Abstract
Cancers harbor significant genetic heterogeneity and patterns of relapse following many therapies are due to evolved resistance to treatment. While efforts have been made to combine targeted therapies, significant levels of toxicity have stymied efforts to effectively treat cancer with multi-drug combinations using currently approved therapeutics. We discuss the relationship between tumor-promoting inflammation and cancer as part of a larger effort to develop a broad-spectrum therapeutic approach aimed at a wide range of targets to address this heterogeneity. Specifically, macrophage migration inhibitory factor, cyclooxygenase-2, transcription factor nuclear factor-kappaB, tumor necrosis factor alpha, inducible nitric oxide synthase, protein kinase B, and CXC chemokines are reviewed as important antiinflammatory targets while curcumin, resveratrol, epigallocatechin gallate, genistein, lycopene, and anthocyanins are reviewed as low-cost, low toxicity means by which these targets might all be reached simultaneously. Future translational work will need to assess the resulting synergies of rationally designed antiinflammatory mixtures (employing low-toxicity constituents), and then combine this with similar approaches targeting the most important pathways across the range of cancer hallmark phenotypes.
Keywords: cancer, tumor, inflammation, hallmarks, phytochemicals
Introduction
In 1863, Rudolf Virchow first proposed the role of inflammation in cancer, after observing the presence of leukocytes in neoplastic tissue [1]. Since Virchow’s initial observation that inflammation and cancer are linked, empirical evidence has underscored inflammation as both a cause and consequence of cancer [2, 3]. The inflammatory milieu promotes a cellular microenvironment that favors the expansion of genomic aberrations and the initiation of carcinogenesis [4]. While acute inflammation is predominantly considered to be a self-limiting process and an important component of the immune system with therapeutic significance, inadequate or incomplete resolution of inflammatory responses frequently leads to various chronic diseases, including cancer [5, 6]. In fact, numerous epidemiological and clinical studies have indicated that chronic unresolved inflammation promotes and exacerbates malignancy [7]. Several types of cancer arise in the setting of chronic inflammation suggesting a strong link between inflammation and cancer [3, 8].
It has been estimated that about 25% of all cancers are etiologically linked to chronic inflammation and infection [9]. For example, the risk of colorectal cancer has been found to be 10-fold higher in inflammatory bowel disease, such as ulcerative colitis and Crohn's disease [10]. The risk for cancer of the respiratory system is positively associated with the severity and duration of inflammatory diseases [11]. Possible associations have also been found between inflammatory diseases, such as esophagitis and Barrett's metaplasia, and esophageal cancer [12] and between chronic pancreatitis and pancreatic cancer [13]. Emerging studies have established a crucial role of chronic, unresolved inflammation in the promotion and progression of breast cancer, including the most aggressive type known as inflammatory breast cancer [14, 15]. The ovarian epithelial inflammation is linked to ovarian cancer [16]. Likewise, foreskin inflammation (phimosis) has been associated with penile cancer [17]. Helicobacter pylori (H. pylori) infection and associated inflammation in the gastrointestinal tract represent the leading cause of adenocarcinoma [12]. Hepatic inflammation, due to exposure to infectious agents including hepatitis B virus and hepatitis C virus as well as toxic compounds, represent an early step in the development of hepatocellular carcinoma [18]. Moreover, chronic prostatitis, due to persistent bacterial infection or noninfective stimuli, has been linked to prostate cancer [19]. All of this evidence supports an association between chronic inflammation and cancer development.
Chronic inflammation is linked to various phases implicated in tumorigenesis, such as cellular proliferation, transformation, apoptosis evasion, survival, invasion, angiogenesis and metastasis [7, 8, 20]. A number of proinflammatory molecules within the tumor microenvironment participate in a complex signaling network that enables extravasations of tumor cells through the stroma, resulting in promotion of tumor progression [21]. Inflammation is known to contribute to the process of carcinogenesis mediated through the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) capable of damaging the DNA at the site of the tumor [22]. Free radicals and aldehydes, produced during chronic inflammation, can induce deleterious gene mutation and posttranslational modifications of key cancer-related proteins [23]. Damage can also occur in tissues that are distant from the tumor [24].
Other procarcinogenic products of inflammation include cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6), as well as chemokines, prostaglandins, oncogenes, inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), 5-lipoxygenase, matrix metalloproteinases (MMPs), vascular endothelial growth factor (VEGF), hypoxia-inducible factor-1α (HIF-1α), nuclear factor-κB (NF-κB), nuclear factor of activated T-cells, signal transducers and activators of transcription 3 (STAT3), activator protein-1 (AP-1), cAMP response binding protein/p300 (CBP/p300), and CCAAT enhancer binding protein (C/EBP) [25–28]. Additionally, activation of various upstream kinases, including IκB kinase (IKK), protein kinase C (PKC), mitogen-activated protein kinase (MAPK), and phosphoinositide-3 kinase/Protein kinase B (PI3K)/AKT, are known to participate in inflammation-driven oncogenesis [28]. The pro-cancerous outcome of chronic inflammation is increased DNA damage, increased DNA synthesis, cellular proliferation, the disruption of DNA repair pathways and cellular milieu, the inhibition of apoptosis, the promotion of angiogenesis and invasion.
As well, chronic inflammation has an influence on immune system constituents that are directly linked with cancer progression. Under normal conditions, immune cells, including macrophages, granulocytes, mast cells, dendritic cells (DCs), innate lymphocytes, and natural killer (NK) cells serve as the front line of defense against pathogens. When tissue disruption occurs, macrophages and mast cells secrete matrix-remodeling proteins, cytokines and chemokines, which activate local stromal cells (fibroblasts, adipocytes, vascular cells and others) to recruit circulating leukocytes into damaged tissue (acute inflammation), to eliminate the pathogens [29]. However, when these processes are initiated in the tumor microenvironment, they are not resolved which leads to chronic inflammation of the “damaged” (tumor) tissue. Thus, while acute inflammation normally supports and balances two opposing needs for the repair of damaged tissues (apoptosis and wound healing), chronic inflammation represents a loss of this balance and the resulting confluence of factors has deleterious implications for the immune system [30].
For example, chronic inflammation is directly associated with immunosuppression mediated primarily by immature myeloid-derived suppressor cells (MDSCs) [31]. Several factors induce MDSC differentiation arrest thus suppressing the host's innate and adaptive immune systems, which are essential for effective antitumor responses [31]. For example, chronically activated leukocytes supply mitogenic growth factors that stimulate proliferation of cancer and stromal cells [29, 32]. Similarly, cluster of differentiation (CD)4+ T helper cells (e.g., subsets TH1, 2, 9, 10, 17, and 22) are key regulators of inflammation in cancer, and these cells secrete cytokines which are needed in immune responses [33] and contribute to tumorigenesis in a variety of ways, depending on context [29]. Indeed, the many effects that these chronically activated immune system constituents have on neoplastic progression have been the subject of intense interest by cancer researchers [3, 34, 35]
Our intent here is not to elaborate on these details, but rather to discuss the relationship between tumor-promoting inflammation and cancer as part of a larger effort to develop a broad-spectrum therapeutic approach aimed at a wide range of therapeutic targets relevant for cancer biology. A nonprofit organization, entitled Getting to Know Cancer launched an initiative called “The Halifax Project” in 2011 with the aim of producing a series of overarching reviews in each of the areas that are widely considered to be cancer hallmarks [36]. The basis of this novel approach is premised on many of the insights of genomic sequencing in cancers. Cancers harbor significant genetic heterogeneity [37], and patterns of relapse following many therapies are due to evolved resistance to treatment. While efforts have been made to combine targeted therapies, a lack of success, rising drug costs and significant levels of toxicity have stymied efforts to effectively treat cancer with multi-drug combinations using currently approved therapeutics [38]. Consequently, this approach aims to target many disease-specific pathways simultaneously - using low-cost chemistry with little to no toxicity - to address this heterogeneity (in contrast to the limited number of actionable targets that have become the norm in combination chemotherapy).
To accomplish this task, the concept of the hallmarks of cancer [36] was used as a broad organizing framework and tumor–promoting inflammation was one of the areas of focus. We were specifically tasked to assess the many target choices that exist for inflammation related to cancer, and identify up to ten important targets as well as prospective non-toxic approaches that could potentially be combined to produce a low-toxicity approach to the suppression of tumor-promoting inflammation. In theory, inclusive investigation towards inflammatory associated carcinogenic pathways and associated therapeutics would also be combined with similar approaches being recommended for the other hallmark areas under review in this special issue. To that end, a list of seven important therapeutic targets was identified by this team along with seven corresponding approaches (i.e., approaches that have been shown to have potential to reach those targets) to support this objective. In addition to looking at the traditional pathways associated with the chosen approaches, we also review the known impact of these approaches on microRNA, a relatively new area of intense interest in cancer research. The following is a description of those targets and approaches.
Therapeutic Targets
The following therapeutic targets are reviewed in relation to inflammation: macrophage migration inhibitory factor (MIF), COX-2, NF-κB, tumor necrosis factor alpha (TNF-α), iNOS, AKT and CXC chemokines.
Macrophage migration inhibitory factor (MIF)
The hypothalamic–pituitary–adrenal (HPA) axis (also known as the stress-axis) sits at the apex of the human inflammatory response. Daily fluctuations of bodily inflammation are managed and regulated in a diurnal pattern [39] by the release of cortisol from the adrenal gland. The hypothalamus is comprised of a diverse group of nuclei at the base of the brain which integrates information from a range of stimuli (e.g., circulating hormone levels in the blood) and generates appropriate responses based on ambient conditions. In the HPA-axis, the secretory neurons within the hypothalamus secrete corticotrophin-releasing hormone (CRH), which in turn stimulates the secretion of adrenocorticotropic hormone (ACTH) from the pituitary gland, which subsequently acts on the adrenal cortex to promote cortisol release [40]. A negative feedback loop completes the HPA circuit resulting in cortisol suppressing the production of CRH and ACTH through feedback to both the hypothalamus and pituitary [40]. The stress-axis is therefore widely recognized for its role in the stress response, but adrenal cortisol is also a vitally important steroid hormone that plays a critical role in the ongoing modulation of the inflammatory and immune responses. Specifically, cortisol achieves this mediation of the inflammatory cascade, in part, by acting on the master immune/inflammatory cytokine MIF.
MIF is released from macrophages and T lymphocytes that have been stimulated by glucocorticoids, and is a potent proinflammatory cytokine that binds to the CD74 molecule on immune cells in an acute immune response, which provides the coupling between the HPA-axis and inflammation [41, 42]. In general, the HPA-axis is able to regulate inflammation with low concentrations of cortisol which induce MIF [41], and higher levels of cortisol which result in decreases in MIF secretions [42]. As proinflammatory cytokine, MIF overcomes the inhibitory effects of glucocorticoids on TNF-α, IL-1β, IL-6, and IL-8 production [43].
In cancer, MIF is frequently elevated [44] and it has been widely implicated in tumor growth and progression. Specifically, the effects of MIF extends to multiple processes fundamental to tumorigenesis such as proliferation, tumor suppressor downregulation, evasion of apoptosis, angiogenesis, and tissue invasion [45, 46]. MIF signaling is involved in COX-2 and PGE2 upregulation, the activation of the extracellular-signal-regulated kinases (ERK)-1/2 and AKT pathways, and the regulation of c-Jun activation domain-binding protein-1 (JAB1), p53, Skp1–Cul1–F-box-protein (SCF) ubiquitin ligases and HIF-1, which are central to growth regulation, apoptosis and cell cycle control [45, 47, 48]. MIF also upregulates TNF-α [49] which is believed to occur via an amplifying proinflammatory loop [50]. In chronic lymphocytic leukemia (CLL) cells, the binding of MIF to CD74 induces NF-κB activation [51]. MIF contributes to the immune escape of malignant gliomas by counteracting NK and cytotoxic T-cell-mediated tumor immune surveillance [52].
Anti-MIF therapeutics are therefore believed to have considerable promise for many types of cancer [53–57], Indeed several MIF-inactivating strategies have proven successful in delaying cancer growth, including ISO-66, a synthetic MIF inhibitor which caused a significant decrease in tumor burden when administered to mice with established syngeneic melanoma or colon cancer [58]. Recently human anti-MIF antibodies have been tested for their ability to influence growth rate and invasion of the human PC3 prostate cancer cell line in vitro, and in a PC3-xenograft mouse model in vivo. Treatment with human anti-MIF antibodies suppressed xenograft tumor growth in a dose-dependent manner [53].
However, it should be noted that MIF may also be crucial for controlling infection. In a Ugandan cohort, genetic low expressers of MIF were 2.4-times more frequently identified among patients with Mycobacterium tuberculosis (TB) bacteremia compared to those without. While MIF-deficient mice have been shown to succumb to infection more quickly (with higher organism burden and decreased innate cytokine production) and MIF-deficient macrophages show a decrease in cytokine production and impaired mycobacterial killing. So MIF is a crucial upstream mediator in the innate immune response to mycobacteria [59], and an increased risk of infection could be a concern in any therapeutic approach aimed a suppressing MIF.
COX-2
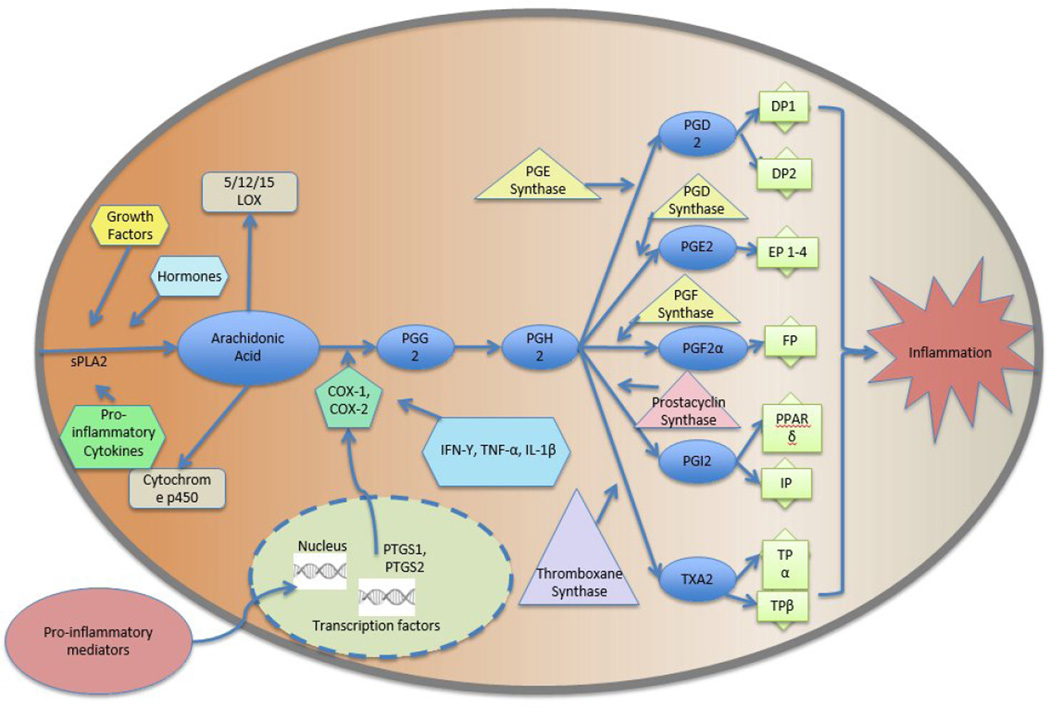
The arachidonic acid (AA) cascade (see Figure 1) plays a vital role in mediating either the suppression or induction of the inflammatory response [60]. COX-1 and COX-2 are the primary regulatory enzymes responsible for the translation of AA into the several prostanoids, lipid mediators involved in many biological functions [61]. While COX-1 is a constitutive enzyme responsible for several house-keeping functions, the inducible form, COX-2, is responsible for various inflammatory events. COX-2 is readily available to perform both oxygenation and reduction of AA [62]. COX-1/COX-2, also known as prostaglandin (PG) H synthase, transforms AA into PGG2, which is then reduced further by PGH synthase to form PGH2 [61]. PGH2 then further metabolizes via PG synthases into PGE2, PGD2, PGI2, PGF2α, and TXA2, which are then paired with distinctive G protein-coupled receptors [61, 63]. The proinflammatory messenger prostaglandin E2 (PGE2) has further been linked to carcinogenesis [64]. PGE2 is an agonist towards prostaglandin E receptors, which are divided into four subtypes, EP1–4 [63, 64]. The binding of PGE2 to four PGE receptors along with heterotrimeric GTP-binding proteins, results in the activation of adenylyl cyclase, stimulated via EP2 and EP4 binding, or phospholipase C, stimulated via EP1 and EP3 binding [65]. This stimulation of the PGE receptors thus results in the formation of cyclic AMP (cAMP) or the mobilization of intracellular calcium [65]. PGE2 has noted tumorigenic properties and contributes to carcinogenesis by promoting insensitivity to antigrowth signals, evasion of apoptosis, sustained angiogenesis, and tissue invasion/metastasis [61].
Figure I.
Arachidonic Acid Cascade
Elevated levels of COX-2 have been associated with both carcinogenesis and cancer progression [66]. Overexpression of COX-2 has been associated with carcinogenesis in animal models, and in several human cancers [67–71]. In human UV-induced skin carcinogenesis, elevation of COX-2 activity is associated with the activation of proinflammatory transcription factors (NF-κB, AP-1, STAT3 and others) [72]. COX-2 is transcriptionally regulated and its promoter is activated by multiple transcription factors, either alone or in combination [73–75]. This leads to breast, gastrointestinal, hematological prostate and oral cancers [68–78]. COX-2 induces carcinogenesis through the aromatase pathway, particularly in estrogen positive breast cancers, and through the COX/lipoxygenase (LOX) pathway in estrogen-independent breast tumors [78]. Recently, elevated activity of COX-2 has been found to be correlated with chemoresistance through altered redox induced EGFR-mediated activation of the cell survival cascade (AKT/c-FLIP/COX-2), which results in diminished drug-induced apoptosis [79].
The indirect role of the COX-2/PGE2 pathway in regulating the tumor immune microenvironment has also been suggested through IL-17 promoting M2 macrophage differentiation [80]. The interplay between cancer and stroma via COX-2 and indoleamine 2,3-dioxygenase (IDO) promotes tumor progression and predicts poor patient survival [81]. COX-2 is also known to promote the development of MDSCs which directly suppress T cell immune responses. Indeed MDSCs accumulate in the blood, lymphoid organs, spleens and tumor tissues of cancer patients [82] and serve as critical mediators of tumor-associated immune suppression [83], but recently it was shown that a COX-2 blockade inhibited accumulation and function of MDSCs and restored T-cell response after traumatic stress [84]. So COX-2 inhibition may also prove to be an attractive target for counteracting MDSC-mediated immune suppression in cancer [83]. However, it should be noted that chronic inhibition of Cox-2 activity or expression, is noted to blunt the ability of B cells to produce antiviral antibodies, thereby possibly increasing susceptibility to viral infection [85], which has relevance for numerous cancers that are virus-related.
COX-2 expression and its activity are inhibited by small molecular inhibitors both synthetic and natural such as NSAIDS, capsaicin and curcumin [86, 87]. Recently, melatonin has also been found to enhance the antitumor effect of fisetin by inhibiting COX-2/iNOS and NF-κB/p300 signaling pathways [88]. However, clinically, the most effective way to inhibit COX-2 is with selective pharmacological inhibitors, notably rofecoxib, valdecoxib and celecoxib. Several clinical trials of COX-2 inhibitors, including rofecoxib and celecoxib were performed and their clinical usage was recommended for prevention of colorectal cancers. These studies showed unequivocally that up to 50% reduction in colonic polyps was achieved by daily use of 800 mg COX-2 inhibitors in patients with familial adenomatous polyposis [89]. However, this is not currently practiced due to the subsequent findings of severe cardiovascular risk associated with COX-2 inhibitors in a small patient subpopulation (resulting in the withdrawal of rofecoxib and valdecoxib in 2004 and 2005, respectively).
The search for more specific inhibitors of COX-2 for long-term preventative use has not been very successful, other than the classic NSAID, aspirin in lower dose. Long-term use of natural COX inhibitors, such as curcumin and capsaicin has significant potential, at least for the prevention of gastrointestinal tumors [90–93]. The low bioavailability of these natural compounds by oral administration is a challenge that has limited their use in other solid tumors.
NF-κB
NF-κB transcription factors are evolutionarily conserved, coordinating regulators of immune and inflammatory responses that play a pivotal role in oncogenesis [94]. NF-κB belongs to a class of transcription factor family designated as p65 (RelA), RelB, c-Rel, NF- κB1 and NF- κB2. NF- κB1 and NF-κB2 are synthesized as pro-forms, p105 and p100, which are proteolytically processed to active p50 and p52 respectively [95, 96].
All NF-κB family members form mono- or heterodimers and share common structural features including a Rel homology domain, which is essential for dimerization and binding to cognate DNA elements [97]. These dimers bind to inhibitory protein IκB family of proteins (inhibitors of NF-κB) preventing their binding to DNA domains and localizing them to the cytoplasm in most quiescent cells [98]. Furthermore, the complexity of this transcriptional regulation system is also amplified by the fact that different NF-κB dimers have differential preferences for variations of the DNA-binding sequence [99]. Therefore distinct NF-κB dimers induce different target genes. Low frequency shuttling between nucleus and cytoplasm is observed which might be the basis for low basal transcriptional activity of NF-κB and indicative of rapid NF-κB /IκB association and re-association events.
NF-κB proteins are activated by phosphorylation and polyubiquitination of IκB and subsequent proteasomal degradation. IκB phosphorylation is catalyzed by an enzyme complex containing IκB kinases (IKK1/IKKα and IKK2/IKKβ)) and at least one non-catalytic accessory protein (NF-κB essential modulator, NEMO, also called IKKγ) [100, 101]. Furthermore, p105 and p100 are cleaved to active p50 and p52 forms respectively by targeted polyubiquitination and proteasomal degradation [102]. IκB and IKK complex bind to other components and interact with other upstream kinases [103]. NF-κB inducing kinase (NIK) phosphorylates and activates IKK1, mitogen-activated protein kinase kinase kinase 1 (MEKK1), MEKK2, MEKK3 and transforming growth factor beta (TGF-β) activating kinase 1 (TAK1) [104–106].
NF-κB is activated by canonical and non-canonical activation pathways. In the canonical activation pathway, ligands interact and activate toll-like receptors (TLRs), the IL-1 receptor (IL-1R), tumor necrosis factor receptor (TNFR) and antigen receptors. TNF-α, lipopolysaccharide (LPS) and IL-1-β are typical stimulating molecules [107, 108]. Alternatively, the non-canonical pathway originates from different classes of receptors including B-cell activation factor, lymphotoxin β-receptor (LTβR), CD40, receptor activator for NF-κB (RANK), TNFR2 and fn14 [109]. These receptors stimulate NF-κB by activation of the kinase NIK and phosphorylation of IKK1. IKK1 subsequently results in phosphorylation, ubiquitination and partial degradation of p100 to p50 [110]. Therefore, the non-canonical activation of NF-κB is independent of the activity of IKK2 and NEMO [111].
Upon activation, NF-κB dimers move to the nucleus and their Rel homology domains are free to bind cognate DNA-sequences in the enhancer elements of target gene promoters. Thousands of different target genes can be transcriptionally activated. Recent reports point to the role of NF-κB in inflammation and induction of cancer. Physical, physiological and/or oxidative stress results in activation of innate immunological processes leading to inflammation which is associated with canonical activation of the NF-κB signaling pathway [112]. NF-κB has a dual effect on inflammation. On one hand, the activation of NF-κB, as part of the acute immune response, activates cytotoxic immune cells against cancer cells [113]. However, the activation of NF-κB also results in up-regulation of antiapoptotic genes and the induced expression of other proinflammatory cytokines (e.g., TNF-α, IL-1, IL-6, and IL-8) and adhesion molecules which leads to the recruitment of leukocytes to the site of inflammation [114]. Both, STAT3 and HIF1 pathways are interconnected with NF-κB signaling and interact with NF-κB. For example, the proinflammatory cytokine IL-6, encoded by NF-κB target genes, is important for STAT3 activation. STAT3 and NF-κB also co-regulate numerous oncogenic and inflammatory genes [115]. These observations suggest that NF-κB and STAT3 alone or in combination induce inflammation and an inflammatory microenvironment.
NF-κB activation is also involved in growth regulation [116], and contributes to tumor progression by controlling vascularization of tumors via upregulation of VEGF and its receptors [117, 118]. The activation of NF-κB also causes an increase in the expression of the transcription factor Snail, which is essential in the TNF-α-induction of the epithelial-mesenchymal transition (EMT) [119], which enables cancer progression and metastasis.
NF-κB-induced transcriptional regulation of HIF-1α is mediated by the recruitment of the NF-κB complex to the HIF-1α promoter [120]. Chronic expression of the proinflammatory protein tissue transglutaminase (TG2) reprograms the transcription regulatory network in epithelial cells via constitutive activation of NF-κB. TG2-induced NF-κB binds the functional NF-κB binding site in HIF-1α promoter and results in its increased expression at transcription and protein levels even under normoxic conditions. Like NF-κB, HIF-1α is also considered a negative prognostic factor because of its ability to promote chemoresistance, angiogenesis, invasiveness, metastasis, resistance to cell death, altered metabolism, and genomic instability [121]. So aberrant activation of NF-κB and its downstream events (HIF-1α, Snail, Twist, and Zeb expression) can induce EMT, stem cell-ness, and endow cancer cells with the ability to disseminate, survive in stressful environments, and regrow at metastatic sites, making NF-κB a very important target.
However, under normal conditions, NF-κB plays an important role in the maintenance of host defense responses so it may not be practical to inhibit NF-κB on a sustained basis. For example, in studies on mice, a prolonged inhibition of NF-κB activity resulted in animals that were more susceptible to bacterial infection [122]. So short-term treatment with specific bioactive inhibitors of IKK activity might be a preferred means to reduce systemic toxicity and avoid broad suppression of innate immunity. Ideally, an IKK/ NF-κB molecular-targeted inhibitor would prevent NF-κB activation without any effects on other signaling pathways, and be differentially active in tumor cells versus in normal cells. But one major shortcoming that will need to be addressed before targeted anti-IKK or NF-κB therapies become successful is the surprising but pronounced ability of NF-κB activation inhibitors to enhance the production of IL-1β and related cytokines (due to excessive inflammasome activation) during bacterial infections [123]. So any strategy that inhibits NF-κB will need to be carefully monitored for immune-related side-effects.
TNF-α
TNF-α is a key proinflammatory cytokine, secreted by inflammatory cells, which is involved in inflammation-associated carcinogenesis. It was named TNF-α because it can induce tumor regression through the induction of cell death [124]. TNF-α is involved in inflammation and immunity, but also in a multitude of biological processes including apoptosis, cell survival, angiogenesis and tumor cell migration and invasion [125].
TNF-α acts primarily via two receptors TNFR1 and TNFR2 [126]. TNF-α is a 17 kDa protein consisting of 157 amino acids that is a homotrimer in solution, and it is primarily produced in macrophages, T lymphocytes, and NK cells. However lower expression levels have been reported in other cells including fibroblasts, smooth muscle cells, and tumor cells. Although TNF-α binds TNFR2 five times higher than TNFR1, TNFR1 initiates the majority of the biological activities resulting from TNF-α [127]. TNFR1 (p60) is expressed in all cell types whereas TNFR2 (p80) is expressed mainly in immune cells [128]. Only TNFR1 contains the death domain (DD) (i.e., TNFR2 does not contain the DD) making it an important member of the death receptor family that is capable of inducing apoptotic cell death [129].
Aside from death inducing activity, TNFR1 also has the ability to transduce cell survival signals. Binding to the homotrimer TNF-α, TNFR1 trimerizes the silencer of death domain (SODD) protein that is released [130]. The TNFR-associated domain (TRADD) binds to the DD of TNFR1 and recruits other adaptor proteins including the receptor interacting protein (RIP), TNFR-associated factor 2 (TRAF-2), and Fas-associated death domain (FADD)[131]. These adaptor proteins, in turn, are responsible for downstream cellular signaling. Apoptotic signaling mediated by TNFR1 results in FADD binding to caspase 8 and its activation. The chain of events leads to proteolytic activation of caspase enzymes and involves the mitochondrial cytochrome c release [132], which leads to the activation of endonucleases and DNA fragmentation.
Alternatively, TNFR1 may signal survival processes by recruiting TRAF-2 to the complex. TRAF-2 inhibits apoptosis by association with the cytoplasmic inhibitor of the apoptosis protein (cIAP). Once TRAF-2 associates with TNFR1, cell survival pathways are initiated through a series of phosphorylation steps resulting to the activation of cFOS/cJun transcription factors by MAPK and cJun N-terminal kinase (JNK) [133, 134]. Activation of TRAF-2 and RIP is associated with activation of the NF-κB transcription factor via a complex of NF-κB-inducing kinase (NIK) and an inhibitor, κB kinase (IKK) [135]. The activation of cFos/cJun and NF-κB transcription factors mediates the transcription of anti-apoptotic, proliferative immunoregulatory, and inflammatory genes. NF-κB is the main survival transcription factor that prevents TNF-α-induced apoptosis, so NF-κB inhibition may be an efficient strategy for apoptosis-inducing cancer therapy [135–137].
Inhibition of NF-κB is known to sensitize cancer cells to TNF-α treatment [138, 139]. Furthermore, it has been shown that NF-κB-induced expression of iNOS increases cancer cell survival [140, 141]. Inhibition of NOS can potentially sensitize cancer cells to TNF-α treatment. ROS are generated by TNF-α-mediated apoptotic events, while NF-κB induces expression of ROS-neutralizing enzymes like superoxide dismutase [142]. Recent data also show that the mRNA-decay protein tristetraprolin (TTP) interacts with TNFR1 in a TRAF2-mediated fashion initiating cJun-kinase activation. Inhibition of TTP ubiquitination results in enhanced TNF-induced apoptosis in cervical cancer cells [143].
The role of TNF-α in carcinogenesis is controversial. While high concentrations of this cytokine display antitumoral response in murine model of sarcoma [144], low sustained TNF-α levels can induce a tumor phenotype [145]. The TNF-α tumor promoting mechanism is based on ROS and RNS which can induce DNA damage and facilitate tumorigenesis [146–148]. TNF-α-mediated inflammation has been linked to cancer; for instance, a recent report shows that H. pylori strains produce TNF-α-inducing protein (Tip-α), a carcinogenic factor in gastric epithelium. H. pylori isolated from gastric cancer patients secreted large amount of Tip-α, which is incorporated into gastric cancer cells by cell surface nucleolin, a Tip-α receptor. The nucleolin-Tip-α binding induces TNF-α and other cytokine genes expression and results in NF-κB activation. Similarly, TNF-α through TNFR1, Noxo1, and Gna14 signaling leads to H. pylori-mediated gastric tumorigenesis [149]. These events are also associated with epithelial to mesenchymal transition (EMT) in human gastric carcinogenesis [150].
Direct evidence also points to the role of TNF-α in the metastatic cascade. Administration of TNF-α leads to significant increase of the number of lung metastases [151]. Conversely, tumor cells activate myeloid cells to generate a microenvironment favorable for metastasis. In Lewis lung carcinoma (LLC) cells-conditioned-medium, high levels of IL-6 and TNF-α were induced in bone marrow-derived macrophages [152], and TNF-α−/− but not IL-6−/− mice injected with LLC cells showed improved survival and reduced lung tumor multiplicity, suggesting a critical role of TNF-α in LLC metastasis [152]. Others report that TNF-α-deficient mice are resistant to tetradecanoyl-phorbol-13-acetate-(TPA) induced skin carcinogenesis [153]. The role of TNF-α in angiogenesis was also studied recently, and Fajardo et al [154] showed that high TNF-α doses inhibited angiogenesis in mice subcutaneously implanted with angiogenesis disc-system, an experimental strategy used to induce new blood vessels, while low loses promoted vascularization of the area. The antiangiogenic action of TNF-α is related to downregulation of αvβ3 and the angiotensin signaling pathway [155], while proangiogenic responses have been associated with increased VEGF, VEFGR, IL-8, and FGF expression [156]. Furthermore, low TNF-α increases tumor growth and induces angiogenesis of diverse tumors in mice [157, 158].
The effect of TNF-α in induction of carcinogenesis, angiogenesis and metastasis and invasion has therefore been supported by several studies, so targeting TNF-α and TNFR may be a viable option for treatment of cancer.
Recently several TNF-α targeting drugs have also been used mostly to treat inflammatory diseases. Examples include infliximab, a recombinant IgG1 monoclonal antibody specific for TNF-α [159], Etanercept, a genetically engineered protein comprising two molecules of the extracellular domain of TNFR2 (p75) and the Fc portion of IgG1 [160], adalimumb, a monoclonal antibody of recombinant IgG1 [161], golimumab, a human anti-TNF-α monoclonal antibody [162], and certolizumab, a humanized anti-TNF-α antibody with high affinity to TNF-α [163]. However, major side effects of these anti-TNF-α agents are infection (tuberculosis, varicella, and other opportunistic infections) and malignancies especially when TNF-α antagonists are used concurrently with other therapies [164, 165]. For example, a subset of patients with inflammatory diseases may also have an increased risk of non-Hodgkin’s lymphoma (NHL) [166], therefore treating these patients with anti-TNF-α may increase the rate of lymphoma [167–169]. Skin cancer has also been reported as a side effect in some studies involving TNF-α blocking [170, 171].
So, although TNF-α is a cytokine with well-known anticancer properties that has been utilized as an anticancer agent for the treatment of some patients with locally advanced solid tumors [172], its promise as a constituent within a multipronged approach aimed at a broad-spectrum of targets will need to be carefully assessed in light of these divergent outcomes.
iNOS
iNOS has been of interest in cancer since the discovery of its metabolite, nitric oxide (NO) in the 1990´s. Over the years, experimental data highlighted iNOS overexpression as a pivotal event ensuring tumor growth [173]. Indeed, more than 2,000 peer-reviewed publications support the iNOS-NO axis as a potential target in cancer. Under normal physiological conditions, NO is produced by the constitutive forms of NOS (cNOS and eNOS) and modulates pivotal cellular processes, such as vasodilatation, cell survival and growth. However, in chronic inflammatory conditions, the iNOS-NO axis is upregulated, and quickly yields NO-derived species with strong nitrosative properties, especially when other reactive species are also produced (such as the superoxide anion). Once formed, NO-derived species can quickly react with all cellular components, including proteins, lipids and DNA. Therefore, the main carcinogenic effect of NO-derived metabolites is related to their capability to potentiate genomic instability, as induced by the RNS peroxynitrite [174].
Experimental data and in vitro studies have supported iNOS as a viable target by demonstrating its overexpression in virtually all types of cancer cells, including glioma [175], hepatoma [176], mastocytoma [177], melanoma [178], B-cell lymphoma [179], neuroblastoma [180], mammary adenocarcinoma [181], and ovarian carcinoma [182], among others. In the same way, iNOS up-regulation has been documented in human cancerous tissues such as glioblastomas [183], brain tumors [184], prostate carcinoma [185], esophageal adenocarcinomas [186], B-cell CLL [187], primary lung cancer [188], transitional cell carcinoma of the bladder [189], pancreatic cancer [190], thyroid papillary carcinomas [191], buccal squamous-cell carcinomas [192], melanoma [193], colon carcinoma [194], gastric cancer [195], breast cancer [196], stomach cancer [197], malignant mesotheliomas and metastatic pleural adenocarcinomas [198], hepatocellular carcinoma [199] and ovarian carcinoma [200]. The enhanced activity and expression of iNOS in cancer cells seems to be a necessary mechanism for generating high levels of NO and its derived species, which promote genomic instability [201], cancer growth [202], and spreading [203]. Therefore interfering with this enhanced NO-iNOS machinery may represent a putative target for pharmacological intervention in cancer.
Interfering with the NO dynamic is not a simple task. In cancer, NO can be derived from both host and tumor cells [204]; therefore, blocking tumor-iNOS has potential implications for healthy cells. The mode of therapeutic delivery therefore needs a degree of specificity for cancerous cells (e.g. nano-carriers targeting membrane receptors unique to cancerous cells). In this context, strategies may be directed against a) iNOS activity, b) iNOS-derived NO and c) mainstream regulators of iNOS expression. Regarding the iNOS-NO axis, experimental approaches have been exploited to either block iNOS or to scavenge NO in cancer models, and interventions include treatment with aminoguanidine [197], N(G)-nitro-L-arginine methyl ester [205], carboxy-PTIO [206], tyrosine-kinase inhibitors [207], TGF-β-like molecules [208], S-methylisothiourea sulfate [173] and some natural compounds [209].
Interventions of the mainstream regulators of iNOS expression may be quite difficult because there are so many molecules involved in inflammation. It has been demonstrated that cancer-relevant mediators could include IL-1β [210], TNF-α [211], NF-κB [209] and STAT-1 [212], among others. In fact, NO blockage has reached promising results in experimental models, inhibiting tumor growth [213], prolonging survival [214], and reducing metastasis [215]. These data indicate that the pharmacological impairment of iNOS functioning may be useful in patients diagnosed with metastatic disease, since sustained high levels of systemic NO are reported in such patients [216–219].
Clinical trials have tested the efficacy and safety of iNOS inhibitors in humans, and have provided support to encourage the use of such drugs in cancer, with no important adverse effects [220–222]. Vital functions such as blood pressure, pulse rate, or respiratory function – all pivotal functions physiologically controlled by NO - did not change after the systemic administration of the iNOS inhibitor L-N6-(1-iminoethyl)lysine 5-tetrazole amide (SC-51) on healthy volunteers [220]. In the same way, the use of nebulized aminoguanidine was tested in healthy individuals and patients with pulmonary diseases, and no adverse effects were reported regarding cardiovascular functioning after NO blocking [221, 222]. Although the evidence is promising, in-depth studies still need to be conducted to confirm that iNOS blockage will stop tumor growth without compromising normal functions that are dependent on NO.
In theory, interfering with the NO-axis could also affect immune function. For example, experimental knockout of iNOS enhances the mortality of mice in sepsis [223]. However, there is no evidence of immunosuppression after iNOS blockage in cancer models and none of the clinical trials using NOblockers have reported on immunosuppressive effects [220–222]
AKT
Protein kinases are an important family of regulatory enzymes required for the growth, division, and differentiation of cells, and they have been closely examined as possible mediators of oncogenesis. In particular, the kinase signaling pathway known as the phosphatidylinositol 3-kinase/protein kinase-B/mammalian target of rapamycin (PI3K/AKT/mTOR) represents one of the intracellular cascades of utmost importance when examining cellular proliferation, differentiation, as well as cytoskeletal reorganization. The dysregulation of this pathway can direct the cell towards a carcinogenesis [224].
AKT was initially defined by three groups in 1991, Bellacosa et al. [225], Coffer et al. [226], and Jones et al. [227]. It possesses tumorigenic potential, which normally remains downregulated via the phosphatase and tensin homologue (PTEN) gene [224, 228, 229]. However, mutations in the PTEN gene, which are found in several human malignancies, lead towards the inhibition of AKT downregulation, which would would normally occur through the dephosphorylation of PIP3, a product of PI3K activation [229, 230]. The increased potential for cellular proliferation leading towards tumorigenesis initiated through PKB activation may also result from a response towards various cellular stimuli, such as heat shock, osmotic, and oxidative stress [229]. Mechanistic research has revealed a wide range of influences [231], including critical roles by AKT in proliferation [232], resistance to apoptosis [233], glucose metabolism [234], cell migration, [235] and the regulation of autophagy [236].
From an inflammation standpoint, studies of the role of AKT in phagocytosis, bacterial infections, LPS tolerance, production of proinflammatory cytokines, and migration during macrophage-mediated innate immunity strongly suggest a pivotal role in the functional activation of macrophages [237]. Evidence suggests that AKT promotes NF-κB activation [238]. In vivo tests on rodents (rat paw edema) also suggest that AKT inhibitors prevent AKT phosphorylation and downregulate the expression of inflammatory factors IL-6, MCP-1,TNFα and iNOS [239]. Similarly, in research on pancreatitis, researchers have found that AKT inhibition mediates a reduction in the activation of NF-κB and p38MAPK activity in SAP rats and a downregulation of NF-κB-dependent proinflammatory genes, including TNF-α, IL-1β and IL-6 [240].
From an immune perspective, PI3K-Akt pathway inhibitors are also attractive for their ability to selectively inhibit regulatory T cells (Tregs) with minimal effect on conventional T cells. In many cancers, an important tumor immune-evasion mechanisms involves the effects of suppressive immune cells, specifically regulatory T cells (Treg). So the depletion of Tregs has been found to be an effective strategy to enhance the immune response, but selective depletion of these suppressive cells (i.e., without affecting other immune cells) has not been very successful. Notably, however, PI3K-Akt pathway inhibitors selectively inhibit Tregs with minimal effect on conventional T cells (this has been shown in both human and murine CD4 T cells) and in vivo treatment with these inhibitors resulted in a significant and selective reduction in Tregs in both naïve and tumor-bearing mice (combined with a significant therapeutic antitumor effect). So PI3K-Akt pathway inhibitors appear to represent a promising approach to deplete Tregs in cancer [241].
Consequently, AKT inhibition is being aggressively pursued as a new therapeutic strategy for a range of cancer types, including ovarian [242], breast [243], lung [244], and bladder [245]. PI3K and AKT inhibitors are still in the early stages of development, but despite three generations of compounds targeting PI3K already having been developed, none have proved efficacious, mainly due to the emergence of therapeutic resistance [246, 247]. It is our opinion that this particular target, which appears to have strong promise, may still prove to be more effective when acted upon with a range of other therapeutic constituents that can address the alternate pathways that might otherwise serve to support this resistance.
CXC Chemokines
Chemokines were originally characterized by their ability to regulate the directional migration of leukocytes to inflammatory sites. This observation has key implications for tumorigenesis, as inflammatory cell infiltration is a common feature of many cancers and has varied functional consequences.
Chemokines or chemotactic cytokines are a group of small (8–14 kDa) heparin-binding proteins that interact with cognate cell-surface receptors and play important roles in a number of physiological processes such as development, host immunity, and cellular trafficking [248]. These functionally-related small secreted proteins constitute the largest cytokine family in humans [249]. Chemokines contain cysteine residues at their N-terminus and the position of these amino acids forms the basis for classification into four major groups: CXC, CC, CX3C or C [248]. Most chemokines harbor a four-cysteine motif internally linked by disulfide bonds at conserved sites.
The mechanism whereby chemokines exert biological effects relies on their ability to bind to the extracellular domain of G protein-coupled chemokine receptors, which leads to production of second messengers, cytoplasmic calcium mobilization, and the activation of multiple downstream signaling cascades, including the PI3K/AKT pathway, the Ras/MAPK axis, and the Janus kinase (JAK)/STAT cascade [250]. Chemokines are produced by leukocytes, endothelial cells, fibroblasts, epithelial cells, and tumor cells [251]. This section will be limited to a discussion of CXC chemokines.
Chemokines produced by neoplastic and/or stromal cells control the nature of the inflammatory infiltrate by actively recruiting cells of the innate and adaptive immune systems [249]. The ability to regulate cell trafficking in and out of the tumor milieu has diverse and complex functional consequences. Some chemokines promote conditions favorable for tumor growth and progression, while others have antitumor activity [252]. For example, IL8/CXCL8 induces leukocyte cell migration during inflammation, and this response can promote tumor growth and development by generating a favorable microenvironment [252, 253].
In contrast, chemokines such as CXCL10 can have angiostatic properties owing to their ability to attract antitumoral lymphocytes via the receptor CXCR3. The extents to which chemokines recruit immune cells to tumor sites have dramatic, often opposite, functional effects. Indeed, chemokines recruit tumor-associated macrophages (TAM) that promote tumor progression, but when TAMs are recruited massively and appropriately activated, they can exert antitumor activity [249]. Neutrophils, lymphocytes and dendritic cells commonly are recruited to tumors such as bronchioloalveolar carcinomas, colon adenocarcinomas, myxofibrosarcomas, gastric carcinomas, and melanomas, where they can have pro- and antitumorigenic effects [254–261]. However, the presence of NK cells is relatively infrequent in tumors and their presence consistently correlates with good prognosis and increased survival [262, 263].
In addition to their role in cell migration and inflammation, the chemokine/chemokine receptor system impacts development and progression of malignant diseases by regulating tumor initiation, growth, survival, migration, adhesion, invasion, angiogenesis, and metastasis [248, 253]. In summary, chemokines and their receptors regulate tumorigenesis directly by acting on tumor cells, and indirectly by regulating the composition of the inflammatory infiltrate. The diversity of the chemokine/chemokine receptor system is such that it can both contribute to, and inhibit, key events relevant to the tumorigenic process.
CXC chemokines and their receptors are often over expressed in a variety of tumors, affecting proliferation, motility, cell survival and resistance to chemotherapeutic drugs [264–266] Chemokine receptors, unlike other cell surface receptors, are also promiscuous as they bind multiple ligands (chemokines), they can function in ligand-independent manners, and they can elicit multiple effects following binding to a single CXC chemokine [264, 267]. For example, each of the two cell surface receptors of IL-8, CXCR1 and CXCR2 has diverse functions. IL-8 binding to CXCR1 results in activation of mitogenic signaling and increased ERK1/2 MAP kinase activity. CXCR2 mediates angiogenesis, motility, invasion and activation of NF-κB mediated cell survival pathways [267, 268]. Some receptors, e.g., the CXCL12 co-receptor CXCR7, also binds CXCL11 and MIF, and activates EGFRs independently of their ligands [269–272]. These complex and diverse functions of CXC chemokines and their receptors present significant challenges for cancer therapy, but also opportunities for investigating novel targeted approaches.
Chemokines and their receptors are regarded as promising molecular targets for therapeutic intervention. Several antagonists of CXCL8-CXCR1/CXCR2-mediated signaling are in development, including neutralizing antibodies, orally active small-molecule antagonists (e.g., SCH-527123, SCH-479833 [273]), and adenoviral-mediated anti-sense gene transfer approaches [274, 275]. Studies have shown that chemokines and their receptors are closely linked to emergence of drug-resistant cancer stem cells following regular chemotherapy exposure [276]. Use of small molecule inhibitors of IL-8 binding to CXCR1, such as repertaxin, has been shown to enhance responses to chemotherapy in breast cancer [277]. Identification of the CXCL12-CXCR4/CXCR7 axis as a novel therapeutic target led to development of several therapeutic approaches [248, 278]. Examples of these are the anti- CXCR4 drug AMD3100 [279], the CXCL12 analog CTCE-9908 [280, 281, 282], the anti-CXCL12 aptamer NOX-A12 [283], the inhibitor of CXCR4 expression chalcone 4 [284], and the CXCR7-specific inhibitors CCX2066 [278, 283], CCX733 [285] and CCX754 [286, 287]. CXCR4 also has been targeted using monoclonal antibodies and small molecule antagonists [288–291]. In addition, administration of recombinant forms of chemokines with angiostatic and/or antitumorigenic effects such as CXCL4, CXCL9, and CXCL10 has been proposed as a potential strategy to inhibit tumor growth and limit spreading [252, 292–295]. Thus, currently there are several chemokines that are targets of therapy, such as CXCL-1, CXCL8 and CXCL12 and others in various stages of development [296, 297]
The intrinsic functional redundancy in the chemokine system suggests that blocking a single receptor upregulated in a particular tumor is unlikely to significantly affect the integrity of protective immune mechanisms. The redundancy of this system itself presents therapeutic challenges related to possible overlapping functions of multiple receptors, but this feature also offers attractive opportunities from a therapeutic standpoint. It may be possible to fine-tune experimental screening studies to identify agents that inhibit certain signaling pathways while sparing others. The ability to bias signaling responses opens the possibility of selectively targeting events that contribute to disease while preserving immunity. In addition, the receptor microenvironment can profoundly affect its function and downstream signaling, and there may be serendipitous and unique specificities built into target cancer cells that can be capitalized upon to maximize beneficial therapeutic action and minimize or block the loss of beneficial responses such as antitumor immunity [298].
Many recent studies have revealed that chemokines can regulate the movement of a wide variety of immune cells including lymphocytes, NK cells, and dendritic cells in both physiological and pathological conditions. So these features endow chemokines with crucial roles in immune responses [299]. But therapeutic approaches that focus on chemokines can produce a range of immune-related effects. For example, a recent study demonstrated in several murine models of anthracycline-based chemotherapy that the inhibition of CCL2 or CCR2 might actually impair the anticancer immune response [300]. On the other hand, there are other chemokines that appear to have the potential to enhance the recruitment of antigen presenting cells and effector cells to sites where they are needed [301]. Given the range of chemokines and the complexity of the immune system, readers who are seeking more detail on this topic are encouraged to peruse several recent reviews that cover this topic in considerable detail [299, 302, 303]. Suffice to say that although the development of therapeutics based on targeting chemokines and their receptors has been challenging, but the lessons learned are leading to advances that should allow us to develop more refined strategies with better chances of success.
Low Toxicity Approaches
Several synthetic antiinflammatory molecules have been tested in cancer research with important preclinical results; however, the translation to clinical practice has been hampered by the abrupt finding of unpredictable serious side effects or by a lack of significant anticancer activity when tested in humans. For example, the use of nonsteroidal antiinflammatory drugs (NSAIDs), in particular aspirin, have been included as a factor in several epidemiological studies, and also clinical trials have been attempted in order to demonstrate chemopreventive activity. While epidemiological data do show association between long term ‘baby aspirin’ intake and colon cancer risk [304], many of the clinical trials designed to look for prevention of the onset of cancer or of pre-cancerous lesions have not reached satisfactory results for a variety of reasons (such as problems with the target population, duration of the study, and more importantly, side effects [305–308] that range from gastrointestinal bleeding to hemorrhagic stroke). Thus, the use of NSAIDs in clinical practice for cancer chemoprevention has always been outweighed by the possibility of serious complications.
At the same time, a wide spectrum of phytochemicals, present in edible, non-edible and medicinal plants, and endowed with potent antiinflammatory properties, have been shown to prevent tumor occurrence in several organs of experimental animals and inhibit the growth of neoplastic cells [309–315]. Indeed, several epidemiological and experimental studies provide convincing evidence that there exists a strong relationship between increased consumption of various vegetables, fruits, whole grains, legumes and spices and a decrease in cancer risk [316–319]. A large number of phytochemicals present in dietary sources are capable of suppressing carcinogenesis through inhibition of inflammatory cascade [320–322] as well as modulation of various signaling pathways implicated in cancer initiation, promotion and progression. We have therefore focused on the following chemicals from plants and foods as promising approaches with therapeutic potential to reach the targets that we have identified: curcumin, resveratrol, epigallocatechin gallate (EGCG), lycopenes, anthocyanins, and genistein.
Curcumin
Curcumin, (diferuloylmethane) is a component of golden spice Curcuma longa (commonly known as turmeric) which has been used for centuries in many Asian countries as part of diet or as a coloring agent. The anticancer and antiinflammatory effects of curcumin have been demonstrated in many cell and animal studies, and recent research has shown that curcumin can also target cancer stem cells [323], which makes it a dietary substance of considerable interest.
In Nepal and India, where daily curcumin uptake in diet has been assessed as high as 50–100 mg per day, no toxicities or adverse effects have been reported at the population level [324, 325]. The National Toxicology Program of the National Institutes of Health evaluated the toxicology and carcinogenic effects of turmeric in 1993 at a dose of 0.2g/kg/day (CAS no. 8024−37−1) for 13 weeks and 2 years on rats and mice. No adverse toxicological effects and no histopathological changes in treated mice were found. Similarly, in a study undertaken by National Cancer Institute in the United States, the oral administration of 3,500mg/kg body weight curcumin for 90 days in rats, dogs, or monkeys did not cause any adverse effects and was well tolerated [326]. In 1996, the Food and Drug Administration of the United States recognized curcumin as a Generally Recognized As Safe (GRAS) compound [327]. Up to 1,000mg/kg/body weight oral administration of curcumin did not have any adverse effect on reproduction of rats, when fed for two successive generations [328]. Finally, in humans, a dose escalation study performed in 24 adults, found that single oral doses up to 12g were well tolerated and the observed adverse effects were not dose-related. Curcumin supplementation up to 8 g/day for three months was well tolerated in the patients with precancerous conditions or non-invasive cancer [329], and in another clinical trial in patients with advanced colorectal cancer, curcumin supplementation ranging from 0.45–3.6 g/day for four months was well tolerated by subjects [330].
However, curcumin may have adverse effect in the following situations: (a) curcumin increases contraction in the gallbladder and potentially could increase the risk of symptoms in people with gallstone. [331, 332]; (b) curcumin can increase the risk of bleeding in subjects taking anticoagulant medicines because it can inhibit platelet aggregation [333, 334]; and (c) curcumin also increases acid output in the stomach and can interfere with acid suppressing drugs in patients with duodenal ulcers [335].
Curcumin has garnered significant interest in cancer research because it can regulate signaling pathways that are dysregulated during tumorigenesis, including proliferation, differentiation, invasion, apoptosis, and cell cycle checkpoints [336]. In vitro studies indicate that curcumin can target numerous kinases, phosphatases, and enzymes [337]. For example, curcumin can inactivate NF-κB [338], and reduce COX-2 expression [339] and downstream targets as well [338]. It promotes apoptosis through interaction with p53 [340] and by increasing caspase expression [341], and it induces cell cycle arrest [342]. In animal models curcumin prevents cancer development through reduction of TNF-α, interferon-γ (IFN-γ), and COX-2 [343]. So the diverse biological effects of curcumin make this compound an attractive constituent therapeutic that has been widely evaluated for its anticancer activity [344].
Indeed, curcumin has been shown to inhibit the development of chemically induced tumors of the oral cavity, forestomach, duodenum, and colon of experimental animals [337]. For example, the combination of 480 mg of curcumin and 20 mg of quercetin (three times daily) for six months reduced the number of polyps in a small number of familial adenomatous polyposis (FAP) patients without major side effects [345]. Similarly, 4 grams of curcumin daily for 1 month prevented the development of aberrant crypt foci in humans [346]. A preclinical study also suggests that curcumin could work as chemotherapeutic agent, by enhancing colon cancer cells sensitivity to oxaliplatin [347]. However, not all trials have been successful [348], and the systemic bioavailability of curcumin is extremely poor [349]. Nonetheless, at the US National Institutes of Health website (https://clinicaltrials.gov), there are 47 ongoing clinical trials with curcumin registered for different types of cancers, but most of them appear to be preclinical or pilot studies. For formal validation of the efficacy of curcumin as a chemopreventive or chemotherapeutic drug, randomized, placebo-controlled, and double-blind trials are required.
Chemical and photochemical instability/degradation, absorption, metabolism, and excretion of the curcumin are considered the reason for low systemic bio-availability in human subjects [350]. When curcumin was administered orally at a dose of 1,000 mg/kg in rats, the majority of the curcumin was excreted in feces and negligible amounts were detected in the urine [351]. Curcumin is biotransformed in the intestine, and the liver converts it into glucuronides and curcumin sulfates [352, 353]. Also, reduction of the curcumin to tetrahydrocurcumin and hexahydrocurcumin has been reported after oral administration in rats, mice, and human [353–355]. Even intravenous and intraperitoneal administration of curcumin in rats resulted in reduced curcumin and subsequently reduced curcumin converted to monoglucuronide conjugates [354]. Transformation of curcumin may result in loss of the biological activity of curcumin [353]. In pharmacokinetic and dynamic studies, serum curcumin concentrations peaked in 1–2 hours [356]. The peak serum concentrations of curcumin were 0.5, 0.6, and 1.8 micromoles/liter following an oral dose of 4, 6, and 8 g of curcumin, respectively. [356]
Although systemic availability of curcumin is very low, it has been shown in some studies that orally administered curcumin accumulates in gastrointestinal tissues [357, 358]. It has been reported that when colorectal cancer patients were administered 3.6 g/d of curcumin orally for seven days, curcumin was detected in normal surgical samples of colorectal tissue [357]. Recent advances that use implantable polymeric micelles as nano-delivery systems or phospholipid-based delivery systems for curcumin increase its accumulation in organs specifically in the gastrointestinal tract, that can target COX-2 as well as prostaglandin synthesis pathway more effectively [359–362]. In vitro, curcumin shows potential as a COX-2 inhibitor, inhibiting the expression of COX-2 mRNA and enzymatic activities of COX-2 protein in colonic epithelial and in macrophages [363, 364]. Curcumin also inhibited the expression of COX-2 mRNA and enzymatic activities of COX-2 protein in colonic epithelial and in macrophages [363, 364].
Because curcumin can target prostaglandin biosynthesis, it can be used in cancers where COX-2 activation plays an important role. New advancements in in vivo delivery systems of curcumin will result in a higher levels of curcumin accumulation in organs (specifically in the gastrointestinal tract) that can target COX-2 as well as prostaglandin synthesis pathway more effectively. Curcumin inhibited bile acid and phorbol ester induced COX-2 mRNA expression in gastrointestinal epithelial cells [365]. In mouse skin cells, curcumin inhibits phorbol ester-induced expression of COX-2 [348]. In a human non-small cell lung cancer ectopic and orthotopic xenograft mouse model, curcumin reduced COX-2 expression in subcutaneous tumors in vivo and caused a 36% decrease in weight of intralung tumors accompanied by a significant survival rate increase [366]. Curcumin inhibition of COX-2 in NSCLC cells was associated with decreased survival [366].
Notably, in vitro treatment of curcumin also suppressed CXCL-8 production by human pancreatic carcinoma cell lines and downregulated the inflammatory cytokines CXCL1 and CXCL2 in breast cancer cells via NF-κB [367, 368]. In a Kras-mediated lung cancer model in mice, curcumin inhibited the expression of neutrophil chemoattractant keratinocyte-derived chemokine CXC-KC and subsequently inhibited progression of the cancer [369].
From an immune perspective, curcumin suppresses the type 1 immune response, which can increase susceptibility to infection [370]. But at the same time curcumin appears to act in a supportive manner for tumor-related immune effects. For example, in in vitro tests aimed at studying the role of curcumin in the prevention of tumor-induced dysfunction of T cell-based immune response, curcumin prevented the loss of T cells, expanded central memory T cell (T(CM))/effector memory T cell (T(EM)) populations, reversed the type 2 immune bias and attenuated the tumor-induced inhibition of T-cell proliferation in tumor-bearing hosts. Curcumin also inhibited the suppressive activity of Treg cells (by downregulating the production of TGF-β and IL-10) and enhanced the ability of effector T cells to kill cancer cells [371]. As well, curcumin significantly inhibited the induction of IDO expression (a key enzyme in T-cell suppression-mediated immune tolerance to tumors) and activity by IFN-γ in bone marrow-derived DCs, which appears to be an important immunomodulatory property of curcumin that may serve to strengthen its therapeutic potential [372].
Resveratrol
Resveratrol (3,5,4'-trihydroxystilbene), a compound found in the skins of red grapes, red wine, berries, peanuts and many other plants, has been shown to possess health-promoting properties. It is a bioactive polyphenol and has antiinflammatory, antioxidant, antimicrobial, anticancer, neuroprotective, and cardioprotective effects. Numerous preclinical animal studies provided encouraging evidence for cancer chemopreventive and chemotherapeutic potential of this phytochemical [373]. In vitro evidence of resveratrol efficacy is well described; however, many concerns regarding its effectiveness in vivo arise from its poor stability and rapid metabolism and bioavailability following oral ingestion. Peak plasma concentrations occur at around 1hr, and levels of the parent compound are very low [374, 375]. Adverse effects are mild, even at high doses (up to 5g daily) [376]. Resveratrol works in animal models [377] and humans; although the data for humans is more sparse and controversial [378, 379].
Resveratrol has been shown to have efficacy in multiple animal models of chronic inflammatory diseases. These diseases include hepatitis [380], esophagitis [381], and in particular, there are many confirmed studies that resveratrol suppresses colitis [382, 383] and pancreatitis [384–386]. Resveratrol targets many of the key players involved in inflammation, prevents DNA damage, and induces apoptosis in a p53-dependent manner [387–389]. Interestingly, resveratrol can induce the expression of the p53 target, NAG-1 [non-steroidal antiinflammatory (NSAID) drug-activated gene-1], a member of the transforming growth factor-beta superfamily, that has pro-apoptotic and antitumorigenesis activities [390]. Also, resveratrol prevents pRb hyperphosphorylation and thus the inactivation of this tumor suppressor protein. Resveratrol also inhibits MMP-2 [391] and MMP-9 [392, 393], COX-1 [394], proinflammatory cytokines [395–397], and growth factors such as hepatocyte growth factor [398].
Additionally, resveratrol has potent NF-κB-dependent antiinflammatory and chemopreventive effects both in vitro and in vivo, and impacts multiple disease phenotypes in a favorable manner. For example, through the inhibition of NF-κB, resveratrol ameliorates diabetic vascular inflammation and macrophage infiltration in diabetic mice, inhibits the epithelial-mesenchymal transition, modulates autophagy, suppresses cell transformation, regulates miRNA levels, and reverses resistance to chemotherapeutic agents [399–405]. Notably, resveratrol has also been shown to inhibit other key modulators of inflammation and cancer discussed in this review, including COX-2 [406–408], MIF [409], TNF-α [410], iNOS [411], AKT [412], and the CXC group of cytokines [413]. For example, Cichocki et al. showed resveratrol inhibited 12-O-tetradecanoylphorbol-13-acetate activated NF-κB, AP-1, COX-2, and iNOS in mouse epidermis [414]. Similarly, Kundu et al. showed that resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-κB in mouse skin by blocking I-κB kinase activity [408]. Dietary resveratrol (50–300 mg/kg) was found to inhibit chemically-induced hepatocarcinogenesis in rats with simultaneous suppression of hepatic iNOS, 3-nitrotyrosine, COX-2 and NF-κB [415–417].
Several recently published clinical trials on resveratrol in humans have shown that it exhibits antioxidant and antiinflammatory activities. It can improve glucose and lipid metabolism, and favorably modify a number of important pathways involved in carcinogenesis (e.g., the insulin-like growth factor system [418], apoptosis [419] and others [420]). However, these effects can vary and depend on the protocols [376]. The plasma pharmacokinetics of resveratrol in humans are also now reasonably well defined, and daily doses up to 1 gram appear to be safe and well tolerated, although gastrointestinal toxicity is observed at higher intakes, and there is potential for drug interactions at higher doses[420].
In some of the earliest research on resveratrol and immune function, Falchetti et al. [421] showed that in vitro exposure to resveratrol produced a biphasic effect on anti-CD3/anti-CD28-induced development of both IFN-γ - IL2- and IL4-producing CD8+ and CD4+ T cells (with stimulation at low resveratrol concentrations and suppression at high concentrations). Similarly, it was found to induce a significant enhancement at low concentrations and suppression at high concentrations of both cytotoxic T lymphocytes and NK cell cytotoxic activity [421], and this biphasic modulation of NK cells has been confirmed in more recent research as well [422]. The administration of low doses of resveratrol also inhibited Renca tumor growth with regulatory T cells being decreased, and a massive amount of activated CD8+ T cells accumulating in the tumor microenvironment. At the same time, the expression of T-helper (Th)-2 cytokines (e.g., IL-6 and IL-10) switched to Th-1 cytokines with dominance of interferon (IFN)-γ, which increases the expression of Fas in Renca cells. [423]. And resveratrol has also been shown to suppress tumor-derived CD4+CD25+ regulatory T cells (which are a negative regulator of the immune system and main obstacles to cancer immunotherapy in tumor-bearing hosts) in mice [424]. And resveratrol at low and noncytotoxic doses has been shown to inactivate Stat3, preventing the generation and function of tumor-evoked regulatory B cells (tBreg), including expression of TGF-β in mice. This frees antitumor effector immune responses by disabling tBreg-induced conversion of forkhead box protein (FOX)p3(+) Tregs (without nonspecific inactivation of effector immune cells), which efficiently inhibited lung metastasis in mice[425]. So the effects of resveratrol on the antitumor capabilities of the immune system appear equally promising, and notably, this is accomplished with no apparent increase in susceptibility to risks of infection.
Epigallocatechin gallate (EGCG)
EGCG is the most abundant catechin in tea, is a potent antioxidant and antiinflammatory agent. It is found mainly in white tea, green tea and, in smaller quantities, black tea. Despite the demonstration of cancer prevention by EGCG in many animal studies, epidemiological studies have found mixed results concerning the effectiveness of EGCG as a superior medicine for prevention and therapy of cancer in humans [426]. Its limited in vivo activities can be attributed to metabolism and bioavailability. Methylation, glucuronidation, sulfation, and ring-fission metabolism represent the major metabolic pathways for tea catechins [427]. It has also been found that efflux transporters P-glycoprotein (Pgp), MRP1 and MRP2 play roles in the absorption and excretion of green tea catechins [428]. Several processes including intestinal metabolism, microbial metabolism, hepatic metabolism and chemical degradation are also involved in the fate of EGCG, resulting in its low availability in animals, and most likely also in humans [429].
Isbrucker et al. conducted toxicity studies on EGCG. An oral dose delivering 2000 mg EGCG preparation/kg was lethal to rats, whereas a dose of 200 mg EGCG/kg induced no toxicity. The dietary administration of EGCG to rats for 13 weeks was not toxic at doses up to 500 mg/kg/day. Similarly, no adverse effects were noted when 500 mg EGCG preparation/kg/day was administered to pre-fed dogs in divided doses. This dose caused morbidity when administered to fasted dogs as a single bolus dose, although this model was considered an unrealistic comparison to the human condition. From these studies a no-observed adverse effect level of 500 mg EGCG/kg/day was established [430].
There are multiple mechanisms that can explain the chemopreventive potentials of EGCG, among which are its ability to affect cancer cell signaling pathways, suppress cellular proliferation and induce apoptosis [426]. The diversified effects of EGCG may explain its broad pharmacologic activities. With regards to chronic inflammatory diseases associated with a high cancer risk, EGCG has been shown to suppress colitis [431], hepatitis [432] (and may have antiviral properties against HBV and HCV [433, 434]), and pancreatitis [435] in animal models. Excitingly, in a pilot study involving patients with mild to moderate ulcerative colitis, EGCG (400–800 mg daily) showed a therapeutic benefit for patients who were refractory to 5-aminosalicylic and/or azathioprine [436].
There is extensive evidence that EGCG targets key players in inflammation, providing a mechanism of its efficacy in vitro and in vivo against chronic inflammatory diseases and associated cancers. Noh et al. showed that EGCG improves Dermatophagoides pteronissinus extract-induced atopic dermatitis-like skin lesions in a mouse model by suppressing MIF [437]. In addition, EGCG can inhibit TNF-α [438], iNOS [439, 440], AKT [441], the CXC group of cytokines [442], and by by reducing the transcriptional activity of NF-κB, COX-2 expression and PGE-2 synthesis [443–448]. Additionally, EGCG activates wild-type p53 [449–451], and protects from p53 mutation [452]. It promotes pRb hypophosphorylation and activation of this tumor suppressor protein [453], and inhibits MMPs such as MMP-9 [454].
In animal models EGCG prevents the development of adenomatous polyps in ApcMin/+ mice [455, 456]. Some epidemiological studies have shown that high consumption of green tea reduces the risk of several types of cancers, including the lung, colorectum, liver, esophagus and stomach [457, 458]. High urinary levels of tea polyphenol epigallocatechin (EGC) have been associated with reduction of colorectal cancer among a Chinese population [459] and a randomized clinical trial has shown a significant reduction in adenoma incidence among patients taking 1.5 g/day of green tea extract [460]. Doses of green tea polyphenols greater than 800 mg/day increase in liver enzymes, and there is possible hepatic toxicity in humans at this level [461–463]. Nonetheless, despite evidence from in vitro and non-human in vivo research on green and black tea as chemopreventive agents for colorectal cancer, data are still insufficient to conclude that either tea type is protective [464]. But EGCG does target and suppress many of the key players involved in the inflammation-to-cancer sequence, and therefore may be quite useful as a constituent within a mixture aimed at inflammation in cancer.
From an immune perspective, EGCG significantly suppressed IFN-γ production and the proliferation of peripheral blood mononuclear cells in vitro [465]. It was also shown to exert antitumor effects on colorectal cancer cells, at least in part by inhibiting the expression and function of IDO through the suppression of STAT1 activation [466]. In leukemic BALB/c mice that received 5, 20 and 40 mg/kg EGCG (orally) for two weeks, it increased the percentage of CD3, T-cell, CD19, B-cell, and Macrophage-3 antigen (Mac-3), and macrophages, but reduced the percentage of CD11b (monocyte) cell surface markers. It also promoted the phagocytosis of macrophages from 5 mg/kg treatment and promoted NK cell activity at 40 mg/kg, increased T-cell proliferation at 40 mg/kg, but also promoted B-cell proliferation at all three doses [467].
At the same time, EGCG appears to have a protective effect against bacterial infection. This was shown in EGCG treatment of nicotine-suppressed macrophages where it reconstituted the resistance to the infection and diminished a nicotine-induced inhibition of cytokine production [468]. It was also demonstrated in research against Pseudomonas aeruginosa and Escherichia coli isolated from skin wounds [469], and against burn wound infection by methicillin-resistant Staphylococcus aureus [470].
Lycopene
Lycopene is a phytochemical that belongs to a group of plant pigments known as carotenoids. Red colored lycopene is lipophilic and naturally occurs in many fruits and vegetables. The richest sources of lycopene are tomatoes and tomato products, however, apricots, guava, watermelon, papaya, and pink grapefruit are also sources of this phytochemical. Some studies suggest that cooking tomatoes in oil may increase the bioavailability of lycopene [471, 472]. Research, dating as far back as the 1920s, has shown that naturally occurring carotenoids, specifically beta-carotene, have anticancer properties. Since the late 1980’s when it was recognized that the antioxidant activity of lycopene was twice that of beta-carotene there has been a growing interest regarding lycopene as a possible anticancer agent.
Only 10–30% of the lycopene in dietary sources can be absorbed via the human digestive system [473]. Although there is conflicting data, it has been suggested that lycopene is better absorbed when taken in conjunction with fats due to its lipophilic properties [474]. Once ingested, lycopene is incorporated into lipid micelles and absorbed by the mucosa of the small intestine. The micelles are then transported to the liver as chylomicrons. Lipoproteins are the carriers of lycopene in the blood stream and the means by which bioactive lycopene gains access to the various organ systems. High concentrations of lycopene have been found in the testes, prostate, adrenal glands and liver [475].
Lycopene is a constituent of human diets that are rich in fruit and vegetables and epidemiological studies suggest that it may have a protective effect against various cancers [476]. Lycopene is a powerful antioxidant that blocks the action of free radicals which are activated oxygen molecules that can damage cells and have been shown to support the development of some cancers. For example, numerous studies suggest that lycopene and lycopene rich natural dietary products, when taken regularly, may decrease the incidence of a variety of malignancies including breast [477], ovarian [478] bladder mouth, esophagus, pancreas [479] and colorectal cancer [480]. There is also great interest regarding lycopene and prostate cancer; about 30 percent of the published human studies (16/54) that have considered lycopene concern prostate cancer. The association of a diet rich in lycopene from tomato-based foods with a lower risk of prostate cancer is supported by multiple studies [481–485].
Thus far, several researchers have investigated lycopene’s mechanism(s) of action as regards its anticancer effects. Oxidative stress is a major factor implicated in chronic diseases and carcinogenesis. Lycopene has been found to increase the effects of deoxification proteins (such as epoxide hydrolase-1) and protective enzymes (such as glutathione-S-transferase-omega-1, peroxiredoxin-1 and sulphide-quinone oxidoreductase) [486]. Other studies have shown that lycopene downregulates the genes that regulate proteins involved in the generation of ROS, including ERO1-like protein-a and CLIC-1 [487]. In addition, lycopene may prevent cancers, especially prostate cancer, via other mechanisms. In vitro studies have shown that lycopene-induced activation of the peroxisome proliferator-activated receptors (PPAR)-gamma-LXR alpha-ABCA1 pathway is associated with decreased proliferation of LNCaP prostate cancer cells [488, 489]. When LNCaP cells were exposed to lycopene, a dose-dependent decrease of the G0/G1 phase-related protein, cyclin D1, and an increase in the cyclin kinase inhibitors, p53, p21 and p27 have been noted and were associated with cell cycle arrest [490]. Other in vitro studies suggest that lycopene may induce apoptosis in human prostatic epithelial cells. A protein expression profiling study revealed that lycopene may upregulate pro-apoptotic proteins as well as downregulate antiapoptotic proteins in human primary prostatic epithelial cells in vitro [487]. Lycopene has also been shown to suppress the invasion and migration of prostate cancer cells by downregulating the expression of integrins [491].
Lycopene has also been shown to have antiinflammatory effects in both in vitro studies that assessed macrophages as well as rodent studies. In particular, lycopene has been associated with downregulation of TNF-α gene expression and/or inhibition of TNF-α secretion in LPS stimulated macrophages [492–494]. Also, in a rat model of pancreatitis, blood levels of TNF-α were notably lower in lycopene-treated versus control animals [495]. Similarly, decreased TNF expression and secretion results have been noted in a number of endothelial cell in vitro studies [496, 497]. Modulation of the following signaling pathways have been proposed as the mechanism of this antiinflammatory effects: ERK, NF-κB, JNK, and HMGB1 [492–494, 496, 497].
It is not clear whether or not lycopene predisposes patients to infections or immune system suppression. There is limited evidence that lycopene and other carotenoids have antiinflammatory effects that may impact native immune function [492] In some of the earliest animal studies, intraperitoneally or intravenously injected lycopene produced prolonged survival times in bacterially infected mice [498]. But according to Medfacts.com, a total of 143 lycopene drug adverse event reports were reported to the FDA between January 2004 and October 2012, including 21 infectious complications, but lycopene was not thought to be the cause of the infection in any of those cases (based on physician opinions - no further details provided).
From an anticancer perspective, lycopene treatment promoted promote spleen lymphocyte proliferation, and NK activity in vivo in mice [499]. But another study on mice showed that lycopene significantly attenuates the maturation of murine bone marrow-derived dendritic cells, and that it downregulated the expression of costimulatory molecules (CD80 and CD86) and major histocompatibility complex type II molecules, suggesting that it has immunosuppressive potential [500].
Studies in which lycopene was orally administered repeatedly, for a period of time, did not identify any clear organ toxicity related to the lycopene in rats or mice, however, in a dog, accumulation of lycopene and vitamin A in the liver, and excess vitamin A in the kidneys were noted. Skin pigmentation and colored fatty deposits in the liver were seen in a person who ingested high large amounts of lycopene daily over a period of years [501]. A study concerning 20 male and 20 female Wistar rats that were given lycopene in their diets (a range of levels were assessed, the highest being 1% of diet) for 90 days showed no evidence of toxicity based on: 1) clinical and neurobehavioral observations; 2) motor activity assessment; 3) body weight and food consumption measurements; 4) ophthalmoscopic examinations; 5) hematology, clinical chemistry, and urinalysis; 6) organ weights, 7) gross pathology, or 7) histopathology [502].
Dietary lycopene, from eating fruits and vegetables, has no known side effects and is thought to be safe for humans. The optimum dosage for lycopene has not been established, but the amount found helpful in studies generally falls in the range of 4 to 8 mg daily. Patients in some studies who took a lycopene-rich tomato supplement of 15 milligrams twice a day had some intestinal side effects such as nausea, vomiting, diarrhea, indigestion, gas, and bloating. Lycopene at higher doses, especially when taken for long periods of time, has been associated with diarrhea, fat buildup under the skin, chest pain, heart attack, skin discoloration, stomach pain, stomach ulcer irritation, vomiting, and worsened hot flashes [503].
Supplements containing antioxidants such as lycopene may interfere with radiation therapy and chemotherapy if taken during cancer treatment [504]. Even though studies have not been done in humans, antioxidants are known to clear free radicals, which could interfere with one of the methods by which chemotherapy and radiation destroy cancer cells. Most of the human studies, thus far, have been case control or other types of observational studies which not as useful or predictive as clinical trials. More evidence from clinical trials is needed to confirm that lycopene-rich foods can help prevent or treat cancer. Further studies are needed to better document the benefits and effects of lycopene supplements and its mechanism of action in vivo.
Anthocyanins
A diet rich in polyphenolic anthocyanins (ACs) has been reported as a chemoprotective agent in in vivo models by regulating inflammatory cytokines. It inhibited the development of N-nitrosomethylbenzylamine- induced esophageal cancer in rats. The inhibition was mediated through decreased expression of inflammatory biomarkers like COX-2, iNOS, p-NF-κB, and soluble epoxide hydrolase (sEH)) and cytokine, pentraxin-3 (PTX3) expression [505]. AC-rich black currant skin extract showed chemopreventive activity through downregulation of abnormal lipid peroxidation, protein oxidation, and expression of iNOS and 3-nitrotyrosine (3-NT) in a dose-responsive fashion (100 and 500 mg/kg) and upregulation of the gene expression of a number of hepatic antioxidant (Nrf2-regulated antioxidant pathway) and carcinogen detoxifying enzymes, such as NAD(P)H:quinone oxidoreductase, glutathione S-transferase, and uridine diphosphate-glucuronosyltransferase isoenzymes in diethylnitrosamine (DENA)-initiated hepatocarcinogenesis in rats [506]. Black currant anthocyanins also abrogated elevated inflammatory markers, such as COX-2 and NF-κB, during DENA hepatocarcinogenesis in rats [507].
ACs also exerted an antiinflammatory effect in H. pylori-infected gastric epithelial cells. The inflammatory cytokine IL-8 and ROS increase in the H. pylori-infected gastric mucosa. First, ACs inhibit the phosphorylation of MAPKs, translocation of NF-κB and IκBα degradation. Secondly, they also inhibit H. pylori-induced iNOS and COX-2 mRNA expression and IL-8 production [508]. Additionally, in vitro studies showed that the anthocyanins inhibit the mRNA and/or protein expression levels of COX-2, NF-κB and various interleukins and exhibit antiinflammatory effects in multiple cell types [509, 510].
These studies suggest that anthocyanins significantly inhibit induced proinflammatory mediators, such as nitric oxide (NO) and prostaglandin E2, as well as proinflammatory cytokines including TNF-α and IL-1β, without significant cytotoxicity. Anthocyanins also downregulated excessive expression of inducible NO synthase, COX-2, TNF-α, and IL-1β in a dose-dependent manner in different cancers. Moreover, anthocyanins inhibited nuclear translocation of NF-κB and IκBα degradation as well as phosphorylating MAPKs.
In addition to these antiinflammatory effects, anthocyanins have been shown to inhibit the growth and invasion of SKHep-1 cells through reduced expression of MMP-9 and urokinase plasminogen activator (u-PA) [511]. Similarly, a MMP-9 and u-PA mediated reduction of migration and invasion was observed in highly metastatic A549 human lung carcinoma cells through cyanidin 3-rutinoside and cyanidin 3-glucoside (anthocyanins). This inhibition was also through the downregulation of activation of c-Jun and NF-κB [512]. Treatment with anthocyanins (such as delphinidin, cyanidin, and pelargonidin) in normal human epidermal keratinocytes inhibited UV-B-mediated degradation and phosphorylation of IκBα and activation of IKKα which further inhibited nuclear translocation and phosphorylation of NF-κB/p65 at Ser (536) [513].
Some caution must be exercised, because anthocyanins are often addressed as a homogenous class of agents, but they represent a group of structurally dissimilar molecules. Some studies also look at anthocyanidins (which are similar to anthocyanins but without sugar moieties). Both anthocyanins and anthocyanidins (especially cyanidin and delphinidin) have been subjected to extensive mechanistic studies in relation to antiproliferation, induction of apoptosis and inhibition of activities of oncogenic transcription factors and protein tyrosine kinases. Water soluble anthocyanins are mostly 3-glucosides of the anthocyanidins. The most common anthocyanidins are pelargonidin, delphinidin, peonidin, petunidin, malvidin and cyanidin. Peonidin 3-glucoside and cyanidin 3-glucoside extracted from black rice (Oryza sativa ssp. indica) inhibit the growth and invasion of SKHep-1 cells through reduced expression of MMP-9 and urokinase plasminogen activator (u-PA) [511]. Similarly, MMP-9 and u-PA mediated reduction of migration and invasion was observed in highly metastatic A549 human lung carcinoma cells through cyanidin 3-rutinoside and cyanidin 3-glucoside (extracted from Morus alba). This inhibition was also through the downregulation of activation of c-Jun and NF-κB [512].
Treatment with pomegranate-derived delphinidin, cyanidin, and pelargonidin in normal human epidermal keratinocytes inhibited UV-B-mediated degradation and phosphorylation of IκBα and activation of IKKα which further inhibited nuclear translocation and phosphorylation of NF-κB/p65 at Ser [513]. Based on the accumulating evidence, pure anthocyanidins as well as berry extracts enriched with anthocyanidin showed higher chemopreventive activities than berry extracts with high anthocyanin. The major points of concern are pH, temperature and light-dependent interconversion of anthocyanins and anthocyanidins, a greater susceptibility of anthocyanidins (in comparison to the glycosides) to chemical decomposition, and shorter half-lives in the biophase.
Notably, a number of immunosuppressive effects of berry extract rich in anthocyanins have been reported by Hushmendy et al [514] who demonstrated that anthocyanidin rich fractions inhibit T-cell proliferation and IL-2 production on anti-CD3 plus anti-CD28-activated primary human T-lymphocytes in culture [514]. However, very little research on anthocyanidins and the immune system in cancer exists, suggesting that this is an area that needs further investigation.
In general, these findings suggest that anthocyanins offer substantial chemopreventative and therapeutic potential, although there is paucity of data regarding the bioavailability of anthocyanin. Only a small portion of orally ingested anthocyanins is absorbed (<1 %). Maximum plasma levels are reached within 2 hours of consumption. About 68 % of absorbed anthocyanins are metabolized, and excreted as monoglucuronides [515]. Low bioavailability of the anthocyanins is due to to their extensive metabolism in the tissues and by the colonic microflora. The gut microflora degrades anthocyanins to release simple phenolics that conjugate in intestine and later in liver and hamper the absorption process. However, some reports contradict this observation and suggest that anthocyanin glycosides remain intact during absorption [516]. Although the bioavailability of cyanidin-3-glucoside and anthocyanin as shown through the above report is low, Mayrczylo et al. demonstrated systemic levels of parent cyanidin-3-glucoside and total anthocyanins as 1.7% and 3.3% respectively in C57BL6J mice that received cyanidin-3-glucoside by oral gavage or tail vein injection [517].
Overall, in most in vitro and in vivo assays anthocyanins are not genotoxic. Some evidence of genotoxicity was provided by a single in vitro study using pure anthocyanidins. However, the genotoxicity of grape seed extract was negative in a bone marrow micronucleus test in vivo. Moreover, in guinea pigs and dogs, no short-term or subchronic toxic effects were observed at 3 g/kg anthocyanins and 15 % of grape-skin extract respectively. In addition, in rats fed with 6 g/day unspecified anthocyanins extract or grape seed extract no toxic effect was observed. But because of a lack of data, no firm conclusion can be drawn with respect to long-term toxicity or carcinogenicity of anthocyanins [515].
Genistein
Genistein (GEN) is a prominent isoflavone which inhibits cell growth and induces apoptosis in vitro and in vivo without toxicity [518, 519]. It inhibits activated AKT, the downstream target of many pathways such as Notch [520], and IGF-1 in pancreatic cancer cells [521], and in osteosarcoma [522] and breast cancer [523]. Additionally, GEN inhibits the activity of Akt-targets like FOXM1 in pancreatic cancer cells [520] and FOXO3 [524] in colon cancer cells. AKT also forms a complex with human TERT, heat shock protein 90, p70S6 kinase and mTOR and GEN restrains the formation of this complex [525]. In pancreatic cancer cells GEN inhibits growth via inactivation of Notch- 1/AKT/FOXM1 [520]. Estrogen receptor-β/AKT mediated inhibition was also observed in DLD-1 human colon adenocarcinoma cells [526]. GEN also targets AKT and p21 WAF1/CIP1 in BRCA1-mutant human breast cancer cell lines [527], GEN induced AKT-mediated enhanced apoptosis/downregulation of AKT has also been reported in combination with compounds like arsenic trioxide in human hepatocellular carcinoma [528], gefitinib in NSCLS [529], gemcitabine in human osteosarcoma [522, 530], cisplatin in cervical cancer cells [531], cetuximab in oral squamous cell carcinoma [532], photoactivated hypericin in breast cancer cells [533] and indole-3-carbinol in human colon cancer HT-29 cells [534]. GEN also inhibits the carcinogenic effect of 17 beta estradiol or bisphenol-A via ER/IGF-1/AKT pathway in BG-1 ovarian cancer cells [535] and also downregulates FOXO3 activity in colon cancer cells [524]. It also modulates MAPKs/AKT in cervical cancer cells. [536]. Repression of breast cancer stem cell-induced mammospheres by GEN was similar to the AKT inhibitor perifosine and was related to enhanced tumor suppressor PTEN expressions [537]. Increased ceramide and lipid raft cholesterol accompanied with genistein inhibited the cell viability of prostate cancer cells via the partial contribution of EGFR-AKT/p70S6k pathway and down-regulation of androgen receptor [538, 539].
Some reports also show a distinct genistein effect whereby it induces PI3K/AKT nongenomic ER signaling to the histone methyltransferase enhancer of zeste homolog 2 (EZH2). As a result, this phosphorylates and represses EZH2 and reduces levels of H3K27me3 repressive mark in chromatin during developmental reprogramming, and promotes uterine tumorigenesis [540]. In colon cancer cells, membrane androgen receptors (mAR) activation inhibits the prosurvival signals AKT/Bad in vitro and in vivo and blocks migration of colon cancer cells via regulation of vinculin (a protein controlling cell adhesion) signaling and actin reorganization, supporting the powerful tumoristatic effect of mAR receptors. GEN inhibited actin reorganization and restored the motility of these cells and reversed the tumoristatic effect of mARs [541].
A number of concerns have been raised about the estrogen-like effects of natural isoflavones (i.e., the possible promotion of estrogen-sensitive cancers) [542–544]. However, a recent nested case-control study and meta-analysis of numerous epidemiological studies show an inverse correlation between GEN intake and breast cancer risk and a number of other clinical studies support the breast and uterine safety of purified naturally derived GEN administered for up to 3 years [545].
Most phase I and phase II clinical trials of GEN have considered normal dietary dose ranges (i.e., 0.3 mg to 1 mg per kg body weight per day [546]. In one study patients were treated with 2 mg GEN per kg body weight and compared against no treatment prior to undergoing radical prostatectomy for localized prostate cancer [547]. After treatment, it was shown that GEN decreased MMP-2 gene expression to 24% of the level seen in control subjects (blood concentrations of free GEN were approximately 140 nM in the GEN-treated cohorts while control group levels were below detection) [547]. Messing et al initiated a Phase 2 randomized, placebo-controlled trial with oral GEN (300 or 600 mg/d) as the purified soy extract G-2535 and found that GEN was more effective at lower dose on bladder cancer tissue through EGFR phosphorylation but the AKT pathway was unaltered in both in vivo conditions [548]. Another phase II clinical trial with GEN administered at a dose of 531 mg twice daily P.O. starting day -7 until the end of study participation with erlotinib, and gemcitabine in advanced pancreatic cancer did not appear to increase the survival of patients with advanced pancreatic cancer [549]. In another phase II trial, subjects with progressive prostate cancer were treated with soy milk three times daily for 12 months (approx 1 mg GEN per kilogram per day) which decreased the rate of increase of serum prostate-specific antigen (PSA) when compared to that which was seen in subjects prior to entering the study [550]. Finally, a third phase II study of GEN in men with various stages of prostate cancer used soy extract (6 mg GEN per kg per day for 6 months) [551] with 17% of the participants experiencing a decrease in their PSA levels.
From an immune perspective, a range of effects have been found. For example, Yellayi et al reported that sub-cutaneous GEN injections (8 mg/kg per day) in ovariectomized adult mice lead to estrogen receptor (ER) and non-ER-mediated inhibition of thymocyte and CD4(+)CD8(−) helper T cell lineage maturation as well as systemic lymphocytopenia [514]. Additionally, GEN produced suppression of humoral immunity. The significant thymic and immune changes in mice produced by serum GEN levels at 8 mg/kg per day was also comparable to those reported in soy-fed human infants [514]. GEN also appears to compete with endogenous 17beta-estradiol for estrogen receptors to suppresses Agspecific immune responses. Specifically 20mg/kg GEN downregulated OVA-specific proliferative responses, interferon-gamma production levels and immunoglobulin (Ig)G1 without reduction in responses to anti-CD3 monoclonal (m)antibody and Ag-presenting activity of CD11c(+) dendritic cells [552]. And GEN has also been shown to potently induce the granzyme B inhibitor, proteinase inhibitor 9 (PI-9) in MCF-7 human breast cancer cells inhibiting the ability of human NK cells to lyse breast cancer cells [553].
By contrast, however, the ingestion of GEN significantly increased lymphocyte proliferation and LDH release, and caused a significant increment in IFN-γ in a mouse model of Human Papillomavirus associated-cervical cancer resulting in a significant therapeutic effect (compared to a control group) [554]. GEN also produced a significant increase in ex vivo cytotoxic T lymphocyte (CTL), a potentiating effect on NK cells (but a decrease in the percentage of CD4(+)CD25(+) T cells), an increase in the production of IFN-γ, and the activation of STAT1 and STAT4 in a 7,12-dimethylbenz[a]anthracene (DMBA)-induced tumor model in mice. This resulted in an antitumor effect and an enhancement to host resistance in this study [555]. So the immunomodulatory potential of GEN appears to be quite nuanced and it may require further investigation before we fully understand how these effects impact various cancers.
MicroRNA (MiR)
In this section we also review the known impact of these approaches on microRNA (miRs), a relatively new area of intense interest in cancer research. miRs are small non-coding RNAs that regulate gene expression (post-transcriptionally) and target about 80% of the protein-coding mRNAs [556, 557]. They are master regulators of multiple cellular pathways, and the deregulation of miRNAs plays a fundamental role in the onset and progression of many cancers [556].
The miRBase database (http://www.mirbase.org) is a searchable database of published miRNA sequences and annotation. miRBase version 16.0 has 1048 miRNA sequences annotated in the human genome, and miRs and a single miR can target approximately 200 transcripts simultaneously. Each miR can target hundreds of messenger RNAs (mRNA)s and a single mRNA is often the target of multiple miRs within a given cell type [557]. Many housekeeping genes have evolved with shorter length of 3’-UTR to avoid miR regulation [558]. About 50% of annotated human miR genes are located in cancer associated genomic regions or fragile sites that are susceptible to amplification, deletion and translocation in a variety of tumors [23, 559]. Because of this, some miRs could act as either tumor suppressors or oncogenes (oncomir) [560–564].
The posttranscriptional fine tuning of mRNA and proteins levels by miR also plays an important role in developmental and immune regulatory processes [565–569]. They are involved in the regulation of nearly all aspects of cellular function including innate and adaptive immune responses [570–573]. Deregulated miR expression has been found in several autoimmune disorders and inflammatory conditions [574–576]. Importantly, miRs have been found to be either upregulated or downregulated in tumors [577–580]. Epidemiological studies suggests about 25% of all cancer may be due to chronic inflammation [3, 8], and several miRs have been implicated in both inflammation and cancer [569, 581–584].
MicroRNA-155
miR-155 is found on chromosome 21 (human) and 16 (mice) [585] [586], and is generally considered to be an oncomir with mostly proinflammatory effects. This miR is upregulated by NF-κB [566, 587, 588], which is pivotal in inflammation and cancer [589]. miR-155 is upregulated/activated in B and T cells, macrophages and dendritic cells [566, 585, 590, 591]. miR-155−/− mice are highly resistant to experimental autoimmune encephalomyelitis (EAE) [592, 593]. Mechanistically, this appears to be due to the role of miR-155 in mediating the production of IL-17 (Th17) and IFN-γ (Th1) producing CD4+ T cells [592].
miR-155 has been found at high levels in human B cell lymphomas and other tumors [585, 590, 594–596]. Enforced overexpression of miR-155 in mouse B cells is sufficient to trigger murine B cell lymphoma [597]. It has also been reported that miR-155 acts as an oncogene by targeting tumor suppressor gene suppressors of cytokine signaling 1 (SOCS1) in breast cancer cells [598]. Additionally, the upregulation of miR-155 by mutant p53 was reported to drive breast cancer invasion [599] and this miR suppressed the expression of tumor protein p53 induced nuclear protein 1 (TP53INP1) [600]. miR-155 may also play a role in multiple sclerosis (MS) and rheumatoid arthritis (RA), where elevated levels have been found in brain lesions of MS patients [601] and in synovial samples of RA patients [602]. Overall, miR-155 is emerging, then, as a key oncomir linking inflammation and cancer.
MicroRNA-146
miR-146 is a miRNA family, consisting of two evolutionarily conserved miRNA genes: miR-146a and miR-146b. miR-146 suppresses inflammation and cancer. The distal region of chromosome 5q, which contains miR-146a gene (5q33) in humans is reported to harbor susceptibility loci for autoimmune diseases such as RA [603], Crohn’s Disease [604], asthma [605] and psoriasis [606]. miR-146a and miR-146b, when expressed in highly metastatic human breast cancer cells, function to negatively regulate NF-κB activity [607]. miR-146a and miR-146b have also been found to be highly expressed in RA synovial tissue [608]. Although RA is not a high cancer risk disease, other auto-immune, chronic inflammatory diseases such as inflammatory bowel disease (IBD) are treated in a similar manner (e.g. TNFα inhibitors). Therefore, it would be interesting to examine the role of this miR in such diseases. miR-146a also directly targets PGE2 synthase and increased expression of miR-146a in bone mesenchymal stem cells (BMSCs) is correlated with the inhibition of PGE2 synthase-2 (Ptges-2) and the inhibition of PGE2 release [609]. In contrast to miR-155, miR-146a limits T cell activation and promotes resolution of inflammatory responses [610]. miR-146a−/− mice develop spontaneous autoimmunity and myeloid cancers upon aging, due to hyperactivation of T cells via de-repression of the proinflammatory proteins, IL-1 receptor-associated kinase 1 (IRAK1) and TNF receptor associated factor (TRAF)6 [610–612]. Finally, Xie et al. recently reported that the inhibition of miR-146 results in increased IL-1β, IL-6 and TNF-α secretion, as well as increased expression of IRAK1 [613]. Such studies, then, again highlight a key role of miR-146 in inflammation and cancer.
MicroRNA-21
miR-21 is an oncomir. Its oncogenic activity has been reported where it targets and represses important tumor suppressor genes such as PTEN [614], programmed cell death 4 (PDCD4) [615], tropomyosin 1 (TMP1) [616], B-cell translocation gene 2 (BTG) [617], components of the p53 pathway [618] and also modulates growth inhibitory and pro-apoptotic cytokine TGF-β signaling [618] to further enhance its tumorigenic effects. miR-21 deregulation is a very early event in the multistep progression of pancreatic ductal adenocarcinoma (PDAC) [619]. miR-21 expression is increased in breast and colorectal cancer and in the serum of patients with hepatocellular carcinoma (HCC) [620, 621]. With regards to its role in inflammation, miR-21 expression has been shown to be induced in macrophages and peripheral blood mononuclear (PBM) cells upon LPS challenge [622] and in mammary epithelial cells by inflammatory signals [582]. Similarly induction of miR-21 by IL-6 is a STAT3 dependent mechanism that is responsible for the survival of multiple myeloma cells [623]. It appears that STAT3 together with miR-21, miR-181b-1, PTEN and cylindromatosis (CYLD) is a part of the epigenetic switch that links inflammation to cancer in several cancer types including breast, colon, prostrate, lung and HCC [581]. Finally, Schetter et al. have reported a positive correlation of IL-6 with miR-21 expression in human colon cancer tissues [624], further supporting the role of miR-21 in linking inflammation and cancer.
MicroRNA-17~92 Cluster
miR-17~92 (OncomiR-1) [562] is a cluster of miRs located on human chromosome 13 and encodes a polycistronic miR gene for six mature functional miRs: miR-17, miR-18a, miR-19a, -20, -19b and -92 [625]. Overall, this cluster of miRs has cancer and inflammation-promoting properties. For example, SOCS1, a gene frequently silenced in multiple myeloma, and a strong antiinflammatory instigator, is targeted by miR-19, elucidating the proinflammatory property of miR-19 and its possible link to tumorigenesis [626, 627]. miR-17~92 clusters weaken TGF-β signaling by functioning both upstream and downstream of phospho-SMAD2 as well as through direct inhibition of TGF-β responsive genes [628]. miR-19b positively regulates NF-κβ signaling for proinflammatory cytokine production, is involved in controlling several negative regulators of NF-κB signaling, and plays a crucial role in the pathology of autoimmune diseases [629]. Additionally, miR-17~92 is a well-established player of oncogenesis and overexpression of this cluster and in a Myc-driven mouse model of B-cell leukemia accelerates tumor development [562]. miR-19 can exert its oncogenic effect through its repression of tumor suppressors PTEN and Protein phosphatase 2 (PP2A), pro-apoptotic molecule B-cell lymphoma 2 interacting mediator of cell death and Protein kinase, AMP-activated, alpha 1 catalytic subunit [630–632]. Overall, the miR-17~92 cluster, based on its role in inflammation and cancer could also serve as a potential therapeutic target.
MicroRNA-196
miR-196 is considered an oncomir, is upregulated in several cancer types [569] and is associated with Barrett’s esophagus-to-adenocarcinoma disease progression [633]. Luthra et al. demonstrated miR-196a directly targets the antiinflammatory player, annexin 1 and has growth promoting and antiapoptotic properties in esophageal adenocarcinoma cell lines [634]. miR-196 is overexpressed in inflamed intestinal epithelial of Crohn's disease patients and downregulates immunity-related GTPase family M protein (IRGM) protective variant (c.313C) but not the risk associated allele (c.313T) [635]. Also, the Rs11614913 SNP in miR-196a-2 may promote susceptibility to breast and lung cancer [636]. These oncogenic and proinflammatory properties of miR-196a support its role in inflammation and cancer.
microRNA-663
miR-663 is currently reported as an antiinflammatory and tumor suppressor miR and impairs the upregulation of miR-155 by inflammatory stimuli [637, 638]. The overexpression of hypomethylated miR-663 induces chemotherapy resistance in human breast cancer cells by targeting heparan sulfate proteoglycan 2 (HSPG2) [639].
Other microRNAs involved in inflammation and cancer
miR-9 is canonically induced by NF-κβ following TLR4 activation in human neutrophils and monocytes and provides feedback to repress NF-κB signaling through direct targeting of p50 mRNA [640]. Overexpression of miR-9 by MYC/MYCN is involved in cancer metastasis [641, 642]. This elucidates a possible link between inflammation and cancer by miR-9. Several studies reported upregulation of miR-210 in hypoxic condition [643–645] and its importance for cell survival [646]. miR-210 is a sensor for hypoxic stress during tumorigenesis, where increased miR-210 expression inhibits tumor growth to provide tumor cells an opportunity to prevail in stressful hypoxic condition [647]. Thus, a possible connection between hypoxia and tumorigenesis is mediated by miR-210. miRNA-16 is a putative tumor suppressor miR, and is downregulated in a variety of human cancers [648–654]. One recognized function of miR-16 is that it controls the cell cycle primarily through a G1 cell cycle checkpoint [649, 655–662].
The finding that miR-16 is upregulated in high colon cancer risk, and chronic inflammatory disease possibly indicates an adaptive upregulation of this tumor suppressor miR in response to inflammatory stress. Finally, inhibiting the peptidyl arginine deiminase (PAD) enzyme, which catalyzes the post-translational conversion of peptidyl-arginine to peptidyl-citrulline (“citrullination”) causes an increase in miR-16 [663]. The fact that citrullination is thought to be an inflammation-dependent process [664] supports the notion that miR-16 is involved in the suppression of inflammation. miR-125b expression is decreased after LPS challenge in macrophage cells [665], and additionally in several inflammatory condition such as psoriasis and atopic eczema [666]. Further down-regulation of miR-125b has been reported in several tumor types such as thyroid anaplastic carcinomas, hepatocarcinomas, oral, bladder cancer, ovarian and breast cancer [569]. Finally, miR-663 is currently reported as antiinflammatory and tumor suppressor microRNA and impairs the upregulation of miR-155 by inflammatory stimuli [637, 638].
Selected approaches that modulate miR involved in inflammation and cancer
Signaling pathways involving inflammation and cancer are clearly regulated by miRs so here we specifically discuss studies that relate to the therapeutic approaches reviewed above. For reference sake, additional details on other dietary components that regulate miRs have been reviewed in detail elsewhere [557, 667].
Resveratrol
Since both resveratrol and miR influence cellular homeostasis and disease conditions, resveratrol could act through miRs in modulating and targeting the factors involved in disease and cellular homeostasis. Tili and Michaille reviewed resveratrol, miRs, inflammation and cancer [668], and note that resveratrol has been shown to induce the expression of miR-663, a tumor-suppressor and antiinflammatory miR, while down-regulating proinflammatory miR-155 and oncogenic miR-21.
Curcumin
Curcumin regulates the expression of genes that are involved in the regulation of cellular inflammatory and cancer signaling pathways, such as NF-κB, AKT, MAPK and other pathways [669, 670]. These signaling pathways are in turn regulated by several miRs. In a spontaneously arising retinal pigment epithelia cell line (ARPE-19 cells), curcumin treatment lowers the expression of miR-17~92 cluster and its pre-treatment attenuates H2O2 induced expression of miR-15b, miR-21, miR-17, miR-196b and miR-9 [671]. The curcumin analog CDF decreases pancreatic cancer cell survival by increasing the expression of the tumor suppressor miRs, Let-7 and miR-146a, which are typically lost in pancreatic cancer [672]. The mesenchymal phenotype of gemcitabine-resistant pancreatic cancer cells has been shown to be reversed by simply treating the cells with either CDF or curcumin which upregulates the expression of miR-200b and miR-200c [673]. Curcumin also reduces miR-21 expression and activity via AP-1, suppresses tumor progression, and stabilizes the tumor suppressor Pdcd4 in colorectal cancer cells [674].
Genistein
Genistein enhances the apoptotic effects of exogenous miR-16 in murine CLL cells [675]. Isoflavones regulate miR function by inducing expression of miR-200 and let-7 to reverse EMT phenotype [676]. Isoflavones have also been shown to upregulate miR-146a and target EGFR and IRAK-1/NF-κB signaling to inhibit pancreatic cancer cell invasion [677]. These studies provide evidence that isoflavones regulate miRs involved in inflammation and cancer which may provide a prevention and/or treatment measure.
EGCG
EGCG is a major catechin in green tea and has been implicated in many pathways involved in inflammation and cancer. EGCG upregulates miR-210 in human and mouse lung cancer cells in culture which leads to reduced cell proliferation mediated by stabilization of HIF-1α [678]. EGCG antagonizes androgen action and down-regulates miR-21 and upregulates tumor suppressor miR-330 in prostate tumors of mice [679]. EGCG has also been shown to decrease expression of oncomirs (miR-92, miR-93, and miR-106b) and increase the expression of tumor suppressor miRs (miR-7-1, miR-34a, and miR-99a) in neuroblastoma cells [680].
Cross-validation for Tumor Promoting Inflammation
Given that the heterogeneity that is present in most cancers, it is our assumption that the complete arrest of the various subpopulations of immortalized cells in any given cancer will require simultaneous actions on mechanisms that are important for several aspects of cancer’s biology. We therefore believe that it is important to be able to anticipate synergies that might be achieved by acting on specific targets and with specific approaches (i.e., when contemplating an approach aimed at a broad-spectrum of targets). Accordingly, in this review the prioritized target sites and the approaches that have been identified (as potential ways to reach those targets) were all cross-validated by conducting a background literature research. A team of researchers consisting of specialists in each area specifically sought to determine the relevance of these targets and the nominated approaches across a number of important areas of cancer’s biology.
In this regard, targets and approaches that were not only relevant for this area of study, but also relevant for other aspects of cancer’s biology (i.e., anticarcinogenic) were noted as having "complementary" effects. Those that were found to have procarcinogenic actions were noted as having "contrary" effects. In instances where reports on relevant actions in other aspects of cancer biology were mixed (i.e., reports showing both procarcinogenic potential and anticarcinogenic potential), the term "controversial" was used. Finally, in instances where no literature support was found to document the relevance of a target site or approach in a particular aspect of cancer's biology, we documented this as "no known relationship". These validation results are shown below in tabular form in Tables I and II.
Table I. Cross validation of Targets.
Prioritized targets evaluated for known effects in other cancer hallmark areas.
| Potential targets for inflammation1 |
Inhibit Cox-2 |
Inhibit NF- κB |
Block MIF |
Block TNFα |
Block iNOS |
Block AKT |
Inhibit CXC chemokines |
|---|---|---|---|---|---|---|---|
| Other hallmarks | |||||||
| Genomic Instability | + [681] | + [682] | 0 | 0 | 0 | + [683–685] | 0 |
| Sustained Proliferative Signaling | + [686–688] | + [689–691] | + [54, 692] | + [693, 694] | + [695] | + [696] | + [697] |
| Evasion of Anti-growth Signaling | + [698, 699] | 0 | + [53, 700] | + [701, 702] | + [703] | + [704] | + [705] |
| Resistance to Apoptosis | + [706] | + [707] | + [708] | + [709] | + [710] | + [711] | + [712] |
| Replicative Immortality | + [713–715] | +/− [716–718] | + [719, 720] | + [721] | 0 | + [711, 722, 723] | 0 |
| Deregulated Metabolism | + [724] | + [725–727] | 0 | + [728–731] | + [732, 733] | + [234, 734–736] | 0 |
| Immune System Evasion | + [737, 738] | + [739] | + [740] | − [44] | 0 | + [741] | +/− [742] |
| Angiogenesis | − [743] | +/− [744, 745] | + [54, 55, 746] | +/− [154] | − [747] | + [748] | +/− [749] |
| Tissue Invasion and Metastasis | + [750–753] | + [754] | + [755, 756] | + [757] | + [758] | + [759] | + [760] |
| Tumor Microenvironment | + [761] | + [762] | + [763] | + [764] | +/− [765] | + [766, 767] | +/− [768, 769] |
Targets that were found to have complementary, anticarcinogenic actions reported in another hallmark area were indicated with “+“, while targets that were found to have procarcinogenic actions in another hallmark area were indicated with “−”. In instances where reports on relevant actions in other hallmark areas were mixed (i.e., reports showing both anticarcinogenic potential and procarcinogenic potential), the symbol “+/− ” was used. Finally, in instances where no literature support was found to document the relevance of a target in a particular aspect of cancer's biology, we documented this as “0”. These cross-hallmark relationships are reported in the first eleven columns of the table. The number of anticarcinogenic, procarcinogenic and mixed cross-hallmark relationships for each target have been summed and are reported in the last three columns of the table.
Table II. Cross validation of Approaches.
Selected approaches evaluated for reported actions in other cancer hallmark areas.
| Approach1 | Curcumin | Resveratrol | EGCG | Lycopene | Anthocyanins | Genistein |
|---|---|---|---|---|---|---|
| Other Hallmarks | ||||||
| Genomic Instability | + [770] | + [682] | + [771] | + [772] | + [770] | + [773] |
| Sustained Proliferative Signaling | + [774] | + [775–777] | + [678, 778] | + [488, 779] | + [780, 781] | +/− [782, 783] |
| Evasion of Anti-growth Signaling | + [784, 785] | + [786, 787] | + [788, 789] | + [790–792] | + [793] | +/− [544, 794, 795] |
| Resistance to Apoptosis | + [796] | + [797] | + [798] | + [799] | + [800] | + [518] |
| Replicative Immortality | + [774, 801, 802] | + [803, 804] | + [805, 806] | 0 | + [807] | + [808, 809] |
| Deregulated Metabolism | + [810] | + [811, 812] | + [813–815] | 0 | 0 | +/− [816] |
| Immune System Evasion | + [371] | +/− [425, 817–819] | + [820] | 0 | 0 | +/− [554, 821] |
| Angiogenesis | + [822] | +/− [823] | + [824] | + [825] | + [826] | + [827] |
| Tissue Invasion and Metastasis | + [774, 828] | + [829] | + [312, 830, 831] | + [832] | + [833] | + [830] |
| Tumor Microenvironment | + [834, 835] | + [836, 837] | + [820, 838] | + [772, 839] | + [840] | + [841] |
Approaches that were found to have complementary, anticarcinogenic actions in a particular hallmark area were were indicated with “+”, while approaches that were found to have procarcinogenic actions in a particular hallmark area were indicated with “ −”. In instances where reports on relevant actions in other hallmarks were mixed (i.e., reports showing both anticarcinogenic and procarcinogenic potential), the symbol “+/−“ was used. Finally, in instances where no literature support was found to document the relevance of an approach in a particular aspect of cancer's biology, we documented this as “0”'. Threse cross-hallmark relationships are reported in the first eleven columns of the table. Finally, the number of anticarcinogenic, procarcinogenic and mixed cross-hallmark relationships for each target have been summed and are reported in the last three columns of the table.
The decision to review priority target sites and approaches for reports of cross-hallmark effects was driven by the fact that many individual studies and reviews fail to account systematically for the spectrum of incidental actions that can result from various forms of therapeutic interventions. It is our belief that this approach constitutes a better way to ensure that we had assembled a reasonably thorough review of the literature (i.e., where any sort of evidence of cross-hallmark activity had been reported).
Because future research on therapeutic combinations will likely involve empirical testing of mixtures of constituents, we wanted to create a starting point for other researchers who might be considering translational projects. We anticipated interest in approaches reported to exhibit a large number of anticarcinogenic actions across the hallmarks and we anticipated that a lack of procarcinogenic potential was important to identify (since targets or approaches that have been shown to exert procarcinogenic actions would potentially represent a confounding and unwanted influence/factor in empirical research). A summary of these reports is also provided in Tables I and II.
Note that, in some instances, the underlying evidence used to support the indication of a cross-hallmark relationship was robust, consisting of multiple studies involving detailed in-vitro and in vivo findings. In other instances, however, the underlying evidence that was used to report the existence of a cross-hallmark relationship was quite weak (e.g., consisting of only a single in vitro study involving a single cell-type). Additionally, there are examples of approaches that are known to exert different effects at different dose levels and in different tissues but dose-levels and cell/tissue types were not used to discriminate when gathering together these reported actions.
Nonetheless, given that the overarching goal in this project was to create a foundation that would allow researchers to look systematically across the literature in each of these areas, the tables should serve as a useful starting point as long as they are approached with caveats in mind and a degree of caution. Essentially, we believe that this heuristic model should be useful to consider synergies that might be anticipated in testing that involves certain targets and/or mixtures of chemical constituents that are being considered for therapeutic effects.
Summary/Conclusions
In sum, it was our goal to explore a series of high priority antiinflammatory targets for therapeutic intervention in cancer as part of a larger effort to develop a broad-spectrum approach aimed at a wide range of targets that are relevant for cancer biology. The selected targets MIF, COX-2, NF-κB, TNF-α, iNOS, AKT and CXC chemokines represent a promising and interrelated set of targets that are pleiotropic, with demonstrated potential not only for inflammation, but also for a wide range of other effects that support the various hallmark phenotypes found in a wide range of cancer types.
At the same time, the approaches that we selected to act on those targets, (curcumin, resveratrol, EGCG, genistein, lycopene, and anthocyanins) are all agents than have demonstrated a range of anticancer effects. While we focused mainly on antiinflammatory effects, many of these approaches have demonstrated a range of anticarcinogenic actions as well. In addition to the most widely reported direct effects of these agents, we have also summarized miR regulated gene expression related to inflammation and cancer, and the known effects of these approaches on these MiRs.
Given the tight coupling between inflammation and the immune system, we also wanted to consider the possibility that proposed actions on important antiinflammatory targets, and/or the chronic administration of the antiinflammatory chemicals might predispose individuals to infection or modulate the immune system in a manner that might be relevant for immune-related antitumor effects. Perhaps not surprisingly, an increased risk of infection appears to be a concern for therapeutic approaches aimed at suppressing MIF, Cox-2, NF-κB, and TNF-α, and in the use of curcumin (as a therapeutic approach). By contrast, EGCG appears to have a protective effect against bacterial infection. Immunomodulation of antitumoral effects is also a nuanced picture. COX-2 inhibition and PI3K-Akt pathway inhibition both appear to be attractive targeting strategies that have antitumoral effects that are immune-related. Similarly, curcumin, resveratrol and EGCG have also been shown to act on the immune system in a favorable manner. However, lycopene and genistein have demonstrated a range of competing effects on the immune system making their utility from this perspective more difficult to discern.
Future research should address the ambiguities posed by the wide range of CXC Chemokines and their various effects, as precise targets are needed to better characterize the range of effects and synergies that might be anticipated. Similarly, within the selected approaches, specific anthocyanins that appear to have the greatest promise should be isolated and better characterized for effects across the range of cancer hallmark phenotypes, and for bioavailability and toxicity.
Ideally, future translational work would utilize the agents that we have identified in this review combined as constituents within a multi-pronged antiinflammatory approach with very little/no toxicity.
However, any multipronged strategy that focuses on these targets and/or approaches will need to carefully consider the potential for increased risks related to infection and anticipate the possibility for a range of immunomodulation that will have relevance for antitumoral effects.
Bioavailability challenges with a number of these agents are starting to be addressed, and foreseeably recent advances that uses implantable polymeric micelles, liposomes, microspheres, nano-delivery systems, phospholipid-based delivery systems and other systems (c.f. [359–362]) will help address this issue.
The cross-validation tables (Table I and II) are offered here as a simple heuristic framework that is intended to help researchers approach the topic of anticipated synergies. Although these initial results do not represent a homogenous set of underlying data, it is hoped that they can serve as a starting point for the translational research that will be needed. Rigorous experimentation will obviously be needed to determine whether or not actual synergies emerge that can be predicted using this approach. Other synergies may emerge depending on the specific constituents and model used.
The key is to recognize that a low-toxicity approach aimed at many important targets to reduce tumor-promoting inflammation is only a stepping stone. Most cancers harbor significant genetic heterogeneity [4], and patterns of relapse following many therapies are due to evolved resistance to treatment. Consequently, an antiinflammatory approach along these lines should be developed and then combined with other similar approaches that aim to target the many disease-specific pathways that have relevance across the range of hallmark phenotypes. A much broader range of targets overall may be the only chance we will have to address this heterogeneity. It is a promising approach, but a considerable amount of encompassing research needs to follow to determine methodological validity
Acknowledgements
Funding
Alexandros G. Georgakilas was supported by an EU Marie Curie Reintegration Grant MC-CIG-303514, Greek National funds through the Operational Program ‘Educational and Lifelong Learning of the National Strategic Reference Framework (NSRF)-Research Funding Program: THALES (Grant number MIS 379346) and COST Action CM1201 ‘Biomimetic Radical Chemistry’; Amr Amin was supported by the Terry Fox Foundation (TF-36), UAEU Program for Advanced Research (UPAR25183), Al-Jalila Foundation (AJF201454) and Zayed Center for Health Sciences (ZCHS2014); Bal L. Lokeshwar was supported by NIH Grant NO. R01 CA 156776-01; VA Merit Award NO. BLR&D 1 01-BX001517-01; Diana M. Stafforini was supported by grant 5P01CA073992 from the National Cancer Institute, by DOD IDEA Award W81XWH-12-1-0515 and by the Huntsman Cancer Foundation; Kanya Honoki was supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan (No. 24590493); Kapil Mehta was supported by Bayer Healthcare System G4T (Grants4Targets); Lorne Hofseth was supported by US NIH National Cancer Institute grants: 1R01CA151304 (LJH), 1R03CA1711326 (LJH), and 1P01AT003961 (LJH); Luigi Ricciardiello Associazione Italiana per la Ricerca sul Cancro (AIRC) was supported by investigator grants n. 10216 and 14281, The European Community’s Seventh Framework Program FP7/2007–2013 under Grant Agreement 311876; Neetu Singh was supported in part by the Fast Track Scheme for Young Scientists, Department of Science and Technology, India (SR/FT/LS-063/2008); Richard L. Whelan was supported in part by philanthropy (grateful patients) and Hospital Start-up funds; Rupesh Chaturvedi was supported by a grant from US NIH National Center for Complementary and Alternative Medicine (K01AT007324)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Kapil Mehta is a Scientific Adviser to Lifecare Innovations, India and is an inventor in United States patent # 8,765,797 (TG2 inhibitors and uses thereof); Luigi Ricciardiello received an unrestricted research grant by SLA Pharma AG
References
- 1.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantovani A. Cancer: Inflaming metastasis. Nature. 2009;457:36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 6.Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Current opinion in genetics & development. 2010;20:65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demaria S, Pikarsky E, Karin M, Coussens LM, Chen YC, El-Omar EM, et al. Cancer and inflammation: promise for biologic therapy. Journal of immunotherapy (Hagerstown, Md : 1997) 2010;33:335–351. doi: 10.1097/CJI.0b013e3181d32e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 9.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 10.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 11.Borm PJ, Driscoll K. Particles, inflammation and respiratory tract carcinogenesis. Toxicol Lett. 1996;88:109–113. doi: 10.1016/0378-4274(96)03725-3. [DOI] [PubMed] [Google Scholar]
- 12.Macarthur M, Hold GL, El-Omar EM. Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. American journal of physiology Gastrointestinal and liver physiology. 2004;286:G515–G520. doi: 10.1152/ajpgi.00475.2003. [DOI] [PubMed] [Google Scholar]
- 13.Whitcomb DC. Inflammation and Cancer V. Chronic pancreatitis and pancreatic cancer. American journal of physiology Gastrointestinal and liver physiology. 2004;287:G315–G319. doi: 10.1152/ajpgi.00115.2004. [DOI] [PubMed] [Google Scholar]
- 14.Baumgarten SC, Frasor J. Minireview: Inflammation: an instigator of more aggressive estrogen receptor (ER) positive breast cancers. Molecular endocrinology (Baltimore, Md. 2012;26:360–371. doi: 10.1210/me.2011-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fouad TM, Kogawa T, Reuben JM, Ueno NT. The role of inflammation in inflammatory breast cancer. Adv Exp Med Biol. 2014;816:53–73. doi: 10.1007/978-3-0348-0837-8_3. [DOI] [PubMed] [Google Scholar]
- 16.Risch HA, Howe GR. Pelvic inflammatory disease and the risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 1995;4:447–451. [PubMed] [Google Scholar]
- 17.Persky L. Epidemiology of cancer of the penis. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 1977:97–109. doi: 10.1007/978-3-642-81095-4_11. [DOI] [PubMed] [Google Scholar]
- 18.Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Annals of the New York Academy of Sciences. 2009;1155:206–221. doi: 10.1111/j.1749-6632.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- 19.Palapattu GS, Sutcliffe S, Bastian PJ, Platz EA, De Marzo AM, Isaacs WB, et al. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis. 2005;26:1170–1181. doi: 10.1093/carcin/bgh317. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 22.Ohnishi S, Ma N, Thanan R, Pinlaor S, Hammam O, Murata M, et al. DNA damage in inflammation-related carcinogenesis and cancer stem cells. Oxidative medicine and cellular longevity. 2013;2013:387014. doi: 10.1155/2013/387014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nature Reviews Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 24.Georgakilas AG, Redon CE, Ferguson NF, Kryston TB, Parekh P, Dickey JS, et al. Systemic DNA damage accumulation under in vivo tumor growth can be inhibited by the antioxidant Tempol. Cancer letters. 2014 doi: 10.1016/j.canlet.2014.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Current opinion in pharmacology. 2009;9:351–369. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balkwill F, Mantovani A. Cancer and inflammation: implications for pharmacology and therapeutics. Clinical pharmacology and therapeutics. 2010;87:401–406. doi: 10.1038/clpt.2009.312. [DOI] [PubMed] [Google Scholar]
- 27.Kundu JK, Surh YJ. Nrf2-Keap1 signaling as a potential target for chemoprevention of inflammation-associated carcinogenesis. Pharmaceutical research. 2010;27:999–1013. doi: 10.1007/s11095-010-0096-8. [DOI] [PubMed] [Google Scholar]
- 28.Bishayee A. The role of inflammation and liver cancer. Adv Exp Med Biol. 2014;816:401–435. doi: 10.1007/978-3-0348-0837-8_16. [DOI] [PubMed] [Google Scholar]
- 29.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khatami M. Inflammation, aging, and cancer: tumoricidal versus tumorigenesis of immunity: a common denominator mapping chronic diseases. Cell biochemistry and biophysics. 2009;55:55–79. doi: 10.1007/s12013-009-9059-2. [DOI] [PubMed] [Google Scholar]
- 31.Baniyash M, Sade-Feldman M, Kanterman J. Chronic inflammation and cancer: suppressing the suppressors. Cancer Immunol Immunother. 2014;63:11–20. doi: 10.1007/s00262-013-1468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Bluestone JA, Mackay CR, O'Shea JJ, Stockinger B. The functional plasticity of T cell subsets. Nature reviews Immunology. 2009;9:811–816. doi: 10.1038/nri2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer metastasis reviews. 2010;29:309–316. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ringash J, Au HJ, Siu LL, Shapiro JD, Jonker DJ, Zalcberg JR, et al. Quality of life in patients with K-RAS wild-type colorectal cancer: the CO.20 phase 3 randomized trial. Cancer. 2014;120:181–189. doi: 10.1002/cncr.28410. [DOI] [PubMed] [Google Scholar]
- 39.Chung S, Son GH, Kim K. Circadian rhythm of adrenal glucocorticoid: its regulation and clinical implications. Biochimica et biophysica acta. 2011;1812:581–591. doi: 10.1016/j.bbadis.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Volden PA, Conzen SD. The influence of glucocorticoid signaling on tumor progression. Brain, behavior, and immunity. 2013;30(Suppl):S26–S31. doi: 10.1016/j.bbi.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 42.Bucala R. MIF rediscovered: cytokine, pituitary hormone, and glucocorticoid-induced regulator of the immune response. Faseb J. 1996;10:1607–1613. doi: 10.1096/fasebj.10.14.9002552. [DOI] [PubMed] [Google Scholar]
- 43.Calandra T, Bucala R. Macrophage migration inhibitory factor (MIF): a glucocorticoid counter-regulator within the immune system. Critical reviews in immunology. 1997;17:77–88. doi: 10.1615/critrevimmunol.v17.i1.30. [DOI] [PubMed] [Google Scholar]
- 44.Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14:e218–e228. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 45.Bifulco C, McDaniel K, Leng L, Bucala R. Tumor growth-promoting properties of macrophage migration inhibitory factor. Current pharmaceutical design. 2008;14:3790–3801. doi: 10.2174/138161208786898608. [DOI] [PubMed] [Google Scholar]
- 46.Bach JP, Deuster O, Balzer-Geldsetzer M, Meyer B, Dodel R, Bacher M. The role of macrophage inhibitory factor in tumorigenesis and central nervous system tumors. Cancer. 2009;115:2031–2040. doi: 10.1002/cncr.24245. [DOI] [PubMed] [Google Scholar]
- 47.Babu SN, Chetal G, Kumar S. Macrophage migration inhibitory factor: a potential marker for cancer diagnosis and therapy. Asian Pacific journal of cancer prevention : APJCP. 2012;13:1737–1744. doi: 10.7314/apjcp.2012.13.5.1737. [DOI] [PubMed] [Google Scholar]
- 48.Conroy H, Mawhinney L, Donnelly SC. Inflammation and cancer: macrophage migration inhibitory factor (MIF)--the potential missing link. QJM : monthly journal of the Association of Physicians. 2010;103:831–836. doi: 10.1093/qjmed/hcq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toh ML, Aeberli D, Lacey D, Yang Y, Santos LL, Clarkson M, et al. Regulation of IL-1 and TNF receptor expression and function by endogenous macrophage migration inhibitory factor. J Immunol. 2006;177:4818–4825. doi: 10.4049/jimmunol.177.7.4818. [DOI] [PubMed] [Google Scholar]
- 50.Lai KN, Leung JC, Metz CN, Lai FM, Bucala R, Lan HY. Role for macrophage migration inhibitory factor in acute respiratory distress syndrome. The Journal of pathology. 2003;199:496–508. doi: 10.1002/path.1291. [DOI] [PubMed] [Google Scholar]
- 51.Shachar I, Haran M. The secret second life of an innocent chaperone: the story of CD74 and B cell/chronic lymphocytic leukemia cell survival. Leukemia & lymphoma. 2011;52:1446–1454. doi: 10.3109/10428194.2011.565437. [DOI] [PubMed] [Google Scholar]
- 52.Mittelbronn M, Platten M, Zeiner P, Dombrowski Y, Frank B, Zachskorn C, et al. Macrophage migration inhibitory factor (MIF) expression in human malignant gliomas contributes to immune escape and tumour progression. Acta neuropathologica. 2011;122:353–365. doi: 10.1007/s00401-011-0858-3. [DOI] [PubMed] [Google Scholar]
- 53.Hussain F, Freissmuth M, Volkel D, Thiele M, Douillard P, Antoine G, et al. Human antimacrophage migration inhibitory factor antibodies inhibit growth of human prostate cancer cells in vitro and in vivo. Molecular cancer therapeutics. 2013;12:1223–1234. doi: 10.1158/1535-7163.MCT-12-0988. [DOI] [PubMed] [Google Scholar]
- 54.Choudhary S, Hegde P, Pruitt JR, Sielecki TM, Choudhary D, Scarpato K, et al. Macrophage migratory inhibitory factor promotes bladder cancer progression via increasing proliferation and angiogenesis. Carcinogenesis. 2013;34:2891–2899. doi: 10.1093/carcin/bgt239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asare Y, Schmitt M, Bernhagen J. The vascular biology of macrophage migration inhibitory factor (MIF). Expression and effects in inflammation, atherogenesis and angiogenesis. Thrombosis and haemostasis. 2013;109:391–398. doi: 10.1160/TH12-11-0831. [DOI] [PubMed] [Google Scholar]
- 56.Borghese F, Clanchy FI. CD74: an emerging opportunity as a therapeutic target in cancer and autoimmune disease. Expert opinion on therapeutic targets. 2011;15:237–251. doi: 10.1517/14728222.2011.550879. [DOI] [PubMed] [Google Scholar]
- 57.Grieb G, Merk M, Bernhagen J, Bucala R. Macrophage migration inhibitory factor (MIF): a promising biomarker. Drug News Perspect. 2010;23:257–264. doi: 10.1358/dnp.2010.23.4.1453629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ioannou K, Cheng KF, Crichlow GV, Birmpilis AI, Lolis EJ, Tsitsilonis OE, et al. ISO-66, a novel inhibitor of macrophage migration, shows efficacy in melanoma and colon cancer models. Int J Oncol. 2014 doi: 10.3892/ijo.2014.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Das R, Koo MS, Kim BH, Jacob ST, Subbian S, Yao J, et al. Macrophage migration inhibitory factor (MIF) is a critical mediator of the innate immune response to Mycobacterium tuberculosis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E2997–E3006. doi: 10.1073/pnas.1301128110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bogatcheva NV, Sergeeva MG, Dudek SM, Verin AD. Arachidonic acid cascade in endothelial pathobiology. Microvascular research. 2005;69:107–127. doi: 10.1016/j.mvr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 61.Chen EP, Smyth EM. COX-2 and PGE2-dependent immunomodulation in breast cancer. Prostaglandins & other lipid mediators. 2011;96:14–20. doi: 10.1016/j.prostaglandins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clinical immunology (Orlando, Fla. 2006;119:229–240. doi: 10.1016/j.clim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 63.Andreasson K. Emerging roles of PGE2 receptors in models of neurological disease. Prostaglandins & other lipid mediators. 2010;91:104–112. doi: 10.1016/j.prostaglandins.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kundu JK, Surh YJ. Emerging avenues linking inflammation and cancer. Free Radic Biol Med. 2012;52:2013–2037. doi: 10.1016/j.freeradbiomed.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 65.Biswas S, Bhattacherjee P, Paterson CA. Prostaglandin E2 receptor subtypes, EP1, EP2, EP3 and EP4 in human and mouse ocular tissues--a comparative immunohistochemical study. Prostaglandins, leukotrienes, and essential fatty acids. 2004;71:277–288. doi: 10.1016/j.plefa.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 66.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 67.Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Annals of clinical and laboratory science. 2000;30:3–21. [PubMed] [Google Scholar]
- 68.Goulet AC, Einsphar JG, Alberts DS, Beas A, Burk C, Bhattacharyya A, et al. Analysis of cyclooxygenase 2 (COX-2) expression during malignant melanoma progression. Cancer biology & therapy. 2003;2:713–718. [PubMed] [Google Scholar]
- 69.Hussain T, Gupta S, Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer letters. 2003;191:125–135. doi: 10.1016/s0304-3835(02)00524-4. [DOI] [PubMed] [Google Scholar]
- 70.Schneider SL, Ross AL, Grichnik JM. Do inflammatory pathways drive melanomagenesis? Experimental dermatology. 2014 doi: 10.1111/exd.12502. [DOI] [PubMed] [Google Scholar]
- 71.Wu KK, Cheng HH, Chang TC. 5-methoxyindole metabolites of L-tryptophan: control of COX-2 expression, inflammation and tumorigenesis. Journal of biomedical science. 2014;21:17. doi: 10.1186/1423-0127-21-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maru GB, Gandhi K, Ramchandani A, Kumar G. The role of inflammation in skin cancer. Adv Exp Med Biol. 2014;816:437–469. doi: 10.1007/978-3-0348-0837-8_17. [DOI] [PubMed] [Google Scholar]
- 73.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annual review of biochemistry. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 74.Marks F, Furstenberger G. Cancer chemoprevention through interruption of multistage carcinogenesis. The lessons learnt by comparing mouse skin carcinogenesis and human large bowel cancer. Eur J Cancer. 2000;36:314–329. doi: 10.1016/s0959-8049(99)00318-4. [DOI] [PubMed] [Google Scholar]
- 75.Subbaramaiah K, Hart JC, Norton L, Dannenberg AJ. Microtubule-interfering agents stimulate the transcription of cyclooxygenase-2. Evidence for involvement of ERK1/2 AND p38 mitogen-activated protein kinase pathways. J Biol Chem. 2000;275:14838–14845. doi: 10.1074/jbc.275.20.14838. [DOI] [PubMed] [Google Scholar]
- 76.Feller L, Altini M, Lemmer J. Inflammation in the context of oral cancer. Oral oncology. 2013;49:887–892. doi: 10.1016/j.oraloncology.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 77.Ramon S, Woeller CF, Phipps RP. The influence of Cox-2 and bioactive lipids on hematological cancers. Current angiogenesis. 2013;2:135–142. doi: 10.2174/2211552802999140131105947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vona-Davis L, Rose DP. The obesity-inflammation-eicosanoid axis in breast cancer. J Mammary Gland Biol Neoplasia. 2013;18:291–307. doi: 10.1007/s10911-013-9299-z. [DOI] [PubMed] [Google Scholar]
- 79.Xu X, Wells A, Padilla MT, Kato K, Kim KC, Lin Y. A signaling pathway consisting of miR-551b, catalase and MUC1 contributes to acquired apoptosis resistance and chemoresistance. Carcinogenesis. 2014 doi: 10.1093/carcin/bgu159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li W, Zhang B, Li H, Zhao C, Zhong Y, Sun J, et al. TGF beta1 Mediates Epithelial Mesenchymal Transition via beta6 Integrin Signaling Pathway in Breast Cancer. Cancer investigation. 2014 doi: 10.3109/07357907.2014.933235. [DOI] [PubMed] [Google Scholar]
- 81.Chen JY, Li CF, Kuo CC, Tsai KK, Hou MF, Hung WC. Cancer/stroma interplay via cyclooxygenase-2 and indoleamine 2,3-dioxygenase promotes breast cancer progression. Breast Cancer Res. 2014;16:410. doi: 10.1186/s13058-014-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang J, Guo W, Liang X. Phenotypes, accumulation, and functions of myeloid-derived suppressor cells and associated treatment strategies in cancer patients. Human immunology. 2014;75:1128–1137. doi: 10.1016/j.humimm.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 83.Obermajer N, Wong JL, Edwards RP, Odunsi K, Moysich K, Kalinski P. PGE(2)-driven induction and maintenance of cancer-associated myeloid-derived suppressor cells. Immunological investigations. 2012;41:635–657. doi: 10.3109/08820139.2012.695417. [DOI] [PubMed] [Google Scholar]
- 84.Li RJ, Liu L, Gao W, Song XZ, Bai XJ, Li ZF. Cyclooxygenase-2 blockade inhibits accumulation and function of myeloid-derived suppressor cells and restores T cell response after traumatic stress. Journal of Huazhong University of Science and Technology Medical sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban. 2014;34:234–240. doi: 10.1007/s11596-014-1264-6. [DOI] [PubMed] [Google Scholar]
- 85.Bernard MP, Bancos S, Chapman TJ, Ryan EP, Treanor JJ, Rose RC, et al. Chronic inhibition of cyclooxygenase-2 attenuates antibody responses against vaccinia infection. Vaccine. 2010;28:1363–1372. doi: 10.1016/j.vaccine.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee YS, Kwon EJ, Jin DQ, Park SH, Kang YS, Huh K, et al. Redox status-dependent regulation of cyclooxygenases mediates the capsaicin-induced apoptosis in human neuroblastoma cells. J Environ Pathol Toxicol Oncol. 2002;21:113–120. [PubMed] [Google Scholar]
- 87.Oyagbemi AA, Saba AB, Azeez OI. Capsaicin: a novel chemopreventive molecule and its underlying molecular mechanisms of action. Indian journal of cancer. 2010;47:53–58. doi: 10.4103/0019-509X.58860. [DOI] [PubMed] [Google Scholar]
- 88.Yi C, Zhang Y, Yu Z, Xiao Y, Wang J, Qiu H, et al. Melatonin Enhances the Anti-Tumor Effect of Fisetin by Inhibiting COX-2/iNOS and NF-kappaB/p300 Signaling Pathways. PloS one. 2014;9:e99943. doi: 10.1371/journal.pone.0099943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 90.Shishodia S. Molecular mechanisms of curcumin action: gene expression. Biofactors. 2013;39:37–55. doi: 10.1002/biof.1041. [DOI] [PubMed] [Google Scholar]
- 91.Wang R, Guo L, Wang P, Yang W, Lu Y, Huang Z, et al. Chemoprevention of cancers in gastrointestinal tract with cyclooxygenase 2 inhibitors. Current pharmaceutical design. 2013;19:115–125. doi: 10.2174/13816128130116. [DOI] [PubMed] [Google Scholar]
- 92.Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Molecular cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cerella C, Sobolewski C, Dicato M, Diederich M. Targeting COX-2 expression by natural compounds: a promising alternative strategy to synthetic COX-2 inhibitors for cancer chemoprevention and therapy. Biochem Pharmacol. 2010;80:1801–1815. doi: 10.1016/j.bcp.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 94.Tornatore L, Thotakura AK, Bennett J, Moretti M, Franzoso G. The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends in cell biology. 2012;22:557–566. doi: 10.1016/j.tcb.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 95.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. [PubMed] [Google Scholar]
- 96.Caamano J, Hunter CA. NF-kappaB family of transcription factors: central regulators of innate and adaptive immune functions. Clinical microbiology reviews. 2002;15:414–429. doi: 10.1128/CMR.15.3.414-429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.May MJ, Ghosh S. Rel/NF-kappa B and I kappa B proteins: an overview. Semin Cancer Biol. 1997;8:63–73. doi: 10.1006/scbi.1997.0057. [DOI] [PubMed] [Google Scholar]
- 98.Marienfeld R, May MJ, Berberich I, Serfling E, Ghosh S, Neumann M. RelB forms transcriptionally inactive complexes with RelA/p65. J Biol Chem. 2003;278:19852–19860. doi: 10.1074/jbc.M301945200. [DOI] [PubMed] [Google Scholar]
- 99.Wong D, Teixeira A, Oikonomopoulos S, Humburg P, Lone IN, Saliba D, et al. Extensive characterization of NF-kappaB binding uncovers non-canonical motifs and advances the interpretation of genetic functional traits. Genome biology. 2011;12:R70. doi: 10.1186/gb-2011-12-7-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tan P, Fuchs SY, Chen A, Wu K, Gomez C, Ronai Z, et al. Recruitment of a ROC1-CUL1 ubiquitin ligase by Skp1 and HOS to catalyze the ubiquitination of I kappa B alpha. Molecular cell. 1999;3:527–533. doi: 10.1016/s1097-2765(00)80481-5. [DOI] [PubMed] [Google Scholar]
- 101.Wu K, Fuchs SY, Chen A, Tan P, Gomez C, Ronai Z, et al. The SCF(HOS/beta-TRCP)-ROC1 E3 ubiquitin ligase utilizes two distinct domains within CUL1 for substrate targeting and ubiquitin ligation. Mol Cell Biol. 2000;20:1382–1393. doi: 10.1128/mcb.20.4.1382-1393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 103.Weigmann B, Lehr HA, Yancopoulos G, Valenzuela D, Murphy A, Stevens S, et al. The transcription factor NFATc2 controls IL-6-dependent T cell activation in experimental colitis. The Journal of experimental medicine. 2008;205:2099–2110. doi: 10.1084/jem.20072484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ling L, Cao Z, Goeddel DV. NF-kappaB-inducing kinase activates IKK-alpha by phosphorylation of Ser-176. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 106.Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H, et al. Differential regulation of IkappaB kinase alpha and beta by two upstream kinases, NF-kappaB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schmid JA, Birbach A. IkappaB kinase beta (IKKbeta/IKK2/IKBKB)--a key molecule in signaling to the transcription factor NF-kappaB. Cytokine & growth factor reviews. 2008;19:157–165. doi: 10.1016/j.cytogfr.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 108.Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-kappaB. Cell death and differentiation. 2006;13:759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- 109.Sun SC. Non-canonical NF-kappaB signaling pathway. Cell research. 2011;21:71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Molecular cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 111.Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, et al. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 112.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nature immunology. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 113.Disis ML. Immune regulation of cancer. J Clin Oncol. 2010;28:4531–4538. doi: 10.1200/JCO.2009.27.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Laveti D, Kumar M, Hemalatha R, Sistla R, Naidu VG, Talla V, et al. Anti-inflammatory treatments for chronic diseases: a review. Inflammation & allergy drug targets. 2013;12:349–361. doi: 10.2174/18715281113129990053. [DOI] [PubMed] [Google Scholar]
- 115.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochimica et biophysica acta. 2010;1799:775–787. doi: 10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xie TX, Xia Z, Zhang N, Gong W, Huang S. Constitutive NF-kappaB activity regulates the expression of VEGF and IL-8 and tumor angiogenesis of human glioblastoma. Oncology reports. 2010;23:725–732. [PubMed] [Google Scholar]
- 118.Yoshida A, Yoshida S, Ishibashi T, Kuwano M, Inomata H. Suppression of retinal neovascularization by the NF-kappaB inhibitor pyrrolidine dithiocarbamate in mice. Investigative ophthalmology & visual science. 1999;40:1624–1629. [PubMed] [Google Scholar]
- 119.Dong R, Wang Q, He XL, Chu YK, Lu JG, Ma QJ. Role of nuclear factor kappa B and reactive oxygen species in the tumor necrosis factor-alpha-induced epithelial-mesenchymal transition of MCF-7 cells. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica [et al] 2007;40:1071–1078. doi: 10.1590/s0100-879x2007000800007. [DOI] [PubMed] [Google Scholar]
- 120.Kumar S, Mehta K. Tissue transglutaminase constitutively activates HIF-1alpha promoter and nuclear factor-kappaB via a non-canonical pathway. PloS one. 2012;7:e49321. doi: 10.1371/journal.pone.0049321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.DeClerck K, Elble RC. The role of hypoxia and acidosis in promoting metastasis and resistance to chemotherapy. Frontiers in bioscience. 2010;15:213–225. doi: 10.2741/3616. [DOI] [PubMed] [Google Scholar]
- 122.Lavon I, Goldberg I, Amit S, Landsman L, Jung S, Tsuberi BZ, et al. High susceptibility to bacterial infection, but no liver dysfunction, in mice compromised for hepatocyte NF-kappaB activation. Nature medicine. 2000;6:573–577. doi: 10.1038/75057. [DOI] [PubMed] [Google Scholar]
- 123.Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pennica D, Nedwin GE, Hayflick JS, Seeburg PH, Derynck R, Palladino MA, et al. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984;312:724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- 125.Waters JP, Pober JS, Bradley JR. Tumour necrosis factor and cancer. The Journal of pathology. 2013;230:241–248. doi: 10.1002/path.4188. [DOI] [PubMed] [Google Scholar]
- 126.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 127.Tartaglia LA, Goeddel DV. Two TNF receptors. Immunology today. 1992;13:151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- 128.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nature reviews Immunology. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 129.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 130.Takada H, Chen NJ, Mirtsos C, Suzuki S, Suzuki N, Wakeham A, et al. Role of SODD in regulation of tumor necrosis factor responses. Mol Cell Biol. 2003;23:4026–4033. doi: 10.1128/MCB.23.11.4026-4033.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rath PC, Aggarwal BB. TNF-induced signaling in apoptosis. Journal of clinical immunology. 1999;19:350–364. doi: 10.1023/a:1020546615229. [DOI] [PubMed] [Google Scholar]
- 132.Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543–8567. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- 133.Karin M, Gallagher E. TNFR signaling: ubiquitin-conjugated TRAFfic signals control stop-andgo for MAPK signaling complexes. Immunological reviews. 2009;228:225–240. doi: 10.1111/j.1600-065X.2008.00755.x. [DOI] [PubMed] [Google Scholar]
- 134.Natoli G, Costanzo A, Ianni A, Templeton DJ, Woodgett JR, Balsano C, et al. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science. 1997;275:200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- 135.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 136.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Archiv : an international journal of pathology. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 137.Lin A, Karin M. NF-kappaB in cancer: a marked target. Semin Cancer Biol. 2003;13:107–114. doi: 10.1016/s1044-579x(02)00128-1. [DOI] [PubMed] [Google Scholar]
- 138.Orlowski RZ, Baldwin AS., Jr NF-kappaB as a therapeutic target in cancer. Trends in molecular medicine. 2002;8:385–389. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- 139.Lee KY, Chang W, Qiu D, Kao PN, Rosen GD. PG490 (triptolide) cooperates with tumor necrosis factor-alpha to induce apoptosis in tumor cells. J Biol Chem. 1999;274:13451–13455. doi: 10.1074/jbc.274.19.13451. [DOI] [PubMed] [Google Scholar]
- 140.Ganster RW, Taylor BS, Shao L, Geller DA. Complex regulation of human inducible nitric oxide synthase gene transcription by Stat 1 and NF-kappa B. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8638–8643. doi: 10.1073/pnas.151239498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Binder C, Schulz M, Hiddemann W, Oellerich M. Induction of inducible nitric oxide synthase is an essential part of tumor necrosis factor-alpha-induced apoptosis in MCF-7 and other epithelial tumor cells. Laboratory investigation; a journal of technical methods and pathology. 1999;79:1703–1712. [PubMed] [Google Scholar]
- 142.Delhalle S, Deregowski V, Benoit V, Merville MP, Bours V. NF-kappaB-dependent MnSOD expression protects adenocarcinoma cells from TNF-alpha-induced apoptosis. Oncogene. 2002;21:3917–3924. doi: 10.1038/sj.onc.1205489. [DOI] [PubMed] [Google Scholar]
- 143.Resch U, Cuapio A, Sturtzel C, Hofer E, de Martin R, Holper-Schichl YM. Polyubiquitinated Tristetraprolin protects from TNF-induced, Caspase-mediated Apoptosis. J Biol Chem. 2014 doi: 10.1074/jbc.M114.563312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Havell EA, Fiers W, North RJ. The antitumor function of tumor necrosis factor (TNF), I. Therapeutic action of TNF against an established murine sarcoma is indirect, immunologically dependent, and limited by severe toxicity. The Journal of experimental medicine. 1988;167:1067–1085. doi: 10.1084/jem.167.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer metastasis reviews. 2006;25:409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 146.Woo CH, Eom YW, Yoo MH, You HJ, Han HJ, Song WK, et al. Tumor necrosis factor-alpha generates reactive oxygen species via a cytosolic phospholipase A2-linked cascade. J Biol Chem. 2000;275:32357–32362. doi: 10.1074/jbc.M005638200. [DOI] [PubMed] [Google Scholar]
- 147.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 148.Xia L, Mo P, Huang W, Zhang L, Wang Y, Zhu H, et al. The TNF-alpha/ROS/HIF-1-induced upregulation of FoxMI expression promotes HCC proliferation and resistance to apoptosis. Carcinogenesis. 2012;33:2250–2259. doi: 10.1093/carcin/bgs249. [DOI] [PubMed] [Google Scholar]
- 149.Oshima H, Ishikawa T, Yoshida GJ, Naoi K, Maeda Y, Naka K, et al. TNF-alpha/TNFR1 signaling promotes gastric tumorigenesis through induction of Noxo1 and Gna14 in tumor cells. Oncogene. 2014;33:3820–3829. doi: 10.1038/onc.2013.356. [DOI] [PubMed] [Google Scholar]
- 150.Watanabe T, Takahashi A, Suzuki K, Kurusu-Kanno M, Yamaguchi K, Fujiki H, et al. Epithelial-mesenchymal transition in human gastric cancer cell lines induced by TNF-alpha-inducing protein of Helicobacter pylori. Int J Cancer. 2014;134:2373–2382. doi: 10.1002/ijc.28582. [DOI] [PubMed] [Google Scholar]
- 151.Orosz P, Echtenacher B, Falk W, Ruschoff J, Weber D, Mannel DN. Enhancement of experimental metastasis by tumor necrosis factor. The Journal of experimental medicine. 1993;177:1391–1398. doi: 10.1084/jem.177.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, et al. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10662–10667. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fajardo LF, Kwan HH, Kowalski J, Prionas SD, Allison AC. Dual role of tumor necrosis factor-alpha in angiogenesis. The American journal of pathology. 1992;140:539–544. [PMC free article] [PubMed] [Google Scholar]
- 155.Weichselbaum RR, Kufe DW, Hellman S, Rasmussen HS, King CR, Fischer PH, et al. Radiation-induced tumour necrosis factor-alpha expression: clinical application of transcriptional and physical targeting of gene therapy. Lancet Oncol. 2002;3:665–671. doi: 10.1016/s1470-2045(02)00900-2. [DOI] [PubMed] [Google Scholar]
- 156.Yoshida S, Ono M, Shono T, Izumi H, Ishibashi T, Suzuki H, et al. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol Cell Biol. 1997;17:4015–4023. doi: 10.1128/mcb.17.7.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li B, Vincent A, Cates J, Brantley-Sieders DM, Polk DB, Young PP. Low levels of tumor necrosis factor alpha increase tumor growth by inducing an endothelial phenotype of monocytes recruited to the tumor site. Cancer Res. 2009;69:338–348. doi: 10.1158/0008-5472.CAN-08-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kulbe H, Thompson R, Wilson JL, Robinson S, Hagemann T, Fatah R, et al. The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res. 2007;67:585–592. doi: 10.1158/0008-5472.CAN-06-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Feldmann M, Elliott MJ, Woody JN, Maini RN. Anti-tumor necrosis factor-alpha therapy of rheumatoid arthritis. Advances in immunology. 1997;64:283–350. doi: 10.1016/s0065-2776(08)60891-3. [DOI] [PubMed] [Google Scholar]
- 160.Moreland LW, Baumgartner SW, Schiff MH, Tindall EA, Fleischmann RM, Weaver AL, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337:141–147. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- 161.Wiens A, Correr CJ, Venson R, Otuki MF, Pontarolo R. A systematic review and meta-analysis of the efficacy and safety of adalimumab for treating rheumatoid arthritis. Rheumatology international. 2010;30:1063–1070. doi: 10.1007/s00296-009-1111-4. [DOI] [PubMed] [Google Scholar]
- 162.Keystone EC, Genovese MC, Klareskog L, Hsia EC, Hall ST, Miranda PC, et al. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Annals of the rheumatic diseases. 2009;68:789–796. doi: 10.1136/ard.2008.099010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nesbitt A, Fossati G, Bergin M, Stephens P, Stephens S, Foulkes R, et al. Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor alpha agents. Inflammatory bowel diseases. 2007;13:1323–1332. doi: 10.1002/ibd.20225. [DOI] [PubMed] [Google Scholar]
- 164.Targownik LE, Bernstein CN. Infectious and malignant complications of TNF inhibitor therapy in IBD. The American journal of gastroenterology. 2013;108:1835–1842. doi: 10.1038/ajg.2013.294. quiz 43. [DOI] [PubMed] [Google Scholar]
- 165.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 166.Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Archives of internal medicine. 2005;165:2337–2344. doi: 10.1001/archinte.165.20.2337. [DOI] [PubMed] [Google Scholar]
- 167.Mariette X, Tubach F, Bagheri H, Bardet M, Berthelot JM, Gaudin P, et al. Lymphoma in patients treated with anti-TNF: results of the 3-year prospective French RATIO registry. Annals of the rheumatic diseases. 2010;69:400–408. doi: 10.1136/ard.2009.117762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Askling J, Baecklund E, Granath F, Geborek P, Fored M, Backlin C, et al. Anti-tumour necrosis factor therapy in rheumatoid arthritis and risk of malignant lymphomas: relative risks and time trends in the Swedish Biologics Register. Annals of the rheumatic diseases. 2009;68:648–653. doi: 10.1136/ard.2007.085852. [DOI] [PubMed] [Google Scholar]
- 169.Pallavicini FB, Caporali R, Sarzi-Puttini P, Atzeni F, Bazzani C, Gorla R, et al. Tumour necrosis factor antagonist therapy and cancer development: analysis of the LORHEN registry. Autoimmunity reviews. 2010;9:175–180. doi: 10.1016/j.autrev.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 170.Mann J, Thomson P, Stevens H, Palamaras I. Malignant melanoma and tumor necrosis factor-alpha inhibitors: a case report and review of the literature. International journal of dermatology. 2013;52:471–474. doi: 10.1111/j.1365-4632.2011.05336.x. [DOI] [PubMed] [Google Scholar]
- 171.Williams GM. Antitumor necrosis factor-alpha therapy and potential cancer inhibition. Eur J Cancer Prev. 2008;17:169–177. doi: 10.1097/CEJ.0b013e3282b6fcff. [DOI] [PubMed] [Google Scholar]
- 172.Bertazza L, Mocellin S. The dual role of tumor necrosis factor (TNF) in cancer biology. Curr Med Chem. 2010;17:3337–3352. doi: 10.2174/092986710793176339. [DOI] [PubMed] [Google Scholar]
- 173.Doi K, Akaike T, Horie H, Noguchi Y, Fujii S, Beppu T, et al. Excessive production of nitric oxide in rat solid tumor and its implication in rapid tumor growth. Cancer. 1996;77:1598–1604. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1598::AID-CNCR27>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 174.Mutamba JT, Svilar D, Prasongtanakij S, Wang XH, Lin YC, Dedon PC, et al. XRCC1 and base excision repair balance in response to nitric oxide. DNA Repair (Amst) 2011;10:1282–1293. doi: 10.1016/j.dnarep.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Syapin PJ. Ethanol inhibition of inducible nitric oxide synthase activity in C6 glioma cells. Alcoholism, clinical and experimental research. 1995;19:262–267. doi: 10.1111/j.1530-0277.1995.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 176.Kurose I, Ebinuma H, Higuchi H, Yonei Y, Saito H, Kato S, et al. Nitric oxide mediates mitochondrial dysfunction in hepatoma cells induced by non-activated Kupffer cells: evidence implicating ICAM-1-dependent process. Journal of gastroenterology and hepatology. 1995;10(Suppl 1):S68–S71. doi: 10.1111/j.1440-1746.1995.tb01803.x. [DOI] [PubMed] [Google Scholar]
- 177.Kitajima I, Kawahara K, Nakajima T, Soejima Y, Matsuyama T, Maruyama I. Nitric oxide-mediated apoptosis in murine mastocytoma. Biochemical and biophysical research communications. 1994;204:244–251. doi: 10.1006/bbrc.1994.2451. [DOI] [PubMed] [Google Scholar]
- 178.Xie K, Huang S, Dong Z, Juang SH, Gutman M, Xie QW, et al. Transfection with the inducible nitric oxide synthase gene suppresses tumorigenicity and abrogates metastasis by K-1735 murine melanoma cells. The Journal of experimental medicine. 1995;181:1333–1343. doi: 10.1084/jem.181.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tamir S, deRojas-Walker T, Gal A, Weller AH, Li X, Fox JG, et al. Nitric oxide production in relation to spontaneous B-cell lymphoma and myositis in SJL mice. Cancer Res. 1995;55:4391–4397. [PubMed] [Google Scholar]
- 180.Ogura T, Esumi H. Nitric oxide synthase expression in human neuroblastoma cell line induced by cytokines. Neuroreport. 1996;7:853–856. doi: 10.1097/00001756-199603220-00003. [DOI] [PubMed] [Google Scholar]
- 181.Orucevic A, Bechberger J, Green AM, Shapiro RA, Billiar TR, Lala PK. Nitric-oxide production by murine mammary adenocarcinoma cells promotes tumor-cell invasiveness. Int J Cancer. 1999;81:889–896. doi: 10.1002/(sici)1097-0215(19990611)81:6<889::aid-ijc9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 182.Rieder J, Jahnke R, Schloesser M, Seibel M, Czechowski M, Marth C, et al. Nitric oxide-dependent apoptosis in ovarian carcinoma cell lines. Gynecologic oncology. 2001;82:172–176. doi: 10.1006/gyno.2001.6242. [DOI] [PubMed] [Google Scholar]
- 183.Ellie E, Loiseau H, Lafond F, Arsaut J, Demotes-Mainard J. Differential expression of inducible nitric oxide synthase mRNA in human brain tumours. Neuroreport. 1995;7:294–296. [PubMed] [Google Scholar]
- 184.Hara E, Takahashi K, Tominaga T, Kumabe T, Kayama T, Suzuki H, et al. Expression of heme oxygenase and inducible nitric oxide synthase mRNA in human brain tumors. Biochemical and biophysical research communications. 1996;224:153–158. doi: 10.1006/bbrc.1996.0999. [DOI] [PubMed] [Google Scholar]
- 185.Klotz T, Bloch W, Volberg C, Engelmann U, Addicks K. Selective expression of inducible nitric oxide synthase in human prostate carcinoma. Cancer. 1998;82:1897–1903. [PubMed] [Google Scholar]
- 186.Wilson KT, Fu S, Ramanujam KS, Meltzer SJ. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett's esophagus and associated adenocarcinomas. Cancer Res. 1998;58:2929–2934. [PubMed] [Google Scholar]
- 187.Zhao H, Dugas N, Mathiot C, Delmer A, Dugas B, Sigaux F, et al. B-cell chronic lymphocytic leukemia cells express a functional inducible nitric oxide synthase displaying anti-apoptotic activity. Blood. 1998;92:1031–1043. [PubMed] [Google Scholar]
- 188.Liu CY, Wang CH, Chen TC, Lin HC, Yu CT, Kuo HP. Increased level of exhaled nitric oxide and up-regulation of inducible nitric oxide synthase in patients with primary lung cancer. British journal of cancer. 1998;78:534–541. doi: 10.1038/bjc.1998.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Swana HS, Smith SD, Perrotta PL, Saito N, Wheeler MA, Weiss RM. Inducible nitric oxide synthase with transitional cell carcinoma of the bladder. The Journal of urology. 1999;161:630–634. [PubMed] [Google Scholar]
- 190.Vickers SM, MacMillan-Crow LA, Green M, Ellis C, Thompson JA. Association of increased immunostaining for inducible nitric oxide synthase and nitrotyrosine with fibroblast growth factor transformation in pancreatic cancer. Archives of surgery. 1999;134:245–251. doi: 10.1001/archsurg.134.3.245. [DOI] [PubMed] [Google Scholar]
- 191.Kitano H, Kitanishi T, Nakanishi Y, Suzuki M, Takeuchi E, Yazawa Y, et al. Expression of inducible nitric oxide synthase in human thyroid papillary carcinomas. Thyroid : official journal of the American Thyroid Association. 1999;9:113–117. doi: 10.1089/thy.1999.9.113. [DOI] [PubMed] [Google Scholar]
- 192.Chen YK, Hsue SS, Lin LM. Increased expression of inducible nitric oxide synthase for human buccal squamous-cell carcinomas: immunohistochemical, reverse transcription-polymerase chain reaction (RT-PCR) and in situ RT-PCR studies. Head & neck. 2002;24:925–932. doi: 10.1002/hed.10131. [DOI] [PubMed] [Google Scholar]
- 193.Tschugguel W, Pustelnik T, Lass H, Mildner M, Weninger W, Schneeberger C, et al. Inducible nitric oxide synthase (iNOS) expression may predict distant metastasis in human melanoma. British journal of cancer. 1999;79:1609–1612. doi: 10.1038/sj.bjc.6690256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kojima M, Morisaki T, Tsukahara Y, Uchiyama A, Matsunari Y, Mibu R, et al. Nitric oxide synthase expression and nitric oxide production in human colon carcinoma tissue. Journal of surgical oncology. 1999;70:222–229. doi: 10.1002/(sici)1096-9098(199904)70:4<222::aid-jso5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 195.Goto T, Haruma K, Kitadai Y, Ito M, Yoshihara M, Sumii K, et al. Enhanced expression of inducible nitric oxide synthase and nitrotyrosine in gastric mucosa of gastric cancer patients. Clin Cancer Res. 1999;5:1411–1415. [PubMed] [Google Scholar]
- 196.Tschugguel W, Schneeberger C, Unfried G, Czerwenka K, Weninger W, Mildner M, et al. Expression of inducible nitric oxide synthase in human breast cancer depends on tumor grade. Breast Cancer Res Treat. 1999;56:145–151. doi: 10.1023/a:1006288526311. [DOI] [PubMed] [Google Scholar]
- 197.Koh E, Noh SH, Lee YD, Lee HY, Han JW, Lee HW, et al. Differential expression of nitric oxide synthase in human stomach cancer. Cancer letters. 1999;146:173–180. doi: 10.1016/s0304-3835(99)00265-7. [DOI] [PubMed] [Google Scholar]
- 198.Soini Y, Kahlos K, Puhakka A, Lakari E, Saily M, Paakko P, et al. Expression of inducible nitric oxide synthase in healthy pleura and in malignant mesothelioma. British journal of cancer. 2000;83:880–886. doi: 10.1054/bjoc.2000.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rahman MA, Dhar DK, Yamaguchi E, Maruyama S, Sato T, Hayashi H, et al. Coexpression of inducible nitric oxide synthase and COX-2 in hepatocellular carcinoma and surrounding liver: possible involvement of COX-2 in the angiogenesis of hepatitis C virus-positive cases. Clin Cancer Res. 2001;7:1325–1332. [PubMed] [Google Scholar]
- 200.Anttila MA, Voutilainen K, Merivalo S, Saarikoski S, Kosma VM. Prognostic significance of iNOS in epithelial ovarian cancer. Gynecologic oncology. 2007;105:97–103. doi: 10.1016/j.ygyno.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 201.Bentz BG, Hammer ND, Radosevich JA, Haines GK., 3rd Nitrosative stress induces DNA strand breaks but not caspase mediated apoptosis in a lung cancer cell line. Journal of carcinogenesis. 2004;3:16. doi: 10.1186/1477-3163-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cheng H, Wang L, Mollica M, Re AT, Wu S, Zuo L. Nitric oxide in cancer metastasis. Cancer letters. 2014 doi: 10.1016/j.canlet.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Luanpitpong S, Iyer AK, Azad N, Wang L, Rojanasakul Y. Nitrosothiol Signaling in Anoikis Resistance and Cancer Metastasis. Forum on immunopathological diseases and therapeutics. 2012;3:141–154. doi: 10.1615/ForumImmunDisTher.2012006115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Thomsen LL, Miles DW. Role of nitric oxide in tumour progression: lessons from human tumours. Cancer metastasis reviews. 1998;17:107–118. doi: 10.1023/a:1005912906436. [DOI] [PubMed] [Google Scholar]
- 205.Jadeski LC, Lala PK. Nitric oxide synthase inhibition by N(G)-nitro-L-arginine methyl ester inhibits tumor-induced angiogenesis in mammary tumors. The American journal of pathology. 1999;155:1381–1390. doi: 10.1016/S0002-9440(10)65240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hajri A, Metzger E, Vallat F, Coffy S, Flatter E, Evrard S, et al. Role of nitric oxide in pancreatic tumour growth: in vivo and in vitro studies. British journal of cancer. 1998;78:841–849. doi: 10.1038/bjc.1998.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Feinstein DL, Galea E, Cermak J, Chugh P, Lyandvert L, Reis DJ. Nitric oxide synthase expression in glial cells: suppression by tyrosine kinase inhibitors. Journal of neurochemistry. 1994;62:811–814. doi: 10.1046/j.1471-4159.1994.62020811.x. [DOI] [PubMed] [Google Scholar]
- 208.Murata J, Corradin SB, Felley-Bosco E, Juillerat-Jeanneret L. Involvement of a transforming-growth-factor-beta-like molecule in tumor-cell-derived inhibition of nitric-oxide synthesis in cerebral endothelial cells. Int J Cancer. 1995;62:743–748. doi: 10.1002/ijc.2910620616. [DOI] [PubMed] [Google Scholar]
- 209.Nishiya T, Uehara T, Nomura Y. Herbimycin A suppresses NF-kappa B activation and tyrosine phosphorylation of JAK2 and the subsequent induction of nitric oxide synthase in C6 glioma cells. FEBS Lett. 1995;371:333–336. doi: 10.1016/0014-5793(95)00933-z. [DOI] [PubMed] [Google Scholar]
- 210.Park SK, Murphy S. Duration of expression of inducible nitric oxide synthase in glial cells. Journal of neuroscience research. 1994;39:405–411. doi: 10.1002/jnr.490390407. [DOI] [PubMed] [Google Scholar]
- 211.Fujisawa H, Ogura T, Hokari A, Weisz A, Yamashita J, Esumi H. Inducible nitric oxide synthase in a human glioblastoma cell line. Journal of neurochemistry. 1995;64:85–91. doi: 10.1046/j.1471-4159.1995.64010085.x. [DOI] [PubMed] [Google Scholar]
- 212.Nishiya T, Uehara T, Edamatsu H, Kaziro Y, Itoh H, Nomura Y. Activation of Stat1 and subsequent transcription of inducible nitric oxide synthase gene in C6 glioma cells is independent of interferon-gamma-induced MAPK activation that is mediated by p21ras. FEBS Lett. 1997;408:33–38. doi: 10.1016/s0014-5793(97)00383-9. [DOI] [PubMed] [Google Scholar]
- 213.Rao CV, Indranie C, Simi B, Manning PT, Connor JR, Reddy BS. Chemopreventive properties of a selective inducible nitric oxide synthase inhibitor in colon carcinogenesis, administered alone or in combination with celecoxib, a selective cyclooxygenase-2 inhibitor. Cancer Res. 2002;62:165–170. [PubMed] [Google Scholar]
- 214.Giliano NY, Konevega LV, Noskin LA. Dynamics of intracellular superoxide and NO content in human endotheliocytes and carcinoma cells after treatment with NO synthase inhibitors. Bulletin of experimental biology and medicine. 2010;149:78–81. doi: 10.1007/s10517-010-0880-9. [DOI] [PubMed] [Google Scholar]
- 215.Mohamad NA, Cricco GP, Sambuco LA, Croci M, Medina VA, Gutierrez AS, et al. Aminoguanidine impedes human pancreatic tumor growth and metastasis development in nude mice. World journal of gastroenterology : WJG. 2009;15:1065–1071. doi: 10.3748/wjg.15.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Panis C, Victorino VJ, Herrera AC, Freitas LF, De Rossi T, Campos FC, et al. Differential oxidative status and immune characterization of the early and advanced stages of human breast cancer. Breast Cancer Res Treat. 2012;133:881–888. doi: 10.1007/s10549-011-1851-1. [DOI] [PubMed] [Google Scholar]
- 217.Hamed EA, Zakhary MM, Maximous DW. Apoptosis, angiogenesis, inflammation, and oxidative stress: basic interactions in patients with early and metastatic breast cancer. Journal of cancer research and clinical oncology. 2012;138:999–1009. doi: 10.1007/s00432-012-1176-4. [DOI] [PubMed] [Google Scholar]
- 218.Herrera AC, Panis C, Victorino VJ, Campos FC, Colado-Simao AN, Cecchini AL, et al. Molecular subtype is determinant on inflammatory status and immunological profile from invasive breast cancer patients. Cancer Immunol Immunother. 2012;61:2193–2201. doi: 10.1007/s00262-012-1283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gaballah HE, Abdel Salam I, Abdel Wahab N, Mansour OM. Plasma bcl-2 and nitric oxide: possible prognostic role in patients with metastatic breast cancer. Medical oncology (Northwood, London, England) 2001;18:171–178. doi: 10.1385/mo:18:3:171. [DOI] [PubMed] [Google Scholar]
- 220.Hansel TT, Kharitonov SA, Donnelly LE, Erin EM, Currie MG, Moore WM, et al. A selective inhibitor of inducible nitric oxide synthase inhibits exhaled breath nitric oxide in healthy volunteers and asthmatics. FASEB J. 2003;17:1298–1300. doi: 10.1096/fj.02-0633fje. [DOI] [PubMed] [Google Scholar]
- 221.Brindicci C, Ito K, Barnes PJ, Kharitonov SA. Effect of an inducible nitric oxide synthase inhibitor on differential flow-exhaled nitric oxide in asthmatic patients and healthy volunteers. Chest. 2007;132:581–588. doi: 10.1378/chest.06-3046. [DOI] [PubMed] [Google Scholar]
- 222.Brindicci C, Ito K, Torre O, Barnes PJ, Kharitonov SA. Effects of aminoguanidine, an inhibitor of inducible nitric oxide synthase, on nitric oxide production and its metabolites in healthy control subjects, healthy smokers, and COPD patients. Chest. 2009;135:353–367. doi: 10.1378/chest.08-0964. [DOI] [PubMed] [Google Scholar]
- 223.Cobb JP, Hotchkiss RS, Swanson PE, Chang K, Qiu Y, Laubach VE, et al. Inducible nitric oxide synthase (iNOS) gene deficiency increases the mortality of sepsis in mice. Surgery. 1999;126:438–442. [PubMed] [Google Scholar]
- 224.Zhu GD, Gandhi VB, Gong J, Luo Y, Liu X, Shi Y, et al. Discovery and SAR of oxindole-pyridine-based protein kinase B/Akt inhibitors for treating cancers. Bioorganic & medicinal chemistry letters. 2006;16:3424–3429. doi: 10.1016/j.bmcl.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 225.Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 226.Coffer PJ, Woodgett JR. Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. European journal of biochemistry/FEBS. 1991;201:475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- 227.Jones PF, Jakubowicz T, Pitossi FJ, Maurer F, Hemmings BA. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Polivka J, Jr, Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacology & therapeutics. 2014;142:164–175. doi: 10.1016/j.pharmthera.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 229.Ma N, Jin J, Lu F, Woodgett J, Liu FF. The role of protein kinase B (PKB) in modulating heat sensitivity in a human breast cancer cell line. International journal of radiation oncology, biology, physics. 2001;50:1041–1050. doi: 10.1016/s0360-3016(01)01596-6. [DOI] [PubMed] [Google Scholar]
- 230.Ciruelos Gil EM. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer treatment reviews. 2014;40:862–871. doi: 10.1016/j.ctrv.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 231.Chen SF, Chen JZ. Development for anticancer therapy: small-molecule inhibitors targeting protein kinase B. Mini reviews in medicinal chemistry. 2013;13:1272–1294. doi: 10.2174/1389557511313090003. [DOI] [PubMed] [Google Scholar]
- 232.Hill MM, Hemmings BA. Inhibition of protein kinase B/Akt. implications for cancer therapy. Pharmacology & therapeutics. 2002;93:243–251. doi: 10.1016/s0163-7258(02)00193-6. [DOI] [PubMed] [Google Scholar]
- 233.Abraham AG, O'Neill E. PI3K/Akt-mediated regulation of p53 in cancer. Biochemical Society transactions. 2014;42:798–803. doi: 10.1042/BST20140070. [DOI] [PubMed] [Google Scholar]
- 234.Liu PP, Liao J, Tang ZJ, Wu WJ, Yang J, Zeng ZL, et al. Metabolic regulation of cancer cell side population by glucose through activation of the Akt pathway. Cell death and differentiation. 2014;21:124–135. doi: 10.1038/cdd.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Stambolic V, Woodgett JR. Functional distinctions of protein kinase B/Akt isoforms defined by their influence on cell migration. Trends in cell biology. 2006;16:461–466. doi: 10.1016/j.tcb.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 236.Noguchi M, Hirata N, Suizu F. The links between AKT and two intracellular proteolytic cascades: Ubiquitination and autophagy. Biochimica et biophysica acta. 2014 doi: 10.1016/j.bbcan.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 237.Lee YG, Lee J, Byeon SE, Yoo DS, Kim MH, Lee SY, et al. Functional role of Akt in macrophage-mediated innate immunity. Frontiers in bioscience. 2011;16:517–530. doi: 10.2741/3702. [DOI] [PubMed] [Google Scholar]
- 238.Rajaram MV, Ganesan LP, Parsa KV, Butchar JP, Gunn JS, Tridandapani S. Akt/Protein kinase B modulates macrophage inflammatory response to Francisella infection and confers a survival advantage in mice. J Immunol. 2006;177:6317–6324. doi: 10.4049/jimmunol.177.9.6317. [DOI] [PubMed] [Google Scholar]
- 239.Erasalo H, Laavola M, Hamalainen M, Leppanen T, Nieminen R, Moilanen E. PI3K Inhibitors LY294002 and IC87114 Reduce Inflammation in Carrageenan-Induced Paw Oedema and Down-Regulate Inflammatory Gene Expression in Activated Macrophages. Basic & clinical pharmacology & toxicology. 2014 doi: 10.1111/bcpt.12284. [DOI] [PubMed] [Google Scholar]
- 240.Xu P, Wang J, Yang ZW, Lou XL, Chen C. Regulatory roles of the PI3K/Akt signaling pathway in rats with severe acute pancreatitis. PloS one. 2013;8:e81767. doi: 10.1371/journal.pone.0081767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Abu-Eid R, Samara RN, Ozbun L, Abdalla MY, Berzofsky JA, Friedman KM, et al. Selective inhibition of regulatory T cells by targeting the PI3K-Akt pathway. Cancer immunology research. 2014;2:1080–1089. doi: 10.1158/2326-6066.CIR-14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Li H, Zeng J, Shen K. PI3K/AKT/mTOR signaling pathway as a therapeutic target for ovarian cancer. Archives of gynecology and obstetrics. 2014 doi: 10.1007/s00404-014-3377-3. [DOI] [PubMed] [Google Scholar]
- 243.Paplomata E, O'Regan R. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Therapeutic advances in medical oncology. 2014;6:154–166. doi: 10.1177/1758834014530023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Beck JT, Ismail A, Tolomeo C. Targeting the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway: An emerging treatment strategy for squamous cell lung carcinoma. Cancer treatment reviews. 2014;40:980–989. doi: 10.1016/j.ctrv.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 245.Houede N, Pourquier P. Targeting the genetic alterations of the PI3K-AKT-mTOR pathway: Its potential use in the treatment of bladder cancers. Pharmacology & therapeutics. 2014 doi: 10.1016/j.pharmthera.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 246.Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Frontiers in oncology. 2014;4:64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tan J, Yu Q. Molecular mechanisms of tumor resistance to PI3K-mTOR-targeted therapy. Chinese journal of cancer. 2013;32:376–379. doi: 10.5732/cjc.012.10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lee HJ, Song IC, Yun HJ, Jo DY, Kim S. CXC chemokines and chemokine receptors in gastric cancer: from basic findings towards therapeutic targeting. World journal of gastroenterology : WJG. 2014;20:1681–1693. doi: 10.3748/wjg.v20.i7.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, Bonecchi R. The chemokine system in cancer biology and therapy. Cytokine & growth factor reviews. 2010;21:27–39. doi: 10.1016/j.cytogfr.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 250.Balkwill FR. The chemokine system and cancer. The Journal of pathology. 2012;226:148–157. doi: 10.1002/path.3029. [DOI] [PubMed] [Google Scholar]
- 251.Verbeke H, Struyf S, Laureys G, Van Damme J. The expression and role of CXC chemokines in colorectal cancer. Cytokine & growth factor reviews. 2011;22:345–358. doi: 10.1016/j.cytogfr.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 252.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer letters. 2008;267:226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 253.Verbeke H, Geboes K, Van Damme J, Struyf S. The role of CXC chemokines in the transition of chronic inflammation to esophageal and gastric cancer. Biochimica et biophysica acta. 2012;1825:117–129. doi: 10.1016/j.bbcan.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 254.Bellocq A, Antoine M, Flahault A, Philippe C, Crestani B, Bernaudin JF, et al. Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. The American journal of pathology. 1998;152:83–92. [PMC free article] [PubMed] [Google Scholar]
- 255.Tazzyman S, Lewis CE, Murdoch C. Neutrophils: key mediators of tumour angiogenesis. International journal of experimental pathology. 2009;90:222–231. doi: 10.1111/j.1365-2613.2009.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Eck M, Schmausser B, Scheller K, Brandlein S, Muller-Hermelink HK. Pleiotropic effects of CXC chemokines in gastric carcinoma: differences in CXCL8 and CXCL1 expression between diffuse and intestinal types of gastric carcinoma. Clinical and experimental immunology. 2003;134:508–515. doi: 10.1111/j.1365-2249.2003.02305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Dhawan P, Richmond A. Role of CXCL1 in tumorigenesis of melanoma. Journal of leukocyte biology. 2002;72:9–18. [PMC free article] [PubMed] [Google Scholar]
- 258.Wislez M, Philippe C, Antoine M, Rabbe N, Moreau J, Bellocq A, et al. Upregulation of bronchioloalveolar carcinoma-derived C-X-C chemokines by tumor infiltrating inflammatory cells. Inflamm Res. 2004;53:4–12. doi: 10.1007/s00011-003-1215-3. [DOI] [PubMed] [Google Scholar]
- 259.Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, et al. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. The Journal of experimental medicine. 1999;190:1417–1426. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hojo S, Koizumi K, Tsuneyama K, Arita Y, Cui Z, Shinohara K, et al. High-level expression of chemokine CXCL16 by tumor cells correlates with a good prognosis and increased tumor-infiltrating lymphocytes in colorectal cancer. Cancer Res. 2007;67:4725–4731. doi: 10.1158/0008-5472.CAN-06-3424. [DOI] [PubMed] [Google Scholar]
- 261.Scarpino S, Stoppacciaro A, Ballerini F, Marchesi M, Prat M, Stella MC, et al. Papillary carcinoma of the thyroid: hepatocyte growth factor (HGF) stimulates tumor cells to release chemokines active in recruiting dendritic cells. The American journal of pathology. 2000;156:831–837. doi: 10.1016/S0002-9440(10)64951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577–583. [PubMed] [Google Scholar]
- 263.Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, et al. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung cancer. 2002;35:23–28. doi: 10.1016/s0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 264.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 265.Salazar N, Castellan M, Shirodkar SS, Lokeshwar BL. Chemokines and chemokine receptors as promoters of prostate cancer growth and progression. Critical reviews in eukaryotic gene expression. 2013;23:77–91. doi: 10.1615/critreveukaryotgeneexpr.2013006905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Murphy C, McGurk M, Pettigrew J, Santinelli A, Mazzucchelli R, Johnston PG, et al. Nonapical and cytoplasmic expression of interleukin-8, CXCR1, and CXCR2 correlates with cell proliferation and microvessel density in prostate cancer. Clin Cancer Res. 2005;11:4117–4127. doi: 10.1158/1078-0432.CCR-04-1518. [DOI] [PubMed] [Google Scholar]
- 267.Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends in molecular medicine. 2010;16:133–144. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Singh RK, Lokeshwar BL. Depletion of intrinsic expression of Interleukin-8 in prostate cancer cells causes cell cycle arrest, spontaneous apoptosis and increases the efficacy of chemotherapeutic drugs. Molecular cancer. 2009;8:57. doi: 10.1186/1476-4598-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. The Journal of experimental medicine. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Goldmann T, Dromann D, Radtke J, Marwitz S, Lang DS, Schultz H, et al. CXCR7 transcription in human non-small cell lung cancer and tumor-free lung tissues; possible regulation upon chemotherapy. Virchows Archiv : an international journal of pathology. 2008;452:347–348. doi: 10.1007/s00428-008-0579-8. [DOI] [PubMed] [Google Scholar]
- 271.Wang J, Shiozawa Y, Wang Y, Jung Y, Pienta KJ, Mehra R, et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283:4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 272.Singh RK, Lokeshwar BL. The IL-8-regulated chemokine receptor CXCR7 stimulates EGFR signaling to promote prostate cancer growth. Cancer Res. 2011;71:3268–3277. doi: 10.1158/0008-5472.CAN-10-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Singh S, Sadanandam A, Nannuru KC, Varney ML, Mayer-Ezell R, Bond R, et al. Small-molecule antagonists for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing tumor cell proliferation, survival, and angiogenesis. Clin Cancer Res. 2009;15:2380–2386. doi: 10.1158/1078-0432.CCR-08-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Inoue K, Wood CG, Slaton JW, Karashima T, Sweeney P, Dinney CP. Adenoviral-mediated gene therapy of human bladder cancer with antisense interleukin-8. Oncology reports. 2001;8:955–964. doi: 10.3892/or.8.5.955. [DOI] [PubMed] [Google Scholar]
- 275.Shi Q, Abbruzzese JL, Huang S, Fidler IJ, Xiong Q, Xie K. Constitutive and inducible interleukin 8 expression by hypoxia and acidosis renders human pancreatic cancer cells more tumorigenic and metastatic. Clin Cancer Res. 1999;5:3711–3721. [PubMed] [Google Scholar]
- 276.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. The Journal of clinical investigation. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Korkaya H, Liu S, Wicha MS. Regulation of cancer stem cells by cytokine networks: attacking cancer's inflammatory roots. Clin Cancer Res. 2011;17:6125–6129. doi: 10.1158/1078-0432.CCR-10-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, Jain RK. CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clin Cancer Res. 2011;17:2074–2080. doi: 10.1158/1078-0432.CCR-10-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Schols D, Struyf S, Van Damme J, Este JA, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. The Journal of experimental medicine. 1997;186:1383–1388. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Gil M, Seshadri M, Komorowski MP, Abrams SI, Kozbor D. Targeting CXCL12/CXCR4 signaling with oncolytic virotherapy disrupts tumor vasculature and inhibits breast cancer metastases. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E1291–E1300. doi: 10.1073/pnas.1220580110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Drenckhan A, Kurschat N, Dohrmann T, Raabe N, Koenig AM, Reichelt U, et al. Effective inhibition of metastases and primary tumor growth with CTCE-9908 in esophageal cancer. The Journal of surgical research. 2013;182:250–256. doi: 10.1016/j.jss.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 282.Wong D, Kandagatla P, Korz W, Chinni SR. Targeting CXCR4 with CTCE-9908 inhibits prostate tumor metastasis. BMC urology. 2014;14:12. doi: 10.1186/1471-2490-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.de Nigris F, Schiano C, Infante T, Napoli C. CXCR4 inhibitors: tumor vasculature and therapeutic challenges. Recent patents on anti-cancer drug discovery. 2012;7:251–264. doi: 10.2174/157489212801820039. [DOI] [PubMed] [Google Scholar]
- 284.Wang Y, Chen Y, Wang J, Chen J, Aggarwal BB, Pang X, et al. Xanthohumol, a prenylated chalcone derived from hops, suppresses cancer cell invasion through inhibiting the expression of CXCR4 chemokine receptor. Current molecular medicine. 2012;12:153–162. doi: 10.2174/156652412798889072. [DOI] [PubMed] [Google Scholar]
- 285.Hattermann K, Held-Feindt J, Lucius R, Muerkoster SS, Penfold ME, Schall TJ, et al. The chemokine receptor CXCR7 is highly expressed in human glioma cells and mediates antiapoptotic effects. Cancer Res. 2010;70:3299–3308. doi: 10.1158/0008-5472.CAN-09-3642. [DOI] [PubMed] [Google Scholar]
- 286.Jin Z, Nagakubo D, Shirakawa AK, Nakayama T, Shigeta A, Hieshima K, et al. CXCR7 is inducible by HTLV-1 Tax and promotes growth and survival of HTLV-1-infected T cells. Int J Cancer. 2009;125:2229–2235. doi: 10.1002/ijc.24612. [DOI] [PubMed] [Google Scholar]
- 287.Odemis V, Boosmann K, Heinen A, Kury P, Engele J. CXCR7 is an active component of SDF-1 signalling in astrocytes and Schwann cells. Journal of cell science. 2010;123:1081–1088. doi: 10.1242/jcs.062810. [DOI] [PubMed] [Google Scholar]
- 288.Rubin JB, Kung AL, Klein RS, Chan JA, Sun Y, Schmidt K, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Yoon Y, Liang Z, Zhang X, Choe M, Zhu A, Cho HT, et al. CXC chemokine receptor-4 antagonist blocks both growth of primary tumor and metastasis of head and neck cancer in xenograft mouse models. Cancer Res. 2007;67:7518–7524. doi: 10.1158/0008-5472.CAN-06-2263. [DOI] [PubMed] [Google Scholar]
- 290.Juarez J, Bradstock KF, Gottlieb DJ, Bendall LJ. Effects of inhibitors of the chemokine receptor CXCR4 on acute lymphoblastic leukemia cells in vitro. Leukemia. 2003;17:1294–1300. doi: 10.1038/sj.leu.2402998. [DOI] [PubMed] [Google Scholar]
- 291.Tamamura H, Hori A, Kanzaki N, Hiramatsu K, Mizumoto M, Nakashima H, et al. T140 analogs as CXCR4 antagonists identified as anti-metastatic agents in the treatment of breast cancer. FEBS Lett. 2003;550:79–83. doi: 10.1016/s0014-5793(03)00824-x. [DOI] [PubMed] [Google Scholar]
- 292.Addison CL, Arenberg DA, Morris SB, Xue YY, Burdick MD, Mulligan MS, et al. The CXC chemokine, monokine induced by interferon-gamma, inhibits non-small cell lung carcinoma tumor growth and metastasis. Human gene therapy. 2000;11:247–261. doi: 10.1089/10430340050015996. [DOI] [PubMed] [Google Scholar]
- 293.Struyf S, Burdick MD, Peeters E, Van den Broeck K, Dillen C, Proost P, et al. Platelet factor-4 variant chemokine CXCL4L1 inhibits melanoma and lung carcinoma growth and metastasis by preventing angiogenesis. Cancer Res. 2007;67:5940–5948. doi: 10.1158/0008-5472.CAN-06-4682. [DOI] [PubMed] [Google Scholar]
- 294.Giese NA, Raykov Z, DeMartino L, Vecchi A, Sozzani S, Dinsart C, et al. Suppression of metastatic hemangiosarcoma by a parvovirus MVMp vector transducing the IP-10 chemokine into immunocompetent mice. Cancer gene therapy. 2002;9:432–442. doi: 10.1038/sj.cgt.7700457. [DOI] [PubMed] [Google Scholar]
- 295.Narvaiza I, Mazzolini G, Barajas M, Duarte M, Zaratiegui M, Qian C, et al. Intratumoral coinjection of two adenoviruses, one encoding the chemokine IFN-gamma-inducible protein-10 and another encoding IL-12, results in marked antitumoral synergy. J Immunol. 2000;164:3112–3122. doi: 10.4049/jimmunol.164.6.3112. [DOI] [PubMed] [Google Scholar]
- 296.Huang J, Li C, Wang Y, Lv H, Guo Y, Dai H, et al. Cytokine-induced killer (CIK) cells bound with anti-CD3/anti-CD133 bispecific antibodies target CD133(high) cancer stem cells in vitro and in vivo. Clinical immunology (Orlando, Fla. 2013;149:156–168. doi: 10.1016/j.clim.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 297.Borsig L, Wolf MJ, Roblek M, Lorentzen A, Heikenwalder M. Inflammatory chemokines and metastasis--tracing the accessory. Oncogene. 2014;33:3217–3224. doi: 10.1038/onc.2013.272. [DOI] [PubMed] [Google Scholar]
- 298.Yoshie O. Chemokine receptors as therapeutic targets. Nihon Rinsho Men'eki Gakkai kaishi = Japanese journal of clinical immunology. 2013;36:189–196. doi: 10.2177/jsci.36.189. [DOI] [PubMed] [Google Scholar]
- 299.Mukaida N, Sasaki S, Baba T. Chemokines in cancer development and progression and their potential as targeting molecules for cancer treatment. Mediators of inflammation. 2014;2014:170381. doi: 10.1155/2014/170381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ma Y, Adjemian S, Galluzzi L, Zitvogel L, Kroemer G. Chemokines and chemokine receptors required for optimal responses to anticancer chemotherapy. Oncoimmunology. 2014;3:e27663. doi: 10.4161/onci.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Bobanga ID, Petrosiute A, Huang AY. Chemokines as Cancer Vaccine Adjuvants. Vaccines. 2013;1:444–462. doi: 10.3390/vaccines1040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Franciszkiewicz K, Boissonnas A, Boutet M, Combadiere C, Mami-Chouaib F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res. 2012;72:6325–6332. doi: 10.1158/0008-5472.CAN-12-2027. [DOI] [PubMed] [Google Scholar]
- 303.Krieg C, Boyman O. The role of chemokines in cancer immune surveillance by the adaptive immune system. Semin Cancer Biol. 2009;19:76–83. doi: 10.1016/j.semcancer.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 304.Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 305.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 306.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 307.Burn J, Gerdes AM, Macrae F, Mecklin JP, Moeslein G, Olschwang S, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081–2087. doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Burn J, Bishop DT, Chapman PD, Elliott F, Bertario L, Dunlop MG, et al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer prevention research. 2011;4:655–665. doi: 10.1158/1940-6207.CAPR-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kim YS, Young MR, Bobe G, Colburn NH, Milner JA. Bioactive food components, inflammatory targets, and cancer prevention. Cancer prevention research. 2009;2:200–208. doi: 10.1158/1940-6207.CAPR-08-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Pan MH, Lai CS, Dushenkov S, Ho CT. Modulation of inflammatory genes by natural dietary bioactive compounds. Journal of agricultural and food chemistry. 2009;57:4467–4477. doi: 10.1021/jf900612n. [DOI] [PubMed] [Google Scholar]
- 311.Aravindaram K, Yang NS. Anti-inflammatory plant natural products for cancer therapy. Planta medica. 2010;76:1103–1117. doi: 10.1055/s-0030-1249859. [DOI] [PubMed] [Google Scholar]
- 312.Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer metastasis reviews. 2010;29:405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Neergheen VS, Bahorun T, Taylor EW, Jen LS, Aruoma OI. Targeting specific cell signaling transduction pathways by dietary and medicinal phytochemicals in cancer chemoprevention. Toxicology. 2010;278:229–241. doi: 10.1016/j.tox.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 314.Bishayee A, Thoppil RJ, Waghray A, Kruse JA, Novotny NA, Darvesh AS. Dietary phytochemicals in the chemoprevention and treatment of hepatocellular carcinoma: in vivo evidence, molecular targets, and clinical relevance. Current cancer drug targets. 2012;12:1191–1232. [PubMed] [Google Scholar]
- 315.Madka V, Rao CV. Anti-inflammatory phytochemicals for chemoprevention of colon cancer. Current cancer drug targets. 2013;13:542–557. doi: 10.2174/15680096113139990036. [DOI] [PubMed] [Google Scholar]
- 316.Benetou V, Orfanos P, Lagiou P, Trichopoulos D, Boffetta P, Trichopoulou A. Vegetables and fruits in relation to cancer risk: evidence from the Greek EPIC cohort study. Cancer Epidemiol Biomarkers Prev. 2008;17:387–392. doi: 10.1158/1055-9965.EPI-07-2665. [DOI] [PubMed] [Google Scholar]
- 317.Freedman ND, Park Y, Subar AF, Hollenbeck AR, Leitzmann MF, Schatzkin A, et al. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer. 2008;122:2330–2336. doi: 10.1002/ijc.23319. [DOI] [PubMed] [Google Scholar]
- 318.Haas P, Machado MJ, Anton AA, Silva AS, de Francisco A. Effectiveness of whole grain consumption in the prevention of colorectal cancer: meta-analysis of cohort studies. International journal of food sciences and nutrition. 2009;60(Suppl 6):1–13. doi: 10.1080/09637480802183380. [DOI] [PubMed] [Google Scholar]
- 319.Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: World Cancer Research Fund/American Institute for Cancer Research; 2007. [Google Scholar]
- 320.Kundu JK, Surh YJ. Breaking the relay in deregulated cellular signal transduction as a rationale for chemoprevention with anti-inflammatory phytochemicals. Mutat Res. 2005;591:123–146. doi: 10.1016/j.mrfmmm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 321.Khor TO, Yu S, Kong AN. Dietary cancer chemopreventive agents - targeting inflammation and Nrf2 signaling pathway. Planta medica. 2008;74:1540–1547. doi: 10.1055/s-0028-1088303. [DOI] [PubMed] [Google Scholar]
- 322.Johnson JJ. Carnosol: a promising anti-cancer and anti-inflammatory agent. Cancer letters. 2011;305:1–7. doi: 10.1016/j.canlet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Li Y, Zhang T. Targeting cancer stem cells by curcumin and clinical applications. Cancer letters. 2014;346:197–205. doi: 10.1016/j.canlet.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 324.Eigner D, Scholz D. Ferula asa-foetida and Curcuma longa in traditional medical treatment and diet in Nepal. J Ethnopharmacol. 1999;67:1–6. doi: 10.1016/s0378-8741(98)00234-7. [DOI] [PubMed] [Google Scholar]
- 325.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa) J Altern Complement Med. 2003;9:161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 326.Institute. NC. Clinical development plan: Curcumin. J Cell Biochem Suppl. 1996;26(26):72–85. [PubMed] [Google Scholar]
- 327.Administration. USFaD. Food Additive Status List. 2005. [Accesses January 13];2009 Available at: http://wwwcfsanfdagov/~dms/opa-appahtml.
- 328.Ganiger S, Malleshappa HN, Krishnappa H, Rajashekhar G, Ramakrishna Rao V, Sullivan F. A two generation reproductive toxicity study with curcumin, turmeric yellow, in Wistar rats. Food Chem Toxicol. 2007;45:64–69. doi: 10.1016/j.fct.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 329.Lao CD, Ruffin MTt, Normolle D, Heath DD, Murray SI, Bailey JM, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 331.Rasyid A, Lelo A. The effect of curcumin and placebo on human gall-bladder function: an ultrasound study. Aliment Pharmacol Ther. 1999;13:245–249. doi: 10.1046/j.1365-2036.1999.00464.x. [DOI] [PubMed] [Google Scholar]
- 332.Rasyid A, Rahman AR, Jaalam K, Lelo A. Effect of different curcumin dosages on human gall bladder. Asia Pac J Clin Nutr. 2002;11:314–318. doi: 10.1046/j.1440-6047.2002.00296.x. [DOI] [PubMed] [Google Scholar]
- 333.Shah BH, Nawaz Z, Pertani SA, Roomi A, Mahmood H, Saeed SA, et al. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem Pharmacol. 1999;58:1167–1172. doi: 10.1016/s0006-2952(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 334.Srivastava KC, Bordia A, Verma SK. Curcumin, a major component of food spice turmeric (Curcuma longa) inhibits aggregation and alters eicosanoid metabolism in human blood platelets. Prostaglandins Leukot Essent Fatty Acids. 1995;52:223–227. doi: 10.1016/0952-3278(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 335.Kim DC, Kim SH, Choi BH, Baek NI, Kim D, Kim MJ, et al. Curcuma longa extract protects against gastric ulcers by blocking H2 histamine receptors. Biol Pharm Bull. 2005;28:2220–2224. doi: 10.1248/bpb.28.2220. [DOI] [PubMed] [Google Scholar]
- 336.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78:2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 337.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 338.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Molecular pharmacology. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 339.Lev-Ari S, Strier L, Kazanov D, Madar-Shapiro L, Dvory-Sobol H, Pinchuk I, et al. Celecoxib and curcumin synergistically inhibit the growth of colorectal cancer cells. Clin Cancer Res. 2005;11:6738–6744. doi: 10.1158/1078-0432.CCR-05-0171. [DOI] [PubMed] [Google Scholar]
- 340.He ZY, Shi CB, Wen H, Li FL, Wang BL, Wang J. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer investigation. 2011;29:208–213. doi: 10.3109/07357907.2010.550592. [DOI] [PubMed] [Google Scholar]
- 341.Guo LD, Chen XJ, Hu YH, Yu ZJ, Wang D, Liu JZ. Curcumin inhibits proliferation and induces apoptosis of human colorectal cancer cells by activating the mitochondria apoptotic pathway. Phytotherapy research : PTR. 2013;27:422–430. doi: 10.1002/ptr.4731. [DOI] [PubMed] [Google Scholar]
- 342.Lee SJ, Langhans SA. Anaphase-promoting complex/cyclosome protein Cdc27 is a target for curcumin-induced cell cycle arrest and apoptosis. BMC cancer. 2012;12:44. doi: 10.1186/1471-2407-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Villegas I, Sanchez-Fidalgo S, de la Lastra CA. Chemopreventive effect of dietary curcumin on inflammation-induced colorectal carcinogenesis in mice. Molecular nutrition & food research. 2011;55:259–267. doi: 10.1002/mnfr.201000225. [DOI] [PubMed] [Google Scholar]
- 344.Wei X, Du ZY, Zheng X, Cui XX, Conney AH, Zhang K. Synthesis and evaluation of curcumin-related compounds for anticancer activity. European journal of medicinal chemistry. 2012;53:235–245. doi: 10.1016/j.ejmech.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 345.Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2006;4:1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 346.Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer prevention research. 2011;4:354–364. doi: 10.1158/1940-6207.CAPR-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Howells LM, Sale S, Sriramareddy SN, Irving GR, Jones DJ, Ottley CJ, et al. Curcumin ameliorates oxaliplatin-induced chemoresistance in HCT116 colorectal cancer cells in vitro and in vivo. Int J Cancer. 2011;129:476–486. doi: 10.1002/ijc.25670. [DOI] [PubMed] [Google Scholar]
- 348.Chun KS, Keum YS, Han SS, Song YS, Kim SH, Surh YJ. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extracellular signal-regulated kinase activity and NF-kappaB activation. Carcinogenesis. 2003;24:1515–1524. doi: 10.1093/carcin/bgg107. [DOI] [PubMed] [Google Scholar]
- 349.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 350.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 351.Ravindranath V, Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980;16:259–265. doi: 10.1016/0300-483x(80)90122-5. [DOI] [PubMed] [Google Scholar]
- 352.Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, et al. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev. 2002;11:105–111. [PubMed] [Google Scholar]
- 353.Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61:1058–1064. [PubMed] [Google Scholar]
- 354.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27:486–494. [PubMed] [Google Scholar]
- 355.Garcea G, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, et al. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer. 2004;90:1011–1015. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 357.Garcea G, Berry DP, Jones DJ, Singh R, Dennison AR, Farmer PB, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005;14:120–125. [PubMed] [Google Scholar]
- 358.Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- 359.Bansal SS, Vadhanam MV, Gupta RC. Development and in vitro-in vivo evaluation of polymeric implants for continuous systemic delivery of curcumin. Pharm Res. 2011;28:1121–1130. doi: 10.1007/s11095-011-0375-z. [DOI] [PubMed] [Google Scholar]
- 360.Song Z, Feng R, Sun M, Guo C, Gao Y, Li L, et al. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J Colloid Interface Sci. 2011;354:116–123. doi: 10.1016/j.jcis.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 361.Shaikh J, Ankola DD, Beniwal V, Singh D, Kumar MN. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci. 2009;37:223–230. doi: 10.1016/j.ejps.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 362.Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007;60:171–177. doi: 10.1007/s00280-006-0355-x. [DOI] [PubMed] [Google Scholar]
- 363.Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 364.Goel A, Boland CR, Chauhan DP. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001;172:111–118. doi: 10.1016/s0304-3835(01)00655-3. [DOI] [PubMed] [Google Scholar]
- 365.Zhang F, Altorki NK, Mestre JR, Subbaramaiah K, Dannenberg AJ. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis. 1999;20:445–451. doi: 10.1093/carcin/20.3.445. [DOI] [PubMed] [Google Scholar]
- 366.Lev-Ari S, Starr A, Katzburg S, Berkovich L, Rimmon A, Ben-Yosef R, et al. Curcumin induces apoptosis and inhibits growth of orthotopic human non-small cell lung cancer xenografts. J Nutr Biochem. 2014;25:843–850. doi: 10.1016/j.jnutbio.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 367.Hidaka H, Ishiko T, Furuhashi T, Kamohara H, Suzuki S, Miyazaki M, et al. Curcumin inhibits interleukin 8 production and enhances interleukin 8 receptor expression on the cell surface:impact on human pancreatic carcinoma cell growth by autocrine regulation. Cancer. 2002;95:1206–1214. doi: 10.1002/cncr.10812. [DOI] [PubMed] [Google Scholar]
- 368.Bachmeier BE, Mohrenz IV, Mirisola V, Schleicher E, Romeo F, Hohneke C, et al. Curcumin downregulates the inflammatory cytokines CXCL1 and-2 in breast cancer cells via NFkappaB. Carcinogenesis. 2008;29:779–789. doi: 10.1093/carcin/bgm248. [DOI] [PubMed] [Google Scholar]
- 369.Moghaddam SJ, Barta P, Mirabolfathinejad SG, Ammar-Aouchiche Z, Garza NT, Vo TT, et al. Curcumin inhibits COPD-like airway inflammation and lung cancer progression in mice. Carcinogenesis. 2009;30:1949–1956. doi: 10.1093/carcin/bgp229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Marathe SA, Dasgupta I, Gnanadhas DP, Chakravortty D. Multifaceted roles of curcumin: two sides of a coin! Expert opinion on biological therapy. 2011;11:1485–1499. doi: 10.1517/14712598.2011.623124. [DOI] [PubMed] [Google Scholar]
- 371.Bhattacharyya S, Md Sakib Hossain D, Mohanty S, Sankar Sen G, Chattopadhyay S, Banerjee S, et al. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cellular & molecular immunology. 2010;7:306–315. doi: 10.1038/cmi.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Jeong YI, Kim SW, Jung ID, Lee JS, Chang JH, Lee CM, et al. Curcumin suppresses the induction of indoleamine 2,3-dioxygenase by blocking the Janus-activated kinase-protein kinase Cdelta-STAT1 signaling pathway in interferon-gamma-stimulated murine dendritic cells. J Biol Chem. 2009;284:3700–3708. doi: 10.1074/jbc.M807328200. [DOI] [PubMed] [Google Scholar]
- 373.Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer prevention research. 2009;2:409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 374.Almeida L, Vaz-da-Silva M, Falcao A, Soares E, Costa R, Loureiro AI, et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Molecular nutrition & food research. 2009;53(Suppl 1):S7–S15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 375.Walle T. Bioavailability of resveratrol. Annals of the New York Academy of Sciences. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- 376.Cottart CH, Nivet-Antoine V, Beaudeux JL. Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans. Molecular nutrition & food research. 2014;58:7–21. doi: 10.1002/mnfr.201200589. [DOI] [PubMed] [Google Scholar]
- 377.Ponzo V, Soldati L, Bo S. Resveratrol: a supplementation for men or for mice? Journal of translational medicine. 2014;12:158. doi: 10.1186/1479-5876-12-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Witte AV, Kerti L, Margulies DS, Floel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci. 2014;34:7862–7870. doi: 10.1523/JNEUROSCI.0385-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Knop FK, Konings E, Timmers S, Schrauwen P, Holst JJ, Blaak EE. Thirty days of resveratrol supplementation does not affect postprandial incretin hormone responses, but suppresses postprandial glucagon in obese subjects. Diabetic medicine : a journal of the British Diabetic Association. 2013;30:1214–1218. doi: 10.1111/dme.12231. [DOI] [PubMed] [Google Scholar]
- 380.Farghali H, Cerny D, Kamenikova L, Martinek J, Horinek A, Kmonickova E, et al. Resveratrol attenuates lipopolysaccharide-induced hepatitis in D-galactosamine sensitized rats: role of nitric oxide synthase 2 and heme oxygenase-1. Nitric Oxide. 2009;21:216–225. doi: 10.1016/j.niox.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 381.Woodall CE, Li Y, Liu QH, Wo J, Martin RC. Chemoprevention of metaplasia initiation and carcinogenic progression to esophageal adenocarcinoma by resveratrol supplementation. Anti-cancer drugs. 2009;20:437–443. doi: 10.1097/CAD.0b013e32832afb95. [DOI] [PubMed] [Google Scholar]
- 382.Cui X, Jin Y, Hofseth AB, Pena E, Habiger J, Chumanevich A, et al. Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prev Res. 2010;3:549–559. doi: 10.1158/1940-6207.CAPR-09-0117. Epub 2010 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Martin AR, Villegas I, La Casa C, de la Lastra CA. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem Pharmacol. 2004;67:1399–1410. doi: 10.1016/j.bcp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 384.Carrasco C, Holguin-Arevalo MS, Martin-Partido G, Rodriguez AB, Pariente JA. Chemopreventive effects of resveratrol in a rat model of cerulein-induced acute pancreatitis. Mol Cell Biochem. 2014;387:217–225. doi: 10.1007/s11010-013-1887-0. [DOI] [PubMed] [Google Scholar]
- 385.Jha RK, Ma Q, Lei Z, Sha H. Resveratrol ameliorates the deleterious effect of severe acute pancreatitis. Cell biochemistry and biophysics. 2012;62:397–402. doi: 10.1007/s12013-011-9313-2. [DOI] [PubMed] [Google Scholar]
- 386.Szabolcs A, Varga IS, Varga C, Berko A, Kaszaki J, Letoha T, et al. Beneficial effect of resveratrol on cholecystokinin-induced experimental pancreatitis. Eur J Pharmacol. 2006;532:187–193. doi: 10.1016/j.ejphar.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 387.Gatz SA, Keimling M, Baumann C, Dork T, Debatin KM, Fulda S, et al. Resveratrol modulates DNA double-strand break repair pathways in an ATM/ATR-p53- and -Nbs1-dependent manner. Carcinogenesis. 2008;3:3. doi: 10.1093/carcin/bgm283. [DOI] [PubMed] [Google Scholar]
- 388.Huang C, Ma WY, Goranson A, Dong Z. Resveratrol suppresses cell transformation and induces apoptosis through a p53-dependent pathway. Carcinogenesis. 1999;20:237–242. doi: 10.1093/carcin/20.2.237. [DOI] [PubMed] [Google Scholar]
- 389.She QB, Bode AM, Ma WY, Chen NY, Dong Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. 2001;61:1604–1610. [PubMed] [Google Scholar]
- 390.Baek SJ, Wilson LC, Eling TE. Resveratrol enhances the expression of non-steroidal anti-inflammatory drug-activated gene (NAG-1) by increasing the expression of p53. Carcinogenesis. 2002;23:425–434. doi: 10.1093/carcin/23.3.425. [DOI] [PubMed] [Google Scholar]
- 391.Gagliano N, Moscheni C, Torri C, Magnani I, Bertelli AA, Gioia M. Effect of resveratrol on matrix metalloproteinase-2 (MMP-2) and Secreted Protein Acidic and Rich in Cysteine (SPARC) on human cultured glioblastoma cells. Biomed Pharmacother. 2005;59:359–364. doi: 10.1016/j.biopha.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 392.Woo JH, Lim JH, Kim YH, Suh SI, Min DS, Chang JS, et al. Resveratrol inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting JNK and PKC delta signal transduction. Oncogene. 2004;23:1845–1853. doi: 10.1038/sj.onc.1207307. [DOI] [PubMed] [Google Scholar]
- 393.Yu H, Pan C, Zhao S, Wang Z, Zhang H, Wu W. Resveratrol inhibits tumor necrosis factor-alpha-mediated matrix metalloproteinase-9 expression and invasion of human hepatocellular carcinoma cells. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2007;22:22. doi: 10.1016/j.biopha.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 394.Szewczuk LM, Forti L, Stivala LA, Penning TM. Resveratrol is a peroxidase-mediated inactivator of COX-1 but not COX-2: a mechanistic approach to the design of COX-1 selective agents. J Biol Chem. 2004;279:22727–22737. doi: 10.1074/jbc.M314302200. Epub 2004 Mar 12. [DOI] [PubMed] [Google Scholar]
- 395.Zhong M, Cheng GF, Wang WJ, Guo Y, Zhu XY, Zhang JT. Inhibitory effect of resveratrol on interleukin 6 release by stimulated peritoneal macrophages of mice. Phytomedicine. 1999;6:79–84. doi: 10.1016/S0944-7113(99)80039-7. [DOI] [PubMed] [Google Scholar]
- 396.Wirleitner B, Schroecksnadel K, Winkler C, Schennach H, Fuchs D. Resveratrol suppresses interferon-gamma-induced biochemical pathways in human peripheral blood mononuclear cells in vitro. Immunol Lett. 2005;100:159–163. doi: 10.1016/j.imlet.2005.03.008. Epub 2005 Apr 7. [DOI] [PubMed] [Google Scholar]
- 397.Lee B, Moon SK. Resveratrol inhibits TNF-alpha-induced proliferation and matrix metalloproteinase expression in human vascular smooth muscle cells. J Nutr. 2005;135:2767–2773. doi: 10.1093/jn/135.12.2767. [DOI] [PubMed] [Google Scholar]
- 398.De Ledinghen V, Monvoisin A, Neaud V, Krisa S, Payrastre B, Bedin C, et al. Trans-resveratrol, a grapevine-derived polyphenol, blocks hepatocyte growth factor-induced invasion of hepatocellular carcinoma cells. Int J Oncol. 2001;19:83–88. [PubMed] [Google Scholar]
- 399.Huang H, Lin H, Zhang X, Li J. Resveratrol reverses temozolomide resistance by downregulation of MGMT in T98G glioblastoma cells by the NF-kappaB-dependent pathway. Oncology reports. 2012;27:2050–2056. doi: 10.3892/or.2012.1715. [DOI] [PubMed] [Google Scholar]
- 400.Guo R, Liu B, Wang K, Zhou S, Li W, Xu Y. Resveratrol ameliorates diabetic vascular inflammation and macrophage infiltration in db/db mice by inhibiting the NF-kappaB pathway. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease. 2014;11:92–102. doi: 10.1177/1479164113520332. [DOI] [PubMed] [Google Scholar]
- 401.Li W, Ma J, Ma Q, Li B, Han L, Liu J, et al. Resveratrol inhibits the epithelial-mesenchymal transition of pancreatic cancer cells via suppression of the PI-3K/Akt/NF-kappaB pathway. Current medicinal chemistry. 2013;20:4185–4194. doi: 10.2174/09298673113209990251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Li L, Hai J, Li Z, Zhang Y, Peng H, Li K, et al. Resveratrol modulates autophagy and NF-kappaB activity in a murine model for treating non-alcoholic fatty liver disease. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2014;63:166–173. doi: 10.1016/j.fct.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 403.Li H, Jia Z, Li A, Jenkins G, Yang X, Hu J, et al. Resveratrol repressed viability of U251 cells by miR-21 inhibiting of NF-kappaB pathway. Mol Cell Biochem. 2013;382:137–143. doi: 10.1007/s11010-013-1728-1. [DOI] [PubMed] [Google Scholar]
- 404.Park SA, Na HK, Surh YJ. Resveratrol suppresses 4-hydroxyestradiol-induced transformation of human breast epithelial cells by blocking IkappaB kinase beta-NF-kappaB signalling. Free radical research. 2012;46:1051–1057. doi: 10.3109/10715762.2012.671940. [DOI] [PubMed] [Google Scholar]
- 405.Altamemi I, Murphy EA, Catroppo JF, Zumbrun EE, Zhang J, McClellan JL, et al. Role of microRNAs in resveratrol-mediated mitigation of colitis-associated tumorigenesis in Apc(Min/+) mice. J Pharmacol Exp Ther. 2014;350:99–109. doi: 10.1124/jpet.114.213306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Subbaramaiah K, Chung WJ, Michaluart P, Telang N, Tanabe T, Inoue H, et al. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J Biol Chem. 1998;273:21875–21882. doi: 10.1074/jbc.273.34.21875. [DOI] [PubMed] [Google Scholar]
- 407.Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62:4945–4954. [PubMed] [Google Scholar]
- 408.Kundu JK, Shin YK, Kim SH, Surh YJ. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-kappaB in mouse skin by blocking IkappaB kinase activity. Carcinogenesis. 2006;27:1465–1474. doi: 10.1093/carcin/bgi349. Epub 2006 Feb 12. [DOI] [PubMed] [Google Scholar]
- 409.Fujita Y, Islam R, Sakai K, Kaneda H, Kudo K, Tamura D, et al. Aza-derivatives of resveratrol are potent macrophage migration inhibitory factor inhibitors. Investigational new drugs. 2012;30:1878–1886. doi: 10.1007/s10637-011-9749-7. [DOI] [PubMed] [Google Scholar]
- 410.Silva AM, Oliveira MI, Sette L, Almeida CR, Oliveira MJ, Barbosa MA, et al. Resveratrol as a natural anti-tumor necrosis factor-alpha molecule: implications to dendritic cells and their crosstalk with mesenchymal stromal cells. PloS one. 2014;9:e91406. doi: 10.1371/journal.pone.0091406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Tsai SH, Lin-Shiau SY, Lin JK. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. British journal of pharmacology. 1999;126:673–680. doi: 10.1038/sj.bjp.0702357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Vergara D, Simeone P, Toraldo D, Del Boccio P, Vergaro V, Leporatti S, et al. Resveratrol downregulates Akt/GSK and ERK signalling pathways in OVCAR-3 ovarian cancer cells. Molecular bioSystems. 2012;8:1078–1087. doi: 10.1039/c2mb05486h. [DOI] [PubMed] [Google Scholar]
- 413.Tao HY, Wu CF, Zhou Y, Gong WH, Zhang X, Iribarren P, et al. The grape component resveratrol interferes with the function of chemoattractant receptors on phagocytic leukocytes. Cellular & molecular immunology. 2004;1:50–56. [PubMed] [Google Scholar]
- 414.Cichocki M, Paluszczak J, Szaefer H, Piechowiak A, Rimando AM, Baer-Dubowska W. Pterostilbene is equally potent as resveratrol in inhibiting 12-O-tetradecanoylphorbol-13-acetate activated NFkappaB, AP-1, COX-2, and iNOS in mouse epidermis. Molecular nutrition & food research. 2008;52(Suppl 1):S62–S70. doi: 10.1002/mnfr.200700466. [DOI] [PubMed] [Google Scholar]
- 415.Bishayee A, Dhir N. Resveratrol-mediated chemoprevention of diethylnitrosamine-initiated hepatocarcinogenesis: inhibition of cell proliferation and induction of apoptosis. Chemico-biological interactions. 2009;179:131–144. doi: 10.1016/j.cbi.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 416.Bishayee A, Barnes KF, Bhatia D, Darvesh AS, Carroll RT. Resveratrol suppresses oxidative stress and inflammatory response in diethylnitrosamine-initiated rat hepatocarcinogenesis. Cancer prevention research. 2010;3:753–763. doi: 10.1158/1940-6207.CAPR-09-0171. [DOI] [PubMed] [Google Scholar]
- 417.Bishayee A, Waghray A, Barnes KF, Mbimba T, Bhatia D, Chatterjee M, et al. Suppression of the inflammatory cascade is implicated in resveratrol chemoprevention of experimental hepatocarcinogenesis. Pharmaceutical research. 2010;27:1080–1091. doi: 10.1007/s11095-010-0144-4. [DOI] [PubMed] [Google Scholar]
- 418.Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, et al. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics. Cancer prevention research. 2011;4:1419–1425. doi: 10.1158/1940-6207.CAPR-11-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Gescher A, Steward WP, Brown K. Resveratrol in the management of human cancer: how strong is the clinical evidence? Annals of the New York Academy of Sciences. 2013;1290:12–20. doi: 10.1111/nyas.12205. [DOI] [PubMed] [Google Scholar]
- 421.Falchetti R, Fuggetta MP, Lanzilli G, Tricarico M, Ravagnan G. Effects of resveratrol on human immune cell function. Life sciences. 2001;70:81–96. doi: 10.1016/s0024-3205(01)01367-4. [DOI] [PubMed] [Google Scholar]
- 422.Li Q, Huyan T, Ye LJ, Li J, Shi JL, Huang QS. Concentration-dependent biphasic effects of resveratrol on human natural killer cells in vitro. Journal of agricultural and food chemistry. 2014;62:10928–10935. doi: 10.1021/jf502950u. [DOI] [PubMed] [Google Scholar]
- 423.Chen L, Yang S, Liao W, Xiong Y. Modification of Antitumor Immunity and Tumor Microenvironment by Resveratrol in Mouse Renal Tumor Model. Cell biochemistry and biophysics. 2015 doi: 10.1007/s12013-015-0513-z. [DOI] [PubMed] [Google Scholar]
- 424.Yang Y, Paik JH, Cho D, Cho JA, Kim CW. Resveratrol induces the suppression of tumor-derived CD4+CD25+ regulatory T cells. International immunopharmacology. 2008;8:542–547. doi: 10.1016/j.intimp.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 425.Lee-Chang C, Bodogai M, Martin-Montalvo A, Wejksza K, Sanghvi M, Moaddel R, et al. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J Immunol. 2013;191:4141–4151. doi: 10.4049/jimmunol.1300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Sang S, Lambert JD, Ho CT, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacological research : the official journal of the Italian Pharmacological Society. 2011;64:87–99. doi: 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 428.Vaidyanathan JB, Walle T. Cellular uptake and efflux of the tea flavonoid (−)epicatechin-3-gallate in the human intestinal cell line Caco- The Journal of pharmacology and experimental therapeutics. 2003;307:745–752. doi: 10.1124/jpet.103.054296. [DOI] [PubMed] [Google Scholar]
- 429.Feng WY. Metabolism of green tea catechins: an overview. Current drug metabolism. 2006;7:755–809. doi: 10.2174/138920006778520552. [DOI] [PubMed] [Google Scholar]
- 430.Isbrucker RA, Edwards JA, Wolz E, Davidovich A, Bausch J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: dermal, acute and short-term toxicity studies. Food Chem Toxicol. 2006;44:636–650. doi: 10.1016/j.fct.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 431.Abboud PA, Hake PW, Burroughs TJ, Odoms K, O'Connor M, Mangeshkar P, et al. Therapeutic effect of epigallocatechin-3-gallate in a mouse model of colitis. Eur J Pharmacol. 2008;579:411–417. doi: 10.1016/j.ejphar.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 432.Wang Y, Mei Y, Feng D, Xu L. (−)-Epigallocatechin-3-gallate protects mice from concanavalin A-induced hepatitis through suppressing immune-mediated liver injury. Clinical and experimental immunology. 2006;145:485–492. doi: 10.1111/j.1365-2249.2006.03137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Ciesek S, von Hahn T, Colpitts CC, Schang LM, Friesland M, Steinmann J, et al. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology (Baltimore, Md) 2011;54:1947–1955. doi: 10.1002/hep.24610. [DOI] [PubMed] [Google Scholar]
- 434.Xu J, Wang J, Deng F, Hu Z, Wang H. Green tea extract and its major component epigallocatechin gallate inhibits hepatitis B virus in vitro. Antiviral research. 2008;78:242–249. doi: 10.1016/j.antiviral.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 435.Asaumi H, Watanabe S, Taguchi M, Tashiro M, Nagashio Y, Nomiyama Y, et al. Green tea polyphenol (−)-epigallocatechin-3-gallate inhibits ethanol-induced activation of pancreatic stellate cells. European journal of clinical investigation. 2006;36:113–122. doi: 10.1111/j.1365-2362.2006.01599.x. [DOI] [PubMed] [Google Scholar]
- 436.Dryden GW, Lam A, Beatty K, Qazzaz HH, McClain CJ. A pilot study to evaluate the safety and efficacy of an oral dose of (−)-epigallocatechin-3-gallate-rich polyphenon E in patients with mild to moderate ulcerative colitis. Inflammatory bowel diseases. 2013;19:1904–1912. doi: 10.1097/MIB.0b013e31828f5198. [DOI] [PubMed] [Google Scholar]
- 437.Noh SU, Cho EA, Kim HO, Park YM. Epigallocatechin-3-gallate improves Dermatophagoides pteronissinus extract-induced atopic dermatitis-like skin lesions in NC/Nga mice by suppressing macrophage migration inhibitory factor. International immunopharmacology. 2008;8:1172–1182. doi: 10.1016/j.intimp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 438.Rasheed Z, Anbazhagan AN, Akhtar N, Ramamurthy S, Voss FR, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate inhibits advanced glycation end product-induced expression of tumor necrosis factor-alpha and matrix metalloproteinase-13 in human chondrocytes. Arthritis research & therapy. 2009;11:R71. doi: 10.1186/ar2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Ahmed S, Rahman A, Hasnain A, Lalonde M, Goldberg VM, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic Biol Med. 2002;33:1097–1105. doi: 10.1016/s0891-5849(02)01004-3. [DOI] [PubMed] [Google Scholar]
- 440.Punathil T, Tollefsbol TO, Katiyar SK. EGCG inhibits mammary cancer cell migration through inhibition of nitric oxide synthase and guanylate cyclase. Biochemical and biophysical research communications. 2008;375:162–167. doi: 10.1016/j.bbrc.2008.07.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Shen X, Zhang Y, Feng Y, Zhang L, Li J, Xie YA, et al. Epigallocatechin-3-gallate inhibits cell growth, induces apoptosis and causes S phase arrest in hepatocellular carcinoma by suppressing the AKT pathway. Int J Oncol. 2014;44:791–796. doi: 10.3892/ijo.2014.2251. [DOI] [PubMed] [Google Scholar]
- 442.Takano K, Nakaima K, Nitta M, Shibata F, Nakagawa H. Inhibitory effect of (−)-epigallocatechin 3-gallate, a polyphenol of green tea, on neutrophil chemotaxis in vitro and in vivo. Journal of agricultural and food chemistry. 2004;52:4571–4576. doi: 10.1021/jf0355194. [DOI] [PubMed] [Google Scholar]
- 443.Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res. 2005;11:2735–2746. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- 444.Shimizu M, Deguchi A, Joe AK, McKoy JF, Moriwaki H, Weinstein IB. EGCG inhibits activation of HER3 and expression of cyclooxygenase-2 in human colon cancer cells. Journal of experimental therapeutics & oncology. 2005;5:69–78. [PubMed] [Google Scholar]
- 445.Yang F, Oz HS, Barve S, de Villiers WJ, McClain CJ, Varilek GW. The green tea polyphenol (−)-epigallocatechin-3-gallate blocks nuclear factor-kappa B activation by inhibiting I kappa B kinase activity in the intestinal epithelial cell line IEC-6. Mol Pharmacol. 2001;60:528–533. [PubMed] [Google Scholar]
- 446.Aktas O, Prozorovski T, Smorodchenko A, Savaskan NE, Lauster R, Kloetzel PM, et al. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J Immunol. 2004;173:5794–5800. doi: 10.4049/jimmunol.173.9.5794. [DOI] [PubMed] [Google Scholar]
- 447.Hussain T, Gupta S, Adhami VM, Mukhtar H. Green tea constituent epigallocatechin-3-gallate selectively inhibits COX-2 without affecting COX-1 expression in human prostate carcinoma cells. Int J Cancer. 2005;113:660–669. doi: 10.1002/ijc.20629. [DOI] [PubMed] [Google Scholar]
- 448.Peng G, Dixon DA, Muga SJ, Smith TJ, Wargovich MJ. Green tea polyphenol (−)-epigallocatechin-3-gallate inhibits cyclooxygenase-2 expression in colon carcinogenesis. Mol Carcinog. 2006;45:309–319. doi: 10.1002/mc.20166. [DOI] [PubMed] [Google Scholar]
- 449.Amin AR, Thakur VS, Paul RK, Feng GS, Qu CK, Mukhtar H, et al. SHP-2 tyrosine phosphatase inhibits p73-dependent apoptosis and expression of a subset of p53 target genes induced by EGCG. Proc Natl Acad Sci U S A. 2007;104:5419–5424. doi: 10.1073/pnas.0700642104. Epub 2007 Mar 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Kuo PL, Lin CC. Green tea constituent (−)-epigallocatechin-3-gallate inhibits Hep G2 cell proliferation and induces apoptosis through p53-dependent and Fas-mediated pathways. J Biomed Sci. 2003;10:219–227. doi: 10.1007/BF02256057. [DOI] [PubMed] [Google Scholar]
- 451.Hofmann CS, Sonenshein GE. Green tea polyphenol epigallocatechin-3 gallate induces apoptosis of proliferating vascular smooth muscle cells via activation of p53. Faseb J. 2003;17:702–704. doi: 10.1096/fj.02-0665fje. Epub 2003 Feb 5. [DOI] [PubMed] [Google Scholar]
- 452.Lu YP, Lou YR, Liao J, Xie JG, Peng QY, Yang CS, et al. Administration of green tea or caffeine enhances the disappearance of UVB-induced patches of mutant p53 positive epidermal cells in SKH-1 mice. Carcinogenesis. 2005;26:1465–1472. doi: 10.1093/carcin/bgi086. Epub 2005 Apr 7. [DOI] [PubMed] [Google Scholar]
- 453.Ahmad N, Adhami VM, Gupta S, Cheng P, Mukhtar H. Role of the retinoblastoma (pRb)-E2F/DP pathway in cancer chemopreventive effects of green tea polyphenol epigallocatechin-3- gallate. Arch Biochem Biophys. 2002;398:125–131. doi: 10.1006/abbi.2001.2704. [DOI] [PubMed] [Google Scholar]
- 454.Yun JH, Pang EK, Kim CS, Yoo YJ, Cho KS, Chai JK, et al. Inhibitory effects of green tea polyphenol (-)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J Periodontal Res. 2004;39:300–307. doi: 10.1111/j.1600-0765.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 455.Sukhthankar M, Yamaguchi K, Lee SH, McEntee MF, Eling TE, Hara Y, et al. A green tea component suppresses posttranslational expression of basic fibroblast growth factor in colorectal cancer. Gastroenterology. 2008;134:1972–1980. doi: 10.1053/j.gastro.2008.02.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Ju J, Hong J, Zhou JN, Pan Z, Bose M, Liao J, et al. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (−)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005;65:10623–10631. doi: 10.1158/0008-5472.CAN-05-1949. [DOI] [PubMed] [Google Scholar]
- 457.Nakachi K, Matsuyama S, Miyake S, Suganuma M, Imai K. Preventive effects of drinking green tea on cancer and cardiovascular disease: epidemiological evidence for multiple targeting prevention. Biofactors. 2000;13:49–54. doi: 10.1002/biof.5520130109. [DOI] [PubMed] [Google Scholar]
- 458.Gao YT, McLaughlin JK, Blot WJ, Ji BT, Dai Q, Fraumeni JF., Jr Reduced risk of esophageal cancer associated with green tea consumption. J Natl Cancer Inst. 1994;86:855–858. doi: 10.1093/jnci/86.11.855. [DOI] [PubMed] [Google Scholar]
- 459.Yuan JM, Gao YT, Yang CS, Yu MC. Urinary biomarkers of tea polyphenols and risk of colorectal cancer in the Shanghai Cohort Study. Int J Cancer. 2007;120:1344–1350. doi: 10.1002/ijc.22460. [DOI] [PubMed] [Google Scholar]
- 460.Shimizu M, Fukutomi Y, Ninomiya M, Nagura K, Kato T, Araki H, et al. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiol Biomarkers Prev. 2008;17:3020–3025. doi: 10.1158/1055-9965.EPI-08-0528. [DOI] [PubMed] [Google Scholar]
- 461.Donovan JL, Chavin KD, Devane CL, Taylor RM, Wang JS, Ruan Y, et al. Green tea (Camellia sinensis) extract does not alter cytochrome p450 3A4 or 2D6 activity in healthy volunteers. Drug metabolism and disposition: the biological fate of chemicals. 2004;32:906–908. doi: 10.1124/dmd.104.000083. [DOI] [PubMed] [Google Scholar]
- 462.Chow HH, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, et al. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin Cancer Res. 2005;11:4627–4633. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- 463.Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, et al. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. European journal of clinical pharmacology. 2009;65:331–341. doi: 10.1007/s00228-008-0610-7. [DOI] [PubMed] [Google Scholar]
- 464.Sun CL, Yuan JM, Koh WP, Yu MC. Green tea, black tea and colorectal cancer risk: a metaanalysis of epidemiologic studies. Carcinogenesis. 2006;27:1301–1309. doi: 10.1093/carcin/bgl024. [DOI] [PubMed] [Google Scholar]
- 465.Saleh F, Raghupathy R, Asfar S, Oteifa M, Al-Saleh N. Analysis of the effect of the active compound of green tea (EGCG) on the proliferation of peripheral blood mononuclear cells. BMC complementary and alternative medicine. 2014;14:322. doi: 10.1186/1472-6882-14-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Ogawa K, Hara T, Shimizu M, Nagano J, Ohno T, Hoshi M, et al. (−)-Epigallocatechin gallate inhibits the expression of indoleamine 2,3-dioxygenase in human colorectal cancer cells. Oncology letters. 2012;4:546–550. doi: 10.3892/ol.2012.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Huang AC, Cheng HY, Lin TS, Chen WH, Lin JH, Lin JJ, et al. Epigallocatechin gallate (EGCG), influences a murine WEHI-3 leukemia model in vivo through enhancing phagocytosis of macrophages and populations of T- and B-cells. In vivo (Athens, Greece) 2013;27:627–634. [PubMed] [Google Scholar]
- 468.Matsunaga K, Klein TW, Friedman H, Yamamoto Y. In vitro therapeutic effect of epigallocatechin gallate on nicotine-induced impairment of resistance to Legionella pneumophila infection of established MH-S alveolar macrophages. The Journal of infectious diseases. 2002;185:229–236. doi: 10.1086/338449. [DOI] [PubMed] [Google Scholar]
- 469.Jeon J, Kim JH, Lee CK, Oh CH, Song HJ. The Antimicrobial Activity of (−)-Epigallocatehin-3-Gallate and Green Tea Extracts against Pseudomonas aeruginosa and Escherichia coli Isolated from Skin Wounds. Annals of dermatology. 2014;26:564–569. doi: 10.5021/ad.2014.26.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Gharib A, Faezizadeh Z, Godarzee M. Therapeutic efficacy of epigallocatechin gallate-loaded nanoliposomes against burn wound infection by methicillin-resistant Staphylococcus aureus. Skin pharmacology and physiology. 2013;26:68–75. doi: 10.1159/000345761. [DOI] [PubMed] [Google Scholar]
- 471.Weisburger JH. Evaluation of the evidence on the role of tomato products in disease prevention. Proc Soc Exp Biol Med. 1998;218:140–143. doi: 10.3181/00379727-218-44281. [DOI] [PubMed] [Google Scholar]
- 472.Sies H, Stahl W. Lycopene: antioxidant and biological effects and its bioavailability in the human. Proc Soc Exp Biol Med. 1998;218:121–124. doi: 10.3181/00379727-218-44285a. [DOI] [PubMed] [Google Scholar]
- 473.Stahl W, Sies H. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. The Journal of nutrition. 1992;122:2161–2166. doi: 10.1093/jn/122.11.2161. [DOI] [PubMed] [Google Scholar]
- 474.Ahuja KD, Pittaway JK, Ball MJ. Effects of olive oil and tomato lycopene combination on serum lycopene, lipid profile, and lipid oxidation. Nutrition. 2006;22:259–265. doi: 10.1016/j.nut.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 475.Parker RS. Absorption, metabolism, and transport of carotenoids. FASEB J. 1996;10:542–551. [PubMed] [Google Scholar]
- 476.Tang L, Lee AH, Su D, Binns CW. Fruit and vegetable consumption associated with reduced risk of epithelial ovarian cancer in southern Chinese women. Gynecologic oncology. 2014;132:241–247. doi: 10.1016/j.ygyno.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 477.Pouchieu C, Galan P, Ducros V, Latino-Martel P, Hercberg S, Touvier M. Plasma Carotenoids and Retinol and Overall and Breast Cancer Risk: A Nested Case-Control Study. Nutrition and cancer. 2014:1–9. doi: 10.1080/01635581.2014.936952. [DOI] [PubMed] [Google Scholar]
- 478.Li X, Xu J. Meta-analysis of the association between dietary lycopene intake and ovarian cancer risk in postmenopausal women. Scientific reports. 2014;4:4885. doi: 10.1038/srep04885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst. 1999;91:317–331. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- 480.Lu MS, Fang YJ, Chen YM, Luo WP, Pan ZZ, Zhong X, et al. Higher intake of carotenoid is associated with a lower risk of colorectal cancer in Chinese adults: a case-control study. European journal of nutrition. 2014 doi: 10.1007/s00394-014-0743-7. [DOI] [PubMed] [Google Scholar]
- 481.Etminan M, Takkouche B, Caamano-Isorna F. The role of tomato products and lycopene in the prevention of prostate cancer: a meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev. 2004;13:340–345. [PubMed] [Google Scholar]
- 482.Kirsh VA, Mayne ST, Peters U, Chatterjee N, Leitzmann MF, Dixon LB, et al. A prospective study of lycopene and tomato product intake and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:92–98. doi: 10.1158/1055-9965.EPI-05-0563. [DOI] [PubMed] [Google Scholar]
- 483.Kristal AR, Till C, Platz EA, Song X, King IB, Neuhouser ML, et al. Serum lycopene concentration and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2011;20:638–646. doi: 10.1158/1055-9965.EPI-10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Peters U, Leitzmann MF, Chatterjee N, Wang Y, Albanes D, Gelmann EP, et al. Serum lycopene, other carotenoids, and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2007;16:962–968. doi: 10.1158/1055-9965.EPI-06-0861. [DOI] [PubMed] [Google Scholar]
- 485.Vogt TM, Mayne ST, Graubard BI, Swanson CA, Sowell AL, Schoenberg JB, et al. Serum lycopene, other serum carotenoids, and risk of prostate cancer in US Blacks and Whites. American journal of epidemiology. 2002;155:1023–1032. doi: 10.1093/aje/155.11.1023. [DOI] [PubMed] [Google Scholar]
- 486.Goo YA, Li Z, Pajkovic N, Shaffer S, Taylor G, Chen J, et al. Systematic investigation of lycopene effects in LNCaP cells by use of novel large-scale proteomic analysis software. Proteomics Clinical applications. 2007;1:513–523. doi: 10.1002/prca.200600511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Qiu X, Yuan Y, Vaishnav A, Tessel MA, Nonn L, van Breemen RB. Effects of lycopene on protein expression in human primary prostatic epithelial cells. Cancer prevention research. 2013;6:419–427. doi: 10.1158/1940-6207.CAPR-12-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Yang CM, Lu IH, Chen HY, Hu ML. Lycopene inhibits the proliferation of androgen-dependent human prostate tumor cells through activation of PPARgamma-LXRalpha-ABCA1 pathway. The Journal of nutritional biochemistry. 2012;23:8–17. doi: 10.1016/j.jnutbio.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 489.Yang CM, Lu YL, Chen HY, Hu ML. Lycopene and the LXRalpha agonist T0901317 synergistically inhibit the proliferation of androgen-independent prostate cancer cells via the PPARgamma-LXRalpha-ABCA1 pathway. The Journal of nutritional biochemistry. 2012;23:1155–1162. doi: 10.1016/j.jnutbio.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 490.Palozza P, Colangelo M, Simone R, Catalano A, Boninsegna A, Lanza P, et al. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis. 2010;31:1813–1821. doi: 10.1093/carcin/bgq157. [DOI] [PubMed] [Google Scholar]
- 491.Bureyko T, Hurdle H, Metcalfe JB, Clandinin MT, Mazurak VC. Reduced growth and integrin expression of prostate cells cultured with lycopene, vitamin E and fish oil in vitro. The British journal of nutrition. 2009;101:990–997. doi: 10.1017/S0007114508051684. [DOI] [PubMed] [Google Scholar]
- 492.Hadad N, Levy R. The synergistic anti-inflammatory effects of lycopene, lutein, beta-carotene, and carnosic acid combinations via redox-based inhibition of NF-kappaB signaling. Free Radic Biol Med. 2012;53:1381–1391. doi: 10.1016/j.freeradbiomed.2012.07.078. [DOI] [PubMed] [Google Scholar]
- 493.Marcotorchino J, Romier B, Gouranton E, Riollet C, Gleize B, Malezet-Desmoulins C, et al. Lycopene attenuates LPS-induced TNF-alpha secretion in macrophages and inflammatory markers in adipocytes exposed to macrophage-conditioned media. Molecular nutrition & food research. 2012;56:725–732. doi: 10.1002/mnfr.201100623. [DOI] [PubMed] [Google Scholar]
- 494.Bonvissuto G, Minutoli L, Morgia G, Bitto A, Polito F, Irrera N, et al. Effect of Serenoa repens, lycopene, and selenium on proinflammatory phenotype activation: an in vitro and in vivo comparison study. Urology. 2011;77:248, e9–e16. doi: 10.1016/j.urology.2010.07.514. [DOI] [PubMed] [Google Scholar]
- 495.Ozkan E, Akyuz C, Dulundu E, Topaloglu U, Sehirli AO, Ercan F, et al. Protective effects of lycopene on cerulein-induced experimental acute pancreatitis in rats. The Journal of surgical research. 2012;176:232–238. doi: 10.1016/j.jss.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 496.Lee W, Ku SK, Bae JW, Bae JS. Inhibitory effects of lycopene on HMGB1-mediated proinflammatory responses in both cellular and animal models. Food Chem Toxicol. 2012;50:1826–1833. doi: 10.1016/j.fct.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 497.Di Tomo P, Canali R, Ciavardelli D, Di Silvestre S, De Marco A, Giardinelli A, et al. beta-Carotene and lycopene affect endothelial response to TNF-alpha reducing nitro-oxidative stress and interaction with monocytes. Molecular nutrition & food research. 2012;56:217–227. doi: 10.1002/mnfr.201100500. [DOI] [PubMed] [Google Scholar]
- 498.Lingen C, Ernster L, Lindberg O. The promoting effect of lycopene on the non-specific resistance of animals. Experimental cell research. 1959;16:384–393. doi: 10.1016/0014-4827(59)90267-8. [DOI] [PubMed] [Google Scholar]
- 499.Pan H, Jiang X, Wan L, Na L, Wang J. Experimental studies of lycopene in inhibiting tumor growth in S180-bearing mice. Wei sheng yan jiu = Journal of hygiene research. 2004;33:456–457. [PubMed] [Google Scholar]
- 500.Kim GY, Kim JH, Ahn SC, Lee HJ, Moon DO, Lee CM, et al. Lycopene suppresses the lipopolysaccharide-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinases and nuclear factor-kappaB. Immunology. 2004;113:203–211. doi: 10.1111/j.1365-2567.2004.01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Toxicity Profile for Lycopene. Surrey, UK: Bibra Toxicology Advice & Consulting; 1999. [Google Scholar]
- 502.Jonker D, Kuper CF, Fraile N, Estrella A, Rodriguez Otero C. Ninety-day oral toxicity study of lycopene from Blakeslea trispora in rats. Regul Toxicol Pharmacol. 2003;37:396–406. doi: 10.1016/s0273-2300(03)00013-8. [DOI] [PubMed] [Google Scholar]
- 503.Clark PE, Hall MC, Borden LS, Jr, Miller AA, Hu JJ, Lee WR, et al. Phase I-II prospective dose-escalating trial of lycopene in patients with biochemical relapse of prostate cancer after definitive local therapy. Urology. 2006;67:1257–1261. doi: 10.1016/j.urology.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 504.Cassileth B. Lycopene. Oncology (Williston Park) 2010;24:296. [PubMed] [Google Scholar]
- 505.Peiffer DS, Zimmerman NP, Wang LS, Ransom BW, Carmella SG, Kuo CT, et al. Chemoprevention of esophageal cancer with black raspberries, their component anthocyanins, and a major anthocyanin metabolite, protocatechuic acid. Cancer Prev Res (Phila) 2014;7:574–584. doi: 10.1158/1940-6207.CAPR-14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Thoppil RJ, Bhatia D, Barnes KF, Haznagy-Radnai E, Hohmann J, Darvesh AS, et al. Black currant anthocyanins abrogate oxidative stress through Nrf2- mediated antioxidant mechanisms in a rat model of hepatocellular carcinoma. Curr Cancer Drug Targets. 2012;12:1244–1257. doi: 10.2174/156800912803987968. [DOI] [PubMed] [Google Scholar]
- 507.Bishayee A, Thoppil RJ, Mandal A, Darvesh AS, Ohanyan V, Meszaros JG, et al. Black currant phytoconstituents exert chemoprevention of diethylnitrosamine-initiated hepatocarcinogenesis by suppression of the inflammatory response. Molecular carcinogenesis. 2013;52:304–317. doi: 10.1002/mc.21860. [DOI] [PubMed] [Google Scholar]
- 508.Kim JM, Kim KM, Park EH, Seo JH, Song JY, Shin SC, et al. Anthocyanins from black soybean inhibit Helicobacter pylori-induced inflammation in human gastric epithelial AGS cells. Microbiology and immunology. 2013;57:366–373. doi: 10.1111/1348-0421.12049. [DOI] [PubMed] [Google Scholar]
- 509.Rodrigo KA, Rawal Y, Renner RJ, Schwartz SJ, Tian Q, Larsen PE, et al. Suppression of the tumorigenic phenotype in human oral squamous cell carcinoma cells by an ethanol extract derived from freeze-dried black raspberries. Nutrition and cancer. 2006;54:58–68. doi: 10.1207/s15327914nc5401_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Reddy MK, Alexander-Lindo RL, Nair MG. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. Journal of agricultural and food chemistry. 2005;53:9268–9273. doi: 10.1021/jf051399j. [DOI] [PubMed] [Google Scholar]
- 511.Chen PN, Kuo WH, Chiang CL, Chiou HL, Hsieh YS, Chu SC. Black rice anthocyanins inhibit cancer cells invasion via repressions of MMPs and u-PA expression. Chemico-biological interactions. 2006;163:218–229. doi: 10.1016/j.cbi.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 512.Chen PN, Chu SC, Chiou HL, Kuo WH, Chiang CL, Hsieh YS. Mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer letters. 2006;235:248–259. doi: 10.1016/j.canlet.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 513.Afaq F, Malik A, Syed D, Maes D, Matsui MS, Mukhtar H. Pomegranate fruit extract modulates UV-B-mediated phosphorylation of mitogen-activated protein kinases and activation of nuclear factor kappa B in normal human epidermal keratinocytes paragraph sign. Photochem Photobiol. 2005;81:38–45. doi: 10.1562/2004-08-06-RA-264. [DOI] [PubMed] [Google Scholar]
- 514.Hushmendy S, Jayakumar L, Hahn AB, Bhoiwala D, Bhoiwala DL, Crawford DR. Select phytochemicals suppress human T-lymphocytes and mouse splenocytes suggesting their use in autoimmunity and transplantation. Nutr Res. 2009;29:568–578. doi: 10.1016/j.nutres.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of anthocyanins (E 163) as a food additive. EFSA Journal. 2013:11. [Google Scholar]
- 516.Miyazawa T, Nakagawa K, Kudo M, Muraishi K, Someya K. Direct intestinal absorption of red fruit anthocyanins, cyanidin-3-glucoside and cyanidin-3,5-diglucoside, into rats and humans. Journal of agricultural and food chemistry. 1999;47:1083–1091. doi: 10.1021/jf9809582. [DOI] [PubMed] [Google Scholar]
- 517.Marczylo TH, Cooke D, Brown K, Steward WP, Gescher AJ. Pharmacokinetics and metabolism of the putative cancer chemopreventive agent cyanidin-3-glucoside in mice. Cancer Chemother Pharmacol. 2009;64:1261–1268. doi: 10.1007/s00280-009-0996-7. [DOI] [PubMed] [Google Scholar]
- 518.Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer letters. 2008;269:226–242. doi: 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Li HQ, Luo Y, Qiao CH. The mechanisms of anticancer agents by genistein and synthetic derivatives of isoflavone. Mini reviews in medicinal chemistry. 2012;12:350–362. doi: 10.2174/138955712799829258. [DOI] [PubMed] [Google Scholar]
- 520.Wang Z, Li Y, Ahmad A, Banerjee S, Azmi AS, Kong D, et al. Down-regulation of Notch-1 is associated with Akt and FoxM1 in inducing cell growth inhibition and apoptosis in prostate cancer cells. Journal of cellular biochemistry. 2011;112:78–88. doi: 10.1002/jcb.22770. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 521.Lee J, Ju J, Park S, Hong SJ, Yoon S. Inhibition of IGF-1 signaling by genistein: modulation of E-cadherin expression and downregulation of beta-catenin signaling in hormone refractory PC-3 prostate cancer cells. Nutrition and cancer. 2012;64:153–162. doi: 10.1080/01635581.2012.630161. [DOI] [PubMed] [Google Scholar]
- 522.Liang C, Li H, Shen C, Lai J, Shi Z, Liu B, et al. Genistein potentiates the anti-cancer effects of gemcitabine in human osteosarcoma via the downregulation of Akt and nuclear factor-kappaB pathway. Anti-cancer agents in medicinal chemistry. 2012;12:554–563. doi: 10.2174/187152012800617867. [DOI] [PubMed] [Google Scholar]
- 523.Whyte J, Bergin O, Bianchi A, McNally S, Martin F. Key signalling nodes in mammary gland development and cancer. Mitogen-activated protein kinase signalling in experimental models of breast cancer progression and in mammary gland development. Breast Cancer Res. 2009;11:209. doi: 10.1186/bcr2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Qi W, Weber CR, Wasland K, Savkovic SD. Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC cancer. 2011;11:219. doi: 10.1186/1471-2407-11-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Sundin T, Hentosh P. InTERTesting association between telomerase, mTOR and phytochemicals. Expert reviews in molecular medicine. 2012;14:e8. doi: 10.1017/erm.2012.1. [DOI] [PubMed] [Google Scholar]
- 526.Bielecki A, Roberts J, Mehta R, Raju J. Estrogen receptor-beta mediates the inhibition of DLD-1 human colon adenocarcinoma cells by soy isoflavones. Nutrition and cancer. 2011;63:139–150. doi: 10.1080/01635581.2010.516867. [DOI] [PubMed] [Google Scholar]
- 527.Privat M, Aubel C, Arnould S, Communal Y, Ferrara M, Bignon YJ. AKT and p21 WAF1/CIP1 as potential genistein targets in BRCA1-mutant human breast cancer cell lines. Anticancer Res. 2010;30:2049–2054. [PubMed] [Google Scholar]
- 528.Ma Y, Wang J, Liu L, Zhu H, Chen X, Pan S, et al. Genistein potentiates the effect of arsenic trioxide against human hepatocellular carcinoma: role of Akt and nuclear factor-kappaB. Cancer letters. 2011;301:75–84. doi: 10.1016/j.canlet.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 529.Zhu H, Cheng H, Ren Y, Liu ZG, Zhang YF, De Luo B. Synergistic inhibitory effects by the combination of gefitinib and genistein on NSCLC with acquired drug-resistance in vitro and in vivo. Molecular biology reports. 2012;39:4971–4979. doi: 10.1007/s11033-011-1293-1. [DOI] [PubMed] [Google Scholar]
- 530.Zhang B, Shi ZL, Liu B, Yan XB, Feng J, Tao HM. Enhanced anticancer effect of gemcitabine by genistein in osteosarcoma: the role of Akt and nuclear factor-kappaB. Anti-cancer drugs. 2010;21:288–296. doi: 10.1097/CAD.0b013e328334da17. [DOI] [PubMed] [Google Scholar]
- 531.Sahin K, Tuzcu M, Basak N, Caglayan B, Kilic U, Sahin F, et al. Sensitization of Cervical Cancer Cells to Cisplatin by Genistein: The Role of NFkappaB and Akt/mTOR Signaling Pathways. Journal of oncology. 2012;2012:461562. doi: 10.1155/2012/461562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Park SJ, Kim MJ, Kim YK, Kim SM, Park JY, Myoung H. Combined cetuximab and genistein treatment shows additive anti-cancer effect on oral squamous cell carcinoma. Cancer letters. 2010;292:54–63. doi: 10.1016/j.canlet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 533.Ferenc P, Solar P, Kleban J, Mikes J, Fedorocko P. Down-regulation of Bcl-2 and Akt induced by combination of photoactivated hypericin and genistein in human breast cancer cells. Journal of photochemistry and photobiology B, Biology. 2010;98:25–34. doi: 10.1016/j.jphotobiol.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 534.Nakamura Y, Yogosawa S, Izutani Y, Watanabe H, Otsuji E, Sakai T. A combination of indol-3-carbinol and genistein synergistically induces apoptosis in human colon cancer HT-29 cells by inhibiting Akt phosphorylation and progression of autophagy. Molecular cancer. 2009;8:100. doi: 10.1186/1476-4598-8-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Hwang KA, Park MA, Kang NH, Yi BR, Hyun SH, Jeung EB, et al. Anticancer effect of genistein on BG-1 ovarian cancer growth induced by 17 beta-estradiol or bisphenol A via the suppression of the crosstalk between estrogen receptor alpha and insulin-like growth factor-1 receptor signaling pathways. Toxicol Appl Pharmacol. 2013;272:637–646. doi: 10.1016/j.taap.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 536.Kim SH, Kim SH, Kim YB, Jeon YT, Lee SC, Song YS. Genistein inhibits cell growth by modulating various mitogen-activated protein kinases and AKT in cervical cancer cells. Annals of the New York Academy of Sciences. 2009;1171:495–500. doi: 10.1111/j.1749-6632.2009.04899.x. [DOI] [PubMed] [Google Scholar]
- 537.Montales MT, Rahal OM, Kang J, Rogers TJ, Prior RL, Wu X, et al. Repression of mammosphere formation of human breast cancer cells by soy isoflavone genistein and blueberry polyphenolic acids suggests diet-mediated targeting of cancer stem-like/progenitor cells. Carcinogenesis. 2012;33:652–660. doi: 10.1093/carcin/bgr317. [DOI] [PubMed] [Google Scholar]
- 538.Ji C, Yang YL, He L, Gu B, Xia JP, Sun WL, et al. Increasing ceramides sensitizes genistein-induced melanoma cell apoptosis and growth inhibition. Biochemical and biophysical research communications. 2012;421:462–467. doi: 10.1016/j.bbrc.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 539.Oh HY, Leem J, Yoon SJ, Yoon S, Hong SJ. Lipid raft cholesterol and genistein inhibit the cell viability of prostate cancer cells via the partial contribution of EGFR-Akt/p70S6k pathway and downregulation of androgen receptor. Biochemical and biophysical research communications. 2010;393:319–324. doi: 10.1016/j.bbrc.2010.01.133. [DOI] [PubMed] [Google Scholar]
- 540.Greathouse KL, Bredfeldt T, Everitt JI, Lin K, Berry T, Kannan K, et al. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Molecular cancer research : MCR. 2012;10:546–557. doi: 10.1158/1541-7786.MCR-11-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Gu S, Papadopoulou N, Nasir O, Foller M, Alevizopoulos K, Lang F, et al. Activation of membrane androgen receptors in colon cancer inhibits the prosurvival signals Akt/bad in vitro and in vivo and blocks migration via vinculin/actin signaling. Molecular medicine (Cambridge, Mass. 2011;17:48–58. doi: 10.2119/molmed.2010.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Wietrzyk J, Mazurkiewicz M, Madej J, Dzimira S, Grynkiewicz G, Radzikowski C, et al. Genistein alone or combined with cyclophosphamide may stimulate 16/C transplantable mouse mammary cancer growth. Med Sci Monit. 2004;10:BR414–BR419. [PubMed] [Google Scholar]
- 543.Allred CD, Allred KF, Ju YH, Virant SM, Helferich WG. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001;61:5045–5050. [PubMed] [Google Scholar]
- 544.Nakamura H, Wang Y, Kurita T, Adomat H, Cunha GR, Wang Y. Genistein increases epidermal growth factor receptor signaling and promotes tumor progression in advanced human prostate cancer. PloS one. 2011;6:e20034. doi: 10.1371/journal.pone.0020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Taylor CK, Levy RM, Elliott JC, Burnett BP. The effect of genistein aglycone on cancer and cancer risk: a review of in vitro, preclinical, and clinical studies. Nutr Rev. 2009;67:398–415. doi: 10.1111/j.1753-4887.2009.00213.x. [DOI] [PubMed] [Google Scholar]
- 546.Pavese JM, Farmer RL, Bergan RC. Inhibition of cancer cell invasion and metastasis by genistein. Cancer metastasis reviews. 2010;29:465–482. doi: 10.1007/s10555-010-9238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Xu L, Ding Y, Catalona WJ, Yang XJ, Anderson WF, Jovanovic B, et al. MEK4 function, genistein treatment, and invasion of human prostate cancer cells. J Natl Cancer Inst. 2009;101:1141–1155. doi: 10.1093/jnci/djp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Messing E, Gee JR, Saltzstein DR, Kim K, diSant'Agnese A, Kolesar J, et al. A phase 2 cancer chemoprevention biomarker trial of isoflavone G-2535 (genistein) in presurgical bladder cancer patients. Cancer prevention research. 2012;5:621–630. doi: 10.1158/1940-6207.CAPR-11-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.El-Rayes BF, Philip PA, Sarkar FH, Shields AF, Ferris AM, Hess K, et al. A phase II study of isoflavones, erlotinib, and gemcitabine in advanced pancreatic cancer. Investigational new drugs. 2011;29:694–699. doi: 10.1007/s10637-010-9386-6. [DOI] [PubMed] [Google Scholar]
- 550.Pendleton JM, Tan WW, Anai S, Chang M, Hou W, Shiverick KT, et al. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC cancer. 2008;8:132. doi: 10.1186/1471-2407-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.deVere White RW, Hackman RM, Soares SE, Beckett LA, Li Y, Sun B. Effects of a genistein-rich extract on PSA levels in men with a history of prostate cancer. Urology. 2004;63:259–263. doi: 10.1016/j.urology.2003.09.061. [DOI] [PubMed] [Google Scholar]
- 552.Kogiso M, Sakai T, Mitsuya K, Komatsu T, Yamamoto S. Genistein suppresses antigen-specific immune responses through competition with 17beta-estradiol for estrogen receptors in ovalbumin-immunized BALB/c mice. Nutrition. 2006;22:802–809. doi: 10.1016/j.nut.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 553.Jiang X, Patterson NM, Ling Y, Xie J, Helferich WG, Shapiro DJ. Low concentrations of the soy phytoestrogen genistein induce proteinase inhibitor 9 and block killing of breast cancer cells by immune cells. Endocrinology. 2008;149:5366–5373. doi: 10.1210/en.2008-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Ghaemi A, Soleimanjahi H, Razeghi S, Gorji A, Tabaraei A, Moradi A, et al. Genistein induces a protective immunomodulatory effect in a mouse model of cervical cancer. Iranian journal of immunology : IJI. 2012;9:119–127. [PubMed] [Google Scholar]
- 555.Guo TL, Chi RP, Hernandez DM, Auttachoat W, Zheng JF. Decreased 7,12-dimethylbenz[a]anthracene-induced carcinogenesis coincides with the induction of antitumor immunities in adult female B6C3F1 mice pretreated with genistein. Carcinogenesis. 2007;28:2560–256. doi: 10.1093/carcin/bgm223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Melo SA, Esteller M. Disruption of microRNA nuclear transport in human cancer. Semin Cancer Biol. 2014;27C:46–51. doi: 10.1016/j.semcancer.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 557.Izzotti A, Cartiglia C, Steele VE, De Flora S. MicroRNAs as targets for dietary and pharmacological inhibitors of mutagenesis and carcinogenesis. Mutat Res. 2012 doi: 10.1016/j.mrrev.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Stark A, Brennecke J, Bushati N, Russell RB, Cohen SM. Animal MicroRNAs confer robustness to gene expression and have a significant impact on 3'UTR evolution. Cell. 2005;123:1133–1146. doi: 10.1016/j.cell.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 559.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 561.Cho WC. OncomiRs: the discovery and progress of microRNAs in cancers. Mol Cancer. 2007;6:60. doi: 10.1186/1476-4598-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 564.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 565.Lodish HF, Zhou B, Liu G, Chen CZ. Micromanagement of the immune system by microRNAs. Nat Rev Immunol. 2008;8:120–130. doi: 10.1038/nri2252. [DOI] [PubMed] [Google Scholar]
- 566.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Ha TY. The Role of MicroRNAs in Regulatory T Cells and in the Immune Response. Immune Netw. 2011;11:11–41. doi: 10.4110/in.2011.11.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Fernando TR, Rodriguez-Malave NI, Rao DS. MicroRNAs in B cell development and malignancy. J Hematol Oncol. 2012;5:7. doi: 10.1186/1756-8722-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Tili E, Michaille JJ, Croce CM. MicroRNAs play a central role in molecular dysfunctions linking inflammation with cancer. Immunol Rev. 2013;253:167–184. doi: 10.1111/imr.12050. [DOI] [PubMed] [Google Scholar]
- 570.Niwa R, Slack FJ. The evolution of animal microRNA function. Curr Opin Genet Dev. 2007;17:145–150. doi: 10.1016/j.gde.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 571.Tili E, Michaille JJ, Costinean S, Croce CM. MicroRNAs, the immune system and rheumatic disease. Nat Clin Pract Rheumatol. 2008;4:534–541. doi: 10.1038/ncprheum0885. [DOI] [PubMed] [Google Scholar]
- 572.O'Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol. 2012;30:295–312. doi: 10.1146/annurev-immunol-020711-075013. [DOI] [PubMed] [Google Scholar]
- 573.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Wu F, Zikusoka M, Trindade A, Dassopoulos T, Harris ML, Bayless TM, et al. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135:1624–1635. e24. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 575.Tang Y, Luo X, Cui H, Ni X, Yuan M, Guo Y, et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009;60:1065–1075. doi: 10.1002/art.24436. [DOI] [PubMed] [Google Scholar]
- 576.Jones SW, Watkins G, Le Good N, Roberts S, Murphy CL, Brockbank SM, et al. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. Osteoarthritis Cartilage. 2009;17:464–4672. doi: 10.1016/j.joca.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 577.Jin Z, Selaru FM, Cheng Y, Kan T, Agarwal R, Mori Y, et al. MicroRNA-192 and-215 are upregulated in human gastric cancer in vivo and suppress ALCAM expression in vitro. Oncogene. 2011;30:1577–1585. doi: 10.1038/onc.2010.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Han Y, Chen J, Zhao X, Liang C, Wang Y, Sun L, et al. MicroRNA expression signatures of bladder cancer revealed by deep sequencing. PLoS ONE. 2011;6:e18286. doi: 10.1371/journal.pone.0018286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Dahiya N, Sherman-Baust CA, Wang TL, Davidson B, Shih Ie M, Zhang Y, et al. MicroRNA expression and identification of putative miRNA targets in ovarian cancer. PLoS ONE. 2008;3:e2436. doi: 10.1371/journal.pone.0002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Tili E, Michaille JJ, Wernicke D, Alder H, Costinean S, Volinia S, et al. Mutator activity induced by microRNA-155 (miR-155) links inflammation and cancer. Proc Natl Acad Sci U S A. 2011;108:4908–4913. doi: 10.1073/pnas.1101795108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Eis PS, Tam W, Sun L, Chadburn A, Li Z, Gomez MF, et al. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci U S A. 2005;102:3627–3632. doi: 10.1073/pnas.0500613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Tam W. Identification and characterization of human BIC, a gene on chromosome 21 that encodes a noncoding RNA. Gene. 2001;274:157–167. doi: 10.1016/s0378-1119(01)00612-6. [DOI] [PubMed] [Google Scholar]
- 587.Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, et al. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 588.O'Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007;104:1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 590.van den Berg A, Kroesen BJ, Kooistra K, de Jong D, Briggs J, Blokzijl T, et al. High expression of B-cell receptor inducible gene BIC in all subtypes of Hodgkin lymphoma. Genes Chromosomes Cancer. 2003;37:20–28. doi: 10.1002/gcc.10186. [DOI] [PubMed] [Google Scholar]
- 591.Haasch D, Chen YW, Reilly RM, Chiou XG, Koterski S, Smith ML, et al. T cell activation induces a noncoding RNA transcript sensitive to inhibition by immunosuppressant drugs and encoded by the proto-oncogene, BIC. Cell Immunol. 2002;217:78–86. doi: 10.1016/s0008-8749(02)00506-3. [DOI] [PubMed] [Google Scholar]
- 592.O'Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, et al. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33:607–619. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Murugaiyan G, Beynon V, Mittal A, Joller N, Weiner HL. Silencing microRNA-155 ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2011;187:2213–2221. doi: 10.4049/jimmunol.1003952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Jiang J, Lee EJ, Schmittgen TD. Increased expression of microRNA-155 in Epstein-Barr virus transformed lymphoblastoid cell lines. Genes Chromosomes Cancer. 2006;45:103–106. doi: 10.1002/gcc.20264. [DOI] [PubMed] [Google Scholar]
- 595.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Tam W, Dahlberg JE. miR-155/BIC as an oncogenic microRNA. Genes Chromosomes Cancer. 2006;45:211–212. doi: 10.1002/gcc.20282. [DOI] [PubMed] [Google Scholar]
- 597.Costinean S, Zanesi N, Pekarsky Y, Tili E, Volinia S, Heerema N, et al. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice. Proc Natl Acad Sci U S A. 2006;103:7024–7029. doi: 10.1073/pnas.0602266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Jiang S, Zhang HW, Lu MH, He XH, Li Y, Gu H, et al. MicroRNA-155 functions as an OncomiR in breast cancer by targeting the suppressor of cytokine signaling 1 gene. Cancer Res. 2010;70:3119–3127. doi: 10.1158/0008-5472.CAN-09-4250. [DOI] [PubMed] [Google Scholar]
- 599.Neilsen PM, Noll JE, Mattiske S, Bracken CP, Gregory PA, Schulz RB, et al. Mutant p53 drives invasion in breast tumors through up-regulation of miR-155. Oncogene. 2012 doi: 10.1038/onc.2012.305. [DOI] [PubMed] [Google Scholar]
- 600.Gironella M, Seux M, Xie MJ, Cano C, Tomasini R, Gommeaux J, et al. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc Natl Acad Sci U S A. 2007;104:16170–16175. doi: 10.1073/pnas.0703942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Junker A, Krumbholz M, Eisele S, Mohan H, Augstein F, Bittner R, et al. MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain. 2009;132:3342–3352. doi: 10.1093/brain/awp300. [DOI] [PubMed] [Google Scholar]
- 602.Stanczyk J, Pedrioli DM, Brentano F, Sanchez-Pernaute O, Kolling C, Gay RE, et al. Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum. 2008;58:1001–1009. doi: 10.1002/art.23386. [DOI] [PubMed] [Google Scholar]
- 603.Tokuhiro S, Yamada R, Chang X, Suzuki A, Kochi Y, Sawada T, et al. An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritis. Nat Genet. 2003;35:341–348. doi: 10.1038/ng1267. [DOI] [PubMed] [Google Scholar]
- 604.Rioux JD, Daly MJ, Silverberg MS, Lindblad K, Steinhart H, Cohen Z, et al. Genetic variation in the 5q31 cytokine gene cluster confers susceptibility to Crohn disease. Nat Genet. 2001;29:223–228. doi: 10.1038/ng1001-223. [DOI] [PubMed] [Google Scholar]
- 605.Palmer LJ, Daniels SE, Rye PJ, Gibson NA, Tay GK, Cookson WO, et al. Linkage of chromosome 5q and 11q gene markers to asthma-associated quantitative traits in Australian children. Am J Respir Crit Care Med. 1998;158:1825–1830. doi: 10.1164/ajrccm.158.6.9804037. [DOI] [PubMed] [Google Scholar]
- 606.Friberg C, Bjorck K, Nilsson S, Inerot A, Wahlstrom J, Samuelsson L. Analysis of chromosome 5q31–32 and psoriasis: confirmation of a susceptibility locus but no association with SNPs within SLC22A4 and SLC22A5. J Investig Dermatol. 2006;126:998–1002. doi: 10.1038/sj.jid.5700194. [DOI] [PubMed] [Google Scholar]
- 607.Bhaumik D, Scott GK, Schokrpur S, Patil CK, Campisi J, Benz CC. Expression of microRNA-146 suppresses NF-kappaB activity with reduction of metastatic potential in breast cancer cells. Oncogene. 2008;27:5643–5647. doi: 10.1038/onc.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Nakasa T, Miyaki S, Okubo A, Hashimoto M, Nishida K, Ochi M, et al. Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Rheum. 2008;58:1284–1292. doi: 10.1002/art.23429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Matysiak M, Fortak-Michalska M, Szymanska B, Orlowski W, Jurewicz A, Selmaj K. MicroRNA-146a Negatively Regulates the Immunoregulatory Activity of Bone Marrow Stem Cells by Targeting Prostaglandin E2 Synthase-2. J Immunol. 2013;190:5102–5109. doi: 10.4049/jimmunol.1202397. [DOI] [PubMed] [Google Scholar]
- 610.Yang L, Boldin MP, Yu Y, Liu CS, Ea CK, Ramakrishnan P, et al. miR-146a controls the resolution of T cell responses in mice. J Exp Med. 2012;209:1655–1670. doi: 10.1084/jem.20112218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M, et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. 2011;208:1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Zhao JL, Rao DS, Boldin MP, Taganov KD, O'Connell RM, Baltimore D. NF-kappaB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. Proc Natl Acad Sci U S A. 2011;108:9184–9189. doi: 10.1073/pnas.1105398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Xie YF, Shu R, Jiang SY, Liu DL, Ni J, Zhang XL. MicroRNA-146 inhibits pro-inflammatory cytokine secretion through IL-1 receptor-associated kinase 1 in human gingival fibroblasts. J Inflamm (Lond) 2013;10:20. doi: 10.1186/1476-9255-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 616.Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J Biol Chem. 2007;282:14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 617.Liu M, Wu H, Liu T, Li Y, Wang F, Wan H, et al. Regulation of the cell cycle gene, BTG2, by miR-21 in human laryngeal carcinoma. Cell Res. 2009;19:828–837. doi: 10.1038/cr.2009.72. [DOI] [PubMed] [Google Scholar]
- 618.Papagiannakopoulos T, Shapiro A, Kosik KS. MicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Res. 2008;68:8164–8172. doi: 10.1158/0008-5472.CAN-08-1305. [DOI] [PubMed] [Google Scholar]
- 619.du Rieu MC, Torrisani J, Selves J, Al Saati T, Souque A, Dufresne M, et al. MicroRNA-21 is induced early in pancreatic ductal adenocarcinoma precursor lesions. Clin Chem. 2010;56:603–612. doi: 10.1373/clinchem.2009.137364. [DOI] [PubMed] [Google Scholar]
- 620.Yan LX, Huang XF, Shao Q, Huang MY, Deng L, Wu QL, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14:2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Slaby O, Svoboda M, Fabian P, Smerdova T, Knoflickova D, Bednarikova M, et al. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology. 2007;72:397–402. doi: 10.1159/000113489. [DOI] [PubMed] [Google Scholar]
- 622.Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O'Leary JJ, Ruan Q, et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 623.Loffler D, Brocke-Heidrich K, Pfeifer G, Stocsits C, Hackermuller J, Kretzschmar AK, et al. Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood. 2007;110:1330–1333. doi: 10.1182/blood-2007-03-081133. [DOI] [PubMed] [Google Scholar]
- 624.Schetter AJ, Nguyen GH, Bowman ED, Mathe EA, Yuen ST, Hawkes JE, et al. Association of inflammation-related and microRNA gene expression with cancer-specific mortality of colon adenocarcinoma. Clin Cancer Res. 2009;15:5878–5887. doi: 10.1158/1078-0432.CCR-09-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Philippe L, Alsaleh G, Bahram S, Pfeffer S, Georgel P. The miR-17 approximately 92 Cluster: A Key Player in the Control of Inflammation during Rheumatoid Arthritis. Front Immunol. 2013;4:70. doi: 10.3389/fimmu.2013.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Pichiorri F, Suh SS, Ladetto M, Kuehl M, Palumbo T, Drandi D, et al. MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. Proc Natl Acad Sci U S A. 2008;105:12885–12890. doi: 10.1073/pnas.0806202105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Qin S, Ai F, Ji WF, Rao W, Zhang HC, Yao WJ. miR-19a Promotes Cell Growth and Tumorigenesis through Targeting SOCS1 in Gastric Cancer. Asian Pac J Cancer Prev. 2013;14:835–840. doi: 10.7314/apjcp.2013.14.2.835. [DOI] [PubMed] [Google Scholar]
- 628.Mestdagh P, Bostrom AK, Impens F, Fredlund E, Van Peer G, De Antonellis P, et al. The miR-17-92 microRNA cluster regulates multiple components of the TGF-beta pathway in neuroblastoma. Mol Cell. 2010;40:762–773. doi: 10.1016/j.molcel.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Gantier MP, Stunden HJ, McCoy CE, Behlke MA, Wang D, Kaparakis-Liaskos M, et al. A miR-19 regulon that controls NF-kappaB signaling. Nucleic Acids Res. 2012;40:8048–8058. doi: 10.1093/nar/gks521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Mavrakis KJ, Wolfe AL, Oricchio E, Palomero T, de Keersmaecker K, McJunkin K, et al. Genome-wide RNA-mediated interference screen identifies miR-19 targets in Notch-induced T-cell acute lymphoblastic leukaemia. Nat Cell Biol. 2010;12:372–379. doi: 10.1038/ncb2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Olive V, Bennett MJ, Walker JC, Ma C, Jiang I, Cordon-Cardo C, et al. miR-19 is a key oncogenic component of mir-17–92. Genes Dev. 2009;23:2839–2849. doi: 10.1101/gad.1861409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Mu P, Han YC, Betel D, Yao E, Squatrito M, Ogrodowski P, et al. Genetic dissection of the miR-17~92 cluster of microRNAs in Myc-induced B-cell lymphomas. Genes Dev. 2009;23:2806–2811. doi: 10.1101/gad.1872909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Maru DM, Singh RR, Hannah C, Albarracin CT, Li YX, Abraham R, et al. MicroRNA-196a is a potential marker of progression during Barrett's metaplasia-dysplasia-invasive adenocarcinoma sequence in esophagus. Am J Pathol. 2009;174:1940–1948. doi: 10.2353/ajpath.2009.080718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Luthra R, Singh RR, Luthra MG, Li YX, Hannah C, Romans AM, et al. MicroRNA-196a targets annexin A1: a microRNA-mediated mechanism of annexin A1 downregulation in cancers. Oncogene. 2008;27:6667–6678. doi: 10.1038/onc.2008.256. [DOI] [PubMed] [Google Scholar]
- 635.Brest P, Lapaquette P, Souidi M, Lebrigand K, Cesaro A, Vouret-Craviari V, et al. A synonymous variant in IRGM alters a binding site for miR-196 and causes deregulation of IRGM-dependent xenophagy in Crohn's disease. Nat Genet. 2011;43:242–245. doi: 10.1038/ng.762. [DOI] [PubMed] [Google Scholar]
- 636.Chu H, Wang M, Shi D, Ma L, Zhang Z, Tong N, et al. Hsa-miR-196a2 Rs11614913 polymorphism contributes to cancer susceptibility: evidence from 15 case-control studies. PLoS ONE. 2011;6:e18108. doi: 10.1371/journal.pone.0018108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Tili E, Michaille JJ, Adair B, Alder H, Limagne E, Taccioli C, et al. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a microRNA targeting JunB and JunD. Carcinogenesis. 2010;31:1561–1566. doi: 10.1093/carcin/bgq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Pan J, Hu H, Zhou Z, Sun L, Peng L, Yu L, et al. Tumor-suppressive mir-663 gene induces mitotic catastrophe growth arrest in human gastric cancer cells. Oncol Rep. 2010;24:105–112. doi: 10.3892/or_00000834. [DOI] [PubMed] [Google Scholar]
- 639.Hu H, Li S, Cui X, Lv X, Jiao Y, Yu F, et al. The Overexpression of Hypomethylated miR-663 Induces Chemotherapy Resistance in Human Breast Cancer Cells by Targeting Heparin Sulfate Proteoglycan 2 (HSPG2) J Biol Chem. 2013;288:10973–10985. doi: 10.1074/jbc.M112.434340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Bazzoni F, Rossato M, Fabbri M, Gaudiosi D, Mirolo M, Mori L, et al. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci U S A. 2009;106:5282–5287. doi: 10.1073/pnas.0810909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Almeida MI, Reis RM, Calin GA. MYC-microRNA-9-metastasis connection in breast cancer. Cell Res. 2010;20:603–604. doi: 10.1038/cr.2010.70. [DOI] [PubMed] [Google Scholar]
- 642.Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12:247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Giannakakis A, Sandaltzopoulos R, Greshock J, Liang S, Huang J, Hasegawa K, et al. miR-210 links hypoxia with cell cycle regulation and is deleted in human epithelial ovarian cancer. Cancer Biol Ther. 2008;7:255–264. doi: 10.4161/cbt.7.2.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Biswas S, Roy S, Banerjee J, Hussain SR, Khanna S, Meenakshisundaram G, et al. Hypoxia inducible microRNA 210 attenuates keratinocyte proliferation and impairs closure in a murine model of ischemic wounds. Proc Natl Acad Sci U S A. 2010;107:6976–6981. doi: 10.1073/pnas.1001653107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Fasanaro P, D'Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, et al. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell. 2009;35:856–867. doi: 10.1016/j.molcel.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Bandi N, Zbinden S, Gugger M, Arnold M, Kocher V, Hasan L, et al. miR-15a and miR-16 are implicated in cell cycle regulation in a Rb-dependent manner and are frequently deleted or down-regulated in non-small cell lung cancer. Cancer Res. 2009;69:5553–5559. doi: 10.1158/0008-5472.CAN-08-4277. [DOI] [PubMed] [Google Scholar]
- 650.Klein U, Lia M, Crespo M, Siegel R, Shen Q, Mo T, et al. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell. 2010;17:28–40. doi: 10.1016/j.ccr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 651.Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, Memeo L, et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med. 2008;14:1271–1277. doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- 652.Porkka KP, Ogg EL, Saramaki OR, Vessella RL, Pukkila H, Lahdesmaki H, et al. The miR-15amiR-16-1 locus is homozygously deleted in a subset of prostate cancers. Genes Chromosomes Cancer. 2011;50:499–509. doi: 10.1002/gcc.20873. [DOI] [PubMed] [Google Scholar]
- 653.Rivas MA, Venturutti L, Huang YW, Schillaci R, Huang TH, Elizalde PV. Downregulation of the tumor-suppressor miR-16 via progestin-mediated oncogenic signaling contributes to breast cancer development. Breast Cancer Res. 2012;14:R77. doi: 10.1186/bcr3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Young LE, Moore AE, Sokol L, Meisner-Kober N, Dixon DA. The mRNA stability factor HuR inhibits microRNA-16 targeting of COX-2. Mol Cancer Res. 2012;10:167–180. doi: 10.1158/1541-7786.MCR-11-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Cai CK, Zhao GY, Tian LY, Liu L, Yan K, Ma YL, et al. miR-15a and miR-16-1 downregulate CCND1 and induce apoptosis and cell cycle arrest in osteosarcoma. Oncol Rep. 2012;28:1764–1770. doi: 10.3892/or.2012.1995. [DOI] [PubMed] [Google Scholar]
- 656.Linsley PS, Schelter J, Burchard J, Kibukawa M, Martin MM, Bartz SR, et al. Transcripts targeted by the microRNA-16 family cooperatively regulate cell cycle progression. Mol Cell Biol. 2007;27:2240–2252. doi: 10.1128/MCB.02005-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Rissland OS, Hong SJ, Bartel DP. MicroRNA destabilization enables dynamic regulation of the miR-16 family in response to cell-cycle changes. Mol Cell. 2011;43:993–1004. doi: 10.1016/j.molcel.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Wu G, Yu F, Xiao Z, Xu K, Xu J, Tang W, et al. Hepatitis B virus X protein downregulates expression of the miR-16 family in malignant hepatocytes in vitro. Br J Cancer. 2011;105:146–153. doi: 10.1038/bjc.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Ofir M, Hacohen D, Ginsberg D. MiR-15 and miR-16 are direct transcriptional targets of E2F1 that limit E2F-induced proliferation by targeting cyclin E. Mol Cancer Res. 2011;9:440–447. doi: 10.1158/1541-7786.MCR-10-0344. [DOI] [PubMed] [Google Scholar]
- 660.Wang F, Fu XD, Zhou Y, Zhang Y. Down-regulation of the cyclin E1 oncogene expression by microRNA-16-1 induces cell cycle arrest in human cancer cells. BMB Rep. 2009;42:725–730. doi: 10.5483/bmbrep.2009.42.11.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Takeshita F, Patrawala L, Osaki M, Takahashi RU, Yamamoto Y, Kosaka N, et al. Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell-cycle genes. Mol Ther. 2010;18:181–187. doi: 10.1038/mt.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Lerner M, Harada M, Loven J, Castro J, Davis Z, Oscier D, et al. DLEU2, frequently deleted in malignancy, functions as a critical host gene of the cell cycle inhibitory microRNAs miR-15a and miR-16-1. Exp Cell Res. 2009;315:2941–2952. doi: 10.1016/j.yexcr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 663.Cui X, Witalison EE, Chumanevich AP, Chumanevich AA, Poudyal D, Subramanian V, et al. The induction of microRNA-16 in colon cancer cells by protein arginine deiminase inhibition causes a p53-dependent cell cycle arrest. PLoS ONE. 2013;8:e53791. doi: 10.1371/journal.pone.0053791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Makrygiannakis D, af Klint E, Lundberg IE, Lofberg R, Ulfgren AK, Klareskog L, et al. Citrullination is an inflammation-dependent process. Ann Rheum Dis. 2006;65:1219–1222. doi: 10.1136/ard.2005.049403. Epub 2006 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179:5082–5089. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 666.Sonkoly E, Stahle M, Pivarcsi A. MicroRNAs: novel regulators in skin inflammation. Clin Exp Dermatol. 2008;33:312–315. doi: 10.1111/j.1365-2230.2008.02804.x. [DOI] [PubMed] [Google Scholar]
- 667.Karius T, Schnekenburger M, Dicato M, Diederich M. MicroRNAs in cancer management and their modulation by dietary agents. Biochem Pharmacol. 2012;83:1591–1601. doi: 10.1016/j.bcp.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 668.Tili E, Michaille JJ. Resveratrol, MicroRNAs, Inflammation, and Cancer. J Nucleic Acids. 2011;2011:102431. doi: 10.4061/2011/102431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Sarkar FH, Li Y. Cell signaling pathways altered by natural chemopreventive agents. Mutat Res. 2004;555:53–64. doi: 10.1016/j.mrfmmm.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 670.Mukhopadhyay A, Bueso-Ramos C, Chatterjee D, Pantazis P, Aggarwal BB. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene. 2001;20:7597–7609. doi: 10.1038/sj.onc.1204997. [DOI] [PubMed] [Google Scholar]
- 671.Howell JC, Chun E, Farrell AN, Hur EY, Caroti CM, Iuvone PM, et al. Global microRNA expression profiling: curcumin (diferuloylmethane) alters oxidative stress-responsive microRNAs in human ARPE-19 cells. Mol Vis. 2013;19:544–560. [PMC free article] [PubMed] [Google Scholar]
- 672.Bao B, Ali S, Banerjee S, Wang Z, Logna F, Azmi AS, et al. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 2012;72:335–345. doi: 10.1158/0008-5472.CAN-11-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, et al. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010;70:3606–3617. doi: 10.1158/0008-5472.CAN-09-4598. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 674.Mudduluru G, George-William JN, Muppala S, Asangani IA, Kumarswamy R, Nelson LD, et al. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci Rep. 2011;31:185–197. doi: 10.1042/BSR20100065. [DOI] [PubMed] [Google Scholar]
- 675.Salerno E, Scaglione BJ, Coffman FD, Brown BD, Baccarini A, Fernandes H, et al. Correcting miR-15a/16 genetic defect in New Zealand Black mouse model of CLL enhances drug sensitivity. Molecular Cancer Therapeutics. 2009;8:2684–2692. doi: 10.1158/1535-7163.MCT-09-0127. [DOI] [PubMed] [Google Scholar]
- 676.Li Y, VandenBoom TG, 2nd, Kong D, Wang Z, Ali S, Philip PA, et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Li Y, Vandenboom TG, 2nd, Wang Z, Kong D, Ali S, Philip PA, et al. miR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 2010;70:1486–1495. doi: 10.1158/0008-5472.CAN-09-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Wang H, Bian S, Yang CS. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1alpha. Carcinogenesis. 2011;32:1881–1889. doi: 10.1093/carcin/bgr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Siddiqui IA, Asim M, Hafeez BB, Adhami VM, Tarapore RS, Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. Faseb J. 2011;25:1198–1207. doi: 10.1096/fj.10-167924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Chakrabarti M, Khandkar M, Banik NL, Ray SK. Alterations in expression of specific microRNAs by combination of 4-HPR and EGCG inhibited growth of human malignant neuroblastoma cells. Brain Res. 2012;1454:1–13. doi: 10.1016/j.brainres.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Singh B, Cook KR, Vincent L, Hall CS, Berry JA, Multani AS, et al. Cyclooxygenase-2 induces genomic instability, BCL2 expression, doxorubicin resistance, and altered cancer-initiating cell phenotype in MCF7 breast cancer cells. The Journal of surgical research. 2008;147:240–246. doi: 10.1016/j.jss.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 682.Wen S, Li H, Wu ML, Fan SH, Wang Q, Shu XH, et al. Inhibition of NF-kappaB signaling commits resveratrol-treated medulloblastoma cells to apoptosis without neuronal differentiation. Journal of neuro-oncology. 2011;104:169–177. doi: 10.1007/s11060-010-0496-y. [DOI] [PubMed] [Google Scholar]
- 683.Liu L, Yang Z, Xu Y, Li J, Xu D, Zhang L, et al. Inhibition of oxidative stress-elicited AKT activation facilitates PPARgamma agonist-mediated inhibition of stem cell character and tumor growth of liver cancer cells. PloS one. 2013;8:e73038. doi: 10.1371/journal.pone.0073038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Sampath D, Malik A, Plunkett W, Nowak B, Williams B, Burton M, et al. Phase I clinical, pharmacokinetic, and pharmacodynamic study of the Akt-inhibitor triciribine phosphate monohydrate in patients with advanced hematologic malignancies. Leukemia research. 2013;37:1461–1467. doi: 10.1016/j.leukres.2013.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Arumugam A, Walsh SB, Xu J, Afaq F, Elmets CA, Athar M. Combined inhibition of p38 and Akt signaling pathways abrogates cyclosporine A-mediated pathogenesis of aggressive skin SCCs. Biochemical and biophysical research communications. 2012;425:177–181. doi: 10.1016/j.bbrc.2012.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Grosch S, Tegeder I, Niederberger E, Brautigam L, Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001;15:2742–2744. doi: 10.1096/fj.01-0299fje. [DOI] [PubMed] [Google Scholar]
- 687.Qin J, Yuan J, Li L, Liu H, Qin R, Qin W, et al. In vitro and in vivo inhibitory effect evaluation of cyclooxygenase-2 inhibitors, antisense cyclooxygenase-2 cDNA, and their combination on the growth of human bladder cancer cells. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2009;63:241–248. doi: 10.1016/j.biopha.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 688.Gowda R, Madhunapantula SV, Desai D, Amin S, Robertson GP. Simultaneous targeting of COX-2 and AKT using selenocoxib-1-GSH to inhibit melanoma. Molecular cancer therapeutics. 2013;12:3–15. doi: 10.1158/1535-7163.MCT-12-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Connelly L, Barham W, Onishko HM, Sherrill T, Chodosh LA, Blackwell TS, et al. Inhibition of NF-kappa B activity in mammary epithelium increases tumor latency and decreases tumor burden. Oncogene. 2011;30:1402–1412. doi: 10.1038/onc.2010.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Li J, Cheng Y, Qu W, Sun Y, Wang Z, Wang H, et al. Fisetin, a dietary flavonoid, induces cell cycle arrest and apoptosis through activation of p53 and inhibition of NF-kappa B pathways in bladder cancer cells. Basic & clinical pharmacology & toxicology. 2011;108:84–93. doi: 10.1111/j.1742-7843.2010.00613.x. [DOI] [PubMed] [Google Scholar]
- 691.Harikumar KB, Sung B, Tharakan ST, Pandey MK, Joy B, Guha S, et al. Sesamin manifests chemopreventive effects through the suppression of NF-kappa B-regulated cell survival, proliferation, invasion, and angiogenic gene products. Molecular cancer research : MCR. 2010;8:751–761. doi: 10.1158/1541-7786.MCR-09-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Lo MC, Yip TC, Ngan KC, Cheng WW, Law CK, Chan PS, et al. Role of MIF/CXCL8/CXCR2 signaling in the growth of nasopharyngeal carcinoma tumor spheres. Cancer letters. 2013;335:81–92. doi: 10.1016/j.canlet.2013.01.052. [DOI] [PubMed] [Google Scholar]
- 693.Tsubaki M, Komai M, Itoh T, Imano M, Sakamoto K, Shimaoka H, et al. Inhibition of the tumour necrosis factor-alpha autocrine loop enhances the sensitivity of multiple myeloma cells to anticancer drugs. Eur J Cancer. 2013;49:3708–3717. doi: 10.1016/j.ejca.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 694.Flores MB, Rocha GZ, Damas-Souza DM, Osorio-Costa F, Dias MM, Ropelle ER, et al. Obesity-induced increase in tumor necrosis factor-alpha leads to development of colon cancer in mice. Gastroenterology. 2012;143:741–753. e1–e4. doi: 10.1053/j.gastro.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 695.Lopez-Rivera E, Jayaraman P, Parikh F, Davies MA, Ekmekcioglu S, Izadmehr S, et al. Inducible nitric oxide synthase drives mTOR pathway activation and proliferation of human melanoma by reversible nitrosylation of TSC2. Cancer Res. 2014;74:1067–1078. doi: 10.1158/0008-5472.CAN-13-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Kunter I, Erdal E, Nart D, Yilmaz F, Karademir S, Sagol O, et al. Active form of AKT controls cell proliferation and response to apoptosis in hepatocellular carcinoma. Oncology reports. 2014;31:573–580. doi: 10.3892/or.2013.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Chen G, Chen SM, Wang X, Ding XF, Ding J, Meng LH. Inhibition of chemokine (CXC motif) ligand 12/chemokine (CXC motif) receptor 4 axis (CXCL12/CXCR4)-mediated cell migration by targeting mammalian target of rapamycin (mTOR) pathway in human gastric carcinoma cells. J Biol Chem. 2012;287:12132–12141. doi: 10.1074/jbc.M111.302299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 699.Axelsson H, Lonnroth C, Andersson M, Lundholm K. Mechanisms behind COX-1 and COX-2 inhibition of tumor growth in vivo. Int J Oncol. 2010;37:1143–1152. doi: 10.3892/ijo_00000766. [DOI] [PubMed] [Google Scholar]
- 700.Meyer-Siegler KL, Iczkowski KA, Leng L, Bucala R, Vera PL. Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J Immunol. 2006;177:8730–8739. doi: 10.4049/jimmunol.177.12.8730. [DOI] [PubMed] [Google Scholar]
- 701.Faurschou A, Gniadecki R, Calay D, Wulf HC. TNF-alpha impairs the S-G2/M cell cycle checkpoint and cyclobutane pyrimidine dimer repair in premalignant skin cells: role of the PI3K-Akt pathway. The Journal of investigative dermatology. 2008;128:2069–2077. doi: 10.1038/jid.2008.19. [DOI] [PubMed] [Google Scholar]
- 702.Pusztai L, Lewis CE, McGee JO. Growth arrest of the breast cancer cell line, T47D, by TNF alpha; cell cycle specificity and signal transduction. British journal of cancer. 1993;67:290–296. doi: 10.1038/bjc.1993.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Liu Q, Chan ST, Mahendran R. Nitric oxide induces cyclooxygenase expression and inhibits cell growth in colon cancer cell lines. Carcinogenesis. 2003;24:637–642. doi: 10.1093/carcin/bgg014. [DOI] [PubMed] [Google Scholar]
- 704.Krystal GW, Sulanke G, Litz J. Inhibition of phosphatidylinositol 3-kinase-Akt signaling blocks growth, promotes apoptosis, and enhances sensitivity of small cell lung cancer cells to chemotherapy. Molecular cancer therapeutics. 2002;1:913–922. [PubMed] [Google Scholar]
- 705.Cho KS, Yoon SJ, Lee JY, Cho NH, Choi YD, Song YS, et al. Inhibition of tumor growth and histopathological changes following treatment with a chemokine receptor CXCR4 antagonist in a prostate cancer xenograft model. Oncology letters. 2013;6:933–938. doi: 10.3892/ol.2013.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Jendrossek V. Targeting apoptosis pathways by Celecoxib in cancer. Cancer letters. 2013;332:313–324. doi: 10.1016/j.canlet.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 707.Wang H, Cho CH. Effect of NF-kappaB signaling on apoptosis in chronic inflammation-associated carcinogenesis. Current cancer drug targets. 2010;10:593–599. doi: 10.2174/156800910791859425. [DOI] [PubMed] [Google Scholar]
- 708.Schulz R, Moll UM. Targeting the heat shock protein 90: a rational way to inhibit macrophage migration inhibitory factor function in cancer. Curr Opin Oncol. 2014;26:108–113. doi: 10.1097/CCO.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 709.Wallach D, Kang TB, Kovalenko A. The extrinsic cell death pathway and the elan mortel. Cell death and differentiation. 2008;15:1533–1541. doi: 10.1038/cdd.2008.41. [DOI] [PubMed] [Google Scholar]
- 710.Xie K, Fidler IJ. Therapy of cancer metastasis by activation of the inducible nitric oxide synthase. Cancer metastasis reviews. 1998;17:55–75. doi: 10.1023/a:1005956721457. [DOI] [PubMed] [Google Scholar]
- 711.Cassinelli G, Zuco V, Gatti L, Lanzi C, Zaffaroni N, Colombo D, et al. Targeting the Akt kinase to modulate survival, invasiveness and drug resistance of cancer cells. Curr Med Chem. 2013;20:1923–1945. doi: 10.2174/09298673113209990106. [DOI] [PubMed] [Google Scholar]
- 712.Billottet C, Quemener C, Bikfalvi A. CXCR3, a double-edged sword in tumor progression and angiogenesis. Biochimica et biophysica acta. 2013;1836:287–295. doi: 10.1016/j.bbcan.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 713.He H, Xia HH, Wang JD, Gu Q, Lin MC, Zou B, et al. Inhibition of human telomerase reverse transcriptase by nonsteroidal antiinflammatory drugs in colon carcinoma. Cancer. 2006;106:1243–1249. doi: 10.1002/cncr.21694. [DOI] [PubMed] [Google Scholar]
- 714.Baoping Y, Guoyong H, Jieping Y, Zongxue R, Hesheng L. Cyclooxygenase-2 inhibitor nimesulide suppresses telomerase activity by blocking Akt/PKB activation in gastric cancer cell line. Digestive diseases and sciences. 2004;49:948–953. doi: 10.1023/b:ddas.0000034553.58554.ab. [DOI] [PubMed] [Google Scholar]
- 715.Hasegawa K, Ohashi Y, Ishikawa K, Yasue A, Kato R, Achiwa Y, et al. Expression of cyclooxygenase-2 in uterine endometrial cancer and anti-tumor effects of a selective COX-2 inhibitor. Int J Oncol. 2005;26:1419–1428. [PubMed] [Google Scholar]
- 716.Nogueira L, Ruiz-Ontanon P, Vazquez-Barquero A, Lafarga M, Berciano MT, Aldaz B, et al. Blockade of the NFkappaB pathway drives differentiating glioblastoma-initiating cells into senescence both in vitro and in vivo. Oncogene. 2011;30:3537–3548. doi: 10.1038/onc.2011.74. [DOI] [PubMed] [Google Scholar]
- 717.Akiyama M, Hideshima T, Hayashi T, Tai YT, Mitsiades CS, Mitsiades N, et al. Nuclear factor-kappaB p65 mediates tumor necrosis factor alpha-induced nuclear translocation of telomerase reverse transcriptase protein. Cancer Res. 2003;63:18–21. [PubMed] [Google Scholar]
- 718.Mowla SN, Perkins ND, Jat PS. Friend or foe: emerging role of nuclear factor kappa-light-chain-enhancer of activated B cells in cell senescence. OncoTargets and therapy. 2013;6:1221–1229. doi: 10.2147/OTT.S36160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Welford SM, Bedogni B, Gradin K, Poellinger L, Broome Powell M, Giaccia AJ. HIF1alpha delays premature senescence through the activation of MIF. Genes & development. 2006;20:3366–3371. doi: 10.1101/gad.1471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Winner M, Leng L, Zundel W, Mitchell RA. Macrophage migration inhibitory factor manipulation and evaluation in tumoral hypoxic adaptation. Methods in enzymology. 2007;435:355–369. doi: 10.1016/S0076-6879(07)35018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Beyne-Rauzy O, Recher C, Dastugue N, Demur C, Pottier G, Laurent G, et al. Tumor necrosis factor alpha induces senescence and chromosomal instability in human leukemic cells. Oncogene. 2004;23:7507–7516. doi: 10.1038/sj.onc.1208024. [DOI] [PubMed] [Google Scholar]
- 722.Wang Y, Wang W, Wang L, Wang X, Xia J. Regulatory mechanisms of interleukin-8 production induced by tumour necrosis factor-alpha in human hepatocellular carcinoma cells. Journal of cellular and molecular medicine. 2012;16:496–506. doi: 10.1111/j.1582-4934.2011.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Zhang Y, Wang L, Zhang M, Jin M, Bai C, Wang X. Potential mechanism of interleukin-8 production from lung cancer cells: an involvement of EGF-EGFR-PI3K-Akt-Erk pathway. J Cell Physiol. 2012;227:35–43. doi: 10.1002/jcp.22722. [DOI] [PubMed] [Google Scholar]
- 724.Wu CT, Wang WC, Chen MF, Su HY, Chen WY, Wu CH, et al. Glucose-regulated protein 78 mediates hormone-independent prostate cancer progression and metastasis through maspin and COX-2 expression. Tumour Biol. 2014;35:195–204. doi: 10.1007/s13277-013-1024-4. [DOI] [PubMed] [Google Scholar]
- 725.Rakib MA, Lee WS, Kim GS, Han JH, Kim JO, Ha YL. Antiproliferative Action of Conjugated Linoleic Acid on Human MCF-7 Breast Cancer Cells Mediated by Enhancement of Gap Junctional Intercellular Communication through Inactivation of NF- kappa B. Evidence-based complementary and alternative medicine : eCAM. 2013;2013:429393. doi: 10.1155/2013/429393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726.Zhang R, Yin X, Shi H, Wu J, Shakya P, Liu D, et al. Adiponectin modulates DCA-induced inflammation via the ROS/NF-kappa B signaling pathway in esophageal adenocarcinoma cells. Digestive diseases and sciences. 2014;59:89–97. doi: 10.1007/s10620-013-2877-5. [DOI] [PubMed] [Google Scholar]
- 727.Zhao Z, Wu MS, Zou C, Tang Q, Lu J, Liu D, et al. Downregulation of MCT1 inhibits tumor growth, metastasis and enhances chemotherapeutic efficacy in osteosarcoma through regulation of the NF-kappaB pathway. Cancer letters. 2014;342:150–158. doi: 10.1016/j.canlet.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 728.Straus DS. TNFalpha and IL-17 cooperatively stimulate glucose metabolism and growth factor production in human colorectal cancer cells. Molecular cancer. 2013;12:78. doi: 10.1186/1476-4598-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Vaughan RA, Garcia-Smith R, Trujillo KA, Bisoffi M. Tumor necrosis factor alpha increases aerobic glycolysis and reduces oxidative metabolism in prostate epithelial cells. The Prostate. 2013;73:1538–1546. doi: 10.1002/pros.22703. [DOI] [PubMed] [Google Scholar]
- 730.Vaughan RA, Garcia-Smith R, Dorsey J, Griffith JK, Bisoffi M, Trujillo KA. Tumor necrosis factor alpha induces Warburg-like metabolism and is reversed by anti-inflammatory curcumin in breast epithelial cells. Int J Cancer. 2013;133:2504–2510. doi: 10.1002/ijc.28264. [DOI] [PubMed] [Google Scholar]
- 731.Lutz NW, Tome ME, Cozzone PJ. Early changes in glucose and phospholipid metabolism following apoptosis induction by IFN-gamma/TNF-alpha in HT-29 cells. FEBS Lett. 2003;544:123–128. doi: 10.1016/s0014-5793(03)00489-7. [DOI] [PubMed] [Google Scholar]
- 732.Almeida A, Moncada S, Bolanos JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nature cell biology. 2004;6:45–51. doi: 10.1038/ncb1080. [DOI] [PubMed] [Google Scholar]
- 733.Das UN. Pyruvate is an endogenous anti-inflammatory and anti-oxidant molecule. Med Sci Monit. 2006;12:RA79–RA84. [PubMed] [Google Scholar]
- 734.Min JW, Kim KI, Kim HA, Kim EK, Noh WC, Jeon HB, et al. INPP4B-mediated tumor resistance is associated with modulation of glucose metabolism via hexokinase 2 regulation in laryngeal cancer cells. Biochemical and biophysical research communications. 2013;440:137–142. doi: 10.1016/j.bbrc.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 735.Guo D, Bell EH, Mischel P, Chakravarti A. Targeting SREBP-1-driven lipid metabolism to treat cancer. Current pharmaceutical design. 2014;20:2619–2626. doi: 10.2174/13816128113199990486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736.Wang H, Zhao L, Zhu LT, Wang Y, Pan D, Yao J, et al. Wogonin reverses hypoxia resistance of human colon cancer HCT116 cells via downregulation of HIF-1alpha and glycolysis, by inhibiting PI3K/Akt signaling pathway. Molecular carcinogenesis. 2014;53(Suppl 1):E107–E118. doi: 10.1002/mc.22052. [DOI] [PubMed] [Google Scholar]
- 737.Garufi A, Pistritto G, Ceci C, Di Renzo L, Santarelli R, Faggioni A, et al. Targeting COX-2/PGE(2) pathway in HIPK2 knockdown cancer cells: impact on dendritic cell maturation. PloS one. 2012;7:e48342. doi: 10.1371/journal.pone.0048342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Rolle CE, Sengupta S, Lesniak MS. Mechanisms of immune evasion by gliomas. Adv Exp Med Biol. 2012;746:53–76. doi: 10.1007/978-1-4614-3146-6_5. [DOI] [PubMed] [Google Scholar]
- 739.Mineharu Y, Muhammad AK, Yagiz K, Candolfi M, Kroeger KM, Xiong W, et al. Gene therapy-mediated reprogramming tumor infiltrating T cells using IL-2 and inhibiting NF-kappaB signaling improves the efficacy of immunotherapy in a brain cancer model. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2012;9:827–843. doi: 10.1007/s13311-012-0144-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Choi S, Kim HR, Leng L, Kang I, Jorgensen WL, Cho CS, et al. Role of macrophage migration inhibitory factor in the regulatory T cell response of tumor-bearing mice. J Immunol. 2012;189:3905–3913. doi: 10.4049/jimmunol.1102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Noh KH, Kang TH, Kim JH, Pai SI, Lin KY, Hung CF, et al. Activation of Akt as a mechanism for tumor immune evasion. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17:439–447. doi: 10.1038/mt.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Lombardi L, Tavano F, Morelli F, Latiano TP, Di Sebastiano P, Maiello E. Chemokine receptor CXCR4: role in gastrointestinal cancer. Critical reviews in oncology/hematology. 2013;88:696–705. doi: 10.1016/j.critrevonc.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 743.Boosani CS, Mannam AP, Cosgrove D, Silva R, Hodivala-Dilke KM, Keshamouni VG, et al. Regulation of COX-2 mediated signaling by alpha3 type IV noncollagenous domain in tumor angiogenesis. Blood. 2007;110:1168–1177. doi: 10.1182/blood-2007-01-066282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nature reviews Immunology. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 745.Jin F, Liu X, Zhou Z, Yue P, Lotan R, Khuri FR, et al. Activation of nuclear factor-kappaB contributes to induction of death receptors and apoptosis by the synthetic retinoid CD437 in DU145 human prostate cancer cells. Cancer Res. 2005;65:6354–6363. doi: 10.1158/0008-5472.CAN-04-4061. [DOI] [PubMed] [Google Scholar]
- 746.Kanzler I, Tuchscheerer N, Steffens G, Simsekyilmaz S, Konschalla S, Kroh A, et al. Differential roles of angiogenic chemokines in endothelial progenitor cell-induced angiogenesis. Basic research in cardiology. 2013;108:310. doi: 10.1007/s00395-012-0310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 747.Kane AJ, Barker JE, Mitchell GM, Theile DR, Romero R, Messina A, et al. Inducible nitric oxide synthase (iNOS) activity promotes ischaemic skin flap survival. British journal of pharmacology. 2001;132:1631–1638. doi: 10.1038/sj.bjp.0703944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Karar J, Maity A. PI3K/AKT/mTOR Pathway in Angiogenesis. Frontiers in molecular neuroscience. 2011;4:51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 749.Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends in immunology. 2004;25:201–209. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 750.Ishizaki T, Katsumata K, Tsuchida A, Wada T, Mori Y, Hisada M, et al. Etodolac, a selective cyclooxygenase-2 inhibitor, inhibits liver metastasis of colorectal cancer cells via the suppression of MMP-9 activity. International journal of molecular medicine. 2006;17:357–362. [PubMed] [Google Scholar]
- 751.Chen WS, Wei SJ, Liu JM, Hsiao M, Kou-Lin J, Yang WK. Tumor invasiveness and liver metastasis of colon cancer cells correlated with cyclooxygenase-2 (COX-2) expression and inhibited by a COX-2-selective inhibitor, etodolac. Int J Cancer. 2001;91:894–899. doi: 10.1002/1097-0215(200102)9999:9999<894::aid-ijc1146>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 752.Wei D, Wang L, He Y, Xiong HQ, Abbruzzese JL, Xie K. Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer Res. 2004;64:2030–2038. doi: 10.1158/0008-5472.can-03-1945. [DOI] [PubMed] [Google Scholar]
- 753.Barlow M, Edelman M, Glick RD, Steinberg BM, Soffer SZ. Celecoxib inhibits invasion and metastasis via a cyclooxygenase 2-independent mechanism in an in vitro model of Ewing sarcoma. Journal of pediatric surgery. 2012;47:1223–1227. doi: 10.1016/j.jpedsurg.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 754.Yan M, Xu Q, Zhang P, Zhou XJ, Zhang ZY, Chen WT. Correlation of NF-kappaB signal pathway with tumor metastasis of human head and neck squamous cell carcinoma. BMC cancer. 2010;10:437. doi: 10.1186/1471-2407-10-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 755.Simpson KD, Cross JV. MIF: metastasis/MDSC-inducing factor? Oncoimmunology. 2013;2:e23337. doi: 10.4161/onci.23337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 756.Morris KT, Nofchissey RA, Pinchuk IV, Beswick EJ. Chronic macrophage migration inhibitory factor exposure induces mesenchymal epithelial transition and promotes gastric and colon cancers. PloS one. 2014;9:e98656. doi: 10.1371/journal.pone.0098656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 757.Jiao SF, Sun K, Chen XJ, Zhao X, Cai N, Liu YJ, et al. Inhibition of tumor necrosis factor alpha reduces the outgrowth of hepatic micrometastasis of colorectal tumors in a mouse model of liver ischemia-reperfusion injury. Journal of biomedical science. 2014;21:1. doi: 10.1186/1423-0127-21-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758.Karadayi N, Kandemir NO, Yavuzer D, Korkmaz T, Gecmen G, Kokturk F. Inducible nitric oxide synthase expression in gastric adenocarcinoma: impact on lymphangiogenesis and lymphatic metastasis. Diagnostic pathology. 2013;8:151. doi: 10.1186/1746-1596-8-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 759.Kim D, Kim S, Koh H, Yoon SO, Chung AS, Cho KS, et al. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 2001;15:1953–1962. doi: 10.1096/fj.01-0198com. [DOI] [PubMed] [Google Scholar]
- 760.Yu ZH, Liu T, Zhao YH, Huang YY, Gao YT. Cisplatin targets the stromal cell-derived factor-1-CXC chemokine receptor type 4 axis to suppress metastasis and invasion of ovarian cancer-initiating cells. Tumour Biol. 2014;35:4637–4644. doi: 10.1007/s13277-014-1607-8. [DOI] [PubMed] [Google Scholar]
- 761.Bocca C, Ievolella M, Autelli R, Motta M, Mosso L, Torchio B, et al. Expression of Cox-2 in human breast cancer cells as a critical determinant of epithelial-to-mesenchymal transition and invasiveness. Expert opinion on therapeutic targets. 2014;18:121–135. doi: 10.1517/14728222.2014.860447. [DOI] [PubMed] [Google Scholar]
- 762.He WA, Berardi E, Cardillo VM, Acharyya S, Aulino P, Thomas-Ahner J, et al. NF-kappaB-mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. The Journal of clinical investigation. 2013;123:4821–4835. doi: 10.1172/JCI68523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Zhang L, Ye SB, Ma G, Tang XF, Chen SP, He J, et al. The expressions of MIF and CXCR4 protein in tumor microenvironment are adverse prognostic factors in patients with esophageal squamous cell carcinoma. Journal of translational medicine. 2013;11:60. doi: 10.1186/1479-5876-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764.Leibovich-Rivkin T, Liubomirski Y, Bernstein B, Meshel T, Ben-Baruch A. Inflammatory factors of the tumor microenvironment induce plasticity in nontransformed breast epithelial cells: EMT, invasion, and collapse of normally organized breast textures. Neoplasia. 2013;15:1330–1346. doi: 10.1593/neo.131688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 765.Singh S, Gupta AK. Nitric oxide: role in tumour biology and iNOS/NO-based anticancer therapies. Cancer Chemother Pharmacol. 2011;67:1211–1224. doi: 10.1007/s00280-011-1654-4. [DOI] [PubMed] [Google Scholar]
- 766.Subramaniam KS, Tham ST, Mohamed Z, Woo YL, Mat Adenan NA, Chung I. Cancer-associated fibroblasts promote proliferation of endometrial cancer cells. PloS one. 2013;8:e68923. doi: 10.1371/journal.pone.0068923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767.Lin BQ, Zeng ZY, Yang SS, Zhuang CW. Dietary restriction suppresses tumor growth, reduces angiogenesis, and improves tumor microenvironment in human non-small-cell lung cancer xenografts. Lung cancer. 2013;79:111–117. doi: 10.1016/j.lungcan.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 768.Sambandam Y, Sundaram K, Liu A, Kirkwood KL, Ries WL, Reddy SV. CXCL13 activation of c-Myc induces RANK ligand expression in stromal/preosteoblast cells in the oral squamous cell carcinoma tumor-bone microenvironment. Oncogene. 2013;32:97–105. doi: 10.1038/onc.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 769.Van Raemdonck K, Gouwy M, Lepers SA, Van Damme J, Struyf S. CXCL4L1 and CXCL4 signaling in human lymphatic and microvascular endothelial cells and activated lymphocytes: involvement of mitogen-activated protein (MAP) kinases, Src and p70S6 kinase. Angiogenesis. 2014;17:631–640. doi: 10.1007/s10456-014-9417-6. [DOI] [PubMed] [Google Scholar]
- 770.Samuels TL, Pearson AC, Wells CW, Stoner GD, Johnston N. Curcumin and anthocyanin inhibit pepsin-mediated cell damage and carcinogenic changes in airway epithelial cells. The Annals of otology, rhinology, and laryngology. 2013;122:632–641. [PubMed] [Google Scholar]
- 771.Weitberg AB, Corvese D. The effect of epigallocatechin galleate and sarcophytol A on DNA strand breakage induced by tobacco-specific nitrosamines and stimulated human phagocytes. Journal of experimental & clinical cancer research : CR. 1999;18:433–437. [PubMed] [Google Scholar]
- 772.Simone RE, Russo M, Catalano A, Monego G, Froehlich K, Boehm V, et al. Lycopene inhibits NF-kB-mediated IL-8 expression and changes redox and PPARgamma signalling in cigarette smoke-stimulated macrophages. PloS one. 2011;6:e19652. doi: 10.1371/journal.pone.0019652. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 773.Gong L, Li Y, Nedeljkovic-Kurepa A, Sarkar FH. Inactivation of NF-kappaB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene. 2003;22:4702–4709. doi: 10.1038/sj.onc.1206583. [DOI] [PubMed] [Google Scholar]
- 774.Ono M, Higuchi T, Takeshima M, Chen C, Nakano S. Antiproliferative and apoptosis-inducing activity of curcumin against human gallbladder adenocarcinoma cells. Anticancer Res. 2013;33:1861–1866. [PubMed] [Google Scholar]
- 775.Zhang M, Zhou X, Zhou K. Resveratrol inhibits human nasopharyngeal carcinoma cell growth via blocking pAkt/p70S6K signaling pathways. International journal of molecular medicine. 2013;31:621–627. doi: 10.3892/ijmm.2013.1237. [DOI] [PubMed] [Google Scholar]
- 776.Amiri F, Zarnani AH, Zand H, Koohdani F, Jeddi-Tehrani M, Vafa M. Synergistic anti-proliferative effect of resveratrol and etoposide on human hepatocellular and colon cancer cell lines. Eur J Pharmacol. 2013;718:34–40. doi: 10.1016/j.ejphar.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 777.Liu B, Zhou Z, Zhou W, Liu J, Zhang Q, Xia J, et al. Resveratrol inhibits proliferation in human colorectal carcinoma cells by inducing G1/Sphase cell cycle arrest and apoptosis through caspase/cyclinCDK pathways. Molecular medicine reports. 2014 doi: 10.3892/mmr.2014.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 778.Deng YT, Lin JK. EGCG inhibits the invasion of highly invasive CL1–5 lung cancer cells through suppressing MMP-2 expression via JNK signaling and induces G2/M arrest. Journal of agricultural and food chemistry. 2011;59:13318–13327. doi: 10.1021/jf204149c. [DOI] [PubMed] [Google Scholar]
- 779.Uppala PT, Dissmore T, Lau BH, Andacht T, Rajaram S. Selective inhibition of cell proliferation by lycopene in MCF-7 breast cancer cells in vitro: a proteomic analysis. Phytotherapy research : PTR. 2013;27:595–601. doi: 10.1002/ptr.4764. [DOI] [PubMed] [Google Scholar]
- 780.Hsu CP, Shih YT, Lin BR, Chiu CF, Lin CC. Inhibitory effect and mechanisms of an anthocyanins- and anthocyanidins-rich extract from purple-shoot tea on colorectal carcinoma cell proliferation. Journal of agricultural and food chemistry. 2012;60:3686–3692. doi: 10.1021/jf204619n. [DOI] [PubMed] [Google Scholar]
- 781.Lee YK, Lee WS, Kim GS, Park OJ. Anthocyanins are novel AMPKalpha1 stimulators that suppress tumor growth by inhibiting mTOR phosphorylation. Oncology reports. 2010;24:1471–1477. doi: 10.3892/or_00001007. [DOI] [PubMed] [Google Scholar]
- 782.Moore AB, Castro L, Yu L, Zheng X, Di X, Sifre MI, et al. Stimulatory and inhibitory effects of genistein on human uterine leiomyoma cell proliferation are influenced by the concentration. Hum Reprod. 2007;22:2623–2631. doi: 10.1093/humrep/dem185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 783.Di X, Yu L, Moore AB, Castro L, Zheng X, Hermon T, et al. A low concentration of genistein induces estrogen receptor-alpha and insulin-like growth factor-I receptor interactions and proliferation in uterine leiomyoma cells. Hum Reprod. 2008;23:1873–1883. doi: 10.1093/humrep/den087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784.Hasima N, Aggarwal BB. Cancer-linked targets modulated by curcumin. International journal of biochemistry and molecular biology. 2012;3:328–351. [PMC free article] [PubMed] [Google Scholar]
- 785.Lee SJ, Krauthauser C, Maduskuie V, Fawcett PT, Olson JM, Rajasekaran SA. Curcumin-induced HDAC inhibition and attenuation of medulloblastoma growth in vitro and in vivo. BMC cancer. 2011;11:144. doi: 10.1186/1471-2407-11-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 786.He X, Wang Y, Zhu J, Orloff M, Eng C. Resveratrol enhances the anti-tumor activity of the mTOR inhibitor rapamycin in multiple breast cancer cell lines mainly by suppressing rapamycin-induced AKT signaling. Cancer letters. 2011;301:168–176. doi: 10.1016/j.canlet.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 787.Kim KH, Back JH, Zhu Y, Arbesman J, Athar M, Kopelovich L, et al. Resveratrol targets transforming growth factor-beta2 signaling to block UV-induced tumor progression. The Journal of investigative dermatology. 2011;131:195–202. doi: 10.1038/jid.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 788.Chang CM, Chang PY, Tu MG, Lu CC, Kuo SC, Amagaya S, et al. Epigallocatechin gallate sensitizes CAL-27 human oral squamous cell carcinoma cells to the anti-metastatic effects of gefitinib (Iressa) via synergistic suppression of epidermal growth factor receptor and matrix metalloproteinase-2. Oncology reports. 2012;28:1799–1807. doi: 10.3892/or.2012.1991. [DOI] [PubMed] [Google Scholar]
- 789.Hwang KC, Lee KH, Jang Y, Yun YP, Chung KH. Epigallocatechin-3-gallate inhibits basic fibroblast growth factor-induced intracellular signaling transduction pathway in rat aortic smooth muscle cells. Journal of cardiovascular pharmacology. 2002;39:271–277. doi: 10.1097/00005344-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 790.Hwang ES, Bowen PE. Cell cycle arrest and induction of apoptosis by lycopene in LNCaP human prostate cancer cells. Journal of medicinal food. 2004;7:284–289. doi: 10.1089/jmf.2004.7.284. [DOI] [PubMed] [Google Scholar]
- 791.Teodoro AJ, Oliveira FL, Martins NB, Maia Gde A, Martucci RB, Borojevic R. Effect of lycopene on cell viability and cell cycle progression in human cancer cell lines. Cancer cell international. 2012;12:36. doi: 10.1186/1475-2867-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 792.Soares Nda C, Teodoro AJ, Oliveira FL, Santos CA, Takiya CM, Junior OS, et al. Influence of lycopene on cell viability, cell cycle, and apoptosis of human prostate cancer and benign hyperplastic cells. Nutrition and cancer. 2013;65:1076–1085. doi: 10.1080/01635581.2013.812225. [DOI] [PubMed] [Google Scholar]
- 793.Wang LS, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer letters. 2008;269:281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794.Wang J, Eltoum IE, Lamartiniere CA. Genistein alters growth factor signaling in transgenic prostate model (TRAMP) Mol Cell Endocrinol. 2004;219:171–180. doi: 10.1016/j.mce.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 795.Shim HY, Park JH, Paik HD, Nah SY, Kim DS, Han YS. Genistein-induced apoptosis of human breast cancer MCF-7 cells involves calpain-caspase and apoptosis signaling kinase 1-p38 mitogen-activated protein kinase activation cascades. Anti-cancer drugs. 2007;18:649–657. doi: 10.1097/CAD.0b013e3280825573. [DOI] [PubMed] [Google Scholar]
- 796.Yu LL, Wu JG, Dai N, Yu HG, Si JM. Curcumin reverses chemoresistance of human gastric cancer cells by downregulating the NF-kappaB transcription factor. Oncology reports. 2011;26:1197–1203. doi: 10.3892/or.2011.1410. [DOI] [PubMed] [Google Scholar]
- 797.Wesolowska O, Wisniewski J, Bielawska-Pohl A, Paprocka M, Duarte N, Ferreira MJ, et al. Stilbenes as multidrug resistance modulators and apoptosis inducers in human adenocarcinoma cells. Anticancer Res. 2010;30:4587–4593. [PubMed] [Google Scholar]
- 798.Sarkar FH, Li Y, Wang Z, Kong D. Cellular signaling perturbation by natural products. Cellular signalling. 2009;21:1541–1547. doi: 10.1016/j.cellsig.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 799.Molnar J, Gyemant N, Mucsi I, Molnar A, Szabo M, Kortvelyesi T, et al. Modulation of multidrug resistance and apoptosis of cancer cells by selected carotenoids. In vivo (Athens, Greece) 2004;18:237–244. [PubMed] [Google Scholar]
- 800.Choe YJ, Ha TJ, Ko KW, Lee SY, Shin SJ, Kim HS. Anthocyanins in the black soybean (Glycine max L.) protect U2OS cells from apoptosis by inducing autophagy via the activation of adenosyl monophosphate-dependent protein kinase. Oncology reports. 2012;28:2049–2056. doi: 10.3892/or.2012.2034. [DOI] [PubMed] [Google Scholar]
- 801.Mosieniak G, Adamowicz M, Alster O, Jaskowiak H, Szczepankiewicz AA, Wilczynski GM, et al. Curcumin induces permanent growth arrest of human colon cancer cells: link between senescence and autophagy. Mechanisms of ageing and development. 2012;133:444–455. doi: 10.1016/j.mad.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 802.Malhotra A, Nair P, Dhawan DK. Premature mitochondrial senescence and related ultrastructural changes during lung carcinogenesis modulation by curcumin and resveratrol. Ultrastructural pathology. 2012;36:179–184. doi: 10.3109/01913123.2011.652765. [DOI] [PubMed] [Google Scholar]
- 803.Fuggetta MP, Lanzilli G, Tricarico M, Cottarelli A, Falchetti R, Ravagnan G, et al. Effect of resveratrol on proliferation and telomerase activity of human colon cancer cells in vitro. Journal of experimental & clinical cancer research : CR. 2006;25:189–193. [PubMed] [Google Scholar]
- 804.Lanzilli G, Fuggetta MP, Tricarico M, Cottarelli A, Serafino A, Falchetti R, et al. Resveratrol down-regulates the growth and telomerase activity of breast cancer cells in vitro. Int J Oncol. 2006;28:641–648. [PubMed] [Google Scholar]
- 805.Wang X, Hao MW, Dong K, Lin F, Ren JH, Zhang HZ. Apoptosis induction effects of EGCG in laryngeal squamous cell carcinoma cells through telomerase repression. Archives of pharmacal research. 2009;32:1263–1269. doi: 10.1007/s12272-009-1912-8. [DOI] [PubMed] [Google Scholar]
- 806.Berletch JB, Liu C, Love WK, Andrews LG, Katiyar SK, Tollefsbol TO. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. Journal of cellular biochemistry. 2008;103:509–519. doi: 10.1002/jcb.21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807.Symonds EL, Konczak I, Fenech M. The Australian fruit Illawarra plum (Podocarpus elatus Endl., Podocarpaceae) inhibits telomerase, increases histone deacetylase activity and decreases proliferation of colon cancer cells. The British journal of nutrition. 2013;109:2117–2125. doi: 10.1017/S0007114512004333. [DOI] [PubMed] [Google Scholar]
- 808.Khaw AK, Yong JW, Kalthur G, Hande MP. Genistein induces growth arrest and suppresses telomerase activity in brain tumor cells. Genes, chromosomes & cancer. 2012;51:961–974. doi: 10.1002/gcc.21979. [DOI] [PubMed] [Google Scholar]
- 809.Jagadeesh S, Kyo S, Banerjee PP. Genistein represses telomerase activity via both transcriptional and posttranslational mechanisms in human prostate cancer cells. Cancer Res. 2006;66:2107–2115. doi: 10.1158/0008-5472.CAN-05-2494. [DOI] [PubMed] [Google Scholar]
- 810.Chang YH, Jiang M, Liu KG, Li XQ. Curcumin inhibited hypoxia induced epithelialmesenchymal transition in hepatic carcinoma cell line HepG2 in vitro. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi = Chinese journal of integrated traditional and Western medicine / Zhongguo Zhong xi yi jie he xue hui. Zhongguo Zhong yi yan jiu yuan zhu ban. 2013;33:1102–1106. [PubMed] [Google Scholar]
- 811.Gomez LS, Zancan P, Marcondes MC, Ramos-Santos L, Meyer-Fernandes JR, Sola-Penna M, et al. Resveratrol decreases breast cancer cell viability and glucose metabolism by inhibiting 6-phosphofructo-1-kinase. Biochimie. 2013;95:1336–1343. doi: 10.1016/j.biochi.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 812.Fouad MA, Agha AM, Merzabani MM, Shouman SA. Resveratrol inhibits proliferation, angiogenesis and induces apoptosis in colon cancer cells: calorie restriction is the force to the cytotoxicity. Human & experimental toxicology. 2013;32:1067–1080. doi: 10.1177/0960327113475679. [DOI] [PubMed] [Google Scholar]
- 813.Zhang S, Yang X, Luo J, Ge X, Sun W, Zhu H, et al. PPARalpha activation sensitizes cancer cells to epigallocatechin-3-gallate (EGCG) treatment via suppressing heme oxygenase-1. Nutrition and cancer. 2014;66:315–324. doi: 10.1080/01635581.2014.868909. [DOI] [PubMed] [Google Scholar]
- 814.Tao L, Forester SC, Lambert JD. The role of the mitochondrial oxidative stress in the cytotoxic effects of the green tea catechin, (-)-epigallocatechin-3-gallate, in oral cells. Molecular nutrition & food research. 2014;58:665–676. doi: 10.1002/mnfr.201300427. [DOI] [PubMed] [Google Scholar]
- 815.Valenti D, de Bari L, Manente GA, Rossi L, Mutti L, Moro L, et al. Negative modulation of mitochondrial oxidative phosphorylation by epigallocatechin-3 gallate leads to growth arrest and apoptosis in human malignant pleural mesothelioma cells. Biochimica et biophysica acta. 2013;1832:2085–2096. doi: 10.1016/j.bbadis.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 816.Pons DG, Nadal-Serrano M, Blanquer-Rossello MM, Sastre-Serra J, Oliver J, Roca P. Genistein modulates proliferation and mitochondrial functionality in breast cancer cells depending on ERalpha/ERbeta ratio. Journal of cellular biochemistry. 2014;115:949–958. doi: 10.1002/jcb.24737. [DOI] [PubMed] [Google Scholar]
- 817.Iwasaki K, Ray PD, Huang BW, Sakamoto K, Kobayashi T, Tsuji Y. Role of AMP-activated protein kinase in ferritin H gene expression by resveratrol in human T cells. Biochemistry. 2013;52:5075–5083. doi: 10.1021/bi400399f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 818.Noh KT, Chae SH, Chun SH, Jung ID, Kang HK, Park YM. Resveratrol suppresses tumor progression via the regulation of indoleamine 2,3-dioxygenase. Biochemical and biophysical research communications. 2013;431:348–353. doi: 10.1016/j.bbrc.2012.12.093. [DOI] [PubMed] [Google Scholar]
- 819.Buttari B, Profumo E, Facchiano F, Ozturk EI, Segoni L, Saso L, et al. Resveratrol prevents dendritic cell maturation in response to advanced glycation end products. Oxidative medicine and cellular longevity. 2013;2013:574029. doi: 10.1155/2013/574029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 820.Jang JY, Lee JK, Jeon YK, Kim CW. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC cancer. 2013;13:421. doi: 10.1186/1471-2407-13-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 821.Sakai T, Kogiso M. Soy isoflavones and immunity. The journal of medical investigation : JMI. 2008;55:167–173. doi: 10.2152/jmi.55.167. [DOI] [PubMed] [Google Scholar]
- 822.Arbiser JL, Klauber N, Rohan R, van Leeuwen R, Huang MT, Fisher C, et al. Curcumin is an in vivo inhibitor of angiogenesis. Molecular medicine (Cambridge, Mass. 1998;4:376–383. [PMC free article] [PubMed] [Google Scholar]
- 823.Chen Y, Tseng SH. Review. Pro- and anti-angiogenesis effects of resveratrol. In vivo (Athens, Greece) 2007;21:365–370. [PubMed] [Google Scholar]
- 824.Jung YD, Ellis LM. Inhibition of tumour invasion and angiogenesis by epigallocatechin gallate (EGCG), a major component of green tea. International journal of experimental pathology. 2001;82:309–316. doi: 10.1046/j.1365-2613.2001.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 825.Chen ML, Lin YH, Yang CM, Hu ML. Lycopene inhibits angiogenesis both in vitro and in vivo by inhibiting MMP-2/uPA system through VEGFR2-mediated PI3K-Akt and ERK/p38 signaling pathways. Molecular nutrition & food research. 2012;56:889–899. doi: 10.1002/mnfr.201100683. [DOI] [PubMed] [Google Scholar]
- 826.Wang LS, Hecht SS, Carmella SG, Yu N, Larue B, Henry C, et al. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer prevention research. 2009;2:84–93. doi: 10.1158/1940-6207.CAPR-08-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 827.Fotsis T, Pepper M, Adlercreutz H, Fleischmann G, Hase T, Montesano R, et al. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:2690–2694. doi: 10.1073/pnas.90.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 828.Lu Y, Wei C, Xi Z. Curcumin suppresses proliferation and invasion in non-small cell lung cancer by modulation of MTA1-mediated Wnt/beta-catenin pathway. In vitro cellular & developmental biology Animal. 2014 doi: 10.1007/s11626-014-9779-5. [DOI] [PubMed] [Google Scholar]
- 829.Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L, et al. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/beta-catenin signal pathway. PloS one. 2013;8:e78700. doi: 10.1371/journal.pone.0078700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 830.Sartor L, Pezzato E, Dell'Aica I, Caniato R, Biggin S, Garbisa S. Inhibition of matrix-proteases by polyphenols: chemical insights for anti-inflammatory and anti-invasion drug design. Biochem Pharmacol. 2002;64:229–237. doi: 10.1016/s0006-2952(02)01069-9. [DOI] [PubMed] [Google Scholar]
- 831.Sartor L, Pezzato E, Dona M, Dell'Aica I, Calabrese F, Morini M, et al. Prostate carcinoma and green tea: (-)epigallocatechin-3-gallate inhibits inflammation-triggered MMP-2 activation and invasion in murine TRAMP model. Int J Cancer. 2004;112:823–829. doi: 10.1002/ijc.20496. [DOI] [PubMed] [Google Scholar]
- 832.Huang CS, Fan YE, Lin CY, Hu ML. Lycopene inhibits matrix metalloproteinase-9 expression and down-regulates the binding activity of nuclear factor-kappa B and stimulatory protein-1. The Journal of nutritional biochemistry. 2007;18:449–456. doi: 10.1016/j.jnutbio.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 833.Neto CC, Amoroso JW, Liberty AM. Anticancer activities of cranberry phytochemicals: an update. Molecular nutrition & food research. 2008;52(Suppl 1):S18–S27. doi: 10.1002/mnfr.200700433. [DOI] [PubMed] [Google Scholar]
- 834.Xu Y, Zhang J, Han J, Pan X, Cao Y, Guo H, et al. Curcumin inhibits tumor proliferation induced by neutrophil elastase through the upregulation of alpha1-antitrypsin in lung cancer. Molecular oncology. 2012;6:405–417. doi: 10.1016/j.molonc.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 835.Vishvakarma NK, Kumar A, Singh SM. Role of curcumin-dependent modulation of tumor microenvironment of a murine T cell lymphoma in altered regulation of tumor cell survival. Toxicol Appl Pharmacol. 2011;252:298–306. doi: 10.1016/j.taap.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 836.Zlotogorski A, Dayan A, Dayan D, Chaushu G, Salo T, Vered M. Nutraceuticals as new treatment approaches for oral cancer: II. Green tea extracts and resveratrol. Oral oncology. 2013;49:502–506. doi: 10.1016/j.oraloncology.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 837.Shamim U, Hanif S, Albanyan A, Beck FW, Bao B, Wang Z, et al. Resveratrol-induced apoptosis is enhanced in low pH environments associated with cancer. J Cell Physiol. 2012;227:1493–1500. doi: 10.1002/jcp.22865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 838.Zgheib A, Lamy S, Annabi B. Epigallocatechin gallate targeting of membrane type 1 matrix metalloproteinase-mediated Src and Janus kinase/signal transducers and activators of transcription 3 signaling inhibits transcription of colony-stimulating factors 2 and 3 in mesenchymal stromal cells. J Biol Chem. 2013;288:13378–13386. doi: 10.1074/jbc.M113.456533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 839.Haddad NF, Teodoro AJ, Leite de Oliveira F, Soares N, de Mattos RM, Hecht F, et al. Lycopene and beta-carotene induce growth inhibition and proapoptotic effects on ACTH-secreting pituitary adenoma cells. PloS one. 2013;8:e62773. doi: 10.1371/journal.pone.0062773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 840.Faria A, Pestana D, Teixeira D, de Freitas V, Mateus N, Calhau C. Blueberry anthocyanins and pyruvic acid adducts: anticancer properties in breast cancer cell lines. Phytotherapy research : PTR. 2010;24:1862–1869. doi: 10.1002/ptr.3213. [DOI] [PubMed] [Google Scholar]
- 841.Dai W, Wang F, He L, Lin C, Wu S, Chen P, et al. Genistein inhibits hepatocellular carcinoma cell migration by reversing the epithelial-mesenchymal transition: Partial mediation by the transcription factor NFAT. Molecular carcinogenesis. 2013 doi: 10.1002/mc.22100. [DOI] [PubMed] [Google Scholar]