Abstract
Moxibustion is under active research as a complementary and alternative treatment for various diseases such as pain. “Heat-sensitization” responses have been reported during suspended moxibustion, whose occurrence is associated with significantly better therapeutic effects. The present study aimed to investigate the cortical activities of this interesting phenomenon by a standardized low-resolution brain electromagnetic tomography. We performed electroencephalography recording in a group of patients with chronic low back pain before, during, and after moxibustion treatment at Yaoyangguan (DU3) areas. 11 out of 21 subjects experienced strong heat-sensitization during moxibustion, which were accompanied with significant decreases of current densities in the beta frequency bands in prefrontal, primary and second somatosensory, and cingulate cortices, as well as increased current densities in the alpha2 band in the left insula. No changes were detected in patients without sensitization responses, or in the post-moxibustion phase of either group. These data indicated widespread activity changes across different frequency bands during heat-sensitization. Cortical oscillatory activities could be used to evaluate the “heat-sensitization” responses during suspended moxibustion.
Keywords: Moxibustion, Heat-sensitization, Chronic low back pain, Cortical activity, sLORETA
Introduction
Acupuncture and moxibustion are traditional Chinese therapeutic techniques used for treating a variety of diseases. Acupuncture treatment is applied by inserting needles at identified acupuncture points to induce “deqi sensations”, which include soreness, aching, pressure, heaviness, warmth, fullness dull pain and numbness. Numerous studies have been carried out to understand therapeutic mechanisms of acupuncture. In contrast, moxibustion uses burning dried plant materials (Artimisia moxa) to treat various diseases, where direct moxibustion is applying a small amount of burning moxa directly on the skin, and suspended (or indirect) moxibustion stimulates the body with heat generated from burning moxa 3–5 cm away from the skin surface. Though the earliest record of moxibustion traced back to over 2000 years ago, even earlier than acupuncture, its therapeutic efficacies and mechanisms have not been sufficiently investigated. But in recent years, an increasing number of randomized controlled clinical trials start to validate the anti-nociceptive, anti-inflammatory, and immuno-modulatory effects of moxibustion (Chen et al. 2012a, b, c, 2013a, b, c). It has been reported that a proportion of patients exhibit “sensitized” responses to suspended moxibustion at certain restricted locations on their bodies (Liao et al. 2014; Xie et al. 2013). Instead of experiencing local warmth as healthy volunteers do, these patients report strong warmth or heat spreading around the stimulating site or penetrating into the body, which quite frequently is accompanied with pleasant feelings. The occurrence of these responses during moxibustion treatment is associated with significantly better clinical outcomes (Chen et al. 2012a, b, c, 2013a, b, c; Xie et al. 2013). Each disease has a specific anatomically stable set of such “heat-sensitized” points. For example, chronic low back pain (CLBP) patients but not healthy subjects most frequently showed heat-sensitization around the acupuncture point Yaoyangguan (DU3) (Chen et al. 2012a, c, 2013c; Liao et al. 2014). These responses were accompanied with increased absolute power spectral densities (PSDs) and phase coherence in the theta and beta bands of the EEG (Liao et al. 2014).
Although detailed topographic distribution of EEG changes has been reported, it is difficult to establish a direct link between the EEG measures and heat-sensitization responses, as the scalp EEG is the summed activity of multiple cortical neuronal generators (Kiebel et al. 2008; Michel et al. 2004; Zhang et al. 2014). The advances in EEG source localization methods have enabled the investigation of electric neuronal activity inside the brain, and provide the possibility to reveal brain areas associated with heat-sensitization responses. Among them, the standardized low-resolution brain electromagnetic tomography (sLORETA) allowing 3-D localization of cortical EEG generators both in the time (Jiang et al. 2014; Ozaki et al. 2012) and frequency domain (Houdayer et al. 2015; Jaiswal et al. 2010; Justen et al. 2014), has been successfully applied to electrophysiological characterization of intracranial responses to pain (Shao et al. 2012; Stern et al. 2006). Recent studies have confirmed sLORETA to accurately localize neural generators of brain electrical activity (Mulert et al. 2004a; Pascual-Marqui 2002a; Walla et al. 2008).
The present study sought to investigate the cortical correlates of heat-sensitization responses with sLORETA, by comparing the rhythmicity and topography of cortical sources before, during, and after moxibustion.
Methods
Participants and EEG recording and pre-processing
Details of the participants and EEG recording information were described in our previous report (Liao et al. 2014). In brief, twenty-five right-handed Chinese patients suffering from CLBP were recruited to receive suspended moxibustion on Yaoyangguan (DU3), which located between the fourth and fifth lumbar vertebra. On the self-report, the patients who experienced strong warmth or heat spreading around or penetrating into the body during moxibustion fell into the heat-sensitized group (n = 12), whereas those who experienced only mild local warmth entered the non-sensitized group (n = 13).
Spontaneous resting EEG was simultaneously recorded via a 128 channel EGI system with eyes closed. Three sessions (15 min each) of resting EEG were recorded for each patient: one before moxibustion (Pre-Moxi session), one during moxibustion (Moxi session), and one immediately after moxibustion (Post-Moxi session). The middle 5 min of each session were chosen for offline preprocessing: The data were resampled to 250 Hz, band-pass filtered in the range of 1.5–30 Hz and subsequently inspected for artifact-rejection; Conspicuous artifacts were eliminated by visual inspection on time series and automatic artifact rejection threshold was set to ±100 µV; Further artifacts caused by eye movements, or body movements were removed with extended informax independent component analysis (ICA). Four patients (two patients for each group) were excluded for sLORETA analysis because of insufficient recording for some sessions.
sLORETA imaging
sLORETA (Pascual-Marqui 2002b) is a linear inverse algorithm which estimates the intracerebral electrical sources of scalp EEG data. Its solution space is restricted to the cortical grey matter, for which the neocortical (including the hippocampus and anterior cingulate cortex) MNI-152 volume is divided and labelled in 6239 voxels of dimension 5 mm3, based on probabilities returned by the Demon Atlas (Lancaster et al. 2000). The sLORETA algorithm has been shown to outperform several other linear inverse algorithms (Pascual-Marqui 2002b). It has been widely used in EEG studies (Jaiswal et al. 2010; Justen et al. 2014; Shao et al. 2012), and demonstrated to be a feasible tool for pain research (Nir et al. 2008).
In the present study, cross-spectra of EEG segments were computed with the sLORETA software for seven frequency bands: delta (1.5–4 Hz), theta (4–8 Hz), alpha1 (8–10 Hz), alpha2 (10–13 Hz), beta1 (13–18 Hz), beta2 (18–21 Hz), and beta3 (21–30 Hz). The EEG cross-spectra matrices for each subject were then averaged as the input for sLORETA source analyses. This procedure resulted in 3D sLORETA images for each subject in Pre-Moxi, Moxi, and Post-Moxi sessions for each frequency range. To localize the neuronal generators responsible for measured EEG phenomena, a normalization of sLORETA solution was obtained by normalizing the sLORETA current density at each image with the power density averaged across all 6239 voxels of the brain volume. This method has been used to explore activation or inhibition state for the brain regions (Theodoropoulou et al. 2011; Wong et al. 2008; Yamashita et al. 2004).
Statistical analysis
The sLORETA performed a voxel-by-voxel t test of the normalized and log-transformed sLORETA images for the different frequency bands for the between-group comparison (non-sensitized versus sensitized in the Pre-Moxi) and the within-group comparison (paired t test: Pre-Moxi versus Moxi, and Pre-Moxi versus Post-Moxi). Statistical non-paramatric mapping (SnPM) was used to compare voxel-by-voxel sLORETA images (Holmes et al. 1996; Nichols and Holmes 2002). The method used a randomization strategy, and the critical significant threshold (a significance level of p < 0.05) for the observed t values was assessed with correction for multiple testing: After each permutation test, the largest t value was kept; Following 5000 permutations, the t value associated with the most extreme 5 % of the distribution was identified for the selected frequency band. Finally, the threshold was used to identify the statistically significant voxels.
Results
Topography of EEG cortical sources with sLORETA
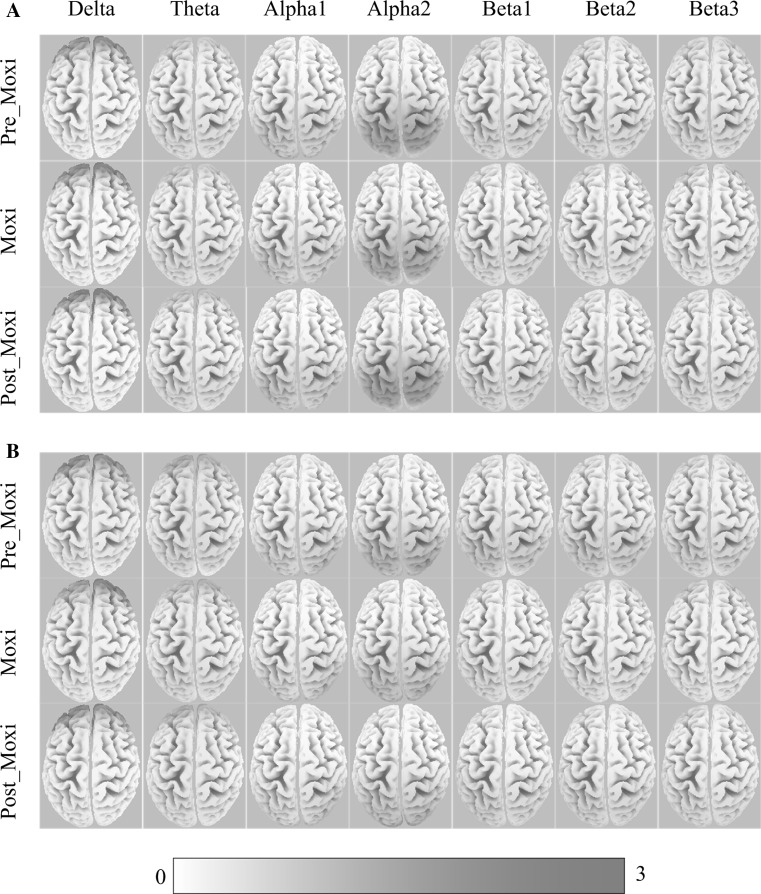
We first modelled the distributed cortical sources for delta, theta, alpha1, alpha2, beta1, beta2, and beta3 bands with relative power current densities at cortical voxels obtained from sLORETA. The global averages of sLORETA solutions for each session (Pre-Moxi, Moxi, and Post-Moxi) in the non-sensitized and sensitized groups were shown in Fig. 1. Visual inspection revealed that the distribution of the delta, alpha1, and alpha2 sources focused on the frontal, occipital, and parietal cortices, while the theta, beta1, beta2, and beta3 sources were more globally distributed in both groups. In addition, for three sessions, the alpha2 sources were prominently activated in the occipital cortex in the sensitized group, but in the parietal-occipital cortex in the non-sensitized group.
Fig. 1.
The grand average of sLORETA solutions for each frequency band in the Pre-Moxi, Moxi and Post-Moxi sessions in the non-sensitized group (a) and sensitized group (b). Color scale all power density estimates were scaled based on the maximal value. (Color figure online)
Comparisons for the EEG cortical sources with sLORETA
We first compared sLORETA images of the Pre-Moxi sessions between the sensitized and the non-sensitized groups. No significant differences were found for any frequency bands, indicating few baseline differences between the two groups.
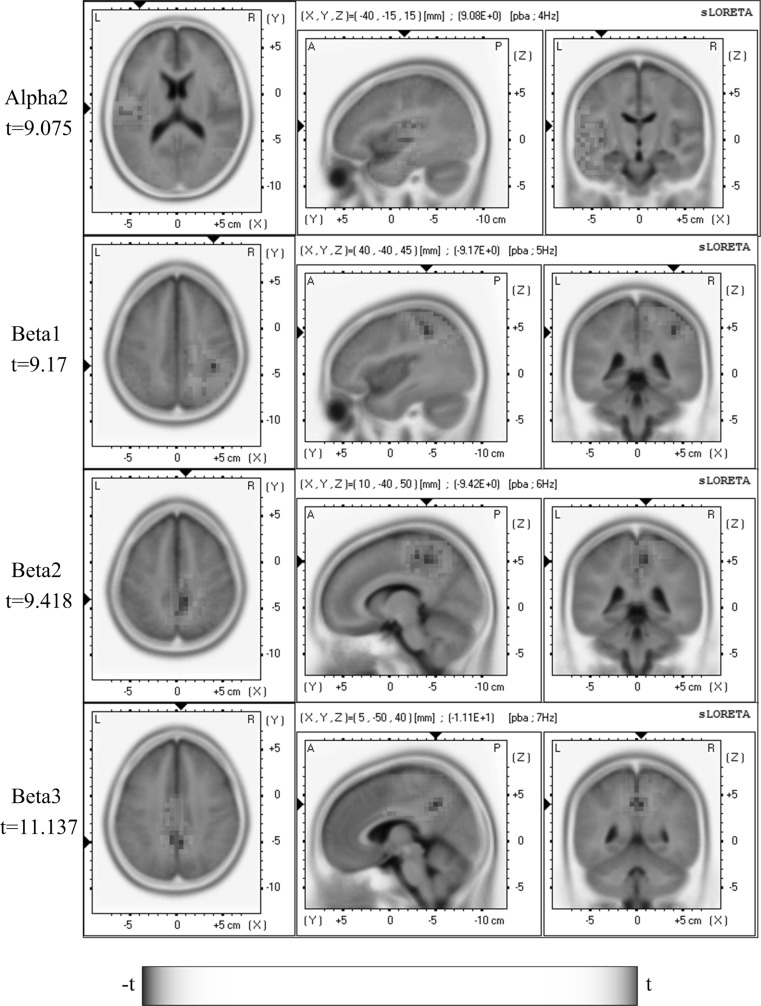
We next sought to reveal the cortical sources with significant power changes correlated with heat-sensitization during moxibustion for each frequency band. Within-group comparisons of sLORETA images were performed between the Pre-Moxi and Moxi or Post-Moxi sessions. In the non-sensitized group, no significant power changes were found during or after moxibustion compared to the Pre-Moxi (p > 0.05, paired t tests). In the sensitized group, in contrast, neuronal sources with significant power changes in alpha2, beta1, beta2, and beta3 bands (p < 0.05, paired t tests) between the Pre-Moxi and Moxi sessions were localized by sLORETA in widespread brain regions as shown in Fig. 2. In particular, significantly higher alpha2 activation for heat-sensitization during moxibustion was found in the left insula. Reduced beta1 activities were revealed in the right parietal and cingulate regions, reduced beta2 activation was detected in bilateral parietal, medial/right frontal regions, and cingulate regions, whereas significantly lower beta3 activities were found in bilateral frontal/parietal and cingulate regions. These results were largely consistent with the changes of frequency-wise normalization spectrum power reported above. No significant changes were detected in Post-Moxi session relative to the Pre-Moxi session (p > 0.05, paired t tests). Table 1 illustrated an overview of statistically significant differences in all tested EEG frequency bands.
Fig. 2.
Comparison of sLORETA activation between Pre-Moxi and Moxi sessions revealed significant changes in alpha2, beta1, beta2 and beta3 bands during moxibustion (blue for decrease and red for increase). Images were color-coded non-parametric statistical maps. Increased activities in the alpha2 band were observed in the insula, whereas reduced activities occurred in the beta1 band in right parietal and cingulate gyrus, in the beta2 band in bilateral parietal, medial/right frontal regions and cingulate gyrus, and in the beta3 band in bilateral frontal/parietal and cingulate gyrus. A scale bar indicated statistical power and color scale linearity equalled to 75. The location of the maximal t value was graphically indicated by black triangles on the coordinate axes. (Color figure online)
Table 1.
The sLORETA localized the regions of interest (ROIs) with significantly different powers between Pre-Moxi and Moxi sessions in the heat-sensitized group
| ROIs | Power | Frequency bands | Brodmann areas |
|---|---|---|---|
| Insula | ↑ | Alpha2 | 13 |
| Inferior parietal lobule | ↓ | Beta1 | 40 |
| Postcentral gyrus | ↓ | Beta1 | 1 2 3 40 |
| Precentral gyrus | ↓ | Beta1/Beta2/Beta3 | 4 6 |
| Precuneus | ↓ | Beta1/Beta2/Beta3 | 7 31 |
| Sub-gyral | ↓ | Beta2/Beta3 | 7 31 40 |
| Cingulate gyrus | ↓ | Beta1/Beta2/Beta3 | 23 24 31 |
| Supramarginal gyrus | ↓ | Beta1 | 40 |
| Paracentral lobule | ↓ | Beta2/Beta3 | 3 4 5 6 31 |
| Medial frontal gyrus | ↓ | Beta2/Beta3 | 6 |
| Postcentral gyrus | ↓ | Beta2/Beta3 | 2 3 4 5 7 40 |
| Superior parietal lobule | ↓ | Beta2/Beta3 | 5 7 |
| Superior frontal gyrus | ↓ | Beta2/Beta3 | 6 |
| Posterior cingulate | ↓ | Beta3 | 23 31 |
Anatomical names and abbreviations of the ROIs, the number of voxels with significant power changes and their corresponding Brodmann areas were listed for each frequency band. ‘↑/↓’ indicated power increase/decrease during moxibustion
Discussion
The present study attempted to characterize moxibustion-induced heat-sensitization responses at the cortical level by frequency-domain EEG source localization using sLORETA. The tomography sLORETA has received considerable validation from studies combining LORETA with other more established localization methods including functional magnetic resonance imaging (fMRI) (Mulert et al. 2004b; Vitacco et al. 2002) and positron emission tomography (Dierks et al. 2000; Pizzagalli et al. 2004; Zumsteg et al. 2005). It is now widely used in studies involving time and frequency domain analysis (Shao et al. 2012; Volpe et al. 2007; Zumsteg et al. 2006).
The major finding was that significant cortical power changes were not observed in all subjects, who received exactly identical manipulations (suspended moxibustion at DU3), but only in a proportion of patients reporting heat-sensitization responses. Source localization analysis revealed significant changes across different frequency bands in multiple brain regions. Bidirectional changes were both present. Increased current densities in the alpha2 range were detected in the left insula, a key area crucial for multi-modal integration at the interface between pain’s sensory-discriminative dimension encoded by the somatosensory cortex and the affective component mediated by the limbic cortical structures (Craig et al. 2000). An interesting point was that this change was restricted to the left but not bilateral insula. Previous studies have shown that the left insula participates in a variety of pain modulatory activities, especially those in the affective and cognitive dimensions (Bos et al. 2015; Fan et al. 2011; La Cesa et al. 2014; Lang et al. 2011; Liu et al. 2013). Alpha activities represent an important EEG correlate of top–down control of incoming sensory information (Klimesch et al. 2007). Under pain conditions, the alpha activity has been repeatedly shown associated with the cognitive–evaluative aspect of pain. In particular, decreased alpha power follows painful stimulation and/or perception (Babiloni et al. 2006; Franciotti et al. 2009; Nir et al. 2012). One study examining the cognitive modulation of pain with EEG revealed correlated analgesic effects and increased alpha activities in left insula, suggesting that ongoing alpha power might actively modulate the perceived pain (Huneke et al. 2013). Our findings here could represent a similar process, and suggested that the heat-sensitization might be associated with affective and cognitive pain modulation. This was consistent with the clinical finding that heat-sensitization responses were frequently accompanied with pleasant feelings (Xie et al. 2013).
In contrast, decreased current densities, mainly in the beta range, were found in widespread prefrontal, primary and second somatosensory, and cingulate cortices, all of which belong to the classic pain matrix involved in pain processing and modulation (Apkarian et al. 2005, 2009; Ohara et al. 2005). Previous imaging studies have shown increased neuronal activities in these regions under chronic pain conditions (Apkarian et al. 2005, 2009; Brooks and Tracey 2005; Ohara et al. 2005), and reduced over-activation during pain relief (Prichep et al. 2011; Stern et al. 2006). Similarly, multiple brain regions in the pain matrix showed stronger oscillatory activities in the higher frequency range (>12 Hz) but decreased low frequency oscillations (<12 Hz) during noxious stimulation (Dowman et al. 2008; Iwata et al. 2005; Ploner et al. 2006; Shao et al. 2012). Thus, it is rational to propose that moxibustion exerts its therapeutic effects through insula-centered cortical circuits. Indeed, previous randomized controlled trials have indicated better therapeutic effects in patients with heat-sensitization responses compared with those without, not only in low back pain (Chen et al. 2012a, c, 2013c), but in knee osteoarthritis (Chen et al. 2012b, 2013b) and persistent asthma (Chen et al. 2013a) as well.
The present study was primarily designed to investigate the cortical correlates of the heat-sensitization responses. One important question not directly addressed here was the clinical relevance of these electrophysiological changes. Previous clinical trials have shown that the presence of heat-sensitization responses during moxibustion treatment is associated with significantly better clinical outcome (Chen et al. 2012a, b, c, Chen et al. 2013a, b, c; Xie et al. 2013). Based on the discussion above, we hypothesize that these EEG changes represent pain modulatory processes. Carefully designed clinical trials tracking heat-sensitized levels, and immediate and long-term clinical measurements including pain ratings are required to validate our hypothesis. An alternative explanation is that the heat-sensitization phenomena represent placebo responses to moxibustion treatment. However, we do not consider it rational, since placebo processes would predict more generalized sensitization responses involving large areas of the body surface or fixed areas local to pain sites. In contrast, the sensitized point is always restricted to small areas not necessarily closed to pain sites (Liao et al. 2014; Xie et al. 2013).
It is still unclear why heat-sensitization occurs in some patients but not others. Clinical data from our previous studies did not support gender, age, and pain duration as crucial factors (Liao et al. 2014; Xie et al. 2013). However, one clear factor affecting its occurrence is the state of the subject. Heat-sensitization could be induced in no more than 30 % of healthy volunteers, but 50–70 % under morbid states (Xie et al. 2013). Further investigation is required to illustrate electrophysiological and behavioural differences between healthy and morbid conditions, as well as heat-sensitized and non-sensitized subjects.
In conclusion, our EEG study provided objective evidence of heat-sensitization responses during suspended moxibustion, which was accompanied with decreased source power at the beta frequency bands in prefrontal, primary and second somatosensory and cingulate cortices, and increased alpha2 oscillations in the insula.
Acknowledgments
This work was supported by the Major State Basic Research Development Program of People’s Republic of China (2014CB548200 and 2015CB554503) and the National Natural Science Foundation of China (31200835 and 81230023). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- CLBP
Chronic low back pain
- fMRI
Functional magnetic resonance imaging
- ROI
Region of interest
- sLORETA
Standardized low-resolution brain electromagnetic tomography
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no competing interests.
Contributor Information
Juan Wang, Email: wangjuan033@163.com.
Ming Yi, Phone: +86 (0)10 8280 5526, Email: mingyi@bjmu.edu.cn.
Xiaoli Li, Phone: +86 (0)10 58802032, Email: xlli@ysu.edu.cn.
References
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Brancucci A, Percio CD, Capotosto P, Arendt-Nielsen L, Chen ACN, Rossini PM. Anticipatory electroencephalography alpha rhythm predicts subjective perception of pain intensity. J Pain. 2006;7:709–717. doi: 10.1016/j.jpain.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Bos PA, Montoya ER, Hermans EJ, Keysers C, van Honk J. Oxytocin reduces neural activity in the pain circuitry when seeing pain in others. NeuroImage. 2015;113:217–224. doi: 10.1016/j.neuroimage.2015.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J, Tracey I. From nociception to pain perception: imaging the spinal and supraspinal pathways. J Anat. 2005;207:19–33. doi: 10.1111/j.1469-7580.2005.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Chen R, Xiong J, Chi Z, Sun J, Su T, Zhou M, Yi F, Zhang B. Evaluation of different moxibustion doses for lumbar disc herniation: multicentre randomised controlled trial of heat-sensitive moxibustion therapy. Acupunct Med. 2012;30:266–272. doi: 10.1136/acupmed-2012-010142. [DOI] [PubMed] [Google Scholar]
- Chen R, Chen M, Xiong J, Chi Z, Zhou M, Su T, Sun J, Yi F, Zhang B. Is there difference between the effects of two-dose stimulation for knee osteoarthritis in the treatment of heat-sensitive moxibustion? Evid Based Complement Altern Med. 2012;2012:696498. doi: 10.1155/2012/696498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Xiong J, Chi Z, Zhang B. Heat-sensitive moxibustion for lumbar disc herniation: a meta-analysis of randomized controlled trials. J Tradit Chin Med. 2012;32:322–328. doi: 10.1016/S0254-6272(13)60032-1. [DOI] [PubMed] [Google Scholar]
- Chen R, Chen M, Xiong J, Chi Z, Zhang B, Tian N, Xu Z, Zhang T, Li W, Zhang W, Rong X, Wang Z, Sun G, Ge B, Yu G, Song N. Curative effect of heat-sensitive moxibustion on chronic persistent asthma: a multicenter randomized controlled trial. J Tradit Chin Med. 2013;33:584–591. doi: 10.1016/S0254-6272(14)60025-X. [DOI] [PubMed] [Google Scholar]
- Chen R, Chen M, Xiong J, Su T, Zhou M, Sun J, Chi Z, Zhang B, Xie D. Comparative effectiveness of the deqi sensation and non-deqi by moxibustion stimulation: a multicenter prospective cohort study in the treatment of knee osteoarthritis. Evid Based Complement Altern Med. 2013;2013:906947. doi: 10.1155/2013/906947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Chen M, Xiong J, Su T, Zhou M, Sun J, Chi Z, Zhang B, Xie D. Influence of the deqi sensation by suspended moxibustion stimulation in lumbar disc herniation: study for a multicenter prospective two arms cohort study. Evid Based Complement Altern Med. 2013;2013:718593. doi: 10.1155/2013/718593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Dierks T, Jelic V, Pascual-Marqui RD, Wahlund L, Julin P, Linden DE, Maurer K, Winblad B, Nordberg A. Spatial pattern of cerebral glucose metabolism (pet) correlates with localization of intracerebral EEG-generators in Alzheimer’s disease. Clin Neurophysiol. 2000;111:1817–1824. doi: 10.1016/S1388-2457(00)00427-2. [DOI] [PubMed] [Google Scholar]
- Dowman R, Rissacher D, Schuckers S. Eeg indices of tonic pain-related activity in the somatosensory cortices. Clin Neurophysiol. 2008;119:1201–1212. doi: 10.1016/j.clinph.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci Biobehav Rev. 2011;35:903–911. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Franciotti R, Ciancetta L, Della Penna S, Belardinelli P, Pizzella V, Romani GL. Modulation of alpha oscillations in insular cortex reflects the threat of painful stimuli. NeuroImage. 2009;46:1082–1090. doi: 10.1016/j.neuroimage.2009.03.034. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Blair RC, Watson JD, Ford I. Nonparametric analysis of statistic images from functional mapping experiments. J Cereb Blood Flow Metab. 1996;16:7–22. doi: 10.1097/00004647-199601000-00002. [DOI] [PubMed] [Google Scholar]
- Houdayer E, Teggi R, Velikova S, Gonzalez-Rosa JJ, Bussi M, Comi G, Leocani L (2015) Involvement of cortico-subcortical circuits in normoacousic chronic tinnitus: A source localization EEG study. Clin Neurophysiol. doi:10.1016/j.clinph.2015.01.027 [DOI] [PubMed]
- Huneke NTM, Brown CA, Burford E, Watson A, Trujillo-Barreto NJ, El-Deredy W, Jones AKP. Experimental placebo analgesia changes resting-state alpha oscillations. PLoS One. 2013;8:e78278. doi: 10.1371/journal.pone.0078278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata K, Kamo H, Ogawa A, Tsuboi Y, Noma N, Mitsuhashi Y, Taira M, Koshikawa N, Kitagawa J. Anterior cingulate cortical neuronal activity during perception of noxious thermal stimuli in monkeys. J Neurophysiol. 2005;94:1980–1991. doi: 10.1152/jn.00190.2005. [DOI] [PubMed] [Google Scholar]
- Jaiswal N, Ray W, Slobounov S. Encoding of visual–spatial information in working memory requires more cerebral efforts than retrieval: evidence from an eeg and virtual reality study. Brain Res. 2010;1347:80–89. doi: 10.1016/j.brainres.2010.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang A, Yang J, Yang Y. Mmn responses during implicit processing of changes in emotional prosody: an erp study using chinese pseudo-syllables. Cogn Neurodyn. 2014;8:499–508. doi: 10.1007/s11571-014-9303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justen C, Herbert C, Werner K, Raab M. Self vs. other: neural correlates underlying agent identification based on unimodal auditory information as revealed by electrotomography (sloreta) Neuroscience. 2014;259:25–34. doi: 10.1016/j.neuroscience.2013.11.042. [DOI] [PubMed] [Google Scholar]
- Kiebel S, Garrido M, Moran R, Friston K. Dynamic causal modelling for eeg and meg. Cogn Neurodyn. 2008;2:121–136. doi: 10.1007/s11571-008-9038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. Eeg alpha oscillations: the inhibition–timing hypothesis. Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- La Cesa S, Tinelli E, Toschi N, Di Stefano G, Collorone S, Aceti A, Francia A, Cruccu G, Truini A, Caramia F. fMRI pain activation in the periaqueductal gray in healthy volunteers during the cold pressor test. Magn Reson Imaging. 2014;32:236–240. doi: 10.1016/j.mri.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S, Yu T, Markl A, Müller F, Kotchoubey B. Hearing others’ pain: neural activity related to empathy. Cogn Affect Behav Neurosci. 2011;11:386–395. doi: 10.3758/s13415-011-0035-0. [DOI] [PubMed] [Google Scholar]
- Liao F, Zhang C, Bian Z, Xie D, Kang M, Li X, Wan Y, Chen R, Yi M. Characterizing heat-sensitization responses in suspended moxibustion with high-density eeg. Pain Med. 2014;15:1272–1281. doi: 10.1111/pme.12512. [DOI] [PubMed] [Google Scholar]
- Liu J, Hao Y, Du M, Wang X, Zhang J, Manor B, Jiang X, Fang W, Wang D. Quantitative cerebral blood flow mapping and functional connectivity of postherpetic neuralgia pain: a perfusion fMRI study. Pain. 2013;154:110–118. doi: 10.1016/j.pain.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R. EEG source imaging. Clin Neurophysiol. 2004;115:2195–2222. doi: 10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Mulert C, Jäger L, Schmitt R, Bussfeld P, Pogarell O, Möller H-J, Juckel G, Hegerl U. Integration of fMRI and simultaneous EEG: towards a comprehensive understanding of localization and time-course of brain activity in target detection. NeuroImage. 2004;22:83–94. doi: 10.1016/j.neuroimage.2003.10.051. [DOI] [PubMed] [Google Scholar]
- Mulert C, Jager L, Schmitt R, Bussfeld P, Pogarell O, Moller HJ, Juckel G, Hegerl U. Integration of fMRI and simultaneous EEG: towards a comprehensive understanding of localization and time-course of brain activity in target detection. Neuroimage. 2004;22:83–94. doi: 10.1016/j.neuroimage.2003.10.051. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir RR, Lev R, Moont R, Granovsky Y, Sprecher E, Yarnitsky D. Neurophysiology of the cortical pain network: revisiting the role of s1 in subjective pain perception via standardized low-resolution brain electromagnetic tomography (sloreta) J Pain. 2008;9:1058–1069. doi: 10.1016/j.jpain.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Nir R-R, Sinai A, Moont R, Harari E, Yarnitsky D. Tonic pain and continuous EEG: prediction of subjective pain perception by alpha-1 power during stimulation and at rest. Clin Neurophysiol. 2012;123:605–612. doi: 10.1016/j.clinph.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Ohara PT, Vit JP, Jasmin L. Cortical modulation of pain. Cell Mol Life Sci. 2005;62:44–52. doi: 10.1007/s00018-004-4283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki T, Sato N, Kitajo K, Someya Y, Anami K, Mizuhara H, Ogawa S, Yamaguchi Y. Traveling eeg slow oscillation along the dorsal attention network initiates spontaneous perceptual switching. Cogn Neurodyn. 2012;6:185–198. doi: 10.1007/s11571-012-9196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sloreta): technical details. Methods and findings in experimental and clinical pharmacology. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sloreta): technical details. Methods Find Exp Clin Pharmacol. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- Pizzagalli DA, Oakes TR, Fox AS, Chung MK, Larson CL, Abercrombie HC, Schaefer SM, Benca RM, Davidson RJ. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry. 2004;9(325):393–405. doi: 10.1038/sj.mp.4001469. [DOI] [PubMed] [Google Scholar]
- Ploner M, Gross J, Timmermann L, Pollok B, Schnitzler A. Pain suppresses spontaneous brain rhythms. Cereb Cortex. 2006;16:537–540. doi: 10.1093/cercor/bhj001. [DOI] [PubMed] [Google Scholar]
- Prichep LS, John ER, Howard B, Merkin H, Hiesiger EM. Evaluation of the pain matrix using EEG source localization: a feasibility study. Pain Med. 2011;12:1241–1248. doi: 10.1111/j.1526-4637.2011.01191.x. [DOI] [PubMed] [Google Scholar]
- Shao S, Shen K, Yu K, Wilder-Smith EPV, Li X. Frequency-domain eeg source analysis for acute tonic cold pain perception. Clin Neurophysiol. 2012;123:2042–2049. doi: 10.1016/j.clinph.2012.02.084. [DOI] [PubMed] [Google Scholar]
- Stern J, Jeanmonod D, Sarnthein J. Persistent EEG overactivation in the cortical pain matrix of neurogenic pain patients. Neuroimage. 2006;31:721–731. doi: 10.1016/j.neuroimage.2005.12.042. [DOI] [PubMed] [Google Scholar]
- Theodoropoulou A, Tei S, Lehmann D, Faber PL, Schlegel F, Milz P. P02-349-EEG frequency band sloreta sources during mental arithmetic compared to resting. Eur Psychiatry. 2011;26(Supplement 1):945. doi: 10.1016/S0924-9338(11)72650-5. [DOI] [Google Scholar]
- Vitacco D, Brandeis D, Pascual-Marqui R, Martin E. Correspondence of event-related potential tomography and functional magnetic resonance imaging during language processing. Hum Brain Mapp. 2002;17:4–12. doi: 10.1002/hbm.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe U, Mucci A, Bucci P, Merlotti E, Galderisi S, Maj M. The cortical generators of p3a and p3b: a loreta study. Brain Res Bull. 2007;73:220–230. doi: 10.1016/j.brainresbull.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Walla P, Duregger C, Greiner K, Thurner S, Ehrenberger K. Multiple aspects related to self-awareness and the awareness of others: an electroencephalography study. J Neural Transm. 2008;115:983–992. doi: 10.1007/s00702-008-0035-6. [DOI] [PubMed] [Google Scholar]
- Wong KK, Galka A, Ozaki T (2008). Frequency-wise inverse solutions to eeg recordings by state space modeling decomposition and dynamical loreta, and its application to changes in slow delta activity during induction of anesthesia. In: Processing of the 14th annual meeting of the organization for human brain mapping (HBM 2008)
- Xie D, Liu Z, Hou X, Zhang B, Xiong J, Yi M, Chen R. Heat sensitisation in suspended moxibustion: features and clinical relevance. Acupunct Med. 2013;31:422–424. doi: 10.1136/acupmed-2013-010409. [DOI] [PubMed] [Google Scholar]
- Yamashita O, Galka A, Ozaki T, Biscay R, Valdes-Sosa P. Recursive penalized least squares solution for dynamical inverse problems of eeg generation. Hum Brain Mapp. 2004;21:221–235. doi: 10.1002/hbm.20000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lei X, Wu T, Jiang T. A review of EEG and MEG for brainnetome research. Cogn Neurodyn. 2014;8:87–98. doi: 10.1007/s11571-013-9274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumsteg D, Wennberg RA, Treyer V, Buck A, Wieser HG. H2(15)o or 13nh3 pet and electromagnetic tomography (loreta) during partial status epilepticus. Neurology. 2005;65:1657–1660. doi: 10.1212/01.wnl.0000184516.32369.1a. [DOI] [PubMed] [Google Scholar]
- Zumsteg D, Lozano AM, Wennberg RA. Depth electrode recorded cerebral responses with deep brain stimulation of the anterior thalamus for epilepsy. Clin Neurophysiol. 2006;117:1602–1609. doi: 10.1016/j.clinph.2006.04.008. [DOI] [PubMed] [Google Scholar]