Abstract
Background
Spontaneous pneumomediastinum (SPM) is a benign disease with a variety degree of severity but definite treatment modality is not clearly identified with its rarity. The purpose of this study was to review our experience and discuss the management of SPM according to the severity of disease.
Methods
From March 1996 to December 2012, total 64 patients were enrolled and classified as mild, moderate and severe groups and subsequent clinical courses were analyzed retrospectively.
Results
Fifty-one were males and 13 were females (M:F =3.9:1) with a mean age of 18 years old (range: 10-30 years old). Thirty-six patients were in mild, 22 in moderate and 6 in severe group. Chief complaints were chest pain (50 cases; 78.1%), neck pain (35 cases; 54.7%), dyspnea (18 cases; 28.1%), odynophagia (9 cases; 14.1%) and precipitating factors were coughing in 12 cases, feeding problems in 9 cases, and vomiting in 7 cases; however, 34 patients (53.1%) had no precipitating signs. All patients received oxygen therapy (100%), prophylactic antibiotics in 57 patients (89.1%), and pain medications in 47 patients (73.4%). The mean hospital stay was 4.6 days (range: 1-10 days). There was an increased linear trend according to time to visit (P=0.023) but clinical course demonstrated no significant trend between groups.
Conclusions
These data demonstrated that there was no difference in symptom, clinical course and SPM was adequately treated with conservative management regardless of the degree of severity of SPM.
Keywords: Pneumomediastinum, mediastinal emphysema, chest pain, subcutaneous emphysema
Introduction
Spontaneous pneumomediastinum (SPM) is defined as the presence of interstitial air in the mediastinum. It is a benign disease entity, which rarely occurs but responds well to conservative treatment (1). SPM is diagnosed with presenting symptoms, physical signs with air in the mediastinum, radiologic confirmation, and the absence of specific pathologic causes that are present in secondary pneumomediastinum (2,3). SPM was first described by Louis Hamman in 1939 (4) and published reports have described the nature of SPM and its clinical significance in only a small number of cases and definite treatment modality is not clearly identified yet. The purpose of this study was to review our clinical experience and detail the clinical approach on the management of SPM according to the severity of the disease with a literature review.
Methods
From March 1996 to December 2012, we retrospectively reviewed the data of patients admitted for treatment of SPM as an initial primary diagnosis in two institutions. We analyzed demographic data, presenting symptoms and signs, radiologic and interventional evaluation studies, and treatment courses. A precipitating factor was defined as a related recent event, which could trigger the development of SPM. Secondary pneumomediastinum due to specific causes were excluded: including trauma, esophageal perforation, recent intervention or surgery involving aero-digestive organs, abnormal findings in tracheobronchial tree, intrathoracic infections by gas forming organisms, mechanical ventilator or tracheostomy related conditions, or combined pneumothorax or emphysematous lung disease.
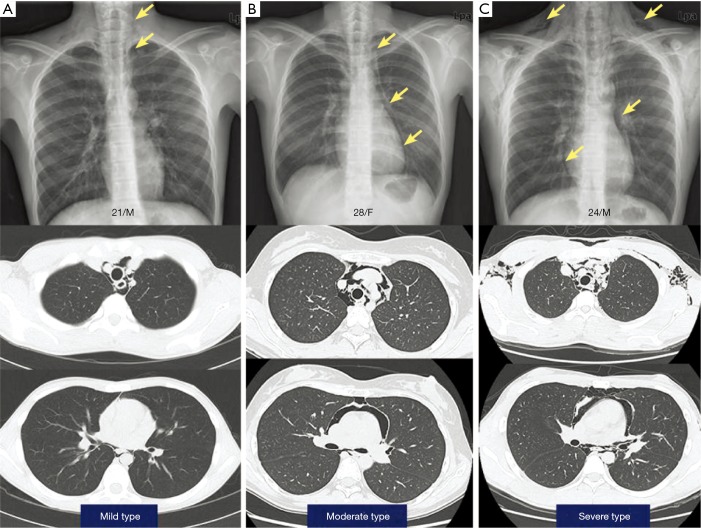
Sixty-four cases were diagnosed with SPM who were older than 10 years of age. We classified the patients three groups according to the extent of disease (mild: presence of minimal air shadow from larynx to carina level plus restricted cervical subcutaneous emphysema; moderate: mild criteria plus involvement of peribronchial tree and pericardial area with subcutaneous emphysema; and severe: moderate criteria plus extensive chest wall subcutaneous emphysema) by initial radiologic evaluation (Figure 1) and clinical course, including lab findings were analyzed. Institutional Review Board approved this study and individual informed consents were waived.
Figure 1.
Representative appearance of spontaneous pneumomediastinum in initial chest X-ray and chest computed tomography by simple classification. Mild type (A), moderate type (B) and severe type (C).
Statistical analysis
Categorical variables are expressed as percentages and continuous variables are reported as mean ± standard deviation (SD). Chi-square test or Fisher’s exact test was used for categorical variables. Patients were divided into three groups (mild, moderate, and severe type) and analyzed with one-way analysis of variance (ANOVA), and performed test for linear trend. The P values <0.05 were considered to indicate statistical significance. All statistical analyses were performed with SAS (version 9.3; SAS Institute, Cary, NC) or SPSS (version 19.0; SPSS Inc., Chicago, IL).
Results
After exclusion of secondary pneumomediastinum cases, 64 patients were enrolled as a SPM. There were 36 (56.3%) patients in mild type, 22 (34.4%) in moderate type and 6 (9.4%) in severe type. Fifty-one were males and 13 were females (M:F =3.9:1); the mean age was 18.5±4 years old (range, 10-30 years old) and the mean body mass index (BMI) was 19.6±2.8 kg/m2 (range, 9.0-24.9 kg/m2). There were 57 (89.1%) non-smokers, 6 (9.4%) current smokers and 1 (1.6%) ex-smoker, but no patients had received steroids or other drugs. There was an increased prevalence in the summer season (28 cases; 43.8%) and 2 (3.1%) with history of pneumothorax patients and 4 (6.3%) with asthmatic patients. The demographic data shows no difference between groups (Table 1). The most frequent chief complaint at presentation was chest pain (50 cases, 78.1%), followed by neck pain (35 cases, 54.7%), dyspnea (18 cases, 28.1%), odynophagia (9 cases, 14.1%). Subcutaneous emphysema was presented by initial assessment in 27 cases (42%). Precipitating factors were coughing (12 cases, 18.8%), feeding difficulties (9 cases, 14.1%), vomiting (7 cases, 10.9%), exercise (4 cases, 6.3%), and playing wind instrument (1 case, 1.6%); however, 34 patients (53.1%) had no precipitating signs. Notably, vomiting and disease severity showed significant relationship (P=0.015, Table 2).
Table 1. Demographics of patients (n=64).
| Variables | Groups, n (%) |
P value | |||
|---|---|---|---|---|---|
| Mild, n=36 | Moderate, n=22 | Severe, n=6 | Total, n=64 | ||
| Age, mean ± SD (years) | 19.06±4.28 | 17.81±3.67 | 18.00±4.05 | 18.53±4.04 | 0.505 |
| Male | 31 (86.1) | 15 (68.2) | 5 (83.3) | 51 (79.7) | 0.262 |
| Height, mean ± SD (cm) | 171.36±8.11 | 167.68±10.83 | 173.50±7.97 | 170.30±9.20 | 0.227 |
| Weight, mean ± SD (kg) | 58.31±10.21 | 56.30±12.09 | 53.17±16.47 | 57.14±11.43 | 0.550 |
| BMI, mean ± SD | 19.78±2.58 | 19.79±2.40 | 17.35±4.71 | 19.56±2.81 | 0.130 |
| Smoking | 0.679 | ||||
| Non-smoker | 30 (83.3) | 21 (95.5) | 6 (100.0) | 57 (89.1) | |
| Smoker | 5 (13.9) | 1 (4.5) | 0 (0.0) | 6 (9.4) | |
| Ex-smoker | 1 (2.8) | 0 (0.0) | 0 (0.0) | 1 (1.6) | |
| Season | 0.244 | ||||
| Spring | 11 (30.6) | 2 (9.1) | 2 (33.3) | 15 (23.4) | |
| Summer | 16 (44.4) | 10 (45.5) | 2 (33.3) | 28 (43.8) | |
| Fall | 8 (22.2) | 7 (31.8) | 1 (16.7) | 16 (25.0) | |
| Winter | 1 (2.8) | 3 (13.6) | 1 (16.7) | 5 (7.8) | |
| Predisposing factor | |||||
| Asthma | 2 (5.6) | 2 (9.1) | 0 (0.0) | 4 (6.3) | 0.753 |
| History of pneumothorax | 2 (5.6) | 0 (0.0) | 0 (0.0) | 2 (3.1) | 0.607 |
| Interstitial lung disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | N/A |
| Steroid use | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | N/A |
| Subcutaneous emphysema | 13 (36.1) | 9 (40.9) | 5 (83.3) | 27 (42.2) | 0.123 |
Categorical variables are reported as frequencies (%), and continuous variables are reported as mean ± SD. Chi-square test or Fischer’s exact test was used for categorical variables. SD, standard deviation; BMI, body mass index; N/A, not applicable.
Table 2. Presenting complaints and precipitating factors (n=64).
| Variables | Groups, n (%) |
P value | |||
|---|---|---|---|---|---|
| Mild, n=36 | Moderate, n=22 | Severe, n=6 | Total, n=64 | ||
| Presenting complaints | |||||
| Chest pain | 26 (72.2) | 18 (81.8) | 6 (100.0) | 50 (78.1) | 0.317 |
| Neck pain | 20 (55.6) | 12 (54.5) | 3 (50.0) | 35 (54.7) | 1.000 |
| Dyspnea | 9 (25.0) | 7 (31.8) | 2 (33.3) | 18 (28.1) | 0.774 |
| Odynophagia | 5 (13.9) | 4 (18.2) | 0 (0.0) | 9 (14.1) | 0.765 |
| Coughing | 0 (0.0) | 1 (4.5) | 0 (0.0) | 1 (1.6) | 0.438 |
| Voice change | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (1.6) | 0.094 |
| Precipitating factors | |||||
| No precipitating factor | 21 (58.3) | 11 (50.0) | 2 (33.3) | 34 (53.1) | 0.490 |
| Coughing | 6 (16.7) | 5 (22.7) | 1 (16.7) | 12 (18.8) | 0.888 |
| Feeding | 4 (11.1) | 4 (18.2) | 1 (16.7) | 9 (14.1) | 0.566 |
| Vomiting | 2 (5.6) | 2 (9.1) | 3 (50.0) | 7 (10.9) | 0.015 |
| Exercise | 2 (5.6) | 1 (4.5) | 1 (16.7) | 4 (6.3) | 0.535 |
| Playing wind instrument | 1 (2.8) | 0 (0.0) | 0 (0.0) | 1 (1.6) | 1.000 |
Categorical variables are reported as frequencies (%). Chi-square test or Fischer’s exact test was used for categorical variables.
Plain chest radiography was initially done in all patients. The plain radiography of the neck was concomitantly checked in 37 (57.8%) patients. Fifty-four (84.4%) chest computed tomography (CT) and 7 (10.9%) neck CT scans were performed. Additional studies of 10 (15.6%) esophagograms, 4 (6.3%) endoscopies and 1 (1.6%) bronchoscopy was conducted; however, they revealed otherwise unremarkable findings. One patient with an abnormal endobronchial tumor was finally diagnosed as secondary pneumomediastinum and was excluded from the study. The mean duration between onset of symptoms and hospital presentation was 14.0±18.6 hours (range, 0.5-96 hours). Conservative management, consisting of bed rest with oxygen inhalation (64 patients; 100%), prophylactic antibiotics (57 patients; 89.1%), and analgesic medications (47 patients; 73.4%) were applied. The mean duration of hospitalization was 4.6±1.8 days (range: 1-10 days). Feeding restriction was conducted in 39 (60.9%) patients. There were no mortality or morbidity cases, such as mediastinitis, tension pneumomediastinum, or tension pneumothorax during hospitalization; furthermore, recurrence of SPM developed in only 1 (1.6%) patient in the moderate type after 12 months, which was also managed conservatively. Primary spontaneous pneumothorax developed 6 and 17 months later in 2 (3.1%) patients in the mild type who had no previous history of pneumothorax. There was no significant difference in clinical course and management between groups (Table 3).
Table 3. Study evaluation and clinical course (n=64).
| Variables | Groups, n (%) |
P value | |||
|---|---|---|---|---|---|
| Mild, n=36 | Moderate, n=22 | Severe, n=6 | Total, n=64 | ||
| Radiologic evaluation | |||||
| Chest X-ray | 36 (100.0) | 22 (100.0) | 6 (100.0) | 64 (100.0) | N/A |
| Neck film | 23 (63.9) | 11 (50.0) | 3 (50.0) | 37 (57.8) | 0.597 |
| Chest CT | 28 (77.8) | 20 (90.9) | 6 (100.0) | 54 (84.4) | 0.332 |
| Neck CT | 4 (11.1) | 2 (9.1) | 1 (16.7) | 7 (10.9) | 0.714 |
| Both | 3 (8.3) | 1 (4.5) | 1 (16.7) | 5 (7.8) | 0.487 |
| Aero-digestive organ study | |||||
| Esophagography | 3 (8.3) | 6 (27.3) | 1 (16.7) | 10 (15.6) | 0.143 |
| Endoscopy | 1 (2.8) | 3 (13.6) | 0 (0.0) | 4 (6.3) | 0.300 |
| Bronchoscopy | 1 (2.8) | 0 (0.0) | 0 (0.0) | 1 (1.6) | 1.000 |
| Treatment modality | |||||
| Initial nothing by mouth | 22 (61.1) | 13 (59.1) | 4 (66.7) | 39 (60.9) | 1 |
| Oxygen inhalation | 36 (100.0) | 22 (100.0) | 6 (100.0) | 64 (100.0) | N/A |
| Empirical antibiotics | 31 (86.1) | 21 (95.5) | 5 (83.3) | 57 (89.1) | 0.392 |
| Pain medication | 27 (75.0) | 15 (68.2) | 5 (83.3) | 47 (73.4) | 0.768 |
| Clinical course | |||||
| Time to visit, mean ± SD (hours) | 12.06±14.15 | 12.68±21.36 | 30.67±25.97 | 14.02±18.60 | 0.068 |
| Hospital days, mean ± SD | 4.11±1.55 | 5.23±2.00 | 5.17±1.33 | 4.59±1.76 | 0.043 |
| Recurrence | 0 (0.0) | 1 (4.5) | 0 (0.0) | 1 (1.6) | 0.438 |
| Spontaneous pneumothorax | 2 (5.6) | 0 (0.0) | 0 (0.0) | 2 (3.1) | 0.607 |
Categorical variables are reported as frequencies (%), and continuous variables are reported as mean ± SD. Chi-square test or Fischer’s exact test was used for categorical variables. N/A, not applicable; CT, computed tomography; SD, standard deviation.
The mean white blood cell (WBC) count was 10,226.6±3,473.2/µL (range, 3,440-19,160/µL) with a mean absolute neutrophil count (ANC) of 7,000.0±3,107.7/µL (range, 1,307-14,774/µL), and an elevated WBC (>10,000) was observed in 29 cases (45.3%). Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were checked in 39 (60.9%) cases and the mean ESR was 6.18±5.43 mm/h (range, 2-24 mm/h) and the mean CRP count was 2.44±8.91 mg/L (range, 0.02-54.72 mg/L). Time to visit has a significant linear trend (P=0.023); however, there was no statistically significant linear trend in hospital days, smoking history, WBC count, ANC count, ESR and CRP (Table 4).
Table 4. Comparison results according to the classification by disease severity.
| Variables | Groups, mean ± SD |
P value# | |||
|---|---|---|---|---|---|
| Mild, n=36 | Moderate, n=22 | Severe, n=6 | Total, n=64 | ||
| Hospital days | 4.11±1.55 | 5.23±2.00 | 5.17±1.33 | 4.59±1.76 | 0.164 |
| Time to visit (hours) | 12.06±14.15 | 12.68±21.36 | 30.67±25.97 | 14.02±18.60 | 0.023 |
| Smoking history, n (%)§ | 6 (16.7) | 1 (4.5) | 0 (0.0) | 7 (10.9) | 0.099 |
| WBC count (/µL) | 9,561.1±3,169.1 | 10,984.5±3,629.7 | 11,440.0±4,344.6 | 10,226.6±3,473.2 | 0.22 |
| Segmented neutrophils (%) | 63.89±11.20 | 68.06±9.49 | 75.28±10.05 | 66.39±10.95 | 0.039 |
| ANC count (/µL) | 6,351.2±2,823.3 | 7,603.5±3,220.5 | 8,679.5±3,782.8 | 7,000.0±3,107.7 | 0.089 |
| ESR (mm/h)* | 5.95±4.74 | 7.29±6.74 | 2.67±1.15 | 6.18±5.43 | 0.402 |
| CRP (mg/L)* | 3.42±11.80 | 1.30±1.96 | 0.63±0.35 | 2.44±8.91 | 0.621 |
#, P values for differences were determined by trend test; §, smoking history includes smoker plus ex-smoker; *, ESR and CRP were checked in 39 (60.9%) cases (i.e., numbers of cases in mild, moderate and severe groups were 22, 14 and 3, respectively). SD, standard deviation; WBC, white blood cell; ANC, absolute neutrophil count; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
Discussion
Pneumomediastinum is a benign disease entity, defined as the presence of free air in the mediastinum and is classified as spontaneous or secondary type (1,2). A secondary pneumomediastinum has several causes: for example, recent interventions, history of aero-digestive organ injury, cervico-thoracic infections, pneumothorax, or mechanical ventilator related injury. These cases may require invasive intervention (3,5,6). On the contrary, SPM is diagnosed without any definite cause. Pneumomediastinum was first reported by René Laennec in 1819 and spontaneous mediastinal emphysema was described by Louis Hamman in 1939 (4). The pathophysiologic mechanism of SPM is uncertain; but Macklin et al. in 1944 suggested that increased intrathoracic pressure induces alveolar rupture with passage of air into the interstitium or bronchovascular tissues of the tracheobronchial tree (7,8). This uncommon disease has been described in several case series but diagnostic and therapeutic guidelines for SPM remain debatable. Kaneki et al. suggested detailed classification could be made by the presence of subcutaneous emphysema on chest X-ray and the degree of free air in the mediastinum imaged by a chest CT (9). We also theorized that the mediastinal air shadow in a radiologic exam and subcutaneous emphysema is indicative of a pneumomediastinum; therefore, we suggested detailed classification made by the presence of subcutaneous emphysema on chest X-ray and the degree of free air in the mediastinum imaged by the chest CT scan.
The most common symptom at presentation is chest pain, as it was found in our study. The other symptoms including dyspnea, sore throat or dysphagia were observed (5,9-13). Possible precipitating events include coughing, vomiting, defecation, parturition, or other physical activity that is associated with increased intrathoracic pressure (e.g., Valsalva maneuver, playing wind instrument); however, it can develop without a triggering event. Many western reports have described a relationship between smoking or drug abuse and the development of SPM. However, we found that the majority of patients were non-smokers (89%) and none used steroids or were drug abusers. According to the literature, precipitating events ranged from 39-100% (2,12). Our study also revealed that chest pain (78%) was the most common symptom; however, more than half of the patients did not experience a precipitating event.
Several studies reported that SPM occurs predominantly in young males, and that minimal, undetectable bulla can be the cause of the pneumomediastinum (2,9-14). Our results also revealed that SPM developed with high prevalence in young males, and it has an ectomorphic appearance similar to that of patients with pneumothorax. However, concomitant pneumothorax cases were excluded initially and definite bullae were not found in all patients who underwent a CT scan. This undefined relationship results in SPM being classified as a unique disease entity; thus, the treatment methodology should be different from that of spontaneous pneumothorax.
A simple diagnostic tool to detect SPM is either a plain chest radiograph or chest CT scan. Previous reports have noted that SPM can be confirmed by chest radiography alone in about 70% of cases; and the other 30% of patients can be diagnosed with a chest CT scan (9,14). In our study, all patients were suspected of suffering from a SPM by an initial chest X-ray; however, chest CT (84.4%) and neck CT (10.9%) scans were also conducted. We performed a CT scan to confirm the diagnosis as well as to exclude secondary pneumomediastinum; moreover, a diagnostic accuracy was achieved up to 100% with chest radiography, including additional CT scans. Further evaluation studies such as an esophagogram, bronchoscopy and endoscopy were performed in several cases to determine another obvious origins; however, as was the case in similar studies (15,16), all but one patient with an incidental endobronchial tumor, exhibited no abnormal finding. Thus, we demonstrated that a plain chest X-ray including a CT scan is an adequate evaluation procedure after a thorough history and physical examination to exclude secondary pneumomediastinum. Further invasive interventional studies such as bronchoscopy or esophagogram should be performed in highly suspicious cases of aero-digestive organ injury, such as Boerhaave’s syndrome or tracheobronchial tree rupture.
Oxygen therapy has been recommended in most previous reports because it is considered that the consumption of oxygen increases the diffusion pressure of nitrogen in the interstitium, promoting absorption of free air in the mediastinum. In our data, oxygen inhalation was applied in all 64 patients (100%). Although an elevated WBC (>10,000) was observed in 29 cases (45.3%) but most of patients (89.1%) were prescribed empirical antibiotics to prevent possible infection or disease aggravation such as mediastinitis. It remains debatable whether antibiotic treatment is essential. We suggest that empirical antibiotics should be minimized and applied in selected patients showing leukocytosis with fever and subsequent infection is likely. Moreover, oral feeding was restricted in 39 patients (60.9%) but a normal diet was given within 24-48 hours in most of cases. Neither evaluation nor clinical course documented digestive organ injury or abnormality in all cases including seven patients with a history of vomiting. We recommend that dietary restriction is unnecessary and early feeding is reasonable, except in the cases of highly suspected patient of Boerhaave’s syndrome. Discharge was determined by relief of subjective symptoms, with regressed pneumomediastinum on follow-up plain radiography. The duration of hospitalization was shorter in the mild type (P=0.043) and it is likely that the early disappearance of subjective symptoms and visible subcutaneous emphysema in a follow-up radiologic examination results in the clinical decision for early discharge without further evaluation.
Recurrence of SPM is rarely reported (5,12). One recurrence (1.6%) occurred in our study after 12 months but it responded well to conservative management. Two other male patients developed a spontaneous pneumothorax; one patient who developed blebs underwent thoracoscopic surgery 6 months later and another patient was managed with a closed thoracostomy 17 months later. We reviewed the chest CT scans in these recurrence and pneumothorax cases just after the SPM was diagnosed but we could not find any evidence of bulla lesion or emphysema. Most published reports demonstrated a very low incidence of recurrence, which responded well to conservative management; similar to our case in which routine outpatient follow-up was not recommended.
According to the literature, the presence of subcutaneous emphysema ranges from 40% to 100% (10,15); 27 cases (42.2%) showed subcutaneous emphysema in this study. There was no difference in demographics, evaluation studies, treatment modality and clinical courses between the groups (Tables 1-3). We also analyzed the relationship between disease groups by classification and laboratory markers of inflammation by test for linear trend. The trend analysis revealed significant result in time to visit only (P=0.023). Progression of the pneumomediastinum according to the increased time interval may show in a radiologic examination as a disease progression. However, there was no significant trend in hospital days, smoking history, WBC count, ANC count including ESR and CRP (Table 4)
Our retrospective review showed good results from conservative management, similar to that described by previous studies (13,17-21). Thus, we suggest that a chest X-ray and CT scan is adequate for diagnostic evaluation and that routine dietary restriction is unnecessary. Empirical antibiotics should be minimized and bed rest with oxygen inhalation or supportive pain control is adequate for the most of cases diagnosed with SPM.
Conclusions
SPM is adequately treated with conservative management regardless of the degree of severity of SPM. Thus, invasive evaluation studies and intensive treatment should be reserved for highly suspicious, selective cases of aero-digestive organ injury.
Acknowledgements
Funding: The statistical consultation was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C1731).
Consents: Written informed consent was obtained from all the patients. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Panacek EA, Singer AJ, Sherman BW, et al. Spontaneous pneumomediastinum: clinical and natural history. Ann Emerg Med 1992;21:1222-7. [DOI] [PubMed] [Google Scholar]
- 2.Caceres M, Ali SZ, Braud R, et al. Spontaneous pneumomediastinum: a comparative study and review of the literature. Ann Thorac Surg 2008;86:962-6. [DOI] [PubMed] [Google Scholar]
- 3.Maunder RJ, Pierson DJ, Hudson LD. Subcutaneous and mediastinal emphysema. Pathophysiology, diagnosis, and management. Arch Intern Med 1984;144:1447-53. [PubMed] [Google Scholar]
- 4.Hamman L. Spontaneous mediastinal emphysema. Bull Johns Hopkins Hosp 1939;64:1-21. [Google Scholar]
- 5.Abolnik I, Lossos IS, Breuer R. Spontaneous pneumomediastinum. A report of 25 cases. Chest 1991;100:93-5. [DOI] [PubMed] [Google Scholar]
- 6.Newcomb AE, Clarke CP. Spontaneous pneumomediastinum: a benign curiosity or a significant problem? Chest 2005;128:3298-302. [DOI] [PubMed] [Google Scholar]
- 7.Macklin MT, Macklin CC. Malignant interstitial emphysema of the lungs and mediastinum as an important occult complication in many respiratory diseases and other conditions: an interpretation of the clinical literature in the light of laboratory experiment. Medicine 1944;23:281-358. [Google Scholar]
- 8.Murayama S, Gibo S. Spontaneous pneumomediastinum and Macklin effect: Overview and appearance on computed tomography. World J Radiol 2014;6:850-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaneki T, Kubo K, Kawashima A, et al. Spontaneous pneumomediastinum in 33 patients: yield of chest computed tomography for the diagnosis of the mild type. Respiration 2000;67:408-11. [DOI] [PubMed] [Google Scholar]
- 10.Miura H, Taira O, Hiraguri S, et al. Clinical features of medical pneumomediastinum. Ann Thorac Cardiovasc Surg 2003;9:188-91. [PubMed] [Google Scholar]
- 11.Campillo-Soto A, Coll-Salinas A, Soria-Aledo V, et al. Spontaneous pneumomediastinum: descriptive study of our experience with 36 cases. Arch Bronconeumol 2005;41:528-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg 2007;31:1110-4. [DOI] [PubMed] [Google Scholar]
- 13.Al-Mufarrej F, Badar J, Gharagozloo F, et al. Spontaneous pneumomediastinum: diagnostic and therapeutic interventions. J Cardiothorac Surg 2008;3:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho AS, Ahmed A, Huang JS, et al. Multidetector computed tomography of spontaneous versus secondary pneumomediastinum in 89 patients: can multidetector computed tomography be used to reliably distinguish between the 2 entities? J Thorac Imaging 2012;27:85-92. [DOI] [PubMed] [Google Scholar]
- 15.Takada K, Matsumoto S, Hiramatsu T, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med 2008;102:1329-34. [DOI] [PubMed] [Google Scholar]
- 16.Iyer VN, Joshi AY, Ryu JH. Spontaneous pneumomediastinum: analysis of 62 consecutive adult patients. Mayo Clin Proc 2009;84:417-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banki F, Estrera AL, Harrison RG, et al. Pneumomediastinum: etiology and a guide to diagnosis and treatment. Am J Surg 2013;206:1001-6; discussion 1006. [DOI] [PubMed] [Google Scholar]
- 18.Chapdelaine J, Beaunoyer M, Daigneault P, et al. Spontaneous pneumomediastinum: are we overinvestigating? J Pediatr Surg 2004;39:681-4. [DOI] [PubMed] [Google Scholar]
- 19.Haam SJ, Lee JG, Kim DJ, et al. Oesophagography and oesophagoscopy are not necessary in patients with spontaneous pneumomediastinum. Emerg Med J 2010;27:29-31. [DOI] [PubMed] [Google Scholar]
- 20.Freixinet J, García F, Rodríguez PM, et al. Spontaneous pneumomediastinum long-term follow-up. Respir Med 2005;99:1160-3. [DOI] [PubMed] [Google Scholar]
- 21.Jougon JB, Ballester M, Delcambre F, et al. Assessment of spontaneous pneumomediastinum: experience with 12 patients. Ann Thorac Surg 2003;75:1711-4. [DOI] [PubMed] [Google Scholar]