Abstract
Vasoconstrictive and vasodilatory eicosanoids generated after cardiac arrest (CA) may contribute to cerebral vasomotor disturbances and neurodegeneration. We evaluated the balance of vasodilator/vasoconstrictor eicosanoids produced by cytochrome P450 (CYP) metabolism, and determined their role on cortical perfusion, functional outcome, and neurodegeneration after pediatric asphyxial CA. Cardiac arrest of 9 and 12 minutes was induced in 16- to 18-day-old rats. At 5 and 120 minutes after CA, we quantified the concentration of CYP eicosanoids in the cortex and subcortical areas. In separate rats, we inhibited 20-hydroxyeicosatetraenoic acid (20-HETE) synthesis after CA and assessed cortical cerebral blood flow (CBF), neurologic deficit score, neurodegeneration, and edema. After 9 minutes of CA, vasodilator eicosanoids markedly increased versus sham. Conversely, after 12 minutes of CA, vasoconstrictor eicosanoid 20-HETE increased versus sham, without compensatory increases in vasodilator eicosanoids. Inhibition of 20-HETE synthesis after 12 minutes of CA decreased cortical 20-HETE levels, increased CBF, reduced neurologic deficits at 3 hours, and reduced neurodegeneration and edema at 48 hours versus vehicle-treated rats. In conclusion, cerebral vasoconstrictor eicosanoids increased after a pediatric CA of 12 minutes. Inhibition of 20-HETE synthesis improved cortical perfusion and short-term neurologic outcome. These results suggest that alterations in CYP eicosanoids have a role in cerebral hypoperfusion and neurodegeneration after CA and may represent important therapeutic targets.
Keywords: arachidonic acid, cerebral blood flow, cardiac arrest, eicosanoids, HET0016, neuroprotection, 20-HETE
Introduction
One of the most common causes of brain injury in children is asphyxial cardiac arrest (CA), resulting in largely unfavorable outcomes.1 Global cerebral ischemia during CA progressively changes the cellular milieu and physiology, rendering the cells susceptible to further damage after reperfusion. Cerebral blood flow dysregulation is commonly observed after cardiopulmonary resuscitation from CA, and may produce secondary neuronal damage in the context of impaired cerebrovascular autoregulation.2, 3, 4 Several studies suggested that cerebral blood flow (CBF) targeted therapies after CA may improve neurologic outcome.5, 6, 7
Our laboratory characterized the regional CBF after CA of moderate and severe durations (9 and 12 minutes of asphyxia, respectively) in postnatal day 16 to 18 rats using arterial spin label magnetic resonance imaging. After pediatric asphyxial CA in our model, CBF alterations are region specific and cerebral perfusion decreases progressively with increasing durations of CA. Cortical areas are characterized by early hypoperfusion, progressing in severity with increased duration of CA, whereas subcortical areas are characterized by early hyperemia after 9 minutes of asphyxia, and the absence of hyperemia after 12 minutes of asphyxia.8 Cerebral blood flow-directed therapies improved outcome in our model.7 Thus, CBF dysregulation after pediatric asphyxial CA is a potential important target for novel therapies.
Among the most potent vasoactive compounds found in the brain are bioactive metabolites of arachidonic acid formed by cytochrome P450 (CYP) mono-oxygenase enzymes: hydroxyeicosatetraenoic acids (HETEs) and epoxyeicosatrienoic acids (EETs).9 Hydroxyeicosatetraenoic acid metabolites, 20-HETE being the most potent vasoactive mediator, are important vasoconstrictors of the microvasculature.10, 11, 12 Conversely, EET metabolites are important vasodilators.13, 14 A normal balance of vasodilators/vasoconstrictors (EETs/HETEs) regulates vascular tone under physiologic conditions. Under conditions of cerebral ischemia, this homeostasis is disrupted, with resulting detrimental consequences on the vasculature and neurons.15 Eicosanoids are also suggested to have direct neuronal effects: inhibition of 20-HETE formation may be neuroprotective, whereas increasing EETs by inhibiting their degradation to dihydroxyeicosatrienoic acids (DHETEs) may be neuroprotective.16, 17, 18
Based on the potent vascular effects of CYP eicosanoids, our primary objective was to determine if imbalances of vasoconstrictor and vasodilator CYP eicosanoids are associated with decreased cortical blood flow in a model of pediatric asphyxial CA in 16- to 18-day-old rats. Our secondary objective was to determine whether inhibiting the synthesis of vasoconstrictor 20-HETE improves cortical perfusion, functional outcome, and neurodegeneration, and decreases brain edema after pediatric asphyxial CA.
Materials and Methods
All animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Animals and Experimental Design
Postnatal day 16 to 18 Sprague–Dawley male rats (30 to 45 g) obtained from Harlan Laboratories (Indianapolis, IN, USA) were used. In one cohort of rats, we quantified a panel of eicosanoids produced by CYP metabolism of arachidonic acid after either 9 or 12 minutes of asphyxial CA (n=6 to 8 per group). In a separate cohort of rats, we inhibited the formation of 20-HETE after 12 minutes of CA and assessed the effect of this inhibition on eicosanoid levels (n=6 to 9 per group), CBF, functional outcome, and neurodegeneration (n=6 per group), and brain tissue water (n=4 to 5 per group).
Asphyxial Cardiac Arrest in Immature Rats
Postnatal day 16 to 18 rats underwent 9 or 12 minutes of asphyxial CA (moderate or severe duration, respectively), followed by resuscitation as described previously.19 Briefly, the rats were initially anesthetized with 3% isoflurane, 50/50 N2O/O2 in a Plexiglas chamber until unconscious, and then the trachea was intubated with an 18-gauge angiocatheter. Mechanical ventilation was initiated, and the PaCo2(partial pressure of arterial carbon dioxide) was maintained between 35 and 45 mm Hg by adjusting the ventilation rate and the tidal volumes. Anesthesia was then reduced to 2.5% isoflurane and 50/50 N2O/O2. Femoral arterial and venous catheters were placed, and intravenous analgesia and neuromuscular blockade were initiated using fentanyl infusion at 50 μg/kg per hour and vecuronium infusion at 5 mg/kg per hour, respectively. Inhalational anesthesia was discontinued to preclude any CBF effects of isoflurane. Baseline CBF recordings were obtained 30 minutes after isoflurane was discontinued. Asphyxial CA was induced by disconnecting the tracheal tube from the ventilator for a duration of 9 or 12 minutes. Resuscitation was initiated by reconnecting the ventilator, 100% oxygen, infusion of epinephrine (0.005 mg/kg) and sodium bicarbonate (1 mEq/kg), and manual chest compressions until return of spontaneous circulation (ROSC). Anesthesia and neuromuscular blockade (using fentanyl and vecuronium) were restarted at 30 minutes after ROSC and continued until the end of the experiment. Mean arterial blood pressure (MAP) and heart rate were continuously monitored via femoral arterial and venous catheters. Arterial blood gases were measured at baseline and at 10, 30, and 60 minutes after ROSC. The oxygen concentration was reduced to 50% at 1 hour after CA. Rectal temperature was continuously monitored and maintained at 37 °C via a heated water blanket system throughout the surgery. Sham control rats underwent anesthesia and surgery without asphyxial CA or resuscitation.
Chromatographic Analysis of CYP Eicosanoids
In the first cohort of rats, we quantified the concentration of CYP eicosanoids in the cortex and subcortical region at 5 or 120 minutes after CA of 9 or 12 minutes and sham surgery. In the second cohort of rats, we quantified the concentration of CYP eicosanoids in the cortex and subcortical region at 5 minutes after the administration of an inhibitor of 20-HETE formation, HET0016 (N-hydroxy-N'-(4-n-butyl-2-methylphenyl)formamidine), at resuscitation from 12 minutes of CA.
We measured the following eicosanoids: HETEs (12-, 15-, and 20-HETE), EETs (8,9-, 11,12-, and 14,15-EET), and dihydroxyeicosatrienoic acids (DHETs) (5,6-, 8,9-, 11,12- and 14,15-DHET) using solid-phase extraction as described previously, with slight modifications.20, 21 Rats were killed by decapitation and the brain tissue was rapidly excised, frozen in liquid nitrogen, and stored at −80 °C until further analysis. The tissue samples were homogenized in deionized water containing 0.113 mmol/L butylated hydroxytoluene and centrifuged for 30 minutes at 10,000 r.p.m. The supernatant was removed and 12.5 ng of 20-HETE-d6 was added as an internal standard. Supernatant samples were then loaded onto Oasis hydrophilic–lipophilic balanced (30 mg) solid-phase extraction cartridges (Waters, Milford, MA, USA) that were conditioned and equilibrated with 1 mL of methanol and 1 mL of water, respectively. Columns were washed with three 1 mL volumes of 5% methanol and were eluted with 100% methanol. A total of 15 μL of 1% acetic acid in methanol was added to the column eluent, which was then dried under nitrogen gas at 37 °C and subsequently reconstituted in 125 μL of 80:20 methanol/deionized water for chromatographic analysis as described previously.20
Hydroxyeicosatetraenoic acids, EETs, and DHETs were separated on a UPLC BEH C-18 column 1.7 μm (2.1 × 100 mm2) protected by a guard column (2.1 × 5 mm2; Waters) of the same packing material. Column temperature was maintained at 55 ºC. Mobile phases consisted of 0.005% acetic acid, 5% acetonitrile in deionized water (A) and 0.005% acetic acid in acetonitrile (B). Hydroxyeicosatetraenoic acids, EETs, and DHETs were separated at a flow rate of 0.5 mL/minute at an initial mixture of 65:35 A and B, respectively. Mobile phase B was increased from 35 to 70% in a linear gradient over 4 min, and again increased to 95% over 0.5 minutes where it remained for 0.3 minutes. This was followed by a linear return to initial conditions over 0.1 minutes with a 1.5 minute pre-equilibration period before the next sample run. Total run time per sample was 6.4 minutes and injection volume was 7.5 μL.
Mass spectrometric analysis of analyte formation was performed using a TSQ Quantum Ultra (Thermo Fisher Scientific, San Jose, CA, USA) triple quadrupole mass spectrometer coupled with heated electrospray ionization operated in negative selective reaction monitoring mode with unit resolutions at both Q1 and Q3 set at 0.70 full-width at half-maximum. Quantitation by selective reaction monitoring analysis on HETEs, EETs, and DHETs was performed by monitoring their m/z transitions. Scan time was set at 0.01 seconds and collision gas pressure was set at 1.3 mTorr. Analytical data was acquired and analyzed using Xcalibur software version 2.0.6 (Thermo Finnigan, San Jose, CA, USA).
Cerebral Blood Flow Assessment Using Laser Speckle Photometry
Cortical CBF was measured noninvasively using laser speckle photometry (PeriCam PSI system; Perimed, Jäfälla, Sweden). The skull of postnatal day 16 to 18 rats is thin and imaging can be performed through the intact skull after deflecting the scalp.22 Rats were placed into a standard stereotaxic frame and the skull was exposed by a midline scalp incision. The skull was cleaned with normal saline solution to remove any traces of blood. Rats were placed under the PeriCam PSI system, which uses a built-in 70 mW laser diode to illuminate the cortical surface. PeriCam PSI camera was adjusted to a distance of 15 cm and at an angle of 90° to the imaging plane with the laser beam targeting the center of the imaging area. The imaging area was set to 2 × 2 cm2. The perfusion imaging was recorded continuously at a frame rate of 10 images per second and a resolution of 0.14 mm. Cerebral blood flow perfusion maps in the somatosensory cortex were analyzed at baseline and at 5, 10, 30, and 60 minutes after ROSC in vehicle- and HET0016-treated rats and perfusion values were generated using the PIMSoft software (Perimed). Additionally, to examine if the CBF reflow was spatially heterogeneous, we quantified the relative variation of %CBF within each animal using the coefficient of variation (COV). Coefficient of variation was calculated by dividing the mean CBF to the standard deviation of pixels CBF values in the somatosensory cortex. We compared the COV at baseline and after CA in vehicle- and HET0016-treated rats to assess if HET0016 attenuates the increase in spatial variability of CBF.
HET0016 Inhibition of 20-Hydroxyeicosatetraenoic Acid Formation
In the second cohort of rats, we inhibited the formation of 20-HETE after resuscitation from 12 minutes duration of CA, and we assessed the effect of this inhibition on eicosanoid levels, CBF, functional outcome, neurodegeneration (n=6 to 9 per group), and percent brain tissue water (n=4 to 5 per group).
We assessed the effect of 20-HETE inhibition using HET0016 on the following five parameters: (1) CBF after CA; (2) CYP eicosanoid levels in the cortex at 5 minutes after resuscitation; (3) short-term neurologic outcome at 3, 24, and 48 hours after resuscitation from CA; (4) neurodegeneration at 48 hours after CA; and (5) percent brain tissue water at 48 hours after CA. For experiments 1, 2, and 5, a single dose of HET0016 (0.9 mg/kg) was administered intravenously at the time of resuscitation from CA.23 For experiments 3 and 4, multiple doses of HET0016 (0.9 mg/kg) were administered every 6 hours for 24 hours after resuscitation: the first dose was administered intravenously, and the subsequent doses were administered intraperitoneally. All hydroxypropyl β-cyclodextrin lyophilized complexes (vehicle or HET0016) were reconstituted in phosphate-buffered saline (pH=7.2) and filtered before administration. The surgeon and the individuals involved in all above experiments were blinded to the treatment groups. One rat that sustained CA and was treated with vehicle and two rats treated with HET0016 died at 24 to 48 hours after CA.
Functional Outcome Assessment
We assessed the effect of inhibition of 20-HETE generation after 12 minutes of CA on functional outcome using a modified neurologic deficit score (NDS) at 3, 24, and 48 hours after resuscitation (n=6 per group).24 This score evaluates the rats' general condition, cranial nerves, motor, sensory systems, and coordination on a cumulative scale of 0 (best score) to 500 (worst score). General consciousness and the respiration pattern were each graded on a scale of 100 points, for a total of 200 points. Cranial nerve function was assessed by evaluating the corneal reflex, whisker motion to stimulation, the olfactory system, and vision and hearing on a graded scale of 20 points each, for a total of 100 points. Motor activity and sensory response to each paw and tail were recorded on a graded scale of 10 points each for a total of 100 points. Finally, coordination was tested by placing the rat on an inclined plane, and travel ledge traverse, placing, and righting reflex, and stop at the table ledge were each recorded on a graded scale of 25 points, for a total of 100 points. A score of zero indicates no damage, whereas highest score on each of the grading systems indicates severe neurologic damage. We calculated the cumulative scores from all the recordings.
Histologic Assessment
We assessed the effect of inhibition of 20-HETE formation after 12 minutes of CA on neurodegeneration at 48 hours after resuscitation. The cohort of rats used for functional outcome testing were used for quantification of neurodegeneration using hematoxylin and eosin staining. At 48 hours from resuscitation after CA, rats receiving either HET0016 or vehicle treatment were anesthetized with 3% isoflurane and 50% N2O/balance O2 and were perfused with 250 mL of ice-cold heparinized saline and 250 mL of 4% paraformaldehyde. The brains were removed and fixed in 4% paraformaldehyde. Coronal sections of paraffin-embedded brains were cut at the level of the parietal cortex at 5 μm using a cryotome. The sections were further processed and stained with hematoxylin and eosin for evaluation of ischemic neuronal changes such as pyknotic neurons. Neurons in the primary motor and sensory parietal cortex and in the thalamus that exhibited neurodegeneration were counted by an observer blinded to the experimental conditions. Neurons were counted in one brain section per rat and six contiguous fields per section.
Assessment of Percent Brain Water
We assessed the change in percent brain water using the brain wet-to-dry weight ratio at 3 hours in the following groups: sham surgery, 12 minutes of CA, and 12 minutes of CA treated with HET0016 (n=4 to 5 per group). Cerebral wet weight was measured immediately with a precision scale. Cerebral dry weight was determined after the brain was dried for 48 hours in a 100 °C oven. Cerebral tissue water content was calculated according to the formula: percent water=(1−dry weight/wet weight)x100.
Statistical Analysis
Data were analyzed with the statistical package Systat software (Systat, San Jose, CA, USA). Eicosanoid levels were analyzed using analysis of variance with Dunnett's posttest when data were normally distributed and Kruskal–Wallis analysis of variance on ranks for non-normally distributed data or if the equal variance assumption was violated. To compare the eicosanoid levels and neurodegeneration in vehicle- versus HET0016-treated rats, we used the Student's t-test for normally distributed data and the Wilcoxon's signed-rank test for non-normally distributed data. Cerebral blood flow changes after resuscitation, NDS scores, and physiologic variables were analyzed using repeated-measures analysis of variance. We calculated that sample size of n=6 per group was needed to achieve 80% power at α=0.05 to detect 3 pmoL/g difference in 20-HETE with a standard deviation equal to 1.5. To detect a difference of 20% in CBF between groups with a standard deviation equal to 10, 80% power at α=0.05, a sample size of n=6 per group was needed. This is also in agreement with our previous studies.8 A P<0.05 was considered significant. All data were represented as mean±s.e.m.
Results
Duration-Dependent Changes of Eicosanoids After Pediatric Asphyxial Cardiac Arrest
Vasoconstrictive eicosanoid 20-hydroxyeicosatetraenoic acids increases in the cortex and subcortical region after a severe duration of pediatric asphyxial cardiac arrest
Early postresuscitation, the cortical levels of 20-HETE were similar to shams after moderate duration of CA (6.79±1.40 versus 3.78±0.41 pmoL/g tissue, 9 minutes versus sham, P=0.1) and increased versus sham after severe durations of CA (12.8±2.78 versus 3.78±0.41 pmoL/g tissue, 12 minutes versus sham, P<0.05). At a delayed time point of 120 minutes after resuscitation from both moderate and severe CA, the cortical levels of 20-HETE were similar to shams (Table 1a).
Table 1. Levels of eicosanoids produced by CYP metabolism in the (a) cortex and (b) subcortical region at 5 and 120 minutes after resuscitation from 9 and 12 minutes of CA.
| Analyte | Sham |
CA (9 minutes) |
CA (12 minutes) |
||
|---|---|---|---|---|---|
| ROSC (minutes) | 5 | 120 | 5 | 120 | |
| (a) Cortex | |||||
| 20-HETE | 3.78±0.41 | 6.79±1.40 | 4.14±0.55 | 12.8±2.78a | 5.35±0.59 |
| 15-HETE | 173±27.2 | 143±19.2 | 291±51.1a | 211±31.5 | 171±61.6 |
| 12-HETE | 1649±366 | 793±61 | 1379±191 | 1732±275 | 1453±478 |
| 8,9-EET | 2.66±0.34 | 39.3±9.32a | 15.9±6.64a | 2.74±0.26 | 2.81±0.56 |
| 11,12-EET | 11.8±4.33 | 20.9±5.2 | 13.9±4.9 | 3.79±0.32 | 5.18±0.87 |
| 14,15-EET | 7.43±2.86 | 44.6±13.3a | 17.8±6.76a | 5.78±1.66 | 3.86±0.62 |
| 5,6-DHETE | 0.63±0.05 | 1.10±0.13 | 0.82±0.07 | 0.84±0.16 | 0.91±0.16 |
| 8,9-DHETE | 0.86±0.08 | 1.31±0.21a | 0.95±0.08 | 1.03±0.15 | 0.81±0.11 |
| 11,12-DHETE | 1.29±0.22 | 2.23±0.57 | 1.18±0.17 | 1.90±0.13 | 1.34±0.44 |
| 14,15-DHETE | 1.41±0.32 | 4.17±0.73a | 1.36±0.34 | 3.39±0.73a | 1.38±0.38 |
| (b) Subcortical region | |||||
| 20-HETE | 4.58±0.87 | 6.34±1.0 | 5.05±0.57 | 7.75±0.79a | 6.30±1.22 |
| 15-HETE | 154±14.3 | 258±39.1 | 375±44a | 144±20.4 | 119±35.3 |
| 12-HETE | 1440±170 | 974±79 | 1263±346 | 1207±173 | 1141±307 |
| 8,9-EET | 2.31±0.51 | 80.3±21.1a | 41.1±7.93a | 3.27±0.61 | 3.05±0.46 |
| 11,12-EET | 20.2±7.71 | 51.4±15.1 | 35.1±6.64 | 4.48±0.87 | 3.66±0.98 |
| 14,15-EET | 14.6±6.01 | 46.5±12.8a | 33.4±5.70a | 4.19±0.74 | 2.53±0.25 |
| 5,6-DHETE | 1.11±0.13 | 1.88±0.30 | 1.33±0.22 | 1.32±0.12 | 1.15±0.13 |
| 8,9-DHETE | 1.59±0.29 | 4.14±0.89a | 3.20±0.51 | 1.18±0.19 | 1.13±0.04 |
| 11,12-DHETE | 1.89±0.55 | 2.94±0.56 | 4.07±0.91 | 1.42±0.25 | 1.32±0.06 |
| 14,15-DHETE | 2.56±0.26 | 2.40±0.24 | 2.79±0.47 | 2.32±0.35 | 1.71±0.54 |
Abbreviations: CA, cardiac arrest; CYP, cytochrome P450; EET, epoxyeicosatrienoic acid; DHETE, dihydroxyeicosatrienoic acid; HET0016, N-hydroxy-N'-(4-n-butyl-2-methylphenyl)formamidine; HETE, hydroxyeicosatetraenoic acid; ROSC, return of spontaneous circulation.
Eicosanoids were quantified in shams and at 5 and 120 minutes after CA, and were reported as pmoL/g tissue. Metabolites from the entire CYP pathway were quantified. Vasoactive metabolites include 20-HETE (vasoconstrictor) and EETs (vasodilators). DHETEs are products of EET degradation by the soluble epoxide hydrolase.
P<0.05 versus sham.
20-Hydroxyeicosatetraenoic acids levels were also increased in the subcortical region at an early time point after severe CA (7.75±0.79 versus 4.58±0.87 pmoL/g, 12 minutes versus sham, P<0.05), and were similar to shams at a delayed time point after both moderate and severe insults (Table 1b).
Vasodilator eicosanoids increase in cortex and subcortical region after moderate duration of pediatric asphyxial cardiac arrest
Cortical levels of vasodilator eicosanoids 8,9-EET and 14,15-EET increased after resuscitation from moderate (9 minutes) CA versus sham (Table 1a). The increase in vasodilator eicosanoids in the cortex after moderate CA was observed at both early and delayed time points (P<0.05 versus sham). In contrast, after severe (12 minutes) CA, there were no increases in the levels of cortical vasodilator eicosanoids compared with shams.
In the subcortical region, the levels of vasodilator eicosanoids followed a similar trend as cortical levels: increased after resuscitation from moderate CA durations at both early and delayed time points, but displayed no increases after severe CA (Table 1b). The levels of respective DHETE, degradation products of EET, were largely similar to shams.
Quantification of Cortical Perfusion Using Laser Speckle After Pediatric Asphyxial Cardiac Arrest
In a previous study, we measured regional CBF after moderate and severe durations of CA using arterial spin label magnetic resonance imaging and found that cortical hypoperfusion was more severe with an increase in the duration of the insult, whereas the subcortical hyperemia was present only after moderate insults.8 In this study, we quantified cortical perfusion continuously at baseline and during the first 120 minutes after CA using the noninvasive method of laser speckle technology. Cortical perfusion measured with laser speckle technology paralleled cortical CBF changes measured by arterial spin label magnetic resonance imaging. The early decrease in the cortical perfusion was more pronounced in the severe duration of CA (86.3±1.2% versus 77.8±1.7% of baseline, 9 versus 12 minutes CA, P<0.05). During the late reperfusion period, CBF was decreased in both durations of CA (64.4±2.7% and 55.4±1.8% for 9 and 12 minutes of CA, respectively, P<0.05 versus baseline).
Inhibition of 20-Hydroxyeicosatetraenoic Acid Formation with HET0016 Inhibits the Reduction in Cortical Perfusion Typically seen After Pediatric Cardiac Arrest
Our evaluation of eicosanoid dysregulation after CA suggests that after severe CA, there is a predominance of the vasoconstrictor 20-HETE, whereas at the same time cortical perfusion is markedly decreased. We evaluated the effect of inhibiting 20-HETE formation using HET0016 on eicosanoid levels, physiologic parameters, cerebral perfusion, functional outcome, neurodegeneration, and percent brain tissue water.
Administration of HET0016 at resuscitation from 12 minutes of CA reduced the 20-HETE levels versus vehicle in the cortex and subcortical region at 5 minutes after resuscitation (8.06±1.33 versus 1.99±0.20 pmoL/g tissue, cortex, vehicle versus HET0016 treatment, P<0.001, and 6.44±0.89 versus 3.18±0.22 pmoL/g tissue, subcortical, vehicle versus HET0016 treatment, P<0.05). To assure that eicosanoid metabolites did not shift toward other pathways after inhibition of 20-HETE formation, we measured the entire panel of CYP metabolites of arachidonic acid after HET0016 administration and compared them with vehicle-treated rats. There were no differences in other CYP eicosanoids in the cortex or subcortical region, confirming the specificity of HET0016 on 20-HETE inhibition (Table 2).
Table 2. Effect of acute HET0016 treatment on eicosanoid levels in the cortex and subcortical areas at 5 minutes after ROSC from 12 minutes of CA.
| Analyte |
CA (12 minutes) |
|||
|---|---|---|---|---|
|
Cortex |
Subcortical region |
|||
| Vehicle | HET0016 | Vehicle | HET0016 | |
| 20-HETE | 8.06±1.33 | 1.99±0.20a | 6.44±0.89 | 3.18±0.22a |
| 15-HETE | 473±118 | 352±42.2 | 495±125 | 336±78 |
| 12-HETE | 3698±725 | 2953±251 | 3932±965 | 2753±539 |
| 8,9-EET | 6.24±1.03 | 5.66±1.02 | 5.62±0.76 | 6.82±0.84 |
| 11,12-EET | 4.32±0.57 | 4.39±0.41 | 3.56±0.40 | 4.70±0.63 |
| 14,15-EET | 6.93±0.93 | 6.55±0.76 | 5.65±0.83 | 6.64±0.52 |
| 5,6-DHETE | 0.64±0.09 | 0.67±0.05 | 0.98±0.07 | 0.93±0.09 |
| 8,9-DHETE | 1.89±0.25 | 2.00±0.29 | 2.56±0.28 | 2.08±0.36 |
| 11,12-DHETE | 1.82±0.13 | 1.54±0.13 | 2.09±0.17 | 2.00±0.18 |
| 14,15-DHETE | 2.88±0.42 | 2.15±0.16 | 3.09±0.16 | 2.29±0.08 |
Abbreviations: CA, cardiac arrest; EET, epoxyeicosatrienoic acid; DHETE, dihydroxyeicosatrienoic acid; HET0016, N-hydroxy-N'-(4-n-butyl-2-methylphenyl)formamidine; HETE, hydroxyeicosatetraenoic acid; ROSC, return of spontaneous circulation.
Eicosanoid levels (pmoL/g tissue) at 5 minutes after ROSC were compared with the vehicle-treated group. HET0016 inhibited 20-HETE levels in the cortex and subcortical region at 5 minutes after ROSC compared with vehicle treatment and did not change the levels of other metabolites, indicating the specificity of HET0016 inhibition for 20-HETE.
P<0.05 HET0016 versus vehicle.
A summary of the physiologic variables at baseline and postresuscitation in rats treated with either vehicle or HET0016 is presented in Table 3. There were no differences in MAP, PaCo2, Pao2(partial pressure of arterial oxygen), or pH between vehicle- and HET0016-treated rats.
Table 3. Physiologic variables at baseline and after resuscitation from 12 minutes of CA in rats treated with HET0016 or vehicle.
| Baseline | 10 Minutes | 30 Minutes | 60 Minutes | |
|---|---|---|---|---|
| MAP | ||||
| Vehicle | 72.2±4.72 | 84.7±4.84a | 57.5±3.68a | 69.2±5.2 |
| HET0016 | 71.0±4.2 | 79.0±4.86 | 50.0±2.24a | 58.3±2.5a |
| PaCo2 | ||||
| Vehicle | 33.9±1.67 | 31.5±4.26 | 30.03±1.91 | 36.4±1.91 |
| HET0016 | 32.5±0.87 | 28.6±1.86 | 29.7±1.90 | 41.9±2.12a |
| Pao2 | ||||
| Vehicle | 209±12.1 | 432±19.6a | 443±11.4a | 378±52.8a |
| HET0016 | 224±1.96 | 423±12.1a | 402±25.1a | 414 ±20.5a |
| pH | ||||
| Vehicle | 7.42±0.01 | 7.29±0.05a | 7.46±0.01 | 7.46±0.03 |
| HET0016 | 7.43±0.01 | 7.31±0.02a | 7.41±0.03 | 7.40±0.03 |
Abbreviations: CA, cardiac arrest; HET0016, N-hydroxy-N'-(4-n-butyl-2-methylphenyl)formamidine; MAP, mean arterial pressure; PaCo2, partial pressure of arterial carbon dioxide; Pao2, partial pressure of arterial oxygen.
P<0.05 versus baseline. There were no differences in the MAP, PaCo2, Pao2, and pH between vehicle- and HET0016-treated rats. P<0.05 versus baseline.
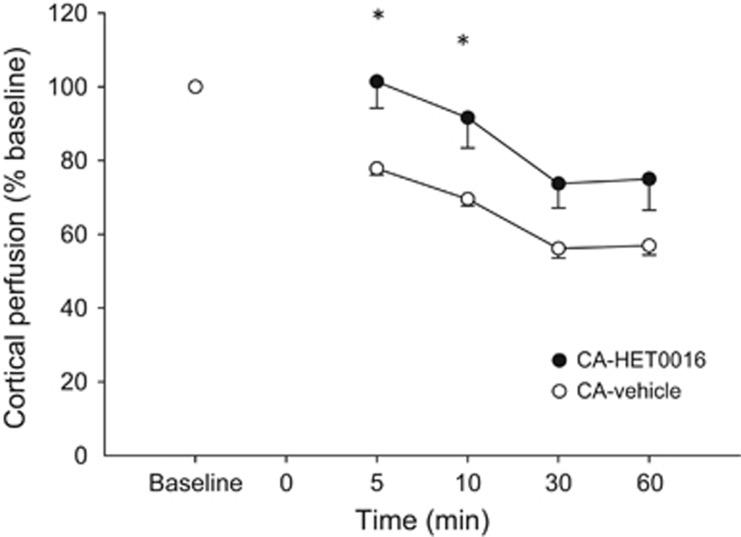
Cortical perfusion in vehicle-treated rats was 77.8±4.2% of baseline at 5 minutes after resuscitation, continued to decrease at 30 minutes to 56.1±2.5% of baseline and was 56.9±2.5% at 60 minutes (Figure 1). After 20-HETE inhibition with HET0016, cortical perfusion was higher versus vehicle at 5 and 10 minutes after resuscitation (101±12.5% versus 77.8±4.2% at 5 minutes, HET0016 versus vehicle, P<0.05, and 91.6±14.2% versus 69.5±4.6% at 10 minutes, HET0016 versus vehicle P<0.05). At 30 and 60 minutes after resuscitation, cortical perfusion was similar in HET0016- and vehicle-treated rats.
Figure 1.
Effect of 20-hydroxyeicosatetraenoic acid (20-HETE) inhibition with HET0016 after resuscitation from 12 minutes of CA on cortical perfusion. Cortical perfusion was higher after 20-HETE inhibition versus vehicle-treated rats 5 and 10 minutes after resuscitation. *P<0.05 HET0016 versus vehicle-treated rats.
The COV of CBF was higher at 30 and 60 minutes after CA versus baseline in both HET0016 (30±1% at baseline versus 37±2%, 37±3%, 40±2%, and 40±1% at 5, 10, 30, and 60 minutes after CA, respectively, P<0.05 at 30 and 60 minutes) and vehicle-treated rats (27±1% at baseline versus 33±2%, 33±2%, 37±1%, and 39±3% at 5, 10, 30, and 60 minutes after CA, respectively, P<0.05 at 30 and 60 minutes), and did not differ between HET0016 and vehicle (P=0.1).
Inhibition of 20-Hydroxyeicosatetraenoic Acid Formation with HET0016 Accelerates Recovery and Reduces Neurodegeneration After Pediatric Cardiac Arrest
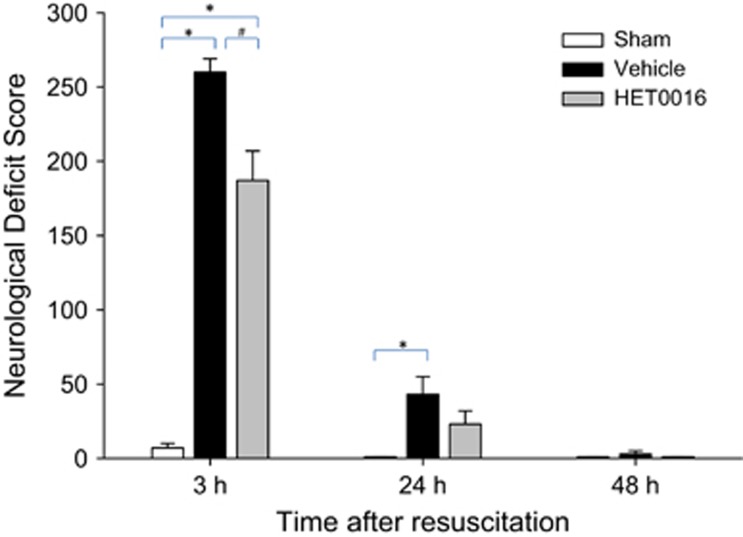
We assessed short-term functional outcome using the NDS score at 3, 24, and 48 hours after resuscitation from 12 minutes of CA in rats treated with HET0016 or vehicle, and rats subjected to sham surgery. At 3 hours after resuscitation, there were moderate to severe neurologic deficits in rats receiving the vehicle (NDS=260±9) and moderate neurologic deficits in rats treated with HET0016 (NDS=187±20, P<0.01 versus vehicle-treated rats). Both treatment groups showed recovery over time. At 24 hours after resuscitation, vehicle-treated rats showed mild neurologic deficits that were worse compared with shams (30±12 versus 0, P<0.05 versus shams), whereas the HET0016-treated rats showed mild neurologic deficits and were not different than shams (23±9 versus 0, P=0.1). By 48 hours after resuscitation, nearly all rats have recovered and NDS scores were similar between groups (Figure 2).
Figure 2.
Effect of 20-hydroxyeicosatetraenoic acid (20-HETE) inhibition with HET0016 after resuscitation from 12 minutes of cardiac arrest (CA) on neurologic deficit score (NDS). HET0016 reduced NDS at 3 hours after resuscitation versus vehicle. *P<0.05 versus sham; #P<0.05 versus vehicle.
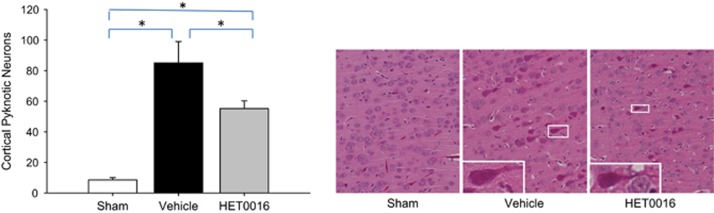
The effect of HET0016 treatment on neurodegeneration at 48 hours after ROSC from 12 minutes of CA was evaluated histologically. HET0016 treatment reduced the number of pyknotic pyramidal neurons in the parietal cortex versus vehicle (55.2±5.1 versus 85±14.1 neurons per section, HET0016 versus vehicle, P<0.05) (Figure 3). There was no difference in the number of pyknotic neurons in the thalamus between vehicle and HET0016-treated rats (data not shown).
Figure 3.
Effect of 20-hydroxyeicosatetraenoic acid (20-HETE) inhibition with HET0016 after resuscitation from 12 minutes of cardiac arrest (CA) on neurodegeneration. Treatment with HET0016 reduced cortical neurodegeneration at 48 hours versus vehicle. Hematoxylin and eosin sections from the parietal cortex of representative sham-, vehicle-, and HET0016-treated rats are illustrated. High power image insets of pyknotic neurons are included. *P<0.05.
Inhibition of 20-HETE Formation with HET0016 Reduces the Percent of Brain Tissue Water After Pediatric Cardiac Arrest
We assessed the percent of brain tissue water at 3 hours after sham surgery, 12 minutes of CA, and 12 minutes of CA treated with HET0016. After 12 minutes of CA, there was an increase in brain tissue water versus sham (83.01±0.1% versus 84±0.13%, sham versus CA, P<0.05). HET0016 treatment decreased the percent of brain tissue water (84±0.13% versus 83.43±0.24%, vehicle versus HET0016, P<0.05).
Discussion
This is the first study assessing a complete array of CYP-dependent eicosanoids in cerebral tissue after moderate and severe pediatric asphyxial CA in rats. Imbalances of the vasoconstrictor and vasodilator eicosanoids are region- and insult-duration-dependent and parallel our previously reported region and insult-duration dependeny of CBF in this model.8 Importantly, treatment with HET0016 attenuated CBF changes, accelerated neurologic recovery, reduced cortical neurodegeneration, and decreased cerebral water content after 12 minutes of CA. These results suggest that CYP-dependent eicosanoids have an important role in CBF homeostasis, the development of brain edema, and possibly neurodegeneration after resuscitation.
20-Hydroxyeicosatetraenoic acid, a powerful vasoactive mediator with important effects on CBF autoregulation, exerts its vasoconstrictive activity via large conductance calcium-dependent K channel and L-type calcium channels.12 20-Hydroxyeicosatetraenoic acid is formed in the cerebral vasculature,25 but may also be formed in neurons,26 and it is suggested that 20-HETE has direct neurotoxic effects based on studies showing that inhibition of 20-HETE formation is neuroprotective in cerebral slice cultures.18 EETs are vasodilators of cerebral circulation, and they also have direct neuroprotective actions.27, 28, 29, 30 The mechanistic underpinnings of 20-HETE-mediated neurodegeneration and EET-mediated neuroprotection and their sites of action on the neurovascular unit remain to be fully characterized.
We observed divergent patterns of eicosanoid generation after moderate and severe insults. After moderate durations of pediatric asphyxial CA, we observed an early increase in vasodilator eicosanoids in both the cortex and subcortical region. Early subcortical hyperemia previously reported by us after moderate durations of CA8 might stem from this vasodilator predominance. Early cortical CBF after moderate CA is similar to baseline, however, despite a slight predominance of vasodilators in the cortex. As multiple vasoactive mediators contribute to CBF regulation, perhaps other vasoconstrictors such as superoxide, or loss of other vasodilators such as nitric oxide and prostaglandin E2,11 oppose the vasodilator eicosanoids after CA in the cortex. Conversely, after severe durations of CA we observed an early increase in vasoconstrictor eicosanoids and a lack of compensatory increase in vasodilator eicosanoids in the cortex, suggesting that the cortical hypoperfusion seen early after severe durations of CA in our model is related to a predominance of vasoconstrictor eicosanoids. Similarly, in the subcortical areas after severe duration of CA, there is an increase in vasoconstrictor eicosanoids, although to a lesser degree compared with the cortex, along with a lack of compensatory increase in vasodilator eicosanoids, which might explain the absence of subcortical hyperemia observed after severe CA in this model.8 During the late reperfusion period after CA, the concentration of CYP450 vasoactive metabolites largely returned to normal levels after both moderate and severe CA, despite ongoing cortical hypoperfusion. Cortical hypoperfusion during the late post-CA syndrome could be a consequence of the early imbalance of eicosanoids producing derangements in the superoxide/nitric oxide homeostasis, other vasoconstrictive mediators, or a reduction in metabolic demands related to dying neurons and other cells. Baseline levels of eicosanoids during late post-CA syndrome might be because of substrate arachidonic acid (AA) depletion, decreased enzyme activity or enzyme depletion, or eicosanoid degradation.
After severe durations of CA, cortical hypoperfusion was associated with increased level of the vasoconstrictor eicosanoid 20-HETE. We hypothesized that inhibition of 20-HETE synthesis using HET0016 would improve CBF and reduce neurodegeneration after CA. HET0016 selectively decreased cortical levels of 20-HETE and increased cortical CBF during the early reperfusion period versus vehicle-treated rats. Short-term functional outcome was improved by HET0016 treatment versus vehicle, and neurodegeneration at 48 hours after CA was reduced by inhibition of 20-HETE formation. Similar to this report, other studies in cerebral ischemia suggest that 20-HETE has deleterious effects on CBF and neurons, and 20-HETE inhibition is beneficial. Cerebral 20-HETE levels were shown to increase after focal ischemia.31 Inhibition of 20-HETE formation led to increase in CBF and decreased neurodegeneration, which was dependent of CBF in some models of cerebral ischemia. For example, treatment with 20-HETE inhibitors improved CBF and reduced lesion volume after focal ischemia.26, 31 In other studies, 20-HETE inhibition reduced lesion volume and neurodegeneration independent of CBF, suggesting a direct neuroprotective effect of 20-HETE inhibition.17, 18, 32 In a global cerebral ischemia model in immature piglets similar in some ways to our model, Yang et al.17 showed direct neuroprotection after CA in piglets treated with HET0016. In that report, focal cortical CBF measured by laser Doppler was not affected by treatment with HET0016 at 5 minutes after resuscitation. However, there are differences between these CA models, CBF measurement technique, and the timing and dose of HET0016 administration. In our model, HET0016 was administered at resuscitation (versus 5 minutes after resuscitation in the other study), suggesting that inhibition of 20-HETE immediately on reperfusion might be necessary to inhibit the cascade of events that occurs at reperfusion. The dose of HET0016 was 10-fold lower in our study,17 and it is possible that a higher dose of HET0016 may lose selectivity for inhibition of CYP4A/4 F enzymes and thus inhibit CYP2C/2 J enzymes responsible for synthesis of vasodilatory EETs. Nevertheless, these two studies in global ischemia suggest neuroprotective effects of HET0016 inhibition. Additionally, cerebral water content was reduced by inhibition of 20-HETE formation in our study. The underlying mechanisms of neuroprotection by 20-HETE inhibition in global ischemia remain to be fully elucidated. Neuroprotection by 20-HETE inhibition may be mediated by (i) improvement of perfusion via a direct effect on the smooth vascular muscle, (ii) improvement of perfusion secondary to a decrease in perivascular edema, (iii) direct cytoprotective effects on neurons, or (iv) a combination of these mechanisms.
There are several limitations of this study. We determined that after CA, there are imbalances of vasoconstrictor (20-HETE) and vasodilator (EET) CYP-dependent eicosanoids, and we assessed the effect of 20-HETE inhibition in our model. The contribution of EETs to a vasodilator component after resuscitation from 9 or 12 minutes of CA was not directly tested in this work. We are planning future studies using inhibitors of EET degradation to address whether EETs contribute to the CBF response after CA. In the current work, we used one dosing paradigm, administration of HET0016 at resuscitation from CA, modeling prehospital treatment of a patient who suffered CA. Other treatment paradigms for 20-HETE inhibition may merit exploration, such as delayed treatment at 1 hour after resuscitation (modeling a patient being treated after the initial stabilization), or continuous intravenous infusion for 120 minutes after resuscitation. In our model, we use 100% oxygen for resuscitation and during the immediate post-CA period, to mimic the clinical condition after asphyxial arrest where 100% oxygen is commonly used. It is not known whether hyperoxia during the early post-CA phase might influence 20-HETE production.
Conclusions
In summary, we show that after pediatric CA there is an imbalance in vasoconstrictive and vasodilatory eicosanoids potentially underlying the pathologic changes in CBF. Further, we have showed that HET0016, a selective inhibitor of 20-HETE, improves cortical CBF during the early reperfusion phase after severe CA. In addition to CBF improvements, we showed a reduction in neurodegeneration, cerebral water content, and accelerated neurologic recovery after administration of HET0016. Future studies are needed to fully elucidate the underlying mechanisms of neuroprotection by HET0016 after pediatric CA with the goal of evaluating the 20-HETE pathway as a potential therapeutic target for CA.
The authors declare no conflict of interest.
Footnotes
Author Contributions
JSBS and MDM: participated in designing and performing the experiments, manuscript preparation, and data analysis; SMP, PMK, and RSBC: designing the experiments and manuscript preparation; HA: designing and performing the experiments, and manuscript preparation; DLT: designing the experiments, data analysis, and manuscript preparation.
This study was supported by NIH R01HD075760 (to MDM), Competitive Medical Research Fund of the UPMC Health System (to MDM), and NIH R01NS084604 (to RSBC).
References
- 1Donoghue AJ, Nadkarni V, Berg RA, Osmond MH, Wells G, Nesbitt L et al. Out-of-hospital pediatric cardiac arrest: an epidemiologic review and assessment of current knowledge. Ann Emerg Med 2005; 46: 512–522. [DOI] [PubMed] [Google Scholar]
- 2Nishizawa H, Kudoh I. Cerebral autoregulation is impaired in patients resuscitated after cardiac arrest. Acta Anaesthesiol Scand 1996; 40: 1149–1153. [DOI] [PubMed] [Google Scholar]
- 3Sundgreen C, Larsen FS, Herzog TM, Knudsen GM, Boesgaard S, Aldershvile J. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke 2001; 32: 128–132. [DOI] [PubMed] [Google Scholar]
- 4Buunk G, van der Hoeven JG, Meinders AE. Cerebral blood flow after cardiac arrest. Netherlands J Med 2000; 57: 106–112. [DOI] [PubMed] [Google Scholar]
- 5Shaffner DH, Eleff SM, Brambrink AM, Sugimoto H, Izuta M, Koehler RC et al. Effect of arrest time and cerebral perfusion pressure during cardiopulmonary resuscitation on cerebral blood flow, metabolism, adenosine triphosphate recovery, and pH in dogs. Crit Care Med 1999; 27: 1335–1342. [DOI] [PubMed] [Google Scholar]
- 6Xu Y, Liachenko S, Tang P. Dependence of early cerebral reperfusion and long-term outcome on resuscitation efficiency after cardiac arrest in rats. Stroke 2002; 33: 837–843. [DOI] [PubMed] [Google Scholar]
- 7Manole MD, Kochanek PM, Foley LM, Hitchens TK, Bayir H, Alexander H et al. Polynitroxyl albumin and albumin therapy after pediatric asphyxial cardiac arrest: effects on cerebral blood flow and neurologic outcome. J Cereb Blood Flow Metab 2012; 32: 560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Manole MD, Foley LM, Hitchens TK, Kochanek PM, Hickey RW, Bayir H et al. Magnetic resonance imaging assessment of regional cerebral blood flow after asphyxial cardiac arrest in immature rats. J Cereb Blood Flow Metab 2009; 29: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Andresen J, Shafi NI, Bryan RM, Jr. Endothelial influences on cerebrovascular tone. J Appl Physiol 2006; 100: 318–327. [DOI] [PubMed] [Google Scholar]
- 10Harder DR, Gebremedhin D, Narayanan J, Jefcoat C, Falck JR, Campbell WB et al. Formation and action of a P-450 4 A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol 1994; 266: H2098–H2107. [DOI] [PubMed] [Google Scholar]
- 11Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem 1997; 272: 27345–27352. [DOI] [PubMed] [Google Scholar]
- 13Iliff JJ, Wang R, Zeldin DC, Alkayed NJ. Epoxyeicosanoids as mediators of neurogenic vasodilation in cerebral vessels. Am J Physiol Heart Circ Physiol 2009; 296: H1352–H1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Leffler CW, Fedinec AL. Newborn piglet cerebral microvascular responses to epoxyeicosatrienoic acids. Am J Physiol 1997; 273: H333–H338. [DOI] [PubMed] [Google Scholar]
- 15Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 2002; 82: 131–185. [DOI] [PubMed] [Google Scholar]
- 16Shaik JS, Ahmad M, Li W, Rose ME, Foley LM, Hitchens TK et al. Soluble epoxide hydrolase inhibitor trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid is neuroprotective in rat model of ischemic stroke. Am J Physiol Heart Circ Physiol 2013; 305: H1605–H1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Yang ZJ, Carter EL, Kibler KK, Kwansa H, Crafa DA, Martin LJ et al. Attenuation of neonatal ischemic brain damage using a 20-HETE synthesis inhibitor. J Neurochem 2012; 121: 168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Renic M, Kumar SN, Gebremedhin D, Florence MA, Gerges NZ, Falck JR et al. Protective effect of 20-HETE inhibition in a model of oxygen-glucose deprivation in hippocampal slice cultures. Am J Physiol Heart Circ Physiol 2012; 302: H1285–H1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Fink EL, Alexander H, Marco CD, Dixon CE, Kochanek PM, Jenkins LW et al. Experimental model of pediatric asphyxial cardiopulmonary arrest in rats. Pediatr Crit Care Med 2004; 5: 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Miller TM, Donnelly MK, Crago EA, Roman DM, Sherwood PR, Horowitz MB et al. Rapid, simultaneous quantitation of mono and dioxygenated metabolites of arachidonic acid in human CSF and rat brain. J Chromatogr 2009; 877: 3991–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Shaik JS, Miller TM, Graham SH, Manole MD, Poloyac SM. Rapid and simultaneous quantitation of prostanoids by UPLC-MS/MS in rat brain. J Chromatogr B 2014; 945–946: 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Ayata C, Dunn AK, Gursoy OY, Huang Z, Boas DA, Moskowitz MA. Laser speckle flowmetry for the study of cerebrovascular physiology in normal and ischemic mouse cortex. J Cereb Blood Flow Metab 2004; 24: 744–755. [DOI] [PubMed] [Google Scholar]
- 23Mu Y, Klamerus MM, Miller TM, Rohan LC, Graham SH, Poloyac SM. Intravenous formulation of N-hydroxy-N'-(4-n-butyl-2-methylphenyl)formamidine (HET0016) for inhibition of rat brain 20-hydroxyeicosatetraenoic acid formation. Drug Metab Dispos 2008; 36: 2324–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Neumar RW, Bircher NG, Sim KM, Xiao F, Zadach KS, Radovsky A et al. Epinephrine and sodium bicarbonate during CPR following asphyxial cardiac arrest in rats. Resuscitation 1995; 29: 249–263. [DOI] [PubMed] [Google Scholar]
- 25Dunn KM, Renic M, Flasch AK, Harder DR, Falck J, Roman RJ. Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 2008; 295: H2455–H2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Marumo T, Eto K, Wake H, Omura T, Nabekura J. The inhibitor of 20-HETE synthesis, TS-011, improves cerebral microcirculatory autoregulation impaired by middle cerebral artery occlusion in mice. Br J Pharmacol 161: 1391–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ et al. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke 2008; 39: 2073–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Simpkins AN, Rudic RD, Schreihofer DA, Roy S, Manhiani M, Tsai HJ et al. Soluble epoxide inhibition is protective against cerebral ischemia via vascular and neural protection. Am J Pathol 2009; 174: 2086–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Dorrance AM, Rupp N, Pollock DM, Newman JW, Hammock BD, Imig JD. An epoxide hydrolase inhibitor, 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA), reduces ischemic cerebral infarct size in stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol 2005; 46: 842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C et al. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab 2007; 27: 1931–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Poloyac SM, Zhang Y, Bies RR, Kochanek PM, Graham SH. Protective effect of the 20-HETE inhibitor HET0016 on brain damage after temporary focal ischemia. J Cereb Blood Flow Metab 2006; 26: 1551–1561. [DOI] [PubMed] [Google Scholar]
- 32Omura T, Tanaka Y, Miyata N, Koizumi C, Sakurai T, Fukasawa M et al. Effect of a new inhibitor of the synthesis of 20-HETE on cerebral ischemia reperfusion injury. Stroke 2006; 37: 1307–1313. [DOI] [PubMed] [Google Scholar]