Abstract
Stroke is one of the leading causes of death. Growing evidence indicates that ketone bodies have beneficial effects in treating stroke, but their underlying mechanism remains unclear. Our previous study showed ketone bodies reduced reactive oxygen species by using NADH as an electron donor, thus increasing the NAD+/NADH ratio. In this study, we investigated whether mitochondrial NAD+-dependent Sirtuin 3 (SIRT3) could mediate the neuroprotective effects of ketone bodies after ischemic stroke. We injected mice with either normal saline or ketones (beta-hydroxybutyrate and acetoacetate) at 30 minutes after ischemia induced by transient middle cerebral artery (MCA) occlusion. We found that ketone treatment enhanced mitochondria function, reduced oxidative stress, and therefore reduced infarct volume. This led to improved neurologic function after ischemia, including the neurologic score and the performance in Rotarod and open field tests. We further showed that ketones' effects were achieved by upregulating NAD+-dependent SIRT3 and its downstream substrates forkhead box O3a (FoxO3a) and superoxide dismutase 2 (SOD2) in the penumbra region since knocking down SIRT3 in vitro diminished ketones' beneficial effects. These results provide us a foundation to develop novel therapeutics targeting this SIRT3-FoxO3a-SOD2 pathway.
Keywords: energy metabolism, focal ischemia, imaging, mitochondria
Introduction
Stroke is the second leading cause of death and a leading cause of disability worldwide.1 Recombinant tissue plasminogen activator has been approved for acute stroke treatment but only 10% of patients can benefit from it.2 Even after blood flow is restored, reperfusion is surprisingly associated with an exacerbation of tissue injury.3 Thus, it remains an urgent need to find a novel therapy that helps not only ischemia but also reperfusion injury.3 Pathologically, the burst generation of reactive oxygen species (ROS) by mitochondria has a critical role in initiating cell death after ischemia and reperfusion.3, 4 Mounting evidence suggests that ketogenic pathway can improve clinical outcomes after stroke,5 but the precise mechanism remains unclear. Two main ketone bodies, beta-hydroxybutyrate (BHB) and acetoacetate (ACA) which we named ketones, are generally regarded as energy carriers.6 Ketones are consumed by the brain as a main source of energy source when glucose is limited.7 Our previous in vitro data showed that ketones decreased the NADH level when neurons were subjected to glutamate excitotoxicity. When NADH converts into NAD+, it donates electrons and can be used as a reducing agent to reduce ROS, thus attenuating glutamate excitotoxicity.8
Sirtuin 3 (SIRT3) is located within mitochondria and is NAD+ dependent. It reduces cellular ROS levels by activating superoxide dismutase 2 (SOD2), which converts toxic superoxide radicals into ordinary oxygen or less toxic hydrogen peroxide.9 It can also activate the forkhead box O3a (FoxO3a) and catalase to reduce ROS.9, 10 However, it is unclear the interaction between ketones and SIRT3 and the roles they play in ischemic stroke. In this study, we hypothesize that SIRT3 medicates ketones' neuroprotective function against ischemic stroke.
Materials and methods
Animals
Three-month-old 129/SvJ male mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). All mice were housed in a temperature and humidity controlled vivarium, kept on a 12-hour dark/light cycle, with free access to food and water. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Barrow Neurological Institute and performed according to the Revised Guide for the Care and Use of Laboratory Animals (USA government) and with the ARRIVE guidelines.
Mice were divided into two groups: ischemia+normal saline (NS group, n=12) and ischemia+ketone treatment (ketones group, n=9). Mice in the ketones group were given ketones (BHB, 0.4 mmol/kg and ACA 0.45 mmol/kg, 100 μL/20 g) subcutaneously at 30 minutes after ischemia. The injection was repeated every hour subsequently for a total of seven doses. Mice in the NS group were give 0.9% NS (100 μL/20 g) as a placebo.
Middle Cerebral Artery Occlusion Model
Mice were subjected to a 90-minute ischemia (occlusion–reperfusion) by middle cerebral artery (MCA) occlusion.11 The mouse was anesthetized and prepared in a sterile manner. Its neck area was exposed to allow a 6-0 nylon monofilament inserted into the right internal carotid artery. The suture blocked the bifurcation of the right internal carotid artery and MCA to achieve occlusion. After the 90-minute occlusion, the suture was removed to allow reperfusion. Successful occlusion was verified by cerebral blood flow (CBF) map and T2-weighted imaging using a 7-Tesla (7T) magnetic resonance imaging (MRI).
Evaluation of Neurologic Function
Three tests were used to assess the neurologic function before and after ischemia. The tests were scored by an examiner who was blinded to the treatment for each animal.
Neurologic score
It is commonly used in a grading system: 0=no deficit; 1=forelimb weakness; 2=circling to affected side; 3=partial paralysis on affected side; 4=no spontaneous motor activity.
Rotarod test
Mice were placed on an elevated rod (Rotarod rod apparatus, IITC life Science, Woodland Hills, CA, USA) from a starting speed of 5 r.p.m./min to a top speed of 40 r.p.m./min for four trials per day x 2 days. The latency to fall from the rod was recorded for each trial.12
Open field test
Mice were placed in a testing chamber. Their activity was tracked by the Activity Monitor software. The time of each testing session was 30 minutes. The total travel distance was calculated.
Magnetic Resonance Imaging Analysis
The 7T MRI designed for small animals was used to acquire T2-weighted images with a multi-shot spin-echo EPI sequence as described previously.11, 13 Apparent diffusion coefficient images were acquired with a rapid acquisition relaxation enhanced sequence with 29 slices. The CBF maps were acquired by a continuous arterial spin labeling method. The MRI technician was blinded to the treatment. The direct infarct area, ipsilateral half brain, and contralateral half brain were manually traced and measured separately in each infarct brain slice using Image J. The total direct infarct volume (DIV), total ipsilateral half brain volume (IBV), and total contralateral half brain volume (CBV) were calculated by the formula: Volume =Area(slices with infarcts) × 0.01 mm2 × 0.5 mm,14 individually. Edema can distort the infarct volume.15, 16, 17 It is known that the two half brain volumes should be the same under health condition and there is no significant swelling change in ipsilateral noninfarct area. Thus, the true infarct volume (corrected infarct volume, CIV) can be calculated by the formula: CIV=CBV−(IBV−DIV). We noted that the inherent technical difficulties in delineating the penumbral zone and ischemic core precisely even high-resolution MRI were used to detect perfusion-diffusion mismatch.
Mitochondrial Complex I and Complex II Activity Test
Mitochondria were isolated from the penumbra, and the activities of complex I and complex II were tested.18 Mitochondrial suspension was mixed with MitoXpress probe solution from MitoXpress fluorescence oxygen probe kit supplemented (Luxcel Biosciences, Cork, Ireland) by either 10% mitochondrial complex I substrates or 10% mitochondrial complex II substrates. The probe fluorescence intensity was recorded immediately using a TECAN infinite M200pro reader (Tecan, Männedorf, Switzerland). The mitochondrial function kinetics was represented as MitoXpress fluorescence intensity time series, which was fitted with a Boltzmann equation using GraphPad Prism4.0 (GraphPad, La Jolla, CA, USA). The person who input the data of this and following tests was blinded to the treatment.
Protein Oxidation Test
After mitochondria were isolated from the penumbra, 20 μg mitochondrial protein was collected to measure oxidation using OxyELISA Oxidized Protein Quantitation Kit (Millipore, Billerica, MA, USA) according to the manufacturer's protocol. The DNP-derivatized protein was separated by electrophoresis followed by western blotting. The blot membrane was then incubated with primary antibody, specific to the DNP moiety. This step was followed by incubation with goat IRDye 800CW secondary antibody (LI-COR, Lincoln, NE, USA). Immunoreactivity was detected and quantified using Odyssey CLx (LI-COR).
NAD+ and NADH Measurement
Brain tissues were collected from ischemic core, penumbra, and contralateral area. NAD+, NADH, and their ratio were detected by using the NADH/NAD Quantification Kit (ab65348, Abcam, Cambridge, MA, USA) according to the manufacturer's protocol.
Western Blotting
Brain tissues from the penumbra were collected, weighted, and homogenized in a fresh cold buffer (total tissue proteins) or a mitochondrial isolation buffer (total mitochondrial proteins). Forty micrograms of total tissue protein or total mitochondrial protein were used for western blotting. Antibodies used were the following: anti-SIRT3 (Cell Signaling, Danvers, MA, USA), anti-FoxO3a, anti-SOD2, anti-Catalase, anti-β-actin (Santa Cruz, Dallas, TX, USA), anti-VDAC1 (Abcam), and IRDye 800CW and IRDye 680CW antibodies (LI-COR). For in vitro studies, all steps were kept the same except they were run on a 96-well plate. Immunoreactive signals were quantified using Odyssey CLx. Protein levels were presented relative to β-actin or VDAC1, a mitochondria housekeeping protein.
Intracellular Adenosine Triphosphate Assay
Intracellular adenosine triphosphate (ATP) concentration was measured using the ATP Bioluminescence Assay (Roche Applied Science, Indianapolis, IN, USA). Cells were plated in 96-well plates and treated for the WST-1 assay. After treatment, the media were aspirated, and the cells were lysed with cell lysis reagent (100 μL/well). It was shaken for 10 minutes at room temperature and triturated repeatedly. The samples were centrifuged at 1,500 g for 5 minutes at 4°C before analysis of ATP in supernatants, with a tube luminometer (Sirius; Berthold Detection Systems, Oak Ridge, TN, USA). Sample protein contents were measured using the BCA protein assay (Pierce, Rockford, IL, USA).
Sirtuin 3 Vector Construction and Transfection
Sirtuin 3 was overexpressed or knocked down for in vitro experiments.18 We subcloned exogenous mouse SIRT3 cDNA sequence into Lenti-CMV-GFP vector to overexpress SIRT3. A short sequence of 19 nucleotides targeting SIRT3 location 764 was constructed into OmicsLink small hairpin RNA (shRNA) expression clone to knock down the expression of SIRT3. The shRNA vector, Lenti-SIRT3 vector, and a control vector were packaged into third-generation Lenti-Virus transfection system. After the cortical neurons matured in petri dishes for 10 days, we added the transfecting viral particles at a multiplicity of infection of 250. All vectors contained sequence of eGFP so they could be visualized after adding the virus particles for 4 days. The level of knockdown and overexpression was confirmed by western blot.
Cell Culture
Primary cortical cultures were prepared from new-born mouse pups according to standard procedures19 with minor modifications. Cortical neurons were plated on poly-d-lysine coated glass coverslips in Neurobasal media supplemented with 0.5% (w/v) l-glutamine, 1% Penicillin-Streptomycin, 5% fetal bovine serum, and 2% B27 supplement (Invitrogen, Grand Island, NY, USA). The medium was partially replaced every 4 days. Cultured neurons were usually matured and could be used for experiments after 14-day incubation.
Statistical Analysis
Data were expressed as mean±s.e.m. Unpaired Student's t test was used for comparison between two groups, while one-way analysis of variance (ANOVA) for multiple groups to determine the significance of the difference. Mann–Whitney U test was used for nonparametric data (such as neurologic scores). P<0.05 was considered as significant using SPSS version 10 for windows (IBM, Armonk, NY, USA).
Results
Ketones Improved Neurologic Function by Reducing Infarct Volume
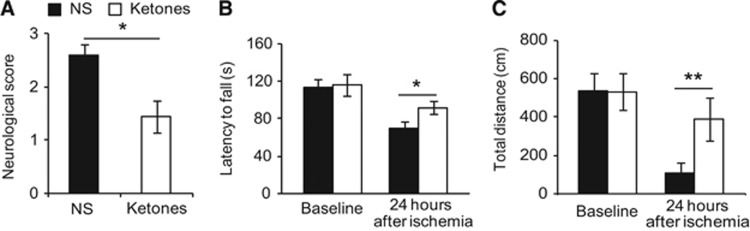
To assess ketones' effects in focal ischemia, we used the combination of BHB and ACA at the ratio of 0.4:0.45. We chose this ratio to mimic natural occurring ketone bodies. Ketone bodies have a third component acetone, which is too volatile to use for injection. Ketones have a half-life of an hour, so we repeated injection every hour to achieve a steady concentration in the blood. Ketones showed improvement in neurologic function at 24 hours after ischemia (Figure 1). The neurologic score is an assessment of paralysis where the lower score represents less paralysis. It was better in the treatment group (1.44±0.21 ketones versus 2.60±0.21 NS, Figure 1A). We used Mann–Whitney U test to compare the mean rank while presented mean score in the figure for clarity. Rotarod test is used to assess motor ability where the longer latency to fall represents better motor function.20 There was no difference before ischemia (113.2±8.3 seconds NS versus 115.8±11.9 seconds ketones). At 24 hours after ischemia, the latency to fall decreased in both groups, but to a much lesser degree in the treatment group (91.7±6.7 seconds versus 69.0±7.2 seconds NS, Figure 1B). The open field test is commonly used to test rodents for general locomotor activity and willingness to explore new areas. The total travel distance was not different in the two groups before ischemia. After ischemia, although both groups traveled less distance than their baseline, the treatment group traveled and explored far more distance than the control group (390±111 cm ketones versus 108±58 cm NS, Figure 1C). Noteworthy, two mice from the NS group died after stroke (mortality rate 2/12) while none of the ketones group died. We reached significance in statistics even when we excluded the two deceased mice from the NS group.
Figure 1.
Ketones improved neurologic function after stroke. At 24 hours after middle cerebral artery (MCA) occlusion. (A) Ketones reduced neurologic score (0 means no deficits; 4 complete paralysis). (B) In the accelerating rotarod test, ketones improved the ability to stay on the rod (latency to fall, in seconds). (C) In the open field test, ketones increased the ability to explore around (total distance, in centimeters). Data were presented as mean±s.e.m., *P<0.05, **P<0.01 compared with NS group. NS=0.9% normal saline treatment, n=10; Ketones=ketones treatment (BHB, 0.4 mmol/kg and ACA 0.45 mmol/kg, n=9). ACA, acetoacetate; BHB, beta-hydroxybutyrate.
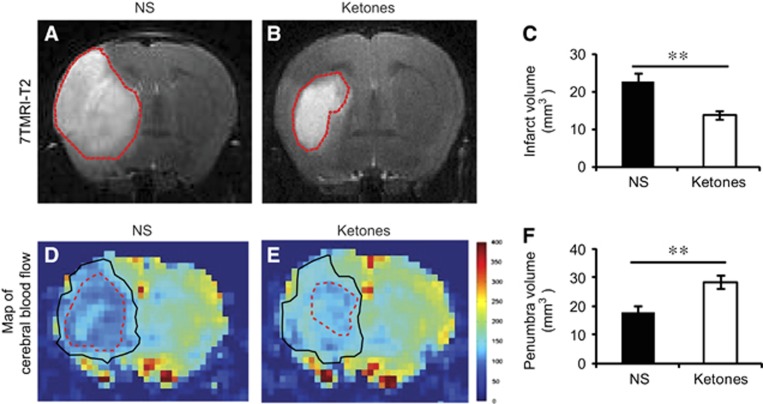
After behavior testing, we used 7T small animal MRI to measure the infarct volume at 24 hours after ischemia. Ketones group showed a reduction of 38.7% in infarct volume (Figures 2A and 2C). The diffusion and perfusion deficit detected by apparent diffusion coefficient and the CBF maps showed a mismatch which approximates ischemic penumbra (the area between the solid line and the dashed line in Figures 2D and 2E). Ketone treatment apparently salvaged the penumbra area (28.5±2.3 mm3 ketones versus 17.7±2.4 mm3 NS, Figure 2F). We focused on the penumbra area in the following studies since it was the area where ketones showed the most protective effects.
Figure 2.
Ketones reduced infarct volume and improved cerebral blood flow (CBF). Representative T2 images of the brain at 24 hours after ischemia. The infarct areas were semi-automatically traced (red lines) and measured in (A) NS group and (B) Ketones group. (C) The infarct volume in both groups was plotted. Representative perfusion-diffusion mismatch of CBF map was shown in (D) NS group and (E) Ketones group. The perfusion areas were traced in black lines, the diffusion area in red lines. The penumbra is the area in between. (F) The penumbra volume in both groups was plotted. Data were presented as mean±s.e.m., n=10 in NS group, n=9 in Ketones group, **P<0.01 compared with NS group.
Ketones Enhanced Mitochondrial Complex I Activity and Reduced Protein Oxidation
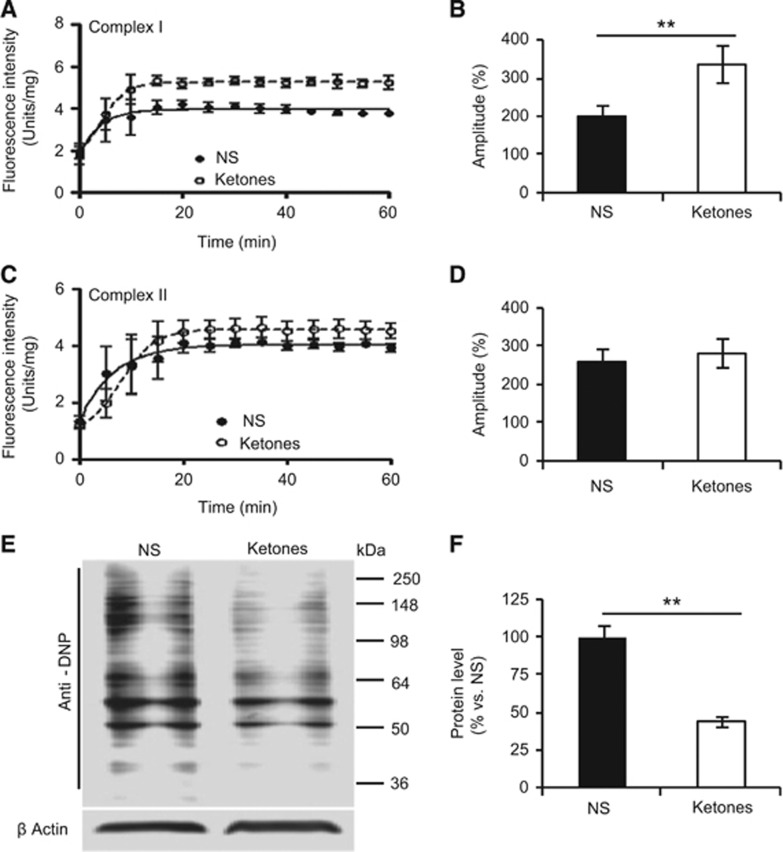
Because ketones are involved in energy metabolism, we examined mitochondrial complex I and complex II function in the penumbra and found an enhanced activity with ketone treatment in complex I (Figures 3A and 3B) but not in complex II (Figures 3C and 3D). The amplitude of complex I activity in the ketones group was 70% higher than the NS group (337.2±26.1% versus 199.3±16.2%, Figure 3B). Since mitochondria are the main source of ROS, which directly causes protein oxidation,4 we examined it in the penumbra. Consistently, ketones reduced protein oxidation by 56% (44.2±4.7% ketones versus 100.0±7.4% NS, Figures 3E and 3F).
Figure 3.
Ketones enhanced mitochondrial complex I activity and reduced protein oxidation. (A) The mitochondrial function kinetics of complex I was represented as MitoXpress fluorescence intensity time series. Mitochondria were isolated from the penumbra. The probe fluorescence intensity indicated oxygen consumption by mitochondria. (B) The amplitude of the time series of complex I was plotted. (C) The mitochondrial function kinetics of complex II was shown. (D) The amplitude of the time series of complex II was plotted. (E) Protein oxidation in the penumbra was analyzed by western blotting. The representative blot of the DNP-derivatized protein (OxyBlot) was shown. β-Actin was used as an internal control. (F) The densitometry quantification of protein levels relative to β-actin was plotted. Data were presented as mean±s.e.m., n=4 per group. **P<0.01 compared with NS group.
Ketones Increased NAD+/NADH, Sirtuin 3, Forkhead Box O3a, and Superoxide Dismutase 2 Expression in the Penumbra
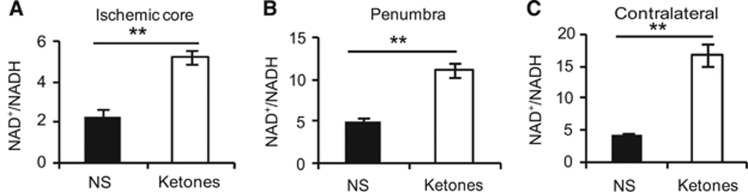
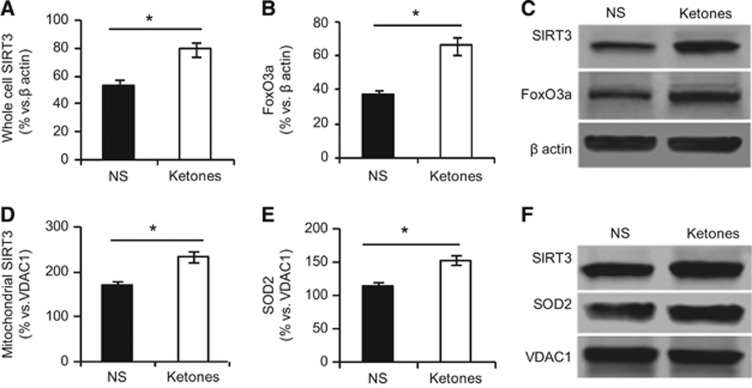
NAD+ is involved in redox reactions, carrying electrons from one reaction to another. Ketones increased the ratio of NAD+/NADH in all three areas in the low to high order: the ischemic core, the penumbra, and the contralateral side (Figures 4A and 4C). Sirtuin 3 is an NAD+-dependent deacetylase. Forkhead box O3a and SOD2 are two main substrates of SIRT3.21 We examined the total protein levels of SIRT3 and FoxO3a from homogenates in the penumbra. Ketones increased SIRT3 (79.0±7.2% ketones versus 53.7±5.5% NS) and FoxO3a levels (66.0±7.8% ketones versus 36.7±2.6% NS) (Figures 5A and 5C). Because SIRT3 and SOD2 target the mitochondria, we isolated mitochondria from the penumbra. Both were increased by ketones (SIRT3: 235.9±12.1% ketones versus 172.1±7.6% NS; SOD2: 152.8±6.2% ketones versus 113.6±7.0% NS group, Figures 5D and 5F).
Figure 4.
Ketones increased NAD+/NADH ratio. NAD+/NADH ratio in all three areas at 24 hours after ischemia was plotted. (A) The ischemic core, (B) the penumbra, and (C) the contralateral side. Data were presented as mean±s.e.m., n=6 per group. **P<0.01 compared with NS group.
Figure 5.
Ketones upregulated Sirtuin 3 (SIRT3), forkhead box O3a (FoxO3a), and superoxide dismutase 2 (SOD2). Homogenates protein was isolated from the penumbra tissue for western blotting. The density was plotted relative to β-actin for (A) SIRT3 and (B) FoxO3a. (C) Representative western blots of SIRT3 and FoxO3a are shown. Mitochondrial protein was isolated from the penumbra tissue for western blotting. The density was plotted relative to VDAC1 for (D) SIRT3 and (E) SOD2. (F) Representative western blots of SIRT3 and SOD2 are shown. Data were presented as mean±s.e.m., n=4 per group. *P<0.05 compared with NS group.
Sirtuin 3 Medicated the Neuroprotective Effects of Ketones
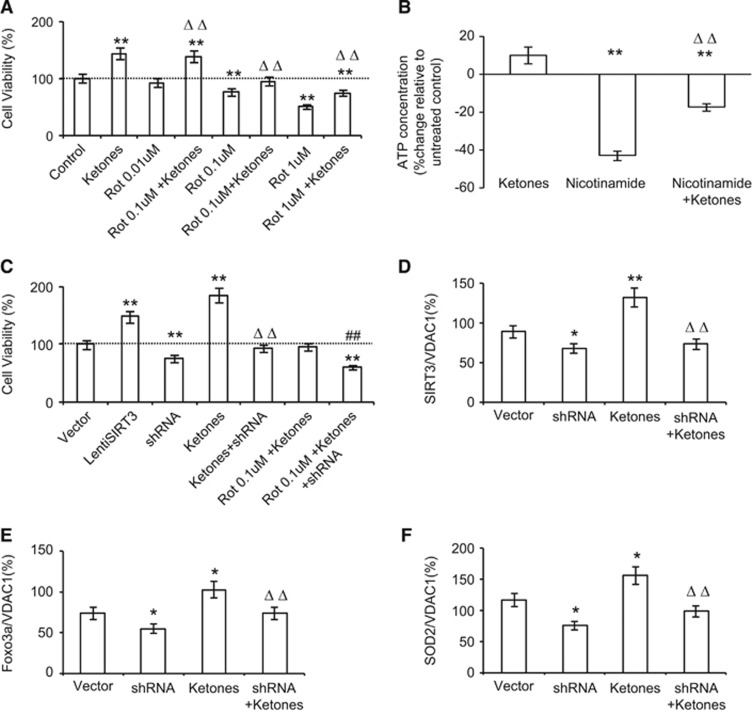
Because ketones' protective effect is on mitochondrial complex I and SIRT3 is a mitochondrial protein, we investigated the interaction of ketones and SIRT3 in mitochondria. Rotenone is a mitochondrial complex I inhibitor. We applied it to primary neuronal culture to mimic conditions in which mitochondria are compromised. Rotenone showed cytotoxicity in a dose-dependent pattern (Figure 6A). At low dose of rotenone (0.01 μmol/L), ketones actually increased cell proliferation by 39.0%. When rotenone was increased to 0.1 μmol/L, ketones exerted its neuroprotection to maintain cell integrity. Further increase in rotenone (1 μmol/L) saw ketones still being able to attenuate cytotoxicity by 23.6% (Figure 6A). Adenosine triphosphate is continuously regenerated from ADP to supply energy for cell proliferation. We measured ATP levels in primary neurons exposed to either ketones or nicotinamide, a broad-spectrum sirtuin inhibitor, for 48 hours (Figure 6B). We found that nicotinamide reversed the ketone-induced increase in ATP levels by 27.5±2.6%, suggesting that SIRT3 is a potential mediator. Consistently, overexpression of SIRT3 increased neuronal survival to 148%, whereas shRNA knocked down SIRT3 and reduced neuronal survival to 76% (Figure 6C). Small hairpin RNA not only reduced ketone-induced cell proliferation, but also weakened ketones' protective effects against rotenone (Figure 6C). Furthermore, ketones increased protein levels of SIRT3, FoxO3a, and SOD2, whereas shRNA caused a reduction in them and mitigated ketone-induced increase (Figures 6D and 6F).
Figure 6.
Sirtuin 3 (SIRT3) mediated the neuroprotective effects of ketones. (A) In primary neuronal cell culture, cells were treated with rotenone at various concentrations. MTT assay for cell viability was plotted with or without ketone treatment. **P<0.01 versus control. ΔΔP<0.01 versus respective rotenone doses. (B) Bioluminescence was measured to represent cellular adenosine triphosphate (ATP) concentration. Neurons were exposed to ketones, nicotinamide or the combination for 48 hours. **P<0.01 versus baseline, ΔΔP<0.01 versus nicotinamide. (C) Primary neuronal cells were transfected by a lentivirus encoding shRNA or SIRT3 cDNA sequence. Cell viability was assessed in cells treated with rotenone, ketones, or both. **P<0.01 versus Vector; ΔΔP<0.01 versus ketones; ##P<0.01 versus Rot 0.1μmol/L+ ketones. Proteins levels of SIRT3 (D), forkhead box O3a (FoxO3a) (E), and superoxide dismutase 2 (SOD2) (F) in neurons treated with ketone or shRNA or the combination were plotted. *P<0.05 versus vector, **P<0.01 versus vector; ΔΔP<0.01 versus ketones. All data were presented as mean±s.e.m., n=8 per group.
Discussion
In this study, we have found that SIRT3-FoxO3a-SOD2 pathway mediates the neuroprotective effects of ketones to enhance mitochondrial complex I activity and to reduce oxidative stress that in turn leads to a reduction in infarct volume and an improvement of neurologic function.
Ischemic stroke disrupts CBF to reduce oxygen and glucose supply to affected areas that lead to mitochondrial dysfunction and oxidative stress. Ketone is reported to increase global CBF.22, 23 Our study on the CBF map showed that ketone increased perfusion to salvage the penumbra area and its effect lasted for at least 24 hours after ischemia. Within minutes after ischemia occurred, the core of ischemic area is fatally damaged and subsequently undergoes necrosis. Although the penumbra is under the threat of irreversible damage, it is potentially salvageable.24, 25 The volume of penumbra that escapes infarction is correlated with the extent of spontaneous neurologic recovery.26 Consistently, we found that ketones saved the penumbra to improve the clinical outcomes.
Our previous in vitro study indicated that ketones enhanced complex I activity and increased mitochondrial NAD+/NADH ratios.8, 27 Complex I is the first enzyme of the respiratory chain and has a central role in cellular ROS production.28 Complex I activity is suppressed during ischemia so enhancing its activity is a potential treatment strategy.29, 30 In this in vivo study, we found ketones improved mitochondrial complex I activity in the penumbra. To restore the mitochondrial function in the penumbra, ketones save it from irreversible damage as evidenced by the reduction in infarct volume.
Sirtuin 3 is synthesized in the nucleus but exerts its function mainly within mitochondria.31, 32 It enhances the activity of FoxO3a33 and SOD2.9, 34 Forkhead box O3a is an important mediator for apoptosis.35 Sirtuin 3 might directly regulate FoxO3a, which in turn regulates cell response to oxidative stress against ROS.33 Superoxide dismutase 2 is a primary mitochondrial antioxidant enzyme that converts O2- to H2O2, which can be further converted into water. Sirtuin 3 can enhance the ability of SOD2 to scavenge ROS.34 Although SIRT1 was shown to protect ischemia by inhibition of p53-induced apoptotic pathway,36 there is no study on the role of SIRT3 in ischemic stroke. In this study, we found ketones upregulated SIRT3, FoxO3a, and SOD2 in the penumbra after ischemia, implying these three interact with each other in salvaging the penumbra. In vitro experiments showed that overexpression of SIRT3 improved cell survival while knocking down SIRT3 diminished neuroprotective effects of ketones, suggesting that SIRT3 is an essential part on complex I for ketone to exert its neuroprotection. In addition, knocking down SIRT3 reduced the levels of Fox3a and SOD2 that were enhanced by ketones. This further suggests that SIRT3-FoxO3a-SOD2 is an integral complex to mediate ketone's protective effects against ischemia.
Taken together, our research supplements the growing literature that ketone protects against ischemia. We provide further evidence to reveal a novel SIRT3-FoxO3a-SOD2 pathway as the underlying mechanism. It sheds light on developing novel therapeutics targeting this pathway.
The authors declare no conflict of interest.
Footnotes
Author Contributions
JY contributed to manuscript draft, experiment design and performance, and data analysis; PH contributed to experiment design and performance, data analysis, and manuscript revision; ZT contributed to experiment design and performance; QL contributed to MRI experiment design and performance, data analysis; JS contributed to study design, data analysis and interpretation, and critical revision.
This study is supported by the Barrow Neurological Foundation.
References
- 1Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 2014; 383: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Radermacher KA, Wingler K, Langhauser F, Altenhofer S, Kleikers P, Hermans JJ et al. Neuroprotection after stroke by targeting NOX4 as a source of oxidative stress. Antioxid Redox Signal 2013; 18: 1418–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med 2011; 17: 1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol 2014; 2: 702–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Gibson CL, Murphy AN, Murphy SP. Stroke outcome in the ketogenic state—a systematic review of the animal data. J Neurochem 2012; 123: 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Dedkova EN, Blatter LA. Role of beta-hydroxybutyrate, its polymer poly-beta-hydroxybutyrate and inorganic polyphosphate in mammalian health and disease. Front Physiol 2014; 5: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S et al. Brain fuel metabolism, aging, and Alzheimer's disease. Nutrition 2011; 27: 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience 2007; 145: 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 2010; 12: 662–667. [DOI] [PubMed] [Google Scholar]
- 10Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 2009; 119: 2758–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Gan Y, Liu Q, Wu W, Yin JX, Bai XF, Shen R et al. Ischemic neurons recruit natural killer cells that accelerate brain infarction. Proc Natl Acad Sci USA 2014; 111: 2704–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Chen Q, Peto CA, Shelton GD, Mizisin A, Sawchenko PE, Schubert D. Loss of modifier of cell adhesion reveals a pathway leading to axonal degeneration. J Neurosci 2009; 29: 118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Yin J, Turner GH, Coons SW, Maalouf M, Reiman EM, Shi J. Association of amyloid burden, brain atrophy and memory deficits in aged apolipoprotein epsilon4 mice. Curr Alzheimer Res 2014; 11: 283–290. [DOI] [PubMed] [Google Scholar]
- 14Yin JX, Turner GH, Lin HJ, Coons SW, Shi J. Deficits in spatial learning and memory is associated with hippocampal volume loss in aged apolipoprotein E4 mice. J Alzheimer's Dis 2011; 27: 89–98. [DOI] [PubMed] [Google Scholar]
- 15Gerriets T, Stolz E, Walberer M, Muller C, Kluge A, Bachmann A et al. Noninvasive quantification of brain edema and the space-occupying effect in rat stroke models using magnetic resonance imaging. Stroke 2004; 35: 566–571. [DOI] [PubMed] [Google Scholar]
- 16Loubinoux I, Volk A, Borredon J, Guirimand S, Tiffon B, Seylaz J et al. Spreading of vasogenic edema and cytotoxic edema assessed by quantitative diffusion and T2 magnetic resonance imaging. Stroke 1997; 28: 419–426, discussion 426-7. [DOI] [PubMed] [Google Scholar]
- 17Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke 1993; 24: 117–121. [DOI] [PubMed] [Google Scholar]
- 18Han P, Tang Z, Yin J, Maalouf M, Beach TG, Reiman EM et al. Pituitary adenylate cyclase-activating polypeptide protects against beta-amyloid toxicity. Neurobiol Aging 2014; 35: 2064–2071. [DOI] [PubMed] [Google Scholar]
- 19Rinetti GV, Schweizer FE. Ubiquitination acutely regulates presynaptic neurotransmitter release in mammalian neurons. J Neurosci 2010; 30: 3157–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Balkaya M, Krober J, Gertz K, Peruzzaro S, Endres M. Characterization of long-term functional outcome in a murine model of mild brain ischemia. J Neurosci Methods 2013; 213: 179–187. [DOI] [PubMed] [Google Scholar]
- 21Pamenter ME, Perkins GA, McGinness AK, Gu XQ, Ellisman MH, Haddad GG. Autophagy and apoptosis are differentially induced in neurons and astrocytes treated with an in vitro mimic of the ischemic penumbra. PLoS One 2012; 7: e51469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol 2006; 17: 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Prins ML. Cerebral metabolic adaptation and ketone metabolism after brain injury. J Cereb Blood Flow Metab 2008; 28: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke 2009; 40: e331–e339. [DOI] [PubMed] [Google Scholar]
- 25Wu CX, Liu R, Gao M, Zhao G, Wu S, Wu CF et al. Pinocembrin protects brain against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress induced apoptosis. Neurosci Lett 2013; 546: 57–62. [DOI] [PubMed] [Google Scholar]
- 26Guadagno JV, Calautti C, Baron JC. Progress in imaging stroke: emerging clinical applications. Br Med Bull 2003; 65: 145–157. [DOI] [PubMed] [Google Scholar]
- 27Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev 2009; 59: 293–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Efremov RG, Sazanov LA. Respiratory complex I: 'steam engine' of the cell? Curr Opin Struct Biol 2011; 21: 532–540. [DOI] [PubMed] [Google Scholar]
- 29Borutaite V, Toleikis A, Brown GC. In the eye of the storm: mitochondrial damage during heart and brain ischaemia. FEBS J 2013; 280: 4999–5014. [DOI] [PubMed] [Google Scholar]
- 30Hardy L, Clark JB, Darley-Usmar VM, Smith DR, Stone D. Reoxygenation-dependent decrease in mitochondrial NADH:CoQ reductase (Complex I) activity in the hypoxic/reoxygenated rat heart. Biochem J 1991; 274: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Sack MN, Finkel T. Mitochondrial metabolism, sirtuins, and aging. Cold Spring Harb Perspect Biol 2012; 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 2007; 27: 8807–8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Peserico A, Chiacchiera F, Grossi V, Matrone A, Latorre D, Simonatto M et al. A novel AMPK-dependent FoxO3A-SIRT3 intramitochondrial complex sensing glucose levels. Cell Mol Life Sci 2013; 70: 2015–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell 2010; 40: 893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Sanphui P, Biswas SC. FoxO3a is activated and executes neuron death via Bim in response to beta-amyloid. Cell Death Dis 2013; 4: e625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Hernandez-Jimenez M, Hurtado O, Cuartero MI, Ballesteros I, Moraga A, Pradillo JM et al. Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke 2013; 44: 2333–2337. [DOI] [PubMed] [Google Scholar]