Abstract
Aim:
A number of evidence shows that the differentiation of B lymphocytes into plasma cells plays an important role in lupus pathogenesis. In this study we investigated how prednisone, a classical therapeutic drug for autoimmune diseases, regulated plasma cell differentiation in MRL/MpSlac-lpr mice.
Methods:
MRL/lpr mice were treated with prednisone (2.5 or 5 mg·kg−1·d−1, ig) for 13 weeks, and the proteinuria levels and survival times were monitored. After the mice were euthanized, blood sample, spleen and thymus were collected. The serum levels of anti-dsDNA antibody, anti-nuclear antibody, IL-21, and IL-10 were detected using ELISA kits. Subsets of splenic B and T lymphocytes were quantified with flow cytometry. Transcription factor Blimp-1 and Bcl-6 expression was determined using qPCR and Western blot.
Results:
Prednisone treatment dose-dependently attenuated the lupus symptoms in MRL/lpr mice with decreased proteinuria levels, prolonged survival times, decreased serum anti-nuclear antibody levels, and reduced spleen and thymus indices. Prednisone treatment also significantly decreased the elevated percentages of plasma cells and plasma cell precursors, decreased the percentages of activated T cells, and increased the frequency of CD4+CD62L+ cells, demonstrated that decreased anti-nuclear antibodies and improvements in lupus symptoms were associated with decreased plasma cells. Furthermore, prednisone treatment decreased serum IL-21 and IL-10 levels and reduced the expression of splenic Blimp-1 and Bcl-6 (two key regulatory factors for plasma cell differentiation) in MRL/lpr mice.
Conclusion:
Prednisone treatment restricts B lymphocyte differentiation into plasma cells in MRL/lpr mice, which may be correlated with the inhibition of IL-21 production and the restoration of the balance between Blimp-1 and Bcl-6.
Keywords: lupus erythematosus, MRL/lpr mice, prednisone, plasma cell differentiation, interleukin-21, interleukin-10, Blimp-1, Bcl-6
Introduction
Autoimmune diseases, such as systematic lupus erythematosus (SLE), are characterized by substantial autoantibodies against various nuclear antigens, the appearance of deposition masses of immune complexes and leukocyte infiltration1. The pathogenesis of SLE remains ambiguous. An antibody-independent role of B cells has been previously identified based on evidence that mice with B cells that lack serum antibodies still develop nephritis and vasculitis2. A previous study has suggested that FasL+ B cells and FasL+ CD4 CD8 double negative (DN) T cells may contribute to the cytotoxic destruction of Fas+ tissues in MRL/lpr mice3. In addition, most studies have reported that the hyper-interaction between T lymphocytes and B lymphocytes is involved4. Moreover, B cell hyperactivity and spontaneous antibody production have been reported in SLE. The important roles of B cells have been confirmed in a previous study in which lupus disease was abrogated in B cell-deficient MRL/lpr mice5,6. In the peripheral blood of SLE patients, the frequency of circulating plasma cells (PCs) correlates with autoantibody production and disease activity, as measured by the SLEDAI scoring system7,8. This finding indicates that the differentiation process of B lymphocytes into plasma cells plays an important role in lupus pathogenesis.
In healthy conditions, plasma cells possess the capacity to combat an extraneous pathogen via the secretion of various antibodies. However, these cells tend to trigger autoimmune disease via the production of autoantibodies against their own tissues. Many studies have demonstrated the hyperactivation of B cells and the presence of excessive plasma cells in autoimmune diseases9,10. Therefore, it is essential to achieve a better understanding of plasma cell differentiation. In addition, the control of plasma cell differentiation may represent a promising direction for the therapy of autoimmune diseases.
There are many regulatory factors involved in plasma cell differentiation. High levels of interleukin-21 (IL-21) and IL-21 mRNA have been identified in the serum of SLE patients11,12. The neutralization of IL-21 with an IL-21R fusion protein markedly inhibited T cell-dependent B cell activation13. Similar to IL-21, IL-10 also promotes plasma cell differentiation at different stages14. Furthermore, both B lymphocyte-induced maturation protein-1 (Blimp-1) and B cell lymphoma-6 (Bcl-6) are important transcription factors that antagonize each other in deciding the fate of B cells, and the balance between these two factors is critical in plasma cell differentiation. Blimp-1 is a key regulator in plasma cell differentiation. It was first identified as a susceptibility loci in SLE patients via genome-wide association studies in 200915. The increased expression of Blimp-1 in MRL/lpr mice and SLE patients is associated with increased plasma cells, autoantibodies, and disease activity16. Mice with B cells that lack Blimp-1 are nearly absent of serum Ig and plasma cells. These findings demonstrate that Blimp-1 is required for plasma cell differentiation and antibody secretion17. However, Bcl-6, which is primarily expressed in germinal center B (GC-B) cells and follicular helper T (TFH) cells, is a crucial inducer of GC-B cell proliferation and an inhibitor of the DNA-damage response18. Bcl-6-deficient mice exhibit difficulties in GC and TFH cell development, which consequently results in a defection in T cell-dependent antibody responses19,20,21. Investigation of the relationship and functions of IL-21, Blimp-1, and Bcl-6 may facilitate our understanding of the plasma cell differentiation process.
Clinically, there are many different types of treatments for autoimmune diseases, such as immunosuppression, hormonotherapy, and B-cell targeted therapies22,23,24,25. However, as a classical and basic therapeutic drug for autoimmune disease, glucocorticoids have been applied for decades because of their strong anti-inflammatory and immunosuppressive effects. However, the mechanism of glucocorticoid regulation of plasma cell differentiation has not been elucidated. The exploration of the detailed mechanisms of glucocorticoid actions on plasma cell differentiation, particularly the relationship with regulatory factors, including IL-21, Bilmp-1, and Bcl-6, is of interest. Prednisone, a classic intermediate-acting glucocorticoid, is a basic drug for SLE treatment in the clinic and has a higher proportion in maintenance therapy. In the present study, MRL/MpSlac-lpr (MRL/lpr) mice, which exhibit systemic autoimmune symptoms similar to human SLE, were used to investigate the regulatory function of prednisone on plasma cell differentiation.
Materials and methods
Animals
MRL/MpSlac-lpr mice (female, 31±3 g, 8±1 weeks of age) were purchased from the Shanghai Laboratory Animal Centre (license: SCXK 2012-0002). Four to five mice were housed per cage under specific pathogen-free (SPF) laboratory conditions; the mice were fed a regular diet and clear water. All experiments were approved by the Ethics Review Committee for Animal Experimentation of the Institute of Clinical Pharmacology of Anhui Medical University.
Reagents
Dulbecco's modified Eagle's medium (DMEM) was purchased from Gibco Co (USA). Anti-mouse FITC-CD4, APC-CD3, PE-CD62L, PE-CD69, FITC-CD19, APC-CR2 (CD21), PE-CD23, PE-CD27, APC-CD38, PE-CD138, APC/Cy7-CD8, and PE-B220 antibodies were purchased from Biolegend, Inc (San Diego, CA, USA). Rat anti-mouse Blimp-1 primary antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit anti-mouse Bcl-6 protein was purchased from Epitomics (Abcam Company, CA, USA). Mouse anti-mouse β-actin was purchased from ZSGQ-Bio Company (Beijing, China). The qPCR products were purchased from Promega Corporation (USA).
Grouping and treatment
The MRL/lpr mice were randomly divided into three groups: one model group (n=10) and two prednisone treatment groups (2.5 and 5.0 mg/kg, n=8 per group). Prednisone (Pre, purchased from Zhejiang Xianju Pharmaceutical Co, Ltd, China) was dissolved in 0.5% sodium carboxymethyl cellulose solution (CMCNa); the drug was intragastrically administered every day for 13 weeks when the proteinuria level of the MRL/lpr mice reached 10 mg/100 mL. The mice in the model group were simultaneously treated with an equal volume of normal saline.
General observation signs
The mice were monitored every day, and the proteinuria levels were detected by the same individual to avoid subjective bias. The proteinuria levels were determined using semi-quantitative Albustix paper (Guangzhou Huadu Biotechnology Co, Ltd, China) twice per week. The results were graded negative, trace, 3–10 mg/100 mL, 10–30 mg/100 mL, 30–100 mg/100 mL, or greater than 100 mg/100 mL and were scored 0, ±, +1, +2, +3, or +4, respectively.
The survival rate of the mice was determined during the 13 weeks of treatment. Humane endpoints were used during the survival study when the mouse met specific clinical criteria. The mouse was anaesthetized with 0.004 mL/g 10% chloral hydrate and was euthanized by cervical dislocation when it exhibited the following symptoms, which indicated that it was on the verge of death: pale lips, lackluster hair, swollen body, sluggish activity, and impaired appetite. These symptoms were typically accompanied by a high level of proteinuria (up to 30 mg/100 mL).
Analysis of the spleen and thymus indices
Following the final administration, the surviving animals were anaesthetized with chloral hydrate and were euthanized by cervical dislocation. Blood was collected from the orbit vein. The mouse spleen and thymus were dissected and weighed under sterile conditions. The ratios of the spleen and thymus weights to the mouse body weight represent the spleen and thymus indices (mg/10 g body weight), respectively.
Measurement of auto-antibodies and cytokines
The mouse blood was collected from the orbit after anesthetization, and the serum was separated from the blood via centrifugation for 10 min at 3000 r/min. The concentrations of serum anti-dsDNA antibodies (IgG), anti-nuclear antibodies (IgG), IL-21, and IL-10 in the individual subjects were determined using an ELISA kit according to the manufacturer's instructions (Cusabio Biotech Co, Ltd, China).
Detection of splenic lymphocyte subsets
The spleen was detached under sterile conditions, and the lymphocytes were collected. The cell suspension was isolated via density-gradient centrifugation using a mouse lymphocyte separation medium (Tianjin Haoyang Biological Manufacture Co, Ltd, China). The cells were then washed three times with PBS buffer. The lymphocyte suspensions (6×105/tube) were stained in duplicate with the antibodies or isotype-matched controls, respectively. The samples were mixed gently, incubated for 20 min at 4°C, and subsequently analyzed using a flow cytometer. All flow cytometric analyses were performed with a FACSCalibur dual-laser cytometer (FC500, Beckman, USA) using Cell QuestTM acquisition and FlowJo V10 (Treestar, Ashland, OR, USA) software.
Blimp-1 and Bcl-6 mRNA expression level detection
The total RNA from the spleen was prepared using TRIzol reagent. Reverse transcription was performed using a MyCyclerTM Terminal Cycler (Bio-Rad Laboratories, Inc, USA). The cDNA was obtained using the following protocol: incubation for 5 min at 70°C, 5 min at 25°C, and 60 min at 42°C. The Blimp-1 specific sense primer is 5′-GACGGGGGTACTTCTGTTCA-3′, and the antisense primer is 5′-GGCATTCTTGGGAACTGTGT-3′. The Bcl-6 specific sense primer is 5′-CTGCAGATGGAGCATGTTGT-3′, and the antisense primer is 5′-CACCCGGGAGTATTTCTCAG-3′. The internal control GAPDH specific sense primer is 5′-GGTGAAGGTCGGTGTGTGAAG-3′, and the antisense primer is 5′-CTCGCTCCTGGAAGATGGTG-3′. qPCR for the two genes was performed using a StepOneTMReal-Time PCR System (Applied Biosystems) following the manufacturer's protocol. The qPCR protocol was performed as follows: 95°C for 2 min, 40 cycles of denaturation at 95°C for 15 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s, reaction with 40 circles, and melting at 95°C for 15 s, 60°C for 1 min, and 95°C for 15 min.
Blimp-1 and Bcl-6 protein detection by Western blot assay
The tissue was homogenized in precooled buffer that contained RIPA lysis buffer [50 mmol/L Tris-HCl, pH 7.4, 150 mmol/l NaCl, 10 mmol/L phenylmethylsulfonyl fluoride (PMSF), 1 mmol/L ethylene diamine tetraacetic acid (EDTA), 0.1% sodium dodecyl sulfate (SDS), 1% Triton X-100, and 1% sodium deoxycholate]. After centrifugation at 15 000 r/min and 4°C for 15 min, the total protein amount in the collected supernatant was measured using the BCA method (Thermo, CA, USA). The protein sample was mixed with 5×SDS-PAGE sample loading buffer (Beyotime Institute of Biotechnology, Shanghai, China) and heated in boiling water for 10 min. The samples were run on a 10% SDS-PAGE gel and transferred to a PVDF membrane (Millipore, Bedford, MA, USA). After blocking with 5% non-fat milk (blocking solution) at room temperature for 2 h, the PVDF membrane was incubated with anti-β-actin primary antibody (1:1000), anti-Blimp-1 primary antibody (1:400), or anti-Bcl-6 primary antibody (1:1000) overnight at 4°C followed by compatible HRP-conjugated secondary antibodies (Biosharp, Hefei, China) for 2 h at room temperature. The bands were visualized with an ECL kit (Thermo, CA, USA), and the multiplication of the intensity and area of the protein bands, which indicates the relative protein expression levels, were determined using ImageJ software.
Statistical analysis
The results are represented as the means±standard deviations (SDs) from an independent experiment and were plotted using GraphPad Prism v4 (San Diego, CA, USA). The statistical comparisons between the groups were evaluated via one-way ANOVA. The survival curves were plotted using the Kaplan-Meier method and examined for significance using the Mantel-Cox log-rank test. P<0.05 was considered significant. SPSS (Statistical Package for the Social Sciences) 10.0 for Windows was used for data analysis.
Results
Effects of prednisone on the survival rate of MRL/lpr mice
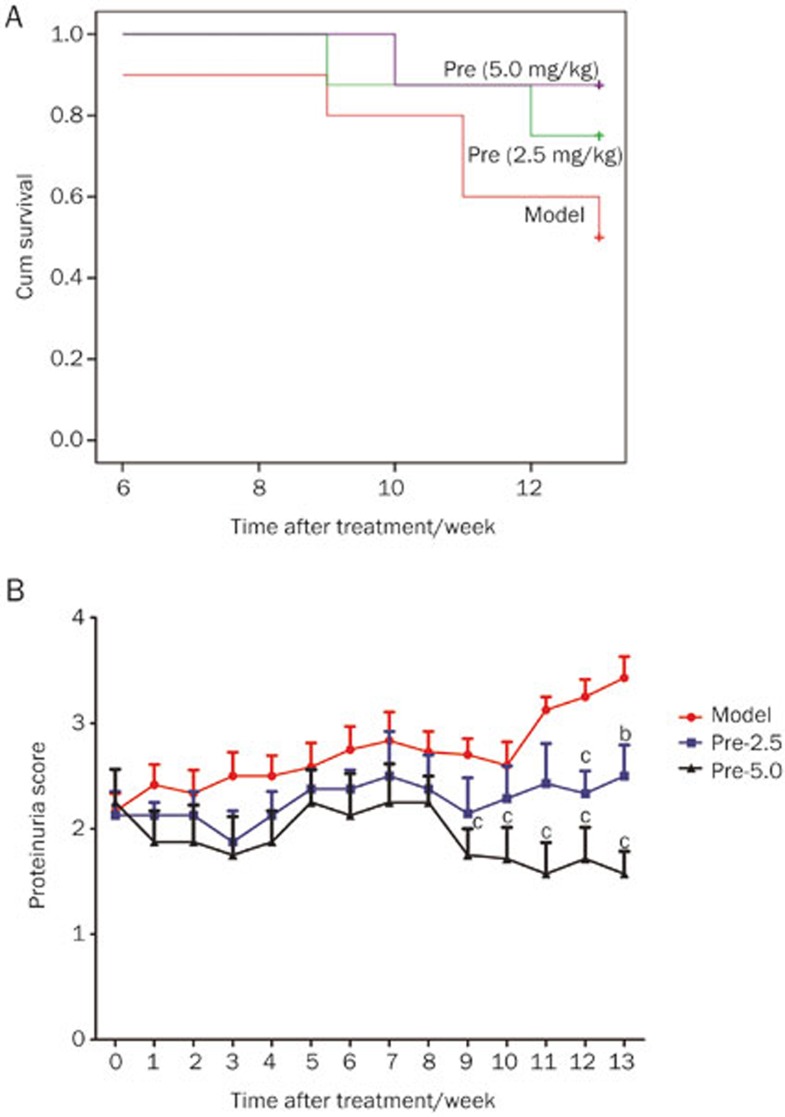
With increased age and exacerbated interstitial nephritis, the MRL/lpr mice gradually exhibited lackluster hair, sluggish activity and a high level of proteinuria. The first mouse death in the model group occurred during the 6th week of treatment, whereas the first mouse death in the prednisone (2.5 mg/kg) treatment group occurred during the 9th week. After 13 weeks of treatment, the survival rate decreased to 50.0% (5/10) in the model group. However, the survival rates of the mice in the treatment groups (2.5 and 5.0 mg/kg prednisone) were 75.0% (6/8) and 87.5% (7/8), respectively. The analysis of the survival rate data indicated that the model group exhibited an earlier mortality than the other groups, and the survival rate of the model group was the lowest at the end of the treatment period. Treatment with prednisone (2.5 and 5.0 mg/kg) was demonstrated to prolong the lifespan of the MRL/lpr mice and increase the survival rate; however, no significant difference was identified (Figure 1A).
Figure 1.
Effects of prednisone on the survival rate and proteinuria level in MRL/lpr mice. (A) Treatment was initiated when the proteinuria level of the MRL/lpr mice reached 10 mg/100 mL urine. The model group mice were intragastrically treated with CMCNa (red line, n=10). The prednisone group mice were intragastrically treated with prednisone at a dose of 2.5 mg/kg body weight (green line, n=8) or 5.0 mg/kg body weight (purple line, n=8). The survival rates during the 13 weeks of treatment are shown, and all mice used in the experiment were female. There was no significant difference between the groups (P=0.076). (B) The changes in the proteinuria level of each group were determined every week from one week before the initiation of prednisone therapy to 13 weeks after treatment initiation (model group, n=10; other groups, n=8; all mice were female). The proteinuria scores are shown. bP<0.05, cP<0.01 vs the model group.
Prednisone treatment improved proteinuria in MRL/lpr mice
When the proteinuria level reached 10 mg/100 mL, prednisone was intragastrically administered to the mice. Figure 1B shows the comparison of the proteinuria scores between the prednisone-treated and model groups. From 9 to 13 weeks of treatment, the mean proteinuria scores of the prednisone (5.0 mg/kg) group were significantly and consistently lower than those of the model mice.
Effects of prednisone on spleen and thymus indices in MRL/lpr mice
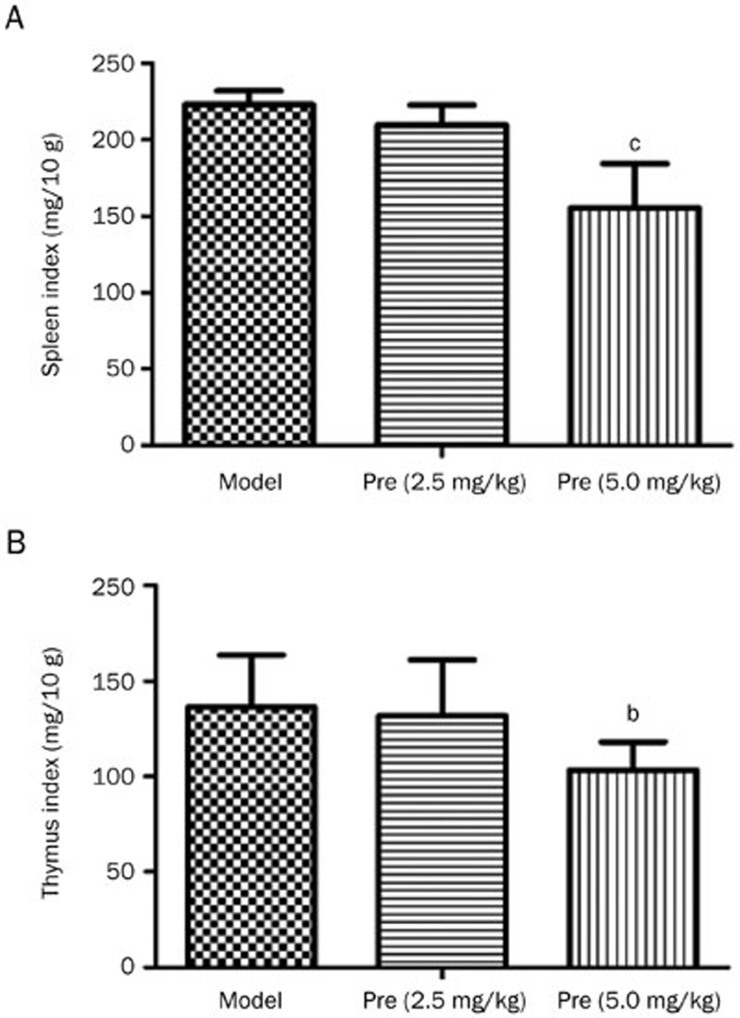
Splenomegaly and lymphadenopathy are typical symptoms in MRL/lpr mice. In this study, the spleen and thymus indices were assessed after 13 weeks of prednisone treatment. The spleens and thymuses of the MRL/lpr mice that received treatments were smaller than the control mice, and the spleen and thymus indices also markedly decreased after treatment with prednisone (5.0 mg/kg) (Figure 2).
Figure 2.
Effects of prednisone on the spleen and thymus indices in MRL/lpr mice. The spleen/thymus indices, which represent the ratios of the spleen/thymus weight to the mouse body weight (mg/10 g body weight), were analyzed (model group, n=5; Pre 2.5 group, n=6; Pre 5.0 group, n=7; all mice were female). bP<0.05, cP<0.01 vs the model group.
Effects of prednisone on serum auto-autoantibodies and cytokines in MRL/lpr mice
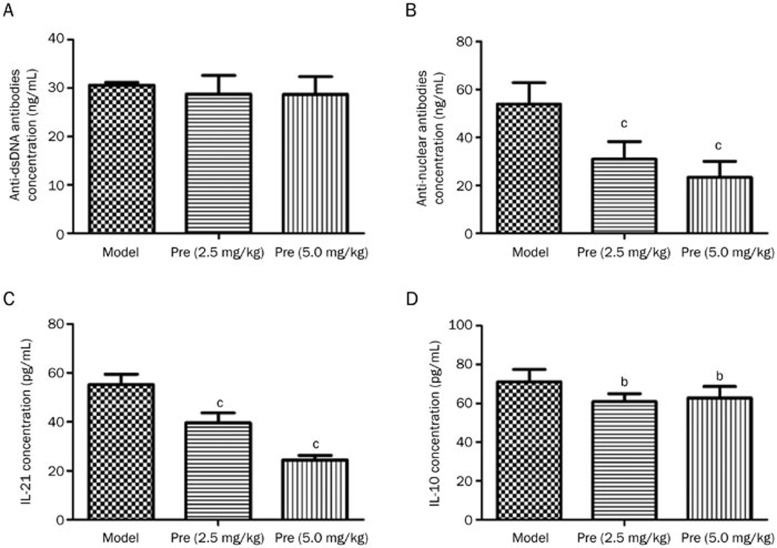
The MRL/lpr mice exhibited a significantly higher level of autoantibodies, including anti-dsDNA antibodies and anti-nuclear antibodies (ANA). Treatment with prednisone (2.5 and 5.0 mg/kg) resulted in a substantial decrease in the serum ANAs; however, it had little effect on the anti-dsDNA antibodies. Importantly, prednisone decreased the high levels of IL-21 and IL-10 in the MRL/lpr mice, which guided the direction of our subsequent research (Figure 3).
Figure 3.
Effects of prednisone on serum anti-dsDNA antibodies, anti-nuclear antibodies (ANA), IL-21, and IL-10 in MRL/lpr mice. Serum levels of autoantibody production (A, B), IL-21 (C), and IL-10 (D) in the model group (n=5), prednisone 2.5 mg/kg group (n=6), and prednisone 5.0 mg/kg group (n=7) were detected via ELISA. bP<0.05, cP<0.01 vs the model group.
Effects of prednisone on the subsets of splenic lymphocytes in MRL/lpr mice
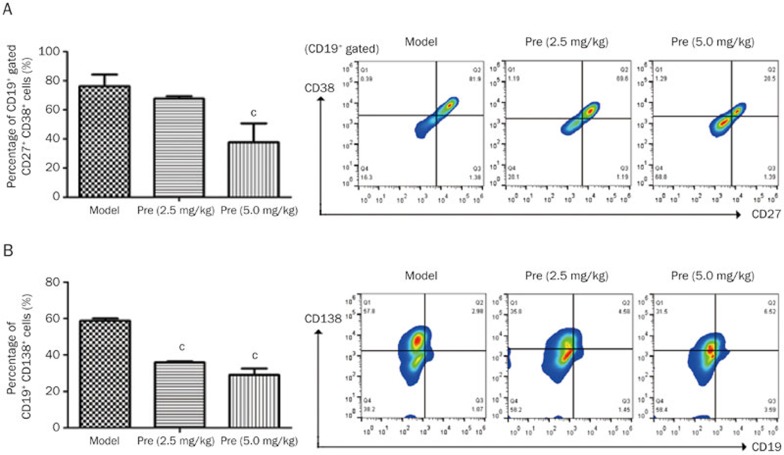
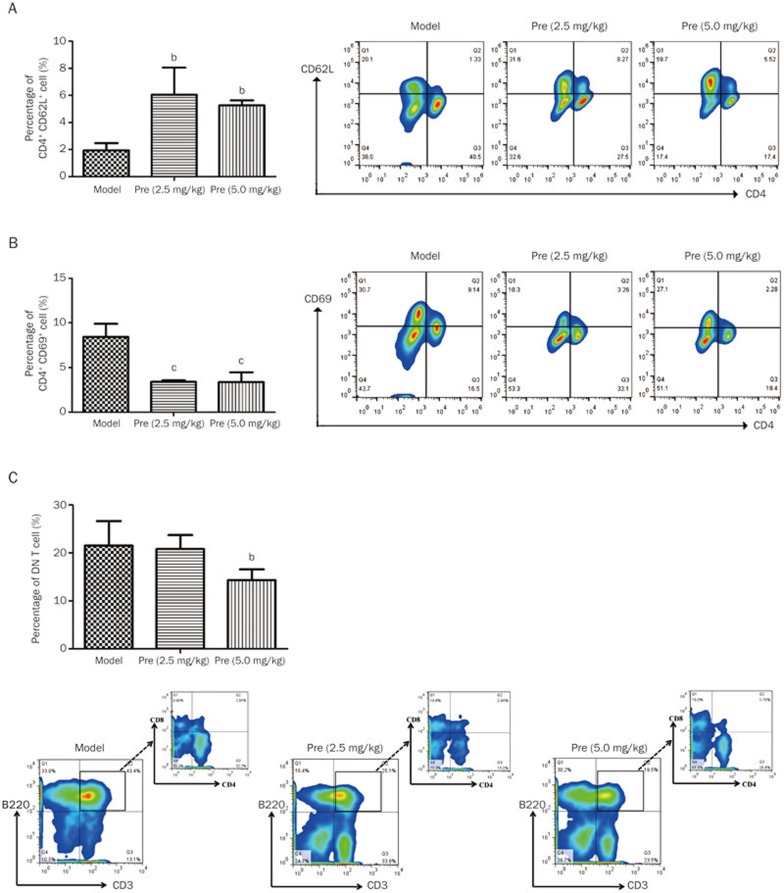
To investigate the effects of prednisone on B cells and plasma cells, the related B cell subsets were detected. The findings indicated that prednisone treatment (2.5 and 5.0 mg/kg) increased CD19 expression on B cells (mean 8.25% and 11.33% vs 5.67%) and significantly decreased the high percentages of plasma cell precursors and plasma cells (Figure 4A, B), which may contribute to the decrease in the ANA level. T–B cell interactions may play an important role in the pathogenesis of SLE. Thus, T cell subsets were also detected in this research, and the findings indicated that large numbers of T cells were activated in the MRL/lpr mice. Following prednisone treatment, the percentage of naïve T cells (CD4+CD62L+) increased (Figure 5A), whereas the percentage of activated T cells (CD4+CD69+) significantly decreased (Figure 5B). Moreover, the high level of DN T cells (CD3+B220+CD4−CD8−) was significantly decreased after prednisone 5.0 mg/kg treatment in the MRL/lpr mice (Figure 5C).
Figure 4.
Effects of prednisone on B cell subsets in MRL/lpr mice. After treatment with prednisone (2.5 and 5.0 mg/kg) for 13 weeks, the splenic mononuclear cell suspension of each MRL/lpr group was separated from the mouse spleen and analyzed via FC. The column diagram indicates the statistical cell frequency results of the indicated group (n=3), and the pseudo-colored flow cytometry picture represents the cellular distribution of one typical sample from the indicated group. (A) The cell suspension was immunofluorescently stained with an anti-CD19, anti-CD27, and anti-CD38 antibody mix, and the total CD27+CD38+ cells in the CD19+ B cell population were statistically analyzed in the column diagram. A typical cell distribution for each group is shown in the Q2 region of the pseudo-colored flow cytometry picture. (B) The percentages of CD19−CD138+ plasma cells in the model, Pre (2.5 mg/kg), and Pre (5.0 mg/kg) groups were analyzed, and the statistical results are shown in the column diagram. cP<0.01 vs the model group.
Figure 5.
Effects of prednisone on T cell subsets in MRL/lpr mice. The column diagram indicates the statistical cell frequency results of the indicated group (n=3), and the pseudo-colored flow cytometry picture represents the cellular distribution of one typical sample from the indicated group. (A) CD4+CD62L+ cell population. (B) CD4+CD69+ cell population. (C) DN T cells population. bP<0.05, cP<0.01 versus the model group.
Effects of prednisone on the level of Blimp-1 and Bcl-6 expression in MRL/lpr mice
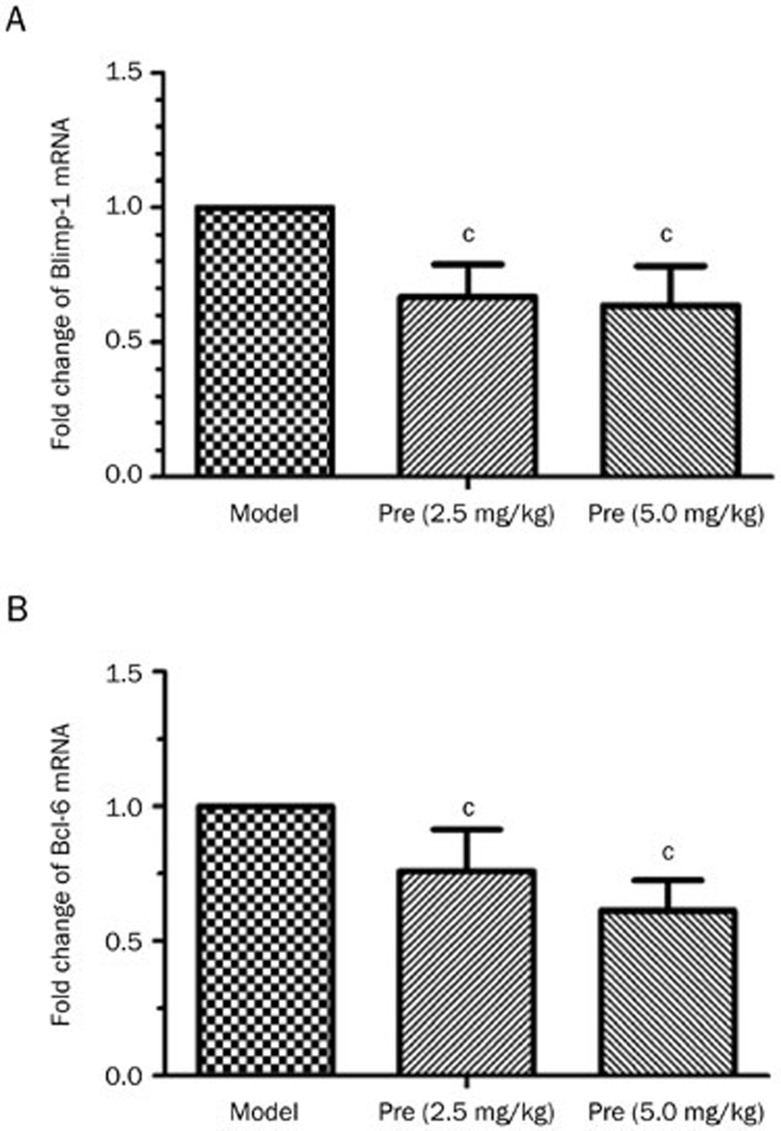
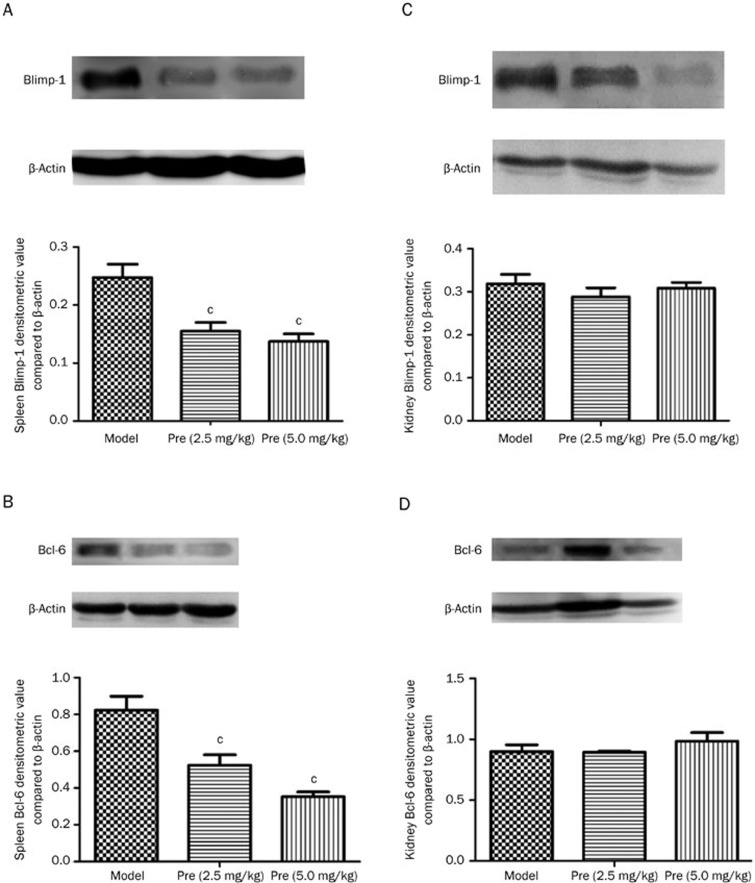
Blimp-1 and Bcl-6 are the pivotal transcription factors in plasma cell differentiation and germinal center development. In this study, the levels of Blimp-1 and Bcl-6 expression were detected via qPCR and Western blot. The findings indicated that Blimp-1 protein was mainly located in the red pulp part between the Malpighian corpuscles of the spleen, and Bcl-6 was also predominately expressed in the red pulp, as well as slightly in the white pulp of the spleen. Both Blimp-1 and Bcl-6 were expressed in the renal capsule of the kidney (data not shown). Prednisone treatment significantly decreased the splenic Blimp-1 and Bcl-6 mRNA levels (Figure 6) and inhibited Blimp-1 and Bcl-6 protein production in the spleen; however, no significant changes in Blimp-1 or Bcl-6 protein expression were identified in the kidney as a result of prednisone treatment (Figure 7).
Figure 6.
Effects of prednisone on Blimp-1 and Bcl-6 mRNA expression in mouse spleen tissue. The total RNA from the mouse spleen (n=3) was extracted, and the cDNA was obtained using a reverse transcription kit. The expression levels of the two genes were detected using qPCR. The comparative analysis of the fold changes in Blimp-1 (A) and Bcl-6 (B) expression are shown. cP<0.01 vs the model group.
Figure 7.
Effects of prednisone on Blimp-1 and Bcl-6 protein expression in mouse spleen and kidney tissues. The Blimp-1 and Bcl-6 protein expression in the spleen and kidney of the study groups was evaluated via Western blot analysis. (A) and (B) indicate Blimp-1 and Bcl-6 expression in the MRL/lpr mouse spleen tissue, whereas (C) and (D) indicate kidney tissue. cP<0.01 vs the model group.
Discussion
Excessive autoantibody secretion as a result of abnormal plasma cell differentiation may comprise the major pathogenesis of autoimmune diseases. In addition, many types of cytokines and transcriptional factors, such as IL-21 and Blimp-1, are involved in the plasma cell differentiation process26. Thus, it was beneficial to remit the disease via the regulation of relevant cytokines and transcriptional factors. Based on these findings, the inhibition of B cell differentiation into plasma cells provides a valid treatment strategy for autoimmune diseases. In MRL/lpr mice, high levels of plasma cell precursors, plasma cells and ANA, which were secreted by plasma cells, indicated that a substantial amount of B lymphocytes were abnormally differentiated into plasma cells. Prednisone treatment prolonged the lifespan of mice, decreased the level of IgG auto-antibody ANA secreted by plasma cells, reduced the high percentages of plasma cell precursors and plasma cells and increased the percentage of CD19+ B cells. These findings indicated that prednisone inhibited B cell differentiation into plasma cells and auto-antibody production. However, more importantly, prednisone exhibited little effect on the high level of IgG anti-dsDNA antibody in the MRL/lpr mice. Similar results have been reported in the treatment of SLE using other drugs, such as infliximab, monoclonal IgM anti-dsDNA antibodies, and the combination of cyclophosphamide and rituximab27,28,29. Additionally, a previous study30 reported that the sensitivity of plasma cells to corticosteroid depends on the site where they reside. In the bone marrow and inflamed kidney, IgG-secreting anti-dsDNA plasma cells are resistant to corticosteroid treatment, which helps explain the unchanged level of anti-dsDNA antibody identified in our study.
Substantial evidence suggests that T lymphocyte and cytokines are involved in the differentiation of plasma cells. In mice with the MRL genetic background, the autosomal recessive gene lpr (Tnfrsf6lpr) is responsible for a syndrome characterized by the progressive accumulation of DN T cell populations in peripheral lymphoid tissues. The lpr mutation engenders substantially less expression of the Fas death receptor, which results in the accumulation, in lymph nodes and spleens, of numerous activated DN T lymphocytes that are not typically regulated by the Fas-mediated mechanism that controls mature T cell apoptosis31. Prednisone treatment could reduce the volumes of the spleen and thymus and decrease the percentage of DN T cells and activated T cells (CD4+CD69+). The improvement of splenomegaly may be correlated with fewer DN T cell infiltration in the tissues32,33. Furthermore, the percentage of the CD4+CD62L+ T subset in the mouse spleens was increased after prednisone treatment, which indicated that the number of naive T cells were significantly increased. This feedback may be correlated with decreased DN T cells and activated T cells (CD4+CD69+).
Previous studies in mice have indicated a role for CD69+ T cells in the pathogenesis of autoimmune disease34. The genetic deletion of CD69 attenuates the secretion of IL-21 by CD4+ T cells35. Additionally, CD69 is a feature of cell accumulation in inflammation sites, and its level is relevant to IL-21 secretion. Thus, a high frequency of CD4+CD69+ T cells suggests a continuous inflammatory process and a hyperactivated T cell stage. In conclusion, the hyper-interaction between T and B cells also accelerates B cell differentiation into plasma cells. Moreover, the high frequency of CD4+CD69+ T cells is also correlated with a high level of the proinflammatory cytokine IL-21.
IL-21 is produced by CD4+ T cells (including Th17 and TFH cells) and mediates the differentiation and function of T, B, and NK cells through binding to its receptor36. Specifically, IL-21 is a pro-inflammatory cytokine that has the capabilities to simulate B cell proliferation, regulate B cell function, promote an Ig isotype switch, and encourage plasma cell differentiation and antibody secretion37. Overexpression of the Th2 cytokine IL-10 is associated with the manifestations of autoimmune lymphoproliferative syndrome and is a potent in vitro inducer of B lymphocyte differentiation, as well as an inhibitor of T helper lymphocyte and antigen-presenting cell function. Both IL-21 and IL-10 induce STAT-3 phosphorylation that leads to Blimp-1 expression, which is essential in the plasma cell differentiation process38. In addition, IL-21 activates JAK/STAT5 signaling to induce Bcl-6 expression39. Decreases in IL-21 and IL-10 after prednisone treatment would downregulate plasma cell differentiation. Substantial studies have suggested that IL-21, Blimp-1, and Bcl-6 play important roles in plasma cell differentiation26,36,40,41,42; thus, the changes and functions of these three factors in MRL/lpr mice were also investigated in our study.
The increased IL-21 level in the serum, as well as the increased Blimp-1 and Bcl-6 expression levels in the spleen clarified the participation of IL-21, Blimp-1, and Bcl-6 in B cell differentiation into plasma cells. As reported by many studies, Blimp-1 is a key regulator of the promotion of plasma cell differentiation, and Bcl-6 is essential for germinal center development. The reciprocal antagonism between these two transcription factors can moderate B cell fate and further influence plasma cell formation. In our study, the Blimp-1 expression decreased after prednisone treatment, which was also analogous to previous studies43 that indicated Blimp-1 siRNA inhibited B cell differentiation to plasma cells and prevented the development of lupus in mice. Thus, prednisone treatment can decrease Blimp-1 expression to inhibit excessive development of plasma cells in the spleen, which thereby contributes to a decrease in the auto-antibody level.
There are controversial opinions regarding Bcl-6 expression in autoimmune diseases. Bcl-6, which is present at high levels in germinal center B cells and TFH cells, moderates the differentiation of both TFH cells and GC-B cells; therefore, it acts as a master transcription factor for T cell-dependent immune responses in the GC44. Specifically, Blimp-1 induces B cell differentiation into plasma cells, and Bcl-6 impels B cells to differentiate into GC-B cells. At the final stage, the decreased GC-B cells and Bcl-6 level and the increased Blimp-1 expression ensures plasma cell differentiation. The balance between the two transcriptional factors is critical for the fate of B cell development45,46,47,48. However, the Bcl-6 expression level in the spleen of MRL/lpr mice was increased, which appears to be contradictory to the mutually antagonistic effects of Blimp-1 and Bcl-649. In the MRL/lpr model mice, the high expression of Bcl-6 may be a result of an excessive amount of CD4+CD69+ activated T cells and a high level of IL-21, which induces JAK/STAT5 signaling. We hypothesized that the effect of IL-21 on plasma cell differentiation is derived from its capacity to increase Blimp-1 expression, whereas the increase in the Bcl-6 level may be crucial for the subsequent differentiation of GC-B cells into post-switched cells. In addition, increased Bcl-6 expression may also explain how IL-21 drives the differentiation of B cells into post-switch cells and plasma cells46. Prednisone regulates Bcl-6 expression to inhibit plasma cell differentiation. However, no significant changes were identified in Blimp-1 or Bcl-6 expression in the kidney after treatment. It is presumable that different tissues have different sensitivities to prednisone treatment32.
In conclusion, prednisone, a basic drug that comprises an intermediate-acting glucocorticoid for SLE treatment, plays an important role in the differentiation of B lymphocytes into plasma cells via regulated T subsets, decreased pro-inflammatory cytokine levels and reduced expression levels of Blimp-1 and Bcl-6 in the spleen. These findings explained the therapeutic effect and potential mechanisms of prednisone for autoimmune disease treatment.
Author contribution
Shang-xue YAN and Wei WEI designed research; Shang-xue YAN and Xiao-mei DENG performed research; Qing-tong WANG contributed analytical tools and reagents; Shang-xue YAN analyzed data; Shang-xue YAN and Xiao-mei DENG wrote the paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No 81173075, 81330081, and 81202541) and the Natural Science Foundation of Anhui Province (No KJ2011A177).
References
- 1Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40: 1725. [DOI] [PubMed] [Google Scholar]
- 2Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med 1999; 189: 1639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Bonardelle D, Benihoud K, Kiger N, Bobé P. B lymphocytes mediate Fas-dependent cytotoxicity in MRL/lpr mice. J Leukocyte Biol 2005; 78: 1052–9. [DOI] [PubMed] [Google Scholar]
- 4Yang P, Li B, Lv P, Zhang Y, Gao XM. Interaction between antigen presenting cells and autoreactive T cells derived from BXSB mice with murine lupus. Cell Res 2007; 17: 556–64. [DOI] [PubMed] [Google Scholar]
- 5Chan O, Shlomchik MJ. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J Immunol 1998; 160: 51–9. [PubMed] [Google Scholar]
- 6Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med 1994; 180: 1295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Bourne T, Zukowska-Cooper M, Salaman MR, Seifert MH, Isenberg DA. Spontaneous immunoglobulin-producing capacity of cultures from lupus patients and normal donors following depletion of cells expressing CD19 or CD38. Clin Exp Immun 1998; 111: 611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Lugar PL, Love C, Grammer AC, Dave SS, Lipsky PE. Molecular characterization of circulating plasma cells in patients with active systemic lupus erythematosus. PLoS One 2012; 7: e44362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9DiLillo DJ, Horikawa M, Tedder TF. B-lymphocyte effector functions in health and disease. Immunol Res 2011; 49: 281–92. [DOI] [PubMed] [Google Scholar]
- 10McQueen F. A B cell explanation for autoimmune disease: the forbidden clone returns. Postgrad Med J 2012; 88: 226–33. [DOI] [PubMed] [Google Scholar]
- 11Li J, Pan HF, Cen H, Tian J, Ma Y, Tao JH, et al. Interleukin-21 as a potential therapeutic target for systemic lupus erythematosus. Mol Biol Rep 2011; 38: 4077–81. [DOI] [PubMed] [Google Scholar]
- 12Nakou M, Papadimitraki ED, Fanouriakis A, Bertsias GK, Choulaki C, Goulidaki N, et al. Interleukin-21 is increased in active systemic lupus erythematosus patients and contributes to generation of plasma B cells. Clin Exp Rheumatol 2012; 31: 172–9. [PubMed] [Google Scholar]
- 13Kuchen S, Robbins R, Sims GP, Sheng C, Phillips TM, Lipsky PE, et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol 2007; 179: 5886–96. [DOI] [PubMed] [Google Scholar]
- 14Yoon SO, Zhang X, Berner P, Choi YS. IL-21 and IL-10 have redundant roles but differential capacities at different stages of plasma cell generation from human germinal center B cells. J Leukocyte Biol 2009; 86: 1311–8. [DOI] [PubMed] [Google Scholar]
- 15Gateva V, Sandling JK, Hom G, Taylor KE, Chung SA, Sun X, et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet 2009; 41: 1228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Luo J, Niu X, Liu H, Zhang M, Chen M, Deng S. Up-regulation of transcription factor Blimp-1 in systemic lupus erythematosus. Mol Immunol 2013; 56: 574–82. [DOI] [PubMed] [Google Scholar]
- 17Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity 2003; 19: 607–20. [DOI] [PubMed] [Google Scholar]
- 18Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol 2008; 8: 22–33. [DOI] [PubMed] [Google Scholar]
- 19Ye BH, Cattoretti G, Shen Q, Zhang J, Hawe N, de Waard R, et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type. Nat Genet 1997; 16: 161–70. [DOI] [PubMed] [Google Scholar]
- 20Tunyaplin C, Shaffer AL, Angelin-Duclos CD, Yu X, Staudt LM, Calame KL. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J Immunol 2004; 173: 1158–65. [DOI] [PubMed] [Google Scholar]
- 21Zhang X, Ing S, Fraser A, Chen M, Khan O, Zakem J, et al. Follicular helper T cells: new insights into mechanisms of autoimmune diseases. Ochsner J 2013; 13: 131–9. [PMC free article] [PubMed] [Google Scholar]
- 22Clarke SH. Anti-Sm B cell tolerance and tolerance loss in systemic lupus erythematosus. Immunol Res 2008; 41: 203–16. [DOI] [PubMed] [Google Scholar]
- 23Ding C, Foote S, Jones G. B-cell-targeted therapy for systemic lupus erythematosus. Biodrugs 2008; 22: 239–49. [DOI] [PubMed] [Google Scholar]
- 24Looney RJ. B cell-targeted therapies for systemic lupus erythematosus. Drugs 2010; 70: 529–40. [DOI] [PubMed] [Google Scholar]
- 25Tieng AT, Peeva E. B-cell-directed therapies in systemic lupus erythematosus. Semin Arthritis Rheum 2008; 38: 218–27. [DOI] [PubMed] [Google Scholar]
- 26Barnes NA, Stephenson S, Cocco M, Tooze RM, Doody GM. BLIMP-1 and STAT3 counter-regulate microRNA-21 during plasma cell differentiation. J Immunol 2012; 189: 253–60. [DOI] [PubMed] [Google Scholar]
- 27AlSonbul A, Al-Mayouf SM. Safety and efficacy of combined cyclophosphamide and rituximab treatment in recalcitrant childhood lupus. Rheumatol Int 2014; 34: 529–33. [DOI] [PubMed] [Google Scholar]
- 28Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum 2004; 50: 2580–9. [DOI] [PubMed] [Google Scholar]
- 29Werwitzke S, Trick D, Kamino K, Matthias T, Kniesch K, Schlegelberger B, et al. Inhibition of lupus disease by anti-double-stranded DNA antibodies of the IgM isotype in the (NZB×NZW) F1 mouse. Arthritis Rheum 2005; 52: 3629–38. [DOI] [PubMed] [Google Scholar]
- 30Anand A, Dean GS, Quereshi K, Isenberg DA, Lydyard PM. Characterization of CD3+ CD4−CD8−(double negative) T cells in patients with systemic lupus erythematosus: activation markers. Lupus 2002; 11: 493–500. [DOI] [PubMed] [Google Scholar]
- 31Bobé P, Bonardelle D, Benihoud K, Opolon P, Chelbi-Alix MK. Arsenic trioxide: A promising novel therapeutic agent for lymphoproliferative and autoimmune syndromes in MRL/lpr mice. Blood 2006; 108: 3967–75. [DOI] [PubMed] [Google Scholar]
- 32Jonsson CA, Erlandsson M, Svensson L, Mölne J, Carlsten H. Mycophenolate mofetil ameliorates perivascular T lymphocyte inflammation and reduces the double-negative T cell population in SLE-prone MRLlpr/lpr mice. Cell Immunol 1999; 197: 136–44. [DOI] [PubMed] [Google Scholar]
- 33Mumtaz IM, Hoyer BF, Panne D, Moser K, Winter O, Cheng QY, et al. Bone marrow of NZB/W mice is the major site for plasma cells resistant to dexamethasone and cyclophosphamide: Implications for the treatment of autoimmunity. J Autoimmun 2012; 39: 180–8. [DOI] [PubMed] [Google Scholar]
- 34Murata K, Inami M, Hasegawa A, Kubo S, Kimura M, Yamashita M, et al. CD69-null mice protected from arthritis induced with anti-type II collagen antibodies. Int Immunol 2003; 15: 987–92. [DOI] [PubMed] [Google Scholar]
- 35Radulovic K, Manta C, Rossini V, Holzmann K, Kestler HA, Wegenka UM, et al. CD69 regulates type I IFN-induced tolerogenic signals to mucosal CD4 T cells that attenuate their colitogenic potential. J Immunol 2012; 188: 2001–13. [DOI] [PubMed] [Google Scholar]
- 36Block KE, Huang H. The cellular source and target of IL-21 in K/BxN autoimmune arthritis. J Immunol 2013; 191: 2948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Bubier JA, Sproule TJ, Foreman O, Spolski R, Shaffer DJ, Morse HC, et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci U S A 2009; 106: 1518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Rodríguez-Bayona B, Ramos-Amaya A, López-Blanco R, Campos-Caro A, Brieva JA. STAT-3 activation by differential cytokines is critical for human in vivo–generated plasma cell survival and Ig secretion. J Immunol 2013; 191: 4996–5004. [DOI] [PubMed] [Google Scholar]
- 39Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol 2005; 5: 688–98. [DOI] [PubMed] [Google Scholar]
- 40Ding BB, Bi E, Chen H, Yu JJ, Ye BH. IL-21 and CD40L synergistically promote plasma cell differentiation through upregulation of Blimp-1 in human B cells. J Immunol 2013; 190: 1827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Lee SK, Rigby RJ, Zotos D, Tsai LM, Kawamoto S, Marshall JL, et al. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J Exp Med 2011; 208: 1377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Rasheed MA, Latner DR, Aubert RD, Gourley T, Spolski R, Davis CW, et al. Interleukin-21 is a critical cytokine for the generation of virus-specific long-lived plasma cells. J Virol 2013; 87: 7737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Zhou Z, Li A, Wang Z, Pei F, Xia Q, Liu G, et al. Blimp-1 siRNA inhibits B cell differentiation and prevents the development of lupus in mice. Hum Immunol 2012; 74: 297–301. [DOI] [PubMed] [Google Scholar]
- 44Ma J, Zhu C, Ma B, Tian J, Baidoo SE, Mao C, et al. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clin Dev Immunol 2012; 2012: 827480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Calame KL, Lin KI, Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells. Annu Rev Immunol 2003; 21: 205–30. [DOI] [PubMed] [Google Scholar]
- 46Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol 2004; 173: 5361–71. [DOI] [PubMed] [Google Scholar]
- 47Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 2002; 17: 51–62. [DOI] [PubMed] [Google Scholar]
- 48Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol 2005; 5: 230–42. [DOI] [PubMed] [Google Scholar]
- 49Garaud JC, Schickel JN, Blaison G, Knapp AM, Dembele D, Ruer-Laventie J, et al. B cell signature during inactive systemic lupus is heterogeneous: toward a biological dissection of lupus. PLoS One 2011; 6: e23900. [DOI] [PMC free article] [PubMed] [Google Scholar]