The discovery of mutations in calreticulin (CALR) in patients with primary myelofibrosis (PMF)1, 2 prompted a reappraisal of the clinical correlates and prognostic impact of the so-called driver mutations that include JAK2V617F, MPLW515L/K/A and CALR in ~60%, 5–10% and 20–25% of patients, respectively. As compared with their JAK2V617F counterpart, PMF patients harboring CALR mutations showed younger age, higher platelet and lower hemoglobin and leukocyte counts. The cumulative incidence of anemia, leukocytosis and thrombocytopenia was significantly lower in CALR-mutated patients who were also less likely to be red cell transfusion-dependent;3, 4 in addition, they had significantly longer large splenomegaly-free survival compared with the other genotypes as well as patients lacking the three driver mutations (triple-negative (TN) patients).3, 4 Interestingly, spliceosome mutations were significantly less represented in CALR-mutated patients; however, no additional molecular or cytogenetic correlate was highlighted.3 These data suggested a milder disease in patients harboring the CALR mutation, and conceivably the presence of CALR mutation was associated with better overall survival (OS) when compared with JAK2V617F- and MPLW515-mutated patients, and particularly TN patients.3, 4 In multivariable analysis, CALR mutations had a favorable impact on survival that was independent of International Prognostic Scoring System (IPSS), Dinamic IPSS (DIPSS)4 or DIPSS-plus risk stratification,3 and also of ASXL1 mutation, known for its dismal impact on survival in PMF.5 At this regard, we found that DIPSS-plus-independent OS was significantly longer in CALR-mutated/ASXL1-unmutated compared with CALR-unmutated/ASXL1-mutated patients.6
Two main types of CALR mutations have been described until now, known as type 1 (a 52-bp deletion; p.L367fs*46) and type 2 (a 5-bp TTGTC insertion; p.K385fs*47); more than 50 indels were subsequently reported that can be grouped as type 1- or type 2-like based on their molecular characteristics. The frequency of type 1 mutation is reported to be higher in PMF (60–80%)4, 7 compared with essential thrombocythemia (39–61%),4, 8, 9, 10, 11 a part for a series including Chinese patients where no difference was noticed.12 Interestingly, known mutations generate a novel amino-acid C terminus of the protein with loss of the KDEL motif and replacement of negatively with positively charged or neutral amino acids, whose proportion however varies according to the type of mutation and might underlie emerging differences in the clinical correlates and prognostic impact associated with the two types of mutations. In this regard, it has been shown recently that the phenotype of mice expressing type 1 or type 2 mutation by retroviral transfer differs in that type 1 was associated with marked thrombocytosis and rapid progression to a myelofibrosis-like disease (with subsequent thrombocytopenia, splenomegaly and anemia), whereas type 2 was a milder phenotype with less thrombocytosis and reduced propensity to evolve.13 In a study of 617 PMF patients, of whom 140 were CALR-mutated, we found that patients harboring type 1 CALR mutation (n=101) had a better OS compared with those harboring JAK2V617F mutation (n=399; P<0.001), whereas no difference was observed between CALR type 1 and type 2 (n=22), and as well as between patients with CALR type 2 mutation and those with JAK2V617F; such differences remained also after adjustment for the DIPSS score.4 A significant impact of CALR type 1 versus type 2 was observed by Tefferi et al.7 in a comparison of 76 and 10 CALR-mutated patients, respectively, that also included 196 patients harboring JAK2V617F mutation. Survival was longer in CALR type 1 compared with both CALR type 2 (hazard ratio (HR) 2.5, 95% confidence interval (CI) 1.1–5.4) and JAK2V617F (HR 2.8, 95% CI 1.9–4.2); in multivariable analysis that included DIPSS and ASXL1 mutational status, JAK2V617F versus CALR type 1 mutation, DIPSS and ASXL1 all remained independently predictive of shortened survival. At variance with the above results, a shorter survival associated with CALR type 1 mutation (HR 36.3, 95% CI 4.0–324.7) was reported by Cabagnols et al.11 in a study that included 45 type 1 and 8 type 2 PMF patients. On the other hand, by comparing 98 type-1/type 1-like with 15 type 2/type 2-like CALR-mutated patients, Tefferi et al.14 showed that the median survival of type 1/type 1-like patients (13.7 years) was significantly better than type 2/type 2-like (3.5 years) ones, that on turn had survival superimposable to patients harboring JAK2V617F mutation (4 years). The difference between the two types of CALR mutation remained significant after adjusting for age, ASXL1 or EZH2 mutations.
Owing to the above conflicting results, the aim of this study was to evaluate the prognostic impact, if any, of the two different types of CALR mutations in a series of 396 PMF patients with a diagnosis of PMF made according to the 2008 World Health Organization criteria seen in our center. The mutational status of CALR was determined at diagnosis using previously described procedure of high-resolution capillary electrophoresis of fluorescent dye-labeled PCR amplicon of CALR exon 9;1 samples with abnormal peaks were subjected to conventional bidirectional Sanger sequencing. The mutated CALR allelic burden was estimated by peak area integration from capillary electrophoresis plot. The JAK2V617F mutational status was determined using real-time polymerase chain reaction, as described.15 Comparisons of quantitative variables between groups of patients were carried out using the nonparametric Wilcoxon rank-sum test. The cumulative probability of survival (OS) was estimated using the Kaplan–Meier method. Differences in OS between the groups were compared by using a log-rank test in univariate analysis. The SPSS package (V.22; Chicago, IL, USA) was used for statistical analysis.
Out of the entire series, we found 251 patients (63.4%) harboring JAK2V617F mutation, 21 (5.3%) with a MPLW515 mutation, 50 (12.6%) lacking any known driver mutation and 74 (18.7%) who harbored a CALR mutation, of whom 53 (71.6%) were type 1/type 1-like and 21 (28.4%) were type 2/type 2-like. There was no statistically significant difference between the two groups harboring CALR mutation as regards the various hematologic and clinical variables reported in Table 1. On the other hand, patients harboring both CALR mutation types differed from the JAK2V617F-mutated counterpart for younger age, lower leukocyte and higher platelet counts; males were more represented among Type 1/type 1-like patients than the other genotypes. The risk stratification of the patients was performed using the IPSS score at the time of diagnosis, coincident with the genotyping. There were less IPSS intermediate-2 and high-risk patients in CALR type 1/type 1-like mutational group (20.8%) as compared with type 2/type 2-like (38% P=0.04) and JAK2V617F mutational groups (44.2% P=0.193).
Table 1. Clinical characteristics and outcome of PMF patients stratified according to their CALR type 1/type 1-like, CALR type 2/type 2-like and JAK2V617F mutational status.
| Variables |
CALR mutated |
JAK2V617F mutated | P-value |
||||
|---|---|---|---|---|---|---|---|
| Type 1/1-like | Type 2/2-like | P | CALR versus JAK2V617F | CALR Type 1/1-like versus JAK2V617F | CALR Type 2/2-like versus JAK2V617F | ||
| N (% of total) | 53 (13.4%) | 21 (5.3%) | — | 251 (63.4%) | — | — | — |
| Age in years; median (range) | 53.3 (21–84) | 61.2 (27–82) | 0.210 | 66.7 (28–90) | <0.0001 | <0.0001 | 0.027 |
| Males; n (%) | 25 (47.2%) | 13 (61.9%) | 0.253 | 159 (63.3%) | 0.090 | 0.022 | 0.534 |
| Hemoglobin, g/l; median (range) | 112 (64–140) | 108 (80–150) | 0.958 | 120 (40–175) | 0.637 | 0.362 | 0.704 |
| Leukocytes, × 109/l; median (range) | 7.0 (1.6–18.9) | 7.7 (4.2–18.1) | 0.159 | 10.0 (1.9–96.2) | <0.0001 | <0.0001 | 0.047 |
| Platelets, × 109/l; median (range) | 441.5 (59–1563) | 629.5 (22–1302) | 0.112 | 308.0 (23–2011) | <0.0001 | 0.024 | <0.0001 |
| Circulating blasts ⩾1% n (%) | 6 (12.8%) | 5 (27.8%) | 0.149 | 45 (18.7%) | 0.356 | 0.227 | 0.252 |
| Constitutional symptoms; n (%) | 14 (26.4%) | 4 (19.0%) | 0.505 | 93 (37.1%) | 0.106 | 0.093 | 0.074 |
| Cytogenetic categories; n (%) 'N' evaluable=231 | |||||||
| Abnormal Unfavorable karyotype | 7 (22.6%) | 3 (23.1%) | 0.971 | 45 (30.6%) | 0.267 | 0.286 | 0.171 |
| High/very high | 4 (12.9%) | 2 (15.4%) | 0.827 | 16 (10.9%) | 0.858 | 0.474 | 0.444 |
| IPSS risk group; n (%) | |||||||
| Low | 26 (49.0%) | 9 (43.0%) | 0.170 | 58 (23.1%) | 0.001 | <0.0001 | 0.193 |
| Intermediate-1 | 16 (30.2%) | 4 (19.0%) | 82 (32.7%) | ||||
| Intermediate-2 | 9 (17.0%) | 4 (19.0%) | 56 (22.3%) | ||||
| High | 2 (3.8%) | 4 (19.0%) | 55 (21.9%) | ||||
| Progression to leukemia; n (%) | 3 (5.8%) | 4 (19.0%) | 0.08 | 17 (6.8%) | 0.105 | 0.540 | 0.066 |
| Death; n (%) | 9 (17.0%) | 10 (47.6%) | 0.007 | 84 (33.5%) | 0.017 | 0.011 | 0.142 |
Abbreviation: PMF, primary myelofibrosis. Bold values denote statistically significant difference.
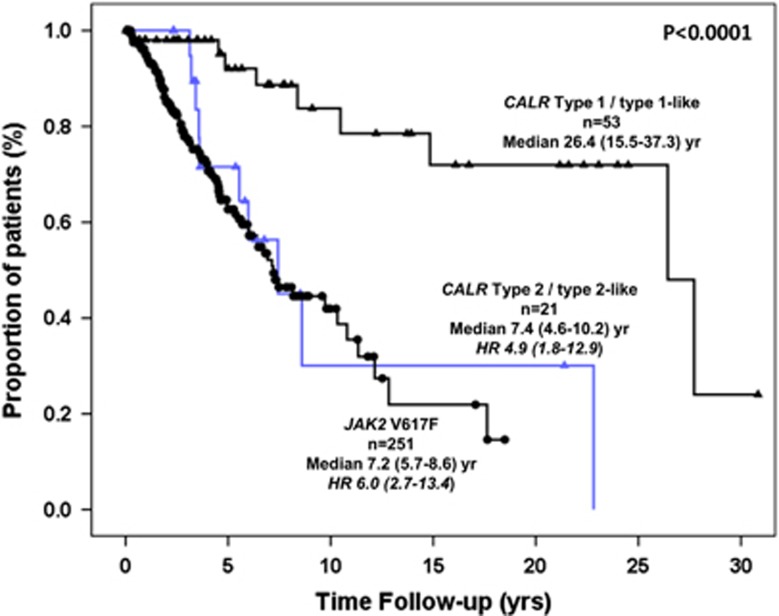
One hundred three patients died (26.0% of total), accounting for 17%, 47.6% and 33.5% of the patients harboring CALR type 1/type 1-like, CALR type 2/type 2-like and JAK2V617F mutation, respectively. The difference was statistically significant for the comparison of type 1/type 1-like versus both type 2/type 2-like (P=0.007) and JAK2V617F (P=0.011), unlike for type 2/type 2-like versus JAK2V617F mutation (P=0.142). Kaplan–Meier estimates of survival are shown in Figure 1. The median survival of patients harboring CALR type 1/type 1-like mutation was 26.4 years (range, 15.5–37.3) versus 7.4 years (4.6–10.2) for CALR type 2/type 2-like and 7.2 years (5.7–8.6) for JAK2V617F. The difference between type 1/type 1-like and the other two groups was highly significant (P<0.0001). The corresponding HR (95% CIs), taking the CALR type 1/type 1-like group as the reference, was 4.9 (95% CI, 1.8–12.9) and 6.0 (95% CI, 2.7–13.4) for CALR type 2/type 2-like and JAK2V617F-mutated patients, respectively. Survival of patients with CALR type 2/type 2-like mutation did not differ from MPLW515-mutated patients (n=21; median survival 14.7 years; HR 6.0, 95% CI, 2.2–16.3), whereas, as previously reported, the worst survival (median, 2.0 years; 1.6–2.4) was observed in TN patients (HR 20.6, 95% CI, 8.9–48.4). In a multivariable Cox proportional hazard regression model considering type of mutation (CALR type 1/1-like, CALR type 2/2-like and JAK2V617F) and the IPSS score, CALR type 2/type 2-like (HR 4.4, 95% CI 1.6–11.7) and JAK2V617F (HR 3.8, 95% CI 1.7–8.7) retained significant IPSS-independent prognostic impact on survival. Finally, there were more leukemia transformations in CALR type 2/type 2-like mutational group than in type 1/type 1-like (19 versus 5.7%) and JAK2V617F (6.8%) one; however, the low number of events warrants caution in the interpretation of the data.
Figure 1.
Survival data of PMF patients stratified according to their CALR type 1/type 1-like, CALR type 2/type 2-like and JAK2V617F mutational status.
In summary, our findings support previous report that the prognostic advantage of CALR mutation in PMF regards only patients harboring type 1/type 1-like mutation, as the survival of those harboring type 2/type 2-like mutation does not differ from JAK2V617F-mutated patients. However, even this study, although including the highest number of patients to date, suffers from a relatively small number of CALR type 2/type 2-like mutated cases, which might hamper a clean understanding of the relevance of such mutational status for survival; furthermore, the mutational landscape of PMF is highly complex because of the occurrence of several other subclonal mutations that exert a strong prognostic impact, which should be taken into an account. Therefore, larger patient series and inclusion of as many as possible mutational data in a multivariable model are needed before such information can be translated to the clinical practice to inform therapy.
Acknowledgments
This work was supported by a grant from Associazione Italiana per la Ricerca sul Cancro (AIRC; Milan, Italy), Special Program Molecular Clinical Oncology 5 × 1000 to AIRC-Gruppo Italiano Malattie Mieloproliferative (AGIMM) project #1005; grant #F11J11000250001 from Fondo per gli Investimenti della Ricerca di Base (FIRB); Progetti di ricerca giovani ricercatori, Ricerca Finalizzata 2011–2012 #GR-2011-02352109; and Progetto AIRC Investigator Grant 2014 Id.15967.
The authors declare no conflict of interest.
References
- 1Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. New Engl J Med 2013; 369: 2379–2390. [DOI] [PubMed] [Google Scholar]
- 2Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. New Engl J Med 2013; 369: 2391–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Tefferi A, Lasho TL, Finke CM, Knudson RA, Ketterling R, Hanson CH et al. CALR vs JAK2 vs MPL mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia 2014; 28: 1472–1477. [DOI] [PubMed] [Google Scholar]
- 4Rumi E, Pietra D, Pascutto C, Guglielmelli P, Martínez-Trillos A, Casetti I et al. Clinical effect of driver mutations of JAK2, CALR or MPL in primary myelofibrosis. Blood 2014; 124: 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Vannucchi AM, Lasho TL, Guglielmelli P, Biamonte F, Pardanani A, Pereira A et al. Mutations and prognosis in primary myelofibrosis. Leukemia 2013; 27: 1861–1869. [DOI] [PubMed] [Google Scholar]
- 6Tefferi A, Guglielmelli P, Lasho TL, Rotunno G, Finke C, Mannarelli C et al. CALR and ASXL1 mutations-based molecular prognostication in primary myelofibrosis: an international study of 570 patients. Leukemia 2014; 28: 1494–1500. [DOI] [PubMed] [Google Scholar]
- 7Tefferi A, Lasho TL, Finke C, Belachew AA, Wassie EA, Ketterling RP et al. Type 1 vs type 2 calreticulin mutations in primary myelofibrosis: differences in phenotype and prognostic impact. Leukemia 2014; 28: 1568–1570. [DOI] [PubMed] [Google Scholar]
- 8Tefferi A, Wassie EA, Guglielmelli P, Gangat N, Belachew AA, Lasho TL et al. Type 1 vs Type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol 2014; 28: 1568–1570. [DOI] [PubMed] [Google Scholar]
- 9Rumi E, Pietra D, Ferretti V, Klampfl T, Harutyunyan AS, Milosevic JD et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood 2014; 123: 1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Rotunno G, Mannarelli C, Guglielmelli P, Pacilli A, Pancrazzi A, Pieri L et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood 2014; 123: 1552–1555. [DOI] [PubMed] [Google Scholar]
- 11Cabagnols X, Defour JP, Ugo V, Ianotto JC, Mossuz P, Mondet J et al. Differential association of calreticulin type 1 and type 2 mutations with myelofibrosis and essential thrombocytemia: relevance for disease evolution. Leukemia 2015; 29: 249–252. [DOI] [PubMed] [Google Scholar]
- 12Qiao C, Sun C, Ouyang Y, Wang JJ, Qian SX, Li JY et al. Clinical importance of different calreticulin gene mutation types in wild-type JAK2 essential thrombocythemia and myelofibrosis patients. Haematologica 2014; 99: e182–e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Marty C, Harini N, Pecquet C, Chachoua I, Gryshkova V, Villeval J-L et al. Calr mutants retroviral mouse models lead to a myeloproliferative neoplasm mimicking an essential thrombocythemia progressing to a myelofibrosis. Blood 2014; 124: 157A.25013158 [Google Scholar]
- 14Tefferi A, Lasho TL, Tischer A, Wassie EA, Finke CM, Belachew AA et al. The prognostic advantage of calreticulin mutations in myelofibrosis might be confined to type 1 or type 1-like CALR variants. Blood 2014; 124: 2465–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Jovanovic JV, Ivey A, Vannucchi AM, Lippert E, Oppliger Leibundgut E, Cassinat B et al. Establishing optimal quantitative-polymerase chain reaction assays for routine diagnosis and tracking of minimal residual disease in JAK2-V617F-associated myeloproliferative neoplasms: a joint European LeukemiaNet/MPN&MPNr-EuroNet (COST action BM0902) study. Leukemia 2013; 27: 2032–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]