Abstract
Background
While phosphatidylethanolamine-binding protein 4 (PEBP4) is a key factor in the malignant proliferation and metastasis of tumor cells, the exact regulatory network governing its roles remains unclear. This study was designed to investigate the effect of PEBP4 on PI3K/Akt/mTOR pathway and explore its molecular network that governs the proliferation and metastasis of tumor cells.
Methods
After the recombinant plasmid pcDNA3.1-PEBP4 was constructed, the recombinant plasmid pcDNA3.1-PEBP4 and PEBP4-targeting siRNA were transfected into lung cancer HCC827 cell line. The expressions of PI3K/Akt/mTOR pathway components in HCC827 cells in each group were determined using Western blotting. In the HCC827 cells, the effect of PI3K pathway inhibitor LY294002 on the expressions of PI3K/Akt/mTOR pathway components under the effect of PEBP4 was determined using Western blotting, and the effects of LY294002 on the cell viability, proliferation, and migration capabilities under the overexpression of PEBP4 were determined using MTT method, flow cytometry, and Transwell migration assay. Furthermore, the effect of mTOR inhibitor rapamycin (RAPA) on the expressions of PI3K/Akt/mTOR pathway components under the effect of PEBP4 was determined using Western blotting, and the effects of RAPA on the cell viability, proliferation, and migration capabilities under the overexpression of PEBP4 were determined using MTT method, flow cytometry, and Transwell migration assay.
Results
As shown by Western blotting, the protein expressions of p-Akt and phosphorylated mTOR (p-mTOR) were significantly higher in the pcDNA3.1-PEBP4-transfected group than in the normal control group and PEBP4 siRNA group (P<0.05); furthermore, the protein expressions of p-Akt and p-mTOR significantly decreased in the PEBP4 targeting siRNA-transfected group (P<0.05). Treatment with LY294002 significantly inhibited the protein expressions of p-Akt and p-mTOR in HCC827 cells (P<0.05). In contrast, treatment with RAPA only significantly inhibited the protein expression of p-mTOR (P<0.05). As shown by MTT, flow cytometry, and Transwell migration assay, both LY294002 and RAPA could significantly lower the viability of HCC827 cells and inhibit their proliferation and invasion (P<0.05); meanwhile, they could reverse the effect of PEBP4 in promoting the proliferation and migration of HCC827 cells (P<0.05).
Conclusions
The overexpression of PEBP4 increases the phosphorylation levels of Akt and mTOR in lung cancer cells. The PI3K/Akt/mTOR signaling axis may be a key molecular pathway via which PEBP4 promotes the proliferation and invasion of non-small cell lung cancer (NSCLC) cells; also, it may serve as a potential therapeutic target.
Keywords: Phosphatidylethanolamine-binding protein 4 (PEBP4), lung cancer, PI3K/Akt/mTOR, LY294002, rapamycin (RAPA)
Introduction
The occurrence and development of tumors is a multiple-step, multiple-stage pathologic process involving multiple genes (1), during which a key mechanism is that the balance between cell proliferation/growth and death/clearance is damaged. On one hand, the excessive stimulation by the growth and proliferation signals causes the uncontrolled growth and excessive proliferation of cells, resulting in tumors (2); on the other hand, the cell apoptosis mechanism is inhibited and thus cannot normally kill or clear the cells. In most cases, however, tumorigenesis is resulted from the effects of both two mechanisms (3,4). Lung cancer is one of the most common malignant tumors worldwide. Although remarkable advances have been made in the management of lung cancer, the survival of lung cancer patients remains suboptimal (5,6). In fact, lung cancers have complex biological features and are often highly malignant. Almost 80% of lung cancers are non-small cell lung cancer (NSCLC), which is often diagnosed in its advanced stages and become metastatic in most cases; as a result, the prognosis is extremely poor (7,8).
Phosphatidylethanolamine-binding protein 4 (PEBP4) is a member of the phosphatidylethanolamine-binding protein family (9-11). Human PEBP4 (hPEBP4) is a novel PEBP family member that is cloned from human bone marrow stromal cells (9). Reverse transcription polymerase chain reaction (RT-PCR) has shown that hPEBP4 is expressed in tumor cells including prostate cancer, ovarian cancer, breast cancer, and lung cancer (9,12-15). Our previous studies also have shown the up-regulated expression of PEBP4 in lung squamous cell carcinoma cells and NSCLC cells (12,16); in addition, it was closely associated with the development, invasion/metastasis, and prognosis of these two types of lung cancer. It was found that miR-34a regulated the cisplatin susceptibility of lung cancer cells by down-regulating PEBP4 expression (17). However, few studies have reported the PEBP4 mechanism.
PI3K/Akt/mTOR is a key survival-signaling pathway in cells and its activation is closely correlated with the proliferation, differentiation, apoptosis, and chemotherapy resistance of tumor cells and with angiogenesis (18,19). The key molecules involved in PI3K/Akt/mTOR pathway include PI3K, PTEN, Akt, and mTOR (20,21). Akt is an evolutionarily conserved serine/threonine kinase, located mainly in the cytoplasm in resting status. The simultaneous phosphorylation of Thr305 and Ser473 is necessary for the activation of Akt. The activated phosphorylated Akt(p-Akt) protein is translocated into cytoplasm or nucleus, where it will further phosphorylate a series of substrates that may regulate mechanisms including protein synthesis and gene transcription and thus regulate the survival and apoptosis of cells (22).
mTOR, also known as FRAP, RAFTI, or RAPTI, is a target of rapamycin (RAPA) in the Akt signaling downstream of cells in mammalians and also a serine/threonine protein kinase (23,24). The activated Akt can directly and indirectly activate the downstream mTOR (25). Akt can activate mTOR by directly phosphorylating the ser2448 site of mTOR; furthermore, by inhibiting tuberous sclerosis complex 2 (TSC2) and TSC1 complex, it can activate mToR signal (26,27).
Many studies have explored the molecular therapies targeting the PI3K/Akt/mTOR pathway. Akt and mTOR are two key kinases in the PI3K downstream of PI3K/Akt/mTOR pathway and are often abnormally activated in many tumors (18,28). PEBP4 is a key factor in the malignant proliferation and multidrug resistance of tumor cells. Searching for the regulatory factors that play key roles in the signaling pathway of tumor development will provide evidences for clinical applications. This study was designed to investigate the effect of PEBP4 on two key kinases—Akt and mTOR—in the PI3K/Akt/mTOR pathway and explore its molecular network that governs the proliferation and metastasis of tumor cells.
Materials and methods
Main reagents
MTT (3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide), Hoechst 33342, PT3K inhibitor LY294002, and mTOR inhibitor RAPA were purchased from Sigma Aldrich (St Louis, MO, USA). PEBP4 targeting siRNA and non-targeting siRNA (siRNA-control) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Propidium iodide (PI) was purchased from Roche (Branchburg, NJ, USA).
The NSCLC cell line HCC827 was purchased from ATCC (Manassas, MA, USA). RPMI 1640 medium, trypsin, and fetal bovine serum (FBS) were purchased from GIBCO (Carlsbad, CA, USA). Cell culture plates and Transwell invasion chamber were purchased from Corning (Corning, NY, USA). RIPA lysis buffer, TBST (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% Tween-20), Trizol kit, lipofectamine 2000 transfection reagent were purchased from Invitrogen (Carlsbad, CA, USA). Bradford protein assay kit and Quantity One software were purchased from Bio-Rad Laboratories Company (Hercules, CA, USA). PVDF membrane was purchased from Millipore Company (Billerica, MA, USA). ECL chemiluminescent substrate was purchased from Pierce Company (Rockford, IL, USA).
Mouse anti-human β-actin primary antibody and rabbit anti-human mTOR and phosphorylated mTOR (p-mTOR) (Ser2448) primary antibodies were purchased from Abcam (Cambridge, MA, USA), and rabbit anti-human Akt and p-Akt (Ser473) primary antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Horseradish peroxidase conjugated goat anti-rabbit IgG polyclonal antibody was obtained from Novus Biologicals (Littleton, CO, USA), and horseradish peroxidase conjugated rabbit anti-mouse IgG polyclonal antibody was purchased from Invitrogen (Carlsbad, CA, USA).
Construction of the recombinant plasmid pcDNA3.1-PEBP4
In accordance with the PEBP4 sequences in GenBank (no.: NM_144962.2), a pair of PCR primers were designed at the two sides of the open reading frame (ORF) of PEBP4 using the Primer Premier 5 software. PEBP4 upstream primer P1: 5’-CG GGATCC ATG GGT TGG ACA ATG AGG CT-3’, which contained the cutting site for BamH I; PEBP4 downstream primer P2: 5’-CC CTCGAG GCA GGC AGC TAT CTC CGC CT-3’, which contained the cutting site for Xho I. Please refer to literature (17) for more details.
Cell treatment
The NSCLC HCC827 cells were cultured in RPMI 1640 medium containing 10% FBS, 1.5 g/l NaHCO3, and 2 mM L-glutamine. The HCC827 cells were cultured in a 37 °C, 5% CO2, and saturated humidity environment. The growth of cells was observed under an inverted microscope. When cells were grown to 70-80% confluence, they were detached with 0.25% trypsin. Medium was changed every other day, and the cells were passaged every 4 to 6 days. Cells in the exponential phase were selected for further experiment. The normally cultured HCC827 cells were uniformly seeded in 6-well culture plates at a density of 3×105/mL (1,000 μL in each well). After the cells reached complete adherence, the transfections of PEBP4 siRNA and control siRNA were performed using Lipofectamine 2000, in accordance with the manufacturer’s instruction. Meanwhile, normal control group (normal) was set. The pcDNA3.1-PEBP4, vector control (pcDNA3.1), and PEBP4 siRNA were diluted using serum-free MEM medium. Then, the liposome Lipofectamine 2000 was diluted in the MEM, gently mixed, and then inoculated at room temperature for 5 min. The diluted Lipofectamine 2000 was separately mixed with the diluted pcDNA3.1-PEBP4 control (pcDNA3.1) and PEBP4 siRNA and then inoculated at room temperature for 20 min to form complexes. The complexes were then added into the culture plate containing the HCC827 cells, which was put inside an incubator at 37 °C in the humidified incubator with 5% CO2. Five hours later, MEM containing 10% FBS was used instead (or, add 50 µm of PI3K inhibitor LY294002 or 1 µm of mTOR inhibitor RAPA in MEM containing 10% FBS), and the cells were cultured for another 48 h.
Relationship between PEBP4 and PI3K/Akt/mTOR pathway
As shown in previous studies, the transfection of pcDNA3.1-PEBP4 into cells could significantly promote PEBP4 expression, whereas PEBP4 siRNA significantly decreased PEBP4 expression (12,17). In HCC827 cells with PEBP4 overexpression of knockdown of PEBP4 expression, the protein expressions of mTOR, p-mTOR (Ser2448), Akt, and p-Akt (Ser473) were determined using Western blotting.
After transfection into HCC827 cells for 48 hours, these cells were placed in 6-well cell culture plates. Add 1 mL of cell lysis buffer in each well. Then, the cell lysates were transferred to a 1.5-mL centrifuge tube and centrifuged at 16,000 rpm for 30 min the supernatants were obtained, and the protein concentration was measured with the Bradford protein assay kit. Electrophoretic separation was conducted using 5% stacking gel and 12% separating gel, with 50 μg total protein added in each lane. After the proteins had been wet transferred onto a PVDF membrane, they were blocked in TBST solution containing 5% skimmed milk powder at room temperature for 1 h, then added with rabbit anti-human mTOR or p-mTOR (Ser2448) polyclonal antibody (1:1000 dilution), rabbit anti-human Akt or p-Akt (Ser473) polyclonal antibody (1:1000 dilution), and mouse anti β-actin monoclonal antibody (1:2000 dilution) and incubated overnight at 4 °C. Wash 3 times with fresh TBST for 5 minutes. Add HRP-labeled rabbit anti-mouse or goat anti-rabbit IgG secondary antibodies and inoculate it at 37 °C for 1 h. Wash 3 times with fresh TBST for 5 minutes. Autoradiography was conducted using ECL chemiluminescent substrate. The change in p-Akt or p-mTOR expression was presented using p-Akt/Akt or p-mTOR/mTOR ratio, and the change of its relative expression was analyzed using PDQuest software (Bio-Rad, Richmond, CA, USA).
Effects of LY294002 on the viability, proliferation, and invasion of PEBP4-transfected HCC827 cells
The cells were divided into the following groups: pcDNA3.1-PEBP4 + LY294002; pcDNA3.1 + LY294002; LY294002; and normal control group. The normal or transfected HCC827 cells were uniformly seeded in 6-well culture plates at a density of 3×105/mL (1,000 μL in each well). After the transfection of pcDNA3.1-PEBP4 or blank control, the cell medium containing LY294002 (50 μM) was used instead; after 48 hours of treatment, the total protein was extracted in each group, and the changes in the expressions of p-Akt/Akt and p-mTOR/mTOR were determined using Western blotting. The effect of LY294002 on the viability of PEBP4-transfected HCC827 cells was detected using MTT. The grouping was performed as described above. The normal or transfected HCC827 cells were uniformly seeded in 96-well culture plates at a density of 3×105/mL (1,000 μL in each well). After the transfection of pcDNA3.1-PEBP4 or blank control, the cell medium containing LY294002 (50 μM) was used instead; after 48 hours of treatment, 100 μL of MTT (0.5 mg/mL) was added in each well. Then, the mixture was incubated at 37 °C in a 5% CO2 incubator for 4 hours. After incubation, cell lysis solution (100 μL/well: 20% SDS, 50% N, N-dimethylformamide) was added to each well, and the plates were placed at 37 °C for 24 h. Record absorbance at 570 nm using a microplate reader (Bio-Tek, USA). Ten duplicate wells were used for each experimental group, and the experiment was repeated 3 times.
The effect of LY294002 on the proliferation of PEBP4-transfected HCC827 cells was determined using flow cytometry. The grouping was performed as described above. The normal or transfected HCC827 cells were uniformly seeded in 6-well culture plates at a density of 3×105/mL (1,000 μL in each well). After the cells were transfected with pcDNA3.1-PEBP4 or control, the cell medium containing LY294002 (50 μM) was used instead; After 48 hours of treatment, the cells were collected by centrifugation and then rinsed with PBS 1-2 times, added with propidium iodide staining solution, and incubated in the dark at 4 °C for 30 minutes. After the mesh filter, the apoptosis was detected using flow cytometry (BD Company, USA). The cells were counted using the CellQuest software (BD, USA), and the data were analyzed using the Macquit software.
The effect of LY294002 on the invasion of PEBP4-transfected HCC827 cells was detected using Transwell invasion chamber. The grouping was performed as described above. After 24 hours of transfection, the HCC827 cells were inoculated in the Transwell invasion chamber, and the cell medium containing LY294002 (50 μM) was used instead; after 48 hours of treatment, the cells washed with PBS 1-2 times and stained with Hoechst 33342. Count the number of cells that had passed through the Transwell polycarbonate membrane using Leica microscope (Leica company, Wetzlar, Germany). These cells were regarded as invasive cells, and eight random fields were observed.
Effects of RAPA on the viability, proliferation, and invasion of PEBP4-transfected HCC827 cells
The cells were divided into the following groups: pcDNA3.1-PEBP4 + RAPA; pcDNA3.1 + RAPA; RAPA; and normal control group. The normal or transfected HCC827 cells were uniformly seeded in 6-well culture plates at a density of 3×105/mL. After the transfection of pcDNA3.1-PEBP4 or blank control, the cell medium containing RAPA (1 μM) was used instead; after 48 hours of treatment, the total protein was extracted in each group, and the changes in the expressions of p-Akt/Akt and p-mTOR/mTOR were determined using Western blotting.
The effect of RAPA on the viability of PEBP4-transfected HCC827 cells was detected using MTT. The grouping was performed as described above. The normal or transfected HCC827 cells were uniformly seeded in 96-well culture plates at a density of 3×105/mL (100 μL in each well). After the transfection of pcDNA3.1-PEBP4 or blank control, the cell medium containing RAPA (1 μM) was used instead; after 48 hours of treatment, 100 μL of MTT (0.5 mg/mL) was added in each well. Then, the mixture was incubated at 37 °C in a 5% CO2 incubator for 4 hours. After incubation, cell lysis solution (100 μL/well: 20% SDS, 50% N, N-dimethylformamide) was added to each well, and the plates were placed at 37 °C for 24 h. Record absorbance at 570 nm using a microplate reader (Bio-Tek, USA). Ten duplicate wells were used for each experimental group, and the experiment was repeated three times.
The effect of RAPA on the proliferation of PEBP4-transfected HCC827 cells was determined using flow cytometry. The grouping was performed as described above. The normal or transfected HCC827 cells were uniformly seeded in 6-well culture plates at a density of 3×105/mL (1,000 μL in each well). After the cells were transfected with pcDNA3.1-PEBP4 or control, the cell medium containing RAPA (1 μM) was used instead; After 48 hours of treatment, the cells were collected by centrifugation and then rinsed with PBS 1-2 times, added with propidium iodide staining solution, and incubated in the dark at 4 °C for 30 minutes. After the mesh filter, the apoptosis was detected using flow cytometry (BD Company, USA). The cells were counted using the CellQuest software (BD, USA), and the data were analyzed using the Macquit software.
The effect of RAPA on the invasion of PEBP4-transfected HCC827 cells was detected using Transwell invasion chamber. The grouping was performed as described above. After 24 hours of transfection, the HCC827 cells were inoculated in the Transwell invasion chamber, and the cell medium containing RAPA (1 μM) was used instead; after 48 hours of treatment, the cells washed with PBS 1-2 times and stained with Hoechst 33342. Count the number of cells that had passed through the Transwell polycarbonate membrane using Leica microscope (Leica company, Wetzlar, Germany). These cells were regarded as invasive cells, and eight random fields were observed.
Statistical analysis
Data were analyzed using chi square test or t-test in Stata 7.0 software. A P value of <0.05 was considered statistically significant.
Results
Relationship between PEBP4 and PI3K/Akt/mTOR pathway
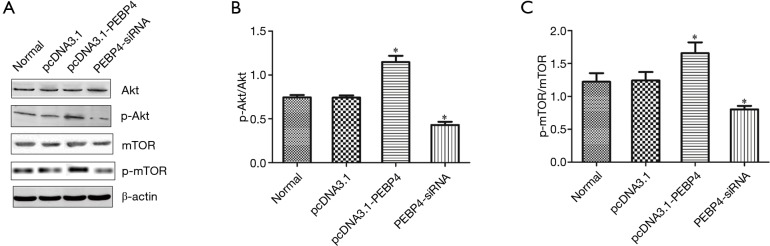
As shown by Western blotting, compared with the normal control group and negative control (pcDNA3.1) group, the pcDNA3.1-PEBP4 group had significantly elevated p-Akt and p-mTOR expressions (P<0.05); in contrast, the p-Akt and p-mTOR expressions were significantly decreased in the PEBP4-siRNA group (P<0.05). Thus, the overexpression of PEBP4 promoted the phosphorylation of Akt and mTOR, whereas the knockdown of PEBP4 expression inhibited the phosphorylation of Akt and mTOR (Figure 1).
Figure 1.
Relationship between PEBP4 and PI3K/Akt/mTOR pathway. (A) Expressions of Akt, p-Akt, mTOR, and p-mTOR in cells in each group; (B) p-Akt/Akt in each group; (C) p-mTOR/mTOR in each group. *P<0.05, versus normal control group or pcDNA3.1 negative control group. PEBP4, phosphatidylethanolamine-binding protein 4; p-mTOR, phosphorylated mTOR.
Effects of LY294002 on the viability, proliferation, and invasion of PEBP4-transfected HCC827 cells
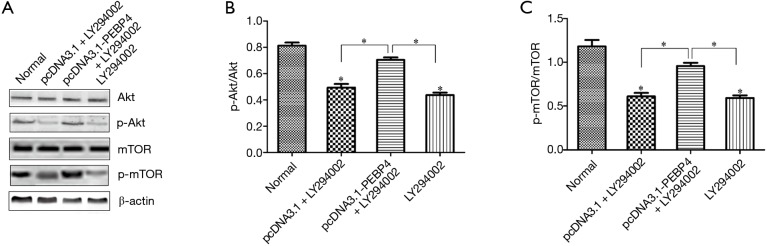
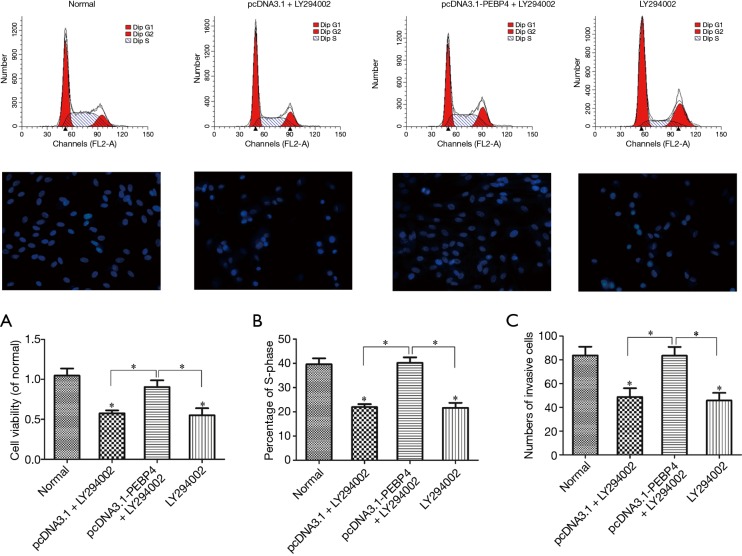
As shown by Western blotting, compared with the normal control group and negative control (pcDNA3.1) group, the pcDNA3.1-PEBP4 group and LY294002 alone group had significantly decreased p-Akt and p-mTOR expressions (P<0.05); in contrast, the p-Akt and p-mTOR expressions in the pcDNA3.1-PEBP4 + LY294002 group were not significantly different from those in the normal control group (P>0.05) (Figure 2). As shown by MTT, compared with the normal control group, the pcDNA3.1 + LY294002 group and LY294002 alone group had significantly decreased cell viability (P<0.05); in contrast, the cell viability in the pcDNA3.1-PEBP4 + LY294002 group was not significantly different from that in the normal control group (P>0.05) (Figure 3A). As shown by flow cytometry, compared with the normal control group, the pcDNA3.1 + LY294002 group and LY294002 alone group had significantly lower proportion of S-phase cells (P<0.05); in contrast, the proportion of S-phase cells in the pcDNA3.1-PEBP4 + LY294002 group was not significantly different from that in the normal control group (P>0.05) (Figure 3B and Figure 3 top row). As shown by Transwell invasion chamber, compared with the normal control group, the pcDNA3.1 + LY294002 group and LY294002 alone group had significantly fewer number of cells that had passed through the Transwell polycarbonate membrane (P<0.05); in contrast, the number of cells that had passed through the Transwell polycarbonate membrane in the pcDNA3.1-PEBP4 + LY294002 group was not significantly different from that in the normal control group (P>0.05) (Figure 3C and Figure 3 middle row).
Figure 2.
Detection of the effect of LY294002 on p-Akt and p-mTOR expressions in PEBP4-treated cells using Western blotting. (A) Expressions of Akt, p-Akt, mTOR, and p-mTOR in cells in each group; (B) p-Akt/Akt in each group; (C) p-mTOR/mTOR in each group. *P<0.05, versus normal control group. PEBP4, phosphatidylethanolamine-binding protein 4; p-mTOR, phosphorylated mTOR.
Figure 3.
Effects of LY294002 on the viability, proliferation, and invasion of PEBP4-treated HCC827 cells. (A) Effect of LY294002 on the viability of PEBP4-treated HCC827 cells (detected using MTT); (B) effect of LY294002 on the proliferation of PEBP4-treated HCC827 cells (determined using flow cytometry); (C) effect of LY294002 on the invasion of PEBP4-treated HCC827 cells (detected using Transwell invasion chamber). *P<0.05, versus normal control group. PEBP4, phosphatidylethanolamine-binding protein 4.
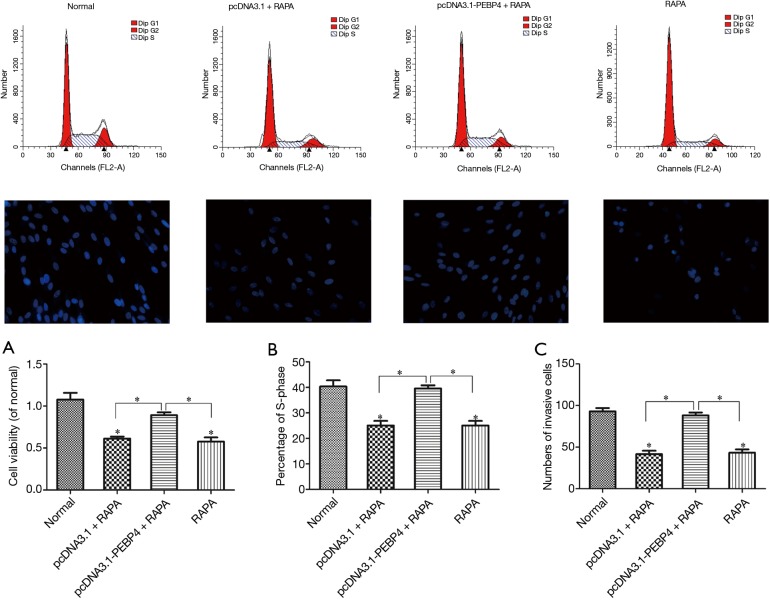
Effects of RAPA on the viability, proliferation, and invasion of PEBP4-transfected HCC827 cells
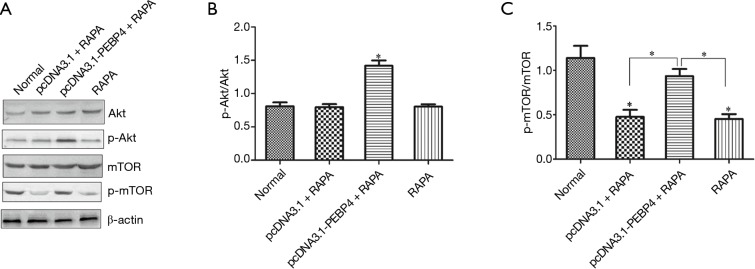
As shown by Western blotting, compared with the normal control group, the pcDNA3.1 + RAPA group and LY294002 alone group had significantly decreased p-mTOR expression (P<0.05), while the p-Akt expression showed no significant change (P>0.05); in contrast, the p-Akt expression significantly increased in the pcDNA3.1-PEBP4 + RAPA group, while the p-mTOR expression showed no significant difference between the pcDNA3.1-PEBP4 + RAPA group and the normal control group (P>0.05) (Figure 4). Thus, RAPA could significantly inhibit the p-mTOR expression in HCC827 cells but could not suppress p-Akt expression; in addition, it could reverse the increased p-mTOR expression due to PEBP4 overexpression. As shown by MTT, compared with the normal control group, the pcDNA3.1 + RAPA group and RAPA alone group had significantly decreased cell viability (P<0.05); in contrast, the cell viability in the pcDNA3.1-PEBP4 + RAPA group was not significantly different from that in the normal control group (P>0.05) (Figure 5A).
Figure 4.
Detection of the effect of RAPA on p-Akt and p-mTOR expressions in PEBP4-treated cells using Western blotting. (A) Expressions of Akt, p-Akt, mTOR, and p-mTOR in cells in each group; (B) p-Akt/Akt in each group; (C) p-mTOR/mTOR in each group. *P<0.05 versus normal control group. RAPA, rapamycin; PEBP4, phosphatidylethanolamine-binding protein 4; p-mTOR, phosphorylated mTOR.
Figure 5.
Effects of RAPA on the viability, proliferation, and invasion of PEBP4-treated HCC827 cells. (A) Effect of RAPA on the viability of PEBP4-treated HCC827 cells (detected using MTT); (B) effect of RAPA on the proliferation of PEBP4-treated HCC827 cells (determined using flow cytometry); (C) effect of RAPA on the invasion of PEBP4-treated HCC827 cells (detected using Transwell invasion chamber). *P<0.05 versus normal control group. RAPA, rapamycin; PEBP4, phosphatidylethanolamine-binding protein 4.
As shown by flow cytometry, compared with the normal control group, the pcDNA3.1 + RAPA group and RAPA alone group had significantly lower proportion of S-phase cells (P<0.05); in contrast, the proportion of S-phase cells in the pcDNA3.1-PEBP4 + RAPA group was not significantly different from that in the normal control group (P>0.05) (Figure 5B and Figure 5 top row). As shown by Transwell invasion chamber, compared with the normal control group, the pcDNA3.1 + RAPA group and RAPA alone group had significantly fewer number of cells that had passed through the Transwell polycarbonate membrane (P<0.05); in contrast, the number of cells that had passed through the Transwell polycarbonate membrane in the pcDNA3.1-PEBP4 + RAPA group was not significantly different from that in the normal control group (P>0.05) (Figure 5C and Figure 5 middle row).
Discussion
PI3K/Akt/mTOR pathway is one of the most important intracellular signaling pathways. By affecting the activation status of multiple effector molecules in its downstream, it regulates series of key physiological activities including the proliferation, apoptosis, differentiation, and metabolism of cells (29,30). In recent years, the abnormal activation of PI3K/Akt/mTOR pathway has been found in many human malignancies; meanwhile, the activation of this pathway may also play a key role in the excessive proliferation and blocked apoptosis of tumor cells (31,32).
In mammals, the most important biological function of mTOR is to regulate the protein translation; after the activation, the p-mTOR can regulate the following two downstream pathways: ribosomal S6-kinase (S6K) and 4E binding protein l (4E-BP1) (each of them controls the translation of specific subunit mRNA), and thus further regulate protein synthesis and affect the proliferation of cells (33-35). LY294002 is a PI3K inhibitor; by inhibiting its downstream Akt and mTOR activities, it can block its downstream p70s6k and 4E-BP1 pathways and induce cell cycle arrest at G phase, leading to the stopping of cell growth (36). RAPA is a macrolide immunosuppressant. As a specific inhibitor of mTOR, it can block the translation of key mRNA of tumor cells by suppressing mTOR activity; by doing so, it can prevent the synthesis of the proteins essential for G1/S-phase transition and thus induce cell phase arrest and cell apoptosis (37,38). In addition, RAPA can also interfere with other cellular processes and thus exert its anti-proliferation effect. Therefore, mTOR and its signaling pathways not only participates in tumor occurrence and development but also may be involved in cell apoptosis (39).
As shown in previous studies, PEBP4 was closely associated with the development, invasion/metastasis, and prognosis of lung cancer, and the transfection of recombinant vector pcDNA3.1-PEBP4 into cells could significantly promote PEBP4 expression, whereas PEBP4 siRNA significantly decreased PEBP4 expression (12,17). Then, how about the effect of the ectopic expression of PEBP4 on the PI3K/Akt/mTOR axis. In our current study, in HCC827 cells with PEBP4 overexpression of knockdown of PEBP4 expression, the protein expressions of mTOR, p-mTOR (Ser2448), Akt, and p-Akt (Ser473) were determined using Western blotting. The results showed that the overexpression of PEBP4 promoted the phosphorylation of Akt and mTOR, whereas the knockdown of PEBP4 expression inhibited the phosphorylation of Akt and mTOR. Thus, PEBP4 may exert its biological effect on lung cancer cells via the PI3K/Akt/mTOR pathway.
The activation of Akt can inhibit cell apoptosis and promote the survival of tumor cells. In many malignant tumors including breast cancer and NSCLC, the activated PI3K/Akt pathway can counteract the cell apoptosis caused by chemotherapy and/or radiotherapy (40,41). mTOR can transfer the mitotic signals to p70S6k, upregulating the translation of the major cell-cycle regulatory proteins such as cyclin and cyclin-dependent kinase 4 (CDK4) and meanwhile inhibiting the expressions of CDK4 inhibitors (e.g., p21CIPI); thus, it can promote G1 progression, accelerate cell cycle, stimulate cell proliferation and differentiation, and thus promote the occurrence and development of tumors (42,43). LY294002 is a PI3K inhibitor, and RAPA is a specific inhibitor of mTOR. In our current study, the possible role of LY294002 or RAPA in reversing the proliferation and invasion of lung cancer cells was explored, with an attempt to further demonstrate whether the biological effect of PEBP4 on lung cancer cells is achieved via the PI3K/Akt/mTOR pathway. It was found that LY294002 could inhibit the phosphorylation of Akt and mTOR and reverse the increased expressions of p-Akt and p-mTOR due to PEBP4 overexpression; in contrast, RAPA could only inhibit the phosphorylation of mTOR and could only reverse the increased expression of p-mTOR due to PEBP4 overexpression. In addition, both LY294002 and RAPA could inhibit the viability of HCC827 cells, lower the proportion of S-phase cells, and suppress the cell invasion; also, they could reverse the biological effects of PEBP4 in promoting the viability, proliferation, and invasion of HCC827 cells. Thus, PEBP4 may exert its biological effects on the proliferation and invasion of lung cancer cells via the PI3K/Akt/mTOR axis.
Conclusions
In conclusion, the overexpression of PEBP4 increases the phosphorylation levels of Akt and mTOR in lung cancer cells. The PI3K/Akt/mTOR signaling axis may be a key molecular pathway via which PEBP4 promotes the proliferation and invasion of NSCLC cells; also, it may serve as a potential therapeutic target.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Fontanella C, Ongaro E, Bolzonello S, et al. Clinical advances in the development of novel VEGFR2 inhibitors. Ann Transl Med 2014;2:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oustimov A, Vu V. Artificial neural networks in the cancer genomics frontier. Transl Cancer Res 2014;3:191-201. [Google Scholar]
- 3.Cooper WA, Lam DC, O’Toole SA, et al. Molecular biology of lung cancer. J Thorac Dis 2013;5 Suppl 5:S479-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poulos RC, Sloane MA, Hesson LB, et al. The search for cis-regulatory driver mutations in cancer genomes. Oncotarget 2015. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton PW, Wang Y, Boyd C, et al. Automated tumor analysis for molecular profiling in lung cancer. Oncotarget 2015;6:27938-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popper HH, Ryska A, Tímár J, et al. Molecular testing in lung cancer in the era of precision medicine. Transl Lung Cancer Res 2014;3:291-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernardo I, Bozinovski S, Vlahos R. Targeting oxidant-dependent mechanisms for the treatment of COPD and its comorbidities. Pharmacol Ther 2015;155:60-79. [DOI] [PubMed] [Google Scholar]
- 8.Vergnenègre A. How many drugs in the maintenance setting for non-small-cell lung cancer? For what benefit? Ann Palliat Med 2013;2:170-2. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Huang F, Fan L, et al. Phosphatidylethanolamine-binding protein 4 is associated with breast cancer metastasis through Src-mediated Akt tyrosine phosphorylation. Oncogene 2014;33:4589-98. [DOI] [PubMed] [Google Scholar]
- 10.Wang K, Jiang Y, Zheng W, et al. Silencing of human phosphatidylethanolamine-binding protein 4 enhances rituximab-induced death and chemosensitization in B-cell lymphoma. PLoS One 2013;8:e56829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harig L, Beinecke FA, Oltmanns J, et al. Proteins from the FLOWERING LOCUS T-like subclade of the PEBP family act antagonistically to regulate floral initiation in tobacco. Plant J 2012;72:908-21. [DOI] [PubMed] [Google Scholar]
- 12.Yu GP, Huang B, Chen GQ, et al. PEBP4 gene expression and its significance in invasion and metastasis of non-small cell lung cancer. Tumour Biol 2012;33:223-8. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Wu J, Keller JM, et al. Transcriptional regulation of RKIP expression by androgen in prostate cells. Cell Physiol Biochem 2012;30:1340-50. [DOI] [PubMed] [Google Scholar]
- 14.Li HZ, Wang Y, Gao Y, et al. Effects of raf kinase inhibitor protein expression on metastasis and progression of human epithelial ovarian cancer. Mol Cancer Res 2008;6:917-28. [DOI] [PubMed] [Google Scholar]
- 15.Zhong WZ, Zhai HR, Wu YL. Clinical trials in lung cancer surgery and research cooperation. Chin Clin Oncol 2014;3:46. [DOI] [PubMed] [Google Scholar]
- 16.Yu GP, Chen GQ, Wu S, et al. The expression of PEBP4 protein in lung squamous cell carcinoma. Tumour Biol 2011;32:1257-63. [DOI] [PubMed] [Google Scholar]
- 17.Yu G, Zhong N, Chen G, et al. Downregulation of PEBP4, a target of miR-34a, sensitizes drug-resistant lung cancer cells. Tumour Biol 2014;35:10341-9. [DOI] [PubMed] [Google Scholar]
- 18.Kishore T KK, Ganugula R, Gade DR, et al. Gedunin abrogates aldose reductase, PI3K/Akt/mToR, and NF-κB signaling pathways to inhibit angiogenesis in a hamster model of oral carcinogenesis. Tumour Biol 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 19.Petrulea MS, Plantinga TS, Smit JW, et al. PI3K/Akt/mTOR: A promising therapeutic target for non-medullary thyroid carcinoma. Cancer Treat Rev 2015;41:707-13. [DOI] [PubMed] [Google Scholar]
- 20.Fransecky L, Mochmann LH, Baldus CD. Outlook on PI3K/AKT/mTOR inhibition in acute leukemia. Mol Cell Ther 2015;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Wu J, Ling MT, et al. The role of the PI3K/Akt/mTOR signalling pathway in human cancers induced by infection with human papillomaviruses. Mol Cancer 2015;14:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo H, German P, Bai S, et al. The PI3K/AKT Pathway and Renal Cell Carcinoma. J Genet Genomics 2015;42:343-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duzgun Z, Eroglu Z, Biray Avci C. Role of mTOR in glioblastoma. Gene 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Liu F, Qu H, et al. 1, 25(OH)2D3 protects β cell against high glucose-induced apoptosis through mTOR suppressing. Mol Cell Endocrinol 2015;414:111-9. [DOI] [PubMed] [Google Scholar]
- 25.Maiese K. Stem cell guidance through the mechanistic target of rapamycin. World J Stem Cells 2015;7:999-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin HP, Lin CY, Huo C, et al. AKT3 promotes prostate cancer proliferation cells through regulation of Akt, B-Raf, and TSC1/TSC2. Oncotarget 2015;6:27097-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabrera-López C, Bullich G, Martí T, et al. Insight into response to mTOR inhibition when PKD1 and TSC2 are mutated. BMC Med Genet 2015;16:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song BQ, Chi Y, Li X, et al. Inhibition of Notch Signaling Promotes the Adipogenic Differentiation of Mesenchymal Stem Cells Through Autophagy Activation and PTEN-PI3K/AKT/mTOR Pathway. Cell Physiol Biochem 2015;36:1991-2002. [DOI] [PubMed] [Google Scholar]
- 29.Wilks ST. Potential of overcoming resistance to HER2-targeted therapies through the PI3K/Akt/mTOR pathway. Breast 2015;24:548-55. [DOI] [PubMed] [Google Scholar]
- 30.Demel HR, Feuerecker B, Piontek G, et al. Effects of topoisomerase inhibitors that induce DNA damage response on glucose metabolism and PI3K/Akt/mTOR signaling in multiple myeloma cells. Am J Cancer Res 2015;5:1649-64. [PMC free article] [PubMed] [Google Scholar]
- 31.Liu M, Qi Z, Liu B, et al. RY-2f, an isoflavone analog, overcomes cisplatin resistance to inhibit ovarian tumorigenesis via targeting the PI3K/AKT/mTOR signaling pathway. Oncotarget 2015;6:25281-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Han L, Zhang J, et al. Down-Regulated NRSN2 Promotes Cell Proliferation and Survival Through PI3K/Akt/mTOR Pathway in Hepatocellular Carcinoma. Dig Dis Sci 2015;60:3011-8. [DOI] [PubMed] [Google Scholar]
- 33.Uno K, Yamada T, Ishigaki Y, et al. A hepatic amino acid/mTOR/S6K-dependent signalling pathway modulates systemic lipid metabolism via neuronal signals. Nat Commun 2015;6:7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong X, Zhang L, Huang T, et al. Activating the translational repressor 4E-BP or reducing S6K-GSK3β activity prevents accelerated axon growth induced by hyperactive mTOR in vivo. Hum Mol Genet 2015;24:5746-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lv T, Wang Q, Cromie M, et al. Twist1-mediated 4E-BP1 regulation through mTOR in non-small cell lung cancer. Oncotarget 2015. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang XL, Wang HS, Liu N, et al. Bisphenol A stimulates the epithelial mesenchymal transition of estrogen negative breast cancer cells via FOXA1 signals. Arch Biochem Biophys 2015;585:10-16. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Kong X, Cui G, et al. Rapamycin restores p14, p15 and p57 expression and inhibits the mTOR/p70S6K pathway in acute lymphoblastic leukemia cells. Int J Hematol 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 38.Thijssen R, Ter Burg J, van Bochove GG, et al. The pan phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor SAR245409 (voxtalisib/XL765) blocks survival, adhesion and proliferation of primary chronic lymphocytic leukemia cells. Leukemia 2015. [Epub ahead of print].26338274 [Google Scholar]
- 39.Singleton KR, Hinz TK, Kleczko EK, et al. Kinome RNAi Screens Reveal Synergistic Targeting of MTOR and FGFR1 Pathways for Treatment of Lung Cancer and HNSCC. Cancer Res 2015;75:4398-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bai H, Li H, Li W, et al. The PI3K/AKT/mTOR pathway is a potential predictor of distinct invasive and migratory capacities in human ovarian cancer cell lines. Oncotarget 2015;6:25520-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong ZF, Zhao WX, Yin ZY, et al. Capsaicin Enhances the Drug Sensitivity of Cholangiocarcinoma through the Inhibition of Chemotherapeutic-Induced Autophagy. PLoS One 2015;10:e0121538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez P, Rojas J. cAMP-Induced Histones H3 Dephosphorylation Is Independent of PKA and MAP Kinase Activations and Correlates With mTOR Inactivation. J Cell Biochem 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 43.Saran U, Foti M, Dufour JF. Cellular and molecular effects of the mTOR inhibitor everolimus. Clin Sci (Lond) 2015;129:895-914. [DOI] [PubMed] [Google Scholar]