Abstract
Dopamine is the predominant catecholamine in the brain and functions as a neurotransmitter. Dopamine is also a potent immune modulator. In this study, we have characterized the expression of dopamine receptors on murine microglia. We found that cultured primary microglia express dopamine D1, D2, D3, D4, and D5 receptors. We specifically focused on the D2 receptor (D2R), a major target of antipsychotic drugs. Whereas D2Rs were strongly expressed on striatal neurons in vivo, we did not detect any D2R expression on resident microglia in the healthy brains of wild-type mice or transgenic mice expressing the green fluorescent protein (GFP) under the control of the Drd2 promoter. However, cerebral ischemia induced the expression of D2R on Iba1-immunoreactive inflammatory cells in the infarct core and penumbra. Notably, D2R expression was confined to CD45hi cells, and GFP BM chimeras revealed that D2R was expressed on activated resident microglia as well as on peripherally derived macrophages in the ischemic brain. Importantly, the D2/3R agonist, pramipexole, enhanced the secretion of nitrite by cultured microglia in response to proinflammatory stimuli. Thus, dopamine may serve as a modulator of microglia function during neuroinflammation.
Keywords: cerebral ischemia, dopamine receptor, inflammation, macrophages, microglia
Introduction
The pathophysiology of cerebral ischemia has been thoroughly studied in search of novel treatments for stroke.1 Neuronal energy deficit, excitotoxicity, and calcium overload are the major factors contributing to immediate cell death after cerebral ischemia.1, 2 The early stages of ischemic injury are followed by a protracted inflammatory reaction that comprises the activation of resident microglia, the infiltration of blood-borne monocytes/macrophages, and finally the recruitment of neutrophils and lymphocytes.3, 4 Notably, the postischemic inflammation contributes to delayed neural injury, but at the same time sets the stage for tissue remodeling and repair.1
Dopamine is the predominant catecholamine neurotransmitter in the central nervous system involved in the control of motor function, cognition, reward, and neuroendocrine secretion.5 Dopaminergic transmission regulates axonal plasticity and modulates adult neurogenesis.6, 7 Treatment with the dopamine precursor, levodopa, not only attenuates the motor symptoms of Parkinson's disease, but also improves motor learning in chronic stroke patients.8 Moreover, levodopa in combination with physiotherapy was found to enhance motor recovery after stroke in a prospective, randomized, placebo-controlled study.9 Importantly, reactive astrocytes de novo express dopamine D1 receptors (D1Rs) and dopamine D2 receptors (D2Rs) after experimental stroke in rats, and respond to levodopa treatment with increased production of glial cell-line-derived neurotrophic factor.10, 11 Thus, glial cells might contribute to the levodopa-induced improvement in sensorimotor function after cerebral ischemia.10
Recent evidence suggests that dopamine also regulates innate immunity in the nervous system. Notably, astroglial D2R tightly controls the expression of αB-crystallin and thereby suppresses neuroinflammation.12 Dopamine induced by sciatic nerve electroacupuncture was found to suppress systemic inflammation and inhibit the production of proinflammatory cytokines via D1R.13 Dopamine can also attenuate inflammation in the postischemic brain. Thus, levodopa treatment after experimental stroke reduced the infiltration of cytotoxic T lymphocytes as well as the expression of endothelial intercellular adhesion molecule 1 in the ischemic brain.14 In this study, we sought to determine the expression of dopamine receptors on microglia and macrophages, the innate immune cells of the central nervous system.15 We found that activated microglia and peripherally derived monocytes/macrophages de novo express D2R after experimental stroke in mice.
Materials and methods
Animals
Wild-type C57BL/6J mice were obtained from the Research Institutes for Experimental Medicine (FEM), Charité—Universitätsmedizin Berlin. All animal experiments were approved by an independent ethics committee (Landesamt für Gesundheit und Soziales, Berlin, Germany) and performed in accordance with national and international guidelines for the care and use of laboratory animals (Tierschutzgesetz der Bundesrepublik Deutschland, European directive 2010/63/EU, as well as GV-SOLAS and FELASA guidelines and recommendations for laboratory animal welfare). The experiments were reported according to the ARRIVE guidelines. Mice were kept under standard housing conditions with a 12-hour light/dark cycle and free access to standard food and water.
Generation of Bone Marrow Chimeras
Bone marrow (BM) chimeric mice were generated as described previously.3 Briefly, BM was harvested from femurs and tibias of 8- to 12-week-old C57BL/6J mice 48 hours after treatment with 150 mg/kg 5-fluoruracil (Sigma-Aldrich, Deisenhofen, Germany). Subsequently, BM cells were transduced with a murine stem cell virus-based retroviral vector encoding the enhanced green fluorescent protein (GFP). After transduction, 5 × 106 unsorted BM cells were transplanted into lethally irradiated (11 Gy) adult male C57BL/6J mice by tail vein injection. Successful reconstitution of hematopoiesis was assessed by flow-cytometric analysis of GFP expression in peripheral blood leukocytes (on average 80%). Transient focal cerebral ischemia was induced 12 weeks after BM transplantation in the GFP BM chimeras.
Middle Cerebral Artery Occlusion
Transient focal cerebral ischemia was induced in 8-week-old male mice by occlusion of the middle cerebral artery (MCAO). During surgery, anesthesia was maintained with 1.5% to 1.0% halothane in 70% N2O/30% O2, and body temperature was kept constant at 37°C to 37.5°C using a heating pad. A silicone-coated monofilament (diameter: 0.21±0.02 mm) was inserted through the common carotid artery and advanced up to within the left internal carotid artery until it occluded the middle cerebral artery. After 60 minutes of occlusion, the filament was withdrawn during a second anesthesia to allow for reperfusion. Animals were killed at 1, 3, 5, 7, or 14 days after MCAO.16
Flow Cytometry
Brain immune cells were harvested using a percoll gradient as described previously.17 Cells were stained with primary antibodies directed against CD45 (1:200; BioLegend, San Diego, CA, USA) and D2R (1:400; Frontier Institute, Hokkaido, Japan) at 4°C for 15 minutes. Cells were washed and then analyzed using a FACSCanto II flow cytometer (Becton Dickinson, Heidelberg, Germany). Viable cells were gated by forward and side scatter pattern. Data were acquired with the FACSdiva software (Becton Dickinson). Postacquisition analysis was performed using the FlowJo software (Tree Star Inc., Ashland, OR, USA).
Neuronal Cell Cultures
Primary murine embryonic cortical neurons were prepared, cultured, and transfected as described previously.18 The human DRD2L cDNA (OpenBiosystems, Lafayette, CO, USA) was subcloned by PCR amplification into a pCAG-mCherry plasmid that we described previously,18 generating a C-terminal fusion of mCherry with hD2RL (pCAG-hD2RL-mCherry). The plasmid was verified by DNA sequencing (LGC Genomics, Berlin, Germany). After transfection, primary cortical neurons were cultured for 7 days and then analyzed by immunocytochemistry.
Glial Cell Cultures
Mixed glial cell cultures were prepared from the cortices of 3-day-old C57BL/6J mice as described previously.19 Cells were cultured in DMEM (4.5 g glucose) supplemented with 10% FBS, 1% L-glutamine and 1% penicillin/streptomycin (Biochrom, Berlin, Germany) for 12 days. Microglia were removed from the astrocyte monolayer by shaking on a dual action shaker (KL-2, Edmund Bühler GmbH, Hechingen, Germany). for 1 hour. Microglia were directly collected into Trizol (Life Technologies, Darmstadt, Germany) for cDNA synthesis and PCR analysis. Alternatively, they were seeded onto uncoated coverslips in 24-well plates at a density of 7 × 104 cells/cm2 and cultured in DMEM supplemented with 10% FBS and L-glutamine for immunocytochemical analysis, or in 48-well plates for pharmacological experiments. The purity of microglial cultures was determined to be >95%. After shaking of mixed glial cultures, astrocytes were harvested by trypsinization and collected into Trizol or seeded onto poly-L-lysine-coated coverslips in 24-well plates at a density of ~105 cells/well.
Pharmacological Assays
Primary microglia (12 to 13 days in vitro) were pretreated for 3 hours with increasing doses of the D2/3R agonist, pramipexole dihydrochloride (Tocris Bioscience, Wiesbaden, Germany), and subsequently stimulated with 100 ng/mL lipopolysaccharide (LPS) and 100 ng/mL interferon (IFN)-γ (Sigma). After 24 hours, nitrite concentration in the supernatant was measured with a Griess reaction. Aliquots of 100 μL were incubated with 100 μL Griess reagent (Sigma), and the absorption was measured spectrophotometrically at 550 nm using a plate reader (Berthold, Bad Wildbad, Germany).
Immunochemical Analysis
For immunohistochemistry, animals were deeply anesthetized with ketamine and xylazine, and transcardially perfused with ice-cold saline, followed by ice-cold 4% paraformaldehyde in 0.1 mol/L phosphate buffer (PB; pH 7.4). The brains were removed from the skull and subsequently transferred to a 30% sucrose solution in PB to ensure cryoprotection. Coronal 30 μm thick sections were cut in a cryostat. Fixed brains from bacterial artificial chromosome transgenic mice (background C57BL/6 and FVB/N) expressing the enhanced green fluorescent protein (EGFP) under the control of the Drd2 promoter (Drd2-EGFP transgenic mice)20 were a kind gift from Dr Paul Bolam (MRC Anatomical Neuropharmacology Unit, Oxford University, UK), and sectioned at 50 μm using a vibratome. Free-floating sections were then incubated for 1 hour in blocking solution (50 mmol/L Tris-buffered saline containing 20% normal goat or donkey serum and 0.3% Triton-X). All further steps were performed in Tris-buffered saline containing 1% normal goat or donkey serum, 0.3% Triton-X. Sections were incubated at 4°C overnight with one or a combination of two of the following primary antibodies: rabbit anti-D2R (1:300 to 1:500; Frontier Institute, Hokkaido, Japan), rabbit anti-GFAP (1:2,000, DAKO, Hamburg, Germany), rat anti-GFAP (1:500; Invitrogen, Darmstadt, Germany), rat anti-GFP (1:500 to 1:1,000; Nacalai Tesque, Kyoto, Japan), goat anti-Iba1 (1:250; Abcam, Cambridge, UK), rabbit anti-Iba-1 (1:1,000; Wako, Düsseldorf, Germany), rat anti-LAMP2 (1:200; Abcam), mouse anti-NeuN (1:100 to 1:250; Chemicon, EMD Millipore, Schwalbach, Germany), and mouse anti-S100B (1:500; Sigma). Sections were washed and incubated with appropriate secondary antibodies conjugated to Alexa dyes (Invitrogen) at room temperature for 4 hours. After washing in Tris-buffered saline and counterstaining of nuclei with 4',6-diamidino-2-phenylindole (DAPI, Life Technologies), sections were mounted onto slides and coverslipped with Vectashield anti-fade medium (Vector Laboratories, Burlingame, CA, USA). Omission of primary antibody or preabsorption with D2R fusion protein (Frontier Institute) served as controls.
Primary cell cultures were fixed with 4% paraformaldehyde in PB for 10 minutes followed by staining with primary and secondary antibodies as described above.
Confocal laser microscopic images were acquired using a Leica TCS SPE confocal imaging system configured around a Leica DM2500 light microscope (Leica, Wtzlar, Germany) with an automated x,y stage and z galvanometer insert, equipped with a solid-state laser (405, 488, 532, and 635 nm) and × 10, × 20, × 40, and × 63 objectives. Fluorescent signals were also visualized using a QImaging EXi Aqua CCD camera on a Leica DMRA2 microscope with × 5, × 10, × 20, × 40, and × 63 (1.5 numerical aperture) objectives and hosted by a computer with QImaging Image-Pro Insight software (QImaging, Surrey, BC, Canada). The quantitative analysis of D2R+Iba1+ cells was performed on conventional fluorescent and confocal images using the ImageJ software (http://rsbweb.nih.gov/ij/). Blinded investigators counted Iba1-immunoreactive cells expressing D2R among all Iba1-immunoreactive cells containing clearly defined DAPI+ nuclei in the infarct core.
PCR and Quantitative Real-Time PCR
PCR and quantitative real-time PCR were used to analyze the expression of Drd1-5 mRNA levels in primary neuronal, astroglial, and microglial cell cultures, as well as in microglia acutely isolated from the mouse brain by fluorescence-activated cell sorting using FITC-conjugated anti-mouse CD45 and APC-conjugated anti-mouse CD11b antibodies (Biolegend, London, UK). Total RNA was extracted using Trizol (Invitrogen) according to the manufacturer's protocol. To eliminate residual genomic DNA, the solution was treated with RNase-free DNase (Promega, Mannheim, Germany). After determination of the RNA content, 2 μg of total RNA was used for corresponding cDNA synthesis using murine myelomonocytic lymphoma virus-reverse transcriptase (Promega). The oligonucleotide primer pairs for the dopamine receptor subtypes and the house-keeping gene, β-actin (Eurofins MWG Operon, Hamburg, Germany), can be found in Supplementary Table I. Conventional PCR was performed on an Eppendorf Mastercycler (Wesseling, Germany). SYBR Green I-based quantitative real-time PCR was performed on a LightCycler 480II (Roche, Mannheim, Germany) to evaluate the relative expression of Drd2 and β-actin mRNAs. The expression of the Drd2 mRNA was normalized against β-actin mRNA, and calculated as fold increase compared with control.
Immunoblotting
Cells were rinsed in phosphate-buffered saline, lysed in 0.7% NP40 lysis buffer (50 mmol/L Tris-HCl, pH 8.0, 0.1 mM EDTA, pH 8.0, 250 mmol/L NaCl, 10% glycerol, 0.2 mmol/L Na3VO4, 50 mmol/L NaF, 1 mmol/L PMSF, 10 mmol/L DTT and a cocktail of protease inhibitors; Roche), and centrifuged at 20,780 g at 4 °C for 15 minutes. The protein concentration of the supernatant was determined according to Bradford method using the Roti-Quant protein quantification assay (Roth, Karlsruhe, Germany). Protein samples were separated on a 10% SDS polyacrylamide gel and electrotransferred onto nitrocellulose membranes (Bio-Rad, Munich, Germany). Thereafter, the nitrocellulose membranes were blocked in Ultrablock (AbD Serotec, Puchheim, Germany) and incubated at 4°C overnight with rabbit anti-D2R primary antibody (1:500; Frontier Institute). After thorough washing, membranes were incubated with horseradish peroxidase-conjugated donkey anti-rabbit immunoglobulin G (Cell Signaling, Leiden, The Netherlands) for 2 hours, and immunoreactive proteins were visualized on the membranes using enhanced chemiluminescence (ECL Kit; Amersham, Geyer, Renningen, Germany) and a cooled charge-coupled device camera (FLI Proline PL09000, PA, USA) for recording the chemiluminescence signal. In all of the experiments, the nitrocellulose membrane was subsequently stripped and analyzed with an anti-β-actin antibody (Sigma) to control for protein loading.
Statistical Analysis
Data are presented as means+standard deviation (s.d.). One-way or two-way analysis of variance (ANOVA) with Bonferroni post-test was performed (see figure legends for details) using GraphPad Prism (Version 5.0 GraphPad Software, La Jolla, CA, USA). A value of P<0.05 was considered to indicate statistical significance.
Results
Cultured Primary Microglia Express Dopamine Receptors
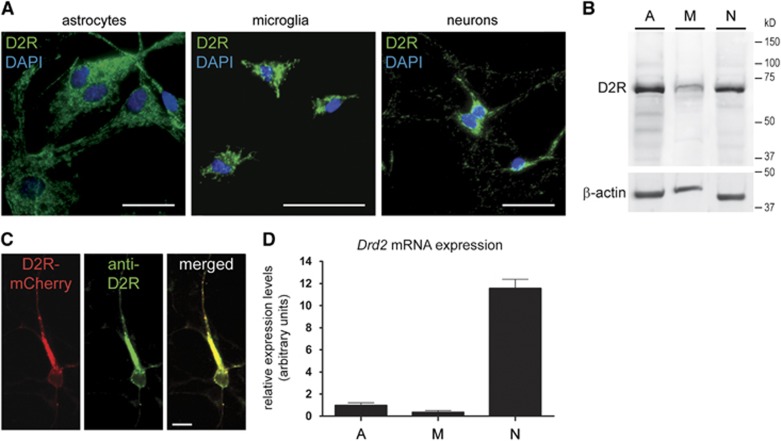
First, we studied the expression of D2R in primary cultures of glial cells and cortical neurons obtained from C57BL/6 mice. We observed D2R immunoreactivity in astrocytes, microglia, and neurons (Figure 1A). Expression of D2R was confirmed by Western blot analysis (Figure 1B). The specificity of the stainings was shown by omission of the primary antibody (data not shown), and by preabsorption of the antibody with recombinant D2R peptide resulting in complete abrogation of the signals in the Western blot analysis and immunocytochemistry (Supplementary Figures 2A and B). Moreover, when we overexpressed D2R-mCherry in cultured cortical neurons, D2R immunoreactivity colocalized with the reporter protein (Figure 1C).
Figure 1.
(A) Representative fluorescence microscopic images of murine primary astroglial, microglial, and neuronal cell cultures show D2 receptor (D2R) immunoreactivity (green) in all cell types. Nuclei are counterstained with 4',6-diamidino-2-phenylindole (DAPI) (blue). Scale bars: 20 μm. (B) Western blot analysis of cell lysates from astroglial (A), microglial (M), and neuronal (N) cultures reveals D2R expression in all cell types. β-Actin serves as a loading control. (C) Laser confocal microscopic images show colocalization of D2R immunoreactivity (green) with D2RL-mCherry fusion protein (red) after transient transfection of primary cortical neurons. The merged image is shown on the right. Scale bar: 10 μm. (D) Relative expression of Drd2 mRNA in astroglial (A), microglial (M), and neuronal (N) cultures. Expression levels are expressed as arbitrary units, normalized against β-actin mRNA and represent means+s.d. from three independent experiments.
Next, we examined the expression of dopamine receptors in cultured glial cells and neurons on a molecular level. Using the primers shown in Supplementary Table I, we detected expression of Drd1, Drd2, Drd3, Drd4, and Drd5 mRNAs by PCR in primary microglia, astrocytes, and cortical neurons (Supplementary Figure 1). Quantitative real-time PCR revealed higher expression of Drd2 mRNA in cortical neurons compared with astrocytes and microglia (Figure 1D).
Resident Microglia Do Not Express D2 Receptor in the Healthy Brain
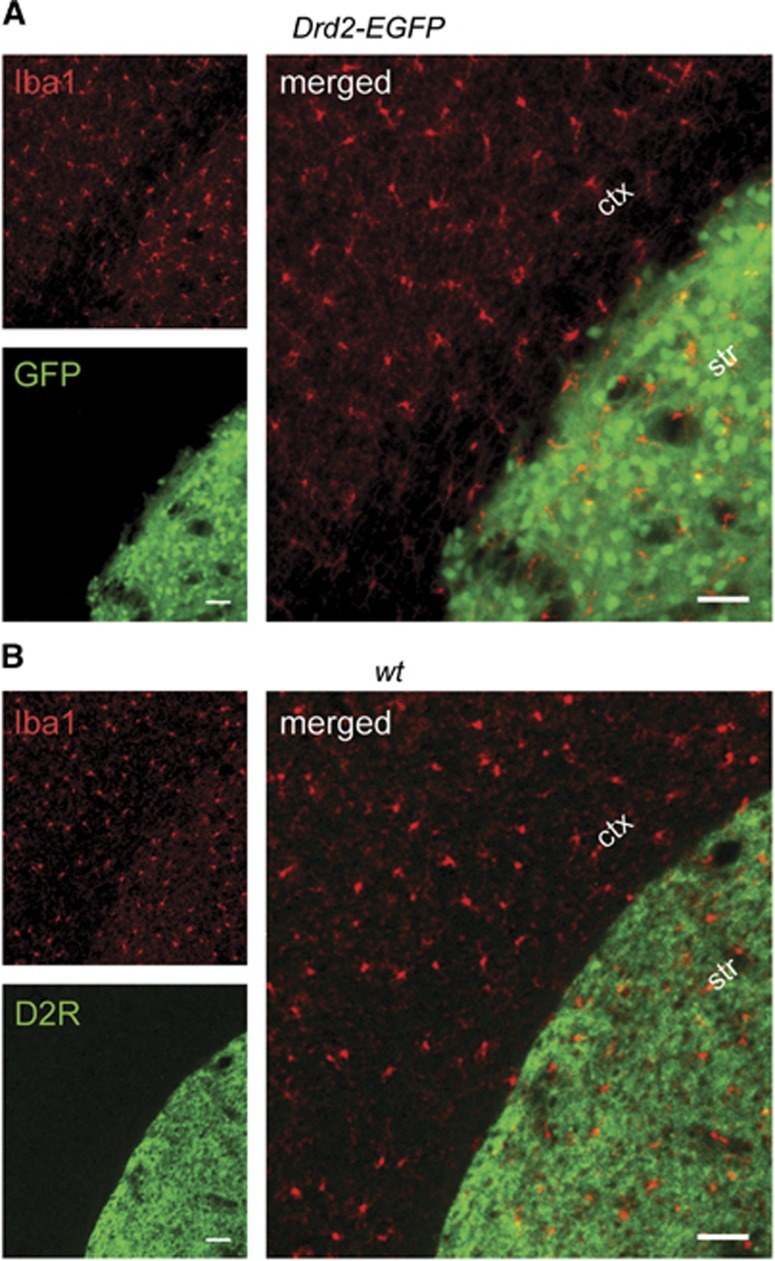
Given the presence of D2R in cultured microglia, we wanted to confirm expression of the dopamine receptor in resident microglia in vivo. To this end, we isolated CD45loCD11b+ microglia from the brains of wild-type mice by fluorescence-activated cell sorting (n=5, pooled from 26 mice). Notably, microglia acutely isolated from the mouse brain in this manner did not express Drd2 mRNA by quantitative real-time PCR (data not shown). However, Drd2 mRNA expression was observed in the CD11b-negative fraction that contains neurons, and served as a positive control (data not shown). To further explore Drd2 expression in the murine brain, we used transgenic mice that express the GFP reporter under the control of the Drd2 promoter.20 Highest GFP expression was detected in the striatum and nucleus accumbens as described previously,20 but subpopulations of cortical neurons (parvalbuminergic interneurons) also expressed GFP (data not shown). However, when we analyzed every tenth section of the entire brains of Drd2-EGFP transgenic mice by immunostaining (n=4), we never found any GFP-positive cell that expressed the microglia/macrophage marker, Iba1 (Figure 2A). Similarly, when we performed double immunofluorescence stainings of the brains of adult wild-type mice with antibodies against D2R and Iba1 (n=3), we never observed colocalization of D2R and Iba1 immunoreactivities (Figure 2B). Our findings suggest that resident microglia do not express D2R in the healthy mouse brain.
Figure 2.
(A) A representative fluorescence microscopic image of the brain of an adult Drd2-EGFP bacterial artificial chromosome transgenic mouse reveals predominant expression of the green fluorescent protein (GFP) reporter (green) in striatal neurons. No colocalization of GFP with Iba1 immunoreactivity in microglia (red) is observed. The small single channel images on the left are merged on the right. (B) A representative fluorescence microscopic image of the brain of an adult C57BL/6 wild-type (wt) mouse double stained with antibodies against Iba1 and D2 receptor (D2R) reveals D2R immunoreactivity in striatal neurons (green) and Iba1 immunoreactivity in microglia (red) that do not colocalize. The small single channel images on the left are merged on the right. Scale bars: 50 μm (ctx, cortex, str, striatum).
Activated Myeloid Cells Express D2 Receptor in the Ischemic Brain
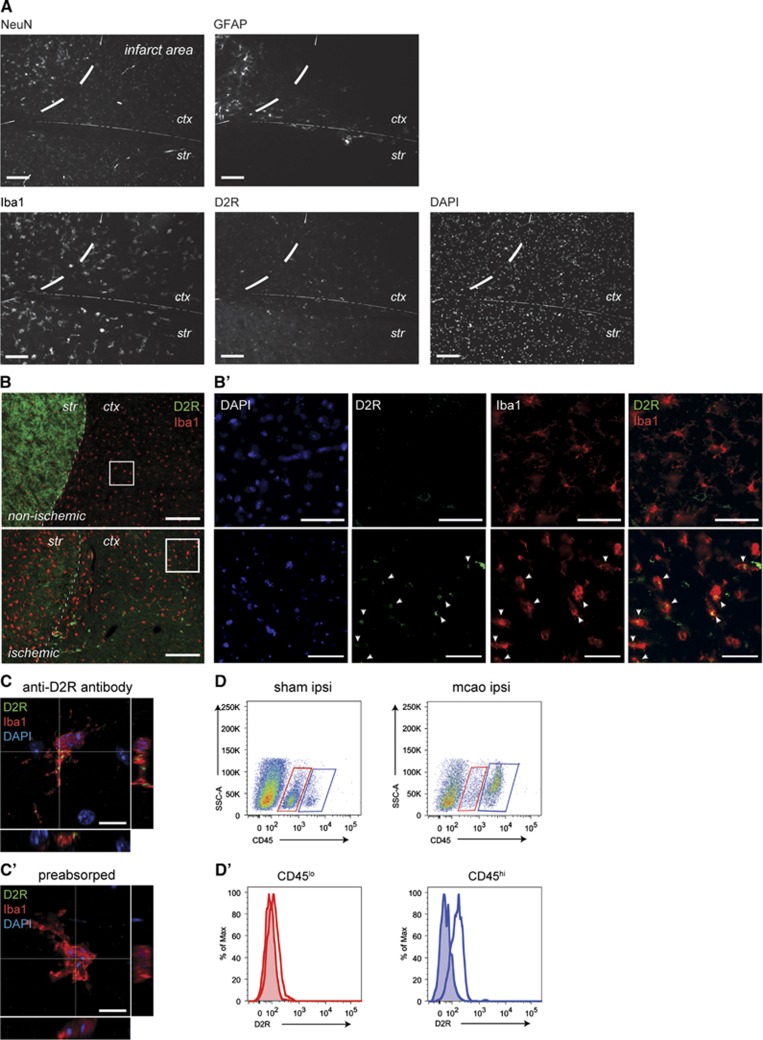
The discrepancy between the absence of D2R expression in microglia in vivo and the presence of D2R in cultured microglia might result from different functional and activation states of microglia. To test this hypothesis, we induced transient focal cerebral ischemia in wild-type C56BL/6 mice, since it has previously been described that reactive astrocytes de novo express D1R and D2R after experimental stroke.10 We examined the brains at 1 and 3 days after MCAO and compared the results with sham-operated mice (n=3 per group). Cerebral ischemia resulted in a marked loss of neurons in the infarct core based on NeuN immunoreactivity (Figure 3A). GFAP staining revealed astroglial scarring at the infarct border (Figure 3A). A massive inflammatory infiltrate of Iba1-immunoreactive cells was observed in the ischemic brain at 3 days after MCAO (Figures 3A and 3B). Notably, D2R immunoreactivity appeared in the infarct core (Figure 3A). We found a significant increase of D2R immunoreactivity in the ischemic cortex from day 1 after MCAO, whereas the contralateral (nonischemic) cortex did not show a concomitant increase in D2R immunoreactivity (Figures 3B and 3B′). In contrast, we observed a profound loss of D2R immunoreactivity in the ischemic striatum (Figure 3B), suggesting different cellular sources of D2R expression after MCAO. Using double immunofluorescence stainings, we were able to detect D2R immunoreactivity in cell bodies and ramified processes of Iba1-expressing cells (Figures 3B′ and 3C). Importantly, D2R immunoreactivity was completely blocked by preabsorption of the primary antibody (Figure 3C′), underscoring the specificity of the staining. Semiquantitative analysis revealed D2R immunoreactivity in 20% to 30% of Iba1-expressing cells in the ischemic brain between 1 and 14 days after MCAO (data not shown). In addition, we found occasional expression of D2R immunoreactivity in reactive astrocytes in the peri-infarct area (data not shown). To better characterize the inflammatory cells expressing D2R in the ischemic brain, we performed flow cytometry. This method also allows us to specifically determine cell surface expression of D2R (no permeabilization). We found a marked increase in the CD45hi cell population at 3 days after MCAO (Figure 3D). D2R fluorescence intensity was increased on CD45lo (i.e., microglia) and more so on CD45hi cells (i.e., macrophages) after MCAO (Figure 3D′).
Figure 3.
(A) Representative fluorescence microscopic images of NeuN, GFAP, Iba1, and D2 receptor (D2R) immunoreactivities in the ischemic brain at 3 days after middle cerebral artery occlusion (MCAO) reveal neuronal loss, astroglial and microglial activation, as well as induction of D2R expression, respectively. Nuclei are counterstained with 4',6-diamidino-2-phenylindole (DAPI). The horizontal line indicates the border between cortex (ctx) and striatum (str), whereas the vertical dashed line indicates the infarct border. Scale bars: 100 μm. (B) Double immunofluorescence staining with antibodies against Iba1 and D2R shows loss of D2R immunoreactivity (green) in striatal neurons, and increased D2R immunoreactivity in the cortex of the ischemic hemisphere (bottom) compared with the contralateral nonischemic hemisphere (top) at 3 days after MCAO. Scale bars: 200 μm. Higher magnifications of the boxed areas in (B) are shown in (B′): Laser confocal microscopy reveals colocalization of D2R immunoreactivity (green) with Iba1-immunoreactive microglia/macrophages (red) in the ischemic cortex. Arrowheads point to myeloid cells that coexpress D2R and Iba1. DAPI staining of nuclei (blue) is shown on the left; the single channel images for D2R and Iba1 are merged on the right. Scale bars: 50 μm. (C) High-resolution single confocal planes and orthogonal reconstructions of the planes marked by lines demonstrate that D2R and Iba1 are coexpressed in myeloid cells in the ischemic brain. (C′) The specificity of the D2R staining is shown by preabsorption of the antibody with D2R peptide, which abolishes D2R immunoreactivity in myeloid cells in the ischemic brain. Scale bars: 10 μm. (D) FACS analysis reveals D2R expression in brain myeloid cells at 3 days after ischemia. The gated viable cells from the ipsilateral hemispheres of sham-operated mice (n=3) and mice with MCAO (n=3) are plotted for side scatter-area (SSC-A) and CD45 expression. Myeloid cell populations are gated according to the expression level of CD45. The microglial population is CD45lo (red boxes), whereas activated microglia and infiltrating leukocytes are gated as CD45hi (blue boxes). The CD45hi population is increased in the ischemic hemispheres of MCAO mice. (D′) The left panel shows D2R expression on gated CD45lo cells from sham-operated mice (shaded red histogram) and MCAO mice (unshaded red histogram). The right panel shows D2R expression on gated CD45hi cells from MCAO mice (unshaded blue histogram) compared with sham-operated mice (shaded blue histogram). Both CD45lo and CD45hi cell populations show a shift in D2R fluorescence intensity after MCAO, but the shift is greater for CD45hi cells.
Activated Microglia and Bone Marrow-Derived Phagocytes Express D2 Receptor in the Ischemic Brain
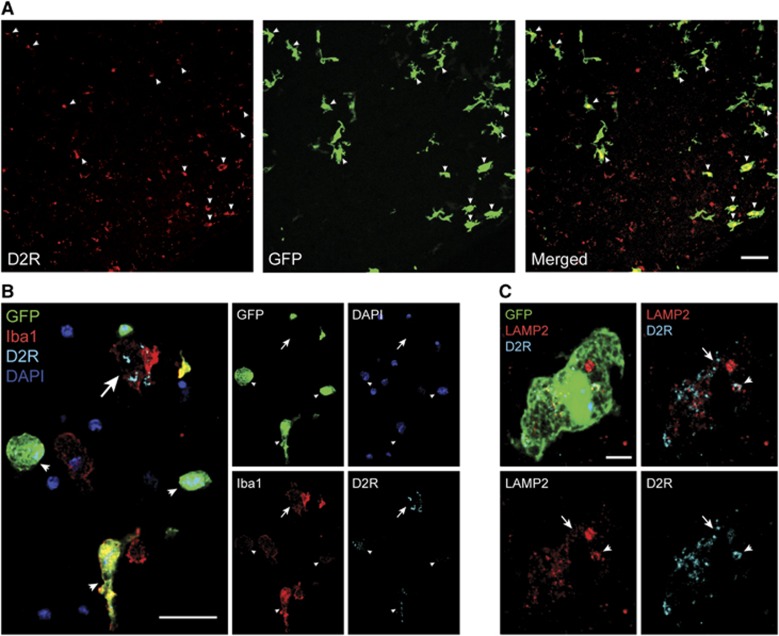
To distinguish yolk sac-derived microglia from peripherally derived monocytes/macrophages after MCAO, we used BM chimeras as described previously.3 After successful reconstitution of hematopoiesis with GFP-expressing peripheral blood cell progeny, BM chimeras were subjected to MCAO (n=4). A massive infiltrate of GFP-expressing BM-derived cells was observed in the ischemic brain at 3 days after MCAO (Figure 4A). Many of these BM-derived cells expressed D2R immunoreactivity (Figure 4A). Further characterization revealed that D2R immunoreactivity was present in activated GFP-negative Iba1+ microglia, as well as in infiltrating GFP+Iba1+ BM-derived phagocytes (Figure 4B). Together with our flow cytometry data, these results suggest that activated resident microglia and infiltrating BM-derived monocytes/macrophages express D2R after cerebral ischemia. To exclude phagocytosis of neuronal debris as the primary source of D2R immunoreactivity in phagocytes, we performed double immunofluorescence stainings for D2R and the lysosome marker, LAMP2. The results suggest only partial colocalization of D2R immunoreactivity with the lysosome marker in BM-derived phagocytes (Figure 4C).
Figure 4.
(A) Laser confocal microscopy reveals expression of D2 receptor (D2R) immunoreactivity (red) in bone marrow (BM)-derived cells (green, arrowheads) that engrafted in the ischemic brain of a green fluorescent protein (GFP) BM chimera. The single channel images for D2R and Iba1 are merged on the right. Scale bar: 40 μm. (B) Triple immunofluorescence staining for GFP (green), D2R (blue), and Iba1 (red) shows D2R immunoreactivity in infiltrating GFP+Iba1+ cells (arrowheads), as well as in activated GFP-negative Iba1+ microglia (arrow) in the ischemic brain of a GFP BM chimera. Nuclei are counterstained with 4',6-diamidino-2-phenylindole (DAPI) (purple). Laser confocal microscopic images are shown; the individual channels are merged on the left. Scale bar: 20 μm. (C) Triple immunofluorescence staining for GFP (green), D2R (blue), and LAMP2 (red) reveals that D2R immunoreactivity in BM-derived macrophages only partially colocalizes with the lysosomal marker, LAMP2 (arrowhead). The majority of D2R immunoreactivity does not colocalize with LAMP2 (arrow). High-resolution laser confocal microscopic images are shown, and the single channels are merged in the top row. Scale bar: 5 μm.
The D2 Receptor Agonist Pramipexole Activates Cultured Microglia
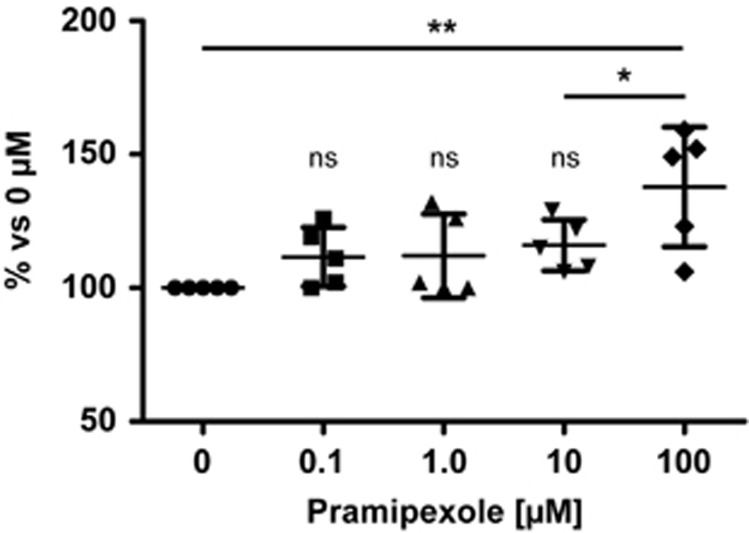
As a final experiment, we wanted to determine whether microglia respond to stimulation with dopamine. To this end, we treated cultured primary microglia with increasing doses of the D2/3R agonist, pramipexole (0.1 to 100 μmol/L; n=5 independent experiments performed in triplicate). At a concentration of 100 μmol/L, pramipexole significantly increased the release of nitrite from cultured microglia stimulated with LPS and IFN-γ for 5 hours (Figure 5). Likewise, doses in the range of 0.1 to 10 μmol/L pramipexole resulted in increased nitrite production, albeit not reaching statistical significance.
Figure 5.
Pretreatment of primary microglial cultures with the D2/3 receptor (D2/3R) agonist, pramipexole, significantly increases the release of nitrite in response to lipopolysaccharide (LPS) and interferon (IFN)-γ stimulation. Data are from five independent experiments normalized to control conditions and graphed as individual data points with means±s.d. **P<0.01, *P<0.05, One-way ANOVA, Newman–Keuls multiple comparison post-test; ns, nonsignificant compared with control conditions.
Discussion
This is the first study to describe D2R expression on activated microglia and peripherally derived monocytes/macrophages after cerebral ischemia. Notably, we find that resident microglia do not express D2R in the healthy brain, whereas cultured microglia express all dopamine receptor subtypes.
The functional importance of neurotransmitter receptors on microglia has long been recognized. Neurotransmitters instruct microglia to perform distinct types of responses, such as triggering an inflammatory cascade or acquiring a neuroprotective phenotype.21 In the case of dopamine, PCR products for Drd1, Drd2, Drd4, and Drd5 were detected in rat primary microglial cultures, whereas only Drd1 and Drd5 were found in murine primary microglial cultures.22 In contrast, we detected all types of dopamine receptors in cultured murine microglia, in particular D2R. This is in line with results from cultured human elderly microglia that express DRD1, DRD2, DRD3, and DRD4 mRNAs.23 Interestingly, a small fraction of human peripheral blood monocytes also express D2R.24 The responses of cultured microglia to dopamine administration are heterogenous, suggesting that dopamine receptor expression may depend on functional and activation states. Thus, dopamine induced an outward current in only about one-third of cultured rat microglia.22 Similarly, only 4% of cultured neonatal microglia from C57BL/6 mice, and 7% of cultured microglia from adult murine brain responded to dopamine.25 Treatment of adult cultured microglia with interleukin-4 significantly reduced the responsiveness to dopamine.25 Importantly, dopamine and the D2R agonist, quinpirole, enhanced the migration of microglia in vitro, and inhibited the release of nitric oxide from microglia in response to LPS stimulation.22 We found that the D2/3R agonist, pramipexole, enhanced the release of nitrite from cultured murine microglia stimulated with LPS and IFN-γ. Thus, dopamine may have an important role in modulating innate immunity in the central nervous system.
Cerebral ischemia induces in a massive release of dopamine into the extracellular space (more than 200-fold increase after MCAO).26 This may sensitize glial cells. Recently, reactive astrocytes were found to de novo express D1R and D2R after MCAO in rats.10 Notably, administration of levodopa improved functional recovery after stroke, and increased glial cell-line-derived neurotrophic factor synthesis in D1R-expressing astrocytes.10, 11 In vivo activation of D2R was found to inhibit astrogliosis and neuroinflammation.13 Here, we describe D2R expression on activated microglia and peripherally derived monocytes/macrophages after MCAO in mice. Interestingly, microglia in striatum sections from patients with Parkinson's disease also express dopamine receptors, including D2R.23 Dopamine agonists and levodopa have been used in the clinical setting to ameliorate motor symptoms of early Parkinson's disease.27 Although levodopa was more effective in treating overall parkinsonian features, the dopamine D2/D3 receptor agonist, pramipexole, reduced the risk of developing early-stage motor complications. Previous studies suggested that pramipexole exerts neuroprotective effects in cerebral ischemia.28 Similarly, the D2R agonists, pergolide, bromocriptine, and lisuride, protected against global ischemia-induced hippocampal neurodegeneration, whereas D1R agonists failed to confer neuroprotection.29
The induction of D2R expression on activated microglia after stroke suggests a role for dopamine in modulating postischemic inflammation.13 Despite ontogenetic and functional differences,15 we also found BM-derived phagocytes to express D2R in the ischemic mouse brain. Interestingly, increased plasma levels of catecholamines, including dopamine, were detected in patients with cerebral infarction and transient ischemic attacks.30 Thus, immune cells might also be sensitized to the effects of dopamine in the periphery. Our data suggest that the expression of dopamine receptors may be a conserved pattern of myeloid cell activation. Whereas we did not detect any D2R expression on resident microglia under physiological conditions, we cannot exclude the possibility that small subsets of microglia express dopamine receptors in the healthy brain.25, 31 Notably, other dopamine receptor subtypes may also be expressed on microglia, and coactivation of these receptors may fine-tune microglial responses. However, isolation procedures and culture conditions need to be taken into consideration when interpreting such findings. In any case, the increasing recognition of dopamine as an immune modulator deserves closer attention in the context of cerebral ischemia. Genetic ablation of Drd2 may help to define the functional relevance of D2R expression on microglia and other myeloid cells in the future.
Acknowledgments
The authors would like to thank Renate Gusinda, Jasmin Jamal El-Din, Peggy Mex, and Katharina Stohlmann for excellent technical assistance. We also thank Amada Costa, Susanne Wolf, and Helmut Kettenmann for calcium imaging experiments.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by the European Union's Seventh Framework Programme (Grant agreements: 201024 and 202213, European Stroke Network; 627951, Marie Curie IOF to PM), Deutsche Forschungsgemeinschaft (SFB/TRR43 A7 and Cluster of Excellence ‘NeuroCure', Exc 257), and Bundesministerium für Bildung und Forschung (Center for Stroke Research Berlin, 01 EO 08 01). JH was supported by a Habilitationsstipendium of the Charité—Universitätsmedizin Berlin. PM received research funding from Sanofi-Aventis GmbH, Germany.
Supplementary Material
References
- 1Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron 2010; 67: 181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci 2013; 36: 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Priller J, Flügel A, Wehner T, Boentert M, Haas CA, Prinz M et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med 2001; 7: 1356–1361. [DOI] [PubMed] [Google Scholar]
- 4Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 2009; 40: 1849–1857. [DOI] [PubMed] [Google Scholar]
- 5Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci 2007; 30: 259–288. [DOI] [PubMed] [Google Scholar]
- 6Mastwal S, Ye Y, Ren M, Jimenez DV, Martinowich K, Gerfen CR et al. Phasic dopamine neuron activity elicits unique mesofrontal plasticity in adolescence. J Neurosci 2014; 34: 9484–9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Höglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci 2004; 7: 726–735. [DOI] [PubMed] [Google Scholar]
- 8Flöel A, Hummel F, Breitenstein C, Knecht S, Cohen LG. Dopaminergic effects on encoding of a motor memory in chronic stroke. Neurology 2005; 65: 472–474. [DOI] [PubMed] [Google Scholar]
- 9Scheidtmann K, Fries W, Müller F, Koenig E. Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomised, double-blind study. Lancet 2001; 358: 787–790. [DOI] [PubMed] [Google Scholar]
- 10Ruscher K, Kuric E, Wieloch T. Levodopa treatment improves functional recovery after experimental stroke. Stroke 2012; 43: 507–513. [DOI] [PubMed] [Google Scholar]
- 11Kuric E, Wieloch T, Ruscher K. Dopamine receptor activation increases glial cell line-derived neurotrophic factor in experimental stroke. Exp Neurol 2013; 247: 202–208. [DOI] [PubMed] [Google Scholar]
- 12Shao W, Zhang SZ, Tang M, Zhang XH, Zhou Z, Yin YQ et al. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature 2013; 494: 90–94. [DOI] [PubMed] [Google Scholar]
- 13Torres-Rosas R, Yehia G, Peña G, Mishra P, del Rocio Thompson-Bonilla M, Moreno-Eutimio MA et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med 2014; 20: 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Kuric E, Ruscher K. Reduction of rat brain CD8(+) T-cells by levodopa/benserazide treatment after experimental stroke. Eur J Neurosci 2014; 40: 2463–2470. [DOI] [PubMed] [Google Scholar]
- 15Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 2014; 15: 300–312. [DOI] [PubMed] [Google Scholar]
- 16Fernández-Klett F, Potas JR, Hilpert D, Blazej K, Radke J, Huck J et al. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J Cereb Blood Flow Metab 2013; 33: 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci 2007; 10: 1544–1553. [DOI] [PubMed] [Google Scholar]
- 18Mergenthaler P, Kahl A, Kamitz A, van Laak V, Stohlmann K, Thomsen S et al. Mitochondrial hexokinase II (HKII) and phosphoprotein enriched in astrocytes (PEA15) form a molecular switch governing cellular fate depending on the metabolic state. Proc Nat Acad Sci USA 2012; 109: 1518–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Priller J, Reddington M, Haas CA, Kreutzberg GW. Stimulation of P2Y-purinoceptors on astrocytes results in immediate early gene expression and potentiation of neuropeptide action. Neuroscience 1998; 85: 521–525. [DOI] [PubMed] [Google Scholar]
- 20Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 2003; 425: 917–925. [DOI] [PubMed] [Google Scholar]
- 21Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci 2007; 30: 527–535. [DOI] [PubMed] [Google Scholar]
- 22Färber K, Pannasch U, Kettenmann H. Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol Cell Neurosci 2005; 29: 128–138. [DOI] [PubMed] [Google Scholar]
- 23Mastroeni D, Grover A, Leonard B, Joyce JN, Coleman PD, Kozik B et al. Microglial responses to dopamine in a cell culture model of Parkinson's disease. Neurobiol Aging 2009; 30: 1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24McKenna F, McLaughlin PJ, Lewis BJ, Sibbring GC, Cummerson JA, Bowen-Jones D et al. Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J Neuroimmunol 2002; 132: 34–40. [DOI] [PubMed] [Google Scholar]
- 25Pannell M, Szulzewsky F, Matyash V, Wolf SA, Kettenmann H. The subpopulation of microglia sensitive to neurotransmitters/neurohormones is modulated by stimulation with LPS, interferon-γ, and IL-4. Glia 2014; 62: 667–679. [DOI] [PubMed] [Google Scholar]
- 26Mitsuyo T, Adachi N, Yorozuya T, Tabo E, Nagaro T, Arai T. Facilitation of ischemia-induced release of dopamine and neuronal damage by dexamethasone in the rat striatum. Eur J Pharmacol 2003; 465: 267–274. [DOI] [PubMed] [Google Scholar]
- 27Parkinson Study Group. Pramipexole vs levodopa as initial treatment for Parkinson disease: A randomized controlled trial. Parkinson Study Group. JAMA 2000; 284: 1931–1938. [DOI] [PubMed] [Google Scholar]
- 28Hall ED, Andrus PK, Oostveen JA, Althaus JS, VonVoigtlander PF. Neuroprotective effects of the dopamine D2/D3 agonist pramipexole against postischemic or methamphetamine-induced degeneration of nigrostriatal neurons. Brain Res 1996; 742: 80–88. [DOI] [PubMed] [Google Scholar]
- 29O'Neill MJ, Hicks CA, Ward MA, Cardwell GP, Reymann JM, Allain H et al. Dopamine D2 receptor agonists protect against ischaemia-induced hippocampal neurodegeneration in global cerebral ischaemia. Eur J Pharmacol 1998; 352: 37–46. [DOI] [PubMed] [Google Scholar]
- 30Myers MG, Norris JW, Hachniski VC, Sole MJ. Plasma norepinephrine in stroke. Stroke 1981; 12: 200–204. [DOI] [PubMed] [Google Scholar]
- 31Schwarz JM, Smith SH, Bilbo SD. FACS analysis of neuronal-glial interactions in the nucleus accumbens following morphine administration. Psychopharmacology (Berl) 2013; 230: 525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.