Abstract
Background
No randomized studies exist comparing pneumonectomy (PN) and sleeve lobectomy (SL). We evaluated surgical results and long-term quality of life in patients operated on for central non-small cell lung cancer (NSCLC) using either SL or PN.
Methods
A total of 641 NSCLC patients underwent surgery 2000-2010. SL was performed in 40 (6.2%) and PN in 67 (10.5%). In 2011, all surviving patients were sent a 15D Quality of Life Questionnaire which 83% replied. Propensity-score-matching analysis was utilized to compare the groups.
Results
Thirty-two bronchial (18 right/14 left), seven vasculobronchial (3 right/4 left), one right wedge SL, and 18 right and 22 left PN were performed. Preoperatively, the Charlson Comorbidity Index (CCI) score, forced expiratory volume in 1 s (FEV1) and diffusion capacity did not differ between groups. The perioperative complication rate and pattern were similar, but SL group had less major complications (P<0.027). One perioperative death (2.5%) occurred in SL group and four (6%) in PN. The 90-day mortality rate was 5% (n=2) for SL and 7.5% (n=5) for PN. In the follow-up total cancer recurrence did not differ (P=0.187). Quality of life measured by 15D showed no significant difference in separate dimensions or total score, except tendency to favor SL in moving or breathing. The 5-year survival did not differ between groups (P=0.458), but no deaths were observed in SL group after 5 years.
Conclusions
Due to less major operative complications and better long-term survival, we would advocate using SL when feasible, but in patients tolerating PN it should be considered if SL seems not to be oncologically sufficiently radical.
Keywords: Bronchial sleeve resection, pneumonectomy (PN), non-small cell lung cancer (NSCLC), analysis, long-term survival, quality of life
Introduction
Surgical resection is the main treatment in local and locally advanced disease. Pneumonectomy (PN) and sleeve lobectomy (SL) are indicated when lung cancer is centrally located, invading the central bronchus and/or vasculature. PN is, however, associated with significant morbidity (1), mortality of 5.6% to 10% (1,2) and decrease in the health-related quality of life (HRQoL) (3). Furthermore, due to poor lung function, PN is not tolerated by a number of non-small cell lung cancer (NSCLC) patients. In our institution first SL in NSCLC was utilized in 1973 (4). In NSCLC bronchial SL has only recently gained greater acceptance as an alternative to PN. The main concerns with SL have been the possible increase in locoregional recurrence(5), complications related to the bronchial anastomosis (6), and the complexity of the operation. According to recent cohort studies (2,7-12) and meta-analyses (13,14) these concerns are not fully realized, and it has been presented that PN should only be implemented when SL is not feasible (5).
No randomized studies exist comparing PN and SL. Only one prospective short-term study evaluating quality of life after SL and PN has been performed by Balduyck et al. in 2008 (15). Because randomized trials that compare the SL and PN patients are difficult perform, we compared our own material with propensity matching. This gives more objective picture than pure retrospective evaluation (16).
Patients and methods
Patients
Between February 2000 and March 2010, a total of 641 patients underwent surgery for NSCLC with curative intent at Helsinki University Central Hospital. From 107 patients with central lung cancer, 67 (10.5%) underwent PN and 40 SL (6.2%). The characteristics of the study patients and their clinical data were collected from patient records. For the study the immediate and basic cause of death and date was acquired from national registry. Preoperative data are presented in Table 1.
Table 1. Patient characteristics at the time of surgery among patient groups.
| Characteristics | SL, n=40 (%) | PN, n=67 (%) | P value | Matched PN, n=40 (%) | P value |
|---|---|---|---|---|---|
| Age, years median [range] | 61.5 [40-78] | 60 [45-83] | 0.560 | 65.5 [46-78] | 0.325 |
| Female | 11 (27.5) | 18 (26.9) | 0.137 | 9 (22.5) | 0.797 |
| Never smokers | 3 (7.5) | 6 (9.0) | 0.734 | 1 (2.5) | 0.615 |
| Pack years, median [range] | 40 [0-150] | 40 [0-100] | 0.547 | 40 [0-100] | 0.944 |
| Mediastinoscopy | 7 (17.5) | 14 (20.9) | 0.010 | 9 (22.5) | 0.781 |
| Neoadjuvant therapy | 11 (27.5) | 14 (20.9) | 0.493 | 11 (27.5) | 0.729 |
| Clinical stage | 0.366 | 0.875 | |||
| IA | 3 (7.5) | 8 (11.9) | 4 (10.0) | ||
| IB | 5 (12.5) | 8 (11.9) | 2 (5.0) | ||
| IIA | 1 (2.5) | 1 (1.5) | 1 (2.5) | ||
| IIB | 18 (45.0) | 25 (37.3) | 17 (42.5) | ||
| IIIA | 6 (15.0) | 9 (13.4) | 8 (20.0) | ||
| IIIB | 7 (17.5) | 16 (23.9) | 8 (20.0) | ||
| IV | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| CCI | 3 [0-6] | 2 [0-6] | 0.135 | 3 [1-5] | 0.657 |
| Preoperative spirometry median [range] | |||||
| FEV1, L/1s | 2.23 [1.21-3.87] | 2.49 [1.41-4.83] | 0.690 | 2.30 [1.41-3.19] | 0.734 |
| FEV1% | 70.5 [29-97] | 77 [42-111] | 0.014 | 71 [42-109] | 0.701 |
| DLCO | 72 [32-101] | 77 [30-125] | 0.037 | 73 [30-115] | 0.256 |
| DL/VA | 84 [48-131] | 89 [25-125] | 0.029 | 88 [25-125] | 0.289 |
| Predicted FEV1, L/1s median [range] | 1.67 [1.07-2.81]; n=14 | 1.47 [0.93-2.39]; n=56 | 0.217 | 1.4 [0.93-2.3]; n=34 | 0.360 |
| Predicted FEV1% median [range] | 54.8 [24-75]; n=40 | 45.66 [32-72]; n=56 | 0.318 | 42.76 [32-68]; n=34 | 0.080 |
| Predicted DLCO median [range] | 59.7 [27-81]; n=29 | 49.77 [31-85]; n=50 | 0.099 | 50.23 [31-85]; n=30 | 1.163 |
SL, sleeve lobectomy; PN, pneumonectomy; FEV1, forced expiratory volume in 1 s; DLCO, diffusing capacity for carbon monoxide; CCI, Charlson Comorbidity Index.
Each patient was preoperatively evaluated with pulmonary function tests (spirometry, diffusing capacity) and computed tomography of the chest and upper abdomen. The additional need for positron emission tomography-computed tomography (PET-CT), bronchoscopy, mediastinoscopy, CT-guided needle biopsy, the stair-climbing test, ventilation-perfusion scanning, and exercise testing was decided on an individual basis. For staging, the 6th edition of the TNM Classification was utilized (American Joint Committee on Cancer, 2002).
All patients completed preoperative spirometry, including forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and FEV1/FVC according to the guidelines of the European Respiratory Society (ERS) and measurements of pulmonary diffusing capacity for carbon monoxide (DLCO) using the single-breath method (American Thoracic Society Guidelines, 1996).
The status of comorbidity was objectively quantified based on the Charlson Comorbidity Index (CCI), which takes into account both the number and seriousness of the comorbid diseases and has been validated in lung cancer patients (17,18).
The sleeve lobectomies included 19 right upper lobes, 1 right lower lobe, 10 left upper lobes, and 8 left lower lobes. Furthermore, two patients underwent right upper sleeve bilobectomy. In seven patients, surgery also included pulmonary artery and in one superior vena cava resection and reconstruction. Of 67 pneumonectomies, 30 were right and 37 left. PN included pericardial resection (n=1), chest wall resection (n=4), and resection of the left atrium (n=1). The rate of PN decreased during the study period and that of SL increased. The death was considered as a cancer related if the immediate cause of death was cancer or the patient had a known metastatic disease (Table 2).
Table 2. Cause of death.
| Disease | PN, n=67 (%) | SL, n=40 (%) | Propensity PN, n=40 (%) |
|---|---|---|---|
| Cancer | 28 (41.8) | 16 (40.0) | 15 (15.0) |
| Other cause | 21 (30.9) | 6 (15.0) | 15 (15.0) |
| >60 months survival, cancer | 6 | 0 | 4 |
| >60 months survival, other cause | 6 | 0 | 5 |
SL, sleeve lobectomy; PN, pneumonectomy.
Propensity matching
Propensity matching was utilized for the PN patient group, using linear regression analysis for the following preoperative parameters: age, gender, CCI, lung functions, smoking status, preoperative histology, and the preoperative TNM stage. After propensity matching, 40 PN patients (18 right and 22 left) were matched.
Patient characteristics
There were no statistically significant differences between SL and PN patients in age, sex, pack years, mediastinoscopy, neoadjuvant therapy, or clinical stage (Table 1). The CCI score was 3 [0-6] for SL patients vs. 2 [0-6] for PN patients (P=0.137). Preoperative FEV1% was worse in the SL group SL 70.5 (range, 29-97) than in PN group 77.0 (range, 42-111), P=0.014. Also DLCO was worse in SL group 72.0 (range, 32-101), PN 77.0 (range, 30-125), P=0.037. The predicted FEV1% was 54.8 (range, 24-75) in the SL and 45.66 (range, 32-72) in the PN group (P=0.318). Compared to the propensity-matched group, no difference was noted for preoperative CCI, FEV1%, or DLCO (P=0.657, 0.701, and 0.256, respectively) (Table 1).
Quality of life instrument, 15D
In June 2011 all surviving patient [273] was send a generic and validated 15D HRQoL questionnaire (3,19), 230 patients (83%) responded, including 13 (32.5%) SL and 18 (26.9%) PN patients (Table 3). The 15D questionnaire has previously been used in operated NSCLC patients (3,19). The 15D HRQoL is a generic, 15-dimensional, standardized, self-administered HRQoL instrument that can serve as both a profile and a single index score measure (20). The 15D questionnaire consists of the following 15 dimensions: moving, seeing, hearing, breathing, sleeping, eating, speech, elimination, usual activities, mental function, discomfort and symptoms, depression, distress, vitality, and sexual activity. A set of utility or preference weights, elicited from the general public through a three-stage valuation procedure, is used in an additive aggregation formula to generate the utility score, i.e., the 15D score (single index number) overall dimensions. The maximum score is 1 (no problems on any dimension), and the minimum score 0 (deceased). A change <0.03 is considered clinically important (20).
Table 3. 15D query activity.
| 15D HRQoL |
Matched PN, n=40 (%) | |
|---|---|---|
| SL, n=40 (%) | PN, n=67 (%) | |
| 13 (32.5) replied | 18 (26.9) replied | 12 (30.0) replied |
| 7 (17.5) no reply | 4 (5.9) no reply | 2 (5.0) no reply |
| 20 (50.0) deceased | 45 (67.2) deceased | 26 (65.0) deceased |
SL, sleeve resection; PN, pneumonectomy; HRQoL, healthrelated quality of life.
Statistical analysis
Statistical analysis was performed using SPSS statistical software (version 21.0, Chicago, IL, USA). Results are reported as the median (range), and 15D as the mean ± standard deviation. The Student’s t-test was used to compare parametric values of groups, while the Mann-Whitney U-test was performed to compare nonparametric data between groups. Comparisons of survival were carried out using the log rank test. A P value less than 0.05 was considered as statistically significant, and 0.03 for the results of 15D. Consent was granted for the study by the Hospital ethics review board.
Results
There was one 30-day perioperative death in the SL group (2.5%) whereas in four patients in the PN group died (6%). The 90-day mortality rate was 5% (n=2) for SL and 7.5% (n=5) for PN patients. The overall morbidity rate (including all complications) was 44.8% in the PN group and 50% in the SL group (P=0.604). A complication was considered major if it was life-threatening or required re-operation. PN group had 16.4% (n=11) life-threatening complications: one acute respiratory distress syndrome, two bronchopleural fistulas, six empyemas, one stroke, and one third-degree atrioventricular block. Seventeen patients (25.4%) in the PN group were re-operated. Major complication rate was in PN group 29.9% (n=20). The amount of major complications was less in SL group (P=0.027). In the SL group life-threatening complication rate was 12.5% (n=5): one stroke, one empyema, two pneumonias, and one postoperative bleeding, while three patients (7.5%) were re-operated in SL group.
Reason for reoperations in PN group was: empyema in seven, postoperative hematoma or bleeding in seven, air leak and suspicion for bronchopleural fistulas in three patients. Reason for reoperations in SL group was: one air-leak, one empyema, one bleeding.
Seven SL patient and four PN patient didn’t return the questionnaire. Of these two SL patients had been re-operated (air-leak and empyema) and two PN-patients (empyema and hematoma). Non-responders had no major complications.
The median hospital stay following surgery was 10 days (range, 4-43 days) in the SL patient group and 9 days (range, 4-219 days) in the PN group. Patients were not routinely observed postoperatively in the intensive care unit (ICU). In the PN patient group, a total of 29 patients were in the ICU for a median of 2 days (range, 9-19 days). After SL, a total of 15 patients were in the ICU for a median of 2 days (range, 1-21 days). No significant differences were observed between patient groups in hospital length of stay.
In long-term follow-up no difference was noted for the rate of distant metastasis or locoregional recurrence (P=0.798) (Table 4). The 5-year survival was similar in the two groups, PN 41.8% and SL 37.5% (P=0.665). The cause of death was other than lung cancer recurrence in 21 PN patients (31.3%) and in six SL patients (15%) (P=0.217). In the PN-group non-cancerous death causes were: for cardiac cause in seven patient, nine in pneumonia, one respiratory insufficiency, one suicide, one urinary sepsis, one cerebral infarction and one patient atherosclerosis. In PN patients, metastases occurred in 13.4% (n=9) of cases in the other lung, 10.4% (n=7) in the brain, and 6% (n=4) in the liver. In SL patients, 10% (n=4) had liver, 7.5% (n=3) brain, and 5% (n=2) bone metastases. No deaths were observed in SL patients after 5 years of follow-up, but 12 patients in PN group died after 5 years of follow-up (Figure 1).
Table 4. Recurrence pattern after SL and PN.
| Recurrence | N=107 |
Matched PN, n=40 (%) | |
|---|---|---|---|
| SL, n=40 (%) | PN, n=67 (%) | ||
| Total recurrence | 14 (35.0) | 25 (37.5), P=0.798 | 11 (27.5), P=0.187 |
| Locoregional | 1 (2.5) | 3 (4.5) | 3 (7.5) |
| Distant | 13 (32.5) | 22 (32.8) | 8 (20.0) |
SL, sleeve lobectomy; PN, pneumonectomy.
Figure 1.
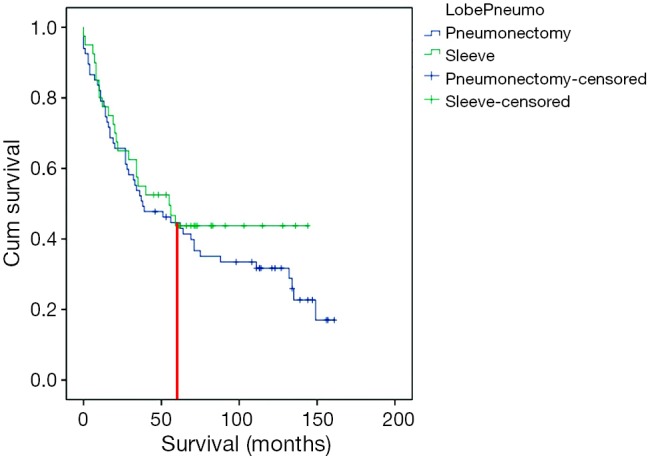
The Kaplan-Meier survival curve. Overall long-term survival after PN or SL. After 5 years, no deaths were observed in SL patients. SL, sleeve lobectomy; PN, pneumonectomy.
Postoperative quality of life
The median time between surgery and completion of the questionnaire was 69 months (range, 24-132 months), being 85 months (range, 25-132 months) in the PN patient group and 44 months (range, 24-115 months) in the SL-group (P=0.007). The response rates for the quality of life questionnaire are presented in Table 3.
Postoperative HRQoL showed no significant difference between groups in the total score (P=0.545) (Figure 2). The only difference is seen for moving and breathing, but it was not statistical significant (P=0.263 and P=0.399).
Figure 2.
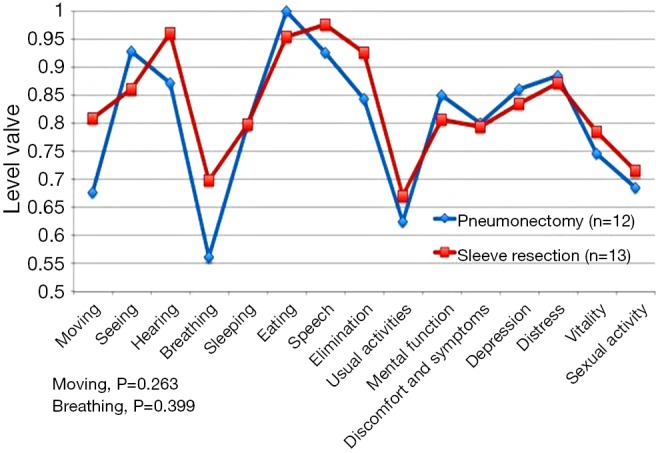
Postoperative quality of life. Comparison of 15D questionnaire scores in PN and SL patients. The only difference is seen for moving and breathing. SL, sleeve lobectomy; PN, pneumonectomy.
Conclusions
The main finding of this study was that the long-term results for PN and SL were comparable in terms of both survival and general HRQoL. In our series, the 5-year survival was similar between the groups. After 5 years of follow-up no deaths were observed among SL patients, but 43% of PN patients died and four patients (14%) cause of death was other than cancer.
A major concern regarding SL for lung cancer is the increased incidence of locoregional recurrence. A recent meta-analysis determined that the pooled locoregional recurrence in SL was 14.4% compared with 26.1% in PN, but there was no statistically significant difference (21). In our study, no differences were detected in the recurrence pattern between patient groups. More distant metastasis was observed in SL group than propensity-matched PN group, but the rate of locoregional recurrence was higher in PN group. In survival, no difference was noted in cancer-related death rate, which highlights the similarity in oncologic results between the two study groups. These findings are consistent with other studies showing a similar local recurrence rate between SL and PN, appearing similar after both procedures (5,8,22).
The overall 5-year survival following SL has ranged from 39% to 53% (2,4,5,8) and after PN from 27% to 49.8% (2). In our study, these figures were 45% for SL and 41.8% for PN (P=0.458). The survival trend appeared similar up to this point, but after 5 years, better survival was observed among SL patients. This finding may serve as an independent prognostic factor for long-term survival. However, further validated multi-center cohort studies are required to validate this finding.
Even with experience, SL is technically more challenging than PN. Ludwig reported that the incidence of bronchopleural fistula in PN patients was 3.6% and the incidence of anastomotic leakage in SL patients was 6.9% (2). In a recent meta-analysis by Shi et al., the pooled incidence of postoperative complications was 32.9% with SL and 27.1% with PN, but the difference was not statistically significant (21). At our institution, in the PN group we had 2/67 (2.9%) bronchopleural fistulas and 6/67 (8.9%) empyemas. No bronchial anastomotic short- or long-term complications occurred in the SL group. The amount of major postoperative complications was also less in SL group (P=0.009).
Pain and impairment of the functional health status can persist for 6-24 months after lung cancer resection (23). Similar morbidity and mortality is noted with SL when compared with PN (5,8,14,24). Better postoperative short term HRQoL (25,26) and better preservation of function have been reported in SL (5,8,14,24), when compared to PN patients. Moreover, SL is associated with better long-term overall survival (9,27). A prospective study by Balduyck et al. revealed that SL was characterized by a 1-month temporary decrease in physical and social functioning scores after surgery (15). Global quality of life, and symptom and pain scores approximated preoperative values 1 month after SL. In the 12-month follow-up period in PN patients, there was no return to the baseline in physical or role functioning (15). Goméz-Caro et al. demonstrated a negative impact of PN on global QoL, but recovery to the baseline did not differ between SL and PN (25). In 2011, Deslauriers et al. reported a follow-up of over 5 years among PN patients, concluding that most PN patients can adjust to living with one lung and can live a near normal life (28). In our study, no difference between SL and PN patients was noted in overall HRQoL. Between our study groups, we only noted non-significant differences were in dimensions of moving and breathing.
The surgery-to-questionnaire interval was shorter for SL patients and the time to recovery was shorter. This could have affected the results in favor of the PN group because of their longer time for adaptation, and the difference between PN and SL could have been more obvious if the time from operation to questionnaires had been equal. Thus, it can assumed that our PN patients may have adjusted to living with one lung, as also Deslauriers et al. reported 2004. If the study had been a prospective study, results might have differed.
Patients with a preoperative FEV1 and DLCO of 60% less than that predicted have been shown to have an increased risk of postoperative complications (29). Brunelli et al. reported that the prediction of postoperative FEV1 and DLCO was fairly accurate at 1 month after major lung resection, but underestimated the actual values at 3 months, particularly for DLCO and after PN (30). Patients with poor DLCO had worse preoperative physical functioning and quality of life, in addition to worse postoperative overall HRQoL (23). In our study, both preoperative FEV1 and DLCO were worse in the SL than the PN group, but compared with the matched PN group there was no statistically significant difference. Even if SL patients were more morbid than PN patients, the preoperative lung function test did not predict postoperative HRQoL after SL.
In our institution, SL was carried out in 6.2% of all lung cancer patients. The relative frequency of PN decreased during the study period, and that of SL increased during the same interval. The main objective in surgery for NSCLC is to achieve good oncologic safety, which includes R0 resection of the tumor and radical lymphadenectomy.
In conclusion, PN is still a valid choice for central tumors when postoperative lung functions will remain reasonable and if with sleeve resection the surgical and oncological results may be compromised. This study was retrospective and lacked some serial data pre- and post-operatively. The relative frequency of PN decreased during the study period and that of SL increased. The small patient cohort could have introduced bias, but multi-center study with this operation type is difficult to organize. In addition patients did not complete the questionnaire at the same time from the operation, but of all operated patients 86% replied to the questionnaire, which is in the same range as in other inquiry studies.
Acknowledgements
The authors thank Ms. Yvonne Sundström for skillful secretarial assistance.
Funding: This study was supported by Helsinki University Hospital Research Funds (EVO V1016SK002).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Shapiro M, Swanson SJ, Wright CD, et al. Predictors of major morbidity and mortality after pneumonectomy utilizing the Society for Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg 2010;90:927-34; discussion 934-5. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig C, Stoelben E, Olschewski M, et al. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non-small cell lung carcinoma. Ann Thorac Surg 2005;79:968-73. [DOI] [PubMed] [Google Scholar]
- 3.Ilonen IK, Räsänen JV, Sihvo EI, et al. Pneumonectomy: post-operative quality of life and lung function. Lung Cancer 2007;58:397-402. [DOI] [PubMed] [Google Scholar]
- 4.Sioris T, Salo J, Mattila S. The role of bronchoplasty in the treatment of lung cancer. Ann Chir Gynaecol 1997;86:31-7. [PubMed] [Google Scholar]
- 5.Yildizeli B, Fadel E, Mussot S, et al. Morbidity, mortality, and long-term survival after sleeve lobectomy for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;31:95-102. [DOI] [PubMed] [Google Scholar]
- 6.Hollaus PH, Wilfing G, Wurnig PN, et al. Risk factors for the development of postoperative complications after bronchial sleeve resection for malignancy: a univariate and multivariate analysis. Ann Thorac Surg 2003;75:966-72. [DOI] [PubMed] [Google Scholar]
- 7.Lausberg HF, Graeter TP, Wendler O, et al. Bronchial and bronchovascular sleeve resection for treatment of central lung tumors. Ann Thorac Surg 2000;70:367-71; discussion 371-2. [DOI] [PubMed] [Google Scholar]
- 8.Deslauriers J, Grégoire J, Jacques LF, et al. Sleeve lobectomy versus pneumonectomy for lung cancer: a comparative analysis of survival and sites or recurrences. Ann Thorac Surg 2004;77:1152-6; discussion 1156. [DOI] [PubMed] [Google Scholar]
- 9.Bagan P, Le Pimpec-Barthes F, Badia A, et al. Bronchial sleeve resections: lung function resurrecting procedure. Eur J Cardiothorac Surg 2008;34:484-7. [DOI] [PubMed] [Google Scholar]
- 10.Takeda S, Maeda H, Koma M, et al. Comparison of surgical results after pneumonectomy and sleeve lobectomy for non-small cell lung cancer: trends over time and 20-year institutional experience. Eur J Cardiothorac Surg 2006;29:276-80. [DOI] [PubMed] [Google Scholar]
- 11.Melloul E, Egger B, Krueger T, et al. Mortality, complications and loss of pulmonary function after pneumonectomy vs. sleeve lobectomy in patients younger and older than 70 years. Interact Cardiovasc Thorac Surg 2008;7:986-9. [DOI] [PubMed] [Google Scholar]
- 12.Bölükbas S, Bergmann T, Fisseler-Eckhoff A, et al. Short- and long-term outcome of sleeve resections in the elderly. Eur J Cardiothorac Surg 2010;37:30-5. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson MK, Durkin AE. A comparison of three scoring systems for predicting complications after major lung resection. Eur J Cardiothorac Surg 2003;23:35-42. [DOI] [PubMed] [Google Scholar]
- 14.Ma Z, Dong A, Fan J, et al. Does sleeve lobectomy concomitant with or without pulmonary artery reconstruction (double sleeve) have favorable results for non-small cell lung cancer compared with pneumonectomy? A meta-analysis. Eur J Cardiothorac Surg 2007;32:20-8. [DOI] [PubMed] [Google Scholar]
- 15.Balduyck B, Hendriks J, Lauwers P, et al. Quality of life after lung cancer surgery: a prospective pilot study comparing bronchial sleeve lobectomy with pneumonectomy. J Thorac Oncol 2008;3:604-8. [DOI] [PubMed] [Google Scholar]
- 16.Blackstone EH. Comparing apples and oranges. J Thorac Cardiovasc Surg 2002;123:8-15. [DOI] [PubMed] [Google Scholar]
- 17.Birim O, Maat AP, Kappetein AP, et al. Validation of the Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur J Cardiothorac Surg 2003;23:30-4. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [DOI] [PubMed] [Google Scholar]
- 19.Ilonen IK, Räsänen JV, Knuuttila A, et al. Quality of life following lobectomy or bilobectomy for non-small cell lung cancer, a two-year prospective follow-up study. Lung Cancer 2010;70:347-51. [DOI] [PubMed] [Google Scholar]
- 20.Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med 2001;33:328-36. [DOI] [PubMed] [Google Scholar]
- 21.Shi W, Zhang W, Sun H, et al. Sleeve lobectomy versus pneumonectomy for non-small cell lung cancer: a meta-analysis. World J Surg Oncol 2012;10:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okada M, Yamagishi H, Satake S, et al. Survival related to lymph node involvement in lung cancer after sleeve lobectomy compared with pneumonectomy. J Thorac Cardiovasc Surg 2000;119:814-9. [DOI] [PubMed] [Google Scholar]
- 23.Handy JR, Jr, Asaph JW, Skokan L, et al. What happens to patients undergoing lung cancer surgery? Outcomes and quality of life before and after surgery. Chest 2002;122:21-30. [DOI] [PubMed] [Google Scholar]
- 24.Martin-Ucar AE, Chaudhuri N, Edwards JG, et al. Can pneumonectomy for non-small cell lung cancer be avoided? An audit of parenchymal sparing lung surgery. Eur J Cardiothorac Surg 2002;21:601-5. [DOI] [PubMed] [Google Scholar]
- 25.Gómez-Caro A, Boada M, Reguart N, et al. Sleeve lobectomy after induction chemoradiotherapy. Eur J Cardiothorac Surg 2012;41:1052-8. [DOI] [PubMed] [Google Scholar]
- 26.Balduyck B, Hendriks J, Lauwers P, et al. Quality of life evolution after lung cancer surgery in septuagenarians: a prospective study. Eur J Cardiothorac Surg 2009;35:1070-5; discussion 1075. [DOI] [PubMed] [Google Scholar]
- 27.Lausberg HF, Graeter TP, Tscholl D, et al. Bronchovascular versus bronchial sleeve resection for central lung tumors. Ann Thorac Surg 2005;79:1147-52; discussion 1147-52. [DOI] [PubMed] [Google Scholar]
- 28.Deslauriers J, Ugalde P, Miro S, et al. Long-term physiological consequences of pneumonectomy. Semin Thorac Cardiovasc Surg 2011;23:196-202. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson MK, Little L, Rizzo L, et al. Diffusing capacity predicts morbidity and mortality after pulmonary resection. J Thorac Cardiovasc Surg 1988;96:894-900. [PubMed] [Google Scholar]
- 30.Brunelli A, Refai M, Salati M, et al. Predicted versus observed FEV1 and DLCO after major lung resection: a prospective evaluation at different postoperative periods. Ann Thorac Surg 2007;83:1134-9. [DOI] [PubMed] [Google Scholar]


