Abstract
Glucagon-like peptide-1 (GLP-1) receptor activation in the brain provides neuroprotection. Exendin-4 (Ex-4), a GLP-1 analog, has seen limited clinical usage because of its short half-life. We developed long-lasting Ex-4-loaded poly(D,L-lactide-co-glycolide) microspheres (PEx-4) and explored its neuroprotective potential against cerebral ischemia in diabetic rats. Compared with Ex-4, PEx-4 in the gradually degraded microspheres sustained higher Ex-4 levels in the plasma and cerebrospinal fluid for at least 2 weeks and improved diabetes-induced glycemia after a single subcutaneous administration (20 μg/day). Ten minutes of bilateral carotid artery occlusion (CAO) combined with hemorrhage-induced hypotension (around 30 mm Hg) significantly decreased cerebral blood flow and microcirculation in male Wistar rats subjected to streptozotocin-induced diabetes. CAO increased cortical O2− levels by chemiluminescence amplification and prefrontal cortex edema by T2-weighted magnetic resonance imaging analysis. CAO significantly increased aquaporin 4 and glial fibrillary acidic protein expression and led to cognition deficits. CAO downregulated phosphorylated Akt/endothelial nitric oxide synthase (p-Akt/p-eNOS) signaling and enhanced nuclear factor (NF)-κBp65/intercellular adhesion molecule-1 (ICAM-1) expression, endoplasmic reticulum (ER) stress, and apoptosis in the cerebral cortex. PEx-4 was more effective than Ex-4 to improve CAO-induced oxidative injury and cognitive deficits. The neuroprotection provided by PEx-4 was through p-Akt/p-eNOS pathways, which suppressed CAO-enhanced NF-κB/ICAM-1 signaling, ER stress, and apoptosis.
Keywords: diabetes, ischemia/reperfusion, neuroinflammation, exendin-4 PLGA microsphere
Introduction
Diabetes mellitus (DM) is a major independent risk factor for stroke. The risk for stroke is doubled in patients with DM in comparison with the general population,1 and these patients are at increased risk of death because of cerebrovascular diseases.2 Hyperglycemia causes oxidative stress and inflammation resulting in neurovascular complications and neuroinflammation.3, 4 The inflammatory cascades evoked by acute traumatic brain injury and stroke lead to an elevation in aquaporin 4 (AQP4) and glial fibrillary acidic protein (GFAP) levels and are correlated with neurologic and cognitive deficits.5, 6
Glucagon-like peptide-1 (GLP-1) is a gut hormone that is secreted by the small intestine, which stimulates insulin release by activating the GLP-1 receptor (GLP-1R)7, leading to β-cell proliferation8 and differentiation of new β-cells.9 GLP-1, and its analog exendin-4 (Ex-4), confer cardiovascular protection in patients with dilated cardiomyopathy, hypertensive heart failure, myocardial infarction,10 and thrombosis.11 GLP-1R is widely located throughout the brain; and GLP-1 and Ex-4 provide neuroprotection against neuronal damage through GLP-1R activation.12, 13
Several neurovascular protective mechanisms are involved in the effects of GLP-1R activation, including activation of phosphorylated Akt/endothelial nitric oxide synthase (p-Akt/p-eNOS) protective signaling,11 protein kinase A-mediated inhibition of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity-induced oxidative stress,14 and inhibition of translocation of the p65 subunit of nuclear factor-κB (NF-κB) and mitochondrial pro-apoptotic Bax.15 Ex-4 attenuates stroke injury in patients with Type 2 diabetes by arresting microglia infiltration, increasing neural stem cell proliferation, and depressing metabolic insults.16 Another GLP-1 agonist, exenatide, suppresses reactive oxygen species production and apoptosis, while inhibiting NADPH oxidase p47-phox and gp91-phox activity.17
In addition to decreasing brain injury by antioxidant, antiapoptotic, and anti-inflammatory actions, another therapeutic strategy for reducing cerebral ischemic injury is to recover cerebral perfusion, leading to decreased cerebral infarction size and neurologic deficits.18 Central injection of long-acting GLP-1R agonists in mice stimulated brown adipose tissue thermogenesis through regulation of the hypothalamic ventromedial nucleus.19 In a longitudinal study involving obese patients with Type 2 diabetes, 1 year of treatment with exenatide and liraglutide increased energy expenditure,19 suggesting the thermoregulatory effect of GLP-1R activation. Ex-4 administered peripherally (intraperitoneal route) and centrally (fourth ventricle route) produced a large and long-lasting hypothermic effect.20 Hypothermic treatment by manipulation of body temperature at 33 °C 30 minutes after traumatic brain injury could provide neuroprotection by decreasing inflammasome signaling-mediated injury.21 It is unknown whether GLP-1R activation affects cerebral microcirculation. Although hypoglycemic, cardiovasoprotective, and neuroprotective effects of Ex-4 have been reported,10, 11, 12, 13 the drug is also limited by a short half-life, high burst release, and lower bioactivity.
Studies show that synthetic biodegradable polyesters such as poly(D,L-lactide-co-glycolide) (PLGA) can be used to increase the bioactivity of administered drugs.22 To overcome the short half-life and limited efficacy of Ex-4, we used a solvent-compatible microfluidic chip based on phenol formaldehyde resin to prepare Ex-4/PLGA (PEx-4) microspheres. We explored whether PEx-4 exerts more effective and long-lasting neurovascular protection than Ex-4 following carotid artery occlusion (CAO), which manifested as increased cerebral blood flow, improvements in neurologic and cognitive deficits, and decreased edema and oxidative injury through p-Akt/p-eNOS signaling.
Materials and methods
Materials
PLGA (cat. no. P2191, lactide/glycolide=50/50, Mw=30 000 to 60 000 Da), poly(vinyl alcohol) (87 to 89% hydrolyzed), Ex-4, and chloroform were purchased from Sigma-Aldrich Chemical (St Louis, MO, USA).
Synthesis of PLGA Microspheres
PLGA (vehicle) or PLGA plus Ex-4 (PEx-4) microspheres were prepared using microfluidics technology (Figure 1A)22 and details of the techniques are described in Supplementary Methods. We prepared some PLGA microspheres containing lipophilic tracer Dil (0.1%w/v, Invitrogen, Grand Island, NY, USA) for evaluation of biodegradation of particles in the brain microcirculation by a Leica DM LFSA microscope with Leica Fluorescence Illuminator LRF4/22 (Leica Microsystems, Wetzlar, Germany). The images were recorded with Zyla sCMOS (Andor Technology, Belfast, UK) and analyzed with Meta Imaging (Molecular Devices, Sunnyvale, CA, USA).
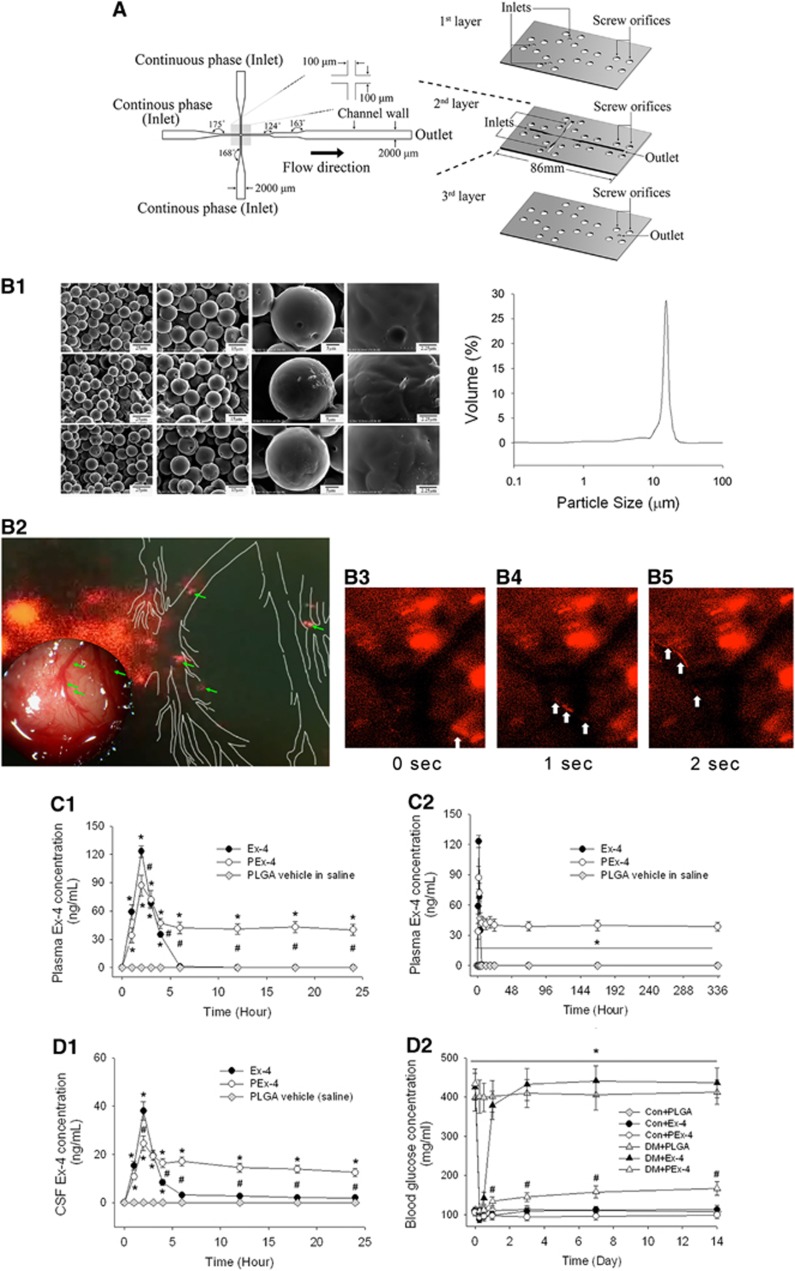
Figure 1.
Exendin-4 (Ex-4)-loaded poly(D,L-lactide-co-glycolide) (PLGA) microspheres (PEx-4) preparation. (A) An expanded view of the phenol formaldehyde resin-based microfluidic device, showing the geometry of the microfluidic channel and chip. (B) The prepared microspheres containing Ex-4 (PEx-4) were examined under a scanning electron microscope. The PEx-4 microspheres were uniform in size (15.0±3.3 μm) and morphology, and their distribution was analyzed by laser diffraction (B-1). After subcutaneous treatment, some of the PEx-4 microspheres were clogged into the brain (left site of red fluorescence, B-2) and some PEx-4 microspheres were degraded into small fragments (indicated by green arrow heads). We traced some degraded microspheres in the cortical microcirculation with white arrow heads within 3 seconds (B-3 to B-5). (C) (C-1) After subcutaneous administration of Ex-4 or PEx-4, both groups of rats (n=5 each) showed maximal plasma concentrations at 2 hours, but PLGA vehicle had no effect on Ex-4 concentration. The maximal plasma Ex-4 value 2 hours following Ex-4 administration was significantly higher (P=0.031) than that following PEx-4 treatment. (C-2) From 6 hours to 14 days, the plasma Ex-4 value of the PEx-4 group was significantly (P=0.001) higher than that of the Ex-4 group. (D) (D-1) The cerebrospinal fluid (CSF) Ex-4 concentration was directly associated with the plasma Ex-4. The maximal CSF Ex-4 value at 2 hours after Ex-4 treatment was significantly higher (P=0.024) than that after PEx-4 treatment. The CSF Ex-4 concentration of the PEx-4 group was significantly (P=0.016) higher than that of the Ex-4 group 6 to 24 hours after subcutaneous administration. (D-2) Hyperglycemic effects of subcutaneously administered Ex-4, PEx-4, or PLGA vehicle in control and diabetes mellitus (DM) rats (n=5 each). In normoglycemic control rats, subcutaneous Ex-4 (P=0.019) or PEx-4 (P=0.032) significantly decreased blood glucose to hypoglycemic levels after 6 hours of treatment, respectively, but PLGA vehicle microspheres did not (P=0.69). PEx-4 significantly (P=0.002 vs. Ex-4) depressed blood glucose levels from 12 hours to 14 days in DM rats, but Ex-4 had no effect on DM-induced hyperglycemia during this period. Group differences were compared by one-way analysis of variance, followed by Tukey's multiple comparison tests. P<0.05 indicates a significant difference. *Different from control (Con); #different from DM.
Microsphere Characterization
A scanning electron microscope (S-2700, Hitachi, Tokyo, Japan) was used to characterize the morphology and size of the PLGA microspheres. The images were analyzed using the ImageJ software (National Institutes of Health, NIH, Bethesda, MD, USA). One-hundred microspheres were counted to ensure adequate statistical representation. The particle size distribution was determined in a water suspension using a laser diffraction particle size analyzer (Mastersizer, Malvern Instruments, Malvern, UK).
In Vivo Drug Release Study
Ex-4 and PEx-4 were suspended in sterile phosphate-buffered saline and subcutaneously administered to male Wistar rats (20 μg/day). Blood was collected by retro-orbital bleeding into tubes containing EDTA at different time points for 14 days, transferred into 1.5-mL centrifuge tubes, and centrifuged at 15 000 g for 5 minutes. The supernatant was stored at −20 °C. Blood glucose levels were determined using a blood glucose meter (One Touch II; LIFESCAN, Milpitas, CA, USA). To measure Ex-4 entry into the brain parenchyma, we measured the Ex-4 concentration in the cerebrospinal fluid (CSF). Under anesthesia, 50 to 100 μl CSF was collected from the medullary cisterna magna of rats as previously described.23 The Ex-4 concentration in CSF and plasma was determined using an Enzyme Immunoassay Kit (EK-070-94, Phoenix Pharmaceuticals, Burlingame, CA, USA).
Animal Preparation for In Vivo Experiments
Animal care and experimental protocols were conducted in accordance with the guidelines of the National Science Council of the Republic of China (NSC1997). The animal studies were approved by the Institutional Animal Care and Use Committee of I-Shou University, and this manuscript was written in accordance with the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines (http://www.nc3rs.org/ARRIVE). Male Wistar rats (250 to 300 g, 8-weeks old) were housed in a temperature-controlled facility at the Experimental Animal Center of I-Shou University (Kaushung, Taiwan) with a 12-hour light cycle. The animals were randomly assigned to 12 groups (n=10 each in groups 1 to 4 and n=30 each in groups 5 to 12): (1) control (PLGA vehicle-treated) group, (2) DM (PLGA vehicle-treated) group, (3) control+Ex-4 group, (4) control+PEx-4 group, (5) DM+Ex-4 group, (6) DM+PEx-4 group, (7) control+CAO group (CAO), (8) DM+CAO group (DM+CAO), (9) control+CAO+Ex-4 group (CAO+Ex-4), (10) control+CAO+PEx-4 group (CAO+PEx-4), (11) DM+CAO+Ex-4-treatment group (DM+CAO+Ex-4), and (12) DM+CAO+PEx-4 treatment group (DM+CAO+PEx-4). The DM rats were intraperitoneally injected with streptozotocin (60 mg/kg, Sigma-Aldrich). The onset of DM occurred rapidly and was associated with a blood glucose concentration >250 mg/dL. After 2 weeks of streptozotocin, Ex-4 or PEx-4 was administered subcutaneously (20 μg/day).
Global Cerebral Ischemia
Our data (Figure 1D-2) suggested that the blood glucose levels were further reduced by systemic PEx-4 and Ex-4 in normoglycemic rats, similar to a previous study.7 Therefore, the neuroprotective effects of Ex-4 and PEx-4 on CAO injury were only investigated in the CAO and DM+CAO rats. In the groups 7 to 12, temporary global cerebral ischemia was induced under avertin (2,2,2-tribromoethanol) anesthesia by 10-minute bilateral common CAO combined with hemorrhage-induced hypotension, according to a previously described method.24 The femoral artery was catheterized with a PE-50 catheter to continuously record arterial blood pressure. After heparinization, blood was quickly withdrawn via the femoral artery. When the mean arterial blood pressure reached 30 mm Hg, the bilateral common carotid arteries were clamped with surgical clips for 10 minutes, after which the clips were removed and blood was reinfused via the femoral vein. In the sham-operated animals, the vessels were exposed, but neither blood withdrawal nor clamping of the carotid arteries was performed. After surgery, the animals were allowed to recover in a facility kept at 20 °C. The mortality rate is indicated in Supplementary Table S1.
In Vivo Measurement of Superoxide Anion (O2−) Production
The brain O2− content in the control and DM rats treated with vehicle, Ex-4, or PEx-4, was measured 1 day after CAO injury. Real-time O2− generation was measured directly from amplified chemiluminescent signals of the cerebral cortex surface in vivo, as described previously (with some modifications) for another organ.25 Briefly, the anesthetized animal was fixed in a holder, and the skin on top of the skull removed to expose the bregma. A cranial window was drilled into the skull (0 to 4 mm posterior, 4 to 7 mm lateral, and to the left and right of the bregma) to expose the cerebral cortex, leaving the dura mater intact. An O2− probe, 2-methyl-6-(4-methoxyphenyl)-3,7-dihydroimidazo-[1,2-a]-pyrazin-3-one-hydrochloride (MCLA; TCI-Ace, Tokyo Kasei Kogyo, Tokyo, Japan) was then infused intravenously throughout the experiment at 0.2 mg/mL/h. The quantity of O2− was detected on the cerebral cortex surface using a Chemiluminescence Analyzing System (CLA-ID3, Tohoku Electronic Industrial, Sendai, Japan). The rat was maintained on a circulating water pad at 37 °C during photon detection. To exclude photon emission from sources other than the brain, the animal was housed in a dark box with a shielded plate. The cerebral cortex was left unshielded and positioned under a reflector, which continuously directed the MCLA-enhanced chemiluminescent signal from the cortical surface onto the detector area. The chemiluminescence counts were recorded every 10 seconds, and total O2− was measured using the area under the detected photons–time curve.
Cerebral Edema Measurement by T2-Weighted Magnetic Resonance Imaging (MRI)
MRI was carried out in the animals using a Bruker Biospec 7-T MRI system as described previously.26 Anesthesia was induced with 5% halothane and maintained with 1.5% halothane. Control and DM rats treated with vehicle, Ex-4, or PEx-4 24 hours after CAO were intubated and mechanically ventilated at a rate of 60 breaths/minute. Heart rates and respiratory rates were monitored throughout the procedure, and body temperature was maintained at 37 °C. A rapid-acquisition relaxation enhancement T2-weighted sequence was used to determine the lesion location, with a rapid-acquisition relaxation enhancement factor (RARE) of 16, a repetition time of 5086 ms, and an echo time of 70.1 ms. The in-plane resolution was 250 × 250 × 250 μm3 and 15 slices were acquired. A second T2-weighted image set of 25 contiguous slices was acquired at the lesion site (RARE factor=16, repetition time=5086 ms, echo time=70.1 ms) with an in-plane resolution of 117 × 117 × 500 μm3. Brain edema areas, outlined in white, were delineated from each MRI image, using the Adobe image analysis software (Adobe Photoshop CS4, Delaware Corporation, San Jose, CA, USA). The percentage of edema was calculated using the equation: Brain edema (%)=edema area/(edema area and unaffected area) × 100%.
Infrared Thermal Imaging (IRT) of Cerebral Blood Flow Intensity
We evaluated the cerebral blood flow responses by IRT in the control and DM rats treated with vehicle, Ex-4, or PEx-4 before, 6 hours, or 12 hours after CAO injury. IRT can detect small temperature changes in the body because of vascular disorders.27 The anesthetized animal was placed in a holder, and the body temperature was maintained at 37 to 38 °C using a heating pad and a rectal thermometer (RET-2, Fine Science Tools, Foster City, CA, USA). The cerebral cortex was exposed as described in a previous section. An F30 IRT camera (Nippon Avionics, Tokyo, Japan) was positioned 20 cm above the assessed area for measurement of the temperature distribution of the cortex. Cerebral blood flow intensity before and after CAO was measured in all the treatment groups. The surface temperature profiles from the same area were continuously recorded and analyzed using the Thermo Tracer software version 1.2 (Nippon Avionics). We used the following formula to calculate the blood flow intensity from the infrared measurements:
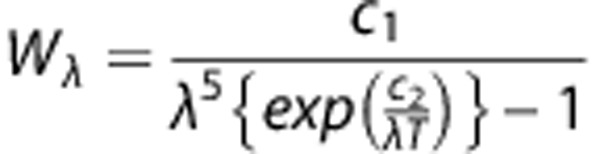 |
After calculating the blood flow intensity, the intensity was classified on a scale from 0 to 5. To obtain the blackbody radiant emission, the Stefan–Boltzmann equation (W=ɛσT4) was used throughout the full range of wavelengths. The surrounding cerebral blood flow was quantified using Pennes' bio-heat equation: kΔ2−cbwb(T−Ta)+qm=0.
Cerebral Cortex Microcirculation Determination
We continuously measured cerebral cortex microcirculation in the control and DM rats with vehicle, Ex-4, or PEx-4 before, 6 hours, or 12 hours after CAO. A full-field laser perfusion imager (MoorFLPI, Moor Instruments, Devon, UK) was used to continuously monitor the microcirculatory blood flow. The microcirculatory blood flow of each region of interest was recorded as flux with perfusion unit, which is related to the product of average speed and the concentration of moving red blood cells in the tissue sample volume. The images were analyzed in real time using the MoorFLPI software version 3.0 (Moor Instruments).
Assessment of Cognition Function
We analyzed the impact of CAO on rat cognitive function using the behavioral analysis. The recognition memory was evaluated in the Ex-4- or PEx-4-treated control and DM rats using a novel sniffing behavior test 1, 3, or 7 days after CAO. On the experimental day, the number of sniffing each object was recorded using the ObjectScan behavior analysis software (CleverSys, Reston, VA, USA). All nose entries within 2 cm of the object were recorded as the number exploring the object. For conditioning, the animals were placed individually in a conditioning chamber (60 × 60 × 60 cm3) for 2 hours. Each chamber contained five objects: sawdust, styrofoam, feed, water, and plastic. Animal movement was recorded by a high throughput-screening top-view camera (STD-CA67D-IR, Sony, Tokyo, Japan). The animal was allowed to explore for 10 minutes, and the amount of number of exploring each object was recorded. Animals that spent <7 minutes exploring the objects during the 10-minute test session were omitted from the analysis. The objects and arenas were thoroughly cleaned with 70% ethanol between trials.
NF-κB p65 Assay
NF-κB p65 assay was determined by real-time reverse transcription–PCR28 and electrophoretic mobility shift assay (EMSA). The nuclear extracts from 0.5 g of fresh cortical samples were obtained with a Nuclear Extraction Kit (Panomics, Redwood City, CA, USA) and analyzed by using a commercial EMSA Kit (Panomics) for identifying NF-κB. The EMSA procedure was followed according to the manual.
In Situ Measurement of AQP-4, Intercellular Adhesion Molecule-1 (ICAM-1), GFAP, Oxidative Stress, Endoplasmic Reticulum Stress, and Apoptosis
The brain tissues from control or DM rats treated with the vehicle, Ex-4, or PEx-4 (24 hours after CAO) were used. A sodium citrate buffer solution (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) was applied to the deparaffinized section for 15 minutes for heat-induced epitope retrieval (antigen retrieval). The sections were then blocked for non-specific binding with 5% bovine serum albumin (Sigma-Aldrich) for 1 hour at room temperature and incubated with the primary antibodies (rabbit anti-GFAP (1:100, Sigma-Aldrich), rabbit anti-AQP-4 (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-ICAM-1 (1:100, R&D Systems, Minneapolis, MN, USA), and mouse anti-CHOP (anti-CCAAT/-enhancer-binding protein homologous protein; 1:100, Cell Signaling Technology, Denver, MA, USA) for 18 hours at 4 °C. Tissue sections were washed thrice with phosphate-buffered saline and subsequently incubated with the secondary antibody (Alexa Fluor 488 (1:200, Abcam, Cambridge, UK) and the nuclear staining dye, DAPI (4,6-diamidino-2-phenylindole; 1:1000, Sigma-Aldrich) for 1 hour each at room temperature. The tissue sections were washed with phosphate-buffered saline and mounted in mounting medium (Leica). The slides were scanned under a Leica TCS SP3 laser confocal microscope (Leica) to obtain the confocal images.
To evaluate brain oxidative stress and apoptosis, the 5-μm sections were stained with gp91phox to assess the levels of reactive oxygen species and subjected to terminal deoxynucleotidyl transferase-mediated nick-end labeling (TUNEL)25 with a methyl green and TUNEL–avidin–biotin complex to assess apoptosis. Control sections were incubated with the normal Immunoglobulin G from the same species at the same protein concentration as the negative controls. Twenty high-power (× 400) fields were randomly selected for each section, and oxidative stress was assessed using a Sonix Image Setup (Sonix Technology Co., Ltd, Hsinchu, Taiwan) with the Carl Zeiss AxioVision Rel.4.8.2 software (Future Optics & Tech., Hangzhou, China).
Western Blotting Analysis
Western blotting was used to detect protein levels in the brain tissue, as described previously.11 Briefly, the total proteins were homogenized, separated, and transferred onto blocked polyvinylidene fluoride membranes (Millipore Corporation, Bedford, MA, USA). The blocked polyvinylidene fluoride membranes then incubated overnight with the following primary antibodies against the indicated proteins in blocking buffer (5% milk in 0.2% Tween 20/TBS) at 4 °C: AQP4 (1:1000, Santa Cruz Biotechnology), Bcl-2 (1:1000, Transduction, Bluegrass-Lexington, KY, USA), Bax (1:1000, Chemicon, Temecula, CA, USA), the activated fragment (17 kDa cleavage product) of caspase 3 (1:1000, Upstate Biotechnology, Lake Placid, NY, USA), N-terminal cleavage product of poly(ADP-ribose) polymerase (PARP; 1:400, Promega, Madison, WI, USA), CHOP (1:1000, Cell Signaling Technology), gp91phox (1:1000, Abcam, Suite Ambridge, MA, USA), ICAM-1 (1:1000, R&D Systems), NF-κB (1:1000, R&D Systems), eNOS (1:1000, BD Transduction Laboratory, BD Biosciences, San Jose, CA, USA), phospho-eNOS (1:1000, Cell Signaling Technology), and β-actin (1:1000, Sigma-Aldrich). The signal was visualized on film using a commercial enhanced chemiluminescence kit (Amersham Biosciences, Piscataway, NJ, USA). The density of the bands was determined semi-quantitatively by densitometry using an image analyzing system (Alpha Innotech, San Leandro, CA, USA).
Statistical Analysis
All data was presented as mean±s.e.m. The data were statistically analyzed by one-way analysis of variance, followed by Tukey's multiple comparison tests. The recognition index was calculated by dividing the number of actions of sniffing specified object by the total number of actions of sniffing all objects and was analyzed separately with respect to time on days 1, 3, and 7 after CAO. The recognition index data were analyzed by a one-way analysis of variance or by Kruskal–Wallis test, followed by the Tukey test for post hoc significant difference test. Results with P<0.05 were considered to be statistically significant. Statistical analyses were performed using the SPSS 18.0 software (IBM, Armonk, NY, USA).
Results
PEx-4 Microspheres
The PEx-4 microspheres were uniform in size (15.0±3.3 μm) and morphology (Figure 1B-1). After subcutaneous treatment in vivo, some of the PEx-4 microspheres appeared and clogged into the brain by its larger diameter than the capillary (Figure 1B-2), whereas some PEx-4 microspheres were degraded into small fragments and freely moved in the cortical microcirculation (Figure 1B–3∼B–5). The pharmacokinetics of PEx-4 and Ex-4 were examined for 14 days. After subcutaneous administration, both groups of rats showed a maximal plasma concentration at 2 hours (Figure 1C-1; F [2,14]=15.33, P<0.0001). The maximal plasma Ex-4 concentration 2 hours following Ex-4 administration was significantly higher (P=0.031) than that following PEx-4 treatment, implicating a high extent of burst release by Ex-4. From 6 hours to 14 days, the plasma Ex-4 concentration of the PEx-4 group was significantly (P=0.0001) higher than that of the Ex-4 group, indicating sustained release of PEx-4 (Figure 1C-2; F [2,14]=14.71, P<0.0001). The CSF Ex-4 concentration was directly associated with plasma Ex-4 (Figure 1D-1, F [2,14]=11.95, P<0.0001). The maximal CSF Ex-4 concentration 2 hours after Ex-4 treatment was significantly higher (P=0.024) than that following PEx-4 treatment. The CSF Ex-4 concentration of the PEx-4 group was significantly (P=0.016) higher than that of the Ex-4 group 6 to 24 hours after subcutaneous administration, indicating sustained release of Ex-4 into the plasma and brain from PEx-4. In normoglycemic control rats, subcutaneous administration of Ex-4 (P=0.019) or PEx-4 (P=0.032) significantly decreased blood glucose to hypoglycemic levels (87 and 92 mg/dL after treatment with Ex-4 and PEx-4, respectively) 6 hours after treatment, but PLGA vehicle microspheres did not produce this effect (P=0.69) (Figure 1D-2; F [5,29]=14.48, P<0.001). PEx-4 significantly (P=0.002 vs. Ex-4) decreased blood glucose levels from 12 hours to 14 days after treatment in DM rats, but Ex-4 had no effect on DM-induced hyperglycemia from 12 hours to 14 days after treatment.
CAO-Induced Forebrain Edema and AQP4 Expression
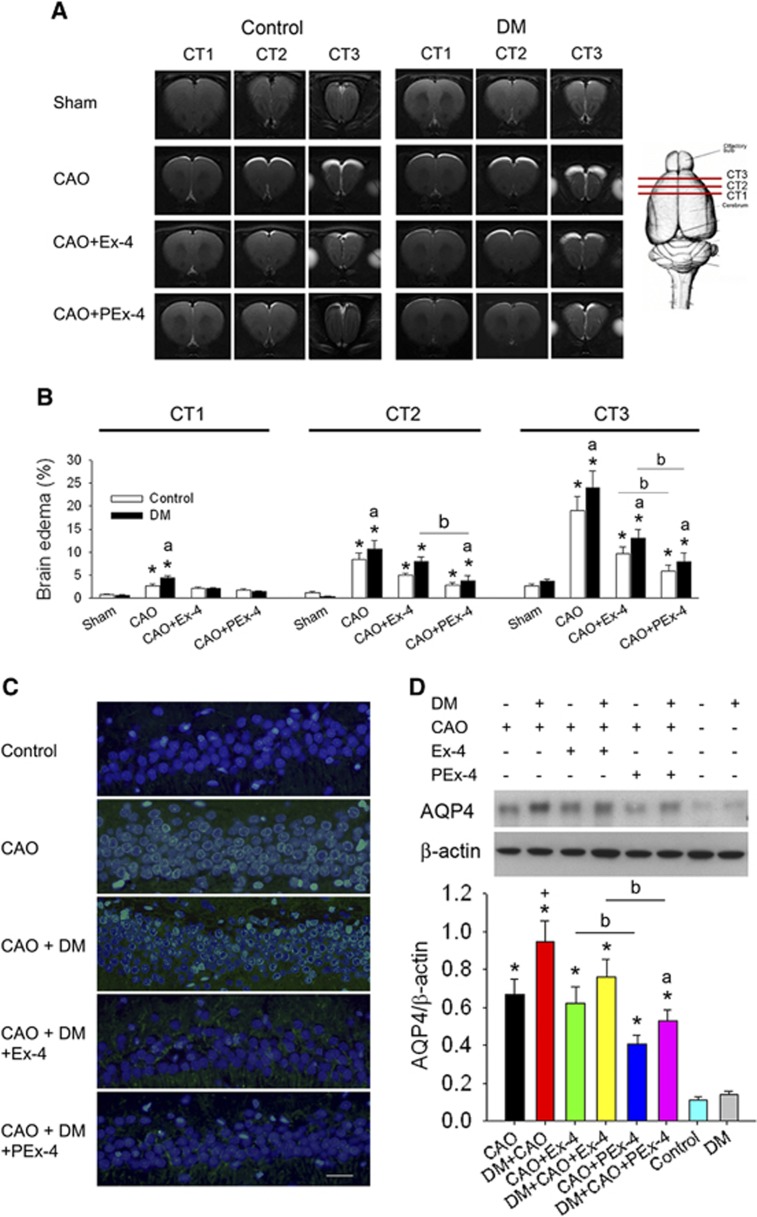
Brain edema was not observed via T2-weighted MRI in control or DM rats. Marked forebrain edema was noted in the CT1, CT2, and CT3 areas 24 hours after CAO (Figure 2A). The percentage of brain edema in these three areas was significantly (P<0.05) increased in the CAO and DM+CAO groups (Figure 2B; F [7,47]=11.69, P<0.0001). The DM+CAO group had a higher (P<0.05) percentage of brain edema in the CT1, CT2, and CT3 areas than the CAO group. Brain edema biomarker AQP4 immunofluorescence was significantly enhanced in the CAO (P=0.015) and DM+CAO (P=0.005) rats compared with control rats. Ex-4 and PEx-4 treatment reduced CAO-induced AQP4 immunofluorescence in the DM rats (Figure 2C). Western blotting determined that AQP4/β-actin ratios at baseline were 0.11±0.02 and 0.14±0.02 in the control and DM rats (Figure 2D; F [7,47]=10.41, P<0.0001). CAO significantly increased AQP4/β-actin ratios in CAO (P=0.022) and CAO+DM (P=0.0041) rats compared with control rats. Ex-4 did not significantly reduce AQP4/β-actin ratios in CAO (P=0.69) and CAO+DM (P=0.57) rats compared with CAO and CAO+DM rats. PEx-4 significantly decreased AQP4/β-actin ratios in CAO (P=0.034) and CAO+DM rats (P=0.041) compared with CAO+Ex-4 and CAO+DM+Ex-4 rats.
Figure 2.
Effect of exendin-4 (Ex-4) and Ex-4-loaded poly(D,L-lactide-co-glycolide) microspheres (PEx-4) on carotid artery occlusion (CAO)-induced brain edema in control and diabetes mellitus (DM) rats. (A) The T2-weighted magnetic resonance imaging series revealed that marked brain edema (high signal intensity area) appeared in the CT1, CT2, and CT3 areas of the CAO and DM+CAO groups but not in the sham-operated control and DM rats. PEx-4 reduced brain edema areas in CAO and DM+CAO rats more effectively than Ex-4. (B) CAO and DM+CAO rats had a significantly (P<0.05) higher percentage of brain edema in the CT1, CT2, and CT3 areas in comparison with sham control rats. PEx-4 treatment decreased the brain edema percentage more significantly (P<0.05) than Ex-4 in the CT2 and CT3 areas of CAO rats and in the CT2 and CT3 areas of DM+CAO rats. (C) Green fluorescence of aquaporin 4 (AQP4) immunostaining in astrocytes was markedly observed in the CAO and CAO+DM groups. PEx-4 and Ex-4 reduced AQP4 expression in the DM+CAO group. (D) Cortical AQP4 expression was significantly enhanced in CAO and DM+CAO rats in comparison with control rats. DM+CAO rats had higher AQP4 expression than CAO rats. Ex-4 showed a non-significant tendency (P=0.37) to reduce AQP4 expression in DM+CAO rats. PEx-4 significantly reduced the cortical AQP4 expression in comparison with Ex-4 treatment. Group differences (n=6 each) were compared by one-way analysis of variance, followed by Tukey's multiple comparison tests. P<0.05 indicates a significant difference. *Different from control (Con); #different from DM; +different from CAO; adifferent from DM+CAO; bdifferent from the Ex-4-treated group.
PEx-4 Attenuated CAO-Induced Oxidative Stress
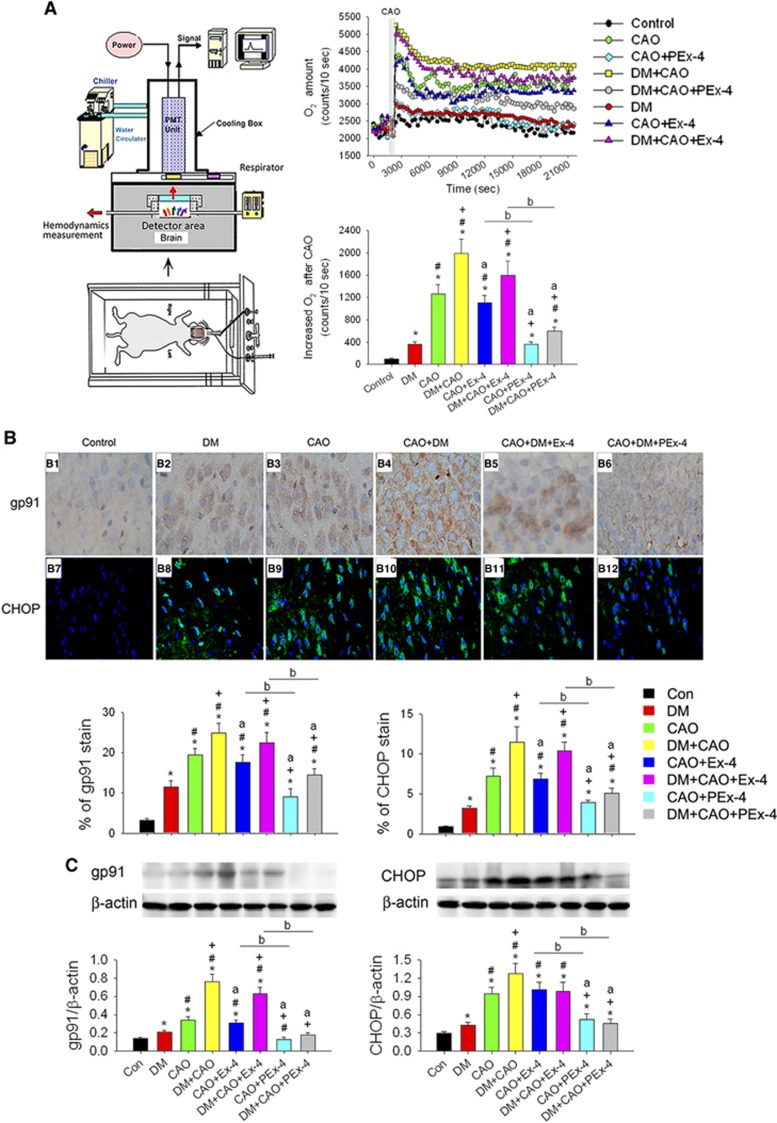
Representative brain O2− levels in the eight groups of rats are shown in Figure 3A. The rank order of the groups for the increase in real-time O2− level following CAO as measured by the area under the curve was: DM+CAO>DM+CAO+Ex-4>CAO>CAO+Ex-4>DM+CAO+PEx-4>CAO+PEx-4=DM>control (F [7,47]=16.90, P<0.0001). Figure 3B shows gp91 (F [7,47]=9.21, P<0.001) and CHOP (F [7,47]=10.02, P<0.001) expression in the rat cerebral cortex after CAO injury. There were low expression levels of gp91 and CHOP in control rat brains. DM and CAO each significantly (P<0.05) increased gp91 and CHOP expression compared with control rats, and gp91 and CHOP expression in the DM+CAO group was significantly higher than that of the DM (P=0.0032) or CAO (P= 0.015) groups. PEx-4 depressed gp91 and CHOP expression more effectively (P<0.05) than Ex-4 treatment in CAO and DM+CAO rats. DM, CAO, and DM+CAO significantly enhanced gp91 (P=0.041, P=0.023, and P=0.0022, respectively) and CHOP expression (P=0.044, P=0.0034, and P=0.0018, respectively) compared with control rats (Figure 3C; F [7,31]=9.89, P<0.001 in gp91 and F [7,31]=11.19, P<0.0001 in CHOP). PEx-4 inhibited gp91 (CAO+PEx-4 vs. CAO+Ex-4, P=0.030; DM+CAO+PEx-4 vs. DM+CAO+PEx-4, P=0.019) and CHOP expression (CAO+PEx-4 vs. CAO+Ex-4, P=0.029; DM+CAO+PEx-4 vs. DM+CAO+PEx-4, P=0.020) more effectively than Ex-4 in CAO and DM+CAO rats.
Figure 3.
The effect of exendin-4 (Ex-4) and Ex-4-loaded poly(D,L-lactide-co-glycolide) microspheres (PEx-4) on carotid artery occlusion (CAO)-induced oxidative stress and endoplasmic reticulum stress in control and diabetes mellitus (DM) rats. (A) The apparatus used for real-time and continuous measurement of cerebral O2− responses in the rat. The exposed rat brain was positioned under the detector for direct measurement of O2−. The resulting in vivo real-time original data and statistical data are shown for the eight groups (n=6 each). When compared with control rats (102±10 counts/10 seconds), the O2− level was significantly increased in DM (358±49 counts/10 seconds, P=0.019), CAO (1259±168 counts/10 s, P<0.001), and DM+CAO (1986±259 counts/10 seconds, P<0.001) rats. The O2− level from the brain surface in DM+CAO rats was higher (P=0.0041) than that of CAO rats. PEx-4 decreased the O2− level more effectively than Ex-4 in CAO and DM+CAO rats. (B) NADPH oxidase gp91 and CCAAT/-enhancer-binding protein homologous protein (CHOP) expression in the control and DM rat cerebral cortex after CAO injury were evaluated by immunohistochemistry and immunofluorescence (n=6 each). Brown gp91 staining (B1–B6) and green fluorescence of the CHOP (B6–B12) were observed in the brain sections from the DM, CAO, and DM+CAO groups. There were low expression levels of gp91 and CHOP in control rat brains. Expression levels of gp91 and CHOP expression were significantly increased in comparison with control rats. PEx-4 treatment depressed gp91 and CHOP expression more effectively than Ex-4 treatment in CAO and DM+CAO rats. (C) Western blotting for gp91 and CHOP protein expression in the eight groups (n=4 each). DM, CAO, and DM+CAO significantly enhanced gp91 and CHOP expression. PEx-4 treatment inhibited gp91 and CHOP expression more effectively than Ex-4 treatment in CAO and DM+CAO rats. Group differences were compared by one-way analysis of variance, followed by Tukey's multiple comparison tests. P<0.05 indicates a significant difference. *Different from control (Con); #different from DM; +different from CAO; adifferent from DM+CAO; bdifferent from the Ex-4-treated group.
PEx-4 Improved Cerebral Blood Flow and Microcirculation After CAO
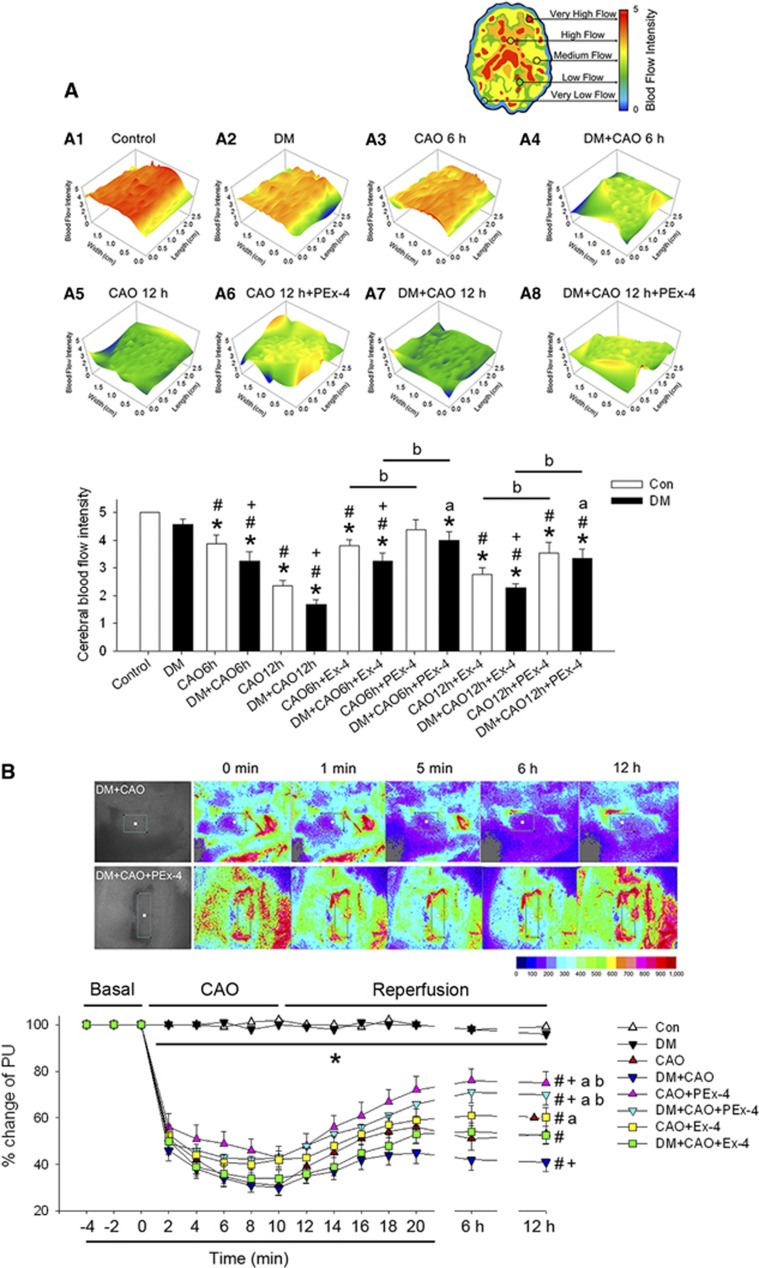
The original IRT image and calculated cerebral blood flow are shown in Figure 4A (F [13,83]=9.31, P<0.001). Compared with control rats, rats treated with DM showed mildly (P=0.059) decreased cerebral blood flow, whereas CAO significantly and time-dependently decreased cerebral blood flow 6 hours after CAO (P=0.039), 12 hours after CAO (P=0.028), 6 hours after DM+CAO (P=0.021), and 12 hours after DM+CAO (P<0.001). DM+CAO significantly (P<0.05) reduced cerebral blood flow compared with CAO. PEx-4 restored CAO-downregulated cerebral blood flow more effectively (P<0.05) than Ex-4 in CAO and DM+CAO rats 6 and 12 hours after CAO.
Figure 4.
Effect of exendin-4 (Ex-4) and Ex-4-loaded poly(D,L-lactide-co-glycolide) (PLGA) microspheres (PEx-4) on cerebral blood flow intensity and microcirculation following carotid artery occlusion (CAO) in control and diabetes mellitus (DM) rats. (A) The blood flow intensity as measured by infrared thermal imaging was scored using color in the indicated groups. In comparison with control rats, DM rats showed mildly (P=0.059) decreased cerebral blood flow, whereas CAO significantly and time-dependently decreased cerebral blood flow 6 hours after CAO (CAO6h, P=0.039), 12 h after CAO (CAO12h, P=0.028), 6 h after CAO in DM rats (DM+CAO6h, P=0.021), and 12 h after CAO in DM rats (DM+CAO12h, P<0.001). DM+CAO significantly (P<0.05) reduced cerebral blood flow in comparison with CAO. PEx-4 restored CAO-downregulated cerebral blood flow more effectively (P<0.05) than Ex-4 in CAO6h, CAO12h, DM+CAO6h, and DM+CAO12h rats. (B) Typical laser speckle imaging of perfusion by MoorFLPI at the indicated time points on a 16-level color palette in two DM+CAO rats receiving either saline containing PLGA vehicle (control) or PEx-4. Laser speckle imaging implicated that cerebral microvascular blood flow was markedly depressed during CAO and partially recovered during the reperfusion stage in control and DM rats. DM+CAO significantly (P<0.05) suppressed cerebral microvascular blood flow to a greater extent than CAO. PEx-4 restored CAO-downregulated cerebral microvascular blood flow more effectively (P<0.05) than Ex-4 in CAO12h and DM+CAO12h. Group differences (n=6 each) were compared by one-way analysis of variance, followed by Tukey's multiple comparison tests. P<0.05 indicates a significant difference. *Different from control (Con); #different from DM; +different from CAO; adifferent from DM+CAO; bdifferent from the Ex-4-treated group.
Laser speckle imaging indicated that cerebral microvascular blood flow was markedly depressed during CAO and partially recovered during the reperfusion stage in control and DM rats (Figure 4B; F [7,47]=7.15, P=0.0024). DM+CAO significantly (P<0.05) suppressed cerebral microvascular blood flow to a greater extent than CAO. PEx-4 restored CAO-downregulated cerebral microvascular blood flow more effectively (P<0.05) than Ex-4 in CAO and DM+CAO rats 12 hours after CAO.
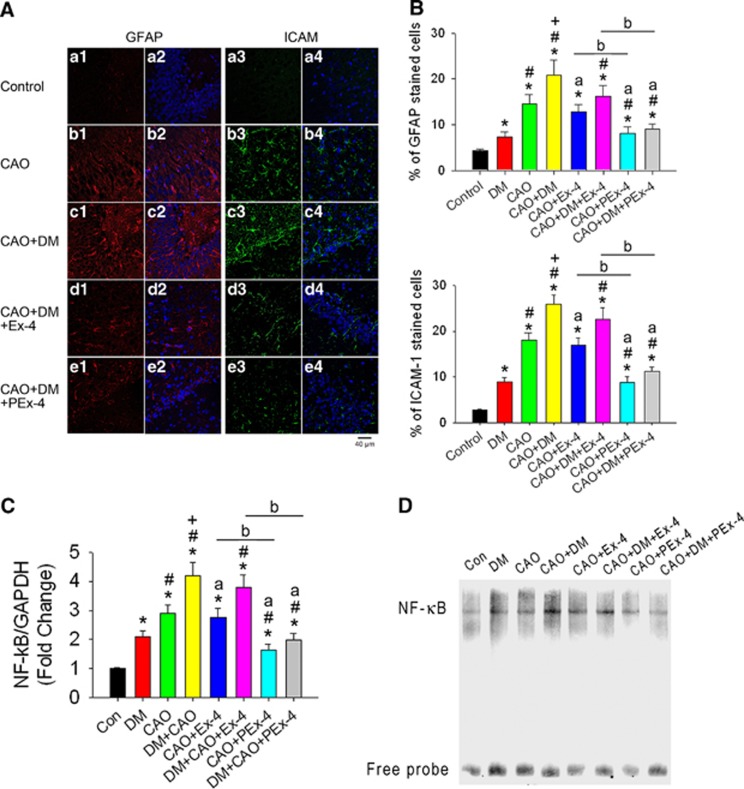
PEx-4 Attenuated CAO-Induced Neuroinflammation and Cognitive Deficits
Figure 5A shows immunofluorescence indicative of GFAP (red), ICAM-1-mediated inflammation (green), and DAPI (blue) in the hippocampal CA1 area. Expression levels of GFAP and ICAM-1 (Figure 5B; F [7,47]=15.96, P<0.001 in GFAP and F [7,47]=13.07, P<0.001 in ICAM-1) were significantly increased in the DM (P=0.039), CAO (P=0.016), and CAO+DM (P=0.0061) groups compared with the control group (Figure 5B). The highest levels of GFAP and ICAM-1 expression were found in the CAO+DM group. PEx-4 inhibited GFAP and ICAM-1 expression more effectively (P<0.05) than Ex-4 in the CAO and CAO+DM groups. Cortical NF-κB mRNA expression level was significantly increased in the DM (P=0.036), CAO (P=0.017), and DM+CAO (P=0.0074) groups compared with control rats. In DM+CAO rats, PEx-4 significantly decreased NF-κB (P=0.042) mRNA expression compared with Ex-4 (Figure 5C; F [7,23]=9.81, P<0.001 in NF-κB and F [7,23]=6.15, P<0.001). Gel shift of nuclear transcription factor NF-κB by EMSA confirmed the similar findings (Figure 5D).
Figure 5.
Effect of exendin-4 (Ex-4) and Ex-4-loaded poly(D,L-lactide-co-glycolide) microspheres (PEx-4) on carotid artery occlusion (CAO)-induced neuroinflammation in control and diabetes mellitus (DM) rats. (A) Positive glial fibrillary acidic protein (GFAP) cells (red fluorescence) and positive intercellular adhesion molecule-1 (ICAM-1) cells (green fluorescence) under × 40 magnification were observed in the CA1 area of the hippocampus in the DM, CAO, DM+CAO, DM+CAO+Ex-4, and DM+CAO+PEx-4 groups (n=6 each). (B) GFAP and ICAM-1 immunoreactivity was significantly (P<0.05) increased in DM rats in comparison with control rats (n=6 each). CAO significantly (P<0.05) enhanced GFAP and ICAM-1 expression in control and DM rats. PEx-4 reduced GFAP (P=0.042 vs. CAO+Ex-4; P=0.023 vs. DM+CAO+Ex-4) and ICAM-1 (P=0.021 vs. CAO+Ex-4; P=0.017 vs. DM+CAO+Ex-4) expression more effectively than Ex-4. (C) Cerebral cortical nuclear factor (NF)-κB mRNA/GAPDH (glyceraldehyde 3-phosphate dehydrogenase) ratios in eight groups (n=3 each) are shown. There were 2.1-, 2.9-, and 4.2-fold increases in NF-κB/GAPDH ratios, respectively, in the DM, CAO, DM+CAO rats in comparison with the control rats. Compared with DM+CAO rats, these increases in NF-κB (P=0.030) mRNA expression were significantly attenuated in DM+CAO+PEx-4 rats, whereas these increases were not significantly attenuated in DM+CAO+Ex-4 rats. (D) Gel shift of nuclear transcription factor NF-κB by electrophoretic mobility shift assay confirmed the similar findings to NF-κB mRNA/GAPDH ratios. Data are presented as mean±s.e.m.; n=6. Group differences were compared by one-way analysis of variance, followed by Tukey's multiple comparison tests. P<0.05 indicates a significant difference. *Different from control (Con); #different from DM; +different from CAO; adifferent from DM+CAO; bdifferent from the Ex-4-treated group.
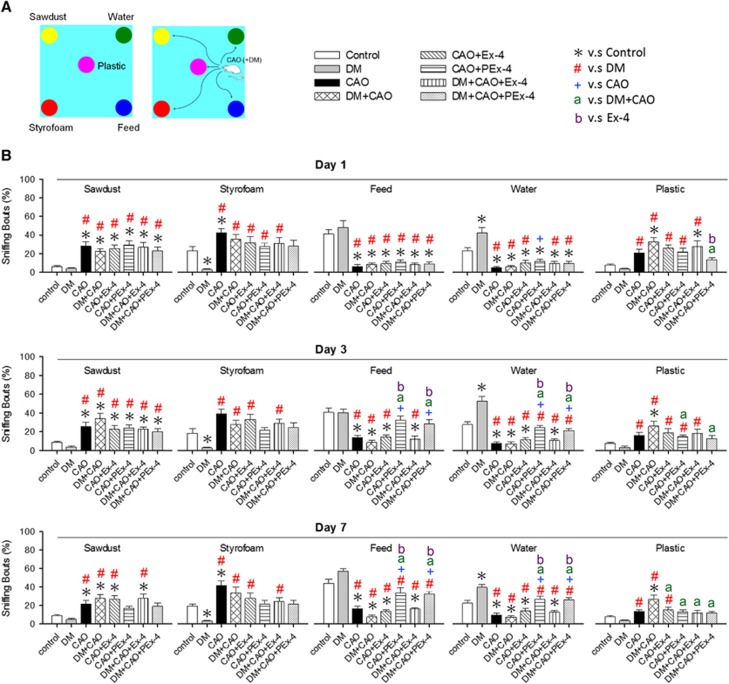
Figure 6 shows the design and results of the cognitive test. Control and DM rats showed a higher preference for feed- and water-sniffing bouts, whereas CAO and DM+CAO rats showed a significantly decreased preference for feed- and water-sniffing bouts compared with controls (F [7,79]=7.96, P<0.001). Tukey post hoc tests showed that, on day 3, the CAO+PEx-4 group had higher (P=0.02) feed-sniffing bouts than the CAO+Ex-4 group and the DM+CAO-PEx-4 group had significantly higher (P=0.047) feed-sniffing bouts than the DM+CAO+Ex-4 group. On day 7, the CAO+PEx-4 group showed higher (P=0.02) feed-sniffing bouts than the CAO+Ex-4 group, and the DM+CAO-PEx-4 group displayed higher (P=0.024) feed-sniffing bouts compared with the DM+CAO+Ex-4 group. On day 3, the CAO+PEx-4 group showed significantly higher (P=0.022) water-sniffing bouts than the CAO+Ex-4 group. On day 7, the CAO+PEx-4 group displayed higher water-sniffing bouts compared with the CAO+Ex-4 (P<0.001) group and the DM+CAO-PEx-4 group showed significantly higher (P=0.023) water-sniffing bouts than the DM+CAO+Ex-4 group.
Figure 6.
Ex-4-loaded poly(D,L-lactide-co-glycolide) microspheres (PEx-4) treatment is significantly more efficient than Ex-4 to improve carotid artery occlusion (CAO)-induced cognitive deficit in rats. The apparatus design used in this cognitive analysis is shown in panel A with five objects, including sawdust, styrofoam, feed, water, and plastic. Statistic data in panel B show that control and diabetes mellitus (DM) rats showed a higher preference for feed- and water-sniffing bouts, whereas CAO and DM+CAO rats showed a significantly decreased preference for feed- and water-sniffing bouts compared with controls (F [7,79]=7.96, P<0.001). Tukey post hoc tests showed that, on day 3, the CAO+PEx-4 group had higher (P=0.02) feed-sniffing bouts than the CAO+Ex-4 group and the DM+CAO-PEx-4 group had significantly higher (P=0.047) feed-sniffing bouts than the DM+CAO+Ex-4 group. On day 7, the CAO+PEx-4 group showed higher (P=0.02) feed-sniffing bouts than the CAO+Ex-4 group, and the DM+CAO-PEx-4 group displayed higher (P=0.024) feed-sniffing bouts compared with the DM+CAO+Ex-4 group. On day 3, the CAO+PEx-4 group showed significantly higher (P=0.022) water-sniffing bouts than the CAO+Ex-4 group. On day 7, the CAO+PEx-4 group displayed higher water-sniffing bouts compared with the CAO+Ex-4 (P<0.001) group and the DM+CAO-PEx-4 group showed significantly higher (P=0.023) water-sniffing bouts than the DM+CAO+Ex-4 group, indicating that PEx-4 treatment seems to partially restore cognitive ability. The recognition index indicated by sniffing bouts (%) data were analyzed separately with respect to time, on days 1, 3, and 7. The recognition index data were analyzed by one-way analysis of variance or by the Kruskal–Wallis test, respectively. Subsequently, the paired analyses were performed by the Tukey post hoc significant difference test. In this study, n=10 in each group is used. P<0.05 indicates a significant difference. *Different from control (Con); #different from DM; +different from CAO; adifferent from DM+CAO; bdifferent from the Ex-4-treated group.
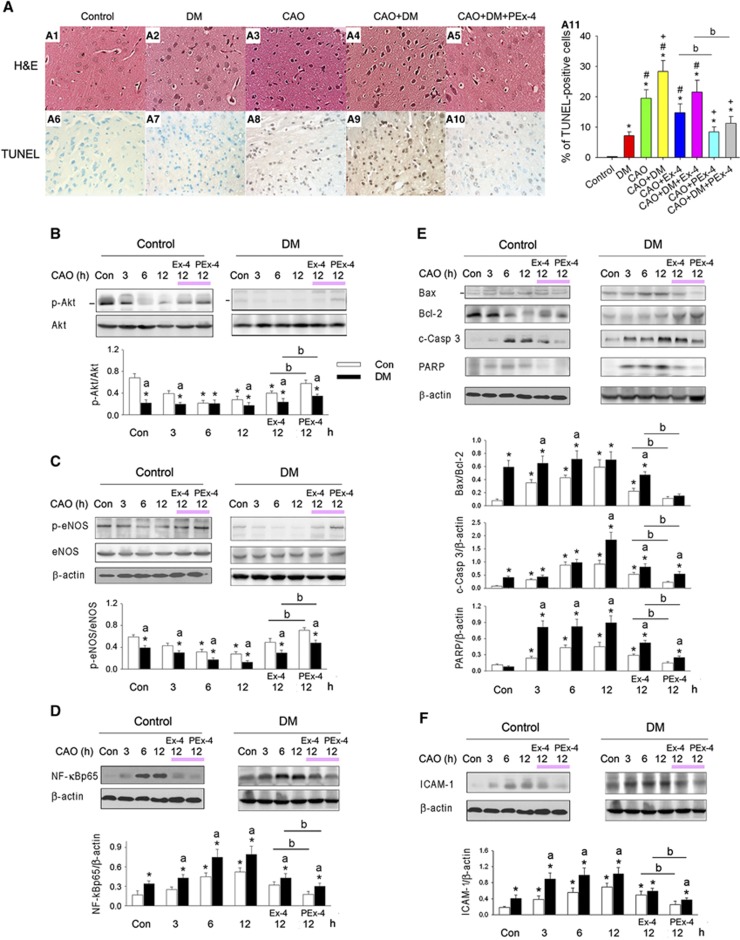
PEx-4 Reduced Cerebral Cortex Oxidative Damage 24 Hours After CAO
Hematoxylin and eosin staining of the cerebral cortex from control and DM rats revealed a clear cell outline, compact structure, and a clearly visible nucleolus, without any interstitial edema (Figure 7A1). Cells in the CAO or DM+CAO brains were sparsely arranged, with a fuzzy cell outline, disordered structure, swelling, interstitial edema, severe cell deformation, nuclear pyknosis, and necrosis (Figures 7A3 and A4). PEx-4 treatment significantly improved brain injury in CAO and DM+CAO rats, as indicated by relatively clear cell outlines and a more compact cellular structure (Figure 7A5). Additionally, swelling and interstitial edema were alleviated by PEx-4, and indices of neuronal cell necrosis were reduced; however, Ex-4 had no significant effect on these symptoms (data not shown).
Figure 7.
Effect of exendin-4 (Ex-4) and Ex-4-loaded poly(D,L-lactide-co-glycolide) microspheres (PEx-4) on carotid artery occlusion (CAO)-induced cerebral cortex injury in control and diabetes mellitus (DM) rats. (A) Representative photomicrographs (400 × magnification) of cerebral astrocyte cell sections stained with hematoxylin and eosin (H&E) (A1 to A5) and terminal deoxinucleotidyl transferase-mediated dUTP-fluorescein nick end labeling (TUNEL) stain (A6 to A10) 24 hours after CAO. Sham-treated rats exhibited a compact neuronal structure with well-characterized round nucleoli (A1). CAO caused neuronal injury that was associated with severe cell deformation, liquefactive necrosis, and triangular, pycnotic nuclei (A3, A4). Rats treated with PEx-4 exhibited significant protection against neuronal injury, with more intact neurons (A5) and reduced TUNEL staining (A10). The rank order for the number of TUNEL-positive cells was: DM+CAO>CAO>DM>control. PEx-4 reduced the number of TUNEL-positive cells in the CAO and DM+CAO rats significantly more effectively than Ex-4 (A11). The representative immunoblots of (B) Akt and p-Akt, (C) endothelial nitric oxide synthase (eNOS) and p-eNOS, (D) nuclear factor (NF)-κBp65, (E) Bcl-2, Bax, cleaved caspase 3 (c-Casp 3), poly ADP-ribose polymerase (PARP), and (F) intercellular adhesion molecule-1 (ICAM-1) in the control and DM rats treated with 20 μg/kg Ex-4 or PEx-4 are displayed. Downregulation in p-Akt and p-eNOS and upregulation in NF-κBp65, Bax/Bcl-2, c-Casp 3, PARP, and ICAM-1 are observed in DM, CAO and DM+CAO rats. PEx-4 restored p-Akt and p-eNOS expression more effectively (P<0.05) than Ex-4 in CAO and DM+CAO rats. PEx-4 reduced NF-κBp65, Bax/Bcl-2, c-Casp 3, PARP, and ICAM-1 expression more effectively (P<0.05) than Ex-4 in CAO and DM+CAO rats. Data are presented as mean±s.e.m.; n=6. Group differences were compared by one-way analysis of variance, followed by Tukey's multiple comparison tests. P<0.05 indicates a significant difference. *Different from control (Con); #different from DM; +different from CAO; adifferent from DM+CAO; bdifferent from the Ex-4-treated group.
TUNEL-positive cells were significantly increased in rats treated with DM (P=0.021), CAO (P<0.001), and DM+CAO (P<0.001) compared with control rats (Figure 7A11; F [7,47]=8.78, P<0.001). DM+CAO rats had more TUNEL-positive cells than DM (P=0.0044) or CAO (P=0.013) rats. Ex-4 did not significantly decrease TUNEL staining in CAO (P=0.070) or DM+CAO (P=0.061) rats, whereas PEx-4 significantly reduced TUNEL staining in CAO (P=0.026) and DM+CAO (P=0.018) rats.
Effect of PEx-4 on DM and CAO-Induced Changes in Protein Levels
p-Akt expression was significantly and time-dependently downregulated in rats treated with DM (P=0.011), after CAO 6 hours (CAO6h, P=0.014), after CAO 12 hours (CAO12h, P=0.023), after CAO 6 hours in DM (DM+CAO6h, P=0.017), or after CAO 12 hours in DM (DM+CAO12h, P=0.005) compared with control rats (Figure 7B; F [11,35]=8.75, P<0.001). PEx-4 restored p-Akt expression more effectively (P<0.05) than Ex-4 in CAO and DM+CAO rats.
p-eNOS expression was significantly and time-dependently downregulated in rats treated with DM (P=0.038), CAO6h (P=0.036), CAO12h (P=0.039), DM+CAO6h (P=0.012), or DM+CAO12h (P=0.003) compared with control rats (Figure 7C; F [11,35]=17.14, P<0.0001). PEx-4 restored p-eNOS expression more effectively (P<0.05) than Ex-4 in CAO and DM+CAO rats.
NF-κBp65 expression was significantly and time-dependently upregulated in rats treated with DM (P=0.032), CAO6h (P=0.024), CAO12h (P=0.019), DM+CAO6h (P=0.0091), or DM+CAO12h (P=0.0074) compared with control rats (Figure 7D; F [11,35]=19.28, P<0.0001). PEx-4 reduced NF-κBp65 expression more effectively (P<0.05) than Ex-4 in CAO and DM+CAO rats.
Bax/Bcl-2 ratios, caspase 3 expression, and PARP expression were significantly (P<0.05) and time-dependently upregulated in rats treated with DM, CAO6h, CAO12h, DM+CAO6h, or DM+CAO12h compared with control rats (Figure 7E; F [11,35]=10.29, P<0.001 in Bax/Bcl-2, F [11,35]=9.38, P<0.001 in capspase 3 and F [11,35]=15.30, P<0.0001 in PARP). PEx-4 reduced Bax/Bcl-2 ratios, caspase 3 expression, and PARP expression more effectively (P<0.05) than Ex-4 in CAO and DM+CAO rats.
ICAM-1 expression was significantly and time-dependently upregulated in rats treated with DM (P=0.037), CAO6h (P=0.028), CAO12h (P=0.019), DM+CAO6h (P=0.011), or DM+CAO12h (P=0.012) compared with control rats (Figure 7F; F [11,35]=16.71, P<0.0001). PEx-4 depressed ICAM-1 expression more effectively (P<0.05) than Ex-4 in CAO and DM+CAO rats.
Discussion
Our prepared PEx-4 microspheres had a narrow size distribution and a simple low-cost production process and throughput. In comparison with Ex-4, PEx-4 showed sustained release of Ex-4 into the plasma and CSF over 14 days, reducing blood glucose levels and CAO injury in DM rats. In comparison with Ex-4, PEx-4 more effectively improved cerebral cortical blood flow intensity, cerebral microcirculation, and neurologic and cognitive deficits and more effectively decreased cerebral cortical reactive oxygen species and oxidative stress, brain edema area, and AQP4 and GFAP expression.
Our prepared PEx-4 (15±3.3 μm), as well as a previously reported Ex-4-loaded PLGA microspheres (11.2±1.2 μm) demonstrating long circulating PEx-4,29 displayed low burst release and high Ex-4-sustained release to decrease the DM-induced hyperglycemia. PLGA microspheres provide a protective steric barrier against nucleophilic reactions between Ex-4 and the polymers; therefore, PLGA degradation in the microspheres leads to substantial Ex-4 levels.29 We observed that PEx-4 displayed higher and sustained levels of CSF Ex-4 compared with Ex-4, 6 to 24 hours after subcutaneous treatment, to ameliorate CAO injury in DM rats. When adjusted for total protein, three times higher quantities of Ex-4 were observed to have released from PEx-4 into the plasma, compared with CSF. CSF is segregated from plasma by the blood–brain barrier (BBB). We detected Ex-4 in the CSF as a result of PEx-4 treatment, indicating that Ex-4 enters the brain parenchyma by directly penetrating the fenestrated endothelium of blood vessels in the choroid plexus because GLP-1 and Ex-4 molecules are relatively small in the central nervous system, with both diffusing readily across the BBB and accessing the brain parenchyma rapidly and directly by their high lipophilicity (not being trapped in the endothelial cells comprising the BBB)30. As the diameter of a brain capillary is approximately 5 to 6 μm31, some of our prepared PEx-4 microspheres were clogged and the others were biodegraded into small fragments in the cortical microcirculation to release Ex-4 possibly by way of diffusion through water-filled pores or polymers, osmotic pumping, and erosion into the brain parenchyma. The degradation of PEx-4 microspheres in vivo may be due to mechanical degradation by blood pressure, chemical digestion, or physical osmotic pressure and so on. However, this must be investigated in detail in further studies. The release of Ex-4 from degradable PEx-4 then activated GLP-1R in the brain, leading to the effects on the brain cerebral blood flow intensity and microcirculation. Under DM and stroke insults, BBB endothelial leakage was enhanced,32, 33 possibly resulting in more PEx-4 microspheres and fragments entry into the brain parenchyma.
CAO initiates a complex cascade to produce excess reactive oxygen species generation and endoplasmic reticulum stress, leading to apoptotic and necrotic brain cell death.34 Ischemia causes a sudden increase in the activity of NOS in neurons and the vascular endothelium, infiltrating neutrophils and macrophages, and activated microglia and astrocytes,5 leading to neuroinflammation associated with BBB dysfunction and cerebral edema. Activation of astrocytes in neuroinflammation has been implicated in the pathogenesis of acute traumatic brain injury and upregulation of AQP4 and GFAP expression involved in neuroinflammation, edema formation, and astrogliosis after stroke injury.5, 6 Downregulation of GFAP expression in hippocampal astrocytes improved hippocampal neuroinflammation and cognitive deficits.35 Increased Ex-4 from PEx-4 provides effective neuroprotection, because it is a small molecule that can cross the BBB,30, 36 and Ex-4 attenuates BBB endothelial leakage partially through decreased ICAM-1 expression.37 In comparison with Ex-4, PEx-4 more effectively reduced CAO-enhanced brain NADPH oxidase gp91, CHOP, AQP4, ICAM-1, and GFAP expression and improved CAO-evoked neurologic and cognitive deficits.
Pretreatment with insulin before CAO significantly increased the number of surviving CA1 pyramidal cells in the hippocampus 5 days after reperfusion.38 Acute and chronic insulin treatment decreased brain lesion volume and the number of apoptotic cells in DM+CAO rats.39 Previous studies have reported that Ex-4 treatment did not influence serum insulin levels.36 Our data showed that PEx-4 did not affect insulin levels, but it significantly reduced blood glucose in DM animals. PEx-4 provides neuroprotection against CAO injury via insulin-independent pathways.
Endothelial dysfunction has been demonstrated to be one of the earliest detectable events in DM-associated ischemic stroke, and impairment of eNOS-dependent vascular function may contribute to a reduction in cerebral blood flow during a stroke. Abnormalities in p-eNOS expression are an important common pathway that links diverse cardiovascular risks (such as DM, obesity, and metabolic syndrome) with endothelial dysfunction to increase the risk for ischemic stroke.40 Insulin and Ex-4 can activate eNOS through Akt-mediated S1179 phosphorylation, thereby increasing blood flow and cell survival.40 Our study indicated that p-eNOS and p-Akt levels were greatly increased in the cerebral cortex after PEx-4 treatment. PEx-4 enhanced p-Akt and p-eNOS expression more effectively than did Ex-4 and depressed NF-κBp65/ICAM-1 and apoptosis-related protein expression in the CAO cerebral cortex. In addition, our results confirmed that DM attenuated increased cerebral eNOS/Akt phosphorylation in parallel with more severe neurologic deficits following CAO. Inhibition of eNOS significantly decreased the protective effects of Ex-4,11, 40 suggesting that normal vascular endothelial function is essential for insulin-mediated protection against CAO injury in diabetic rats.
Ex-4 upregulated cAMP and triggered phosphorylation of cAMP response element-binding protein, which increased Bcl-2 expression and inhibited apoptosis.36 The activation of the Akt pathway in ischemic regions is a protective event that is triggered to protect the tissue from the damage induced by hypoxia or ischemia/reperfusion through regulation of eNOS signaling.11 Our data showed that PEx-4 enhanced protective p-Akt/p-eNOS signaling more effectively than Ex-4.
In summary, PEx-4 provided more neuroprotection compared with Ex-4 against CAO-induced oxidative stress, inflammation, cerebral cortex edema, and neurologic deficits in diabetic rats. These protective effects of PEx-4 were associated with inhibition of gp91 and CHOP-mediated endoplasmic reticulum stress, Bax/Bcl-2/caspase 3/PARP-mediated apoptosis, NF-κB/ICAM-1-mediated inflammation, and GFAP-induced neurodegeneration, possibly through p-AKT/p-eNOS-mediated pathways. These results were encouraging for the possible therapeutic use of GLP-1R agonist-loaded PLGA microspheres to treat neuronal and cardiovascular complications of DM.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Author Contributions
Chiang-Ting Chien and Ping-Chia Li substantially contributed to conception and design, acquisition of data, and analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. Ming-Jia Jou, Tai-Yu Cheng, Chih-Hui Yang, and Tzu-Ying Yu substantially contributed to acquisition of data and analysis and interpretation of data.
This work was supported by grants from the National Science Council of the Republic of China (NSC102-2320-B-003-001-MY3, NSC98-2314-B-214-001-MY3) and from I-Shou University (ISU102-07-02, ISU104-0704A).
Supplementary Material
References
- 1Jeerakathil T, Johnson JA, Simpson SH, Majumdar SR. Short-term risk for stroke is doubled in persons with newly treated type 2 diabetes compared with persons without diabetes: a population-based cohort study. Stroke 2007; 38: 1739–1743. [DOI] [PubMed] [Google Scholar]
- 2Laing SP, Swerdlow AJ, Carpenter LM, Slater SD, Burden AC, Botha JL et al. Mortality from cerebrovascular disease in a cohort of 23000 patients with insulin-treated diabetes. Stroke 2003; 34: 418–421. [DOI] [PubMed] [Google Scholar]
- 3Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke 2007; 38: 1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813–820. [DOI] [PubMed] [Google Scholar]
- 5Fukuda AM, Badaut J. Aquaporin 4: a player in cerebral edema and neuroinflammation. J Neuroinflammation 2012; 9: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Brahmachari S, Fung YK, Pahan K. Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J Neurosci 2006; 26: 4930–4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Wu L, Olverling A, Huang Z, Jansson L, Chao H, Gao X et al. GLP-1, exendin-4 and C-peptide regulate pancreatic islet microcirculation, insulin secretion and glucose tolerance in rats. Clin Sci (Lond) 2012; 122: 375–384. [DOI] [PubMed] [Google Scholar]
- 8Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Bonner-Weir S, Habener JF et al. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein idx-1 and increase islet size in mouse pancreas. Diabetes 2000; 49: 741–748. [DOI] [PubMed] [Google Scholar]
- 9Zhou J, Wang X, Pineyro MA, Egan JM. Glucagon-like peptide 1 and exendin-4 convert pancreatic ar42j cells into glucagon- and insulin-producing cells. Diabetes 1999; 48: 2358–2366. [DOI] [PubMed] [Google Scholar]
- 10Grieve DJ, Cassidy RS, Green BD. Emerging cardiovascular actions of the incretin hormone glucagon-like peptide-1: potential therapeutic benefits beyond glycaemic control? Br J Pharmacol 2009; 157: 1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Chien CT, Fan SC, Lin SC, Kuo CC, Yang CH, Yu TY et al. Glucagon-like peptide-1 receptor agonist activation ameliorates venous thrombosis-induced arteriovenous fistula failure in chronic kidney disease. Thromb Haemost 2014; 112: 1051–1064. [DOI] [PubMed] [Google Scholar]
- 12Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther 2002; 302: 881–888. [DOI] [PubMed] [Google Scholar]
- 13Teramoto S, Miyamoto N, Yatomi K, Tanaka Y, Oishi H, Arai H et al. Exendin-4, a glucagon-like peptide-1 receptor agonist, provides neuroprotection in mice transient focal cerebral ischemia. J Cereb Blood Flow Metab 2011; 31: 1696–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Hendarto H, Inoguchi T, Maeda Y, Ikeda N, Zheng J, Takei R et al. Glp-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase a-mediated inhibition of renal nad(p)h oxidases. Metabolism 2012; 61: 1422–1434. [DOI] [PubMed] [Google Scholar]
- 15Wei Q, Sun YQ, Zhang J. Exendin-4: a glucagon-like peptide-1 receptor agonist, inhibits cell apoptosis induced by lipotoxicity in pancreatic beta-cell line. Peptides 2012; 37: 18–24. [DOI] [PubMed] [Google Scholar]
- 16Darsalia V, Mansouri S, Ortsater H, Olverling A, Nozadze N, Kappe C et al. Glucagon-like peptide-1 receptor activation reduces ischaemic brain damage following stroke in type 2 diabetic rats. Clin Sci (Colch) 2012; 122: 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Wang D, Luo P, Wang Y, Li W, Wang C, Sun D et al. Glucagon-like peptide-1 protects against cardiac microvascular injury in diabetes via a camp/pka/rho-dependent mechanism. Diabetes 2013; 62: 1697–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Favilla CG, Mesquita RC, Mullen M, Durduran T, Lu X, Kim MN et al. Optical bedside monitoring of cerebral blood flow in acute ischemic stroke patients during head-of-bed manipulation. Stroke 2014; 45: 1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014; 63: 3346–3358. [DOI] [PubMed] [Google Scholar]
- 20Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology 2008; 149: 4059–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Tomura S, de Rivero Vaccari JP, Keane RW, Bramlett HM, Dietrich WD. Effects of therapeutic hypothermia on inflammasome signaling after traumatic brain injury. J Cereb Blood Flow Metab 2012; 32: 1939–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Lin YS, Yang CH, Wu CT, Grumezescu AM, Wang CY, Hsieh WC et al. A microfluidic chip using phenol formaldehyde resin for uniform-sized polycaprolactone and chitosan microparticle generation. Molecules 2013; 18: 6521–6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Pegg CC, He C, Stroink AR, Kattner KA, Wang CX. Technique for collection of cerebrospinal fluid from the cisterna magna in rat. J Neurosci Methods 2010; 187: 8–12. [DOI] [PubMed] [Google Scholar]
- 24Sugawara T, Lewen A, Noshita N, Gasche Y, Chan PH. Effects of global ischemia duration on neuronal, astroglial, oligodendroglial, and microglial reactions in the vulnerable hippocampalca1 subregion in rats. J Neurotrauma 2002; 19: 85–98. [DOI] [PubMed] [Google Scholar]
- 25Chien CT, Lee PH, Chen CF, Ma MC, Lai MK, Hsu SM. De novo demonstration and co-localization of free-radical production and apoptosis formation in rat kidney subjected to ischemia/reperfusion. J Am Soc Nephrol 2001; 12: 973–982. [DOI] [PubMed] [Google Scholar]
- 26Lecrux C, McCabe C, Weir CJ, Gallagher L, Mullin J, Touzani O et al. Effects of magnesium treatment in a model of internal capsule lesion in spontaneously hypertensive rats. Stroke 2008; 39: 448–454. [DOI] [PubMed] [Google Scholar]
- 27Bagavathiappan S, Saravanan T, Philip J, Jayakumar T, Raj B, Karunanithi R et al. Infrared thermal imaging for detection of peripheral vascular disorders. J Med Phys 2009; 34: 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Cheng J, Alkayed NJ, Hurn PD. Deleterious effects of dihydrotestosterone on cerebral ischemic injury. J Cereb Blood Flow Metab 2007; 27: 1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Lim SM, Eom HN, Jiang HH, Sohn M, Lee KC. Evaluation of PEGylated exendin-4 released from poly (Lactic-co-Glycolic Acid) microspheres for antidiabetic therapy. J Pharm Sci 2015; 104: 72–80. [DOI] [PubMed] [Google Scholar]
- 30Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord 2003; 27: 313–318. [DOI] [PubMed] [Google Scholar]
- 31Duelli R, Kuschinsky W. Changes in brain capillary diameter during hypocapnia and hypercapnia. J Cereb Blood Flow Metab 1993; 13: 1025–1028. [DOI] [PubMed] [Google Scholar]
- 32Kuntz M, Mysiorek C, Pétrault O, Pétrault M, Uzbekov R, Bordet R et al. Stroke-induced brain parenchymal injury drives blood-brain barrier early leakage kinetics: a combined in vivo/in vitro study. J Cereb Blood Flow Metab 2014; 34: 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Ye X, Chopp M, Cui X, Zacharek A, Cui Y, Yan T et al. Niaspan enhances vascular remodeling after stroke in type 1 diabetic rats. Exp Neurol 2011; 232: 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Love S. Oxidative stress in brain ischemia. Brain Pathol 1999; 9: 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Chin Y, Kishi M, Sekino M, Nakajo F, Abe Y, Terazono Y et al. Involvement of glial P2Y1 receptors in cognitive deficit after focal cerebral stroke in a rodent model. J Neuroinflammation 2013; 10: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Teramoto S, Miyamoto N, Yatomi K, Tanaka Y, Oishi H, Arai H et al. Exendin-4, a glucagon-like peptide-1 receptor agonist, provides neuroprotection in mice transient focal cerebral ischemia. J Cereb Blood Flow Metab 2011; 31: 1696–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Fan Y, Liu K, Wang Q, Ruan Y, Ye W, Zhang Y. Exendin-4 alleviates retinal vascular leakage by protecting the blood-retinal barrier and reducing retinal vascular permeability in diabetic goto-kakizaki rats. Exp Eye Res 2014; 127: 104–116. [DOI] [PubMed] [Google Scholar]
- 38Hui L, Pei DS, Zhang QG, Guan QH, Zhang GY. The neuroprotection of insulin on ischemic brain injury in rat hippocampus through negative regulation of jnk signaling pathway by pi3k/akt activation. Brain Res 2005; 1052: 1–9. [DOI] [PubMed] [Google Scholar]
- 39Rizk NN, Rafols JA, Dunbar JC. Cerebral ischemia-induced apoptosis and necrosis in normal and diabetic rats: effects of insulin and c-peptide. Brain Res 2006; 1096: 204–212. [DOI] [PubMed] [Google Scholar]
- 40Huang SS, Lu YJ, Huang JP, Wu YT, Day YJ, Hung LM. The essential role of endothelial nitric oxide synthase activation in insulin-mediated neuroprotection against ischemic stroke in diabetes. J Vasc Surg 2014; 59: 483–491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.