Abstract
We examined the performance of healthy young (n=57) and older adults (n=43) genotyped as apolipoprotein E-ε4 (APOE-ε4) carriers or APOE-ε4 non-carriers on a delayed match-to-sample task involving varying degrees of spatial interference hypothesized to assess spatial pattern separation. Older adult ε4 carriers were further divided into “impaired” and “unimpaired” groups based on their performance on a standardized test of verbal memory. We found that performance on the spatial pattern separation test increased as a function of decreased spatial interference across all groups. The older ε4 carriers in the impaired group performed significantly worse (p < .05) than unimpaired ε4 carriers, ε4 non-carriers, and young adults. The data suggest that spatial pattern separation may be less efficient in a subset of healthy older adults with subtle memory decline who are carriers of the ε4 allele. However, pattern separation performance may be comparable to that of young adults in a subset of older adult ε4 carriers and more broadly among non-carriers. Our findings offer additional evidence that pattern separation may vary in older adults, and they provide novel insight into pattern separation efficiency in ε4-positive older adults.
Keywords: Aging, Pattern Separation, Spatial Memory, Interference, Apolipoprotein E
1.1 Introduction
Age-related differences on tasks thought to tax pattern separation have been well documented in recent studies (Doxey & Kirwan, 2014; Holden, Hoebel, Loftis, & Gilbert, 2012; Leal & Yassa, 2014; Ly, Murray, & Yassa, 2013; Pidgeon & Morcom, 2014; Reagh et al., 2014; Roberts et al., 2014; Stark, Yassa, Lacy, & Stark, 2013; Stark, Yassa, & Stark, 2010; Tolentino, Pirogovsky, Luu, Toner, & Gilbert, 2012; Toner, Pirogovsky, Kirwan, & Gilbert, 2009; Yassa, Lacy, et al., 2011; Yassa, Mattfeld, Stark, & Stark, 2011). Pattern separation is a mechanism that separates partially overlapping patterns of neural activation so that one pattern may be retrieved as separate from other similar patterns. Pattern separation may reduce potential interference or similarity among memory representations, thereby increasing the likelihood of accurate encoding and subsequent retrieval (Holden & Gilbert, 2012). There is considerable evidence that the dentate gyrus (DG) and CA3 hippocampal subregions play a critical role in pattern separation (for reviews see Gilbert & Brushfield 2009; Kesner & Rolls, 2014; Schmidt et al. 2012; Yassa & Stark 2011).
The human hippocampus has been shown to undergo structural and functional changes as a result of aging (Allen, Bruss, Brown, & Damasio, 2005; Driscoll & Sutherland, 2005; Good et al., 2001; Raz et al., 2005; Small, Tsai, DeLaPaz, Mayeux, & Stern, 2002; Walhovd et al., 2010). However, the DG subregion may be particularly susceptible to these age-related changes (Small et al., 2002). A recent study by Doxey and Kirwan (2014) used functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI) to measure functional and structural correlates of behavioral pattern separation in the hippocampus and medial temporal lobe. They found that the size of left hemisphere DG/CA3 regions was the strongest predictor of performance, other than age, on a task hypothesized to tax pattern separation. Diffusion in white matter tracts and resting function connection strengths did not significantly predict task performance (Doxey & Kirwan, 2014). However, age-related changes are evident in the perforant pathway input to the DG (Yassa, Mattfeld, et al. 2011), and a study that used high-resolution fMRI and ultrahigh-resolution DTI showed that decreased pattern separation activity in the DG/CA3 regions of older adults was associated with structural changes in the perforant pathway. It was hypothesized that these changes weaken the processing of new information and strengthen the processing of stored information (Yassa, Mattfeld, et al., 2011), which may result in less efficient pattern separation.
There is a growing body of evidence that older adults are impaired relative to young adults on behavioral tasks hypothesized to tax pattern separation for visual objects, spatial locations, temporal order, verbal stimuli, and emotional information (Doxey & Kirwan, 2014; Holden et al., 2012; Leal & Yassa, 2014; Ly et al., 2013; Pidgeon & Morcom, 2014; Reagh et al., 2014; Roberts et al., 2014; Stark et al., 2010; Stark et al., 2013; Tolentino et al., 2012; Toner et al., 2009; Yassa, Lacy, et al., 2011; Yassa, Mattfeld, et al., 2011). However, numerous studies have demonstrated that pattern separation efficiency varies among older adults. These studies borrowed an approach commonly used in animal studies of aging (e.g., Gallagher et al., 2006), whereby aged subjects are dichotomized into “impaired” and “unimpaired” groups based on performance on a well-characterized test (such as the water maze test in animals) and then the subjects are tested on a different test to examine group differences in performance. Numerous recent studies have modeled this approach to dichotomize older humans into “older-impaired” and “older-unimpaired” groups based on performance on standardized serial list learning tests and then investigate differences between the two groups on pattern separation tasks (Holden et al., 2012; Holden, Toner, Pirogovsky, Kirwan, & Gilbert, 2013; Reagh et al., 2014; Roberts et al., 2014; Stark et al., 2010; Stark et al., 2013).
Stark and colleagues (2010) were the first to assess potential age-related variability on a task designed to measure spatial pattern separation. In the initial comparison of young and older adults, no group differences were found. However, when the older adult group was divided into aged-impaired and aged-unimpaired groups based on performance on a standardized verbal list learning task, the young adults and aged-unimpaired older adults performed significantly better than the aged-impaired older adults on the trials that taxed pattern separation. Holden and colleagues (2012) replicated these findings using a different task to assess spatial pattern separation and found that the pattern of deficits was remarkably similar to those of Stark et al. (2010). The older-impaired group showed pattern separation deficits relative to the young adults and older-unimpaired adults (Holden et al., 2012). A more recent study reported similar results using an incidental encoding task involving objects presented in various locations (Reagh et al., 2014). In addition to spatial tasks, studies have produced similar results on tests hypothesized to assess pattern separation for temporal order memory (Roberts et al., 2014) and visual object information (Holden et al., 2013; Stark et al., 2013). Therefore, this approach has provided unique insight into the variability among older adults when performing behavioral tasks designed to measure pattern separation.
One factor that might contribute to differences on cognitive tasks among older adults is the presence of the apolipoprotein E-ε4 (APOE-ε4) allele on chromosome 19. The APOE gene codes for a protein called apolipoprotein E, which plays a role in the transfer of cholesterol and other lipids between cells and organs. The ε4 allele of the APOE gene also has been linked to Alzheimer’s disease (AD). Although a number of risk factors for AD have been discussed—such as increased age, a diagnosis of mild cognitive impairment (MCI), and a positive family history—one of the most significant risk factors is possession of the ε4 variant of the APOE gene (Combarros, Alvarez-Arcaya, Sánchez-Guerra, Infante, & Berciano, 2002; Holmes, 2002; Roses & Saunders, 1997; Saunders et al., 2000; Selkoe, 2001). Cognitive differences have been well documented between healthy older adults who carry the ε4 allele versus those who do not (Bondi et al., 1995; Caselli et al., 2001; Lehmann et al., 2006; Lind et al., 2006; Mayeux, Small, Tang, Tycko, & Stern, 2001; Rosen et al., 2005; Seeman et al., 2005; Swan, Lessov-Schlagger, Carmelli, Schellenberg, & La Rue, 2005; Tupler et al., 2007; Wilson et al., 2002). In addition, the allele has been implicated in functional and structural brain changes observed across the aging spectrum (Bookheimer et al., 2000; Bondi, Houston, Eyler, & Brown, 2005; Filbey, Chen, Sunderland, & Cohen, 2010; Han et al., 2007; Johnson et al., 2007; Lind et al., 2006; Mondadori et al., 2007; Wierenga et al., 2012; Yang et al., 2014). Hippocampal atrophy is greater in nondemented older adults who are ε4 carriers compared to ε4 non-carriers (Cohen, Small, Lalonde, Friz, & Sunderland, 2001; den Heijer et al., 2002; Lind et al., 2006; Plassman et al., 1997; Soininen et al., 1995) and longitudinal comparisons reveal that the APOE ε4 allele is linked to a greater reduction in hippocampal volume (Crivello et al., 2010; Jak, Houston, Nagel, Corey-Bloom, & Bondi, 2007; Stewart et al., 2011) and a decline in white matter integrity in the posterior corpus callosum and medial temporal lobes (Nierenberg et al., 2005; Persson et al., 2006). Critically, neuroimaging studies examining subregions of the hippocampus have implicated the DG/CA3 subregions as being particularly susceptible to volumetric (Mueller & Weiner, 2009; Mueller, Schuff, Raptentsetsang, Elman, & Weiner, 2008; cf. Lyall et al., 2013) and functional (Bookheimer et al., 2000; Han et al., 2007; Suthana et al., 2010) changes in older adults who are carriers of the ε4 allele. Given the association between APOE-ε4 genotype and changes in the DG subregion, and the importance of this subregion in pattern separation, we hypothesized that pattern separation would be impaired in older adult carriers of the APOE-ε4 allele.
To the authors’ knowledge, only one published study has examined the association between pattern separation and possession of the APOE-ε4 allele. Wesnes, Annas, Basun, Edgar, and Blennow (2014) examined pattern separation abilities of older adults diagnosed with mild to moderate AD with and without the ε4 allele. Their results indicated that, compared to ε4 non-carriers, ε4 carriers were impaired in their ability to discriminate between pictures with features that were similar to those presented previously—a condition hypothesized to tax pattern separation. However, no study to our knowledge has examined whether pattern separation performance is affected in older adult ε4 carriers without a neurocognitive disorder. The present study sought to test young adults and healthy older adults with and without the APOE-ε4 allele on a previously published behavioral task hypothesized to measure spatial pattern separation.
1.2 Materials and Methods
1.2.1 Participants
The participant sample for the current study consisted of 57 young adults, ranging in age from 18–25 years old, and 43 healthy older adults over 65 years of age. The young adults were recruited from a pool of college students at San Diego State University and the older adults were community dwelling individuals recruited from the San Diego community. All procedures were approved by the Institutional Review Boards at San Diego State University and the University of California at San Diego, and all participants provided written informed consent. The older adult participants were genotyped for APOE using a polymerase chain reaction-based method (Saunders et al., 1993). The older adult group was separated into two demographically similar groups based on the presence (ε4 carriers; n=24) or absence (ε4 non-carriers; n=19) of the APOE ε4 allele. We selected demographically comparable ε4 carrier and ε4 non-carrier samples from existing studies.
A growing number of studies report that pattern separation efficiency varies among older adults (Holden et al., 2012; Holden et al., 2013; Reagh et al., 2014; Roberts et al., 2014; Stark et al., 2010; Stark et al., 2013). As discussed previously, these studies have utilized a method of classifying older adults as “impaired” or “unimpaired” based on performance on a standardized measure of memory in order to investigate differences in performance on pattern separation tasks (Holden et al., 2012; Holden et al., 2013; Reagh et al., 2014; Roberts et al., 2014; Stark et al., 2010; Stark et al., 2013). Thus, participants in the current study were divided into a total of four groups: (1) impaired older ε4 carrier adults, (2) unimpaired older ε4 carrier adults, (3) older ε4 non-carrier adults, and (4) young adults. The older ε4 carrier adults were classified as impaired or unimpaired based on delayed recall performance on the Hopkins Verbal Learning Test Revised (HVLT-R; Benedict, Schretlen, Groninger, & Brandt, 1998; Brandt & Benedict, 2001) or on long-delay free recall performance on the California Verbal Learning Test-Second Edition (CVLT-II; Delis, Kaplan, Kramer, & Ober, 2000). Specifically, unimpaired older ε4 carriers scored within the normal range for young adults (ages 20–29), whereas impaired older ε4 carriers scored more than 1 standard deviation below the normal range for young adults. None of the older ε4 non-carriers scored more than 1 standard deviation below the normal range for young adults. All young adults completed the HVLT-R and older adults completed either the HVLT-R (n=24) or the CVLT-II (n=19). Age-corrected z-scores for the long delay free recall index (LDFR) are reported in Table 1 for each group. A summary of demographic characteristics for the sample also is included in Table 1. One-way analysis of variance (ANOVA) revealed no differences in age (p = .386) or education (p = .193) among the three older adult groups. The average Dementia Rating Scale (DRS; Mattis, 1976) scores for the older adults also are shown in Table 1. None of the older adults had been diagnosed with a neurocognitive disorder and all older adults scored above 130 on the DRS.
Table 1.
Mean (standard deviation) demographic data, total Dementia Rating Scale (DRS) scores, and Long Delay Free Recall (LDFR) age-corrected z-scores for young, older e4−, unimpaired older e4+, and impaired older e4+ adults.
| Young | Older ε4− | Unimpaired Older ε4+ | Impaired Older ε4+ | |
|---|---|---|---|---|
| n | 57 | 19 | 13 | 11 |
| Age (years) | 19.61 (2.02) | 74.72 (6.25) | 73.00 (3.89) | 76.89 (9.36) |
| Gender (%female) | 64.91 | 57.89 | 46.15 | 54.55 |
| Education (years) | 13.07 (.96) | 15.84 (2.36) | 16.54 (2.18) | 14.73 (2.96) |
| DRS Total | N/A | 140.53 (2.86) | 140.77 (2.62) | 138.00 (3.61) |
| LDFR | −.49 (1.36) | .95 (1.00) | 1.12 (.93) | −1.03 (.70) |
1.2.2 Spatial Pattern Separation Test
Participants completed a delayed match-to-sample for spatial location task developed in our laboratory (Holden et al., 2012), which utilized a metric manipulation of spatial interference to assess spatial pattern separation. The participant was seated in front of a computer monitor with a 15 cm black border affixed around the outside of the screen. The border was utilized to eliminate the possibility of using visual cues on the computer monitor to aid in remembering the spatial location of the stimuli.
Each trial consisted of a sample phase, an intratrial interval, and a choice phase. In the sample phase, a gray circle measuring 1.7 cm in diameter appeared on the screen for 5 s. The circle appeared in one of 18 possible locations across the middle of the screen. As part of the task instructions, the participant was told to “remember the location of the circle on the screen.” During the choice phase, two colored circles appeared on the screen, one red and one blue. One of the colored circles (target) was in the same location as the sample phase circle (correct choice) and the other colored circle (foil) was in a location that was either to the left or the right of the original gray circle (incorrect choice). The target and foil circles were separated by one of four possible spatial separations: 0 cm (edges of circles touched), 0.5 cm, 1.0 cm, and 1.5 cm. When the two colored circles were presented during the choice phase, the participant was asked to indicate which one was in the same location as the gray circle from the sample phase by stating its color. Participants were given 10 s to respond during the choice phase. It is hypothesized that choice circles that were closer together would result in heightened interference and thus an increased need for pattern separation. During a 10 s delay between the sample and the choice phases, participants were asked to look away from the screen and read a string of random letters from a laminated card on the table in front of them. The purpose of this activity was to prevent the participant from fixating the eyes on the location of the sample phase circle.
There were 48 total trials for the task, consisting of 12 trials for each of the four spatial separations. Each group of trials for a particular spatial separation was balanced across the width of the screen to ensure there was not an unintended bias toward a particular section of the screen. To counter fatigue effects, the 48 total trials were split into two sets of 24 trials with a 5 min break separating them. The two sets were identical in design and each set was pseudo-randomized, with the restriction that there were not more than two consecutive trials of the following: (1) spatial separation, (2) correct circle of a certain color, or (3) correct circle on a particular side.
1.3 Results
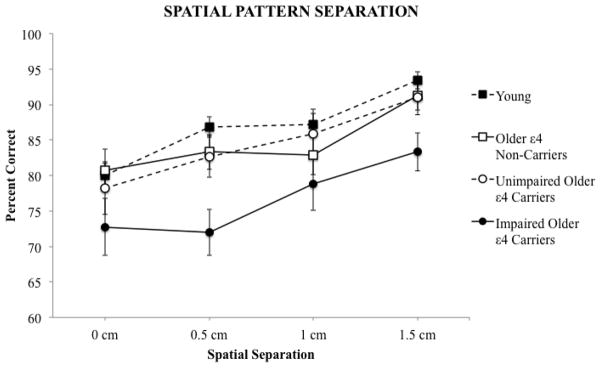
A 4 × 4 mixed model ANOVA was conducted to investigate the effects of group (impaired older ε4 carriers, unimpaired older ε4 carriers, older ε4 non-carriers, young) and spatial separation (0 cm, 0.5 cm, 1.0 cm, 1.5 cm) on spatial pattern separation task performance (percent correct). The analysis revealed a significant main effect of group, F(3, 96) = 5.10, p < .01. As shown in Figure 1, a Student Newman-Keuls post-hoc analysis revealed that impaired older ε4 carrier adults performed significantly worse (p < .05) than unimpaired older ε4 carrier adults (Cohen’s d = 0.83), older ε4 non-carrier adults (d = 0.83), and young adults (d = 1.05). However, significant differences were not found among the unimpaired older ε4 carrier adults, the older ε4 non-carrier adults, and the young adults (p > .05).
Figure 1.
Mean (standard error) performance of impaired older ε4 carriers, unimpaired older ε4 carriers, older ε4 non-carriers, and young adults as a function of spatial separation on the spatial pattern separation task.
Additionally, the mixed model ANOVA revealed a significant main effect of spatial separation on performance, F(3, 288) = 18.64, p < .001. Planned polynomial contrasts revealed a significant linear effect of spatial separation on performance, F(1, 96) = 48.08, p < .001. Specifically, greater spatial separation (i.e., decreased interference) was associated with better performance. No group x spatial separation interaction was detected (p = .77).
1.4 Discussion
The aim of the current study was to compare the performance of healthy older adult ε4 carriers, with and without subtle cognitive impairment, to non-carriers on a spatial memory task involving varying degrees of spatial interference. The task is hypothesized to assess spatial pattern separation. Older adult ε4 carriers were classified as “impaired” or “unimpaired” based on standardized verbal memory test performance (CVLT-II or HVLT-R) relative to standardized norms for young adults. Therefore, the impaired older ε4 carriers were impaired relative to young adults, not relative to age-corrected norms; hence we characterized them as experiencing subtle cognitive impairment. All older adults scored above 130 on the DRS and none were diagnosed with a neurocognitive disorder. We found that older ε4 carriers classified as impaired performed significantly worse on a spatial pattern separation task compared to unimpaired older ε4 carriers, older non-carriers, and young adults. These group differences were robust given the large effect sizes (d = 0.83 – 1.05) in the context of relatively small sample sizes. However, no significant differences were found among the latter three groups. In addition, we found that performance increased as a function of increased spatial separation across all groups, which is consistent with a pattern separation effect. The impaired older E4 carriers show a similar increase in performance across separations relative to the other groups but poorer accuracy, which suggests that pattern separation may be occurring in this group but is less efficient. We hypothesize that as spatial separation increased (e.g. 1 cm and 1.5 cm separation trials), interference was likely to decrease resulting in less demand for pattern separation. It is possible that general memory impairment also may contribute to the observed deficits in the impaired older ε4 carriers. However, we feel there are two pieces of evidence that suggest the current findings do not reflect solely a general memory deficit. First, the general memory demands of the task are identical on each trial. On the sample phase, the participant views one circle on the screen and has to remember its location across the delay. During this time there is no indication of the level of interference that will occur on the ensuing choice phase. On the choice phase, the participant then views two circles and has to indicate which occurred in the same location as the sample phase circle. One could argue that a deficit in general memory (i.e. inability to remember location of sample phase circle) would result in comparable performance across spatial separations. However, we find that all groups improve as spatial separation increases, which is consistent with a pattern separation effect. In addition, the older adults performed on average in the normal range on a standardized test of verbal memory (CVLT-II or HVLT-R) and none of the older adults performed in the impaired range on this measure. Although general memory decline cannot be ruled out completely, these findings provide evidence that the deficit observed in the impaired older ε4 carriers may reflect less efficient pattern separation rather than a general memory deficit. To our knowledge, the present findings offer the first evidence suggesting that spatial pattern separation may be less efficient in a subset of healthy older adults who are carriers of the APOE-ε4 allele. However, pattern separation may be comparable to young adults in a subset of older adults who are carriers of the APOE-ε4 allele or among non-carriers.
A prior study from our laboratory used this same behavioral task to examine spatial pattern separation in young and older adults (Holden et al., 2012). We reported that young adults significantly outperformed older adults on the task across all spatial separations. In addition, we found that a subset of older adults classified as “impaired” on a verbal memory test performed significantly worse on the pattern separation task than older adults classified as “unimpaired” and young adults. Similar findings have been reported in studies using different behavioral tasks hypothesized to measure pattern separation for spatial information (Reagh et al., 2014; Stark et al., 2010), temporal order memory (Roberts et al., 2014), and visual object information (Holden et al., 2013; Stark et al., 2013). Although various factors may have contributed to the observed differences between the impaired and unimpaired older adults in these studies, the present findings offer preliminary evidence that the APOE-ε4 allele further modifies this relationship and represents a factor that warrants further examination.
Recent studies have reported that individuals diagnosed with AD and amnestic MCI (aMCI) demonstrate deficits on pattern separation tests. Stark and colleagues (2013) utilized an incidental encoding task to examine pattern separation for visual object information in healthy older adults divided into aged-unimpaired and aged-impaired groups in addition to a group of individuals diagnosed with aMCI. The aged-unimpaired group outperformed both the aged-impaired group and the aMCI group on trials that taxed visual object pattern separation; however, the aged-impaired group and the aMCI group did not differ on these trials. Individuals with aMCI were impaired relative to both of the other groups on a measure of recognition memory, but there were no recognition memory differences between the aged-unimpaired and aged-impaired groups. Based on the findings of Stark and colleagues (2013), it may be possible to further characterize impairment in particular mnemonic processes in older adults through specific patterns of impairment in individuals with aMCI (impaired recognition and pattern separation), cognitively normal individuals with subtle cognitive decline (intact recognition and impaired pattern separation), and those who are aging successfully (intact recognition and intact pattern separation). A previous study reported that the deficits observed on a continuous recognition task that taxed visual object pattern separation in both cognitively normal older adults and individuals with aMCI were associated with structural and functional changes in the DG/CA3 region of the hippocampus (Yassa et al., 2010).
Another recent study (Ally, Hussey, Ko, & Molitor, 2013) used a similar continuous recognition memory task to behaviorally examine pattern separation in individuals diagnosed with aMCI or mild AD. In addition, the study examined how performance changed as function of the lag between the study and test objects. The data revealed that behavioral pattern separation rates decreased as a function of increasing lag between interfering objects in individuals diagnosed with aMCI. Performance of the aMCI group matched controls at the shortest lag of 4 interfering objects; however, the group performed comparably to the AD group at the largest lag of 40 interfering objects. The AD group was significantly impaired relative to controls across all lags. The data provide additional evidence for impaired visual object pattern separation associated with aMCI and provided some of the first behavioral evidence that pattern separation may be further impaired in those diagnosed with mild AD (Ally et al., 2013). A more recent study examined pattern separation abilities of older adults diagnosed with mild to moderate AD with and without the ε4 allele (Wesnes et al., 2014). Compared to ε4 non-carriers, ε4 carriers were impaired on trials hypothesized to tax pattern separation. Studies have begun to examine the relationship between standardized memory test performance and specific hippocampal subregion functioning (Brickman, Stern, & Small, 2011). As reviewed by Holden & Gilbert (2012), tests that measure pattern separation may be highly sensitive to subtle age-related changes in the DG region. It is hoped that one day these tests may be used in conjunction with standardized neuropsychological measures to help differentiate neurocognitive changes associated with normal aging, MCI, and AD. They also may help to differentiate increasingly nuanced distinctions between the cognitive decline of MCI from the ‘subtle cognitive decline’ (i.e., not of sufficient severity for the diagnosis of MCI) of the NIA-AA criteria for preclinical AD (Sperling et al., 2011).
Although future studies are needed with larger samples, the present findings provide evidence that spatial pattern separation may be less efficient in a subset of older adults who carry the APOE-ε4 allele. However, pattern separation may be comparable to young adults in a subset of older adults who are ε4 carriers or non-carriers. More research is needed into the moderators that may underlie the differences between these two subsets of older adults with the ε4 allele. For example, prior studies have reported sex differences in the association between age-related cognitive decline and the ε4 allele (Lehmann et al., 2006; Mortensen & Hogh, 2001). Future studies with larger samples should examine differences in pattern separation between male and female carriers and non-carries for the ε4 allele. In addition, neuroimaging studies have reported that ε4 carriers evidence increased hippocampal activity compared to non-carriers, even though neuropsychological and generalized memory performance was in the normal range (Bookheimer et al., 2000; Han et al., 2007). Specifically, Han et al. (2007) found that activity in the hippocampus was increased in ε4 carriers during a verbal learning task; however, these individuals had neuropsychological performance profiles that were no different than those of non-carriers. Future studies utilizing fMRI techniques should examine whether older ε4 carriers classified as impaired using verbal memory performance evidence different hippocampal activity levels when performing a spatial pattern separation task compared to older ε4 carriers classified as unimpaired. Furthermore, it would be of interest to test older ε4 carriers and non-carriers on tests designed to assess nonspatial pattern separation in order to examine whether the current findings may be specific to spatial pattern separation. As reviewed previously, the DG subregion of the hippocampus has been shown to play a critical role in pattern separation. Given that changes in the region have been documented in both healthy older adults and older adult carriers for the ε4 allele, we hypothesize that the presently observed deficits in older adults who carry the ε4 allele may stem from changes in the DG subregion. Finally, there has been discussion in the literature about the importance of linking behavior on tasks hypothesized to require pattern separation in humans to the neural/computational process of pattern separation (Roberts et al., 2014; Stark et al., 2013). As noted by Roberts et al. (2014), pattern separation only can be directly observed by recording neural input/output computations. However, behavioral tests that may be sensitive to age-related changes in the neural process of pattern separation can provide important insight into their functional significance. The present task was created based on a task developed for use in rodents, which was found to be sensitive to complete hippocampal lesions (Gilbert et al., 1998) and lesions restricted to the dorsal dentate gyrus (Gilbert et al., 2003) or dorsal CA3 (Gilbert et al., 2006) subregions. Future functional neuroimaging studies are needed to gain insight into the association between performance on the present task and activity in the hippocampus and its subregions.
The present findings add to a growing literature reporting that pattern separation may be less efficient in subsets of healthy older adults (Holden et al., 2012; Holden et al., 2013; Reagh et al., 2014; Roberts et al., 2014; Stark et al., 2010; Stark et al., 2013). Our findings provide novel insight into one potential moderator of these differences—the APOE-ε4 allele. These findings may have important implications for designing behavioral interventions for older adults, such as structuring daily living tasks in order to reduce interference, thus potentially improving memory function.
Highlights.
Examined performance of young and older adults on a spatial pattern separation task
Older adults were genotyped as apolipoprotein E-ε4 carriers or non-carriers
ε4 carriers classified as impaired or unimpaired using a standardized memory test
Older ε4 carriers in impaired group displayed spatial pattern separation deficits
Pattern separation may be less efficient in ε4 carriers with subtle memory decline
Acknowledgments
This research was supported by a National Institutes of Health Grants awarded to Paul E. Gilbert (R01 AG034202) and Mark W. Bondi (R01 AG012674 and K24 AG026431). We also thank all of the participants for their contributions to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David P. Sheppard, Email: dpsheppard@uh.edu.
Lisa V. Graves, Email: lvgraves@gmail.com.
Heather M. Holden, Email: hholden365@gmail.com.
Lisa Delano-Wood, Email: ldelano@ucsd.edu.
Mark W. Bondi, Email: mbondi@ucsd.edu.
Paul E. Gilbert, Email: pgilbert@mail.sdsu.edu.
1.6 References
- Aimone JB, Deng W, Gage FH. Adult neurogenesis: integrating theories and separating functions. Trends in Cognitive Sciences. 2010;14(7):325–337. doi: 10.1016/j.tics.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiology of Aging. 2005;26(9):1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. discussion 1279–1282. [DOI] [PubMed] [Google Scholar]
- Ally BA, Hussey EP, Ko PC, Molitor RJ. Pattern separation and pattern completion in Alzheimer’s disease: evidence of rapid forgetting in amnestic mild cognitive impairment. Hippocampus. 2013;23(12):1246–1258. doi: 10.1002/hipo.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Hwang KS, Andrawis JP, Green AE, Babakchanian S, Morra JH Alzheimer’s Disease Neuroimaging Initiative. 3D PIB and CSF biomarker associations with hippocampal atrophy in ADNI subjects. Neurobiology of Aging. 2010;31(8):1284–1303. doi: 10.1016/j.neurobiolaging.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CEL. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science (New York, NY) 2008;319(5870):1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RHB. The brief visuospatial memory test – revised: Professional manual. Odessa: Psychological Assessment Resources; 1997. [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. The Hopkins verbal learning test – revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- Bondi MW, Salmon DP, Monsch AU, Galasko D, Butters N, Klauber MR, Saitoh T. Episodic memory changes are associated with the APOE-epsilon 4 allele in nondemented older adults. Neurology. 1995;45(12):2203–2206. doi: 10.1212/wnl.45.12.2203. [DOI] [PubMed] [Google Scholar]
- Bondi Mark W, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64(3):501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer’s disease. The New England Journal of Medicine. 2000;343(7):450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Benedict RHB. Professional manual. Lutz: Psychological Assessment Resources; 2001. Hopkins verbal learning test-revised. [Google Scholar]
- Brickman AM, Stern Y, Small SA. Hippocampal subregions differentially associate with standardized memory tests. Hippocampus. 2011;21(9):923–928. doi: 10.1002/hipo.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Osborne D, Reiman EM, Hentz JG, Barbieri CJ, Saunders AM, Alexander GE. Preclinical cognitive decline in late middle-aged asymptomatic apolipoprotein E-e4/4 homozygotes: a replication study. Journal of the Neurological Sciences. 2001;189(1–2):93–98. doi: 10.1016/s0022-510x(01)00577-9. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Small C, Lalonde F, Friz J, Sunderland T. Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology. 2001;57(12):2223–2228. doi: 10.1212/wnl.57.12.2223. [DOI] [PubMed] [Google Scholar]
- Combarros O, Alvarez-Arcaya A, Sánchez-Guerra M, Infante J, Berciano J. Candidate gene association studies in sporadic Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2002;14(1):41–54. doi: 10.1159/000058332. 58332. [DOI] [PubMed] [Google Scholar]
- Crivello F, Lemaître H, Dufouil C, Grassiot B, Delcroix N, Tzourio-Mazoyer N, Mazoyer B. Effects of ApoE-epsilon4 allele load and age on the rates of grey matter and hippocampal volumes loss in a longitudinal cohort of 1186 healthy elderly persons. NeuroImage. 2010;53(3):1064–1069. doi: 10.1016/j.neuroimage.2009.12.116. [DOI] [PubMed] [Google Scholar]
- Delis D, Kaplan E, Kramer J, Ober B. California verbal learning test–II. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Den Heijer T, Oudkerk M, Launer LJ, Van Duijn CM, Hofman A, Breteler MMB. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59(5):746–748. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- Doxey CR, Kirwan CB. Structural and functional correlates of behavioral pattern separation in the hippocampus and medial temporal lobe. Hippocampus. 2014 doi: 10.1002/hipo.22389. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Sutherland RJ. The aging hippocampus: navigating between rat and human experiments. Reviews in the Neurosciences. 2005;16(2):87–121. doi: 10.1515/revneuro.2005.16.2.87. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Chen G, Sunderland T, Cohen RM. Failing compensatory mechanisms during working memory in older apolipoprotein E-epsilon4 healthy adults. Brain Imaging and Behavior. 2010;4(2):177–188. doi: 10.1007/s11682-010-9097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Colantuoni C, Eichenbaum H, Haberman RP, Rapp PR, Tanila H, Wilson IA. Individual differences in neurocognitive aging of the medial temporal lobe. Age (Dordrecht, Netherlands) 2006;28(3):221–233. doi: 10.1007/s11357-006-9017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Brushfield AM. The role of the CA3 hippocampal subregion in spatial memory: a process oriented behavioral assessment. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2009;33(5):774–781. doi: 10.1016/j.pnpbp.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. The role of the dorsal CA3 hippocampal subregion in spatial working memory and pattern separation. Behavioural Brain Research. 2006;169(1):142–149. doi: 10.1016/j.bbr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. Recognition memory for complex visual discriminations is influenced by stimulus interference in rodents with perirhinal cortex damage. Learning & Memory (Cold Spring Harbor, NY) 2003;10(6):525–530. doi: 10.1101/lm.64503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, DeCoteau WE. Memory for spatial location: role of the hippocampus in mediating spatial pattern separation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1998;18(2):804–810. doi: 10.1523/JNEUROSCI.18-02-00804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Han SD, Houston WS, Jak AJ, Eyler LT, Nagel BJ, Fleisher AS, Bondi MW. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiology of Aging. 2007;28(2):238–247. doi: 10.1016/j.neurobiolaging.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden HM, Gilbert PE. Less efficient pattern separation may contribute to age-related spatial memory deficits. Frontiers in Aging Neuroscience. 2012;4:9. doi: 10.3389/fnagi.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden HM, Hoebel C, Loftis K, Gilbert PE. Spatial pattern separation in cognitively normal young and older adults. Hippocampus. 2012;22(9):1826–1832. doi: 10.1002/hipo.22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden HM, Toner C, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation varies in older adults. Learning & Memory (Cold Spring Harbor, NY) 2013;20(7):358–362. doi: 10.1101/lm.030171.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C. Genotype and phenotype in Alzheimer’s disease. The British Journal of Psychiatry: The Journal of Mental Science. 2002;180:131–134. doi: 10.1192/bjp.180.2.131. [DOI] [PubMed] [Google Scholar]
- Jak AJ, Houston WS, Nagel BJ, Corey-Bloom J, Bondi MW. Differential cross-sectional and longitudinal impact of APOE genotype on hippocampal volumes in nondemented older adults. Dementia and Geriatric Cognitive Disorders. 2007;23(6):382–389. doi: 10.1159/000101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Ries ML, Hess TM, Carlsson CM, Gleason CE, Alexander AL, Sager MA. Effect of Alzheimer disease risk on brain function during self-appraisal in healthy middle-aged adults. Archives of General Psychiatry. 2007;64(10):1163–1171. doi: 10.1001/archpsyc.64.10.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Rolls ET. A computational theory of hippocampal function, and tests of the theory: New developments. Neuroscience and Biobehavioral Reviews. 2014;48C:92–147. doi: 10.1016/j.neubiorev.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Leal SL, Yassa MA. Effects of aging on mnemonic discrimination of emotional information. Behavioral Neuroscience. 2014;128(5):539–547. doi: 10.1037/bne0000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann DJ, Refsum H, Nurk E, Warden DR, Tell GS, Vollset SE, Smith AD. Apolipoprotein E epsilon4 and impaired episodic memory in community-dwelling elderly people: a marked sex difference. The Hordaland Health Study. Journal of Neurology, Neurosurgery, and Psychiatry. 2006;77(8):902–908. doi: 10.1136/jnnp.2005.077818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind J, Larsson A, Persson J, Ingvar M, Nilsson LG, Backman L, Nyberg L. Reduced hippocampal volume in non-demented carriers of the apolipoprotein E epsilon4: relation to chronological age and recognition memory. Neuroscience Letters. 2006;396(1):23–27. doi: 10.1016/j.neulet.2005.11.070. [DOI] [PubMed] [Google Scholar]
- Ly M, Murray E, Yassa MA. Perceptual versus conceptual interference and pattern separation of verbal stimuli in young and older adults. Hippocampus. 2013;23(6):425–430. doi: 10.1002/hipo.22110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall DM, Royle NA, Harris SE, Bastin ME, Maniega SM, Murray C, Deary IJ. Alzheimer’s disease susceptibility genes APOE and TOMM40, and hippocampal volumes in the Lothian birth cohort 1936. PloS One. 2013;8(11):e80513. doi: 10.1371/journal.pone.0080513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellack L, Katsau TB, editors. Geriatric psychiatry: A handbook for psychiatrists and primary care physicians. New York: Grune & Stratton; 1976. pp. 77–121. [Google Scholar]
- Mayeux R, Small SA, Tang M, Tycko B, Stern Y. Memory performance in healthy elderly without Alzheimer’s disease: effects of time and apolipoprotein-E. Neurobiology of Aging. 2001;22(4):683–689. doi: 10.1016/s0197-4580(01)00223-8. [DOI] [PubMed] [Google Scholar]
- Mondadori CRA, De Quervain DJ-F, Buchmann A, Mustovic H, Wollmer MA, Schmidt CF, Henke K. Better memory and neural efficiency in young apolipoprotein E epsilon4 carriers. Cerebral Cortex (New York, NY: 1991) 2007;17(8):1934–1947. doi: 10.1093/cercor/bhl103. [DOI] [PubMed] [Google Scholar]
- Mortensen EL, Høgh P. A gender difference in the association between APOE genotype and age-related cognitive decline. Neurology. 2001;57(1):89–95. doi: 10.1212/wnl.57.1.89. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Schuff N, Raptentsetsang S, Elman J, Weiner MW. Selective effect of Apo e4 on CA3 and dentate in normal aging and Alzheimer’s disease using high resolution MRI at 4 T. NeuroImage. 2008;42(1):42–48. doi: 10.1016/j.neuroimage.2008.04.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller Susanne G, Weiner MW. Selective effect of age, Apo e4, and Alzheimer’s disease on hippocampal subfields. Hippocampus. 2009;19(6):558–564. doi: 10.1002/hipo.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierenberg J, Pomara N, Hoptman MJ, Sidtis JJ, Ardekani BA, Lim KO. Abnormal white matter integrity in healthy apolipoprotein E epsilon4 carriers. Neuroreport. 2005;16(12):1369–1372. doi: 10.1097/01.wnr.0000174058.49521.16. [DOI] [PubMed] [Google Scholar]
- Persson J, Lind J, Larsson A, Ingvar M, Cruts M, Van Broeckhoven C, Nyberg L. Altered brain white matter integrity in healthy carriers of the APOE epsilon4 allele: a risk for AD? Neurology. 2006;66(7):1029–1033. doi: 10.1212/01.wnl.0000204180.25361.48. [DOI] [PubMed] [Google Scholar]
- Pidgeon LM, Morcom AM. Age-related increases in false recognition: the role of perceptual and conceptual similarity. Frontiers in Aging Neuroscience. 2014;6:283. doi: 10.3389/fnagi.2014.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Welsh-Bohmer KA, Bigler ED, Johnson SC, Anderson CV, Helms MJ, Breitner JC. Apolipoprotein E epsilon 4 allele and hippocampal volume in twins with normal cognition. Neurology. 1997;48(4):985–989. doi: 10.1212/wnl.48.4.985. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral Cortex (New York, NY: 1991) 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Reagh ZM, Roberts JM, Ly M, DiProspero N, Murray E, Yassa MA. Spatial discrimination deficits as a function of mnemonic interference in aged adults with and without memory impairment. Hippocampus. 2014;24(3):303–314. doi: 10.1002/hipo.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JM, Ly M, Murray E, Yassa MA. Temporal discrimination deficits as a function of lag interference in older adults. Hippocampus. 2014;24(10):1189–1196. doi: 10.1002/hipo.22303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen VM, Sunderland T, Levy J, Harwell A, McGee L, Hammond C, Lefkowitz C. Apolipoprotein E and category fluency: evidence for reduced semantic access in healthy normal controls at risk for developing Alzheimer’s disease. Neuropsychologia. 2005;43(4):647–658. doi: 10.1016/j.neuropsychologia.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Roses AD, Saunders AM. Apolipoprotein E genotyping as a diagnostic adjunct for Alzheimer’s disease. International Psychogeriatrics / IPA. 1997;9(Suppl 1):277–288. doi: 10.1017/s1041610297005012. discussion 317–321. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Alberts MJ. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Trowers MK, Shimkets RA, Blakemore S, Crowther DJ, Mansfield TA, Roses AD. The role of apolipoprotein E in Alzheimer’s disease: pharmacogenomic target selection. Biochimica Et Biophysica Acta. 2000;1502(1):85–94. doi: 10.1016/s0925-4439(00)00035-1. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Marrone DF, Markus EJ. Disambiguating the similar: the dentate gyrus and pattern separation. Behavioural Brain Research. 2012;226(1):56–65. doi: 10.1016/j.bbr.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Huang MH, Bretsky P, Crimmins E, Launer L, Guralnik JM. Education and APOE-e4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. The Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 2005;60(2):74–83. doi: 10.1093/geronb/60.2.p74. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiological Reviews. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Small SA, Tsai WY, DeLaPaz R, Mayeux R, Stern Y. Imaging hippocampal function across the human life span: is memory decline normal or not? Annals of Neurology. 2002;51(3):290–295. doi: 10.1002/ana.10105. [DOI] [PubMed] [Google Scholar]
- Soininen H, Partanen K, Pitkänen A, Hallikainen M, Hanninen T, Helisalmi S, Riekkinen P. Decreased hippocampal volume asymmetry on MRIs in nondemented elderly subjects carrying the apolipoprotein E epsilon 4 allele. Neurology. 1995;45(2):391–392. doi: 10.1212/wnl.45.2.391. [DOI] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CEL. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013;51(12):2442–2449. doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Stark CEL. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learning & Memory (Cold Spring Harbor, NY) 2010;17(6):284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R, Godin O, Crivello F, Maillard P, Mazoyer B, Tzourio C, Dufouil C. Longitudinal neuroimaging correlates of subjective memory impairment: 4-year prospective community study. The British Journal of Psychiatry: The Journal of Mental Science. 2011;198(3):199–205. doi: 10.1192/bjp.bp.110.078683. [DOI] [PubMed] [Google Scholar]
- Suthana NA, Krupa A, Donix M, Burggren A, Ekstrom AD, Jones M, Bookheimer SY. Reduced hippocampal CA2, CA3, and dentate gyrus activity in asymptomatic people at genetic risk for Alzheimer’s disease. NeuroImage. 2010;53(3):1077–1084. doi: 10.1016/j.neuroimage.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN, Carmelli D, Schellenberg GD, La Rue A. Apolipoprotein E epsilon4 and change in cognitive functioning in community-dwelling older adults. Journal of Geriatric Psychiatry and Neurology. 2005;18(4):196–201. doi: 10.1177/0891988705281864. [DOI] [PubMed] [Google Scholar]
- Tolentino JC, Pirogovsky E, Luu T, Toner CK, Gilbert PE. The effect of interference on temporal order memory for random and fixed sequences in nondemented older adults. Learning & Memory (Cold Spring Harbor, NY) 2012;19(6):251–255. doi: 10.1101/lm.026062.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learning & Memory (Cold Spring Harbor, NY) 2009;16(5):338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- Tupler LA, Krishnan KRR, Greenberg DL, Marcovina SM, Payne ME, MacFall JR, Doraiswamy PM. Predicting memory decline in normal elderly: genetics, MRI, and cognitive reserve. Neurobiology of Aging. 2007;28(11):1644–1656. doi: 10.1016/j.neurobiolaging.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Dale AM, McEvoy LK, Brewer J, Karow DS Alzheimer’s Disease Neuroimaging Initiative. Multi-modal imaging predicts memory performance in normal aging and cognitive decline. Neurobiology of Aging. 2010;31(7):1107–1121. doi: 10.1016/j.neurobiolaging.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesnes KA, Annas P, Basun H, Edgar C, Blennow K. Performance on a pattern separation task by Alzheimer’s patients shows possible links between disrupted dentate gyrus activity and apolipoprotein E ε 4 status and cerebrospinal fluid amyloid-β42 levels. Alzheimer’s Research & Therapy. 2014;6(2):20. doi: 10.1186/alzrt250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Dev SI, Shin DD, Clark LR, Bangen KJ, Jak AJ, Bondi MW. Effect of mild cognitive impairment and APOE genotype on resting cerebral blood flow and its association with cognition. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32(8):1589–1599. doi: 10.1038/jcbfm.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Barnes LL, Beckett LA, Aggarwal NT, Cochran EJ, Bennett DA. The apolipoprotein E epsilon 4 allele and decline in different cognitive systems during a 6-year period. Archives of Neurology. 2002;59(7):1154–1160. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- Yang AC, Huang CC, Liu ME, Liou YJ, Hong CJ, Lo MT, Tsai SJ. The APOE ε4 allele affects complexity and functional connectivity of resting brain activity in healthy adults. Human Brain Mapping. 2014;35(7):3238–3248. doi: 10.1002/hbm.22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011;21(9):968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CEL. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(21):8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends in Neurosciences. 2011;34(10):515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CEL. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. NeuroImage. 2010;51(3):1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]