynia with vehicle pretreatment followed by icilin compared to L-NAME followed by icilin in both the face and hind paws. Facial: treatment F(5, 120) = 3.107, P = 0.0113, time F(3, 120) = 16.85, P < 0.0001; Hind paw: time F(15, 78) = 5.172, P < 0.0001, treatment F(5, 120) = 4.008, P = 0.0021.
Discussion
Migraine is a debilitating disorder that affects a population of otherwise healthy individuals and for which current treatments are often ineffective. Despite the prevalence of migraine, the underlying pathophysiology is not well understood and thus identification of novel targets for migraine therapies is challenging. Although pain signaling from the meninges has been proposed to play a role in the development of the pain of migraine, little is known about the mechanisms by which afferents are activated during a migraine event. We and others have described numerous mechanisms capable of initiating nociceptive signaling from the meninges in animals (26,27,37) but human data supporting a role for many of these mechanisms are largely absent. In contrast, human data implicating TRPM8 in migraine has been reported in several recent GWAS of migraine patients (21–25,38) but preclinical data supporting a role for TRPM8 in migraine do not yet exist.
Using a preclinical model of headache, we now demonstrate that stimulation of the dura with the TRPM8 agonist icilin evokes cutaneous allodynia, a common feature of migraine.
Previous preclinical work has shown cutaneous allodynia after dural afferent activation with numerous compounds (39–41) and thus these data are consistent with other pronociceptive stimuli applied to the dura. An interesting observation from these studies is the development of mechanical allodynia following TRPM8 activation, a type of hypersensitivity not typically observed after activation of this channel (cold allodynia is usually the phenotype associated with TRPM8). However, the current studies are not testing the primary site of activation (i.e. the dura mater) and are dependent on referred allodynia of the facial and hindpaw regions. It is thus not known whether thermal thresholds are altered in the dura mater where TRPM8 is activated. Allodynia was nonetheless attenuated by pretreatment with the TRPM8 antagonist AMG1161, a compound that shows several hundred-fold selectivity over related TRP channels, indicating this affect was due to specific activation of TRPM8 in the dura. Additionally, both sumatriptan and L-NAME attenuated the cutaneous facial and hindpaw allodynia due to dural application of icilin. This is presumably due to attenuation of afferent input from the meninges and inhibition of subsequent central sensitization necessary to establish referred facial and hindpaw allodynia (27), although our data do not prove this mechanism. The ability of sumatriptan and L-NAME to attenuate icilin-induced allodynia in this model suggests that the behavioral responses due to activation of TRPM8 within the dura are consistent with headache. Triptans are one of the most common abortive agents for migraine and NOS inhibitors have also been shown to be efficacious in inhibiting migraine pain in humans (35,36). Taken together, these data suggest that activation of TRPM8 on meningeal afferents contributes to headache.
Although several GWAS now exist implicating SNPs in TRPM8 in migraine, the actual effects of the mutations on channel expression and function are not yet known. Consequently, it is difficult to determine whether patients with these mutations have increased or decreased TRPM8 function. Activation of TRPM8 on sensory neurons can initiate afferent signaling but, interestingly in the case of this channel, the sensation that is ultimately perceived may be pronociceptive or anti-nociceptive, possibly depending on the context. In animals, TRPM8 is essential for both neural and behavioral responses to noxious cold, as well as cold mimetics (42). Deletion or antagonism of TRPM8 reduces inflammation-induced cold hypersensitivity (43–45), indicating that this channel is necessary for cold allodynia in pain states. But TRPM8 can also produce analgesia in states of inflammation (8,46) and menthol is widely used as a topical analgesic. Ultimately, it may depend on whether TRPM8 is activated alone or in the presence of other stimuli. In support of this concept, it was recently shown that, while activation of TRPM8 alone is nociceptive, TRPM8 activation can decrease nociception due to stimulation of other TRP channels (47). Our data demonstrate that activation of TRPM8 alone in the meninges is pronociceptive but whether TRPM8 is activated alone during migraine in humans is not clear. Future studies will ideally determine how migraine-associated TRPM8 SNPs impact channel function. These studies will also shed light on whether patients are more likely to have increased or decreased channel expression and function and will help further uncover how TRPM8 may contribute to migraine (i.e. in a protective or causative).
It should be noted that prior studies on TRPM8 expression in meningeal afferents found little to no channel expression on these neurons (48). This is seemingly at odds with our current findings that activation of TRPM8 in the dura is pronociceptive. Differences in species could account for the discrepancy in findings as our studies were performed in rats while the prior work was in mice and expression of TRPM8 on dural afferents may be species dependent. Additionally, other studies using mice have found TRPM8 expression in the meninges but found that the expression is restricted to specific regions (49). TRPM8 may thus be expressed in the meninges in mice, but not equally across the tissue. Another possibility is that TRPM8 is not expressed on meningeal afferents but is found on other cell types in the dura. Mast cells have been implicated in migraine (50) and TRPM8 is expressed on this cell type (51). Additionally, we recently reported that dural fibroblasts may also contribute to headache pathology (52) and TRPM8 is expressed on several types of fibroblasts (53). Thus, activation of the channel on non-neuronal cells could indirectly initiate afferent signaling. Future work is necessary to more conclusively determine the expression pattern of the channel in the meninges and how its activation may ultimately contribute to headache.
These studies provide the first preclinical evidence that TRPM8 can play a role in headache disorders such as migraine and they provide a potential mechanism for how the channel may contribute to migraine pain, i.e. activation within the meninges and subsequent initiation of afferent signaling. Although it still remains to be determined how TRPM8 is impacted by the mutations in human migraine patients, these data suggest that meningeal TRPM8 is capable of contributing to headache. Importantly, these findings imply that TRPM8 antagonists may have efficacy as novel migraine therapeutics.
Article highlights.
Activation of TRPM8 in the dura mater produces behavior in rats that is consistent with headache.
Behavioral responses to dural TRPM8 activation are blocked by a TRPM8 antagonist, sumatriptan, and a NOS inhibitor.
These findings suggest that meningeal TRPM8 may play a role in the pathophysiology of headache.
Acknowledgments
Funding
This work was supported by The National Institutes of Health [NS072204, GM008718] & funding from Amgen Inc.
Footnotes
Conflict of interest
None declared.
References
- 1.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strassman AM, Levy D. Response properties of dural nociceptors in relation to headache. J Neurophysiol. 2006;95:1298–1306. doi: 10.1152/jn.01293.2005. [DOI] [PubMed] [Google Scholar]
- 3.Stucky CL, Dubin AE, Jeske NA, et al. Roles of transient receptor potential channels in pain. Brain Res Rev. 2009;60:2–23. doi: 10.1016/j.brainresrev.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nillus B, Flockerzi V, editors. Handbook of experimental pharmacology. Cham: Springer International Publishing; 2014. Channels and thermosensation; pp. 729–741. [DOI] [PubMed] [Google Scholar]
- 5.Bandell M, Macpherson LJ, Patapoutian A. From chills to chilis: mechanisms for thermosensation and chemesthesis via thermoTRPs. Curr Opin Neurobiol. 2007;17:490–497. doi: 10.1016/j.conb.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nilius B, Szallasi A. Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol Rev. 2014;66:676–814. doi: 10.1124/pr.113.008268. [DOI] [PubMed] [Google Scholar]
- 7.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 8.Knowlton WM, Palkar R, Lippoldt EK, et al. A sensorylabeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci. 2013;33:2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peier AM, Moqrich A, Hergarden AC, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 10.McKemy DD. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol Pain. 2005;1:16. doi: 10.1186/1744-8069-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuang H-H, Neuhausser WM, Julius D. The supercooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron. 2004;43:859–869. doi: 10.1016/j.neuron.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 12.Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci. 2008;28:566–575. doi: 10.1523/JNEUROSCI.3976-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung M-K, Caterina MJ. TRP channel knockout mice lose their cool. Neuron. 2007;54:345–347. doi: 10.1016/j.neuron.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Mukerji G, Yiangou Y, Corcoran S, et al. Cool and menthol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urol. 2006;6:6. doi: 10.1186/1471-2490-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrington AM, Hughes PA, Martin CM, et al. A novel role for TRPM8 in visceral afferent function. PAIN. 2011;152:1459–1468. doi: 10.1016/j.pain.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 16.Nealen ML. TRPM8 mRNA is expressed in a subset of cold-responsive trigeminal neurons from rat. J Neurophysiol. 2003;90:515–520. doi: 10.1152/jn.00843.2002. [DOI] [PubMed] [Google Scholar]
- 17.Lippoldt EK, Elmes RR, McCoy DD, et al. Artemin, a glial cell line-derived neurotrophic factor family member, induces TRPM8-dependent cold pain. J Neurosci. 2013;33:12543–12552. doi: 10.1523/JNEUROSCI.5765-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sousa-Valente J, Andreou AP, Urban L, et al. Transient receptor potential ion channels in primary sensory neurons as targets for novel analgesics. Br J Pharmacol. 2014;171:2508–2527. doi: 10.1111/bph.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freilinger T, Anttila V, de Vries B, et al. Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat Genet. 2012;44:777–782. doi: 10.1038/ng.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esserlind AL, Christensen AF, Le H, et al. Replication and meta-analysis of common variants identifies a genome-wide significant locus in migraine. Eur J Neurol. 2013;20:765–772. doi: 10.1111/ene.12055. [DOI] [PubMed] [Google Scholar]
- 21.Chasman DI, Schürks M, Anttila V, et al. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat Genet. 2011;43:695–698. doi: 10.1038/ng.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan X, Wang J, Fan W, et al. Replication of migraine GWAS susceptibility loci in Chinese Han population. Headache. 2014;54:709–715. doi: 10.1111/head.12329. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh J, Pradhan S, Mittal B. Genome-wide-associated variants in migraine susceptibility: a replication study from North India. Headache. 2013;53:1583–1594. doi: 10.1111/head.12240. [DOI] [PubMed] [Google Scholar]
- 24.An X-K, Ma Q-L, Lin Q, et al. PRDM16 rs2651899 variant is a risk factor for chinese common migraine patients. Headache. 2013;53:1595–1601. doi: 10.1111/head.12212. [DOI] [PubMed] [Google Scholar]
- 25.Chasman DI, Anttila V, Buring JE, et al. Selectivity in genetic association with sub-classified migraine in women. PLoS Genet. 2014;10:e1004366. doi: 10.1371/journal.pgen.1004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan J, Edelmayer RM, Wei X, et al. Dural afferents express acid-sensing ion channels: a role for decreased meningeal pH in migraine headache. PAIN. 2011;152:106–113. doi: 10.1016/j.pain.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edelmayer RM, Vanderah TW, Majuta L, et al. Medullary pain facilitating neurons mediate allodynia in headache- related pain. Ann Neurol. 2009;65:184–193. doi: 10.1002/ana.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 29.Horne DB, Tamayo NA, Bartberger MD, et al. Optimization of potency and pharmacokinetic properties of tetrahydroisoquinoline transient receptor potential melastatin 8 (TRPM8) antagonists. J Med Chem. 2014;57:2989–3004. doi: 10.1021/jm401955h. [DOI] [PubMed] [Google Scholar]
- 30.Edelmayer RM, Le LN, Yan J, et al. Activation of TRPA1 on dural afferents: A potential mechanism of headache pain. PAIN. 2012;153:1949–1958. doi: 10.1016/j.pain.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Poul E, Hisada S, Mizuguchi Y. Adaptation of aequorin functional assay to high throughput screening. J Biomol Screen. 2002;7(1):57–65. doi: 10.1177/108705710200700108. [DOI] [PubMed] [Google Scholar]
- 32.Gavva NR, Davis C, Lehto SG, et al. Transient receptor potential melastatin 8 (TRPM8) channels are involved in body temperature regulation. Mol Pain. 2012;8:36. doi: 10.1186/1744-8069-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almeida MC, Hew-Butler T, Soriano RN, et al. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci. 2012;32:2086–2099. doi: 10.1523/JNEUROSCI.5606-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Felice M, Ossipov MH, Wang R, et al. Triptaninduced latent sensitization: A possible basis for medication overuse headache. Ann Neurol. 2010;67:325–337. doi: 10.1002/ana.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lassen LH, Christiansen I, Iversen HK, et al. The effect of nitric oxide synthase inhibition on histamine induced headache and arterial dilatation in migraineurs. Cephalalgia. 2003;23:877–886. doi: 10.1046/j.1468-2982.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- 36.Lassen LH, Ashina M, Christiansen I, et al. Nitric oxide synthase inhibition: a new principle in the treatment of migraine attacks. Cephalalgia. 1998;18:27–32. doi: 10.1046/j.1468-2982.1998.1801027.x. [DOI] [PubMed] [Google Scholar]
- 37.Burstein R, Jakubowski Mand Rauch SD. The science of migraine. J Vestib Res. 2011;21:305–314. doi: 10.3233/VES-2012-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schürks M. Genetics of migraine in the age of genomewide association studies. J Headache Pain. 2012;13:1–9. doi: 10.1007/s10194-011-0399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: A model for recurrent headache. Headache. 2007;47:1026–1036. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan J, Dussor G. Ion channels and migraine. Headache. 2014;54:619–639. doi: 10.1111/head.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wieseler J, Ellis A, Sprunger D, et al. A novel method for modeling facial allodynia associated with migraine in awake and freely moving rats. J Neurosci Methods. 2010;185:236–245. doi: 10.1016/j.jneumeth.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knowlton WM, Bifolck-Fisher A, Bautista DM, et al. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. PAIN. 2010;150:340–350. doi: 10.1016/j.pain.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colburn RW, Lubin ML, Stone DJ, Jr, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 44.Bautista DM, Siemens J, Glazer JM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 45.Knowlton WM, Daniels RL, Palkar R, et al. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS ONE. 2011;6:e25894. doi: 10.1371/journal.pone.0025894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Premkumar LS, Abooj M. TRP channels and analgesia. Life Sc. 2013;92:415–424. doi: 10.1016/j.lfs.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson EM, Jenkins AC, Caudle RM, et al. The effects of a co-application of menthol and capsaicin on nociceptive behaviors of the rat on the operant orofacial pain assessment device. PLoS ONE. 2014;9:e89137. doi: 10.1371/journal.pone.0089137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang D, Li SY, Dhaka A, et al. Expression of the transient receptor potential channels TRPV1, TRPA1 and TRPM8 in mouse trigeminal primary afferent neurons innervating the dura. Mol Pain. 2012;8:66. doi: 10.1186/1744-8069-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newsom J, Holt JL, Neubert JK, et al. A high density of trpm8 expressing sensory neurons in specialized structures of the head. Abstract retrieved from Society for Neuroscience. 2012:1–8. [Google Scholar]
- 50.Levy D, Burstein R, Kainz V, et al. Mast cell degranulation activates a pain pathway underlying migraine headache. PAIN. 2007;130:166–176. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho Y, Jang Y, Yang YD, et al. TRPM8 mediates cold and menthol allergies associated with mast cell activation. Cell Calcium. 2010;48:202–208. doi: 10.1016/j.ceca.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Wei X, Melemedjian OK, Ahn DD-U, et al. Dural fibroblasts play a potential role in headache pathophysiology. PAIN. 2014;155:1238–1244. doi: 10.1016/j.pain.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El Karim IA, Linden GJ, Curtis TM, et al. Human dental pulp fibroblasts express the “cold-sensing” transient receptor potential channels TRPA1 and TRPM8. J Endod. 2011;37:473–478. doi: 10.1016/j.joen.2010.12.017. [DOI] [PubMed] [Google Scholar]