Abstract
Hepatocellular adenomas (HCA) are rare benign liver tumors. Recent technological advancements have helped in the early identification of such lesions. However, precise diagnosis of hepatocellular incidentalomas remains challenging. Studies at the molecular level have provided new insights into the genetics and pathophysiology of these lesions. These in turn have raised questions over their existing management modalities. However, the rarity of the tumor still restricts the quality of evidence available for current recommendations and guidelines. This article provides a comprehensive review on the etiology, molecular biology, patho-physiology, clinical manifestations, and complications associated with HCA. It also elaborates on the genetic advancements, existing diagnostic tools and current guidelines for management for such lesions.
Keywords: Liver adenoma, Focal liver lesion, Benign liver lesion
Core tip: Hepatocellular adenomas despite being benign liver neoplasms often pose diagnostic and therapeutic challenges. Studies at the molecular level have provided new insights into the genetics and pathophysiology of these lesions. These in turn have raised questions over their existing management modalities. This article provides a comprehensive review and update on the topic.
INTRODUCTION
Hepatocellular adenomas (HCA) are rare monoclonal benign liver tumors of presumable epithelial origin that usually develops in healthy liver. It constitutes 2% of all liver neoplasms; with an incidence of 3/1000000 per year in Europe and North America[1]. In spite of recent technological and radiological advances, these seemingly benign lesions often pose diagnostic challenges. The advancements at molecular levels that predict the clinical courses of these lesions on the basis of their genotypic and phenotypic characteristics have further complicated the management of such lesions.
ETIOLOGY AND PRESENTATION
The true prevalence of hepatocellular adenoma is not easy to access as uncomplicated adenomas often lack symptoms. Often they are encountered as incidental findings in patients undergoing radiological work up for unrelated or non-specific symptoms. Majority (70% to 80%) of HCA are solitary, and are usually located in the right liver lobe[2]. They vary in size from 1 to 30 cm. Patients with larger tumors are more likely to report with symptoms such as vague abdominal pain. A palpable intra-abdominal mass or enlarged liver may be noted in < 30% of the cases[2]. Jaundice if present is usually due to pressure effect of the tumor on intrahepatic biliary system. Elevation levels of gamma glutamyl transferase and alkaline phosphatase may be noted in some patients. High serum alpha-fetoprotein should raise suspicion for malignant changes. Hemorrhage and malignant transformation remain the most notorious complications associated with these lesions. Bleeding from a ruptured HCA is more anticipated with large sub capsular lesions, particularly during pregnancy.
With the widespread use of oral contraceptives (OCPs) in the 70’s the annual incidence of HCAs had reached approximately 3-4 women per 100000 users[3]. Over the years, a reduction in drug potency has reduced their frequency to about 1/100000. In comparison, the annual incidence in women who have never used OCPs is nearly 1/1000000[3]. The role of estrogen in the development of these lesions and its causal relationship has been well established through multiple studies. The natural history of HCAs closely corresponds with the dose and duration of oral contraceptive used. HCAs tend to regress with the withdrawal of estrogen therapy. However those that don’t regress can be explained by the variable expression of estrogen receptors (26%-73%) in these lesions. Recent reports have noted epidemiological differences in HCA patients between East and West. The female preponderance and degree of association with oral contraceptive use was reportedly absent in the Eastern population[4].
Similar to OCPs, use of anabolic steroids predisposes the emergence and growth of HCA, as well as, tends to regress with their withdrawal. Anabolic steroids are used primarily for the treatment of Fanconi syndrome, impotence, to gain muscle mass and in transsexuals. In general, adenomas secondary to exogenous hormone therapy are single, large and encapsulated. In addition to exogenous hormone therapy, high levels of endogenous androgens or estrogens puts both sexes at risk of developing HCA.
Type I and type III Glycogen storage diseases (GSD) result in impaired glycogenesis and excessive hepatic intracellular glycogen deposits. Both are inherited autosomal recessive disorders. HCAs are seen in nearly 22%-75% of patients with type I disease compared to 4.4%-25% of the patients with type III disease[5]. They usually occur in second decade, with a higher proportion among males (2:1). They are typically small, multiple and un-encapsulated. Type I GSD is usually associated with inflammatory subtype of HCA[6]. The possibility of malignant conversion is minimal in type III as compared to nearly 10% in type I GSD[5]. Disease regression has been noted in patients adhering to specific diet (continuous nocturnal feeding) with correction of insulin, glucose, and glucagon levels[7].
In the Western population, obesity and related metabolic syndrome manifestations such as diabetes mellitus, insulin resistance, dyslipidemia and high blood pressure are becoming increasingly postulated as risk factors for development and progression of HCA[8]. Men with metabolic syndrome are at a much higher risk (10 times more likely than females) for malignant transformation[9].
GENETICS AND PATHOPHYSIOLOGY
Advancements in molecular biology have enabled classification of hepatic adenomas into 4 major subgroups based on genotypic and phenotypic characteristics[10]: (1) Adenomas inactivated for hepatocyte nuclear factor 1α: They account for 35%-50% of HCA’s and are considered the least likely to undergo malignant transformation (15%)[10,12]. This subtype involves biallelic inactivation of TCF1 gene[11]. Mutations are predominantly somatic (85%) with remaining 15% being in part somatic and in part hereditary. It is associated with marked steatosis, metabolic disease and absence of liver fatty acid binding protein expression. These also lack cytologic abnormalities or inflammatory infiltrates[10,12]; (2) β-catenin activated adenomas (β-HCA): These mutations account for 15%-18% of HCA’s and is highly correlated with malignant transformation into hepatocellular carcinoma (HCC)[10,12]. CTNNB1 gene alterations, Exon 3 deletions and changes in amino acid chain mostly characterize these mutations. Their development has been linked to male sex, androgenic hormone administration or glycogenesis. These mutated liver cell adenomas overexpress β-catenin (nuclear and cytoplasmic) and glutamine synthetase. They have cytoplasmic changes and lower rates of steatosis; (3) Inflammatory adenomas: These represent the most common subtype (40%-55%). Hepatocellular proliferations noted in these lesions are secondary to sustained activation of janus kinase (involved in the JAK-STAT pathway). It is characterized by inflammatory changes such as sinusoidal dilatation, dystrophic arteries and dystrophic vessel ductular reaction and immunohistochemically positivity for serum amyloid A and C reactive protein[13]. It is more common in females on OCPs, but has also been associated with obesity and alcohol abuse. Telangiectatic hepatocellular adenoma which was once referred as telangiectatic focal nodular hyperplasia has now been reclassified under inflammatory HCA. Ten percent of these HCAs have been reported to harbour mutations in the β-catenin gene are hence also at danger of malignant changes; and (4) Unclassified Adenomas: The unclassified type (< 10%) includes patients whose tumors did not exhibit genetic or inflammatory diseases[13]. It usually is a diagnosis of exclusion.
Several other genetic markers are being studied to clarify their roles in the pathogenesis of hepatic adenomas. These include micro RNAs (miR-224, miR-122a, miR-107, miR-375), tumor suppressor proteins (p16INK4a, p14ARF), APC gene and interleukin-6-Gp130 signaling pathway[14].
DIAGNOSIS AND ROLE OF IMAGING
Accurate diagnosis of HCA is sometimes not feasible with a single diagnostic study. Often the clinical setting is combined with imaging studies and/or surgical resection to enable a comprehensive diagnosis. When lesions do not exhibit typical radiological features characteristic for an adenoma, a definitive diagnosis is impractical; it can be, at most, strongly suspected. The definitive diagnosis then demands tissue examination either by biopsy or resection. Herman et al[15] reported a radiological diagnostic yield of 90% for HCA vs 77% for FNH.
Percutaneous biopsy of a suspected liver adenoma is not favored as this can induce bleeding and tumor dissemination. Further, it is often inaccurate and absence of abnormal tissue does not exclude malignancy. Charny et al[16] in his study reported about 33% accuracy rate for biopsies taken for suspicious liver lesions. Biopsy should be considered only if imaging is inconclusive and a biopsy would possibly bring about a change in management. With advancement at the molecular level, the role of preoperative biopsy could influence management of adenomas in the future to a greater degree based on their genetic pathophysiological outcome[17]. In the near future, the molecular biology of the tumor might guide management of such lesions.
Open/laparoscopic excision biopsy remains the gold standard method for diagnosis[1]. However, this modality is only used if there are doubts regarding the diagnosis after magnetic resonance imaging (MRI) and/or percutaneous biopsy. A common dilemma is the differentiation of an adenoma from focal nodular hyperplasia (FNH) and well differentiated HCC. Considering varying management strategies for the several liver lesions, the implications of a prompt and accurate diagnosis cannot be emphasized more.
Accurate tissue diagnosis may at times be tricky and challenging. Incorporating more recent tissue studies such as QBend 10- and erbB2-immunostaining, comparative genomic in situ hybridization, and fluorescence in-situ hybridization may help overcome this issue[18-21]. Microscopically adenomas typically appear as monotonous sheets of hepatocytes, which lack biliary structures, dysplasia or fibrosis.
Ultrasound
The sonographic features of HCAs lack specificity and could mimic other benign or malignant liver lesions[22]. Adenomas may appear well-demarcated and present as iso echoic, hypo echoic (20%-40%), or hyper echoic in up to 30%, often due to high lipid content of hepatocytes[22,23]. Central necrosis, intratumoral bleeding and calcifications may give rise to heterogeneous echogenicity. Necrosis may appear as hyperechoic areas with acoustic shadows. Focal fat sparing usually appear as a hypo echoic halo. Color Doppler may show the presence of intra tumoral vessels and peri lesional sinusoids in the absence of a central arterial signal, which can aid in distinguishing HCA from FNH[23]. Contrast-enhanced ultrasonography, when available can augment ultrasonic diagnosis.
Computed tomography
Multi-phase computed tomography (CT) angiography enables dynamic sequencing by obtaining images during both contrast and non-contrast phases. HCA characteristically shows early phase peripheral contrast enhancement and subsequent centripetal contrast enhancement during the portal venous phase[23,24]. Adenomas are typically well-demarcated and are isodense on non-contrast images. Post contrast, the lesion may become isodense and then hypodense. They are often heterogeneous due to hemorrhage, necrosis and fibrosis. Previous hemorrhagic areas may appear as calcifications in less than 10% of cases[24].
Magnetic resonance imaging
Most adenomas are heterogeneous on both T1 and T2 weighted sequences. Presence of enhanced T2 weight images that further enhance on gadolinium administration is highly characteristic of HCA[25]. Studies of late have tried to characterize these lesions into its four genotypic types based on MRI features. However their specificity is debatable, owing to the highly variable appearance of HCA. In comparison to the existing gadolinium-based contrast agents for MRI, gadobenate dimeglumine or gadoxetate disodium enhanced liver MRI (hepatobiliary specific contrast agents) have both renal and hepatobiliary clearance. Owing to variable contrast uptake observed in hepatobiliary phase, HCA (being hypointense) can be differentiated from FNH (being iso- or hyperintense on delayed imaging)[25].
Isotope scanning (Technetium Tc-99m sulfur colloid)
HCAs usually have very few to no Kupffer cells, which are mostly nonfunctional. Hence most adenomas do not take up technetium Tc-99m sulfur colloid leaving a “cold” spot in the liver[26]. However upto 23% of adenomas take up colloid uptake, making them indistinguishable from FNH (vivid tracer uptake).
Angiography
This modality is sparingly used to diagnose hepatic adenomas. HCAs demonstrate pattern of perfusion starting at the periphery in contrast to the central vessel seen in FNH[23]. However necrosis and hemorrhage within the HCA may make these features less obvious.
COMPLICATIONS
Rupture/bleeding - How to deal?
Of all the complications associated with HCAs, bleeding is most common. Adenomas morphologically consist of dilated sinusoids, thin-walled blood vessels with minimal connective tissue support[1]. These compounded by the high pressure arterial flow and lack of substantial fibrous capsule make them prone to bleed. More recent studies have associated pregnancy, adenoma size > 3.5 cm, visualization of lesional arteries, left lateral lobe location, and exophytic growth as risk factors for spontaneous bleeding[27].
Bleeding can be subclassified as intra-tumoral, intra-hepatic or extra-hepatic (hemoperitoneum). The risk of spontaneous bleeding in HCA is ill-defined; however the reported rates range between 20% and 40%[1]. Bleeding in most cases are contained and < 10% report with life threatening hemodynamic instability. Intratumoral bleeding accounts for most patients who present with abdominal pain.
Over the past few decades, management of bleeding adenomas has changed drastically. Earlier, the threshold for emergency laparotomy and hepatic resection was very low, however with poor outcomes. Now, the trend is for a multidisciplinary approach to such events. Management of such patients is often dictated by the severity of the bleeding and hemodynamic status of the patient. Options include emergency resection, resection post hemodynamic stabilization or a delayed resection.
Emergency resection of ruptured hepatic adenomas is associated with a mortality of 5% to 10%, which drops to 1% when resection is elective[28]. With the development of minimally invasive locoregional treatments, selective arterial embolisation has increasingly been applied as initial treatment[29]. Apart from reducing mortality, this therapy facilitates hemodynamic stabilization and reduces extent of definitive elective resection.
Patients who present with hemodynamic instability secondary to ruptured HCA carry mortality risk of up to 20%. Severe hemodynamic instability or failure of interventional radiology may warrant emergency laparotomy for damage control and control of hemorrhage. Emergency hepatic resection in this setting is often debated with opponents of this advocating time for hematoma to resorb before undertaking formal resection[30].
Malignant transformation
The precise risk of HCA transforming into carcinoma is little known. Stoot et al[31] in a systematic review noted an overall risk of transformation to HCC to be 4.2%. Prolonged use of exogenous steroid therapy, male gender, increasing size, type I GSD, and adenomas harboring β-catenin mutations are high risk factors for malignant transformation[12,32]. Elevated α-fetoprotein levels may raise suspicion for malignant conversion; however serve as poor indicators of tumor progression.
There is considerable debate on the pathophysiology and genetics involved in HCC arising from hepatic adenomas. Histopathologic evidence of HCCs occurring within regions of otherwise typical adenomas and molecular level evidence of the same nucleotide mutation of β-catenin in the adenoma and HCC part the adenoma supports adenoma-carcinoma sequence hypothesis[12,33,34]. This is further reinforced by the idea of “foci of dysplasia” within hepatic adenoma. A focus of dysplasia once developed in a hepatocellular adenoma is debated to invariably progress into transformation to HCC[14]. This is reasoned through inability to prevent development of HCC in patients with hepatic adenoma secondary to prolonged exogenous hormone therapy inspite of discontinuation of offending agent[33].
Genomic studies have revealed chromosomal aberrations [loss of heterozygosity (LOH) for M6P/IGFRII receptor, LOH in hMLH1] in both HCA and HCC[35,36]. However these aberrations are to a lesser degree in HCA compared to HCC. Scientists are still in search of the initial critical genetic event involved in malignant transformation. There is also little evidence to prove a common origin of HCA and HCC from same stem cells or committed progenitor cells[14].
MANAGEMENT
Owing to inconclusiveness in imaging, possible lethal complications that included bleeding, malignant transformation and the long term disease free guarantee that surgery offers; surgeons of the past classically preferred to operate on all hepatic adenomas. Recent advancements in molecular biology has substantially increased our understanding on pathophysiology of the disease. With greater technological expertise and development of surgical techniques, current surgeons have a more comprehensive outlook towards the management of such tumors (Figure 1). However, the rarity of the tumor has still restricted the quality of evidence available for current recommendations and guidelines.
Figure 1.
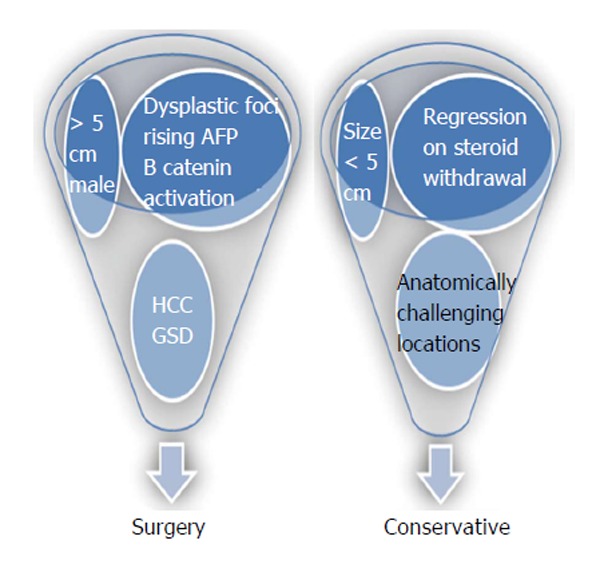
Indications of surgical and conservative approach in management of hepatocellular adenomas. HCC: Hepatocellular carcinoma; GSD: Glycogen storage diseases; AFP: Alpha fetoprotein.
Management options for HCA include surgery for: (1) all adenomas larger than five cm; (2) adenomas harboring HCC or dysplastic foci; (3) β-catenin activated HCA; (4) increasing size or imaging features of malignant transformation; (5) rising alpha fetoprotein (AFP); (6) HCA in males; and (7) HCA in GSD[14]. Minimally invasive options including laparoscopic liver resection has been proven to safe with equivalent or superior outcome when compared to open procedures[37]. However, this approach in anatomically unfavorable segments 7 and 8 need high technical expertise and control.
Adenomas that are < 5 cm, which present in anatomically challenging locations and those that undergo regression on steroid withdrawal can be managed conservatively[38]. All adenoma patients (surgical and nonsurgical candidates) who consume exogenous hormone therapy should be advised to discontinue their use. Malignant transformation has been reported infrequently in adenomas < 3.5 cm and in tumors that have regressed in size over time. Hence such lesions treated conservatively needs to be followed closely with imaging (CT/MRI) at 6- to 12-mo intervals for first two years. Following this, annual imaging may be modulated based on lesion stability and growth patterns[38]. The role of AFP in surveillance is still not established.
The role of selective arterial embolization in management of HCA is not very clear. However, multifocal lesions or those deemed un-resectable due to their location may be managed by such interventional radiology techniques[39]. Further, embolization may enable possibility of elective hepatic resection in cases of bleeding HCAs[34]. The role of preoperative embolization to facilitate safe hepatic resection in very large or hyper vascular adenomas is still controversial. Radiofrequency ablation might be useful in cases not amenable to surgery or in patients who would require major hepatic resection otherwise[40]. However, large sizes of adenomas may demand multiple sessions of the same.
Liver transplantation should be reserved for patients in whom hepatic resection is not technically feasible due to tumor size/location/recurrence/multiplicity and those with adenomatosis or glycogen storage disease. Owing to the complexities associated with liver transplantation, this mode of management should be used as sparingly as possible; when all other modalities of treatment may fail to provide cure. The use of liver transplantation for spontaneous intra-partum rupture of a hepatocellular adenoma has been reported in literature[41].
Pregnancy poses a varied challenge in the management of HCAs. An increased level of endogenous hormones during pregnancy has been postulated towards the increase in size of adenomas during pregnancy. Pregnancy induced hyper dynamic circulation combined with increased liver vascularity may further increase the risk of adenoma rupture, most seen in the third trimester[42]. In the past, owing to high maternal and fetal mortality rates (44% and 38% respectively), fertile females with HCA were discouraged from pregnancy[42]. However, of late due to increased awareness of the disease entity and better imaging modalities, pregnancy associated morbidities and mortalities have been greatly reduced.
Pregnancy is no longer a contraindication in hepatocellular adenoma < 5 cm[43]. All fertile females with adenomas, aspiring pregnancy should discontinue use of exogenous hormone therapy if any. Some still advocate resection for these lesions before pregnancy. However, the present trend is to allow conservative management of these smaller lesions with ultrasound monitoring of the adenoma every 6 wk. However those with adenomas > 5 cm or those who experienced adenoma related complications in previous pregnancies should undergo resection prior to pregnancy. Radiofrequency ablation or embolization might be useful in cases not amenable to surgery[39,40].
Adenomas > 5 cm detected incidentally during pregnancy often calls for an individualized approach. Surgery is most tolerated during second trimester, during which the risks to the mother and the fetus are minimized. Radiofrequency ablation during pregnancy has been described before in literature during first and second trimesters[44]. However their safety and efficacy still needs to be proven. Angioembolisation during pregnancy is questionable owing to the radiation risk to the fetus early in pregnancy. Their use during various trimesters of pregnancy warrants extensive studies before conclusion can be drawn.
LIVER CELL ADENOMATOSIS
Flejou et al[45] in 1985 was the first to propose adenomatosis as a varied clinical entity from HCA. Liver cell adenomatosis is characterized by the presence of multiple adenomas (> 10), involving both lobes of the liver, in absence of glycogen storage disease or exogenous hormone therapy. They account for 10%-24% patients with HCA. Histologic and radiologic features of these lesions are identical to that in adenomas. These lesions however have a higher risk of impaired liver function and bleeding. Flejou et al[45] reported bleeding in 46% of patients in his series. Malignant degeneration risk however does not correspondingly increase with the number of lesions. Chiche et al[46] proposed two different lesion patterns found in liver cell adenomatosis - “massive” and “multifocal”. Lesions in massive type tend to be larger (2 to 10 cm) and often result in gross hepatomegaly with deformed liver contour. In comparison, the multifocal type has smaller lesions (≤ 4 cm) and rarely deform liver contour[1]. The lesions in massive type tend to progress fast and are more likely to be symptomatic.
Management for these lesions remains challenging. The extensive distribution of these lesions in both lobes makes anatomical/non-anatomical hepatic resection impractical. All females should discontinue use of exogenous hormone therapy and avoid future pregnancies. The massive variant of this disease with predominant large lesions (> 5 cm) in a single lobe may warrant hemi hepatectomy. The role for radiofrequency ablation or embolization in these patients is debatable[40].
Liver transplantation presents the lone potential curative measure for these lesions. Evidence of malignant transformation or symptomatic patients with recurrent adenoma complications warrants transplantation[45]. However transplantation in turn increases the risk of de-novo tumors in addition to other complications that include peri-operative morbidities, infections, graft rejection and renal failure[47,48]. Owing to the rarity of the disease entity and lack of substantial clinical evidence, the effectiveness of transplantation is still controversial. Considering the complexities associated with transplantation, the trend of late is to treat such lesions conservatively. Such patients are to be followed on a regular basis with imaging to identify growth patterns and malignant transformation.
CONCLUSION
HCA despite being benign liver neoplasms often pose diagnostic and therapeutic challenges. Further characterization of the genetics and pathophysiology of such lesions and their radiological correlations may trigger changes in the existing management guidelines. We believe larger prospective studies that focus on the management of hepatic adenomas can aid overcome the current short-comings on the quality of evidence available.
Footnotes
P- Reviewer: Cosmi E S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Conflict-of-interest statement: No conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 12, 2015
First decision: July 25, 2015
Article in press: October 27, 2015
References
- 1.Barthelmes L, Tait IS. Liver cell adenoma and liver cell adenomatosis. HPB (Oxford) 2005;7:186–196. doi: 10.1080/13651820510028954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dokmak S, Paradis V, Vilgrain V, Sauvanet A, Farges O, Valla D, Bedossa P, Belghiti J. A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009;137:1698–1705. doi: 10.1053/j.gastro.2009.07.061. [DOI] [PubMed] [Google Scholar]
- 3.Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Hill AP, Tyler CW. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA. 1979;242:644–648. [PubMed] [Google Scholar]
- 4.Lin H, van den Esschert J, Liu C, van Gulik TM. Systematic review of hepatocellular adenoma in China and other regions. J Gastroenterol Hepatol. 2011;26:28–35. doi: 10.1111/j.1440-1746.2010.06502.x. [DOI] [PubMed] [Google Scholar]
- 5.Demo E, Frush D, Gottfried M, Koepke J, Boney A, Bali D, Chen YT, Kishnani PS. Glycogen storage disease type III-hepatocellular carcinoma a long-term complication? J Hepatol. 2007;46:492–498. doi: 10.1016/j.jhep.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakellariou S, Al-Hussaini H, Scalori A, Samyn M, Heaton N, Portmann B, Tobal K, Quaglia A. Hepatocellular adenoma in glycogen storage disorder type I: a clinicopathological and molecular study. Histopathology. 2012;60:E58–E65. doi: 10.1111/j.1365-2559.2011.04153.x. [DOI] [PubMed] [Google Scholar]
- 7.Parker P, Burr I, Slonim A, Ghishan FK, Greene H. Regression of hepatic adenomas in type Ia glycogen storage disease with dietary therapy. Gastroenterology. 1981;81:534–536. [PubMed] [Google Scholar]
- 8.Bioulac-Sage P, Taouji S, Possenti L, Balabaud C. Hepatocellular adenoma subtypes: the impact of overweight and obesity. Liver Int. 2012;32:1217–1221. doi: 10.1111/j.1478-3231.2012.02786.x. [DOI] [PubMed] [Google Scholar]
- 9.Farges O, Ferreira N, Dokmak S, Belghiti J, Bedossa P, Paradis V. Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2011;60:85–89. doi: 10.1136/gut.2010.222109. [DOI] [PubMed] [Google Scholar]
- 10.Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa Cunha A, Rullier A, Cubel G, Couchy G, Imbeaud S, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–748. doi: 10.1002/hep.21743. [DOI] [PubMed] [Google Scholar]
- 11.Bluteau O, Jeannot E, Bioulac-Sage P, Marqués JM, Blanc JF, Bui H, Beaudoin JC, Franco D, Balabaud C, Laurent-Puig P, et al. Bi-allelic inactivation of TCF1 in hepatic adenomas. Nat Genet. 2002;32:312–315. doi: 10.1038/ng1001. [DOI] [PubMed] [Google Scholar]
- 12.Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, Bacq Y, Leteurtre E, Paradis V, Michalak S, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515–524. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 13.Bioulac-Sage P, Laumonier H, Laurent C, Zucman-Rossi J, Balabaud C. Hepatocellular adenoma: what is new in 2008. Hepatol Int. 2008;2:316–321. doi: 10.1007/s12072-008-9075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liau SS, Qureshi MS, Praseedom R, Huguet E. Molecular pathogenesis of hepatic adenomas and its implications for surgical management. J Gastrointest Surg. 2013;17:1869–1882. doi: 10.1007/s11605-013-2274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herman P, Pugliese V, Machado MA, Montagnini AL, Salem MZ, Bacchella T, D’Albuquerque LA, Saad WA, Machado MC, Pinotti HW. Hepatic adenoma and focal nodular hyperplasia: differential diagnosis and treatment. World J Surg. 2000;24:372–376. doi: 10.1007/s002689910059. [DOI] [PubMed] [Google Scholar]
- 16.Charny CK, Jarnagin WR, Schwartz LH, Frommeyer HS, DeMatteo RP, Fong Y, Blumgart LH. Management of 155 patients with benign liver tumours. Br J Surg. 2001;88:808–813. doi: 10.1046/j.0007-1323.2001.01771.x. [DOI] [PubMed] [Google Scholar]
- 17.Dhingra S, Fiel MI. Update on the new classification of hepatic adenomas: clinical, molecular, and pathologic characteristics. Arch Pathol Lab Med. 2014;138:1090–1097. doi: 10.5858/arpa.2013-0183-RA. [DOI] [PubMed] [Google Scholar]
- 18.Scott FR, el-Refaie A, More L, Scheuer PJ, Dhillon AP. Hepatocellular carcinoma arising in an adenoma: value of QBend 10 immunostaining in diagnosis of liver cell carcinoma. Histopathology. 1996;28:472–474. doi: 10.1046/j.1365-2559.1996.t01-3-297345.x. [DOI] [PubMed] [Google Scholar]
- 19.Brunt EM, Swanson PE. Immunoreactivity for c-erbB-2 oncopeptide in benign and malignant diseases of the liver. Am J Clin Pathol. 1992;97:S53–S61. [PubMed] [Google Scholar]
- 20.Wilkens L, Bredt M, Flemming P, Becker T, Klempnauer J, Kreipe HH. Differentiation of liver cell adenomas from well-differentiated hepatocellular carcinomas by comparative genomic hybridization. J Pathol. 2001;193:476–482. doi: 10.1002/path.825. [DOI] [PubMed] [Google Scholar]
- 21.Wilkens L, Bredt M, Flemming P, Schwarze Y, Becker T, Mengel M, von Wasielewski R, Klempnauer J, Kreipe H. Diagnostic impact of fluorescence in situ hybridization in the differentiation of hepatocellular adenoma and well-differentiated hepatocellular carcinoma. J Mol Diagn. 2001;3:68–73. doi: 10.1016/S1525-1578(10)60654-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGahan JP, Goldberg BB. Diagnostic Ultrasound. 2nd Edition. New York: Informa Healthcare; 2008. [Google Scholar]
- 23.Grazioli L, Federle MP, Brancatelli G, Ichikawa T, Olivetti L, Blachar A. Hepatic adenomas: imaging and pathologic findings. Radiographics. 2001;21:877–892; discussion 892-894. doi: 10.1148/radiographics.21.4.g01jl04877. [DOI] [PubMed] [Google Scholar]
- 24.Faria SC, Iyer RB, Rashid A, Whitman GJ. Hepatic adenoma. AJR Am J Roentgenol. 2004;182:1520. doi: 10.2214/ajr.182.6.1821520. [DOI] [PubMed] [Google Scholar]
- 25.Bieze M, van den Esschert JW, Nio CY, Verheij J, Reitsma JB, Terpstra V, van Gulik TM, Phoa SS. Diagnostic accuracy of MRI in differentiating hepatocellular adenoma from focal nodular hyperplasia: prospective study of the additional value of gadoxetate disodium. AJR Am J Roentgenol. 2012;199:26–34. doi: 10.2214/AJR.11.7750. [DOI] [PubMed] [Google Scholar]
- 26.Lubbers PR, Ros PR, Goodman ZD, Ishak KG. Accumulation of technetium-99m sulfur colloid by hepatocellular adenoma: scintigraphic-pathologic correlation. AJR Am J Roentgenol. 1987;148:1105–1108. doi: 10.2214/ajr.148.6.1105. [DOI] [PubMed] [Google Scholar]
- 27.Bieze M, Phoa SS, Verheij J, van Lienden KP, van Gulik TM. Risk factors for bleeding in hepatocellular adenoma. Br J Surg. 2014;101:847–855. doi: 10.1002/bjs.9493. [DOI] [PubMed] [Google Scholar]
- 28.Kammula US, Buell JF, Labow DM, Rosen S, Millis JM, Posner MC. Surgical management of benign tumors of the liver. Int J Gastrointest Cancer. 2001;30:141–146. doi: 10.1385/IJGC:30:3:141. [DOI] [PubMed] [Google Scholar]
- 29.Huurman VA, Schaapherder AF. Management of ruptured hepatocellular adenoma. Dig Surg. 2010;27:56–60. doi: 10.1159/000268427. [DOI] [PubMed] [Google Scholar]
- 30.Darnis B, Rode A, Mohkam K, Ducerf C, Mabrut JY. Management of bleeding liver tumors. J Visc Surg. 2014;151:365–375. doi: 10.1016/j.jviscsurg.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Stoot JH, Coelen RJ, De Jong MC, Dejong CH. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford) 2010;12:509–522. doi: 10.1111/j.1477-2574.2010.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bioulac-Sage P, Laumonier H, Sa Cunha A, Balabaud C. Hepatocellular adenomas. Liver Int. 2009;29:142. doi: 10.1111/j.1478-3231.2008.01884.x. [DOI] [PubMed] [Google Scholar]
- 33.Tao LC. Oral contraceptive-associated liver cell adenoma and hepatocellular carcinoma. Cytomorphology and mechanism of malignant transformation. Cancer. 1991;68:341–347. doi: 10.1002/1097-0142(19910715)68:2<341::aid-cncr2820680223>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 34.Micchelli ST, Vivekanandan P, Boitnott JK, Pawlik TM, Choti MA, Torbenson M. Malignant transformation of hepatic adenomas. Mod Pathol. 2008;21:491–497. doi: 10.1038/modpathol.2008.8. [DOI] [PubMed] [Google Scholar]
- 35.De Souza AT, Hankins GR, Washington MK, Fine RL, Orton TC, Jirtle RL. Frequent loss of heterozygosity on 6q at the mannose 6-phosphate/insulin-like growth factor II receptor locus in human hepatocellular tumors. Oncogene. 1995;10:1725–1729. [PubMed] [Google Scholar]
- 36.Macdonald GA, Greenson JK, Saito K, Cherian SP, Appelman HD, Boland CR. Microsatellite instability and loss of heterozygosity at DNA mismatch repair gene loci occurs during hepatic carcinogenesis. Hepatology. 1998;28:90–97. doi: 10.1002/hep.510280114. [DOI] [PubMed] [Google Scholar]
- 37.Herman P, Coelho FF, Perini MV, Lupinacci RM, D’Albuquerque LA, Cecconello I. Hepatocellular adenoma: an excellent indication for laparoscopic liver resection. HPB (Oxford) 2012;14:390–395. doi: 10.1111/j.1477-2574.2012.00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marrero JA, Ahn J, Rajender Reddy K. ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol. 2014;109:1328–1347; quiz 1348. doi: 10.1038/ajg.2014.213. [DOI] [PubMed] [Google Scholar]
- 39.Lee SH, Hahn ST. Treatment of multiple hepatic adenomatosis using transarterial chemoembolization: a case report. Cardiovasc Intervent Radiol. 2004;27:563–565. doi: 10.1007/s00270-003-2758-y. [DOI] [PubMed] [Google Scholar]
- 40.Ahn SY, Park SY, Kweon YO, Tak WY, Bae HI, Cho SH. Successful treatment of multiple hepatocellular adenomas with percutaneous radiofrequency ablation. World J Gastroenterol. 2013;19:7480–7486. doi: 10.3748/wjg.v19.i42.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santambrogio R, Marconi AM, Ceretti AP, Costa M, Rossi G, Opocher E. Liver transplantation for spontaneous intrapartum rupture of a hepatic adenoma. Obstet Gynecol. 2009;113:508–510. doi: 10.1097/AOG.0b013e318187ff42. [DOI] [PubMed] [Google Scholar]
- 42.Cobey FC, Salem RR. A review of liver masses in pregnancy and a proposed algorithm for their diagnosis and management. Am J Surg. 2004;187:181–191. doi: 10.1016/j.amjsurg.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 43.Bröker ME, Ijzermans JN, van Aalten SM, de Man RA, Terkivatan T. The management of pregnancy in women with hepatocellular adenoma: a plea for an individualized approach. Int J Hepatol. 2012;2012:725735. doi: 10.1155/2012/725735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noels JE, van Aalten SM, van der Windt DJ, Kok NF, de Man RA, Terkivatan T, Ijzermans JN. Management of hepatocellular adenoma during pregnancy. J Hepatol. 2011;54:553–558. doi: 10.1016/j.jhep.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 45.Flejou JF, Barge J, Menu Y, Degott C, Bismuth H, Potet F, Benhamou JP. Liver adenomatosis. An entity distinct from liver adenoma? Gastroenterology. 1985;89:1132–1138. [PubMed] [Google Scholar]
- 46.Chiche L, Dao T, Salamé E, Galais MP, Bouvard N, Schmutz G, Rousselot P, Bioulac-Sage P, Ségol P, Gignoux M. Liver adenomatosis: reappraisal, diagnosis, and surgical management: eight new cases and review of the literature. Ann Surg. 2000;231:74–81. doi: 10.1097/00000658-200001000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mueller J, Keeffe EB, Esquivel CO. Liver transplantation for treatment of giant hepatocellular adenomas. Liver Transpl Surg. 1995;1:99–102. doi: 10.1002/lt.500010205. [DOI] [PubMed] [Google Scholar]
- 48.Pichlmayr R, Weimann A, Ringe B. Indications for liver transplantation in hepatobiliary malignancy. Hepatology. 1994;20:33S–40S. doi: 10.1016/0270-9139(94)90271-2. [DOI] [PubMed] [Google Scholar]


