Abstract
The current study examined efficacy of a small Tat (trans-activator of transcription)-conjugated peptide activator of the Nrf2 (nuclear factor-E2-related factor-2) antioxidant/cell-defense pathway as a potential injury-specific, novel neuroprotectant against global cerebral ischemia (GCI). A competitive peptide, DEETGE-CAL-Tat, was designed to facilitate Nrf2 activation by disrupting interaction of Nrf2 with Keap1 (kelch-like ECH-associated protein 1), a protein that sequesters Nrf2 in the cytoplasm and thereby inactivates it. The DEETGE-CAL-Tat peptide contained the critical sequence DEETGE for the Nrf2–Keap1 interaction, the cell transduction domain of the HIV-Tat protein, and the cleavage sequence of calpain, which is sensitive to Ca2+ increase and allows injury-specific activation of Nrf2. Using an animal model of GCI, we demonstrated that pretreatment with the DEETGE-CAL-Tat peptide markedly decreased Nrf2 interaction with Keap1 in the rat hippocampal CA1 region after GCI, and enhanced Nrf2 nuclear translocation and DNA binding. The DEETGE-CAL-Tat peptide also induced Nrf2 antioxidant/cytoprotective target genes, reduced oxidative stress, and induced strong neuroprotection and marked preservation of hippocampal-dependent cognitive function after GCI. These effects were specific as control peptides lacked neuroprotective ability. Intriguingly, the DEETGE-CAL-Tat peptide effects were also injury specific, as it had no effect upon neuronal survival or cognitive performance in sham nonischemic animals. Of significant interest, peripheral, postischemia administration of the DEETGE-CAL-Tat peptide from days 1–9 after GCI also induced robust neuroprotection and strongly preserved hippocampal-dependent cognitive function. Based on its robust neuroprotective and cognitive-preserving effects, and its unique injury-specific activation properties, the DEETGE-CAL-Tat peptide represents a novel, and potentially promising new therapeutic modality for the treatment of GCI.
SIGNIFICANCE STATEMENT The current study demonstrates that DEETGE-CAL-Tat, a novel peptide activator of a key antioxidant gene transcription pathway in the hippocampus after global cerebral ischemia, can exert robust neuroprotection and preservation of cognitive function. A unique feature of the peptide is that its beneficial effects are injury specific. This feature is attractive as it targets drug activation specifically in the site of injury, and likely would lead to a reduction of undesirable side effects if translatable to the clinic. Due to its injury-specific activation, robust neuroprotection, and cognitive-preserving effects, this novel peptide may represent a much-needed therapeutic advance that could have efficacy in the treatment of global cerebral ischemia.
Keywords: cardiac arrest, cognitive defect, hippocampus CA1 region, neuroprotection, peptide, reactive oxidative species
Introduction
Cardiac arrest is a leading cause of death and disability in the United States, with 350,000–500,000 cardiac arrests occurring each year in the United States (Roger et al., 2011). A major disability feared by cardiac arrest survivors is cognitive impairment. A review of several high-quality prospective studies found that cognitive problems occurred in approximately half of the survivors of out-of-hospital cardiac arrest (Roine et al., 1993; Sauvé et al., 1996; Moulaert et al., 2009). Memory problems were the most common cognitive impairment after cardiac arrest, followed by impairments in attention and executive functioning (Moulaert et al., 2009). Pathologically, the risk of cognitive impairment is primarily due to global cerebral ischemia (GCI) during cardiac arrest, which can induce delayed neuronal cell death in the hippocampal CA1 region and consequent cognitive decline (Kirino, 1982; Pulsinelli et al., 1982; Brillman, 1993; Swain et al., 1993; Wolman et al., 1999). Experimental GCI induced in animals has proven to be a valuable model for the study of cardiac arrest-induced neuronal cell death and cognitive deficits, as it results in selective and delayed neuronal death of pyramidal neurons in the hippocampal CA1 region, similar to the situation in humans (Kirino, 1982; Pulsinelli et al., 1982; Kirino and Sano, 1984; Chen et al., 1996).
Unfortunately, there are currently no effective drugs available that protect the brain from cardiac arrest-induced neurological impairment. Therapeutic hypothermia is the only strategy to date that has been reported to have neurological benefit in cardiac arrest (Harukuni and Bhardwaj, 2006). However, its beneficial effect is not without controversy, as a recent meta-analysis did not find a strong benefit for hypothermia on survival or neurological outcome (Nielsen et al., 2011; Kim et al., 2012). Thus, new therapeutic strategies for protection of the brain from GCI after cardiac arrest are urgently needed.
In the current study, we tested a novel strategy to enhance a key endogenous defense mechanism present in cells, the Nrf2 (nuclear factor E2-related factor 2) transcriptional system for induction of cytoprotective, antioxidant, and detoxifying genes. Nrf2 is a transcription factor that is normally sequestered in the cytoplasm by binding of the adaptor protein, Keap1 (kelch-like ECH-associated protein-1), which leads to degradation of Nrf2 by the proteosome (McMahon et al., 2004; Villeneuve et al., 2010). Cell stress and/or injury can lead to dissociation of Nrf2 from Keap1, and its translocation to the nucleus, where it induces a battery of cytoprotective, antioxidant, and detoxifying genes that can protect the cell, such as heme oxygenase-1 (HO-1), glutathione-S-transferase, glutathione peroxidase (GPx1), glutathione synthetase, NADPH quinone oxidase-1, epoxide hydrolase, and brain-derived growth factor (Kam et al., 2011; Chen et al., 2012; Guerrero-Beltrán et al., 2012).
Recent work has shown that a DEETGE sequence in Nrf2 is critical for the Nrf2–Keap1 interaction, and that DEETGE-CAL-Tat synthetic peptides that contain the DEETGE sequence, a calpain cleavage site, and a HIV-Tat cell transduction domain, can disrupt the Nrf2–Keap1 interaction and induce Nrf2 genes in vitro and in vivo in animals subjected to traumatic brain injury (Zhao et al., 2011). We thus hypothesized that the DEETGE-CAL-Tat peptide may have efficacy as a novel therapeutic agent in GCI. We therefore performed preclinical studies using a four-vessel occlusion (4-VO) animal model of GCI to test the hypothesis. The results of our study demonstrated that administration of the DEETGE-CAL-Tat peptide in an animal model of GCI strongly enhanced both nuclear translocation and DNA binding of Nrf2, as well as expression of known Nrf2-regulated target antioxidant/cell-defense proteins in the hippocampal CA1 region, and significantly reduced GCI-induced oxidative damage. The study also revealed that intracerebroventricular pre-treatment or peripheral post-treatment with the DEETGE-CAL-Tat peptide induced robust neuroprotection in the hippocampal CA1 region and strongly preserved cognitive function after GCI.
Materials and Methods
Antibodies and reagents.
The following antibodies were used: NeuN (Millipore Biotechnology, MAB377), GPx1 (ab22604), β-actin (sc-81178), Nrf2 (sc-722), HO-1 (sc-1797), NAD(P)H dehydrogenase, quinone 1 (NQO1; sc-376023), superoxide dismutase 2 (SOD2; sc-18503), 4-hydroxy-2-nonenal (4HNE; ab46545), 8-hydroxy-2-deoxyguanosine (8OHdG; ab62623), Keap1 (#4617), and Nrf2 (sc-30915). Alexa-conjugated secondary antibodies and DAPI were from Invitrogen. Polyvinylidene difluoride (PVDF) membranes with pore size of 0.45 μm were from Millipore. BCIP (5-bromo-4-chloro-3-indolyl-phosphate) and NBT (nitroblue tetrazolium) were from Promega. Nrf2 transcription factor ELISA kits (catalog #50296) were from Active Motif. Unless indicated otherwise, all the other chemicals were from Sigma-Aldrich.
Animal model of ischemia and administration of drugs.
Adult male Sprague Dawley rats (Beijing HFK Bioscience; weight, 250–300 g) were housed in a temperature-controlled (22–24°C) room with water and food available ad libitum. All procedures were approved by local legislation for ethics of experiments on animals. GCI was induced by the 4-VO method as described previously (Sun and Gilboe, 1994; Zhang et al., 2011; Wang et al., 2013). Briefly, anesthetization was induced in the rats with chloral hydrate (20%; 350 mg/kg, i.p.), both vertebral arteries were electrocauterized through the alar foramen of the first cervical vertebra and both common carotid arteries (CAAs) were exposed followed by closing of the incision with a suture. Rats were allowed to recover for 24 h and fasted overnight. The animals were lightly anesthetized to re-expose and occlude the CAAs for 15 min with aneurysm clips. Rats that lost their righting reflex within 30 s and whose pupils were dilated and unresponsive to light during ischemia were selected for the experiments. Resumption of carotid artery blood flow was verified visually by releasing the clips. Rectal temperature was maintained at 36.5–37.5°C during surgery with a feedback-regulated heating pad. For sham-operated rats, the surgeries were performed exactly as for ischemic animals except that the CAAs were not clamped.
Administrations of DEETGE-CAL-Tat peptide and control peptide.
The following peptide was synthesized by SBS Genentech. The DEETGE-CAL-Tat peptide (NH2-RKKRRQRRR-PLFAER-LDEETGEFLP-CONH2) was designed according to Zhao et al. (2011) with a minor difference in the N-terminal. Rather than amino acids 47–57 of the cell transduction domain of the HIV-Tat, we used amino acids 49–57 (RKKRRQRRR), which have been identified as the minimal protein transduction domain of the HIV-Tat (Wender et al., 2000; Beerens et al., 2003). The other key structural motifs of the DEETGE-CAL-Tat peptide included (1) the critical sequence DEETGE for the Nrf2–Keap1 interaction and (2) the cleavage sequence of calpain (PLFAER), which is highly sensitive to Ca2+ increase and allows injury-specific activation of Nrf2. In pretreatment studies, the DEETGE-CAL-Tat peptide at doses of 30, 50, or 100 μg was dissolved in 5 μl of 0.9% saline and was injected by unilateral intracerebroventricular administration 30 min before ischemia. For intracerebroventricular injection, the rats were placed on ear bars of a stereotaxic instrument under anesthesia and the peptide was injected into the left lateral ventricle (anteroposterior, 0.8 mm; lateral, 1.5 mm; depth, 3.5 mm; from bregma) through a stepper-motorized microsyringe (Stoelting) at a rate of 1 μl/min. To further confirm the effective neuroprotection, the DEETGE-CAL-Tat peptide dissolved at doses 1 or 2 mg in 100 μl of saline (120 and 240 μg/d) was subcutaneously administrated using a minipump (0.5 μl/h for 14 d; Alzet 2002) beginning at 1 d after reperfusion and continued until the end of the experiment. Vehicle-treated control rats received an equal volume of 0.9% saline. The Morris water maze (MWM) test was performed on day 7, 8, and 9 after ischemia, as described previously by our group and others (Wang et al., 2013; Zhang et al., 2013).
To show specificity of the DEETGE-CAL-Tat effects, several groups of controls were administrated by unilateral intracerebroventricular administration (50 μg, in 5 μl of 0.9% saline) 30 min before ischemia, including (1) DEETGE-Tat (to show it was indeed inactive), (2) scrambled-CAL-Tat (GEEDTE-CAL-Tat, to demonstrate the requirement of DEETDE for neuroprotection), (3) CAL-Tat (to rule out the possibility that the neuroprotection was not a result of CAL sequence-mediated inhibition of calpain's ability to cleave its substrate proteins), and (4) Tat (trans-activator of transcription) sequence alone (to rule out a neuroprotective effect of Tat alone).
Histological analysis.
The rats were killed under general anesthesia at various time points as mentioned in the figures, and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1 m phosphate buffer (PB), pH 7.4. Brains were postfixed in the same fixative at 4°C for 12 h, cryoprotected in 30% sucrose in PB, and then were cut longitudinally into 25 or 10 μm sections with a cryostat. Coronal sections were collected through the entire dorsal hippocampus (2.5–4.5 mm posterior from bregma) to investigate the neuroprotective effect of DEETGE-CAL-Tat peptide following GCI by performing double immunofluorescence staining for NeuN, a neuronal marker, and TUNEL, a DNA damage and apoptosis marker (TUNEL kit, lot #1639496, Life Technologies). Briefly, the sections (25 μm) were washed using 0.1 m PBS for 30 min, permeabilized with 0.4% Triton X-100-PBS for 1 h, blocked in 10% donkey serum for 1 h, and then incubated in anti-NeuN antibody (1:800) overnight at 4°C. After rinsing three times over 40 min with 0.1% Triton X-100-PBS, the sections were incubated with secondary antibodies (Alexa Fluor 488 nm donkey anti-mouse IgG) at room temperature for 1 h followed by washing for 4 × 10 min in 0.1% Triton X-100-PBS. The sections were incubated in terminal deoxynucleotidyl transferase (TdT) reaction buffer A for 10 min at 37°C and then in TdT reaction mixture, including enzyme solution, for 1 h at 37°C. After 5 min washing with distilled water, the sections were incubated in Click-iT Plus TUNEL reaction mixture for 30 min at 37°C, washed with 0.1% Triton 100-PBS over 20 min, and then mounted on slides covered with water-based mounting medium. Images were captured under laser scanning confocal microscopy (LSCM, Olympus FV1000) and analysis was performed using digital imaging software (FV10-ASW 1.5). For cresyl violet (CV) staining, sections (10 μm) were mounted on a slide and immersed in 0.1% CV solution for 30 min after washing in 0.1 m PBS 15 min. Then the slide was immersed in a container with distilled water for 2 min followed by dipping in 70 and 90% ethanol for 2 min each, followed by two immersions in 100% ethanol for 5 min each. The final two immersions were in 100% xylene solution for 5 min each. The slide was then coverslipped with permanent mounting medium. The slides were examined with a microscope (Olympus DP80) equipped with image-capture software (Cellsens 1.8). Well-rounded cells with no pyknosis stand for survival cells.
The number of NeuN-positive or TUNEL-positive neurons in immunofluorescent staining, or survival cells in CV staining per 250 μm length of the medial CA1 pyramidal cell layer was bilaterally counted in five sections per animal and was averaged to provide the mean value. A mean ± SD was calculated from the data in each group and statistical analysis performed as described below.
Immunofluorescence staining.
The coronal sections (25 μm) were washed for 30 min with 0.1 m PBS, permeabilized using 0.4% Triton X-100-PBS for 1 h, and then incubated with 10% normal donkey serum for 1 h at room temperature. The sections were incubated with corresponding primary antibodies [anti-4HNE (1:200), anti-8OHdG (1:200), anti-Nrf2 (1:100), and anti-NeuN (1:800)] overnight at 4°C, washed for 3 × 10 min with 0.1% Triton X-100-PBS, and then incubated with secondary antibodies (Alexa Fluor 568 nm donkey anti-mouse, Alexa Fluor 568 nm donkey anti-rabbit, and Alexa Fluor 488 nm donkey anti-mouse) at room temperature for 1 h, followed by a final washing for 5 ×10 min in 0.1% Triton X-100-PBS. DAPI (1 ng/μl) was incubated for counterstaining of the nucleus. Sections were mounted with water-based mounting medium containing anti-fading agents (Biomeda, Fisher Scientific). The confocal images were captured on an LSCM (Olympus FV1000) and digital imaging software (FV10-ASW 1.5 Viewer).
Duolink In Situ hybridization.
Interaction of Nrf2 and Keap1 in hippocampal CA1 region was determined by the Duolink II proximity ligation assay kit (PLA-probe anti-rabbit plus, catalog #DUO92002-30RXN; PLA-probe anti-goat minus, catalog #DUO92006-30RXN; Detection Kit Orange, catalog #DUO92008-100RXN). The Duolink proximity ligation assay (DPLA) probe anti-rabbit plus binds to the Nrf2 antibody, whereas the PLA probe anti-goat minus binds to Keap1 antibody. The DPLA secondary antibodies generate only a signal when the two DPLA probes are bound, which only takes place if both proteins are closer than 40 nm, indicating their interaction. Briefly, the coronal sections were pretreated the same as indicated above for immunofluorescence staining. After blocking in 10% donkey serum, the sections were coincubated with primary antibodies of Nrf2 (1:50) and Keap1 (1:50) overnight at 37°C in a preheated humidity chamber. After washing three times in buffer A for 10 min, Duolink II PLA probes detecting rabbit or goat secondary antibodies were diluted in the blocking agent in a concentration of 1:5 and applied to the sections, followed by incubation for 1 h in a preheated humidity chamber at 37°C. Unbound DPLA probes were removed by washing three times in buffer A for 5 min. The sections were incubated with the ligation solution consisting of Duolink II Ligation stock (1:5) and Duolink Ligase (1:40) diluted in high purity water for 30 min at 37°C. After ligation, the Duolink Amplification and Detection stock was diluted 1:5 by the addition of polymerase (1:80), and then applied to the sections for 100 min. After washing three times with buffer B for 2 min, the sections were mounted on the slides and incubated with DAPI for the identification of nuclei. Images were captured using a Zeiss Icore-510 upright confocal scanning laser microscope, using a 40× oil-immersion objective and 1.0× digital zoom. Confocal z-stacks of 2 μm thicknesses were taken with a z-step of 0.1 μm at 512 × 512 pixels, and a frame average of four. All z-stacks were captured from the depth of 10–12 μm below the surface to ensure that sampling was done from the same level of antibody penetration for all animals. We quantified the number of Duolink puncta per image using ImageJ software (V1.47H) as described previously (Trifilieff et al., 2011; Calabrese et al., 2014).
Brain homogenates and subcellular fractionations.
The rats were killed under anesthesia at 3, 6, 12, 24, and 72 h after ischemia. The hippocampal CA1 region was quickly dissected and the cytosolic or nuclear extraction was performed as described by previously (Wang et al., 2013). Briefly, the tissues were homogenized in ice-cold homogenization medium consisting of (in mm) the following: 50 HEPES, pH 7.4, 150 NaCl, 1 β-glycerophosphate, 3 dithiotheitol (DTT), 2 sodium orthovanadate (Na3VO4), 1 EGTA, 1 NaF, 1 phenylmethylsulfonyl fluoride (PMSF), 1% Triton X-100, and inhibitors of proteases and enzymes (Thermo, LI150825) with a Teflon-glass homogenizer. The homogenates were centrifuged at 15,000 × g for 30 min at 4°C, and then supernatants were collected and stored at −80°C for use. When necessary, cytosol fractions and nuclear fractions were extracted. Briefly, tissues were homogenized in ice-cold buffer A containing (in mm) the following: 10 HEPES, pH 7.9, 1 DTT, 1 Na3VO4, 1,4-nitrophenyl phosphate (PNPP), and inhibitors of proteases and enzymes. The homogenates were allowed to swell on ice for 10 min. Then, tubes were vigorously vortexed for 30 s and centrifuged at 800 × g for 10 min after the addition of NP-40 (0.6% of total solution). Supernatants were centrifuged at 15,000 × g for 30 min at 4°C. The nuclear pellets were washed three times with buffer A and resuspended in buffer B [(in mm) 20 HEPES, pH 7.9, 400 NaCl, 20% glycerine, 1 DTT, 1 Na3VO4, 1 PNPP] with inhibitors of proteases and enzymes, and then the tubes were vigorously rocked at 4°C for 30 min. After centrifugation at 12,000 × g for 15 min, the nuclear extracts were aliquoted and frozen in liquid nitrogen and stored at −80°C until use. An enhanced BCA protein assay kit with bovine serum albumin (BSA) as the standard was used to determine the protein concentrations.
Western blotting analysis.
Protein from each sample was heated at 100°C for 5 min with loading buffer containing 0.125 m Tris-HCl, pH 6.8, 20% glycerol, 4% SDS, 10% mercaptoethanol, and 0.002% bromphenol blue, then separated by SDS-PAGE using 10% acrylamide gels (50 μg per lane). Then proteins were transferred into a PVDF membrane using a wet transfer system at 100 V for 40 min. PVDF membranes were blocked using 3% BSA, 0.2% Tween 20 in Tris-buffered saline for ≥30 min at room temperature and incubated overnight at 4°C with following primary antibodies: Nrf2 (1:200), HO-1 (1:200), NQO1 (1:200), GPx1 (1:500), SOD2 (1:200), NeuN (1:1000), and β-actin (1:500). Alkaline phosphatase-conjugated IgG was used as secondary antibody and BCIP/NBT was used as color substrate. NeuN and β-actin were used as loading controls for the nuclear and cytosol or total protein, respectively. The bands on the membranes were scanned and analyzed with the ImageJ analysis software (version 1.30v; Wayne Rasband, National Institutes of Health). To quantitate hippocampal protein abundance, band densities for the indicated phosphoprotein were normalized to the corresponding band densities for total protein signals, and the indicated total proteins were expressed relative to β-actin or NeuN signals. Normalized means were then expressed as fold changes of the corresponding value for control (sham operated) animals. A mean ± SD was calculated from the data from all of the animals for graphical presentation and statistical comparison.
Coimmunoprecipitation analysis.
For immunoprecipitation (IP), tissue homogenates (400 μg of protein) were diluted fourfold with HEPES buffer containing 50 mm HEPES, pH 7.4, 150 mm NaCl, 10% glycerol, 1% Triton X-100, and 1 mm each of EGTA, EDTA, PMSF, and Na3VO4. Samples were preabsorbed for 1 h with 20 μl of protein A/G, and then centrifuged to remove any protein adhered nonspecifically to the protein A/G. The supernatant was incubated with 2 μg of Keap1 (#4617) antibody or nonspecific IgG with gentle rotation for overnight at 4°C. After the addition of protein A/G-Sepharose, the mixture was incubated at 4°C for an additional 4 h. Samples were washed with IP buffer 2 min × 3. After centrifuge at 4°C, 12,000 × g × 2 min, the samples were diluted with 2× loading buffer and boiled for 5 min. SDS-PAGE for Nrf2 or IgG was carried out.
Nrf2 DNA-binding activity assay.
Nrf2 DNA-binding activity was detected using a Trans-AM Nrf2 assay kit following the protocol of the manufacturer (catalog #50296, Active Motif). Briefly, 40 μl of complete binding buffer was mixed with 10 μl of protein sample (containing 20 μg of protein) and incubated for 1 h at room temperature with mild agitation. For positive or blank control, 10 μl of the provided nuclear extract or complete binding buffer was used instead of the protein samples. After washing, 100 μl of diluted Nrf2 antibody (1:1000) was added to each well and incubated for 1 h, followed by incubation with 100 μl of diluted HRP-conjugated antibody (1:1000) for another 1 h. Developing solution (100 μl) was added to each well and incubated at room temperature until the blue color turned medium to dark blue. Stop solution was then added and absorbance was read on a spectrophotometer at 450 nm with a reference wavelength of 655 nm. Nrf2 DNA-binding activity was expressed as fold changes in absorbance compared with sham groups.
MWM test.
The spatial learning and memory of the rats were evaluated by the MWM test as described previously by our laboratory and others (Wang et al., 2013; Zhang et al., 2013). The apparatus consisted of a black circular pool (diameter, 180 cm; height 80 cm) filled with water at 22–24°C to a height of 50 cm. The pool was divided into four quadrants with a hidden platform (9 cm in diameter, 1.5–2.0 cm below the water line) placed in one of the quadrants. The rat was placed in the water facing the wall at one of four random start locations (first, second, third, and fourth quadrant, located at equal distances from each other on the pool rim). Each rat was allowed to find the submerged platform within 90 s, and rest on it for 20 s. If the rat failed to find the hidden platform within 90 s, it was guided to the platform and placed on it for 20 s. The procedure was repeated for all the four start locations. The latency time, representing the average of the four trails, to reach the platform and distance were recorded. Two sessions of four trials were conducted on the first testing day, and the interval was 4 h. The first session was considered as a training procedure. The second session was formal testing and was conducted daily for the next 2 d. Four hours after the last trial, a probe trial was performed within 90 s in which the platform was removed from the tank. The rat was placed in the water at the same random start location, and time spent in the quadrant that previously contained the platform was used to assess performance of learning and memory of the rats.
Statistical analysis.
Data were expressed as mean ± SD and analyzed using the one-way ANOVA program. Differences were considered significant at p < 0.05 as determined using the Student–Newman–Keuls methods for pair-wide multiple comparisons.
Results
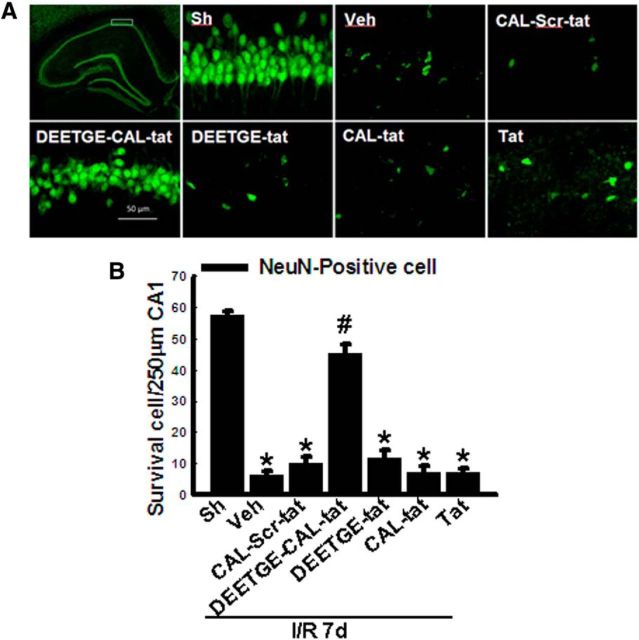
We first examined the ability of the DEETGE-CAL-Tat peptide (50 μg) to exert neuroprotection in the hippocampal CA1 region when administered intracebroventrically into the lateral ventricle of adult male rats 30 min before GCI. To determine specificity of the effect, we also examined the effect of several control peptides (CAL-Scr-Tat, DEETGE-Tat, CAL-Tat, Tat; 50 μg). All animals were killed 7 d after GCI and hippocampal sections collected for immunohistochemistry. Immunostaining with the neuronal marker NeuN was used to examine the number of surviving cells in the CA1 region 7 d after 15 min GCI. Representative photomicrographs of NeuN (green) immunostaining for all groups are shown in Figure 1A, while quantification of results from all animals in each group is presented in Figure 1B (NeuN, surviving cells). As shown in Figure 1A,B, 15 min GCI [ischemia/reperfusion (I/R)] caused a profound decrease in surviving cells in the CA1 region at 7 d, compared with the sham control. Administration of the DEETGE-CAL-Tat peptide 30 min before GCI was profoundly neuroprotective, while the control peptides (CAL-Scr-Tat, DEETGE-Tat, CAL-Tat, Tat) had no significant effect upon the number of surviving cells in the hippocampal CA1 region, indicating the specificity of the DEETGE-CAL-Tat peptide effect.
Figure 1.
Examination of the neuroprotective ability of the DEETGE-CAL-Tat peptide and various control peptides following GCI. Adult male rats were injected intracebroventrically with either DEETGE-CAL-Tat peptide or control peptides (CAL-Scr-Tat, DEETGE-Tat, CAL-Tat, Tat) at 30 min before GCI and the neuroprotective effect was examined in the hippocampal CA1 region. A, Representative hippocampal CA1 sections from sham-operated (Sh), DEETGE-CAL-Tat-treated (50 μg), vehicle-treated (Veh; 0.9% NaCl), or control-treated (CAL-Scr-Tat, DEETGE-Tat, CAL-Tat, Tat; 50 μg) animals were subjected to immunofluorescent staining for NeuN (green, indicating surviving cells) at 7 d after GCI reperfusion. B, Quantification was performed by counting the number of NeuN-positive neurons per 250 μm length in the medial CA1 pyramidal cell layer (n = 5–6). *p < 0.05 compared with Sh group, #p < 0.05 compared with Veh group. Scale bar, 50 μm. Magnification, 40×.
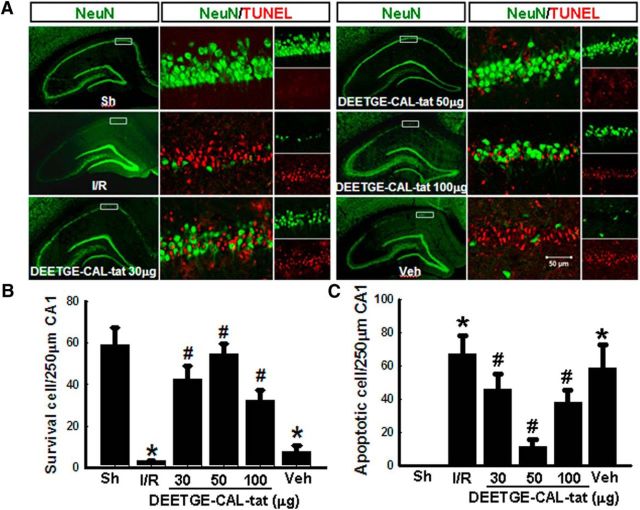
We next performed a dose–response analysis for the DEETGE-CAL-Tat peptide in the GCI model using three doses (30, 50, and 100 μg, i.c.v.). Staining with the neuronal marker NeuN was used to examine the number of surviving cells in the CA1 region at 7 d after 15 min GCI. TUNEL staining was also used to determine the number of apoptotic cells in the CA1 region at 7 d after GCI. Representative photomicrographs of NeuN (green) and TUNEL (red) staining for all groups are shown in Figure 2A, while quantification of results from all animals in each group is presented in Figure 2B (NeuN, surviving cells) and Figure 2C (TUNEL, apoptotic cells). As shown in Figure 2A–C, 15 min GCI (I/R) caused a profound decrease in surviving cells and increase in apoptotic cells in the CA1 region at 7 d, compared with the sham control. Administration of the DEETGE-CAL-Tat peptide 30 min before GCI was profoundly neuroprotective and antiapoptotic at all doses tested, compared with the vehicle control group. Of the three doses, the 50 μg dose of the DEETGE-CAL-Tat peptide was most effective, followed by the 30 μg dose. The 100 μg dose, while effective, had the lowest mean number of surviving neurons compared with the other doses. Its ability to reduce the number of apoptotic cells was also moderate compared with the 50 μg dose. Based on these findings, we chose to use the 50 μg dose of the DEETGE-CAL-Tat peptide for all of the remaining experiments in the study (except for the final experiment where post-treatment and peripheral administration of the DEETGE-CAL-Tat peptide was examined).
Figure 2.
Dose–response characteristics for DEETGE-CAL-Tat peptide neuroprotection of hippocampal CA1 neurons following GCI. Adult male rats were injected intracebroventrically with various doses of the DEETGE-CAL-Tat peptide at 30 min before GCI and the neuroprotective effect examined in the hippocampal CA1 region at 7 d after GCI. A, Representative hippocampal CA1 sections from sham (Sh), I/R, DEETGE-CAL-Tat (30, 50, 100 μg), or vehicle (Veh; 0.9% NaCl) were subjected to double staining for NeuN (green, indicating surviving cells) and TUNEL (red, indicating apoptotic cells). B, C, Quantification was performed by counting the number of NeuN-positive neurons (B) and TUNEL-positive neurons (C) per 250 μm length in the medial CA1 pyramidal cell layer (n = 5–6). *p < 0.05 compared with Sh group, #p < 0.05 compared with Veh groups. Scale bar, 50 μm. Magnification, 40×.
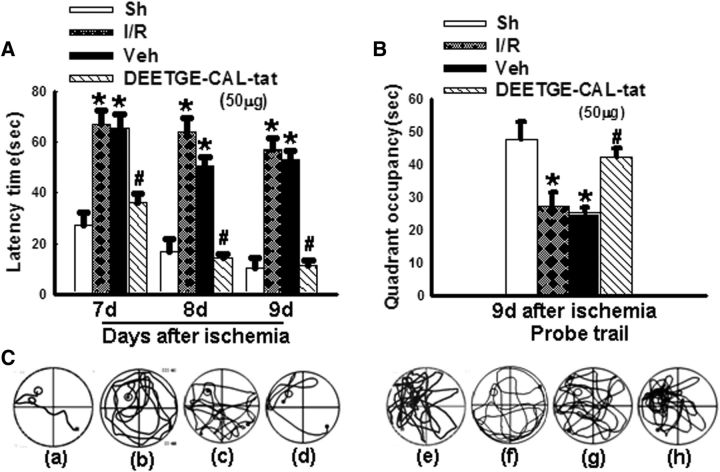
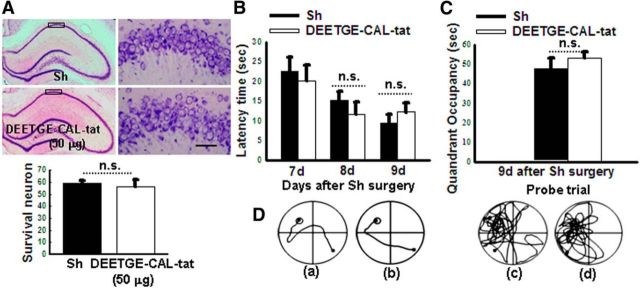
We next examined the effect of the DEETGE-CAL-Tat peptide on preserving cognitive function following GCI. This was accomplished by intracerebroventricular administration of the DEETGE-CAL-Tat peptide 30 min before GCI and then examining spatial learning and memory in the rats at days 7, 8, and 9 after GCI reperfusion using the MWM test. As shown in Figure 3A–C, compared with sham control, animals that underwent 15 min GCI displayed a significant increase in latency to find the platform (Fig. 3A) in the MWM, and a significant decrease in time spent in the platform quadrant (Fig. 3B). As shown in Figure 3A–C, DEETGE-CAL-Tat peptide-treated animals displayed significant cognitive improvements as evidenced by DEETGE-CAL-Tat peptide-treated animals exhibiting a significantly decreased latency to find the platform (Fig. 3A), and a significantly increased time spent in the platform quadrant (Fig. 3B), compared with the I/R and vehicle controls. Representative tracings indicating sample paths of the rats from the maze latency trials and swimming traces from probe trials are shown in Figure 3C. Figure 4A–D shows the effect of the DEETGE-CAL-Tat peptide on neuronal survival and cognitive function in sham, nonischemic rats examined using CV staining or the MWM test, respectively. As shown in Figure 4A–D, the DEETGE-CAL-Tat peptide had no significant effect in sham nonischemic animals upon neuronal survival (Fig. 4A), latency to find the platform (Fig. 4B), time spent in the platform quadrant (Fig. 4C), or swim tracings (Fig. 4D), compared with sham controls.
Figure 3.
DEETGE-CAL-Tat peptide treatment preserves hippocampal-dependent cognitive function following GCI. A, B, Latency trial (A) and probe trial (B) results of sham (Sh), I/R, vehicle (Veh, 0.9% saline), and DEETGE-CAL-Tat (50 μg) pretreatment animals in the MWM. A, Time [in seconds (sec)] spent finding the submerged platform at day 7, 8, and 9 after ischemic injury. B, Exploration time spent in the quadrant that initially contained the platform at day 9 following reperfusion. C, Representative traces indicating the sample paths of the rats from the maze latency trials (a–d) and the swimming traces from probe trials (e–h). a, e: Sh; b, f: I/R; c, g: Veh; d, h: DEETGE-CAL-Tat). Data are expressed as mean ± SD from five different animals. *p < 0.05 vs Sh group; #p < 0.05 vs Veh group.
Figure 4.
DEETGE-CAL-Tat peptide treatment does not affect neuronal survival or cognitive function in nonischemic animals. A, Representative hippocampal CA1 sections from sham and DEETGE-CAL-Tat (50 μg) treatment (nonischemic) groups were subjected to CV staining. B, C, Latency trial (B) and probe trial (C) results in the MWM for the two groups. B, Time [in seconds (sec)] spent finding the submerged platform at 7, 8, and 9 d after sham surgery. C, Exploration time spent in the quadrant that initially contained the platform at 9 d following reperfusion. D, Representative traces indicating the sample paths of the rats from the maze latency trials (a, b), and the swimming traces from probe trials (c, d). a, c: sham; b, d: DEETGE-CAL-Tat. Data are expressed as mean ± SD from five different animals. n.s., No significant change between the two groups. Magnification: 4× in left line and 20× in right line. Scale bar, 50 μm.
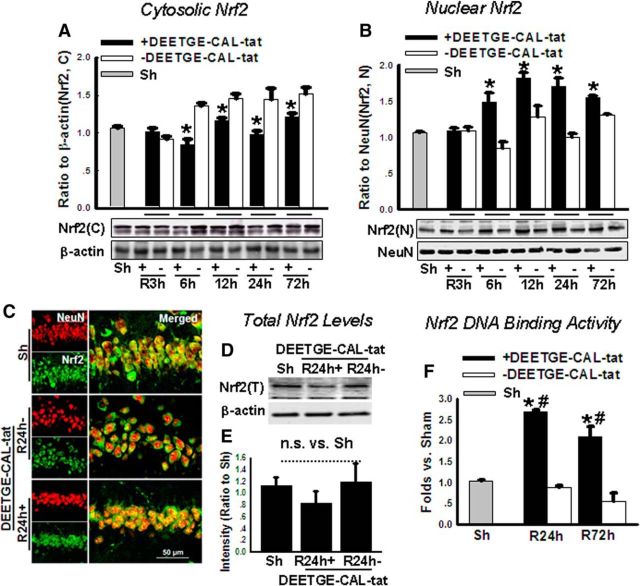
We next examined the efficacy of the DEETGE-CAL-Tat peptide to induce activation of Nrf2 in the hippocampal CA1 region after GCI by examining three endpoints: (1) nuclear translocation of Nrf2 (Fig. 5A–C), (2) total Nrf2 protein levels (Fig. 5D,E), (3) DNA binding of Nrf2 (Fig. 5F), and (4) induction of Nrf2-regulated proteins (Fig. 6). As shown in Figure 5A,B, hippocampal CA1 nuclear and cytoplasmic extracts were subjected to Western blot analysis for Nrf2 at various time points after GCI and in sham animals. The results revealed that Nrf2 protein levels decreased in the cytosolic fractions at 6, 12, 24, and 72 h after GCI reperfusion (Fig. 5A,B). In contrast, Nrf2 levels in the CA1 region increased in the nuclear fraction at 6, 12, 24, and 72 h after GCI reperfusion, but not at 3 h, compared with vehicle controls (Fig. 5A,B). The increase in Nrf2 levels in the nuclear fractions correlates well with the temporal pattern of Nrf2 decrease in the cytosolic fractions. The results suggest that the DEETGE-CAL-Tat peptide strongly increases Nrf2 translocation to the nucleus in ischemic animals from 6 to 72 h after GCI reperfusion. We also used double immunohistochemistry for Nrf2 and NeuN, a nuclear neuronal marker, to determine whether DEETGE-CAL-Tat peptide treatment induced Nrf2 expression and nuclear translocation in neurons at 24 h after GCI reperfusion. As shown in Figure 5C, Nrf2 expression in the hippocampal CA1 region of sham and vehicle animals was primarily cytoplasmic in neuronal fibers and did not colocalize in the nucleus with NeuN. In contrast, DEETGE-CAL-Tat peptide-treated animals showed high Nrf2 translocation to the nucleus of neurons at 24 h after GCI reperfusion, as evidenced by Nrf2 staining being highly colocalized with NeuN. While DEETGE-CAL-Tat peptide treatment enhanced Nrf2 translocation to the nucleus after GCI, it did not significantly affect total Nrf2 levels in the hippocampal CA1 region at 24 h after GCI (Fig. 5D,E). Translocation of Nrf2 to the nucleus is typically associated with it assuming its well known transcriptional role. To explore this possibility, we examined Nrf2 binding to DNA in hippocampal CA1 samples using an Nrf2/ARE-binding assay. As shown in Figure 5F, DEETGE-CAL-Tat peptide-treated animals had a ∼2.0–2.7-fold increase in DNA-binding activity of Nrf2 at 24 and 72 h after GCI reperfusion, compared with vehicle and sham controls. This agrees well with the nuclear translocation of Nrf2 observed in DEETGE-CAL-Tat peptide-treated animals at these same times (Fig. 5A–C).
Figure 5.
DEETGE-CAL-Tat peptide treatment enhances Nrf2 nuclear translocation and DNA binding in the hippocampal CA1 region following GCI. The cytoplasm or nuclear extracts were subjected to Western blot analysis for Nfr2 at indicated time points after reperfusion with or without DEETGE-CAL-Tat pretreatment. A, B, Data analyses of Nrf2 protein from the cytoplasmic extracts (A) or nuclear extracts (B) were expressed as ratios to β-actin or NeuN and the analysis was represented by means ± SD from four independent animals. C, Confocal analysis for NeuN (green), Nrf2 (red), and merged images in the hippocampal CA1 region following sham (Sh) treatment, 24 h after reperfusion with DEETGE-CAL-Tat treatment, or 24 h after reperfusion without DEETGE-CAL-Tat treatment. D, Western blot analysis of DEETGE-CAL-Tat effect upon total Nrf2 protein levels in the hippocampal CA1 region after GCI. E, Quantification of Western blot data shown in D. F, Effect of DEETGE-CAL-Tat upon DNA-binding activity of Nrf2, as determined using a TransAM Nrf2 assay kit. *p < 0.05 compared with the same time point for groups without DEETGE-CAL-Tat treatment, #p < 0.05 compared with Sh group. Magnification, 40×. Scale bar, 50 μm.
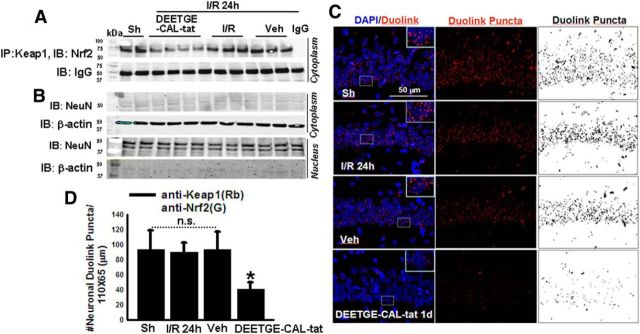
Figure 6.
DEETGE-CAL-Tat peptide decreases interaction of Keap1 with Nrf2 in the cytoplasm at 24 h after GCI reperfusion. A, IP for Keap1 and immunoblotting for Nrf2 reveals that DEETGE-CAL-Tat peptide treatment markedly reduces the interaction of Keap1 with Nrf2 in the cytoplasm of hippocampal CA1 region samples. B, Immunoblotting of the samples for either β-actin, a cytoplasmic marker, or for NeuN, a nuclear marker, showing the purity of the cytoplasmic and nuclear fractions. C, Duo-Link II in situ proximity ligation assay using Keap1 and Nrf2 antibodies reveals that Duolink puncta in the hippocampal CA1 regions at 24 h after GCI is markedly decreased by DEETGE-CAL-Tat peptide treatment, compared with vehicle and I/R controls. For clarity, both color and black-and-white images of the Duolink puncta are provided. D, Quantification of the Duolink puncta in C confirms that DEETGE-CAL-Tat peptide treatment significantly reduced interaction of Keap1 and Nrf2 in the hippocampal CA1 region at 24 h after GCI, compared with vehicle and I/R controls. *p < 0.05 compared with all other groups. N = 5–6/group. Scale bar, 50 μm, Magnification, 40×.
We next performed coimmunoprecipitation (Co-IP) to examine the efficacy of the DEETGE-CAL-Tat peptide to decrease Keap1–Nrf2 protein interaction in the hippocampal CA1 region. Cytosolic fractions collected from the hippocampal CA1 region at 24 h after GCI were used for the Co-IP studies. Sham and vehicle controls were also included. As shown in Figure 6A, DEETGE-CAL-Tat treatment markedly decreased Keap1 interaction with Nrf2 in the cytoplasm of hippocampal CA1 region at 24 h after GCI, compared with I/R, vehicle, and sham controls. Figure 6B demonstrates that the subfractionation method we used to obtain the cytosolic and nuclear fractions produced highly purified cytosolic and nuclear fractions, as determined by use of a specific cytosolic marker, β-actin, and nuclear marker NeuN. To confirm the in vitro Co-IP results, we performed an in vivo Duo-Link II in situ proximity ligation assay using primary antibodies for Keap1 and Nrf2. The Duolink procedure is based on the use of two probes that consist of a secondary antibody attached to a unique synthetic oligonucleotide, which acts as a reporter. When the two target antigens come into sufficient proximity to interact with one another, it generates a fluorescent signal. Amplification is provided by DNA ligation and polymerization, which allows detection of single events. The number of Duolink puncta per image field was quantified as described previously (Trifilieff et al., 2011; Calabrese et al., 2014) and as described in the Materials and Methods. Figure 6C shows representative photomicrographs for Duolink puncta in the hippocampal CA1 region, while Figure 6D shows quantification of the number of Duolink puncta. Light staining with DAPI was used to show cell nuclei. As shown in Figure 6C,D, the results of the Duo-Link II in situ proximity ligation assay revealed that while the number of Duolink puncta between sham and vehicle and I/R controls did not differ significantly in the hippocampal CA1 regions at 24 h after GCI. However, DEETGE-CAL-Tat peptide treatment significantly decreased the number of Duolink puncta, as compared with the vehicle and I/R controls.
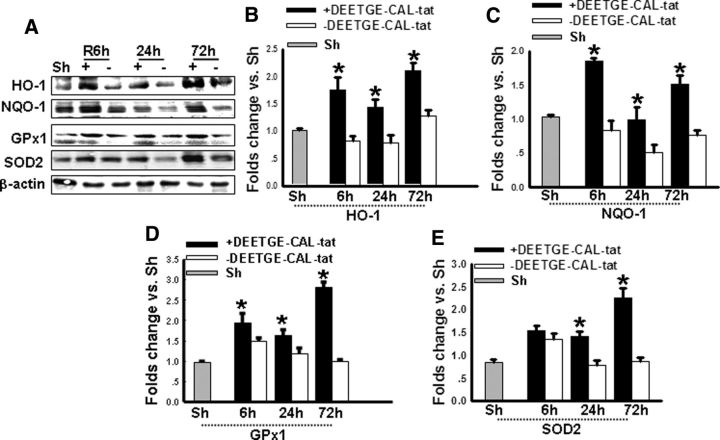
To confirm the functional importance of the increased Nrf2 DNA binding, we examined protein expression of four known cytoprotective and antioxidative Nrf2 transcriptional targets: NQO1, HO-1, GPx1, and SOD2. As shown in Figure 7A–E, Western blot analysis of hippocampal CA1 protein samples revealed that DEETGE-CAL-Tat peptide-treated animals had a significant increase of HO-1 (Fig. 7A,B), NQO1 (Fig. 7A,C), and GPx1 (Fig. 7A,D) protein levels at 6, 24, and 72 h after GCI reperfusion, compared with vehicle controls. SOD2 protein levels were also significantly increased in DEETGE-CAL-Tat peptide-treated animals at 24 and 72 h, but not at 6 h after GCI reperfusion (Fig. 7A,E).
Figure 7.
DEETGE-CAL-Tat peptide treatment enhances expression of Nrf2-regulated antioxidant genes in the hippocampal CA1 region following GCI. A, Total protein from sham (Sh), I/R, and DEETGE-CAL-Tat treatment rats were subjected to Western blotting for the Nrf2-regulated genes: HO-1, NQO1, GPx1, SOD2. β-Actin expression was used for the loading control. B–E, Quantification of blots depicted in A. Data are expressed as fold changes versus Sh (means ± SD, n = 4 in each group). *p < 0.05 compared with the same time point of group without DEETGE-CAL-Tat treatment (I/R).
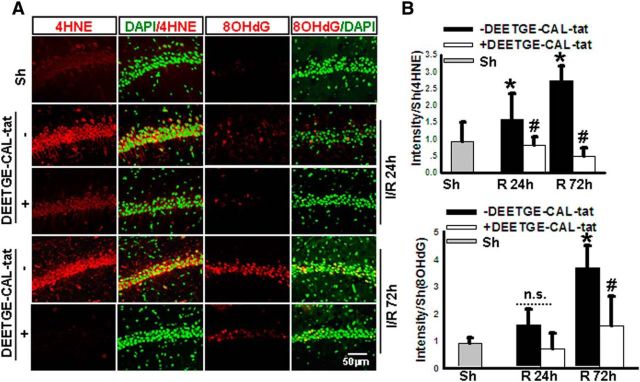
Since Nrf2 activation is well known to exert an antioxidant effect in cells through induction of the above genes, as well as other target genes, we examined whether DEETGE-CAL-Tat peptide treatment could attenuate GCI-induced oxidative damage in the hippocampal CA1 region. Toward this end, we used immunofluorescent staining of hippocampal CA1 sections to determine immunoreactive levels of the oxidative stress markers 4HNE and 8OHdG (red). DAPI staining (green) was also used to visualize the nucleus of cells. As shown in Figure 8A,B, as expected, sham (nonischemic) animals had very low to undetectable immunostaining for the oxidative stress markers 4HNE and 8OHdG in the hippocampal CA1 region at 24 and 72 h after sham surgery, respectively. In contrast, ischemic animals (vehicle group) showed an increase in 4HNE immunostaining in the hippocampal CA1 region at 24 and 72 h after GCI reperfusion, which was markedly attenuated by DEETGE-CAL-Tat peptide treatment. A pattern for increase of 8OHdG levels was also evident at both time points after GCI, but only the 72 h time point increase was statistically significant compared with the sham control. DEETGE-CAL-Tat peptide treatment markedly inhibited the increase of 8OHdG levels at 72 h.
Figure 8.
DEETGE-CAL-Tat peptide treatment attenuates oxidative stress damage in the hippocampal CA1 region following GCI. A, Coronal hippocampal CA1 sections obtained from sham-treated (Sh), I/R-treated (Veh), or DEETGE-CAL-Tat (50 μg)-treated animals at reperfusion 24 or 72 h after GCI were subjected to immunostaining for the oxidative stress markers 4HNE or 8OHdG (red). DAPI was converted to green for better viewing. B, Fluorescence intensity for 4HNE or 8OHdG staining, expressed as arbitrary units ratio to Sh, was measured in hippocampal CA1 region, and data shown are representative as mean ± SD from five independent animals (n = 5 in each group). *p < 0.05 versus Sh groups, #p < 0.05 versus I/R (Veh) without DEETGE-CAL-Tat treatment (−DEETGE-CAL-Tat). Magnification, 40×. Scale bar, 50 μm.
Finally, while the above studies show that pretreatment is strongly neuroprotective and able to preserve cognitive function, it would be more therapeutically relevant if DEETGE-CAL-Tat peptide treatment could be administered postischemia, and peripherally rather than centrally, to protect the brain and maintain cognitive function after GCI. We therefore sought to determine the neuroprotective and cognitive-preserving effect of peripheral, postischemia administration of DEETGE-CAL-Tat peptide following GCI. DEETGE-CAL-Tat peptide (120 and 240 μg/d) or vehicle was administered subcutaneously via miniosmotic pumps implanted under the skin in the midback region. DEETGE-CAL-Tat peptide was administered beginning 1 d after GCI reperfusion, and continued until the end of the experiment (day 9 after reperfusion). As shown in Figure 9B, GCI reperfusion led to a dramatic loss of NeuN-positive cells in the CA1 region and a robust increase of apoptotic cells at 9 d after reperfusion, as evidenced by TUNEL staining, compared with the sham control. Furthermore, DEETGE-CAL-Tat peptide administration peripherally from 1 to 9 d after GCI was strongly neuroprotective at the 240 μg/d dose, but not at the 120 μg/d dose, as evidenced by the increase in the number of NeuN-positive cells, while simultaneously decreasing the number of TUNEL-positive cells in the CA1 region at the 240 μg/d dose at 9 d after GCI.
Figure 9.
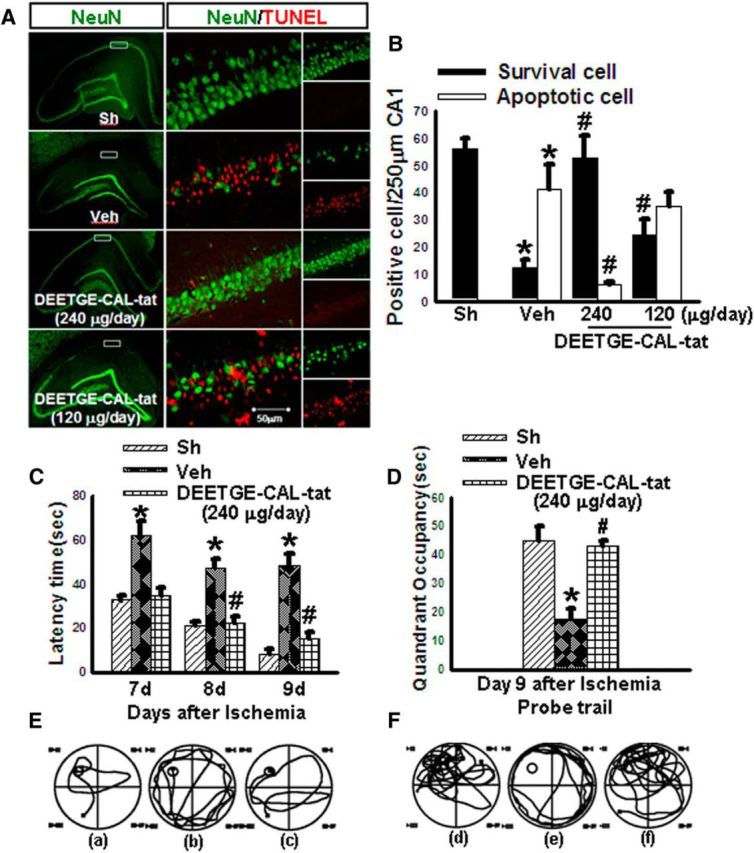
Post-treatment with the DEETGE-CAL-Tat peptide enhances neuronal survival and hippocampal-dependent cognitive function following GCI. DEETGE-CAL-Tat (120 or 240 μg/d) or vehicle (Veh; 0.9% NaCl) was administrated subcutaneously using a minipump (Alzet 2002, 0.5 μl/h for 14 d) beginning at 1 d after reperfusion. The MWM was performed during days 7–9, and double staining for NeuN (green) and TUNEL (red) at 9 d after ischemia. A, Representative hippocampal CA1 area stained with NeuN (green) and TUNEL (red). B, Quantification was performed by counting the number of NeuN-positive cells and TUNEL-positive cells per 250 μm length in the medial CA1 pyramidal cell layer (n = 5. Scale bar, 50 μm). *p < 0.05 compared with sham (Sh) group, #p < 0.05 compared with vehicle (Veh) groups. C, MWM results. Time [in seconds (sec)] spent finding the submerged platform at day 7, 8, and 9 after ischemic injury. D, Exploration time spent in the quadrant that initially contained the platform at day 9 following reperfusion. E–F, Representative traces indicating the sample paths of the rats from the maze latency trials (a–c) and the swimming traces from probe trials (d–f). a, d: Sh; b, e: Veh; c, f: 240 μg/d DEETGE-CAL-Tat treatment. Data are expressed as mean ± SD from five different animals. *p < 0.05 versus Sh group, and #p < 0.05 versus Veh group.
We next examined the ability of postischemia peripheral administration of the 240 μg/d dose of DEETGE-CAL-Tat peptide to preserve spatial learning and memory in rats at days 7, 8, and 9 after GCI reperfusion. In these studies, the MWM was used to access spatial learning and memory. As shown in Figure 9C,D, GCI resulted in a significant increase in latency to find the platform (Fig. 9C) in the MWM, and a significant decrease in time spent in the platform quadrant (Fig. 9D), compared with sham controls. Intriguingly, peripheral, postischemia treatment with the 240 μg/d dose of DEETGE-CAL-Tat peptide strongly preserved cognitive function after GCI, as evidenced by DEETGE-CAL-Tat peptide-treated animals exhibiting a significantly decreased latency to find the platform (Fig. 9C), and a significantly increased time spent in the platform quadrant (Fig. 9D), compared with the vehicle controls. Representative tracings indicating sample paths of the rats from the maze latency trials and swimming traces from probe trials are shown in Figure 9E,F.
Discussion
The current study provides evidence that a novel cell-permeable Nrf2 activation peptide, DEETGE-CAL-Tat, has efficacy as a potential therapy for hippocampal neuroprotection and cognitive preservation following GCI. Both centrally administered pre-treatment, as well as peripherally administered post-treatment, exerted robust neuroprotection of the vulnerable hippocampal CA1 region and preserved cognitive function in adult rats following GCI. While previous work had shown that the DEETGE-CAL-Tat peptide could reduce blood–brain barrier compromise after traumatic brain injury, our study is the first to demonstrate a neuroprotective and cognitive-enhancing effect of the DEETGE-CAL-Tat peptide in the ischemic brain. Our findings have potentially important translational relevance because current therapies for GCI have been reported to have poor efficacy, and thus there is a strong need for new therapeutic approaches (Nielsen et al., 2011; Kim et al., 2012).
The neuroprotective effect of the DEETGE-CAL-Tat peptide in our study appeared specific, as the control peptides (DEETGE-Tat, CAL-Scrambled-Tat, CAL-Tat, and Tat) showed no significant neuroprotective effect. The lack of a significant neuroprotection by the control DEETGE-Tat peptide, which might have been expected to have some activity, is most likely due to inefficient release of the transduction domain (Tat), and a suboptimal DEETGE accumulation in the cytoplasm. A similar conclusion was reached by Zhao et al. (2011) when they tested the DEETGE-Tat peptide for ability to activate Nrf2 target genes in the brain following traumatic brain injury and found no effect, while in contrast, the DEETGE-CAL-Tat peptide strongly induced Nrf2 target genes in their study. We believe the reason the DEETGE-CAL-Tat peptide was effective while the DEETGE-Tat peptide was not is that the calpain cleavage site between the Tat and DEETGE motifs in the DEETGE-CAL-Tat peptide allows for the removal of the protein transduction domain (Tat) and facilitates cytoplasmic accumulation of the DEETGE-containing peptide. This suggestion is also supported by the fact that the DEETGE-CAL-Tat peptide was inactive in the noninjured brain where calpain activity would be low and no or little cleavage of the transduction domain would occur. From a therapeutic standpoint, the injury-specific activation of the DEETGE-CAL-Tat peptide is a unique and attractive feature, as it restricts Nrf2 nuclear activation to brain areas that are injured, thereby providing for less chance of unwanted side effects.
An additional important point to consider is that a higher dose of DEETGE-CAL-Tat peptide was needed for peripheral, postischemia treatment (240 μg/d) compared with centrally administered pretreatment (50 μg). The higher dose needed for postischemia treatment is most likely due to the peripheral route of administration in which higher concentrations are needed to reach an optimal level in the brain. Other studies have used a similar dose range for peripheral administration of Tat-fusion peptides after ischemia, and demonstrated effective uptake into brain neurons (Cao et al., 2002; Yin et al., 2006; Krautwald et al., 2010). While there is controversy on whether Tat-fused peptides are significantly taken up by brain cells in the basal noninjured state, ischemic injury has been shown to significantly enhance Tat-fusion peptide entry into the brain (Simon et al., 2010). The increased uptake of Tat-fused peptides after ischemic injury is reportedly due to disruption of the blood–brain barrier, which undergoes a prolonged disruption after GCI (Mossakowski et al., 1994; Preston and Webster, 2004).
While our study showed the effectiveness of the DEETGE-CAL-Tat peptide administered continuously beginning 1 d after GCI, more detailed work will be needed to carefully define the exact therapeutic window for post-treatment effectiveness. For instance, it remains to be determined whether DEETGE-CAL-Tat peptide administration can be delayed even further beyond the 1 d post-GCI used in our study and still be effective. It might also be advantageous to test other administration routes for post-treatment, such as the intranasal administration route, which has recently emerged as a potentially effective route for delivery of drugs to the brain. Future studies in our laboratory will address these issues.
With respect to the molecular mechanisms underlying the beneficial effects of the DEETGE-CAL-Tat peptide, our study supports induction of downstream Nrf2 antioxidant target genes as a potential key mediator of its beneficial neuroprotective and cognitive-enhancing effects. In support of this contention, our study revealed that the DEETGE-CAL-Tat peptide markedly decreased interaction of Keap1 with Nrf2 in the cytoplasm, and correspondingly enhanced nuclear translocation and DNA binding of Nrf2 in the hippocampal CA1 region. Further work revealed that the nuclear translocation of Nrf2 occurred primarily in neurons in the hippocampal CA1 region, suggesting that beneficial effects of DEETGE-CAL-Tat peptide are exerted directly on neurons. Importantly, our study further showed that Nrf2 activation following DEETGE-CAL-Tat peptide treatment was correlated with strong induction of several known Nrf2 target antioxidant genes, such as NQO1, HO-1, GPx1, and SOD2.
In accordance with its induction of Nrf2 activation, the DEETGE-CAL-Tat peptide exerted a robust attenuation of oxidative DNA damage and lipid peroxidative damage in hippocampal CA1 neurons after GCI, as measured by immunofluorescence staining of the oxidative stress markers 8OHdG and 4-HNE. The antioxidant effects of the DEETGE-CAL-Tat peptide are likely due to the upregulation of the Nrf2-regulated antioxidant genes NQO1, GPx1, and SOD2 by the DEETGE-CAL-Tat peptide. Of these Nrf2-regulated antioxidant factors, HO-1 has been implicated to be particularly important in neuroprotection against cerebral ischemia, as evidenced by the fact that pharmacological induction of HO-1 protects hippocampal CA1 neurons from GCI-induced delayed neuronal cell death (Zhang et al., 2012), and by the finding that HO-1 knock-out mice exhibit greater ischemic damage compared with wild-type mice (Shah et al., 2011). An important role of the other Nrf2-regulated factors is also supported, as overexpression of GPx1 has been shown to markedly inhibit cell death and to reduce astrocyte activation after cerebral ischemia (Ishibashi et al., 2002). Correspondingly, GPx1 knock-out mice have been reported to exhibit increased infarct size and exacerbated apoptosis after cerebral ischemia (Crack et al., 2001). Finally, overexpression of SOD2 has also been shown to reduce neuronal vulnerability to forebrain ischemia (Xu et al., 2010), further supporting the importance of Nrf2 antioxidant defensive mechanisms against ROS-induced injury. The fact that the DEETGE-CAL-Tat peptide can upregulate all these antioxidant factors, as well as potentially a whole battery of other antioxidant, neuroprotective, and metabolism-related genes that are known to be target genes for Nrf2, suggests that a collective effect to regulate a broad array of Nrf2 genes is likely to underlie its robust neuroprotection and cognitive-preserving effects in GCI.
In conclusion, the current study demonstrates that the DEETGE-CAL-Tat peptide can enhance Nrf2 activation and antioxidant gene induction in the hippocampus after GCI, resulting in robust neuroprotection and preservation of cognitive function. A unique feature of the peptide, which is not present in other Nrf2 activators or other Tat-fused peptides, is that its beneficial effects are injury specific. This feature is attractive as it targets drug activation specifically in the site of injury, and likely would lead to a reduction of undesirable side effects if translatable to the clinic. Due to its injury-specific activation, robust neuroprotection, and cognitive-preserving effects, the DEETGE-CAL-tat peptide may represent a much-needed therapeutic advance that could have efficacy in the treatment of GCI.
Footnotes
This work was supported by the Natural Science Foundation of China (30970664; 31171354), Natural Science Foundation of Hebei Province (C2014209277), and the U.S. National Institutes of Neurological Disorders and Stroke, National Institutes of Health (NS050730).
The authors declare no competing financial interests.
References
- Beerens AM, Al Hadithy AF, Rots MG, Haisma HJ. Protein transduction domains and their utility in gene therapy. Curr Gene Ther. 2003;3:486–494. doi: 10.2174/1566523034578258. [DOI] [PubMed] [Google Scholar]
- Brillman J. Central nervous system complications in coronary artery bypass graft surgery. Neurol Clin. 1993;11:475–495. [PubMed] [Google Scholar]
- Calabrese B, Saffin JM, Halpain S. Activity-dependent dendritic spine shrinkage and growth involve downregulation of cofilin via distinct mechanisms. PloS One. 2014;9:e94787. doi: 10.1371/journal.pone.0094787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Pei W, Ge H, Liang Q, Luo Y, Sharp FR, Lu A, Ran R, Graham SH, Chen J. In vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J Neurosci. 2002;22:5423–5431. doi: 10.1523/JNEUROSCI.22-13-05423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Chen YT, Huang YW, Tsai HJ, Kuo CC. 4-Ketopinoresinol, a novel naturally occurring ARE activator, induces the Nrf2/HO-1 axis and protects against oxidative stress-induced cell injury via activation of PI3K/AKT signaling. Free Radic Biol Med. 2012;52:1054–1066. doi: 10.1016/j.freeradbiomed.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhu RL, Nakayama M, Kawaguchi K, Jin K, Stetler RA, Simon RP, Graham SH. Expression of the apoptosis-effector gene, Bax, is up-regulated in vulnerable hippocampal CA1 neurons following global ischemia. J Neurochem. 1996;67:64–71. doi: 10.1046/j.1471-4159.1996.67010064.x. [DOI] [PubMed] [Google Scholar]
- Crack PJ, Taylor JM, Flentjar NJ, de Haan J, Hertzog P, Iannello RC, Kola I. Increased infarct size and exacerbated apoptosis in the glutathione peroxidase-1 (Gpx-1) knockout mouse brain in response to ischemia/reperfusion injury. J Neurochem. 2001;78:1389–1399. doi: 10.1046/j.1471-4159.2001.00535.x. [DOI] [PubMed] [Google Scholar]
- Guerrero-Beltrán CE, Calderón-Oliver M, Pedraza-Chaverri J, Chirino YI. Protective effect of sulforaphane against oxidative stress: recent advances. Exp Toxicol Pathol. 2012;64:503–508. doi: 10.1016/j.etp.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Harukuni I, Bhardwaj A. Mechanisms of brain injury after global cerebral ischemia. Neurol Clin. 2006;24:1–21. doi: 10.1016/j.ncl.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Ishibashi N, Prokopenko O, Weisbrot-Lefkowitz M, Reuhl KR, Mirochnitchenko O. Glutathione peroxidase inhibits cell death and glial activation following experimental stroke. Brain Res Mol Brain Res. 2002;109:34–44. doi: 10.1016/S0169-328X(02)00459-X. [DOI] [PubMed] [Google Scholar]
- Kam KY, Yu SJ, Jeong N, Hong JH, Jalin AM, Lee S, Choi YW, Lee CK, Kang SG. p-Hydroxybenzyl alcohol prevents brain injury and behavioral impairment by activating Nrf2, PDI, and neurotrophic factor genes in a rat model of brain ischemia. Mol Cells. 2011;31:209–215. doi: 10.1007/s10059-011-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Yim HW, Jeong SH, Klem ML, Callaway CW. Does therapeutic hypothermia benefit adult cardiac arrest patients presenting with nonshockable initial rhythms?: A systematic review and meta-analysis of randomized and nonrandomized studies. Resuscitation. 2012;83:188–196. doi: 10.1016/j.resuscitation.2011.07.031. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Kirino T, Sano K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984;62:201–208. doi: 10.1007/BF00691853. [DOI] [PubMed] [Google Scholar]
- Krautwald S, Ziegler E, Rölver L, Linkermann A, Keyser KA, Steen P, Wollert KC, Korf-Klingebiel M, Kunzendorf U. Effective blockage of both the extrinsic and intrinsic pathways of apoptosis in mice by TAT-crmA. J Biol Chem. 2010;285:19997–20005. doi: 10.1074/jbc.M110.122127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J Biol Chem. 2004;279:31556–31567. doi: 10.1074/jbc.M403061200. [DOI] [PubMed] [Google Scholar]
- Mossakowski MJ, Lossinsky AS, Pluta R, Wisniewski HM. Abnormalities of the blood–brain barrier in global cerebral ischemia in rats due to experimental cardiac arrest. Acta Neurochir Suppl (Wien) 1994;60:274–276. doi: 10.1007/978-3-7091-9334-1_73. [DOI] [PubMed] [Google Scholar]
- Moulaert VR, Verbunt JA, van Heugten CM, Wade DT. Cognitive impairments in survivors of out-of-hospital cardiac arrest: a systematic review. Resuscitation. 2009;80:297–305. doi: 10.1016/j.resuscitation.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Nielsen N, Friberg H, Gluud C, Herlitz J, Wetterslev J. Hypothermia after cardiac arrest should be further evaluated—a systematic review of randomised trials with meta-analysis and trial sequential analysis. Int J Cardiol. 2011;151:333–341. doi: 10.1016/j.ijcard.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Preston E, Webster J. A two-hour window for hypothermic modulation of early events that impact delayed opening of the rat blood–brain barrier after ischemia. Acta Neuropathol. 2004;108:406–412. doi: 10.1007/s00401-004-0905-4. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roine RO, Kajaste S, Kaste M. Neuropsychological sequelae of cardiac arrest. JAMA. 1993;269:237–242. doi: 10.1001/jama.1993.03500020071034. [DOI] [PubMed] [Google Scholar]
- Sauvé MJ, Doolittle N, Walker JA, Paul SM, Scheinman MM. Factors associated with cognitive recovery after cardiopulmonary resuscitation. Am J Crit Care. 1996;5:127–139. [PubMed] [Google Scholar]
- Shah ZA, Nada SE, Doré S. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience. 2011;180:248–255. doi: 10.1016/j.neuroscience.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MJ, Kang WH, Gao S, Banta S, Morrison B., 3rd Increased delivery of TAT across an endothelial monolayer following ischemic injury. Neurosci Lett. 2010;486:1–4. doi: 10.1016/j.neulet.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Gilboe DD. Ischemia-induced changes in cerebral mitochondrial free fatty acids, phospholipids, and respiration in the rat. J Neurochem. 1994;62:1921–1928. doi: 10.1046/j.1471-4159.1994.62051921.x. [DOI] [PubMed] [Google Scholar]
- Swain JA, Anderson RV, Siegman MG. Low-flow cardiopulmonary bypass and cerebral protection: a summary of investigations. Ann Thorac Surg. 1993;56:1490–1492. doi: 10.1016/0003-4975(93)90737-3. [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Rives ML, Urizar E, Piskorowski RA, Vishwasrao HD, Castrillon J, Schmauss C, Slättman M, Gullberg M, Javitch JA. Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. BioTechniques. 2011;51:111–118. doi: 10.2144/000113719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve NF, Lau A, Zhang DD. Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin proteasome system: an insight into cullin-ring ubiquitin ligases. Antioxid Redox Signal. 2010;13:1699–1712. doi: 10.1089/ars.2010.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Tu J, Zhang Q, Zhang X, Zhu Y, Ma W, Cheng C, Brann DW, Yang F. Genistein attenuates ischemic oxidative damage and behavioral deficits via eNOS/Nrf2/HO-1 signaling. Hippocampus. 2013;23:634–647. doi: 10.1002/hipo.22126. [DOI] [PubMed] [Google Scholar]
- Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc Natl Acad Sci U S A. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolman RL, Nussmeier NA, Aggarwal A, Kanchuger MS, Roach GW, Newman MF, Mangano CM, Marschall KE, Ley C, Boisvert DM, Ozanne GM, Herskowitz A, Graham SH, Mangano DT. Cerebral injury after cardiac surgery: identification of a group at extraordinary risk. Multicenter Study of Perioperative Ischemia Research Group (McSPI) and the Ischemia Research Education Foundation (IREF) Investigators. Stroke. 1999;30:514–522. doi: 10.1161/01.STR.30.3.514. [DOI] [PubMed] [Google Scholar]
- Xu L, Emery JF, Ouyang YB, Voloboueva LA, Giffard RG. Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia. 2010;58:1042–1049. doi: 10.1002/glia.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Cao G, Johnnides MJ, Signore AP, Luo Y, Hickey RW, Chen J. TAT-mediated delivery of Bcl-xL protein is neuroprotective against neonatal hypoxic-ischemic brain injury via inhibition of caspases and AIF. Neurobiol Dis. 2006;21:358–371. doi: 10.1016/j.nbd.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang S, Zhang M, Weng Z, Li P, Gan Y, Zhang L, Cao G, Gao Y, Leak RK, Sporn MB, Chen J. Pharmacological induction of heme oxygenase-1 by a triterpenoid protects neurons against ischemic injury. Stroke. 2012;43:1390–1397. doi: 10.1161/STROKEAHA.111.647420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QG, Han D, Wang RM, Dong Y, Yang F, Vadlamudi RK, Brann DW. C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-alpha and the critical period hypothesis of estrogen neuroprotection. Proc Natl Acad Sci U S A. 2011;108:E617–E624. doi: 10.1073/pnas.1104391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QG, Wang RM, Scott E, Han D, Dong Y, Tu JY, Yang F, Reddy Sareddy G, Vadlamudi RK, Brann DW. Hypersensitivity of the hippocampal CA3 region to stress-induced neurodegeneration and amyloidogenesis in a rat model of surgical menopause. Brain. 2013;136:1432–1445. doi: 10.1093/brain/awt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Redell JB, Moore AN, Dash PK. A novel strategy to activate cytoprotective genes in the injured brain. Biochem Biophys Res Commun. 2011;407:501–506. doi: 10.1016/j.bbrc.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]