Abstract
AIM: To evaluate the effect of orally administered plecanatide or dolcanatide, analogs of uroguanylin, on amelioration of colitis in murine models.
METHODS: The cyclic guanosine monophosphate (cGMP) stimulatory potency of plecanatide and dolcanatide was measured using a human colon carcinoma T84 cell-based assay. For animal studies all test agents were formulated in phosphate buffered saline. Sulfasalazine or 5-amino salicylic acid (5-ASA) served as positive controls. Effect of oral treatment with test agents on amelioration of acute colitis induced either by dextran sulfate sodium (DSS) in drinking water or by rectal instillation of trinitrobenzene sulfonic (TNBS) acid, was examined in BALB/c and/or BDF1 mice. Additionally, the effect of orally administered plecanatide on the spontaneous colitis in T-cell receptor alpha knockout (TCRα-/-) mice was also examined. Amelioration of colitis was assessed by monitoring severity of colitis, disease activity index and by histopathology. Frozen colon tissues were used to measure myeloperoxidase activity.
RESULTS: Plecanatide and dolcanatide are structurally related analogs of uroguanylin, which is an endogenous ligand of guanylate cyclase-C (GC-C). As expected from the agonists of GC-C, both plecanatide and dolcanatide exhibited potent cGMP-stimulatory activity in T84 cells. Once-daily treatment by oral gavage with either of these analogs (0.05-0.5 mg/kg) ameliorated colitis in both DSS and TNBS-induced models of acute colitis, as assessed by body weight, reduction in colitis severity (P < 0.05) and disease activity index (P < 0.05). Amelioration of colitis by either of the drug candidates was comparable to that achieved by orally administered sulfasalazine or 5-ASA. Plecanatide also effectively ameliorated colitis in TCRα-/- mice, a model of spontaneous colitis. As dolcanatide exhibited higher resistance to proteolysis in simulated gastric and intestinal juices, it was selected for further studies.
CONCLUSION: This is the first-ever study reporting the therapeutic utility of GC-C agonists as a new class of orally delivered and mucosally active drug candidates for the treatment of inflammatory bowel diseases.
Keywords: Guanylate cyclase-C, Inflammatory bowel disease, Uroguanylin, Plecanatide, Dolcanatide
Core tip: Plecanatide (SP-304) and dolcanatide (SP-333) are structurally close analogs of the human endogenous natriuretic peptide uroguanylin, a ligand of guanylate cyclase-C (GC-C). Here we report that oral treatment with plecanatide or dolcanatide effectively ameliorates colitis in acute and chronic models of murine experimental colitis. The anti-inflammatory activity of plecanatide and dolcanatide was comparable to that achieved after treatment with sulfasalazine or 5-amino salicylic acid. This is the first-ever study reporting the therapeutic utility of GC-C agonists as a new class of orally delivered and mucosally active drug candidates for the treatment and management of inflammatory bowel diseases in humans.
INTRODUCTION
Chronic inflammatory bowel diseases (IBD) such as Crohn’s disease (CD) and ulcerative colitis (UC) are multifactorial gastrointestinal (GI) diseases with high incidences worldwide[1]. While the etiology of these diseases remains largely unknown, one of the contributing factors may be poorly regulated immune response against the enteric microbiota in genetically predisposed individuals[2]. Existing therapies for symptomatic relief of IBD include anti-inflammatory drugs, immunosuppressants, biologic agents, antibiotics, and aminosalicylic acid (5-ASA) based drugs. However, these treatments are not entirely satisfactory due to limited effectiveness and associated side effects[3-6]. Thus there is a need to identify drugs that provide safer, convenient and efficient means of therapeutic intervention for IBD. An ideal conceptual advance would be to develop oral drugs that act locally at the site of inflammation to maximize efficacy and to minimize systemic side effects.
Uroguanylin (UG) and guanylin (GN) activate guanylate cyclase-C (GC-C) receptors expressed on the epithelial cells lining the GI mucosa to stimulate production of cyclic GMP (cGMP), which in turn sequentially activates protein kinase G-IIand cystic fibrosis transmembrane conductance regulator to regulate ion and fluid transport, epithelial cell homeostasis and to maintain barrier function in the GI mucosa[7-10]. The expression of mRNAs encoding UG and GN are markedly suppressed in human colonic polyps, tumors and in inflamed tissues from UC and CD patients[10-13]. These findings raise the possibility that the deficiency of UG and GN might be associated with the pathogenesis of these diseases. Consistent with this notion, we have demonstrated that oral administration with UG not only inhibits polyp formation but also delays their progression to adenocarcinomas[10]. Similar findings have been reported by other researchers underscoring the evolving role of GC-C signaling in suppression of GI inflammation and prevention of colorectal tumorigenesis[13-16].
Maintenance of the intestinal epithelial cell barrier function is considered to be crucial for host immune defense. Thus, dysfunctional barrier function and increased gut permeability might be crucial pathophysiological factors underlying the etiology of IBD. Recent studies with the GC-C-/- and UG-/- knockout mice further illustrate the involvement of GC-C signaling in the maintenance of homeostatic intestinal barrier function, permeability and intestinal epithelial cell proliferation[17,18]. Moreover, disruption in GC-C signaling is associated with a reduced number of colonic goblet cells, resulting in decreased production of mucin and intestinal trefoil factor[13], which are the principal components of the gut-coating mucus layer for maintenance of epithelial barrier protection and for post-injury restitution. Importantly, a recent study indicated that colitis in GC-C-/- and IL-10-/- double knockout mice was significantly more severe as compared to that in GC-C+/+ and IL-10-/- mice, suggesting a role of GC-C as a suppressor of spontaneous T-cell-driven intestinal inflammation[19]. In this regard, it has been reported that cell-permeable analogs of cGMP exhibit anti-inflammatory activity, possibly via inhibition of NF-κB activation[20]. In addition, atrial natriuretic peptide, an agonist of natriuretic peptide receptor A (NPR-A), also exhibits anti-inflammatory activity in both in vitro and in vivo models through stimulation of cGMP production[21]. Consistent with these observations, disruption of the NPR-A gene leads to augmented production of pro-inflammatory cytokines and growth factors[22]. Collectively, these studies suggest that activation of GC-C/cGMP signaling may have therapeutic potential in the management of GI inflammatory diseases.
Based on results from thermal bond energy calculations, 3-D structure modeling, molecular simulation and structure-activity relationship studies, we discovered plecanatide and dolcanatide as novel analogs of UG that bind and activate GC-C receptors to stimulate production of cGMP in a pH-dependent manner. Thus, these agonists are expected to mimic UG function in the GI tract[23-25]. This is the first study demonstrating that oral treatment with plecanatide or dolcanatide ameliorates GI inflammation in acute as well as in chronic models of experimental colitis in mice.
MATERIALS AND METHODS
Materials
Human colonic carcinoma T84 cells obtained from Leonard Forte, University of Missouri, MO, were cultured as described earlier[10]. Plecanatide and dolcanatide were chemically synthesized by the procedures as described previously[24,26]. All other chemicals and other reagents were obtained from commercially available vendors.
Methods
Animal studies: Studies employing BALB/c and T-cell receptor alpha knockout (TCRα-/-)mice were performed at the University of Pittsburgh School of Medicine (Pittsburg, PA). Animals obtained from Jackson Laboratories (Bar Harbor, ME) were housed in accordance with guidelines from the American Association for Laboratory Animal Care and Research Protocols as approved by the Institutional Animal Care and Use Committee. Epistem Ltd (Manchester, United Kingdom) conducted dextran sulfate sodium (DSS) and TNBS-induced colitis studies in BDF1 mice. Animals obtained from Harlan Laboratories, United Kingdom, were held in individually ventilated cages in a specific pathogen-free barrier unit in compliance with animal welfare regulations. The day-night cycle was constant, with light and dark phases of 12 h each. At the end of experimental protocols, mice were euthanized by CO2 followed by cervical dislocation.
Cyclic GMP stimulation assay in T84 cells: Potencies of plecanatide and dolcanatide to stimulate cGMP synthesis in T84 cells was assayed as described previously[10]. Briefly, confluent monolayers of T84 cells were pre-incubated with 1 mmol/L isobutylmethylxanthine, a phosphodiesterase inhibitor, in DMEM for 10 min at 37 °C followed by incubation with test peptide for 30 min. The reaction was terminated by adding 3% perchloric acid. Following centrifugation and neutralization with 0.5 N NaOH, supernatants were used for measurements of intracellular cGMP using an ELISA kit (Cayman Chemical Co., Ann Arbor, MI). Results are expressed as pmol of cGMP/mg of protein in the cell extracts.
TNBS-induced colitis in BALB/c mice: BALB/c mice (n = 5-8/group) were used to evaluate the anti-inflammatory effects of plecanatide on TNBS-induced colitis by previously described procedures[27,28]. Briefly, colitis was induced in 2-4 mo old female BALB/c mice by administering 2.5 mg TNBS in 50% ethanol into the lumen of the colon (injection volume 100 μL). Plecanatide (0, 0.5 and 2.5 mg/kg) formulated in PBS was administered by oral gavage for 7 d, with the first dose given the same day as TNBS challenge. After 7 d of treatment animals were euthanized, GI tissues were collected for histopathological examination, and inflammation scoring was performed[29].
TNBS-induced colitis in BDF-1 mice: A TNBS-induced colitis study in 10-12 wk old BDF-1 mice (n = 10/group) was conducted at the Epistem Ltd., United Kingdom, using a procedure essentially similar to that described above except that mice were dosed with plecanatide (0.005-5 mg/kg) a day before TNBS treatment. Daily administration of plecanatide was continued until 7th day when the mice were euthanized. Colon tissues were removed and weighed. Distal sections were fixed, stained with H and E, and evaluated for histopathology and visual severity scores[27,28]. Scoring of the H and E-stained tissue sections employed the following criteria: Normal-appearing crypts (score 0); abnormal crypt pathology without ulceration (score 1); depleted crypts with some ulceration/ inflammation (score 2); 20%-70% depleted crypts and increased ulceration/inflammation (score 3); > 70% depleted crypts with substantial ulceration/inflammation (score 4); and totally ulcerated/inflamed colon with no crypts remaining (score 5). All slides were scored in a blinded manner. Scoring criteria were similar to that described below for DSS induced colitis studies.
DSS induced colitis in BDF-1 mice: This study was conducted by the Epistem Ltd., United Kingdom. BDF1 mice (n = 8-10; age 10-12 wk) were given 5% DSS in the drinking water on day 0 to induce colitis. Plecanatide or dolcanatide (0.005-5 mg/kg) in 0.1 M phosphate buffer (pH7) was administered daily by oral gavage starting a day prior to DSS administration (day-1) through 7th day. Oral gavage with sulfasalazine (80 mg/kg) or 5-ASA (100 mg/kg) served as a positive control. Mice were euthanized at the end of the treatment and tissues from large intestines were removed and weighed. The distal sections were fixed for histopathology evaluations. Colitis severity was assessed by histopathological evaluation of the H and E-stained tissue sections as described above. Disease activity index (DAI) was calculated by determining body weight, stool consistency, and the presence of overt blood in stools or around the anus, substantially similar to that reported earlier[30].
Spontaneous colitis in TCRα-/- knockout mice: This study was conducted at the University of Pittsburgh School of Medicine, PA. The TCRα-/- mice were matched for age and sex in all groups. Sixteen-week-old mice were administered plecanatide (0.5 or 2.5 mg/kg) or vehicle by oral gavage for 14 d (6 mice/group). Mice were euthanized 12 h after the final dose and GI tissues collected for histopathological evaluation of colitis severity. Scoring of colitis severity was as previously described[31].
Myeloperoxidase activity: Myeloperoxidase (MPO) activity in colonic tissue samples was measured according to methods described previously[32]. Briefly, the rate of change in absorbance at 450 nm was recorded when 20 μL of tissue extract was incubated with 150 μL of reaction buffer containing 0.26 mg/mL o-dianisidine and 0.6 μL/mL H2O2. Each reaction was performed in triplicate. The rate of reaction was determined (initial slope) and normalized to the protein concentration of the sample.
Statistical analysis
Statistical significance was performed by comparing mean values of the control with that of the treated samples, using 2-way unpaired Student t-tests, assuming unequal variance, in Microsoft Excel and PRISM. A P value < 0.05 was considered to be statistically significant.
RESULTS
Plecanatide and dolcanatide are analogs of human UG
Plecanatide is structurally similar to UG, differing only in the substitution of Asp3 with Glu3. Dolcanatide is similar to plecanatide in structure except that L-Asn1 and L-Leu16 are replaced by D-Asn1 and D-Leu16 at the N and C-termini, respectively (Figures 1 and 2). In T84 cells assay, both plecanatide and dolcanatide activate GC-C receptors to stimulate cGMP synthesis in a dose-dependent manner with EC50 values of 1.9 × 10-7 mol/L and 2.8 × 10-7 mol/L, respectively (Figure 2).
Figure 1.
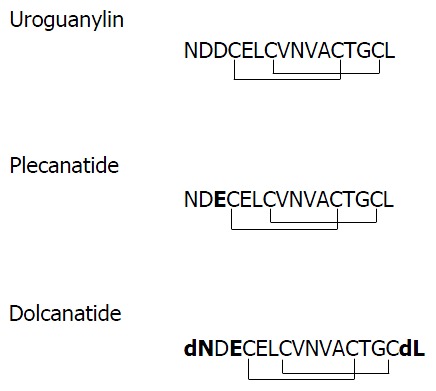
Primary structures of uroguanylin, plecanatide, and dolcanatide. Single-letter abbreviations for amino acids are depicted. Plecanatide is similar to UG except for the substitution of aspartic acid (D) at position 3 from the N-terminus of UG with glutamic acid (E). The structure of dolcanatide is similar to plecanatide except that the amino acids at both termini are replaced with their respective D-stereoisomers. Uroguanylin as well as its analogs have four cysteines (C) enabling the formation of two intramolecular disulfide bonds. Substituted amino acids in plecanatide and dolcanatide are shown in bold type. UG: Uroguanylin.
Figure 2.
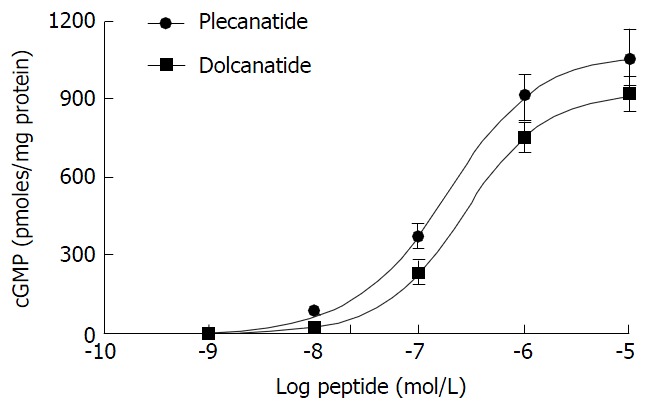
Plecanatide and dolcanatide mediated stimulation of cyclic guanosine monophosphate production in T84 cells. The potency of plecanatide and dolcanatide to stimulate cGMP production was evaluated using the T84 cell bioassay (see Materials and Methods). The data presented are an average of three determinations and are expressed as pmoles cGMP/mg protein ± SD. cGMP: Cyclic guanosine monophosphate.
Plecanatide ameliorates spontaneous and chemically induced colitis
In a preliminary study, the ability of orally-administered plecanatide to ameliorate colitis was evaluated in TNBS-induced colitis in BALB/c mice (Figure 3A). Treatment with plecanatide at 0.5 and 2.5 mg/kg for 7 d effectively reduced colitis severity scores as compared to vehicle treatment. Oral treatment with plecanatide was also evaluated in TCRα-/- mice that develop chronic colitis spontaneously. In this model, oral treatment with 0.5 and 2.5 mg/kg continuously for two weeks reduced colitis scores as compared to those in the vehicle-treated group (Figure 3B). Taken together, these preliminary results prompted a further evaluation of plecanatide and dolcanatide in DSS and TNBS-induced colitis with larger cohorts.
Figure 3.
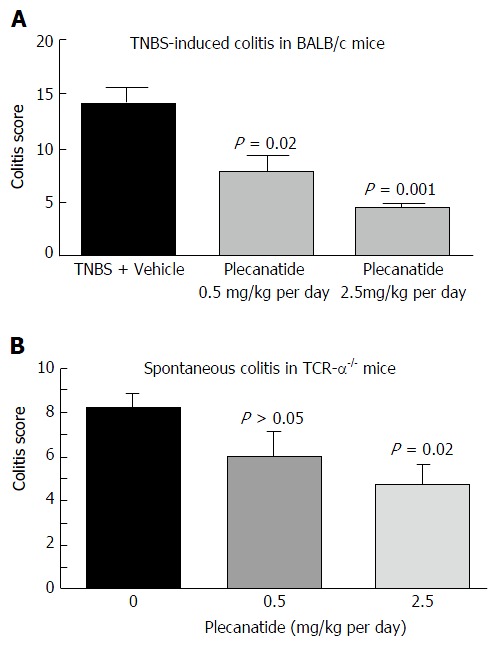
Oral treatment with plecanatide ameliorates colitis in acute and chronic mouse models. A: Acute colitis examined in BALB/c mice (n = 5-8/group) was induced by rectal instillation of TNBS. Mice were administered an oral gavage of vehicle or plecanatide (0.5 and 2.5 mg/kg per day) starting on 0 d; animals were euthanized on 7th day and colitis scores were determined; B: TCRα-/- mice, a model for chronic spontaneous colitis, were administered an oral gavage of plecanatide (0.5 and 2.5 mg/kg) or vehicle for 14 d. At the end of the study, colon tissues were harvested and used for histopathological analysis to assess colitis. Results are depicted as mean colitis score ± SD. TCRα: T-cell receptor alpha; TNBS: 2, 4, 6-trinitrobenzenesulfonic acid sol.
Oral treatment with either vehicle, sulfasalazine (80 mg/kg) or plecanatide (0.005, 0.05, 0.5 and 5.0 mg/kg), was evaluated in both DSS and TNBS-induced colitis in mice. In BDF1 mice, colitis was induced by including 5% DSS in drinking water, and mice were randomly divided into 6 groups. Colitis severity was determined by the histopathological evaluation of H and E-stained colonic tissue sections employing the criteria described under Materials and Methods. Consistent with the results from the preliminary studies described above, orally administered plecanatide even at a dose as low as 0.005 mg/kg was as effective as sulfasalazine (80 mg/kg) to ameliorate DSS induced colitis in BDF-1 mice (Figure 4). However, doses higher than 0.005 mg/kg per day did not produce an incremental effect on the amelioration of colitis, possibly due to the saturation of available GC-C receptors in the gut lumen.
Figure 4.
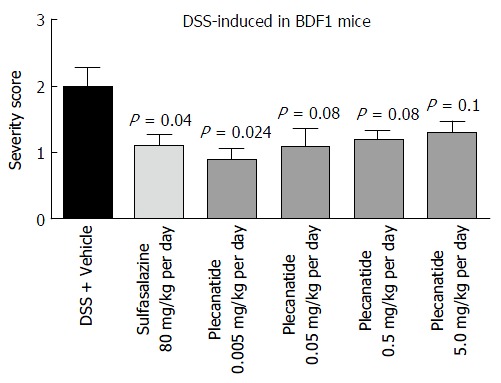
Oral treatment with plecanatide ameliorates gastrointestinal inflammation in the dextran sulfate sodium-induced colitis in BDF1 mice. BDF1 mice (n = 8/group) were administered 5% DSS in drinking water. An oral gavage was administered once daily containing 0.005 to 5.0 mg/kg of plecanatide beginning a day prior to initiating DSS regimen. Sulfasalazine (80 mg/kg) and vehicle (phosphate buffer) served as positive and negative controls, respectively. At the end of the study mice were euthanized; distal sections of the colon were fixed, embedded in paraffin, sectioned, stained with H and E and visualized to assign colitis severity scores. All slides were scored in a blinded manner. The mean histological severity of colitis score ± SEM was plotted for the indicated treatment groups. Statistical significance was calculated by comparing severity scores observed for the plecanatide or sulfasalazine treated group vs corresponding values in the vehicle treated group. DSS: Dextran sulfate sodium; SEM: Standard error of the mean.
The anti-inflammatory activity of plecanatide was further examined in the TNBS-induced colitis in BDF1 mice. Once-daily administration of plecanatide at 0.005 mg/kg was not effective, but all of the higher doses (0.05-5 mg/kg) produced statistically significant reduction in severity of colitis as compared to TNBS plus vehicle treated animals (Figure 5). Again, there was no further reduction in colitis severity with incremental doses above 0.05 mg/kg, suggesting that this dose might be at the saturation level of the available GC-C binding sites in the GI lumen. Notably, the efficacy of plecanatide at 0.05 mg/kg per day dose was comparable to that of sulfasalazine at the dose 80 mg/kg per day.
Figure 5.
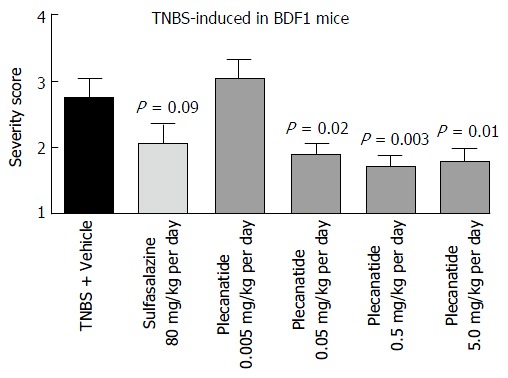
Oral treatment with plecanatide ameliorates gastrointestinal inflammation in 2, 4, 6-trinitrobenzenesulfonic acid-induced colitis in BDF1 mice. BDF1 mice (n = 10/group) were administered TNBS via rectal instillation on 0 d as described under Materials and Methods. An oral gavage was administered once daily containing 0.005 to 5.0 mg/kg plecanatide beginning a day prior to TNBS treatment. Sulfasalazine (80 mg/kg) and vehicle (phosphate buffer) served as positive and negative controls, respectively. At the end of the study mice were euthanized; distal sections of the colon were fixed, embedded in paraffin, sectioned, stained with H and E and visualized to assign colitis severity scores. All slides were scored in a blinded manner. The mean histological severity of the colitis score ± SEM was plotted for the indicated treatment groups. Statistical significance was calculated by comparing the severity score observed for the plecanatide or sulfasalazine-treated group vs the corresponding values in the vehicle-treated group. TNBS: 2, 4, 6-trinitrobenzenesulfonic acid sol; SEM: Standard error of the mean.
Dolcanatide ameliorates DSS induced colitis in BDF-1 mice
To further confirm the anti-inflammatory activity of this class of GC-C agonists, the effect of oral treatment with dolcanatide was evaluated in DSS induced colitis in BDF-1 mice. Except for the 0.5 mg/kg dose of dolcanatide, treatment with all other doses produced statistically significant reduction in colitis severity scores as compared to those in DSS + vehicle control. The reduction in colitis severity was comparable to that observed in 100 mg/kg dose of 5-ASA (Figure 6A). Similarly, treatment with dolcanatide also produced considerable reduction in the DAI score (Figure 6B), although the statistical significance (P = 0.04) was achieved only with a dose of 0.05 mg/kg. The effect of dolcanatide treatment on levels of MPO activity in colon tissues was measured as an indirect way to assess the severity of GI inflammation. As expected, the colon tissues from DSS plus vehicle-treated mice exhibited the highest levels of MPO (0.048 ± 0.004 units/min). A considerable reduction in MPO activity was observed following treatment either with 5-ASA (100 mg/kg) or with dolcanatide at all doses (Figure 6C). Even at the dose as low as (0.05 mg/kg per day) of dolcanatide, showing approximately 50% reduction in total MPO activity, was comparable to that achieved with 5-ASA at 100 mg/kg dose.
Figure 6.
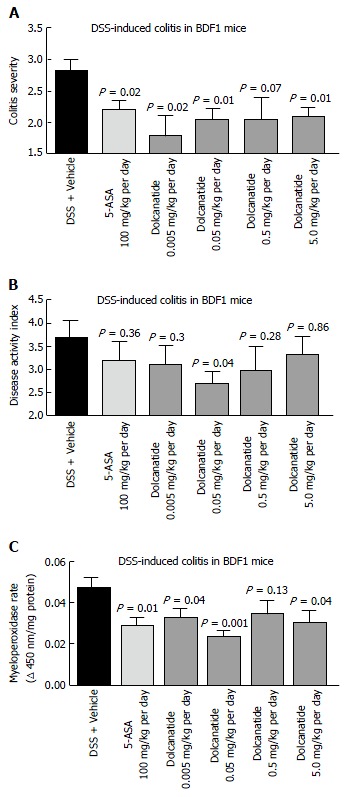
Oral treatment with dolcanatide ameliorates gastrointestinal inflammation in the dextran sulfate sodium induced colitis in BDF1 mice. BDF1 mice (n = 8-10/group) were administered 5% DSS in drinking water to induce colitis. Dolcanatide was administered via oral gavage beginning a day before initiating the DSS regimen. Oral gavage with 5-ASA (100 mg/kg) and vehicle (phosphate buffer) served as positive and negative controls, respectively. At the end of the study, mice were euthanized and colon tissues were removed for histopathological analyses (see Materials and Methods section). Additional groups of mice were treated under identical conditions for measurement of MPO activity in colon tissues. The Figure depicts results for colitis severity (A), disease activity index (B), and MPO activity (C). Data represent mean ± SEM for each group. Statistical significance was calculated by comparing the values observed for the dolcanatide or 5-ASA-treated group vs the corresponding scores for the vehicle-treated group. DSS: Dextran sulfate sodium; GI: Gastrointestinal; MPO: Myeloperoxidase; SEM: Standard error of the mean; 5-ASA: 5-amino salicylic acid.
Colon tissues from mice treated with dolcanatide at all doses or with 5-ASA showed a considerable reduction in histopathological scoring. Representative slides of H and E-stained sections of colon tissues used for histopathological evaluation are shown in Figure 7. Substantial loss of crypts, changes in crypt architecture, ulceration, and localized infiltration of inflammatory cells was observed in tissue slides from DSS plus-vehicle-treated mice (panel B; score = 3) as compared to that in the slides from naïve mice exhibiting normal mucosal architecture and evenly-spaced crypts of uniform length (panel A; score 0). Treatment with dolcanatide at 0.05 mg/kg (panel C; score 2) or with 5-ASA (panel D; score 2) exhibited minimal loss of crypts, changes in crypt architecture, ulceration, and localized infiltration of inflammatory cells, which is indicative of improvement in colitis severity.
Figure 7.
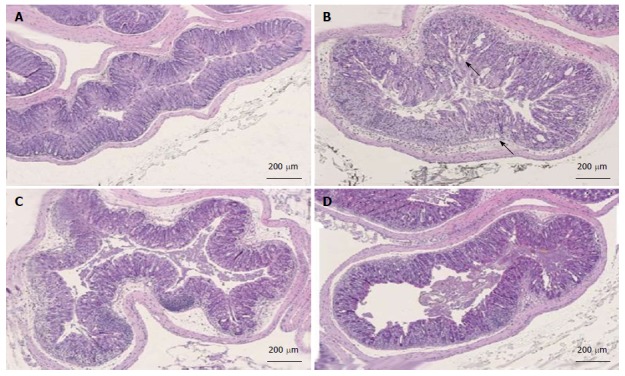
Histopathology analysis of colon tissues after treatment with dolcanatide. Representative histopathological images of the large bowel from the DSS-induced colitis study are depicted. Criteria for scoring colitis severity were as described under Materials and Methods. A: Untreated naïve mice, histopathology score = 0; B: DSS + vehicle treated, histopathology score = 3; C: DSS + dolcanatide (0.05 mg/kg), histopathology score = 2; D: DSS + 5-ASA (100 mg/kg), histopathology score = 2. Arrows in panel B indicate morphological deterioration in crypts and villi of GI mucosa. DSS: Dextran sulfate sodium; 5-ASA: 5-amino salicylic acid; GI: Gastrointestinal.
DISCUSSION
This is the first-ever study reporting that oral treatment with either plecanatide or dolcanatide, analogs of UG, ameliorate colonic inflammation in both acute and chronic models of murine colitis. Oral treatment with plecanatide or dolcanatide at a dose range between 0.05-2.5 mg/kg per day was as effective as once-daily treatment with 5-ASA (100 mg/kg) or sulfasalazine (80 mg/kg). However, the amelioration of colitis by the treatment with plecanatide or dolcanatide was not dose-dependent. This lack of dose response may be attributable to the saturation of the available GC-C receptors on the epithelial cells lining the GI mucosa. Interestingly, the lowest effective dose of plecanatide varied from 0.005 to 2.5 mg/d in different models examined at the three different research facilities used. These apparent differences in the effective dose of plecanatide may be due to the differences in external factors, such as diet and animal husbandry conditions of contract research organizations in the United States and the United Kingdom. These external factors are known to impact the composition of gut microflora influencing the severity of colitis in different species of mice[33,34].
The mechanism of actions of the endogenous natriuretic peptides UG and GN are known to be through activation of GC-C expressed on the apical surface of epithelial cells lining the GI mucosa[8]. Thus, orally administered GC-C agonists enhance cGMP, which mediates their pharmacological actions resulting in increased fluid secretion to promote GI transit and bowel movement. Previously, we reported that orally administered plecanatide acts primarily in the lumen of the proximal intestine to facilitate bowel movement in mice and monkeys[35]. In addition, orally administered plecanatide not only ameliorated GI inflammation but also delayed its progression to colorectal carcinogenesis via enhancement of cGMP production through activation of GC-C signaling in Apc+/min-FCCC mice[36]. Importantly, recent clinical studies confirm that orally administered GC-C agonists are minimally absorbed into systemic circulation and they act locally in the gut lumen[25,37]. Taken together, these studies suggest that the pharmacological actions of orally administered plecanatide and dolcanatide are primarily through activation of GC-C receptors in the gut lumen.
During the renewal process, the intestinal epithelium goes through a cycle (initiated in the crypt) of proliferation, migration, differentiation, apoptosis and ultimately loss of epithelial cells into the lumen[38]. This process is crucial for maintaining the integrity of the intestinal mucosa. UG and GN are secreted in a gradient along this vertical axis. Maximal secretion of UG is in the villus region and minimum in the crypt[39]. GC-C receptor signaling is believed to be important in maintaining the balance between apoptosis and regeneration[14]. Decreased expression of UG and GN in colon polyps, tumors and in inflamed tissues from UC and CD patients[10-12], can potentially impair intestinal homeostasis, suggesting a pathophysiological significance of GC-C signaling in the etiology of these diseases. Consistent with this notion, studies with GC-C-/- and UG-/- knockout mice revealed a functional role of GC-C signaling in the maintenance of homeostatic intestinal barrier function, intestinal permeability, and epithelial cell proliferation[18,40]. Of relevance, intestinal permeability is higher in the GC-C and UG knockout mice than in the wild mice. Expression of the major tight junction proteins is also reduced in the intestines of GC-C deficient mice. Luminal antigens permeate the defective barrier, promote inflammation, and damage the intestinal mucosal architecture[18,41]. These studies suggest a critical role of GC-C signaling in preserving the intestinal integrity. Histological analyses reported here lend support to this argument. Mucosal damage following DSS treatment results in loss of crypts, changes in crypt architecture, ulceration, and infiltration of inflammatory cells into colonic tissues. Treatment with dolcanatide resulted in minimal loss of crypts, low distortions in crypt morphology, and a significant reduction in myeloperoxidase activity. These results suggest less infiltration of inflammatory cells, possibly by strengthening the barrier function. Consistent with this notion, we recently reported that treatment with dolcanatide attenuated LPS-mediated enhancement in cellular permeability in T84 and Caco-2 cells[42].
Following the loss of barrier function, this regulatory balance is disrupted by the massive recruitment of leucocytes and macrophages, resulting in augmented levels of destructive inflammatory cytokines in the intestinal tissues[43]. In a preliminary study, we previously reported that treatment with plecanatide inhibited secretion of pro-inflammatory cytokines such as IL-12p40, IL-23, and TNF in explant cultures of colon tissues from TNBS-treated BALB/c mice. Plecanatide treatment similarly reduced production of RANTES, IL-17 and MIP-1α, with a concomitant increase in IL-10 in colon explants from TCRα-/- mice[36]. Our results using T84 cells further suggest that treatment with dolcanatide inhibited LPS-mediated activation of NF-κB activation, presumably via a cGMP-mediated mechanism[23]. Oral treatment with plecanatide also reduced the formation of colon dysplasia in DSS-treated ApcMin/+-FCCC, possibly through down-regulation of some pro-inflammatory cytokines and growth factors[44]. Taken together, these results suggest that orally-administered plecanatide or dolcanatide ameliorates colitis, possibly through suppression of pro-inflammatory cytokines production. However, this is a simplistic view on the possible mechanism of action for the anti-inflammatory activity of GC-C agonists. Additional studies are necessary to define the precise mechanism by which GC-C agonists promote intestinal barrier function, suppress production of cytokines, and exert their anti-inflammatory activity.
Oral treatment with analogs of the endogenous natriuretic peptide UG is thus an attractive approach with a unique mechanism of action, enabling restoration of homeostatic signaling responsible for maintenance of colonic mucosa integrity. This study may have the following significant implications for treatment and maintenance of IBD in humans: First, oral treatment with locally acting GC-C agonist may eliminate toxicity concerns associated with the existing systemic therapies of IBD; second, the evolving paradigm implies that the reduced production of UG and/or GN might be involved in the pathologies of UC and CD in humans. If so, oral therapy with UG analogs can be considered as a replacement therapy to overcome the deficiency underlying the etiology of IBD. Finally, it is now well established that patients suffering from chronic UC and CD are at higher risk of developing colon cancer[45]. Chronic treatment with orally-safe drug candidates such as plecanatide or dolcanatide could be useful as maintenance therapy to delay the onset of IBD to colon carcinogenesis. In this regard, we recently reported that oral treatment with plecanatide considerably reduced colonic dysplasia in DSS treated Apcmin/+FCCC mice[44].
Although the potency of plecanatide to stimulate cGMP in T84 cells, and to ameliorate colitis in animal studies was comparable to that of dolcanatide, the latter drug candidate was advanced further for clinical development because of its enhanced stability against proteolysis in simulated intestinal fluid[23]. The non-clinical safety and toxicology studies conducted in rodents and monkeys suggest that dolcanatide is an orally-safe and minimally absorbed drug candidate. Synergy Pharmaceuticals Inc. has successfully completed Phase I single-ascending-dose and multiple ascending dose safety studies with dolcanatide in healthy volunteers. The drug candidate is well-tolerated and is currently being further evaluated as an orally safe and mucosally-active drug candidate for treatment in patients with ulcerative colitis.
ACKNOWLEDGMENTS
Authors thank Dr. Melvin Spigelman, Dr. Alan Joslyn, Dr. E Priya Eddy for their constructive suggestions and Sue Nagele for assisting in the preparation of the manuscript.
COMMENTS
Background
There is an unmet need to develop a safer and effective therapeutic intervention for inflammatory bowel diseases (IBD). Existing therapies are of limited effectiveness and often associated with side effects. Biologic injectables are costly and effective in less than 40% of patients, with a potential for systemic side effects. An important conceptual advance would be to develop drugs that act locally at the site of inflammation to maximize efficacy and minimize systemic side effects. Plecanatide and dolcanatide are analogs of the human endogenous peptide uroguanylin (UG), a guanylate cyclase-C (GC-C) agonist that regulates fluid/ion and epithelial cell homeostasis and maintains the barrier function within the gastrointestinal (GI) tract. Given its multitude of functions, GC-C agonists are potentially useful therapeutic candidates for treating IBD.
Research frontiers
Therapeutic intervention with locally acting, minimally absorbed analogs of UG, an endogenous natriuretic peptide, represents a novel and safe approach for treating IBD. This therapy could potentially be useful as a maintenance therapy to delay the onset of IBD into colon carcinogenesis.
Innovations and breakthroughs
This is the first report highlighting the therapeutic potential of orally administered and mucosally active GC-C agonists for treating inflammatory bowel diseases in humans.
Applications
UG is an endogenous peptide hormone that regulates fluid/ion homeostasis and epithelial cell homeostasis and maintains the barrier function within the GI tract. Several studies have demonstrated that transcript levels of UG and related peptide guanylin are markedly reduced in inflamed colonic tissues from ulcerative colitis and Crohn’s patients, as well as in human colonic polyps and tumors, implying that the pathogenesis of these diseases might be associated with the deficiency of UG and guanylin. Oral therapy with UG analogs, therefore, could be considered as a replacement therapy to overcome the deficiency underlying the etiology of IBD.
Peer-review
The manuscript presents some points of concern, and additional experiments should be performed, particularly regarding the evaluation of cyclic guanosine monophosphate production in intestinal mucosa. Nevertheless, the manuscript displays novel findings, which sustain the possible use of GC-C agonists for the treatment of IBDs.
Footnotes
Institutional review board statement: Experiments reported in this manuscript did not involve human samples and hence institutional review is not applicable.
Institutional animal care and use committee statement: Studies employing BALB/c and TCRα-/- mice were performed under the direct supervision of Doctor Scott Plevy at the University of Pittsburgh School of Medicine (Pittsburg, PA). Animals obtained from Jackson Laboratories (Bar Harbor, ME) were housed in accordance with guidelines from the American Association for Laboratory Animal Care and Research. Institutional Animal Care and Use Committee of the University of Pittsburgh approved all protocols. Epistem Ltd (Manchester, United Kingdom) conducted DSS and TNBS-induced colitis studies employing BDF1 mice. Animals obtained from Harlan Laboratories, United Kingdom, were housed individually in ventilated cages in a specific pathogen-free barrier unit in compliance with animal welfare regulations. All procedures were certified according to the United Kingdom Home Office (Animal Procedures) Act 1986.
Conflict-of-interest statement: Shailubhai K, Foss JA, Comiskey S, Palejwala V and Jacob GS are employees of Synergy Pharmaceuticals. Nefsky B, Arjunan KP, Saykhedkar S have no conflict of interest. Plevy SE received compensation as a consultant from Synergy Pharmaceuticals Inc.
Data sharing statement: Authors agree to share the raw data included in the manuscript with other researchers.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 21, 2015
First decision: July 1, 2015
Article in press: August 14, 2015
P- Reviewer: Fornai M S- Editor: Ji FF
L- Editor: A E- Editor: Li D
References
- 1.Loftus EV, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:1–20. doi: 10.1016/s0889-8553(01)00002-4. [DOI] [PubMed] [Google Scholar]
- 2.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn WJ, Targan SR. Biologic therapy of inflammatory bowel disease. Gastroenterology. 2002;122:1592–1608. doi: 10.1053/gast.2002.33426. [DOI] [PubMed] [Google Scholar]
- 4.Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 5.Ross AS, Cohen RD. Medical therapy for ulcerative colitis: the state of the art and beyond. Curr Gastroenterol Rep. 2004;6:488–495. doi: 10.1007/s11894-004-0071-9. [DOI] [PubMed] [Google Scholar]
- 6.Engel MA, Neurath MF. New pathophysiological insights and modern treatment of IBD. J Gastroenterol. 2010;45:571–583. doi: 10.1007/s00535-010-0219-3. [DOI] [PubMed] [Google Scholar]
- 7.London RM, Krause WJ, Fan X, Eber SL, Forte LR. Signal transduction pathways via guanylin and uroguanylin in stomach and intestine. Am J Physiol. 1997;273:G93–105. doi: 10.1152/ajpgi.1997.273.1.G93. [DOI] [PubMed] [Google Scholar]
- 8.Forte LR. Uroguanylin and guanylin peptides: pharmacology and experimental therapeutics. Pharmacol Ther. 2004;104:137–162. doi: 10.1016/j.pharmthera.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Basu N, Arshad N, Visweswariah SS. Receptor guanylyl cyclase C (GC-C): regulation and signal transduction. Mol Cell Biochem. 2010;334:67–80. doi: 10.1007/s11010-009-0324-x. [DOI] [PubMed] [Google Scholar]
- 10.Shailubhai K, Yu HH, Karunanandaa K, Wang JY, Eber SL, Wang Y, Joo NS, Kim HD, Miedema BW, Abbas SZ, et al. Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer Res. 2000;60:5151–5157. [PubMed] [Google Scholar]
- 11.Cohen MB, Hawkins JA, Witte DP. Guanylin mRNA expression in human intestine and colorectal adenocarcinoma. Lab Invest. 1998;78:101–108. [PubMed] [Google Scholar]
- 12.Wu F, Dassopoulos T, Cope L, Maitra A, Brant SR, Harris ML, Bayless TM, Parmigiani G, Chakravarti S. Genome-wide gene expression differences in Crohn’s disease and ulcerative colitis from endoscopic pinch biopsies: insights into distinctive pathogenesis. Inflamm Bowel Dis. 2007;13:807–821. doi: 10.1002/ibd.20110. [DOI] [PubMed] [Google Scholar]
- 13.Li P, Lin JE, Chervoneva I, Schulz S, Waldman SA, Pitari GM. Homeostatic control of the crypt-villus axis by the bacterial enterotoxin receptor guanylyl cyclase C restricts the proliferating compartment in intestine. Am J Pathol. 2007;171:1847–1858. doi: 10.2353/ajpath.2007.070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shailubhai K. Therapeutic applications of guanylate cyclase-C receptor agonists. Curr Opin Drug Discov Devel. 2002;5:261–268. [PubMed] [Google Scholar]
- 15.Steinbrecher KA. The multiple roles of guanylate cyclase C, a heat stable enterotoxin receptor. Curr Opin Gastroenterol. 2014;30:1–6. doi: 10.1097/MOG.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 16.Blomain ES, Lin JE, Kraft CL, Trela UT, Rock JM, Aing AS, Snook AE, Waldman SA. Translating colorectal cancer prevention through the guanylyl cyclase C signaling axis. Expert Rev Clin Pharmacol. 2013;6:557–564. doi: 10.1586/17512433.2013.827406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin JE, Snook AE, Li P, Stoecker BA, Kim GW, Magee MS, Garcia AV, Valentino MA, Hyslop T, Schulz S, et al. GUCY2C opposes systemic genotoxic tumorigenesis by regulating AKT-dependent intestinal barrier integrity. PLoS One. 2012;7:e31686. doi: 10.1371/journal.pone.0031686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han X, Mann E, Gilbert S, Guan Y, Steinbrecher KA, Montrose MH, Cohen MB. Loss of guanylyl cyclase C (GCC) signaling leads to dysfunctional intestinal barrier. PLoS One. 2011;6:e16139. doi: 10.1371/journal.pone.0016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harmel-Laws E, Mann EA, Cohen MB, Steinbrecher KA. Guanylate cyclase C deficiency causes severe inflammation in a murine model of spontaneous colitis. PLoS One. 2013;8:e79180. doi: 10.1371/journal.pone.0079180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vellaichamy E, Sommana NK, Pandey KN. Reduced cGMP signaling activates NF-kappaB in hypertrophied hearts of mice lacking natriuretic peptide receptor-A. Biochem Biophys Res Commun. 2005;327:106–111. doi: 10.1016/j.bbrc.2004.11.153. [DOI] [PubMed] [Google Scholar]
- 21.Ladetzki-Baehs K, Keller M, Kiemer AK, Koch E, Zahler S, Wendel A, Vollmar AM. Atrial natriuretic peptide, a regulator of nuclear factor-kappaB activation in vivo. Endocrinology. 2007;148:332–336. doi: 10.1210/en.2006-0935. [DOI] [PubMed] [Google Scholar]
- 22.Das S, Periyasamy R, Pandey KN. Activation of IKK/NF-κB provokes renal inflammatory responses in guanylyl cyclase/natriuretic peptide receptor-A gene-knockout mice. Physiol Genomics. 2012;44:430–442. doi: 10.1152/physiolgenomics.00147.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang G, Arjunan KP, Foss J, Comiskey S, Shailubhai K. SP-333, a proteolysis-resistant agonist of guanylate cyclase-C, inhibits activation of NF-kB and suppresses production of inflammatory cytokines to ameliorate DSS induced colitis in mice (Abstract 1555) Am J Gastroenterol. 2012;107(Supplement 1):S626. [Google Scholar]
- 24.Shailubhai K, Jacob G, inventors; Synergy Pharmaceuticals Inc. assignee. Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders. Patent US 2009/0048175 A1. Supplement 1 2009. [Google Scholar]
- 25.Shailubhai K, Comiskey S, Foss JA, Feng R, Barrow L, Comer GM, Jacob GS. Plecanatide, an oral guanylate cyclase C agonist acting locally in the gastrointestinal tract, is safe and well-tolerated in single doses. Dig Dis Sci. 2013;58:2580–2586. doi: 10.1007/s10620-013-2684-z. [DOI] [PubMed] [Google Scholar]
- 26.Shailubhai K, Jacob GS, inventors; Synergy Pharmaceuticals Inc (New York, NY, US), assignee. Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders. United States patent US 7, 879, 802 B2. 2011. [Google Scholar]
- 27.Davé SH, Tilstra JS, Matsuoka K, Li F, DeMarco RA, Beer-Stolz D, Sepulveda AR, Fink MP, Lotze MT, Plevy SE. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. J Leukoc Biol. 2009;86:633–643. doi: 10.1189/jlb.1008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hegazi RA, Rao KN, Mayle A, Sepulveda AR, Otterbein LE, Plevy SE. Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. J Exp Med. 2005;202:1703–1713. doi: 10.1084/jem.20051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheikh SZ, Hegazi RA, Kobayashi T, Onyiah JC, Russo SM, Matsuoka K, Sepulveda AR, Li F, Otterbein LE, Plevy SE. An anti-inflammatory role for carbon monoxide and heme oxygenase-1 in chronic Th2-mediated murine colitis. J Immunol. 2011;186:5506–5513. doi: 10.4049/jimmunol.1002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamamoto N, Maemura K, Hirata I, Murano M, Sasaki S, Katsu K. Inhibition of dextran sulphate sodium (DSS)-induced colitis in mice by intracolonically administered antibodies against adhesion molecules (endothelial leucocyte adhesion molecule-1 (ELAM-1) or intercellular adhesion molecule-1 (ICAM-1)) Clin Exp Immunol. 1999;117:462–468. doi: 10.1046/j.1365-2249.1999.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berg DJ, Davidson N, Kühn R, Müller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- 33.Friswell MK, Gika H, Stratford IJ, Theodoridis G, Telfer B, Wilson ID, McBain AJ. Site and strain-specific variation in gut microbiota profiles and metabolism in experimental mice. PLoS One. 2010;5:e8584. doi: 10.1371/journal.pone.0008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gill N, Finlay BB. The gut microbiota: challenging immunology. Nat Rev Immunol. 2011;11:636–637. doi: 10.1038/nri3061. [DOI] [PubMed] [Google Scholar]
- 35.Comiskey S, Foss J, Jacob G, Shailubhai K. Orally administered plecanatide, a guanylate cyclase-C agonist, acts in the lumen of the proximal intestine to facilitate normal bowel movement in mice and monkeys (Abstract 1725) Am J Gastroenterol. 2012;107(Supplement 1):S700. [Google Scholar]
- 36.Shailubhai K, Nefsky B, Masih S, Foss J, Comiskey SC, Jacob GS, Plevy SE. Guanylate cyclase C agonists, a new class of drug candidates for treatment of inflammatory bowel disease. Am J Gastroenterol. 2011;S455:1208. [Google Scholar]
- 37.Miner PB, Surowitz R, Fogel R, Koltun W, Drossman DA, Camilleri M, Mangel A, Barrow L, Jacob G, Shailubhai K. Plecanatide, a novel guanylate cyclase-C (GC-C) receptor agonist, is efficacious and safe in patients with chronic idiopathic constipation (CIC): results from a 951 patient, 12 week, multi-center trial (Abstract 925g) Gastroenterology. 2013;144(5, Supplement 1):S–263. [Google Scholar]
- 38.Eastwood GL. Epithelial renewal in premalignant conditions of the gastrointestinal tract: a review. J Clin Gastroenterol. 1992;14 Suppl 1:S29–S33. doi: 10.1097/00004836-199206001-00005. [DOI] [PubMed] [Google Scholar]
- 39.Pitari GM, Li P, Lin JE, Zuzga D, Gibbons AV, Snook AE, Schulz S, Waldman SA. The paracrine hormone hypothesis of colorectal cancer. Clin Pharmacol Ther. 2007;82:441–447. doi: 10.1038/sj.clpt.6100325. [DOI] [PubMed] [Google Scholar]
- 40.Steinbrecher KA, Harmel-Laws E, Garin-Laflam MP, Mann EA, Bezerra LD, Hogan SP, Cohen MB. Murine guanylate cyclase C regulates colonic injury and inflammation. J Immunol. 2011;186:7205–7214. doi: 10.4049/jimmunol.1002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Shailubhai K, Boulete IM, Foss J, Eddy EP, Joshi A, Patwa V, Theodorou V, Palejwala V, Bueno L. Plecanatide and SP-333, Novel Agonists of Guanylate Cyclase-C, Attenuate Visceral Hypersensitivity in Rat Models. Am J Gastroenterol. 2014;109:S532–S532. [Google Scholar]
- 43.Neuman MG. Immune dysfunction in inflammatory bowel disease. Transl Res. 2007;149:173–186. doi: 10.1016/j.trsl.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Shailubhai K, Chang WC, Masih S, Cooper HS, Clapper ML. Enhancement of cyclic GMP production by guanylate cyclase-C agonists delays progression of colitis into colon cancer though downregulation of pro-inflammatory cytokines. Cancer Res. 2012;72:Abstract 1636. [Google Scholar]
- 45.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–1816. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]


