Abstract
Small molecule compounds promoting the neuronal differentiation of stem/progenitor cells are of pivotal importance to regenerative medicine. We carried out a high-content screen to systematically characterize known bioactive compounds, on their effects on the neuronal differentiation and the midbrain dopamine (mDA) neuron specification of neural progenitor cells (NPCs) derived from the ventral mesencephalon of human fetal brain. Among the promoting compounds three major pharmacological classes were identified including the statins, TGF-βRI inhibitors, and GSK-3 inhibitors. The function of each class was also shown to be distinct, either to promote both the neuronal differentiation and mDA neuron specification, or selectively the latter, or promote the former but suppress the latter. We then carried out initial investigation on the possible mechanisms underlying, and demonstrated their applications on NPCs derived from human pluripotent stem cells (PSCs). Our study revealed the potential of several small molecule compounds for use in the directed differentiation of human NPCs. The screening result also provided insight into the signaling network regulating the differentiation of human NPCs.
Stem/progenitor cells hold great promise for the treatment of various neurological diseases1. A key challenge to realize their potential is to identify small molecule compounds effectively inducing the neuronal differentiation and the further functional specification. Numerous studies have been carried out on the directed neuronal differentiation of stem/progenitor cells. Prevailing protocols are mostly designed based on the principles learned from developmental biology2, by mimicking the appearance order of morphogens to recapitulate the developmental process and achieve the lineage specification3,4,5. However, the complexity of mammalian embryo development has prevented the faithful in vitro recapitulation, resulting in low neuronal yield with the undesired cell types representing a clinical concern such as the formation of tumors6. Techniques to further improve the neuronal differentiation efficiency remain to be developed. Moreover, current methods typically rely on the use of human recombinant proteins which makes the cost prohibitively expensive and impractical for routine use. The identification of small molecule compounds that can improve the differentiation efficiency, and reduce the use of recombinant proteins thus is highly desired for the clinical application of stem/progenitor cells.
In order to identify efficient and cost-effective compounds, as well as gain insight into the possible mechanism underlying, we carried out a chemical genetic screen7,8 to systematically characterize a comprehensive library of known bioactive small molecules, for their effects on the neuronal differentiation as well as mDA neuron specification from human ventral mesencephalon derived NPCs. Our study revealed major pharmacological classes effectively increase the rates of neuronal and/or mDA neuronal differentiation of human NPCs, and provided insight into the signaling network governing the differentiation of NPCs.
Results
Bioactive compounds promoting the neuronal differentiation from human NPCs
There are thousands of small molecule compounds which are known to be bioactive and have been previously used in clinic or research. We first collected more than 5,000 such compounds (Materials and Methods) to systematically characterize their effects on human NPCs by high-content screen9 following the procedure illustrated in Supplementary Fig. 1. In the experiment we used the human NPC line ReNCell VM, which was derived from the ventral mesencephalon of human fetal brain10. The origin of the cells enabled us to carry out the chemical screens on both the neuronal differentiation and the further mDA neuron specification of human NPCs, which occurs spontaneously in ReNCell VM cells upon the removal of mitogens bFGF and EGF (Supplementary Fig. 2). The spontaneous differentiation occurs at a low but consistent rate10, providing an ideal model for assessing the compound effect.
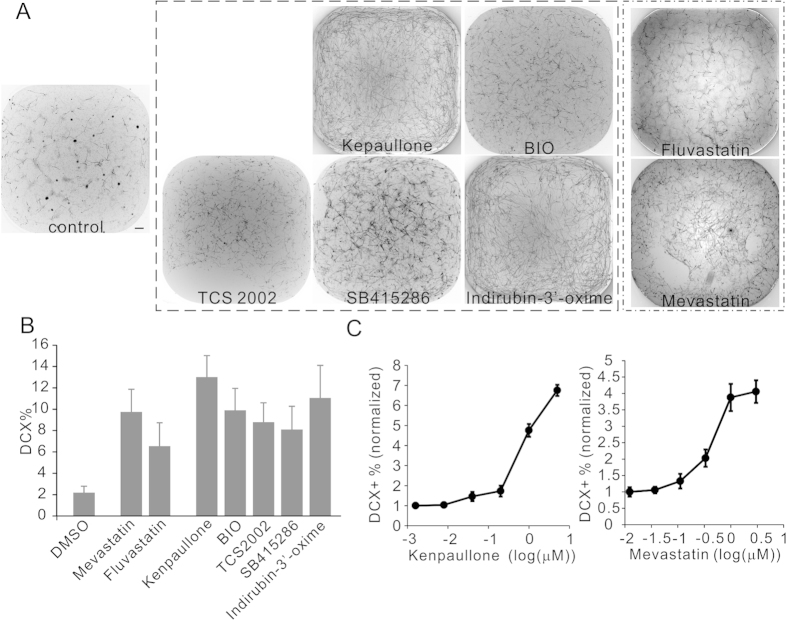
To start the neuronal differentiation screen undifferentiated ReNCell VM cells were seeded in laminin coated 384-well plates at a density of 1.8 × 104 cells/cm2. Twenty-four hours later after the cells attached the medium was changed to remove the mitogens. Then to each well an individual compound from our collected library of bioactive compounds was added to a final concentration of 2 μM. Cells were differentiated in the presence of compound for 2 weeks with medium changed twice a week, before they were processed for immunoreactivity against Doublecortin (DCX), a widely used biomarker for early neuronal precursors to indicate the level of neuronal linage specification11. Each compound was tested 2 to 4 times and the result mean was used to select the primary hits. In this way over 5,000 compounds were scored on its promoting (positive scores from 1 to 3) or suppressing (negative scores from −1 to −3) effect on the neuronal differentiation from human NPCs based on the percentage of DCX+ cells generated in the differentiation (Supplementary Table 1). Further testing of the promoting compounds confirmed the effects of 21 compounds (Table 1), among which major effects were detected on two pharmacological classes, the GSK-3 inhibitors and the statins. For each class multiple chemicals were identified to significantly promote the percentage of DCX+ cells by more than 3 folds (Fig. 1A,B). Of these compounds kenpaullone and mevastatin stood out to be the most effective and therefore were used in the following up experiments (Fig. 1B). Titration experiment showed that they function in a dose-response manner with EC50 around 1 μM (Fig. 1C), suggesting their effects to be via specific pharmacological activities.
Table 1. List of compounds promoting the neuronal differentiation of ReNCell VM cells.
| Compound name | Compound description |
|---|---|
| 4F 4PP oxalate | selective 5-HT2A antagonist |
| BIO | ATP-competitive GSK-3 inhibitor. |
| BWB70C | selective inhibitor of 5-lipoxygenase |
| Canrenone | aldosterone antagonist |
| DMPO | neuroprotective agent, radical spin trap |
| Ezetimibe | Inhibit cholesterol absorption in the small intestine |
| Flunixin meglumine | non-steroidal anti-inflammatory |
| Fluvastatin | HMG-CoA reductase inhibitor |
| Indirubin-3′-oxime | GSK-3 inhibitor; also inhibits other protein kinases |
| Isoproterenol hydrocloride | selective β-adrenoceptor agonist |
| Kenpaullone | GSK-3 inhibitor; CDK inhibitor |
| Mevastatin | HMG-CoA reductase inhibitor |
| n-methyl-metacryloyl-lupinine | quinolizidine alkaloid extracted from plant |
| Phenoxybenzamine hydrochloride | selective α-adrenoceptor blocking agent; calmodulin antagonist |
| SB 203580 | selective inhibitor of p38 MAPK |
| SB 239063 | potent, selective p38 MAPK inhibitor; orally active |
| SB 415286 | competitive GSK-3 inhibitor. |
| Sotalol hydrochloride | β-adrenoceptor agonist |
| Spermine tetrahydrochloride | modulatory on the NMDA glutamate receptor |
| TCS 2002 | GSK-3 inhibitor |
| Tolazoline hydrochloride | non-selective competitive α-adrenoceptor antagonist |
Figure 1. Major pharmacological classes promoting the neuronal differentiation of human NPCs.
(A) The primary screen results of 5 GSK-3 inhibitors (dash line boxed) and 2 statin molecules (dash and dot line boxed), shown are the whole well images of 384-well plate stained with DCX antibody. (B) Quantification of the percentages of DCX+ cells (DCX+%) as the results of effective compounds treatment. The DCX+ % was calculated as the number of DCX+ cells divided by the number of total cells. Compounds were tested at 2 μM concentration. Controls received equal volume of DMSO treatment. (C) Dose responsive curves of the example compounds from the two pharmacological classes. Scale bar, 200 μm. The differentiation rates were normalized to the DCX+% in the DMSO control wells.
Bioactive compounds promoting the mDA neuron specification from human NPCs
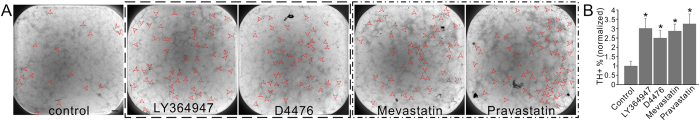
Similarly, high-content screen was carried out to test the compounds’ effects on promoting the mDA neuron specification of ReNCell VM cells, which is potentially valuable for the study of Parkinson’s disease12. Cells were grown in laminin coated 384-well plates at an initial density of 1.8 × 104 cells/cm2, and differentiated in the presence of 2 μM compound for 4 weeks, then processed for immunoreactivity against tyrosine hydroxylase (TH), the most commonly used biomarker for mDA neurons13. Again each compound was tested 2 to 4 times and the result mean was used to select the primary hits. Over 5,000 compounds were scored based on their effect on the percentage of TH+ cells generated in the differentiation (Supplementary Table 2). As the spontaneous differentiation rate is low the compounds were only scored for their promoting effects (positive scores from 1 to 3). Re-test of the primary hits in 96-well plate format confirmed the effects of 30 compounds (Table 2), among which major effects were detected on two pharmacological classes, the TGF-βRI inhibitors and the statins. For each two chemicals were identified to significantly promote the number of TH+ cells generated from differentiation by more than 2 folds (Fig. 2A,B, high quality images are provided in Supplementary Fig. 3). Compounds LY364947 and mevastatin stood out to be the most effect of each pharmacological classes and were used in further experiments.
Table 2. List of compounds promoting the dopaminergic neuronal differentiation of ReNCell VM cells.
| Compound name | Compound description |
|---|---|
| 1-Naphthyl PP1 | Src family kinase inhibitor; also inhibits c-Abl |
| 7-NINA | non-selective NOS inhibitor |
| AG 490 | EGFR-kinase inhibitor; JAK2/3 inhibitor |
| Bifonazole | inhibit ergosterol synthesis; inhibit HMG-CoA |
| Cilostamide | selective inhibitor of PDE III |
| Clobenpropit dihydrobromide | highly potent H3 antagonist and H4 partial agonist |
| D 4476 | CK1 inhibitor; TGF-βRI inhibitor |
| DAPT | γ-secretase inhibitor |
| HA14-1 | Bcl-2 inhibitor |
| ICI 118,551 hydrochloride | highly selective β2-adrenoceptor antagonist |
| ICI-185,282 | potent thromboxane receptor antagonist |
| Kynurenic acid | broad spectrum EAA antagonist |
| LY 364947 | selective inhibitor of TGF-βRI |
| Mevastatin | HMG-CoA reductase inhibitor |
| MK-912 | selective α2A-adrenoreceptor agonist |
| Naltrindole hydrochloride | non-peptide δ opioid antagonist |
| Nefazodone HCl | 5-HT2, 5-HT1A receptor antagonist |
| ODQ | selective inhibitor of NO-sensitive guanylyl cyclase |
| Pranoprofen | non-steroidal anti-inflammatory |
| Pravastatin | inhibit HMG-CoA reductase |
| Prochlorperazine edisylate | D2 receptor antagonist |
| Purpurin | xanthin oxidase inhibitor |
| R(–)-Isoproterenol (+)-bitartrate | selective β-adrenoceptor agonist |
| R(+)-Terguride | dopamine receptor agonist. |
| SLV 320 | potent and selective A1 antagonist |
| S-Sulfo-L-cysteine sodium salt | EAA receptor agonist |
| SU 5416 | potent and selective VEGFR PTK inhibitor |
| Tyrphostin AG 112 | protein tyrosine kinase inhibitor |
| Tyrphostin AG 494 | protein tyrosine kinase inhibitor |
| Tyrphostin B44 | EGFR-kinase inhibitor |
Figure 2. Major pharmacological classes promoting the dopaminergic neuronal differentiation of human NPCs.
(A) The primary screen results of 2 TGF-βRI inhibitors (dash line boxed) and 2 statin molecules (dash and dot line boxed), shown are the whole well images of 384-well plate stained with TH antibody. The positive stained cells were pointed with red arrow heads for clarity as the image sizes are greatly reduced from the original. (B) Quantification of the cell differentiation rate in the validation experiments. The TH+ % was calculated as the number of TH+ cells divided by the number of total cells. The rate in the control wells treated with DMSO was normalized to 1. The compound concentrations are: LY364947 3 μM; D4476 2 μM; mevastatin 1 μM; pravastatin 1 μM. Controls received equal volume of DMSO treatment. *P < 0.05. Scale bar, 200 μm.
Mechanisms of the major effective compound classes
In summary through the bioactive compound high-content screen approach we identified three major pharmacological classes, the GSK-3 inhibitors, statins and TGF-βRI inhibitors, to cause the significant enrichment of neuronal and/or mDA neuronal populations in ReNCell VM cell differentiation. The enrichment can possibly be attributed to the compounds’ promoting effects on signaling pathways driving the neuronal differentiation, or the proliferation/survival selection on the neuronal and non-neuronal cell types. To distinguish the mechanism experiments were carried out to test their effects on cell proliferation, apoptosis and lineage specification.
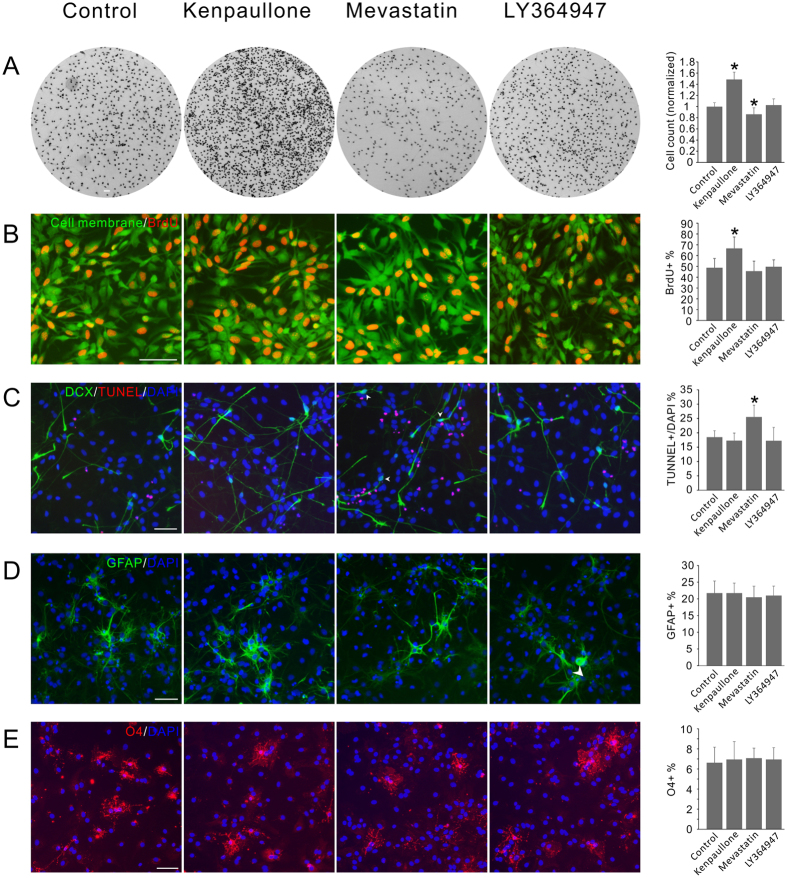
During the screen we consistently observed changes of total cell numbers in the GSK-3 inhibitor and statin treated wells. To confirm the observation we seeded equal numbers of ReNCell VM cells in 96-well plates and incubated with individual compound selected from the three pharmacological classes, and counted the total cell numbers after differentiation for two weeks. While the TGF-βRI inhibitor LY364947 had no effect, the GSK-3 inhibitor kenpaullone significant increased the total cell number and the statin compound mevastatin decreased it (Fig. 3A). To distinguish whether the effects were achieved via changes in the number of proliferative cells or cell survival rate, we then carried out BrdU incorporation experiment. The result (Fig. 3B) indicated that kenpaullone treatment led to significantly increased number of proliferative cells, while mevastatin and LY364947 had no effect. As kenpaullone treatment caused the total cell number to increase by >30% (Fig. 3A), the increased population cannot be exclusively the neuronal cells which only constitute <15% of the total cells. Therefore we reasoned that the pro-proliferation effect of kenpaullone is not selectively on the neuronal population, and the possibility of kenpaullone to directly promote the neuronal differentiation pathways cannot be excluded.
Figure 3. Effects of the Compounds on the cell proliferation, survival and glial differentiation.
(A) Total cell count of the ReNCell VM cells after the treatment of various compounds. Kenpaullone significantly increased the cell number while mevastatin decreased it. (B) BrdU incorporation assay of the compound treated ReNCell VM cells. Kenpaullone significantly increased the number of proliferative cells while the other compounds had no effect. (C) Cell survival assay of the differentiated ReNCell VM cells treated with various compounds. Mevastatin significantly increase the number of apoptosis cells. Notably, apoptosis only occurred in undifferentiated cells, while not detected in the DCX+ neuronal cells (arrow head pointed). (D) Compound effect on the astrocyte differentiation of primary rat NPCs. None of the compounds significantly affected the differentiation rate. (E) Compound effect on the oligodendrocyte differentiation of primary rat NPCs. None of the compounds significantly affected the differentiation rate. In all the experiment the compound concentrations are: kenpaullone 3 μM; mevastatin 1 μM; LY364947 3 μM. Controls received equal volume of DMSO treatment. *P < 0.05. Scale bars, 50 μm.
Experiments were also carried out for cell survival measurement using the TUNEL assay, and the result showed that mevastatin elevated apoptosis rate while the other two compounds had no effect (Fig. 3C). Notably, apoptosis was exclusively detected in the non-neuronal cells, therefore the increased apoptosis level would contribute to the increase of the neuronal cell rate and at least partially accounted for the effect of mevastatin.
NPCs are tripotent cells which can differentiate into neurons, astrocytes and oligodendrocytes. In order to test whether the differentiation inducing capability of the compounds are selective to the neuronal lineage, experiments were carried to determine whether other lineages of cell differentiation were also altered by the compound treatment, using primary cultured NPCs isolated from the fetal rat ventral mesencephalon. The result showed that neither the astrocyte nor the oligodendrocyte differentiation was changed by the three compounds. Cells retained the differentiation capabilities and the glial cell differentiation rates remained the same as untreated cells (Fig. 3D,E). Taking together, our result indicated that the GSK-3 inhibitor increased both the expansion of total cells and the number of differentiated neurons, suggesting that the enrichment of neuronal cells is likely the result of increased neuronal differentiation rate, and a possible role of GSK-3 pathway in controlling the neuronal lineage specification. No effect on cell proliferation or survival was detected for the TGF-βRI inhibitors, so that their effect on mDA neuron enrichment is also a possible result of increased directed differentiation. While an effect on the cell differentiation capability cannot be excluded for the statins, at least part of their effect may be achieved through the selective survival of neuronal cells.
Next we tested how the known signaling pathways controlling NPC differentiation were affected by the compounds. Previously GSK-3 inhibitors14,15 and a different statin molecule16 have been reported to promote the neurogenesis of rodent NPCs. In these studies the enhancing of Wnt/β-catenin signaling was shown to account for the compound effects. We carried out western blot experiment and showed that the effect on Wnt/β-catenin signaling was replicated in human NPCs, with increased intact β-catenin level as a result of kenpaullone and mevastatin treatment (Fig. 4A). Moreover, the western blot result (Fig. 4B) also indicated an increase of Mash117 expression by kenpaullone treatment, confirming a direct role of the GSK-3 inhibitors in promoting the neurogenesis pathway. Mash1 increase was not detected in mevastatin treated cells. However, treatment of mevastatin resulted in a significant increase of the expression of LMX1a, the intrinsic determinant of mDA neurons18. A weak LMX1a increase was also detected as a result of LY364947 treatment, consistent with their promoting effects on the mDA neuron specification of NPCs (Fig. 4C), suggesting the compounds’ effect to be achieved through direct activation of the differentiation pathway. LMX1a overexpression was not detected in the kenpaullone treated cells, consistent with the specific function on the neuronal differentiation but not the mDA neuron specification for the GSK-3 inhibitors (Fig. 4C). Recently TGF-β inhibition was shown to promote Wnt signaling, through the repression of the secreted frizzled related protein 1 (Sfrp1)19 which is a Wnt antagonist20. When this pathway was tested the result (Fig. 4D) indicated that this mechanism was also employed in the cultured ReNCell VM cells, leading to increased Wnt1 expression as a result of LY364947 treatment, to possibly account for the promotion of mDA neuron differentiation from NPCs.
Figure 4. Compound effects on the signaling pathways controlling the NPC differentiation.
(A) The effects of kenpaullone and mevastatin on Wnt/β-catenin signaling. Both compounds increase the amount of intact β-catenin. (B) The effects of kenpaullone and mevastatin on Mash1 expression. Kenpaullone increased Mash1 but not mevastatin. (C) The effects of kenpaullone, LY364947 and mevastatin on the Lmx1a expression. Increases of Lmx1a were detected for LY364947 and mevastatin. (D) The effect of LY364947 on Wnt signaling. LY364947 suppressed Sfrp1 and promoted Wnt1 expression. Full length blots are included in the Supplementary Information.
Functional differences of the major effective compounds and their combinatorial effects
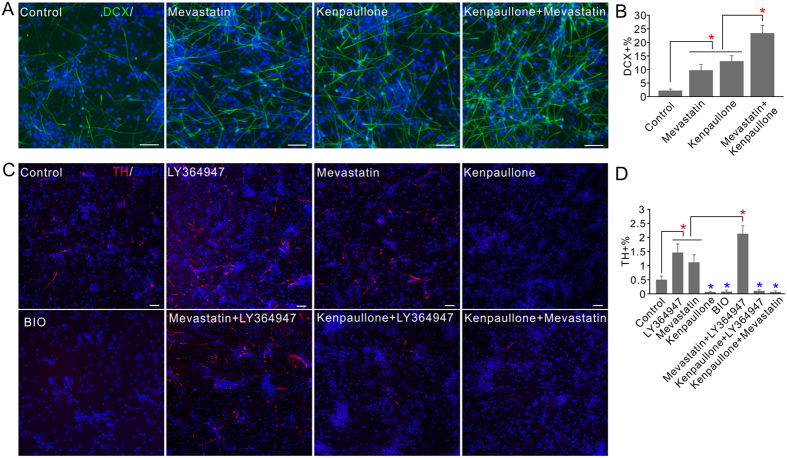
The western blot results indicated that there might be common mediators, e.g., β-catenin and LMX1a, for the promoting effects of the different bioactive compounds identified in our screen. However, the actions of different compound classes on NPCs are also distinct as indicated by their differential effects on the neuronal differentiation and mDA neuron specification, as well as on the cell proliferation and survival. Therefore through complementary mechanisms, their effects may be additive or synergistic. In both screens two major pharmacological classes were identified, we thus examined whether higher efficiency could be achieved through the combinations of them. Significant further enhancement was observed when the two neuronal differentiation promoting compounds kenpaullone and mevastatin were applied together (Fig. 5A,B). When the two dopaminergic differentiation promoting compounds, LY364947 and mevastatin were used in combination, significant enhancement were also observed (Fig. 5C,D).
Figure 5. The effects of compound combinations on human NPC differentiation.
(A) The effects of compound combinations on the neuronal differentiation of ReNCell VM cells. Cells were treated with 0.5 μm mevastatin; 3 μm kenpaullone; combination; or DMSO control during differentiation. Neurons were stained with DCX antibody. (B) Quantification of the percentage of DCX+ cells with or without treatment. (C) The effects of compound combinations on the dopaminergic neuronal differentiation of ReNCell VM cells. Cells were treated with 3 μM LY364947, 1 μM mevastatin, 3 μM kenpaullone, 1 μM BIO or the combinations of two compounds. Cells were stained with TH antibody. (D) Quantification of the percentage of TH+ cells after the treatments in (C). In all the experiment control received equal volumes of DMSO treatment. *P < 0.05 (red, significantly increased; blue, significantly decreased). Scale bars, 50 μm.
The GSK-3 inhibitors were identified in the neuronal differentiation screen but not the mDA neuron specification screen, and increase in LMX1a expression was not detected in the GSK-3 inhibitor treated cells (Fig. 4C). We carried out experiments to examine their effect on the dopaminergic neuron differentiation, and the effect when used in combination with other promoting chemicals. Surprisingly, kenpaullone treatment led to the significant inhibition of the generation of TH+ dopaminergic neurons. Similar effect was also detected for a structurally different GSK-3 inhibitor BIO (Fig. 5C,D). Not only that, using of kenpaullone together with dopaminergic promoting compounds completely abolished their enhancing effects (Fig. 5C,D).
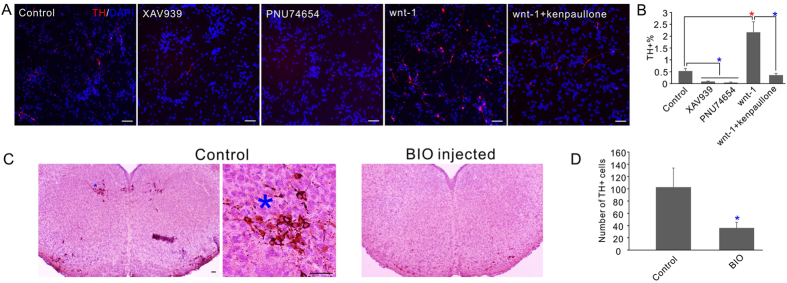
Since GSK-3 inhibition activates Wnt/β-catenin signaling (Fig. 4A), the above result is seemingly contradictory to the known role of Wnt signaling on the mDA neuron development21,22,23. In order to confirm the eligibility of our in vitro assay and clarify the role of GSK-3 inhibition and Wnt signaling, we first directly tested the effect of Wnt-1 and showed that the recombinant protein significantly enhanced the number of TH+ cell differentiated from ReNCell VM cells (Fig. 6A,B). Consistently, treatment of the cells with Wnt inhibitors XAV939 and PNU74654 almost completely eliminated the TH+ cells (Fig. 6A,B), confirming the enhancing effect of Wnt on the development of dopaminergic neurons and the eligibility of our in vitro assay system. Interestingly, when the Wnt-1 recombinant protein is used together with kenpaullone, its enhancing effect was significantly inhibited (Fig. 6A,B). Moreover, injection of a brain permeable GSK-3 inhibitor BIO which also inhibited mDA neuron differentiation in vitro (Fig. 5C,D) significantly decreased the number of TH neurons in vivo in the developing mouse midbrain (Fig. 6C,D). Together these results confirmed the inhibitory effect of GSK-3 inhibitors on mDA neuron specification and suggested that the effect was achieved through pathways that are not related to Wnt signaling. In summary our results showed that the initial neuronal lineage differentiation and the later mDA neuron specification are individually regulated, and the same protein machinery may be utilized in these processes with distinct roles. These results also suggested that the three major compound classes identified in the screen act on NPCs differently, that may be selectively used individually or in combination to regulate the neuronal differentiation and mDA neuronal specification of human NPCs.
Figure 6. The effects of Wnt and GSK-3 inhibitor on mDA neuron differentiation.
(A) The effects of wnt signaling on the dopaminergic neuronal differentiation of ReNCell VM cells. Cells were treated with 1 μM XAV939, 2 μM PNU74654, 20 ng/ml human recombinant Wnt-1 protein, 3 μm kenpaullone or the indicated combination. Cells were stained with TH antibody. (B) Quantification of the percentage of TH+ cells after the treatments in A. In all the experiment control received equal volumes of DMSO treatment. (C) Injection of cell permeable GSK-3 inhibitor BIO significantly inhibited the development of TH+ dopamine neuron in the developing mouse midbrain. Left, an example slice of the control animals; middle, high resolution image of the *region in left image to confirm the TH+ neuron morphology; right, an example slice of the BIO injected animals. (D) Quantification of the number of TH+ cells in the midbrain slices. Numbers shown are the averages of 9 slices from 3 different animals. Scale bars, 50 μm. *P < 0.05 (red, significantly increased; blue, significantly decreased).
Compound effects on human PSCs
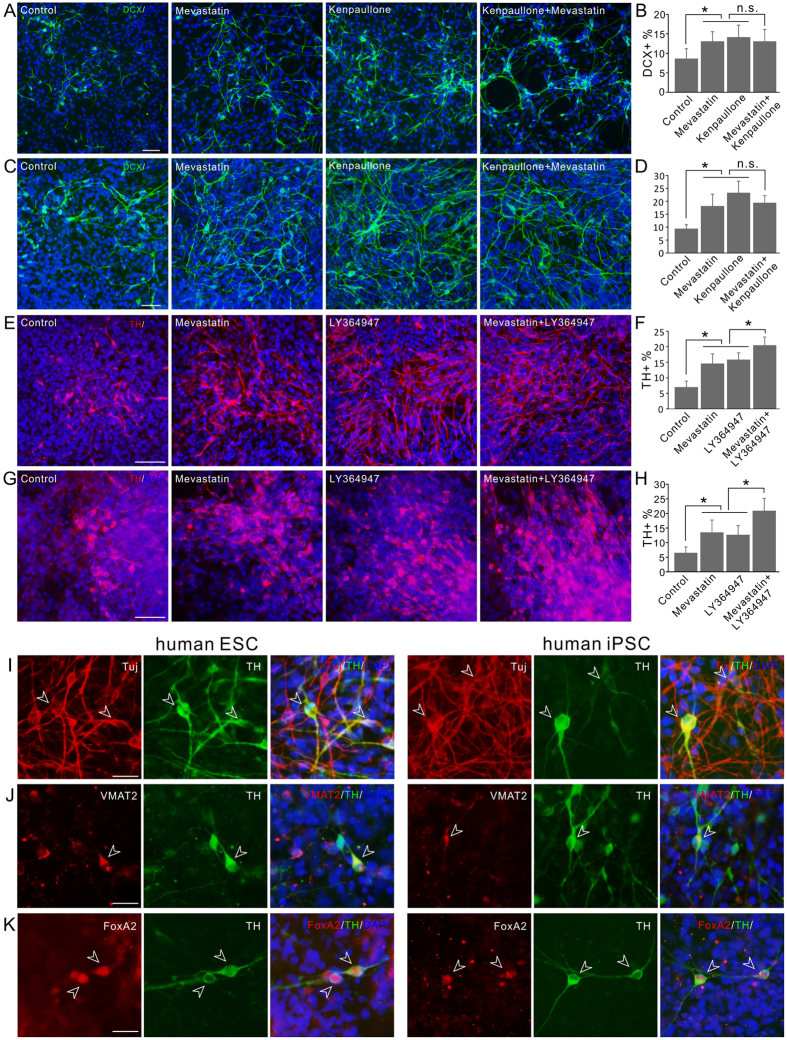
Human PSCs, including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), hold great promise as unlimited transplantation source material for treating neurological disorders24. To determine whether the bioactive compounds we identified can be applied to direct the differentiation of human PSCs, we tested their effects on the neuronal and mDA neuronal differentiation of human ESCs and iPSCs. Although to a less extent compared to the ReNCell VM cells, both kenpaullone and mevastatin were shown to significantly increase the neuronal differentiation of the NPCs derived from both human ESCs and iPSCs. (Fig. 7A–D). However, the combination of the two compounds did not lead to further increase of the neuronal differentiation rates (Fig. 7A–D), possibly due to the significantly increased toxicity especially affecting the differentiated DCX+ cells (Supplementary Fig. 4), which was not observed in the ReNCell VM cells. When the human PSCs were induced to differentiate, dopaminergic neurons were generated upon the patterning with FGF-8 and SHH. Both mevastatin and LY364947 were able to significantly increase the dopaminergic differentiation level, and their combination led to further increase of the number of TH+ dopaminergic neurons (Fig. 7E–H). The compound combination induced TH+ cells from PSCs exhibited typical neuronal cell morphology and were confirmed to express the classical neuronal marker βIII-tubulin (Tuj) (Fig. 7I). Many of the TH+ cells also expressed VMAT2, a critical transporter for the dopamine function of the monoamine neurons (Fig. 7J). Dopamine neurons can originate from several brain areas, of particular interest are the mDA neurons for their role in Parkinson’s Disease. FGF-8 treatment is able to direct the PSC differentiation to midbrain cells25, our result showed that mevastatin and LY364947 treatment did not interfere with the patterning and the resulting TH+ cells retained the expression of midbrain floor plate marker FoxA213 (Fig. 7K). Together these results highlighted the potential of the compounds for the directed differentiation of human PSCs.
Figure 7. The effect of compounds on human PSC differentiation.
(A) Images of DCX+ neuronal cells differentiated from human ESCs. Cells were treated with 0.5 μm mevastatin; 3 μm kenpaullone; combination; or DMSO control during differentiation. (B) Quantification of the percentage of human ESC differentiated DCX+ neuronal cells with or without treatment. (C) Images of DCX+ neuronal cells differentiated from human iPSCs. Cells were treated with 0.5 μM mevastatin; 3 μM kenpaullone; combination; or DMSO control during differentiation. (D) Quantification of the percentage of human iPSC differentiated DCX+ neuronal cells with or without treatment. (E) Images of TH+ dopaminergic neuronal cells differentiated from human ESCs. Cells were treated with 0.5 μm mevastatin; 3 μm LY364947; combination; or DMSO control during differentiation. (F) Quantification of the percentage of human ESC differentiated TH+ neuronal cells with or without treatment. (G) Images of TH+ dopaminergic neuronal cells differentiated from human iPSCs. Cells were treated with 0.5 μM mevastatin; 3 μM LY364947; combination; or DMSO control during differentiation. (H) Quantification of the percentage of human iPSC differentiated TH+ neuronal cells with or without treatment. (I–K) Characterization of the TH+ cells differentiated from mevastatin LY364947 combination treated human PSCs (left panels, human ESCs; right panels, human iPSCs). All the TH+ cells expressed neuronal marker Tuj (I), most of them also coexpressed VMAT2 (J). All the TH+ cells also expressed midbrain floor plate marker FoxA2 (K). Example cells were pointed with arrow heads. *P < 0.05. Scale bars, 50 μm in (A–G); 20 μm in (I–K).
Discussion
Previously studies have been carried out for identifying compounds inducing the neuronal differentiation from rodent SCs14,26,27, but the screening on NPCs derived from human brain tissue has not been reported. Here we reported a chemical screen on the NPCs originated from the ventral mesencephalon of human brain. It is noteworthy that our screen was carried out using small molecule compounds with known pharmacological activities, many of which are clinical used as drugs in USA or worldwide28, or widely used in biological research. Therefore the compounds identified in this study may help to improve the current protocol for the directed differentiation of human NPCs, to achieve the safe and cost-effective production of neuronal cells.
In both neuronal differentiation and mDA neuron specification screens statins were identified to be effective. Since consistent result was observed for several different statin members, including mevastatin, fluvastatin and pravastatin, the promoting effect is highly likely to be associated with the specific bioactivity of statins instead of an individual compound off-target. Statins are best known as HMG-CoA reductase inhibitors which inhibit the cholesterogenesis29. However, cholesterol inhibition is unlikely to directly promote the neuronal differentiation pathway, as cholesterol has been shown to be essential for the normal function of NPCs30. Cholesterol depletion caused massive defects in neural tube development31. Cholesterol was also known to be critical for the function of key NPC biomarker proteins such like prominin-1 (CD133), which directly interact with membrane cholesterol to form the microdomains in the apical plasma membrane32. Statins may have multiple pharmacological activities. Besides cholesterol control, statins were also known to improve the outcome following traumatic brain injury and stroke33,34 but the mechanism remains unclear. A recent study on simvastatin has associated this clinical effect with NPCs, and showed that simvastatin regulated isoprenoid synthesis, but not cholesterol, to enhance the Wnt signaling and adult neurogenesis16. The currently reported effect of statins on the neuronal differentiation of fetal NPCs is likely associated with the same pathway. And the result is consistent with our finding that GSK-3 inhibitors, which also enhance Wnt signaling by preventing β-catenin degradation, significantly promoted neurogenesis and were identified in the screen. However, statins (at least mevastatin) may also have additional biochemical function which further increases the neuronal cell rate when used in combination with the GSK-3 inhibitor kenpaullone. One likely explanation is that mevastatin contributed to reduce the number of undifferentiated cells as shown in Fig. 3C, possibly through cholesterol ablation. The result that statins also promoted the mDA neuron specification from NPCs was not reported before. The effect can be reasonably explained by its promotion on Wnt signaling.
Of the statin molecules identified in our screening, mevastatin showed the outstanding effects in both promoting the neuronal lineage differentiation and mDA neuron specification, on both the NPCs from human ventral mesencephalon and human PSCs. Therefore it may have potential in the directed differentiation of NPCs for the study of Parkinson’s Disease. In addition, differentiation inducing compounds may also find its application in cancer therapy, by targeting the brain tumors that are initiated by cancer stem cells35. Mevastatin is particularly of interest as it simultaneously induce the differentiation of stem cells and the apoptosis of undifferentiated cells, thereby help to eliminate brain tumor cancer stem cells. Our result also provided a possible explanation why long-term prediagnostic statin use may improve the survival following glioblastoma36.
The discovery of GSK-3 inhibitors to promote the neurogenesis is consistent with literature reports14,37. Recently kenpaullone was also identified through a compound screen to prolong the survival of stem cell derived motor neurons38. The effect of kenpaullone on motor neurons is not likely associated with its GSK-3 inhibition activity as other GSK-3 inhibitors failed to promote motor neuron survival. Instead, the inhibition of a different kinase, HPK1/GCK-like kinase (HGK), was suggested to be responsible for its effect motor neurons. In our study, at least five different GSK-3 inhibitors have been shown to enhance the neuronal differentiation efficiency. Therefore the effect is highly likely to be specifically through the GSK-3 inhibition, which then promote the Wnt signaling to enhance the neurogenesis.
Two findings in this study were surprising and seemingly contradictory to existing evidences. The first is the result that GSK-3 inhibitors promoted DCX+ neuronal differentiation but inhibited TH+ mDA neuron specification, as GSK-3 inhibition enhances Wnt signaling which is known to play a critical role in the neurogenesis in the midbrain floor plate22,23. Although the mechanism remains unclear, our result indicated that the effect was achieved through Wnt independent pathway(s). These results clarified the function of GSK-3 inhibition in mDA neuron development and added to the complexity of the role of GSK-3 in brain cell fate determination39. The other surprising result is the identification of TGF-βRI inhibitors to promote the dopaminergic neuronal differentiation, as TGF-β has been shown to be essential for the development of mDA neuron in vitro and in vivo40. In recent years, dual SMAD inhibition (TGF- β inhibitor combined with BMP inhibitor) protocol has been increasingly used to generate mDA neurons from human PSCs41. While the inhibitors was originally thought to enhance the generation of neural progenitors by inhibiting mesenchymal differentiation, a recent study revealed that TGF-β inhibition might up-regulate the SMAD-interacting protein 1 (S1P1), resulting in greater repression of Sfrp1 and the rise of Wnt1-Lmx1a levels, thereby directly promote mDA neuron differentiation19. Our result is consistent with this study, and provided more direct evidence that TGF- β inhibitor may promote the dopamine neuron differentiation from the embryonic midbrain NPCs.
Methods
NPC culture and differentiation
Human NPC line ReNCell VM was purchased from Millipore (Millipore, Billerica, MA, USA), which were derived from the ventral mesencephalon of human fetal brain and immortalized with the myc oncogene10. Primary NPCs were cultured from the ventral mesencephalon from the E14 rat embryos42. Tissues were dissected and dissociated with accutase to single cells, and grown in suspension to form neurospheres first. The spheres were then digested with Accutase (StemCell Technologies, Vancouver, Canada) to single cells and grown adherently as monolayer on laminin (Life Technologies, Grand Island, NY, USA) coated surface. The culture medium consists of knockout DMEM/F12 and StemPro neural supplement (Life Technologies, Grand Island, NY, USA) with 10 ng/ml bFGF and 10 ng/ml EGF (StemCell Technologies, Vancouver, Canada)43,44. To start the neuronal or glial differentiation, the cells were gradually switched to the differentiation medium consisting of Neurobasal and B27 supplement without bFGF or EGF, by changing half medium every two days for two weeks.
Human PSC culture and differentiation
The H9 (WA09) human ESC derived NPCs was obtained from Life Technologies (Life Technologies, Grand Island, NY, USA)45. The human iPSC was purchased from ATCC (ATCC, Manassas, VA, USA). The iPSCs were cultured in feeder-free condition in Essential 8 medium (Life Technologies, Grand Island, NY, USA) with bFGF. Neural induction was started by culturing the cells in STEMdiff neural induction medium (StemCell Technologies, Vancouver, Canada) on Matrigel coated surface for 9 days. Cells were passaged in the same culture condition twice and then the derived NPCs were maintained in STEMdiff neural progenitor medium (StemCell Technologies, Vancouver, Canada) before they were used for the neuronal differentiation experiment. The neuronal differentiation was done following the same procedure as described above for the ReNCell VM and primary rat NPCs, in the absence or presence of indicated compounds.
The dopaminergic neuron differentiation was carried out following a procedure modified from published protocol46. Briefly, human PSC derived NPCs were first dissociated and grown in suspension to form neurospheres. The neurospheres were attached on laminin coated surface in neural differentiation medium consisting of DMEM/F12 and B27 (Life Technologies, Grand Island, NY, USA), and patterned with 50 ng/ml FGF-8 (Peprotech, Rocky Hill, NJ, USA) for 1 week followed by 100 ng/ml SHH (Peprotech, Rocky Hill, NJ, USA) and 50 ng/ml FGF-8 for 1 week, then SHH and FGF-8 were withdrawn to continue the dopamine neuron differentiation for another week. Cells were treated with indicated compounds starting from the patterning.
Chemical Libraries
The compounds libraries used for this study included: the LOPAC library consisting of 1,280 pharmacologically active compounds (LOPAC, Sigma-Aldrich, St Louis, MO, USA); the Tocriscreen Mini library consisting of 1,120 biologically active compounds (Tocriscreen, Tocris Bioscience, Bristol, UK); the Spectrum Collection consisting of 2,320 compounds (Spectrum, MicroSource, Gaylordsville, CT, USA); the Prestwick Chemical library consisting of 1,200 compounds (Prestwick, Prestwick Chemical, Parc dinnovation, France); the Prestwick Phytochemicals Library consisting of 320 phytochemical compounds (Phytochemical, Prestwick Chemical, Parc dinnovation, France); the Prestwick Natural Compounds Library consisting of 240 natural compounds (Natural, Prestwick Chemical, Parc dinnovation, France).
Compound screening
Undifferentiated ReNCell VM cells were seeded at a density of 1.8 × 104 cells/cm2 in 384-well plate pre-coated with laminin. After 24 hours the medium were changed to remove the mitogens and each well was treated with a different compound at 2 μM concentration. Half of the medium was refreshed every two days with the compound level maintained at 2 μM. For neuronal differentiation screening cells were fixed after 2 weeks, and stained DCX antibody to determine the neurogenesis level. For dopaminergic neuronal differentiation screening, the medium were also supplemented with 10 ng/ml BDNF and 10 ng/ml GDNF, and the plates were fixed after 4 weeks of differentiation and stained with TH antibody to count the number of dopaminergic neurons. In both screenings, each compound was tested 2 to 4 times and the result averages were used to select the primary hits.
Primary screening hits selection
Screening images were first subjected to a double blind manual analysis, in which each image was scored by an independent analyzer as 0 (absent of effect as untreated); or positive scores of 1 (minor enhancing effect), 2 (medium enhancing effect) or 3 (major enhancing effect); or negative scores of −1 (minor inhibiting effect), −2 (medium inhibiting effect) or –3 (major inhibiting effect). The results from all repeats and both analyzers were averaged and the compounds of scores ≥2 were selected to further test in 96-well plate format. Images acquired on the 96-well plates were quantified using the NeuriteIQ software47, to determine the percentages of neuronal differentiation (DCX+%, number of DCX positive cells/number of total cells), or dopaminergic neuronal differentiation (TH+%, number of TH positive cells/number of total cells). Statistical analysis was carried out to select the compounds with significant promoting effects (p < 0.05), which were designated as the primary screening hits and subjected to further analysis.
Cell proliferation and survival assay
For the cell proliferation assay, ReNCell VM cells were seeded into laminin coated 96-well plates at a density of 4 × 103 cells/well (1.25 × 104 cells/cm2). Cells were treated with indicated compound or DMSO control for 48 hours before they are fixed and stained with Hoechst 33342 dye (Life Technologies, Grand Island, NY, USA). The plate was scanned with an ImageXpress Micro microscope (Molecular Devices, Sunnyvale, CA, USA) using a 4× objective to take an image covering the whole well for each well. Cell numbers were counted using the ImageJ software with an automatic nuclei counter plug-in. Cell proliferation was also measured by BrdU assay. Cells were grown in laminin coated 96-well plates, and treated with indicated compound for 48 hours. After that cells were pulsed with 10 μM BrdU for 2 hours, then stained with BrdU antibody (1:1000, Cell Signaling, Danvers, MA, USA). For the survival assay, cells were differentiated in the presence of indicated compounds. Then the cells were fixed, permeabilized and assayed with the Roche in situ Cell Death detection kit following the manufacturer’s instruction. Cells were also blocked and stained with additional markers as indicated.
Immunostaining and western blots
The immunofluorescence staining of the cells were done with Alexa-fluor-488 or −568-conjugated secondary antibodies ((Life Technologies, Grand Island, NY, USA), and the cell nuclei were counterstained with DAPI. Antibodies against the following proteins were used at the following dilutions: DCX (1:500, Cell Signaling, Danvers, MA, USA), GFAP (1:100, Millipore, Billerica, MA, USA), O4 (1:100, R&D Systems, Minneapolis, MN, USA), TH (1:500, Cell Signaling, Danvers, MA, USA; 1:200, Millipore, Billerica, MA, USA). Fluorescence images were taken using an IX81 inverted microscope (Olympus, Tokyo, Japan). Image quantification was performed using the ImageJ software.
For western blot, cells were lysed in RIPA buffer (Fisher Scientific, Pittsburgh, PA, USA) in the presence of Xpert protease inhibitor cocktail and Xpert phosphatase inhibitor cocktail (GenDEPOT, Barker, TX, USA). Thirty microgram protein was loaded in each well and separated by 4–15% Mini-PROTEAN TGX precast gel (Bio-rad, Hercules, CA, USA) and transferred to a nitrocellulose membrane. The antibodies tested included β-catenin (1:1000, Cell Signaling, Danvers, MA, USA), Mash1 (1:1000, Abcam, Cambridge, MA, USA), Lmx1a (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), Sfrp1(1:1000, Cell Signaling, Danvers, MA, USA), Wnt1 (1:500, Abcam, Cambridge, MA, USA), and actin (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Animal injection and brain slice immunohistochemistry
Animal experiments were carried out in accordance with the protocol approved by the Institutional Animal Care and Use Committee of Houston Methodist Research Institute. Groups of four C57BL/6 mice were injected intraperitoneally (i.p.) with 1 mg/kg brain permeable GSK-3 inhibitor BIO (Tocris Bioscience, Bristol, UK) or DMSO control daily for 4 days from E13 to E16. Forty-eight hours later the animals were sacrificed and brains were dissected and postfixed in 4% PFA overnight at 4 °C. Then the brains were dehydrated and embedded in paraffin. Sections were made from the midbrain at 10 μm thickness on a microtome. Slides from histologically comparable positions as confirmed by the H&E staining were used in the immunostainings. Immunohistochemistry was carried out with a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) following the manufacturer’s manual. The rabbit anti-TH antibody was purchased from Millipore (1:200, Millipore, Billerica, MA, USA).
Statistics
Statistical analyses were carried out using a two-tailed Student’s t-test. Data presented in the graphs are the mean values with the error bars representing the standard deviations (s.d.).
Additional Information
How to cite this article: Rhim, J. et al. A High-content screen identifies compounds promoting the neuronal differentiation and the midbrain dopamine neuron specification of human neural progenitor cells. Sci. Rep. 5, 16237; doi: 10.1038/srep16237 (2015).
Supplementary Material
Acknowledgments
We thank Lilian Tsou for providing help in laboratory management. This work was supported by the NIH grant (R21AG042585) and HMRI cornerstone award to X.X.(Xia); and the TT & WF Chao Foundation grant to S.T.W.
Footnotes
Author Contributions X.X.(Xia) and S.T.W. conceived the studies; X.X.(Xia) designed the experiments; J.R., X.L., D.G., T.Z. and X.X.(Xia) performed the chemical screening; J.R., X.L., X.X.(Xu) and D.G. performed the in vitro cell studies, biochemistry studies, and animal experiments; F.L., P.W. and X.X.(Xia) analyzed and interpreted the data; L.Q., P.W., S.T.W. and X.X.(Xia) supervised the research; P.W. and X.X.(Xia) wrote the manuscript.
References
- Lunn J. S., Sakowski S. A., Hur J. & Feldman E. L. Stem cell technology for neurodegenerative diseases. Ann Neurol 70, 353–361 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry C. E. & Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132, 661–680 (2008). [DOI] [PubMed] [Google Scholar]
- Li X. J. et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol 23, 215–221 (2005). [DOI] [PubMed] [Google Scholar]
- Zhang S. C., Wernig M., Duncan I. D., Brustle O. & Thomson J. A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 19, 1129–1133 (2001). [DOI] [PubMed] [Google Scholar]
- Perrier A. L. et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA 101, 12543–12548 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham J. J., Ulbright T. M., Pera M. F. & Looijenga L. H. Lessons from human teratomas to guide development of safe stem cell therapies. Nat Biotechnol 30, 849–857 (2012). [DOI] [PubMed] [Google Scholar]
- Diamandis P. et al. Chemical genetics reveals a complex functional ground state of neural stem cells. Nat Chem Biol 3, 268–273 (2007). [DOI] [PubMed] [Google Scholar]
- Walsh D. P. & Chang Y. T. Chemical genetics. Chem Rev 106, 2476–2530 (2006). [DOI] [PubMed] [Google Scholar]
- Xia X. & Wong S. T. Concise review: a high-content screening approach to stem cell research and drug discovery. Stem Cells 30, 1800–1807 (2012). [DOI] [PubMed] [Google Scholar]
- Donato R. et al. Differential development of neuronal physiological responsiveness in two human neural stem cell lines. BMC Neurosci 8, 36 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillard-Despres S. et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci 21, 1–14 (2005). [DOI] [PubMed] [Google Scholar]
- Freed C. R. et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med 344, 710–719 (2001). [DOI] [PubMed] [Google Scholar]
- Xi J. et al. Specification of midbrain dopamine neurons from primate pluripotent stem cells. Stem Cells 30, 1655–1663 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S. et al. Synthetic small molecules that control stem cell fate. Proc Natl Acad Sci USA 100, 7632–7637 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Garcia J. A. et al. Glycogen synthase kinase 3 inhibition promotes adult hippocampal neurogenesis in vitro and in vivo. ACS Chem Neurosci 3, 963–971 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin N. C. et al. Simvastatin promotes adult hippocampal neurogenesis by enhancing Wnt/beta-catenin signaling. Stem Cell Reports 2, 9–17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarosa S., Fode C. & Guillemot F. Mash1 regulates neurogenesis in the ventral telencephalon. Development 126, 525–534 (1999). [DOI] [PubMed] [Google Scholar]
- Andersson E. et al. Identification of intrinsic determinants of midbrain dopamine neurons. Cell 124, 393–405 (2006). [DOI] [PubMed] [Google Scholar]
- Cai J. et al. BMP and TGF-beta pathway mediators are critical upstream regulators of Wnt signaling during midbrain dopamine differentiation in human pluripotent stem cells. Dev Biol 376, 62–73 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquelajauregui A. et al. Smad-interacting protein-1 (Zfhx1b) acts upstream of Wnt signaling in the mouse hippocampus and controls its formation. Proc Natl Acad Sci USA 104, 12919–12924 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo-Branco G. et al. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc Natl Acad Sci USA 100, 12747–12752 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo-Branco G., Rawal N. & Arenas E. GSK-3beta inhibition/beta-catenin stabilization in ventral midbrain precursors increases differentiation into dopamine neurons. J Cell Sci 117, 5731–5737 (2004). [DOI] [PubMed] [Google Scholar]
- Joksimovic M. & Awatramani R. Wnt/beta-catenin signaling in midbrain dopaminergic neuron specification and neurogenesis. J Mol Cell Biol 6, 27–33 (2014). [DOI] [PubMed] [Google Scholar]
- Fox I. J. et al. Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science 345, 1247391 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y. et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells 23, 781–790 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warashina M. et al. A synthetic small molecule that induces neuronal differentiation of adult hippocampal neural progenitor cells. Angew Chem Int Ed Engl 45, 591–593 (2006). [DOI] [PubMed] [Google Scholar]
- Saxe J. P. et al. A phenotypic small-molecule screen identifies an orphan ligand-receptor pair that regulates neural stem cell differentiation. Chem Biol 14, 1019–1030 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong C. R. & Sullivan D. J. Jr. New uses for old drugs. Nature 448, 645–646 (2007). [DOI] [PubMed] [Google Scholar]
- Endo A., Kuroda M. & Tsujita Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J Antibiot (Tokyo) 29, 1346–1348 (1976). [DOI] [PubMed] [Google Scholar]
- Saito K. et al. Ablation of cholesterol biosynthesis in neural stem cells increases their VEGF expression and angiogenesis but causes neuron apoptosis. Proc Natl Acad Sci USA 106, 8350–8355 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozawa R. et al. Embryonic lethality and defective neural tube closure in mice lacking squalene synthase. J Biol Chem 274, 30843–30848 (1999). [DOI] [PubMed] [Google Scholar]
- Roper K., Corbeil D. & Huttner W. B. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat Cell Biol 2, 582–592 (2000). [DOI] [PubMed] [Google Scholar]
- Chen J. et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol 53, 743–751 (2003). [DOI] [PubMed] [Google Scholar]
- Lu D. et al. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma 24, 1132–1146 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danovi D. et al. Imaging-based chemical screens using normal and glioma-derived neural stem cells. Biochem Soc Trans 38, 1067–1071 (2010). [DOI] [PubMed] [Google Scholar]
- Gaist D., Hallas J., Friis S., Hansen S. & Sorensen H. T. Statin use and survival following glioblastoma multiforme. Cancer Epidemiol 38, 722–727 (2014). [DOI] [PubMed] [Google Scholar]
- Lange C. et al. Small molecule GSK-3 inhibitors increase neurogenesis of human neural progenitor cells. Neurosci Lett 488, 36–40 (2011). [DOI] [PubMed] [Google Scholar]
- Yang Y. M. et al. A small molecule screen in stem-cell-derived motor neurons identifies a kinase inhibitor as a candidate therapeutic for ALS. Cell Stem Cell 12, 713–726 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, A. R. In Trends in cell signaling pathways in neuronal fate decision. (ed. S. Wislet-Gendebien) 153–177. (InTech, 2013). [Google Scholar]
- Farkas L. M., Dunker N., Roussa, E., Unsicker K. & Krieglstein K. Transforming growth factor-beta(s) are essential for the development of midbrain dopaminergic neurons in vitro and in vivo. J Neurosci 23, 5178–5186 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S. M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27, 275–280 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszak J., Just L., Isacson O. & Nikkhah G. Isolation and culture of ventral mesencephalic precursor cells and dopaminergic neurons from rodent brains. Curr Protoc Stem Cell Biol Chapter 2, Unit 2D 5 (2009). [DOI] [PubMed] [Google Scholar]
- Conti L. et al. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol 3, e283 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard S. M., Conti L., Sun Y., Goffredo D. & Smith A. Adherent neural stem (NS) cells from fetal and adult forebrain. Cereb Cortex 16 Suppl 1, i112–120 (2006). [DOI] [PubMed] [Google Scholar]
- Thomson J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998). [DOI] [PubMed] [Google Scholar]
- Zhang X. Q. & Zhang S. C. Differentiation of neural precursors and dopaminergic neurons from human embryonic stem cells. Methods Mol Biol 584, 355–366 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofengeim D. et al. Identification of small molecule inhibitors of neurite loss induced by Abeta peptide using high content screening. J Biol Chem 287, 8714–8723 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.