Abstract
The mechanism of action of ribavirin (RBV) as an immunomodulatory and antiviral agent and its clinical significance in the future treatment of patients with hepatitis C virus (HCV) infection are reviewed. RBV up-regulates type 1 and/or 2 cytokines to modulate the T helper (Th) 1/2 cell balance to Th1 dominance. Examination of co-stimulatory signaling indicated that RBV down-modulates inducible co-stimulator on Th cells, which contributes to differentiating naïve Th cells into Th2 cells while reducing their interleukin-10 production. The effects on T-regulatory (Treg) cells were also investigated, and RBV inhibited the differentiation of naïve Th cells into adaptive Treg cells by down-modulating forkhead box-P3. These findings indicate that RBV mainly down-regulates the activity of Th2 cells, resulting in the maintenance of Th1 activity that contributes to abrogating HCV-infected hepatocytes. Although an interferon-free treatment regimen exhibits almost the same efficacy without serious complications, regimens with RBV will be still be used because of their ability to facilitate the cellular immune response, which may contribute to reducing the development of hepatocellular carcinogenesis in patients infected with HCV.
Keywords: Ribavirin, Forkhead box-P3, T helper 1/2 cell balance, T-regulatory lymphocytes, Inducible co-stimulator, Interleukin-10, Hepatitis C virus infection
Core tip: Ribavirin has the potential to regulate the T-helper (Th) 1/2 cell balance into Th1 dominance by modulating the co-stimulatory signaling between antigen-presenting cells and naïve Th cells as well as the inhibitory activity of T-regulatory cells. These are considered useful in treating hepatitis C virus infection, especially to inhibit hepatocellular carcinoma development.
INTRODUCTION
About 185 million people worldwide are estimated to be infected with hepatitis C virus (HCV)[1]. Eighty percent of HCV-infected patients will progress to persistent infection[2,3], and 15%-30% of these will develop cirrhosis over a 25- to 30-year period[4]. In addition, hepatocellular carcinoma (HCC) occurs in 8% of cirrhotic patients annually[5-7]. Because persistent HCV infection is closely related to the development of HCC[8,9], the elimination of HCV contributes markedly to preventing the development of this form of cancer[10,11].
The treatment strategy for HCV infection has improved during the 25 years since the introduction of interferon (IFN) therapy. In particular, the rate of persistent elimination of the HCV genotype 1, which was considered to be IFN resistant, improved markedly from 8% to 45% with the introduction of pegylated (PEG)-IFN treatment in combination with ribavirin (RBV)[12]. In addition, the administration of direct antiviral agents (DAAs) with PEG-IFN plus RBV therapy greatly improved treatment efficacy[13-15]. Currently, about 90% of persistent HCV infection can be eliminated by administering the IFN/RBV/DAA regimen.
In 2014, an IFN-free regimen featuring a combination of the HCV-NS5A inhibitor daclatasvir (DCV) and NS3/4A protease inhibitor asnaprevir (ASV) was approved. This IFN-free regimen appears to have efficacy similar to that of the previous standard regimen without serious side effects[16,17]. Thus, IFN-free regimens will play a leading role in future HCV treatment. However, the potential of RBV to modulate the immune response is considered useful in treating HCV infection, especially to inhibit HCC development[18-22]. This paper reviews the immunological activity of RBV and considers the clinical significance of this antiviral agent in future HCV treatment.
IMPORTANCE OF THE HOST CELLULAR IMMUNE RESPONSE IN ELIMINATING HCV
Abrogation of infected cells is necessary to eliminate persistent viral infection, and up-regulation of the host cellular immune responses triggered by the activation of T helper (Th) 1 cells is thought to be essential for eliminating persistent HCV infection[23-26]. Among the various mechanisms by which IFN eradicates viruses, modulation of the host immune system may be critical, along with its antiviral activity[27]. IFN could enhance host immune responses via the activation of natural killer cells, CD4+ Th cells, and CD8+ cytotoxic T cells and the up-regulation of major histocompatibility complex molecule expression to stabilize the presentation of antigeneic epitopes from the infected cells[28]. Unfortunately, although it has these abilities for modulating immune systems, IFN monotherapy shows only limited efficacy against HCV infection. Numerous investigations have attempted to elucidate why IFN alone fails to eliminate HCV, and it appears that HCV can escape[29] or inhibit[30] the host immune response to establish persistent infection. Hence, additional techniques were needed to enhance the host cellular immune response.
EFFECTS OF RBV ON THE TH1/2 CELL BALANCE
The synthesized purine nucleotide analogue RBV, developed as antiviral reagent[31,32], is well known to contribute to HCV elimination in combination with IFN[33]. The mechanism of action of RBV is not fully understood, and it has been reported to: (1) induce viral RNA-error catastrophe[34]; (2) inhibit RNA polymerase[35]; (3) reduce RNA pooling via nicotinamide adenine dinucleotide phosphate inhibition[36,37]; and (4) alter the Th1/2 balance to Th1 dominance[38,39]. Among the putative mechanisms of the enhancement of viral elimination by RBV, it is notable that RBV polarizes the Th cell balance into Th1 cell dominance because this supports the importance of the activation of the host cellular immune response in eliminating HCV. However, it remains unclear how RBV modulates the Th1/2 balance. Many groups examined the effects of RBV on type 1 and 2 cytokine production from T lymphocytes. Some reported that RBV directly up-regulates Th1 cells through the activation of type 1 cytokines, such as interleukin (IL)-12[40-42]. In contrast, others indicated that RBV may maintain Th1 cell activity through interference with immunosuppressive cytokines such as IL-4 or IL-10[43-46]. From the viewpoint of type 1 and 2 cytokine profiles, it remains controversial whether RBV up-regulates Th1 cells directly or indirectly through the inhibition of Th2 cell activity.
POTENTIAL OF RBV TO MODULATE CO-STIMULATORY SIGNALING
The importance of co-stimulatory signaling to determine the differentiation of naïve Th cells into Th1 or 2 cells is well established[47]. The signaling from CD80 on professional antigen presenting cells (APCs) to its counterreceptor CD28 on CD4+ T cells promotes differentiation of naïve Th into Th1 cells[48]. On the other hand, the signaling from B7-H2 on APCs to its counterreceptor inducible co-stimulator (ICOS) on CD4+ T cells promotes differentiation into Th2[49]. It would be interesting to determine whether RBV exerts specific effects on co-stimulatory signaling, although only a few reports have addressed this aspect. Cheng et al[50] reported that CD28 was up-regulated by IFN plus RBV therapy in both treatment responders and nonresponders. Atsukawa et al[51] demonstrated that RBV down-modulates ICOS on human CD4+ T cells, which is associated with decreased IL-10 production. They also examined the modulation of type 1/2 cytokine fluctuations in the small cohort of patients who received IFN plus RBV treatment and their results indicated that IL-10 production from CD4+ T-cells was decreased in patients whose ICOS were down-modulated by the therapy, which was closely associated with persistent HCV elimination without changing CD28 expression and IFN-γ secretion levels. These results indicated that RBV mainly contributes to inhibiting the differentiation of naïve Th cells into Th2 cells to maintain the activity of Th1 cells by inhibiting stimulation-inducible molecules on the surface of CD4+ T cells. However, these results do not fully explain the role of RBV because other important factors play a role in Th1/2 cell modulation.
POTENTIAL OF RBV FOR MODULATING T-REGULATORY CELL ACTIVITY
It is well known that the activation of host T-regulatory (Treg) cells is critical to allow persistent HCV infection[52]. Treg cells, found at first as antigen-specific inhibitors of autoreactive T lymphocytes[53,54], can identify as CD4+CD25+, and intracellular forkhead box-P3 (FOXP3)-expressing T cells. Subsequent detailed examinations revealed that the Treg family includes various subpopulations such as naturally occurring Treg (Trnat), adaptive Treg (Tradapt), Treg, and Th3 cells[55-58]. Trnat cells differentiate in the thymus and exhibit inhibitory activity against autoreactive Th cells in a cell contact-dependent fashion, which plays an important role in regulating the autoimmune response[59]. Tradapt cells differentiate from naïve Th cells under the influence of Trnat cells in the periphery and exhibit inhibitory activity in a humoral element-dependent fashion[60]. The orchestration of these Treg cells could modulate antigen-specific Th1 activity in the later phase of exogenous pathogen infections to switch the dominant immune response from cellular to humoral[61]. These phenomena play a role in the termination of excessive activation of the Th1 response against exogenous antigens[62]. In addition, Tr1 and Th3 cells exhibit inhibitory activity against Th1 cells in a humoral element-dependent fashion[56-58].
According to previous reports, the effect of antiviral therapy on the activity of Treg cells remains uncertain[63,64]. Recently, Kobayashi et al[65] examined the effects of RBV on the inhibitory activity of Treg cells in vitro and found that it down-modulates the inhibitory activity of peripheral CD4+CD25+CD127- T cells (= FOXP3+ Treg cells). Intracellular FOXP3 expression of CD4+CD25- T cells decreased when they were incubated with RBV-preincubated Treg cells. In addition, RBV reduced the inhibitory effect of Treg cells in an IL-10-dependent, but not tumor growth factor (TGF)-β-dependent, manner[65]. These data indicate that RBV-treated Treg cells would lose their ability to differentiate naive Th cells into Tradapt cells. Moreover, the activity of IL-10-dependent Treg cells such as Tradapt and Tr1 was mostly inhibited in the presence of RBV. Although that in vitro study clearly indicated the effects of RBV against Treg cells, it remained controversial whether RBV could regulate Treg cells in clinical studies. Langhans et al[66] showed that the activity of Treg cells was down-modulated in the clinical course of HCV treatment with PEG-IFN and RBV. In contrast, Lee et al[67] found that RBV did not impair the inhibitory activity of Treg cells. More detailed studies are needed to confirm the effects of RBV on Treg-cell activity in vivo.
Based on these findings, RBV may indirectly maintain and/or up-regulate Th1 activity by inhibiting the differentiation of naive Th cells into Th2 cells. The potential to down-modulate the inhibitory activity of Treg cells would be closely associated with this regulatory cascade. The potential mechanism by which RBV modulates the Th1/2 balance-regulatory cascade is shown in Figure 1. Because both ICOS and FOXP3 are enhanced after cell stimulation, it is also possible that RBV affects the expression of these inducible factors. Further studies are needed to elucidate how RBV is associated with these intracellular signaling pathways.
Figure 1.
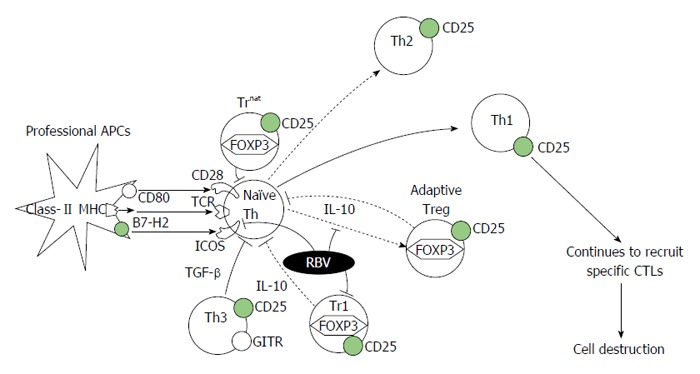
Schema of the potential mechanism of action of ribavirin on T-regulatory cells in the T helper 1/2-regulatory cascade. RBV interferes with FOXP3 expression in naïve Th cells, making them unable to differentiate into adaptive Treg cells. RBV also disrupts the inhibitory activities of adaptive Treg and Treg 1 cells by suppressing their IL-10 production. In addition, RBV down-modulates ICOS, expressed on naïve Th cells after stimulation, to inhibit the differentiation of naïve Th cells in to Th2 cells. The combination of these affects may contribute to maintain Th1 activity against exogenous antigens, which would contribute to the elimination of HCV-infected cells via the activation of specific CTLs. RBV: Ribavirin; ICOS: Inducible co-stimulator; IL: Interleukin; HCV: Hepatitis C virus; CTL: Cytotoxic T cell; FOXP3: Forkhead box-P3; Treg: T-regulatory; Th: T helper; TGF: Tumor growth factor; MHC: Major histocompatibility complex; TCR: T cell receptor; GITR: Glucocorticoid-induced tumour-necrosis-factor-receptor-related protein; APC: Antigen presenting cells.
RBV MAY MODULATE INTRACELLULAR SIGNALING TO INHIBIT EXPRESSION OF FOXP3
The results of various studies indicated that RBV affects intracellular signaling, contributing to the elimination of HCV. However, it remains unclear how RBV modulates intracellular signaling to inhibit the differentiation of Th cells into Tradapt cells. Some investigators reported that RBV promotes signal transducer and activator of transcription (STAT) 1 and 3 phosphorylation[68,69]. In addition, overexpression of STAT-3 in suppressor of cytokine signaling (SOCS)-1-knockout murine Treg cells led to the down-modulation of FOXP3 expression[70]. Although no report directly demonstrated the relationship between SOCS-1 and FOXP3, one possible hypothesis is that RBV promotes STAT3 phosphorylation via interference with SOCS1, which leads to the suppression of FOXP3, with resultant inhibition of the differentiation of naïve Th cells into Tradapt cells. RBV can also reduce intracellular RNA pooling by suppressing inosine-5’-monophosphate dehydrogenase activity[71], which appears to support this hypothesis.
CLINICAL USEFULNESS OF RBV IN THE FUTURE TREATMENT OF HCV
As described above, the agonistic effects of RBV on the cellular immune response plays a major role in the elimination of HCV-infected hepatocytes in combination with IFN and protease inhibitors. Because this effect will promote the autoimmune response, therapeutic regimens featuring RBV may be not recommended for patients with certain autoimmune disorders. On the other hand, because the role of the cellular immune response is important in cancer immunological surveillance, this ability of RBV may be indispensable to protect HCC development after eliminating HCV. However, IFN-free regimens will be the main strategy for the treatment of HCV infection in the near future because their efficacy in eliminating the virus is equivalent to that of IFN-based regimens without serious complications. Although it is still controversial whether IFN-free regimens can protect against HCC to the same degree as IFN and RBV-based regimens, serum alpha-fetoprotein levels were reduced in patients administered the DAA regimen featuring DCV and ASV, indicating that IFN-free regimens could also decrease the rate of HCC development. Therefore, the clinical significance of RBV-featuring regimens will be limited. However, it is still interesting whether combining RBV and DAA therapy can decrease the development of HCC more. Large-scale clinical studies will be needed to establish the efficacy of RBV for protecting HCC.
CONCLUSION
This review of the potential of RBV as a modulator of the Th1/2 balance cascade indicates that the agonistic effects of this antiviral agent on Th1 activity contributes greatly to the elimination of HCV in combination with IFN and protease inhibitors. In addition, it is possible that RBV may assist in the prevention of HCC development by accelerating cancer immunological surveillance.
ACKNOWLEDGMENTS
We are grateful to Dr. Tamaki Kobayashi, MD, PhD, Division of Gastroenterology and Hepatology, Department of Internal Medicine, and Dr. Eiji Shinya, MD, PhD, Microbiology and Immunology, Nippon Medical School, Tokyo, Japan, for contributing to the investigations reviewed here. We are also grateful to Dr. Hideto Tamura, MD, PhD, Division of Hematology, Department of Internal Medicine, and Professor Choitsu Sakamoto, MD, PhD, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Nippon Medical School, Tokyo, Japan, for their helpful suggestions.
Footnotes
P- Reviewer: Shier MK S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Conflict-of-interest statement: Authors declare no conflict of interest for this review article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 1, 2015
First decision: August 4, 2015
Article in press: October 13, 2015
References
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 2.Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ulsenheimer A, Schraut WW, Schirren CA, Waechtler M, Backmund M, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80–88. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
- 3.Page K, Hahn JA, Evans J, Shiboski S, Lum P, Delwart E, Tobler L, Andrews W, Avanesyan L, Cooper S, et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis. 2009;200:1216–1226. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yatsuhashi H, Yano M. Natural history of chronic hepatitis C. J Gastroenterol Hepatol. 2000;15 Suppl:E111–E116. doi: 10.1046/j.1440-1746.2000.02122.x. [DOI] [PubMed] [Google Scholar]
- 7.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 8.McGivern DR, Lemon SM. Tumor suppressors, chromosomal instability, and hepatitis C virus-associated liver cancer. Annu Rev Pathol. 2009;4:399–415. doi: 10.1146/annurev.pathol.4.110807.092202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamashita T, Honda M, Kaneko S. Molecular mechanisms of hepatocarcinogenesis in chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2011;26:960–964. doi: 10.1111/j.1440-1746.2011.06723.x. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174–181. doi: 10.7326/0003-4819-131-3-199908030-00003. [DOI] [PubMed] [Google Scholar]
- 11.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 12.Namiki I, Nishiguchi S, Hino K, Suzuki F, Kumada H, Itoh Y, Asahina Y, Tamori A, Hiramatsu N, Hayashi N, et al. Management of hepatitis C; Report of the Consensus Meeting at the 45th Annual Meeting of the Japan Society of Hepatology (2009) Hepatol Res. 2010;40:347–368. doi: 10.1111/j.1872-034X.2010.00642.x. [DOI] [PubMed] [Google Scholar]
- 13.Kumada H, Toyota J, Okanoue T, Chayama K, Tsubouchi H, Hayashi N. Telaprevir with peginterferon and ribavirin for treatment-naive patients chronically infected with HCV of genotype 1 in Japan. J Hepatol. 2012;56:78–84. doi: 10.1016/j.jhep.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi N, Izumi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Ki R, Komada Y, Seto C, et al. Simeprevir with peginterferon/ribavirin for treatment-naïve hepatitis C genotype 1 patients in Japan: CONCERTO-1, a phase III trial. J Hepatol. 2014;61:219–227. doi: 10.1016/j.jhep.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Izumi N, Hayashi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Ki R, Komada Y, Seto C, et al. Once-daily simeprevir with peginterferon and ribavirin for treatment-experienced HCV genotype 1-infected patients in Japan: the CONCERTO-2 and CONCERTO-3 studies. J Gastroenterol. 2014;49:941–953. doi: 10.1007/s00535-014-0949-8. [DOI] [PubMed] [Google Scholar]
- 16.Kumada H, Suzuki Y, Ikeda K, Toyota J, Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K, et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083–2091. doi: 10.1002/hep.27113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chayama K, Takahashi S, Toyota J, Karino Y, Ikeda K, Ishikawa H, Watanabe H, McPhee F, Hughes E, Kumada H. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology. 2012;55:742–748. doi: 10.1002/hep.24724. [DOI] [PubMed] [Google Scholar]
- 18.Papatheodoridis GV, Papadimitropoulos VC, Hadziyannis SJ. Effect of interferon therapy on the development of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis: a meta-analysis. Aliment Pharmacol Ther. 2001;15:689–698. doi: 10.1046/j.1365-2036.2001.00979.x. [DOI] [PubMed] [Google Scholar]
- 19.Braks RE, Ganne-Carrie N, Fontaine H, Paries J, Grando-Lemaire V, Beaugrand M, Pol S, Trinchet JC. Effect of sustained virological response on long-term clinical outcome in 113 patients with compensated hepatitis C-related cirrhosis treated by interferon alpha and ribavirin. World J Gastroenterol. 2007;13:5648–5653. doi: 10.3748/wjg.v13.i42.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasegawa E, Kobayashi M, Kawamura Y, Yatsuji H, Sezaki H, Hosaka T, Akuta N, Suzuki F, Suzuki Y, Arase Y, et al. Efficacy and anticarcinogenic activity of interferon for hepatitis C virus-related compensated cirrhosis in patients with genotype 1b low viral load or genotype 2. Hepatol Res. 2007;37:793–800. doi: 10.1111/j.1872-034X.2007.00140.x. [DOI] [PubMed] [Google Scholar]
- 21.Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, De Santo JL, Lee WM, Di Bisceglie AM, Bonkovsky HL, Dienstag JL, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52:833–844. doi: 10.1002/hep.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velosa J, Serejo F, Marinho R, Nunes J, Glória H. Eradication of hepatitis C virus reduces the risk of hepatocellular carcinoma in patients with compensated cirrhosis. Dig Dis Sci. 2011;56:1853–1861. doi: 10.1007/s10620-011-1621-2. [DOI] [PubMed] [Google Scholar]
- 23.Spengler U, Nattermann J. Immunopathogenesis in hepatitis C virus cirrhosis. Clin Sci (Lond) 2007;112:141–155. doi: 10.1042/CS20060171. [DOI] [PubMed] [Google Scholar]
- 24.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–952. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 25.Kanto T, Hayashi N, Takehara T, Tatsumi T, Kuzushita N, Ito A, Sasaki Y, Kasahara A, Hori M. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584–5591. [PubMed] [Google Scholar]
- 26.Dolganiuc A, Chang S, Kodys K, Mandrekar P, Bakis G, Cormier M, Szabo G. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol. 2006;177:6758–6768. doi: 10.4049/jimmunol.177.10.6758. [DOI] [PubMed] [Google Scholar]
- 27.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 28.Brassard DL, Grace MJ, Bordens RW. Interferon-alpha as an immunotherapeutic protein. J Leukoc Biol. 2002;71:565–581. [PubMed] [Google Scholar]
- 29.Korenaga M, Hino K, Katoh Y, Yamaguchi Y, Okuda M, Yoshioka K, Okita K. A possible role of hypervariable region 1 quasispecies in escape of hepatitis C virus particles from neutralization. J Viral Hepat. 2001;8:331–340. doi: 10.1046/j.1365-2893.2001.00305.x. [DOI] [PubMed] [Google Scholar]
- 30.Osna N, Silonova G, Vilgert N, Hagina E, Kuse V, Giedraitis V, Zvirbliene A, Mauricas M, Sochnev A. Chronic hepatitis C: T-helper1/T-helper2 imbalance could cause virus persistence in peripheral blood. Scand J Clin Lab Invest. 1997;57:703–710. doi: 10.3109/00365519709105232. [DOI] [PubMed] [Google Scholar]
- 31.Reyes GR. Ribavirin: recent insights into antiviral mechanisms of action. Curr Opin Drug Discov Devel. 2001;4:651–656. [PubMed] [Google Scholar]
- 32.Wu JZ, Lin CC, Hong Z. Ribavirin, viramidine and adenosine-deaminase-catalysed drug activation: implication for nucleoside prodrug design. J Antimicrob Chemother. 2003;52:543–546. doi: 10.1093/jac/dkg405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 34.Crotty S, Maag D, Arnold JJ, Zhong W, Lau JY, Hong Z, Andino R, Cameron CE. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat Med. 2000;6:1375–1379. doi: 10.1038/82191. [DOI] [PubMed] [Google Scholar]
- 35.Vo NV, Young KC, Lai MM. Mutagenic and inhibitory effects of ribavirin on hepatitis C virus RNA polymerase. Biochemistry. 2003;42:10462–10471. doi: 10.1021/bi0344681. [DOI] [PubMed] [Google Scholar]
- 36.Sintchak MD, Nimmesgern E. The structure of inosine 5’-monophosphate dehydrogenase and the design of novel inhibitors. Immunopharmacology. 2000;47:163–184. doi: 10.1016/s0162-3109(00)00193-4. [DOI] [PubMed] [Google Scholar]
- 37.Zhou S, Liu R, Baroudy BM, Malcolm BA, Reyes GR. The effect of ribavirin and IMPDH inhibitors on hepatitis C virus subgenomic replicon RNA. Virology. 2003;310:333–342. doi: 10.1016/s0042-6822(03)00152-1. [DOI] [PubMed] [Google Scholar]
- 38.Hultgren C, Milich DR, Weiland O, Sällberg M. The antiviral compound ribavirin modulates the T helper (Th) 1/Th2 subset balance in hepatitis B and C virus-specific immune responses. J Gen Virol. 1998;79(Pt 10):2381–2391. doi: 10.1099/0022-1317-79-10-2381. [DOI] [PubMed] [Google Scholar]
- 39.Tam RC, Pai B, Bard J, Lim C, Averett DR, Phan UT, Milovanovic T. Ribavirin polarizes human T cell responses towards a Type 1 cytokine profile. J Hepatol. 1999;30:376–382. doi: 10.1016/s0168-8278(99)80093-2. [DOI] [PubMed] [Google Scholar]
- 40.Ning Q, Brown D, Parodo J, Cattral M, Gorczynski R, Cole E, Fung L, Ding JW, Liu MF, Rotstein O, et al. Ribavirin inhibits viral-induced macrophage production of TNF, IL-1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J Immunol. 1998;160:3487–3493. [PubMed] [Google Scholar]
- 41.Shiina M, Kobayashi K, Satoh H, Niitsuma H, Ueno Y, Shimosegawa T. Ribavirin upregulates interleukin-12 receptor and induces T cell differentiation towards type 1 in chronic hepatitis C. J Gastroenterol Hepatol. 2004;19:558–564. doi: 10.1111/j.1440-1746.2003.03329.x. [DOI] [PubMed] [Google Scholar]
- 42.Fang SH, Hwang LH, Chen DS, Chiang BL. Ribavirin enhancement of hepatitis C virus core antigen-specific type 1 T helper cell response correlates with the increased IL-12 level. J Hepatol. 2000;33:791–798. doi: 10.1016/s0168-8278(00)80312-8. [DOI] [PubMed] [Google Scholar]
- 43.Souvignet C, Zarski JP. Combination treatment for chronic hepatitis C: what is the role of ribavirin? Fundam Clin Pharmacol. 2000;14:321–325. doi: 10.1111/j.1472-8206.2000.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 44.Tam RC, Lim C, Bard J, Pai B. Contact hypersensitivity responses following ribavirin treatment in vivo are influenced by type 1 cytokine polarization, regulation of IL-10 expression, and costimulatory signaling. J Immunol. 1999;163:3709–3717. [PubMed] [Google Scholar]
- 45.Cramp ME, Rossol S, Chokshi S, Carucci P, Williams R, Naoumov NV. Hepatitis C virus-specific T-cell reactivity during interferon and ribavirin treatment in chronic hepatitis C. Gastroenterology. 2000;118:346–355. doi: 10.1016/s0016-5085(00)70217-4. [DOI] [PubMed] [Google Scholar]
- 46.Torre F, Rossol S, Pelli N, Basso M, Delfino A, Picciotto A. Kinetics of soluble tumour necrosis factor (TNF)-alpha receptors and cytokines in the early phase of treatment for chronic hepatitis C: comparison between interferon (IFN)-alpha alone, IFN-alpha plus amantadine or plus ribavirin. Clin Exp Immunol. 2004;136:507–512. doi: 10.1111/j.1365-2249.2004.02476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 48.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 49.Vieira PL, Wassink L, Smith LM, Nam S, Kingsbury GA, Gutierrez-Ramos JC, Coyle AJ, Kapsenberg ML, Wierenga EA. ICOS-mediated signaling regulates cytokine production by human T cells and provides a unique signal to selectively control the clonal expansion of Th2 helper cells. Eur J Immunol. 2004;34:1282–1290. doi: 10.1002/eji.200324417. [DOI] [PubMed] [Google Scholar]
- 50.Cheng PN, Wei YL, Chang TT, Chen JS, Young KC. Therapy with interferon-alpha and ribavirin for chronic hepatitis C virus infection upregulates membrane HLA-ABC, CD86, and CD28 on peripheral blood mononuclear cells. J Med Virol. 2008;80:989–996. doi: 10.1002/jmv.21192. [DOI] [PubMed] [Google Scholar]
- 51.Atsukawa M, Nakatsuka K, Kobayashi T, Shimizu M, Tamura H, Harimoto H, Takahashi H, Sakamoto C. Ribavirin downmodulates inducible costimulator on CD4+ T cells and their interleukin-10 secretion to assist in hepatitis C virus clearance. J Gastroenterol Hepatol. 2012;27:823–831. doi: 10.1111/j.1440-1746.2011.06882.x. [DOI] [PubMed] [Google Scholar]
- 52.Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, Nelson DR. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062–1071. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- 53.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 54.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 55.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 56.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujio K, Okamura T, Yamamoto K. The Family of IL-10-secreting CD4+ T cells. Adv Immunol. 2010;105:99–130. doi: 10.1016/S0065-2776(10)05004-2. [DOI] [PubMed] [Google Scholar]
- 58.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 59.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 60.Mahic M, Yaqub S, Bryn T, Henjum K, Eide DM, Torgersen KM, Aandahl EM, Taskén K. Differentiation of naive CD4+ T cells into CD4+CD25+FOXP3+ regulatory T cells by continuous antigen stimulation. J Leukoc Biol. 2008;83:1111–1117. doi: 10.1189/jlb.0507329. [DOI] [PubMed] [Google Scholar]
- 61.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 63.Burton JR, Klarquist J, Im K, Smyk-Pearson S, Golden-Mason L, Castelblanco N, Terrault N, Rosen HR. Prospective analysis of effector and regulatory CD4+ T cells in chronic HCV patients undergoing combination antiviral therapy. J Hepatol. 2008;49:329–338. doi: 10.1016/j.jhep.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 64.Kanto T, Inoue M, Oze T, Miyazaki M, Sakakibara M, Kakita N, Matsubara T, Higashitani K, Hagiwara H, Iio S, et al. Dynamics of regulatory T cells and plasmacytoid dendritic cells as immune markers for virological response in pegylated interferon-α and ribavirin therapy for chronic hepatitis C patients. J Gastroenterol. 2012;47:169–178. doi: 10.1007/s00535-011-0466-y. [DOI] [PubMed] [Google Scholar]
- 65.Kobayashi T, Nakatsuka K, Shimizu M, Tamura H, Shinya E, Atsukawa M, Harimoto H, Takahashi H, Sakamoto C. Ribavirin modulates the conversion of human CD4(+) CD25(-) T cell to CD4(+) CD25(+) FOXP3(+) T cell via suppressing interleukin-10-producing regulatory T cell. Immunology. 2012;137:259–270. doi: 10.1111/imm.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Langhans B, Nischalke HD, Arndt S, Braunschweiger I, Nattermann J, Sauerbruch T, Spengler U. Ribavirin exerts differential effects on functions of Cd4+ Th1, Th2, and regulatory T cell clones in hepatitis C. PLoS One. 2012;7:e42094. doi: 10.1371/journal.pone.0042094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee J, Choi YS, Shin EC. Ribavirin Does Not Impair the Suppressive Activity of Foxp3(+)CD4(+)CD25(+) Regulatory T Cells. Immune Netw. 2013;13:25–29. doi: 10.4110/in.2013.13.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stevenson NJ, Murphy AG, Bourke NM, Keogh CA, Hegarty JE, O’Farrelly C. Ribavirin enhances IFN-α signalling and MxA expression: a novel immune modulation mechanism during treatment of HCV. PLoS One. 2011;6:e27866. doi: 10.1371/journal.pone.0027866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao LJ, Wang W, Liu Y, Ren H, Qi ZT. Interference with ERK and STAT signaling pathways and inhibition of hepatitis C virus replication by ribavirin. Antiviral Res. 2012;96:260–268. doi: 10.1016/j.antiviral.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi R, Nishimoto S, Muto G, Sekiya T, Tamiya T, Kimura A, Morita R, Asakawa M, Chinen T, Yoshimura A. SOCS1 is essential for regulatory T cell functions by preventing loss of Foxp3 expression as well as IFN-{gamma} and IL-17A production. J Exp Med. 2011;208:2055–2067. doi: 10.1084/jem.20110428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mori K, Ikeda M, Ariumi Y, Dansako H, Wakita T, Kato N. Mechanism of action of ribavirin in a novel hepatitis C virus replication cell system. Virus Res. 2011;157:61–70. doi: 10.1016/j.virusres.2011.02.005. [DOI] [PubMed] [Google Scholar]


