Abstract
Implementing endovascular stroke care often impedes neurologic assessment in patients who need sedation or general anesthesia. Cerebral near-infrared spectroscopy (NIRS) may help physicians monitor cerebral tissue viability, but data in hyperacute stroke patients receiving endovascular treatment are sparse. In this observational study, the NIRS index regional oxygen saturation (rSO2) was measured noninvasively before, during, and after endovascular therapy via bilateral forehead NIRS optodes. During the study period, 63 patients were monitored with NIRS; 43 qualified for analysis. Before recanalization, 10 distinct rSO2 decreases occurred in 11 patients with respect to time to intubation. During recanalization, two kinds of unilateral rSO2 changes occurred in the affected hemisphere: small peaks throughout the treatment (n=14, 32.6%) and sustained increases immediately after recanalization (n=2, 4.7%). Lower area under the curve 10% below baseline was associated with better reperfusion status (thrombolysis in cerebral infarction ≥ 2b, P=0.009). At the end of the intervention, lower interhemispheric rSO2 difference predicted death within 90 days (P=0.037). After the intervention, higher rSO2 variability predicted poor outcome (modified Rankin scale > 3, P=0.032). Our findings suggest that bi-channel rSO2-NIRS has potential for guiding neuroanesthesia and predicting outcome. To better monitor local revascularization, an improved stroke-specific set-up in future studies is necessary.
Keywords: acute ischemic stroke, medial cerebral artery occlusion, near-infrared spectroscopy, perfusion, regional oxygen saturation, thrombectomy
Introduction
In acute ischemic stroke, neurointerventionalists are increasingly using endovascular procedures to treat intracranial large-vessel occlusion.1, 2, 3 General anesthesia or other sedation facilitates implementation of the endovascular procedure, but may be harmful.4, 5 Importantly, it impedes the repeated clinical assessment of patients.6 Therefore, it would be desirable to establish a periprocedural means of monitoring the viability of brain tissue at the bedside.
Near-infrared light can penetrate the human skull and—using methods of spectroscopy (near-infrared spectroscopy (NIRS))—provides noninvasive access to determine regional oxygen saturation (rSO2) of the brain. This derived index reflects approximately 70% venous and 30% arterial cortical blood, but varies considerably between individuals and because of extracerebral signal contribution,7 emphasizing that relative changes rather than absolute values are of interest.8, 9, 10, 11
NIRS-guided cerebral oximetry is associated with regional cerebral perfusion and/or oxygenation in tissue underlying the optodes.12, 13, 14 However, this concept has only gained little attention in stroke.15 So far, the small observational studies investigated NIRS predominantly in the subacute phase of ischemic stroke.16, 17, 18, 19, 20, 21, 22, 23, 24 In contrast, only three patients have been examined during the hyperacute phase of stroke,25 when the infarct core has not yet been determined and viable tissue is still present.
We aimed to determine whether rSO2 measured by NIRS can help clinicians monitor those patients receiving endovascular treatment for acute ischemic stroke caused by major intracranial vessel occlusion. In detail, we investigated whether rSO2 (1) reflects relevant circulation and respiration disorders, (2) reflects local reperfusion, and (3) predicts the patients' outcome.
Materials and methods
Study Design, Setting, and Recruitment
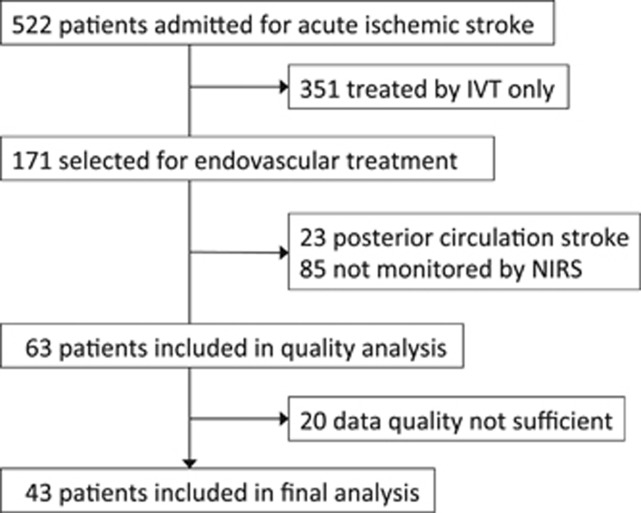
This study was approved by an independent ethics committee and consent was waived because only routine monitoring parameters were analyzed in a purely observational manner (University of Heidelberg, Medical Faculty, Ethics Committee, ID S-189/2013). Patients were eligible if they were suffering from acute ischemic stroke caused by large intracerebral vessel occlusion in the anterior cerebral circulation and were designated to receive endovascular treatment. Data were collected at the stroke center of the Heidelberg University. In a run-in phase of 6 months, we adapted to the new technology and handling the device, gained experience, and implemented NIRS into our standard operating procedure for periinterventional management of ischemic stroke patients. From July 2010 to July 2012, patients who were admitted (95% of all patients) were enrolled prospectively (5% run-in phase). A flowchart displays patient recruitment (Figure 1).
Figure 1.
Flowchart of patients' screening and recruitment. IVT, intravenous thrombolysis; NIRS, near-infrared spectroscopy.
Endovascular treatment was performed under general anesthesia after intubation (using etomidate and fentanyl, then maintained by propofol and remifentanil). Mechanical ventilation (Primus, Dräger, Drägerwerk AG KGaA, Lübeck, Germany) during the procedure was pressure-controlled, targeting physiologic levels of arterial blood gases (PaO2 80 to 110 mm Hg, PaCO2 35 to 45 mm Hg). After the intervention, patients were transferred to the neurocritical care unit, extubated as soon as feasible, and further managed according to international standards of ischemic stroke management.26
Data Acquisition
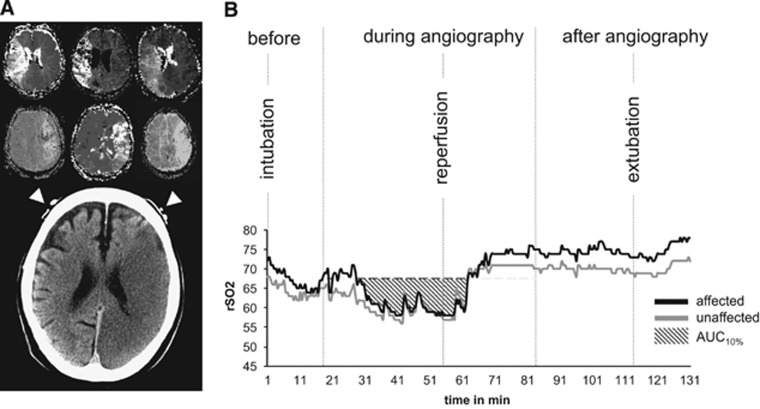
The NIRS signal was acquired noninvasively by using forehead adhesives that were placed on the upper outer forehead, as close to the hairline as possible without prior shaving. Optodes were connected to the NIRS device INVOS 5100 (Somanetics, Detroit, MI, USA). Being aware of the spatial limitation of NIRS in general,15 we aimed at a signal over the crucial border zone between the middle and anterior cerebral arteries territories. As reported previously,27 this location may have potential for NIRS—emphasizing that blood flow is redirected using important anastomotic channels. We hypothesized that it enhances the possibility of NIRS covering penumbral tissue instead of covering ‘only' viable tissue (in case of positioning above nonaffected anterior cerebral artery). In addition, it may not only reflect perfusion changes in tissue directly beneath the optode, but also capture upstream changes. Sampling rate was 0.03 Hz.
NIRS monitoring was started as early as possible and continued until patients were extubated or until 6 hours after the intervention (whatever came first). Blood pressure, peripheral oxygen saturation (SpO2), heart rate, and electrocardiography were monitored every 5 to 15 minutes (Dräger Medical Systems, Infinity Delta, Drägerwerk AG, KGaA, Lübeck, Germany).
Neuroimaging data were derived from the image archiving and communicating system (computed tomography, magnetic resonance imaging, and digital subtraction angiography, respectively). We analyzed images from the computed tomography and magnetic resonance imaging perfusion studies to examine whether NIRS optodes were overlying a presumably hypoperfused area. If perfusion could not be detected (n=24), we examined on follow-up neuroimaging studies whether the infarct was located in the proximity of the optodes. A binary variable indicated the alleged presence (status=1) or absence (status=0) of hypoperfusion of the tissue underneath the NIRS optodes. Reperfusion at the end of intervention was measured by the thrombolysis in cerebral infarction (TICI) score.28 We considered a TICI score ≥2b to indicate a successful reperfusion and dichotomized at that cut-off for analysis. Carotid-T occlusions are known to correlate with a worse probability of reperfusion, and therefore a binary variable indicating presence of carotid-T occlusion served as confounder in our regression analysis.
Data Quality and Data Preparation
One investigator (CH) assessed the data quality. If >80% of the NIRS readings in the corresponding, affected hemisphere were available, the data from the respective patients were included in the analysis. Vital signs and time of predefined events, including endotracheal intubation, recanalization, and extubation, were synchronized with the NIRS reading (Figure 2B).
Figure 2.
(A) Illustration of NIRS optode positioning at the border zone of the two territories of the middle and anterior cerebral arteries; the upper part shows six individual examples of time-to-peak magnetic resonance perfusion maps; the lower part is taken from a follow-up computed tomography scan—white arrow heads indicate NIRS optode positions. (B) Individual curve of one patient demonstrating the NIRS monitoring workflow, in principle including the predefined events for analysis. AUC10%, area under the curve 10% below baseline; NIRS, near-infrared spectroscopy.
Analysis
Two types of analyses were performed.
(A) Descriptive analysis
We inspected the NIRS curves, categorizing three predefined time periods—before, during, and after the intervention—with defined events of intubation, recanalization, and extubation. In each time period for each patient, data were assessed for correlations between rSO2 changes and events and for correlations between rSO2 and blood pressure, oxygen saturation, and ventilation. Special emphasis was placed on detecting unilateral alterations of rSO2. Data are presented descriptively by counts and percentages for the number of available readings and the number of detected events. To further determine whether unilateral findings were influenced by known limitations of NIRS, we correlated them with the alleged absence or presence of NIRS covering a hypoperfused tissue and with the skull thickness of the patients.
(B) Correlation analysis
Baseline rSO2 readings were averaged over the first 5 minutes from the start of monitoring. Area under the curve 10% below baseline (AUC10%) was calculated for the duration of the intervention as a marker of decrease in cerebral oxygenation over time. Previous studies that evaluated rSO2 AUC used a 20% reduction from baseline.29 However, those studies investigated patients undergoing heart or abdominal surgery, in whom changes in hemodynamics are often greater. As only smaller local changes in deoxygenation were expected, we chose a 10% reduction from baseline. The concept of interhemispheric rSO2 difference has been used before.16, 23 We calculated interhemispheric rSO2 differences before and after the intervention as the difference in the averaged 5-minute rSO2 values of the affected hemisphere minus values in the nonaffected hemisphere, yielding positive or negative values. As a new index, average successive rSO2 variability (ASVrSO2-aff) was introduced and calculated for rSO2 values of the affected hemisphere as follows:
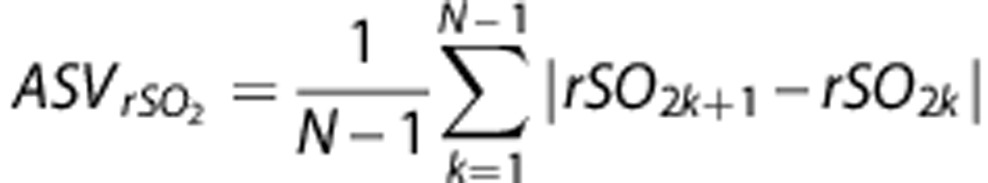 |
where N denotes the number of valid 30-second rSO2 values corresponding to each patient (similar to reported blood pressure variability).30 We chose the period after revascularization for this analysis because patients are physiologically more stable than during the intervention.
RSO2 values correlate with major blood pressure changes as demonstrated in cardiothoracic surgery.11 Arterial vessels only contribute 20% to 30% to the cortical rSO2 signal,31 extracerebral skin blood flow contaminates this signal 7 and cerebral autoregulation usually stabilizes cerebral blow flow over a wide range of cerebral perfusion pressure, although this can be impaired in patients with acute ischemic stroke. Therefore, we expected only changes in mean arterial pressure (MAP) as great as at least 30 mm Hg within up to 20 minutes to be reflected by NIRS. We analyzed the correlation of Δ MAP with Δ rSO2 using Spearman rank test to confirm this known correlation in our sample.
Functional outcome as measured by modified Rankin scale (mRS) was obtained at 90 days after stroke by an independent mRS-certified physician. Owing to the relatively small sample size, we chose two dichotomizations for statistical analysis: poor functional outcome (mRS >3) and death (mRS >5). To explore an association of short-term neurologic deficit with variability of rSO2, we analyzed the National Institutes of Health Stroke Scale (NIHSS) score at discharge for correlations with the ASV using Spearman rank test.
We used SPSS (IBM Corp. Released 2012, IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY, USA), Graphpad Prism (GraphPad Prism version 6.00 for Windows, GraphPad Software, San Diego, CA, USA, www.graphpad.com) and R (a language and environment for statistical computing, Vienna, Austria) for statistical analysis. For nonnormal distributions of continuous variables Mann–Whitney U-test was applied, and for categorical distributions the Fisher's exact test. A P-value ≤0.05 was considered significant. Binomial regression included a maximum of three adjusting confounders and a Firth correction algorithm was chosen to avoid overfitting.32 To improve internal validity and for more robust confidence intervals, we performed bootstrapping based on 9999 samples. Regression analyses were considered exploratory. We present results as common odds ratios and 95% confidence intervals.
Results
Patient Characteristics
Of 522 stroke patients admitted during the study period, 32.8% received endovascular treatment. Of these 171 patients, NIRS monitoring was performed in 63, of whom 43 qualified for analysis (Figure 1). Patients had a mean age of 73 and were severely affected by stroke as determined by a median NIHSS score of 19 (interquartile range (IQR) 15, 20.3). Sex was nearly equally distributed (48.8% females). Further baseline characteristics of the patients, surrogate outcomes, and functional outcomes at day 90 after stroke are presented (Table 1).
Table 1. Patient characteristics.
| N | 43 |
| Median age (IQR)—years | 73 (65–79) |
| Sex—no. of women (%) | 21 (48.8) |
| Hemisphere—no. of left (%) | 24 (55.8) |
| Premorbid mRS—no. (%) | |
| 0 | 22 (51.2) |
| 1 | 9 (20.9) |
| 2 | 7 (16.3) |
| 3 | 3 (7) |
| 4 | 1 (2.3) |
| Risk factor status (on admission)—no. (%) | |
| Atrial fibrillation | 23 (53.5) |
| Coronary heart disease | 16 (37.2) |
| Congestive heart failure | 4 (9.3) |
| Arterial hypertension | 38 (88.4) |
| Diabetes mellitus | 11 (25.6) |
| Glucose (mg/dL) | 125 (30) |
| Current smoking | 9 (20.9) |
| Hyperlipidemia | 20 (46.5) |
| Median NIHSS on admission (IQR) | 19 (15–20.25) |
| Vessel occlusion—no. (%) | |
| M1 | 20 (46.5) |
| ICA + M1 | 18 (41.9) |
| M1 + M2 | 2 (4.7) |
| M2 | 1 (2.3) |
| Intravenous rtPA—no. (%) | 29 (67.4) |
| Intraarterial rtPA—no. (%) | 8 (18.6) |
| Reperfusion result (TICI)—no. (%) | |
| 0 | 7 (16.3) |
| 1 | 0 (0) |
| 2a | 11 (25.6) |
| 2b | 7 (16.3) |
| 3 | 18 (41.8) |
| Etiology (TOAST classification)—no. (%) | |
| Large artery atherosclerosis | 5 (11.6) |
| Cardioembolism | 29 (67.4) |
| Small artery occlusion | 0 (0) |
| Other etiology | 3 (7) |
| Undetermined etiology | 6 (14) |
| Median NIHSS at discharge (IQR) | 13 (8.75–19.5) |
| mRS at day 90a—no. (%) | |
| 0 | 2 (4.7) |
| 1 | 2 (4.7) |
| 2 | 3 (7.0) |
| 3 | 10 (23.2) |
| 4 | 7 (16.3) |
| 5 | 5 (11.6) |
| 6 | 14 (32.6) |
ICA, internal carotid artery; IQR, interquartile range; M1, main branch of medial cerebral artery; M2, distal branch of medial cerebral artery; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale; rtPA, recombinant tissue plasminogen activator; TICI, thrombolysis in cerebral infarction; TOAST, Trial of Org 10172. Data are presented as counts in absolute numbers and percentage, if not stated otherwise.
In one patient, follow-up data at day 90 were missing; this value was imputed after the worst-case principle. As a result of rounding, percentages may not exactly sum up to 100%.
Descriptive Analysis of Curves of Regional Oxygen Saturation (A)
Before the intervention
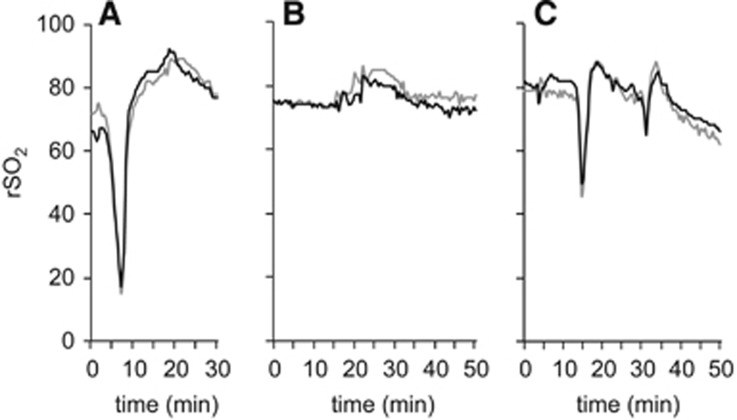
In 11 patients (25% of analyzed patients), the NIRS monitoring period included the intubation procedure. In 10 of those patients (90.9%), rSO2 decreased bilaterally during the intubation period by a median of 15 absolute points (IQR: 13.3, 19; min: 10, max: 68, Figure 3A); MAP (n=10) decreased in the median by 25.5 mm Hg (IQR: 18.25 to 30.75). During intubation, pulse oximetry showed poor signal quality in 6 of 11 patients; the remaining 5 presented normal values (SpO2 ≥ 95%). Median heart rate (n=9) decreased by 10 beats per minute (IQR: 3 to 17). For individual patient data, please see Supplementary Table I. During the preparation period, we did not notice any relevant unilateral, event-related change in the NIRS signal.
Figure 3.
Individual examples of changes in regional oxygen saturation (rSO2) are given: (A) before the intervention, a distinct bilateral decrease in rSO2 with respect to time of intubation is followed by a recovery. (B) After the intervention, small transient increases in rSO2 time with respect to time of extubation, and (C) relevant transient decreases in rSO2 with respect to time of sudden loss of positive end-expiratory pressure are shown.
During the intervention
Data from all patients included in the analysis were available (n=43). We observed two different unilateral patterns over the affected hemisphere: rSO2 peaks and steady, sustained increases in rSO2. Fourteen patients (32.6%) showed rSO2 peaks (median five-point absolute rSO2 increase, IQR 4 to 6) throughout the whole intervention; half of the patients had more than one rSO2 peak, and up to four (Figure 4A). The presence of rSO2 peaks during the intervention was not associated with the NIRS optode localization above hypoperfused tissue (P>0.999) or with the influence of skull thickness (U=175, Z=−0.713, P=0.476). Two patients (4.7%) showed a steady, sustained increase that started directly after successful recanalization (Figure 4B). One patient showed severe bilateral rSO2 decreases while requiring pharmacological resuscitation (using norepinephrine, amiodarone, and atropine) during the revascularization procedure (Figure 4C).
Figure 4.
Individual examples of changes in regional oxygen saturation (rSO2) during the intervention. The black line indicates the affected hemisphere: (A) throughout the whole intervention occurrence of small short peaks in rSO2 predominantly in the affected hemisphere. (B) After successful reperfusion (time of recanalization indicated by the dotted vertical line), a sustained rSO2 increase in the affected hemisphere was observed. (C) Pharmacological resuscitation during angiography: ‘N' indicates administration of norepinephrine; ‘A', amiodarone; ‘a', atropine; the area between the two dotted lines indicates a time of rSO2 signal loss.
After the intervention
In seven patients (16.3% of all analyzed patients), NIRS monitoring was performed during extubation. Most other patients either underwent further necessary procedures, such as hemicraniectomy, or timely extubation was not feasible because of insufficient spontaneous breathing. Associated with extubation, six patients (85.7%) showed bilateral rSO2 increases (median eight-point absolute rSO2 increase, IQR 5.5, 9; Figure 3B). One patient experienced two sequential episodes with a sudden loss of positive end-expiratory pressure (from 5 to 1 and 5 to 3), corresponding rSO2 readings showing a decrease by 31 and 11 absolute points (Figure 3C). We did not notice any relevant unilateral, event-related change in the NIRS signal.
Correlation Analysis of Indices of Regional Oxygen Saturation (B)
Regional oxygen saturation and mean arterial blood pressure
Twenty-six patients (60.5%) experienced at least one substantial change in MAP; we found 55 samples that showed a significant association between Δ MAP and Δ rSO2 (Spearman's ρ=0.289, P=0.032; Supplementary Figure I).
Area under the curve 10 % below baseline and reperfusion
Area under the curve 10% below baseline of rSO2 values in the corresponding, affected hemisphere was available for all patients (n=43). Patients in whom reperfusion was successful had a lower median AUC10% than patients in whom it was not (TICI ≥2b: 0.0 versus TICI <2b: 15.03, U=135.5, Z=−2.323, P=0.02, r=−0.52; Figure 5A). Binomial regression analysis adjusting for the confounders baseline NIHSS and presence of a carotid-T occlusion suggested AUC10% as an independent negative predictor of successful reperfusion (odds ratio 0.998, 95% confidence interval 0.996 to 1.000, P=0.023; Supplementary Table II).
Figure 5.
(A) Association of regional oxygen saturation (rSO2)–area under the curve 10% below baseline (AUC10%) with revascularization (measured by thrombolysis in cerebral infarction score (TICI)), (B) association of end-revascularization interhemispheric difference with death 90 days after stroke, (C) association of variability measure ASVrSO2 after revascularization with poor functional outcome (modified Rankin scale 4 to 6). Asterisks (*) indicate P-value <0.05 in Mann–Whitney U-test. ASVrSO2, average successive rSO2 variability.
Interhemispheric difference in regional oxygen saturation and mortality at day 90
Median rSO2|diff at the beginning of the intervention (n=43) was nonsignificantly different in patients who died by day 90 compared with those who survived (mRS6: 18.04, mRS0-5: 22.38, U=143.5, Z=−1.079, P=0.281, r=−0.169).
Median rSO2|diff at the end of the intervention (n=43) showed significantly lower absolute values in patients who died by day 90 compared with those who survived (mRS6: –1.35, mRS0-5: 2.1, U=113, Z=−2.09, P=0.037, r=−0.326; Figure 5B). Binomial regression analysis to predict death at day 90 and adjusting for confounders of death (age, baseline NIHSS) revealed lower values of end-intervention rSO2|diff as an independent predictor (rSO2|diff odds ratio 0.144, 95% confidence interval 0.027 to 0.618, P=0.008; Supplementary Table III).
Variability in regional oxygen saturation and functional outcome at day 90
Assessing ASVrSO2 during the complete 6-hour period after intervention, a median time period per patient of 349 minutes was registered for 38 patients (IQR 304, 367). Variability in rSO2 was significantly lower in patients in whom functional outcome was poor (ASVrSO2=0.557) than in patients with acceptable functional outcome (ASVrSO2=0.717), U=97, Z=−2.149, P=0.032, r=−0.38; Figure 5C). Binomial regression analysis to predict poor functional outcome at 90 days, adjusting for the confounder age and NIHSS, revealed ASVrSO2 as a significant, independent predictor (odds ratio 0.06, 95% confidence interval 0.002 to 0.948, P=0.046; Supplementary Table IV). Patients who died within 90 days after stroke had lower median variability scores (ASVrSO2=0.542) than patients who survived (ASVrSO2=0.69); however, this was not significant (U=117, Z=−1.543, P=0.123, r=−0.25). The NIHSS at discharge (as a surrogate short-term outcome measure) correlated negatively with the ASVrSO2—patients with lower ASVrSO2 (measured after the intervention) scored higher on the NIHSS (Spearman's ρ=−0.434, P=0.006; Supplementary Figure II), that is, remained more severely affected.
Discussion
In this study, regional cerebral oxygen saturation measured by NIRS reflected systemic disorders of circulation and respiration. The ability to monitor local revascularization appeared limited, though. RSO2 indices served as a composite measure for predicting outcome.
In part A of our analysis (descriptive NIRS curve analysis), we found rSO2 decreases reflecting >90% of recorded intubations, a detrimental rSO2 course in a patient undergoing pharmacological resuscitation, and two episodes of respirator failure-associated rSO2 decreases (in one patient). In a randomized trial on cardiac surgery, Murkin et al.29 demonstrated almost a decade ago that cardiac patients benefited when management was guided by NIRS. There, rSO2 convincingly reflected systemic circulation disorders (e.g., caused by misplacing cannulas in aortic branches). In acute stroke treatment, such guidance is not nearly as advanced, although it would be highly desirable to establish whether brain tissue is viable. One study examining nine awake patients on the stroke unit found NIRS to respond well to hypotension and desaturation.24 Another study investigating sleep-disordered breathing of subacute stroke also reported that NIRS could identify profound deoxygenation.33 In more severely affected patients requiring hemicraniectomy, the authors described NIRS as an aid in judging events such as cardiopulmonary resuscitation or herniation.23 To this body of knowledge, this study adds that—in hyperacute ischemic stroke patients undergoing endovascular treatment—NIRS-derived rSO2 reflects major systemic changes in circulation and respiration.
Furthermore, we observed small unilateral rSO2 peaks in one-third of patients throughout the whole intervention over the affected hemisphere.
Wolf et al.,34 investigating NIRS in focal cerebral ischemia of the rat, coregistered a differential voltage potential by a calomel electrode and found ‘NIRS fingerprints of peri-infart depolarization (PID)'.34 Originating from the border of the infarct core, these depolarizations typically spread over the whole hemisphere35 and evoke an increased local blood flow in viable tissue and a suppressed or even reversed one in penumbral tissue.35 The morphology of peaks and their duration (see Figure 4 in Wolf et al.34) appear similar to the rSO2 peaks in our observations; however, a different NIRS device was used and the large sampling volume in our study may not be ideal to capture peri-infart depolarizations. We did not perform electroencephalography at the same time; therefore, we do not know whether the observed finding originates from peri-infart depolarizations. Other putative explanations for the small peaks observed may include recovered patency of small vessel by procedural steps, introduction of catheter devices, or manipulation thereof, the influence of intravenous (67.4%) and intraarterial (18.6%) recombinant tissue plasmin activator, and the influences of NIRS limitations—for example, extracerebral skin blood or ambient light. To further investigate and elucidate this interesting finding, a coregistered electroencephalography EEG-NIRS approach (preferably multichannel) including a synchronized correction of NIRS influencing factors—primarily blood pressure, carbon dioxide, and skin blood flow—is needed in future studies.
We observed a sustained rSO2 increase correlating to the time of recanalization in two cases (4.7%). One NIRS study investigating carotid endarterectomy for long-standing carotid stenosis reported increases of that kind to be more frequent in patients with hyperperfusion syndrome,36 but the authors did not specify whether this was bilateral or unilateral. Cerebral hyperperfusion follows carotid endarterectomy and stenting in approximately 3% to 5%.37, 38 One of our patients required additional stenting of the internal carotid artery after intracranial thrombectomy, another suffered an iatrogenic dissection with subarachnoidal bleeding followed by balloon dilatation of the carotid artery. In both cases, an impaired autoregulation caused by a dysfunctional endothelium and injured sympathetic nerves38 may have led to a unilateral increase in perfusion. These findings may support further investigating NIRS as an adjunctive tool to guide postintervention blood pressure management in endovascular stroke patients, especially involving stenting.
The NIRS-derived index of rSO2 is difficult to interpret per se: It is based on the ratio between arterial and venous blood in the measured area in an individual and is associated with many inherent, possible sources of bias (see below). Absolute values appear problematic, and only relative changes or changes over time may produce interpretable results. Previous studies tried to overcome this limitation by calculating the integral below a certain reduction of the baseline value of rSO2 over time (AUC). Cardiac NIRS studies established that a value of 20% below baseline is critical.29 For stroke patients, however, this value may be insensitive because a change in local perfusion may not contribute to a great extent to the composite value of rSO2.
In part B of our analysis (correlation analysis), we used AUC10% to assess changes in local perfusion during the endovascular intervention. rSO2 values of the affected hemisphere in unsuccessfully reperfused patients more often declined below 10% of rSO2 baseline (=higher AUC10% values) when compared with those in whom reperfusion was successful. This finding may emphasize the need for different rSO2 AUC cut-off values in different disciplines with different outcome settings (in this case, the surrogate outcome of cerebral reperfusion).
Another option for expressing changes in rSO2 is to use the nonaffected contralateral hemisphere as reference. Using the interhemispheric difference as a prognostic indicator, Damian and Schlosser23 found that stroke patients with higher values in the affected hemisphere tended to have better outcomes. This appears to be in line with our results (lower values for median interhemispheric difference (mRS6: –1.35, mRS0-5: 2.1) after recanalization was associated with death within 90 days), although the two populations cannot be compared directly. Damian and Schlosser investigated large, advanced infarcts treated by hemicraniectomy for brain edema, whereas our patients were monitored in the first several hours after stroke. Great caution is advised when interpreting the underlying cause of the measured interhemispheric difference in different stroke phases: the mainly venous NIRS index rSO2 does not allow for differentiation between perfusion changes or oxygen extraction changes (or both). As hypothesized by Damian and Schlosser23 (i) less oxygen extraction in infarcted (dead) tissue, or (ii) edema in the infarcted hemisphere may contribute to an rSO2 increase (i.e., more O2 remaining in the cortical veins) may be relevant in the phase of advanced, edematous infarcts. In hyperacute stroke, the first hypothesis may also apply if measurements are taken from an underlying infarct core. However, a (iii) hypoperfusion in underlying tissue is just as likely. Therefore, the marker of interhemispheric difference may be supportive as an additional prognostic marker in different stroke phases. One advantage of this measure is that it automatically adjusts for systemic changes because both hemispheres are affected simultaneously and it only shows the difference to normal.
Continuous physiological measures such as heart rate and blood pressure reflect autonomic cardiovascular control,39 especially when successive measures of variability are applied.30 Lower variability or even a loss of variability as a consequence of autonomic failure is evidently disadvantageous in cerebrovascular diseases.40, 41 Therefore, a third way to analyze rSO2 considers the concept of average successive variability. Used in the most stable period of this study (after the intervention), lower ASVrSO2 was associated with higher values of stroke severity (as measured by NIHSS) at discharge and indicated poor functional outcome of patients at day 90. This possibly indicates localized neural circulatory dysfunction.
For all of these indices, an external validation in a larger sample is clearly necessary, particularly because there has been little testing in the setting of acute ischemic stroke.
Our study has several limitations: first, the NIRS signal is influenced by factors such as ambient light, skin wetness, or extracerebral blood. Although the device used in this study intends to account for the latter using a two-distance approach, effectiveness of this correction is controversial. Our study set-up lacked separate monitoring of skin blood flow of the scalp, therefore we were not able to control for this potential source of inaccuracy. Second, several parameters such as status of cerebral collateralization, arterial carbon dioxide, continuous blood pressure, and cardiac output were not systematically assessed. Third, a considerable severity-related selection bias may have been associated with selecting only large-vessel strokes for endovascular treatment. In addition, 58 % of our anterior circulation patients were not monitored and we had 32% data quality dropouts. There are several reasons for this: sometimes the device was being used in one patient and was not available on arrival of another eligible patient. In addition, owing to higher priority matters the treating physician may have decided not to apply the device at all, the optodes may have been misplaced, or technical dysfunction alarms not corrected for. However, the strength here is that the treating physician used the device independently, which thus reflected a more real-life scenario for the use of this technology in its current form. Furthermore, data quality in this study—despite its emergency character—compared well with other NIRS studies of subacute stroke, which also deemed 27%,33 31%,23 and 32%22 of their data unsuitable. Finally, although the intervals during and after the intervention were well defined, the onset of stroke symptoms to intervention was highly variable, which means a heterogeneous penumbra state. The strengths of this largest NIRS study in early acute ischemic stroke to date are its prospective design, a typical, severely affected stroke population, standardized endovascular treatment, and the availability of outcome parameters.
To improve our ability to detect changes in the microvasculature of stroke patients in the future, technology should adapt to the designated population—in our case emergency stroke patients. This may mean improving signal quality and reliability. One way would be to synchronously correct for factors that influence the signal most—such as blood pressure, carbon dioxide, and blood flow of the scalp skin. To improve the practical aspects, a lighter monitor, a long-lasting, independent power source, and a wireless set-up would be desirable.
We conclude that rSO2 monitoring of anesthetized acute ischemic stroke patients receiving endovascular treatment is in principle feasible, but needs to be optimized in terms of application and data acquisition. (1) It detected important systemic events and may help guide neuroanesthesia in the future if NIRS-based treatment protocols should prove to be beneficial in randomized trials. (2) The ability to monitor revascularization appeared to be limited in the current set-up and may require an improved stroke-specific technological adjustment (e.g., more optodes in particular positions, simultaneous perfusion assessment) in future studies. (3) We could calculate the indices area under the curve (10% below baseline), interhemispheric rSO2 difference, and rSO2 variability to predict reperfusion, mortality, and functional outcome, respectively. These findings should be confirmed in larger patient cohorts.
Acknowledgments
The authors thank Peter Ringleb for independent outcome evaluation at day 90 after stroke, Sheryll Sundell for language editing, Matthias Gondan for early statistical guidance and Werner Hacke for providing the research environment and continuing support.
Author contributions
All authors have made substantial contribution to the manuscript: JB and RV conceived and designed the research, and made critical revision of the manuscript for important intellectual content. CH acquired the data, analyzed, and interpreted the data, performed statistical analysis, and drafted the manuscript. PS acquired and analyzed the data and made critical revision of the manuscript for important intellectual content. SS and SR acquired data and made critical revision of the manuscript for important intellectual content.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Christian Hametner received congress fee support from the company (Covidien) that manufactures the near-infrared spectroscopy device used in this study.
Roland Veltkamp received consulting honoraria from Covidien.
Julian Bösel received travel support and speaker honoraria from Covidien.
Covidien had no influence whatsoever on the design, the conduct, the data selection, or the data analysis in this study. The remaining authors declare no conflict of interest.
Supplementary Material
References
- 1Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 2Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 3Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 4Brinjikji W, Murad MH, Rabinstein AA, Cloft HJ, Lanzino G, Kallmes DF. Conscious sedation versus general anesthesia during endovascular acute ischemic stroke treatment: a systematic review and meta-analysis. Am J Neuroradiol 2015; 36: 525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Froehler MT, Fifi JT, Majid A, Bhatt A, Ouyang M, McDonagh DL. Anesthesia for endovascular treatment of acute ischemic stroke. Neurology 2012; 79: S167–S173. [DOI] [PubMed] [Google Scholar]
- 6Davis MJ, Menon BK, Baghirzada LB, Campos-Herrera CR, Goyal M, Hill MD et al. Anesthetic management and outcome in patients during endovascular therapy for acute stroke. Anesthesiology 2012; 116: 396–405. [DOI] [PubMed] [Google Scholar]
- 7Davie SN, Grocott HP. Impact of extracranial contamination on regional cerebral oxygen saturation: a comparison of three cerebral oximetry technologies. Anesthesiology 2012; 116: 834–840. [DOI] [PubMed] [Google Scholar]
- 8Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 1977; 198: 1264–1267. [DOI] [PubMed] [Google Scholar]
- 9Cope M, Delpy DT. System for long-term measurement of cerebral blood and tissue oxygenation on newborn infants by near infra-red transillumination. Med Biol Engineer Comput 1988; 26: 289–294. [DOI] [PubMed] [Google Scholar]
- 10Ghosh A, Elwell C, Smith M. Review article: cerebral near-infrared spectroscopy in adults: a work in progress. Anesthesia Analgesia 2012; 115: 1373–1383. [DOI] [PubMed] [Google Scholar]
- 11Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesthesia 2009; 103: i3–13. [DOI] [PubMed] [Google Scholar]
- 12Terborg C, Groschel K, Petrovitch A, Ringer T, Schnaudigel S, Witte OW et al. Noninvasive assessment of cerebral perfusion and oxygenation in acute ischemic stroke by near-infrared spectroscopy. Eur Neurol 2009; 62: 338–343. [DOI] [PubMed] [Google Scholar]
- 13Taussky P, O'Neal B, Daugherty WP, Luke S, Thorpe D, Pooley RA et al. Validation of frontal near-infrared spectroscopy as noninvasive bedside monitoring for regional cerebral blood flow in brain-injured patients. Neurosurg Focus 2012; 32: E2. [DOI] [PubMed] [Google Scholar]
- 14Steinkellner O, Gruber C, Wabnitz H, Jelzow A, Steinbrink J, Fiebach JB et al. Optical bedside monitoring of cerebral perfusion: technological and methodological advances applied in a study on acute ischemic stroke. J Biomed Optics 2010; 15: 061708. [DOI] [PubMed] [Google Scholar]
- 15Obrig H. NIRS in clinical neurology - a 'promising' tool? NeuroImage 2014; 85: 535–546. [DOI] [PubMed] [Google Scholar]
- 16Terborg C, Bramer S, Harscher S, Simon M, Witte OW. Bedside assessment of cerebral perfusion reductions in patients with acute ischaemic stroke by near-infrared spectroscopy and indocyanine green. J Neurol Neurosurg Psychiatry 2004; 75: 38–42. [PMC free article] [PubMed] [Google Scholar]
- 17Terborg C, Gora F, Weiller C, Rother J. Reduced vasomotor reactivity in cerebral microangiopathy: a study with near-infrared spectroscopy and transcranial Doppler sonography. Stroke 2000; 31: 924–929. [DOI] [PubMed] [Google Scholar]
- 18Vernieri F, Rosato N, Pauri F, Tibuzzi F, Passarelli F, Rossini PM. Near infrared spectroscopy and transcranial Doppler in monohemispheric stroke. Eur Neurol 1999; 41: 159–162. [DOI] [PubMed] [Google Scholar]
- 19Palazzo P, Tibuzzi F, Pasqualetti P, Altamura C, Silvestrini M, Passarelli F et al. Is there a role of near-infrared spectroscopy in predicting the outcome of patients with carotid artery occlusion? J Neurol Sci 2010; 292: 36–39. [DOI] [PubMed] [Google Scholar]
- 20Bonoczk P, Panczel G, Nagy Z. Vinpocetine increases cerebral blood flow and oxygenation in stroke patients: a near infrared spectroscopy and transcranial Doppler study. Eur J Ultrasound 2002; 15: 85–91. [DOI] [PubMed] [Google Scholar]
- 21Hargroves D, Tallis R, Pomeroy V, Bhalla A. The influence of positioning upon cerebral oxygenation after acute stroke: a pilot study. Age Ageing 2008; 37: 581–585. [DOI] [PubMed] [Google Scholar]
- 22Aries MJ, Elting JW, Stewart R, De Keyser J, Kremer B, Vroomen P. Cerebral blood flow velocity changes during upright positioning in bed after acute stroke: an observational study. BMJ Open 2013; 3: e002960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Damian MS, Schlosser R. Bilateral near infrared spectroscopy in space-occupying middle cerebral artery stroke. Neurocrit Care 2007; 6: 165–173. [DOI] [PubMed] [Google Scholar]
- 24Aries MJ, Coumou AD, Elting JW, van der Harst JJ, Kremer BP, Vroomen PC. Near infrared spectroscopy for the detection of desaturations in vulnerable ischemic brain tissue: a pilot study at the stroke unit bedside. Stroke 2012; 43: 1134–1136. [DOI] [PubMed] [Google Scholar]
- 25Ritzenthaler T, Cho TH, Luis D, Berthezene Y, Nighoghossian N. Usefulness of near-infrared spectroscopy in thrombectomy monitoring. J Clin Monit Comput. advance online publication, 4 November 2014 (e-pub ahead of print). [DOI] [PubMed]
- 26Jauch EC, Saver JL, Adams HP, Jr., Bruno A, Connors JJ, Demaerschalk BM et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 870–947. [DOI] [PubMed] [Google Scholar]
- 27Moritz S, Kasprzak P, Arlt M, Taeger K, Metz C. Accuracy of cerebral monitoring in detecting cerebral ischemia during carotid endarterectomy: a comparison of transcranial Doppler sonography, near-infrared spectroscopy, stump pressure, and somatosensory evoked potentials. Anesthesiology 2007; 107: 563–569. [DOI] [PubMed] [Google Scholar]
- 28Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013; 44: 2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Murkin JM, Adams SJ, Novick RJ, Quantz M, Bainbridge D, Iglesias I et al. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesthesia Analgesia 2007; 104: 51–58. [DOI] [PubMed] [Google Scholar]
- 30Mena L, Pintos S, Queipo NV, Aizpurua JA, Maestre G, Sulbaran T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens 2005; 23: 505–511. [DOI] [PubMed] [Google Scholar]
- 31McCormick PW, Stewart M, Goetting MG, Balakrishnan G. Regional cerebrovascular oxygen saturation measured by optical spectroscopy in humans. Stroke 1991; 22: 596–602. [DOI] [PubMed] [Google Scholar]
- 32Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993; 80: 27–38. [Google Scholar]
- 33Pizza F, Biallas M, Kallweit U, Wolf M, Bassetti CL. Cerebral hemodynamic changes in stroke during sleep-disordered breathing. Stroke 2012; 43: 1951–1953. [DOI] [PubMed] [Google Scholar]
- 34Wolf T, Lindauer U, Reuter U, Back T, Villringer A, Einhaupl K et al. Noninvasive near infrared spectroscopy monitoring of regional cerebral blood oxygenation changes during peri-infarct depolarizations in focal cerebral ischemia in the rat. J Cereb Blood Flow Metab 1997; 17: 950–954. [DOI] [PubMed] [Google Scholar]
- 35Strong AJ, Anderson PJ, Watts HR, Virley DJ, Lloyd A, Irving EA et al. Peri-infarct depolarizations lead to loss of perfusion in ischaemic gyrencephalic cerebral cortexBrain 2007; 130: 995–1008. [DOI] [PubMed]
- 36Pennekamp CW, Immink RV, den Ruijter HM, Kappelle LJ, Ferrier CM, Bots ML et al. Near-infrared spectroscopy can predict the onset of cerebral hyperperfusion syndrome after carotid endarterectomy. Cerebrovasc Dis 2012; 34: 314–321. [DOI] [PubMed] [Google Scholar]
- 37Lieb M, Shah U, Hines GL. Cerebral hyperperfusion syndrome after carotid intervention: a review. Cardiol Rev 2012; 20: 84–89. [DOI] [PubMed] [Google Scholar]
- 38van Mook WN, Rennenberg RJ, Schurink GW, van Oostenbrugge RJ, Mess WH, Hofman PA et al. Cerebral hyperperfusion syndrome. Lancet Neurol 2005; 4: 877–888. [DOI] [PubMed] [Google Scholar]
- 39Omboni S, Parati G, Di Rienzo M, Wieling W, Mancia G. Blood pressure and heart rate variability in autonomic disorders: a critical review. Clin Auton Res 1996; 6: 171–182. [DOI] [PubMed] [Google Scholar]
- 40Budohoski KP, Czosnyka M, Kirkpatrick PJ, Reinhard M, Varsos GV, Kasprowicz M et al. Bilateral failure of cerebral autoregulation is related to unfavorable outcome after subarachnoid hemorrhage. Neurocrit Care 2014; 22: 65–73. [DOI] [PubMed] [Google Scholar]
- 41Sykora M, Diedler J, Poli S, Rizos T, Kellert L, Turcani P et al. Association of non-diabetic hyperglycemia with autonomic shift in acute ischaemic stroke. Eur J Neurol 2012; 19: 84–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.