Abstract
Background
Use of computed tomography (CT) for diagnostic evaluation has increased dramatically over the past two decades. Even though CT is associated with substantially higher radiation exposure than conventional x-rays, typical clinical doses are not known. We sought to estimate the radiation dose associated with common CT studies in clinical practice; assess variation in dose across types of studies, patients, and institutions; and quantify the potential cancer risk associated with these examinations.
Methods
Retrospective cross-sectional study describing radiation dose associated with the 11 most common types of diagnostic CT studies performed on 1,119 consecutive adult patients at four San Francisco Bay Area institutions between January 1 and May 30, 2008. We estimated lifetime attributable risks of cancer by study type from these measured doses.
Results
Radiation doses varied significantly between the different types of CT studies. The overall median effective doses ranged from 2.1 milli-Sieverts (mSv) for a routine head CT (interquartile range [IQR] 1.8–2.8) to 31 mSv (IQR 21–43) for a multiphase abdomen and pelvis CT. Within each type of CT study, effective dose varied significantly within and across institutions, with a mean 13-fold variation between the highest and lowest dose for each study type. The estimated number of CTs that will lead to the development of a cancer varied widely depending on the specific type of CT examination and the patient’s age and sex. An estimated 1 in 270 women who underwent a coronary angiography CT at age 40 will develop cancer from that CT (1 in 600 men), compared with an estimated 1 in 8,100 women who had routine head CT at the same age (1 in 11, 080 men). For 20-year olds the risks were approximately doubled, and for 60-year olds, the risks were approximately 50% lower.
Conclusion
Radiation doses from commonly performed diagnostic CT examinations were higher and more variable than generally quoted, highlighting the need for greater standardization across institutions. For individuals, and especially younger female patients, the benefits of CT imaging must be balanced against the potential harm from its associated radiation.
INTRODUCTION
Utilization of computed tomography (CT) has increased dramatically over the past several decades. 1 The total number of CT examinations performed annually in the United States has risen from approximately 3 million in 1980 to nearly 70 million in 20072, 3 Integrating CT into routine care has improved patient health care dramatically, and CT is widely considered among the most important advances in medicine. However, CT delivers much higher radiation doses than do conventional diagnostic x-rays. For example, a chest CT typically delivers more than a hundred times the radiation dose of a routine frontal and lateral chest x-ray 4, 5 Further, radiation exposure from CT examinations has also increased, in part due to the increased speed of image acquisition allowing vascular, cardiac, and multiphase examinations, all associated with higher doses. Thus, greater utilization of CT has resulted in a concurrent increase in the medical exposure to ionizing radiation. 2, 6
Exposure to ionizing radiation is of concern, because evidence has linked exposure to low-level ionizing radiation at doses used in medical imaging to the development of cancer. The National Academy of Sciences’ National Research Council comprehensively reviewed biological and epidemiological data related to health risks from exposure to ionizing radiation, recently published as the Biological Effects of Ionizing Radiation (BEIR) VII Phase 2 report.7 The epidemiologic data described atomic bomb survivors, populations who lived near nuclear facilities during accidental releases of radioactive materials such as Chernobyl, workers with occupational exposures, and populations who received exposures from diagnostic and therapeutic medical studies. Radiation doses associated with commonly used CT examinations resemble doses received by individuals in whom an increased risk of cancer was documented. For example, an increased risk of cancer has been identified among long-term survivors of the Hiroshima and Nagasaki atomic bombs who received exposures of 10–100 milliSieverts (mSv).8–11 A single CT scan can deliver an equivalent radiation exposure, 12 and patients may receive multiple CT scans over time. 13
Even though the risk to an individual patient may be small, the increasingly large number of people exposed, coupled with the increasingly high exposure per examination, could translate into many cases of cancer resulting directly from the radiation exposure from CT. It is important to understand how much radiation medical imaging delivers, so this potential for harm can be balanced against the potential for benefit. This is particularly important as the threshold for using CT has declined, and CT is increasingly being used among healthy individuals, where the potential for carcinogenesis could outweigh its diagnostic value. To date, relatively few data describe how much radiation is received through the most common types of CT examinations when applied in clinical practice, as most published studies focused on phantom studies. CT coronary angiography is the only examination that has been studied in detail. Our study aimed to estimate how much radiation exposure is associated with the types of CT examinations performed most commonly in the United States; to estimate variation across study types, patients, and institutions; and to use these data to estimate the lifetime attributable risk of cancer associated with these tests.
METHODS
Data were collected at four institutions in the San Francisco Bay Area: the University of California, San Francisco (UCSF), a 600-bed academic medical center in San Francisco; Alta Bates Summit Medical Center, a 555-bed private, community-based medical center in Berkeley; Marin General Hospital, a 235-bed acute care hospital serving Marin County; and California Pacific Medical Center, a private, community-based hospital with 1300 beds in San Francisco. These facilities were selected because of their relatively large size, diverse San Francisco Bay area locations which allow for geographic diversity, availability of Picture Archiving and Communications Systems (PACS) that let us select particular types of CT examinations on consecutive patients at each institution, and reporting systems that allowed us to query the clinical reasons studies were ordered. Further, each institution used the same manufacturer’s CT scanners, letting us collect dose information consistently across sites. The institutional review boards at each participating institution approved the study.
Selection of Specific CT Study Types
We abstracted radiation dose information on the most commonly performed types of diagnostic CT examinations. To determine the most frequent CT study types, we queried the UCSF Radiology Information System for all CT examinations performed in a single month (March 2008) and defined common study types as the 11 composing at least 1% of the total number of CT examinations (Table 1). We excluded examinations performed in association with a therapeutic procedure, such as CT-guided abscess drainage.
Table 1.
Types of CT examinations included in our report and the typical clinical indications that led to these examinations
| Anatomic Area | Protocol | Clinical Indications |
|---|---|---|
| Head and Neck | Routine Head | Focal neurologic signs or symptoms suspicious of hydrocephalus, hemorrage or neoplasia; trauma |
| Routine Neck | Pain; trauma; mass; suspect abscess | |
| Suspected Stroke | Focal neurological signs or symptoms suspicious for stroke; acute headache with risk factors for aneurysm | |
| Chest | Routine Chest, no contrast | Pain; trauma; hypoxia; suspect neoplasia |
| Routine Chest, with contrast | Pain; trauma; hypoxia; suspect neoplasia | |
| Suspected Pulmonary Embolism | Pain; tachycardia; shorteness of breath; hypoxia; suspect PE | |
| Coronary Angiogram | Ischemia, suspicion of stenosis; assess bypass grafts, coronary artery anomalies; acute chest pain | |
| Abdomen and Pelvis | Routine Abdomen-Pelvis, no contrast | Pain; trauma; suspect abscess, appendicitis, neoplasia |
| Routine Abdomen-Pelvis, with contrast | Pain; trauma; suspect neoplasia; fever of unknown origin; suspect abscess, appendicitis or diverticulitis | |
| Multiphase Abdomen-Pelvis | Suspect liver, pancrease or renal neoplasia; suspect hepatitis or pancreatitis; suspicion of renal stone | |
| Suspected Aneurysm or Dissection | Acute or radiating chest or back pain; trauma, vasculitis | |
Selection of Patient Studies
We sampled 20–30 consecutive patients aged 18 and older from each of the four institutions for each of the study types, yielding a sample of 80–120 subjects who underwent each study type between January 1, 2008 and May 31, 2008 for a total sample of 1,119 patients. Our assessment of the dose associated with CT coronary angiography is limited to two institutions that routinely saved radiation dose data from this study type. For each patient, the technical parameters and dose report data (scan area, scan length, slice thickness, kVp, mAs, pitch, and dose length product) were abstracted from the CT images.
Radiation Dose
It is impractical to directly measure the radiation dose absorbed by individual patients even when the radiation emitted by a machine is precisely known. Instead, radiation exposure may be quantified using various methods. We used effective dose to quantify the radiation exposure associated with each CT examination, as this is one of the most frequently reported measurements. 14 Further, effective dose allows comparison across the different types of CT studies and between CT and other imaging tests, facilitating comparison of CT to the most common radiology studies patients undergo. The effective dose accounts for the amount of radiation, the exposed organs, and each organ’s sensitivity to developing cancer from radiation exposure. An explanation and glossary is included in Appendix 1. We estimated the effective dose using the dose length product (DLP), which is recorded as part of the CT scan. The DLP is an approximation of the total energy a patient absorbs from the scan. We combined the DLP with organ-specific conversion factors to account for the sensitivities of different organs to developing radiation-induced cancer. 15, 16 A comparison of our approach to a more detailed approach based on organ-specific dose estimates using a computer software program (ImPACT CT Patient Dosimetry Calculator version 0.99×)17 is included in Appendix 2.
Analysis
Descriptive statistics of the effective doses were calculated for each CT study type, and differences within and across institutions were assessed using analysis of variance (ANOVA). Because the distributions of doses were right-skewed, we modeled the log-transformation of dose to better satisfy ANOVA’s assumption of normally distributed outcomes. To calculate the variation in dose, for each CT study type, we calculated the difference between the highest and lowest dose observed. To put the dose estimates in context that patients and physicians can readily understand, the effective dose for each CT study type was compared with the effective doses for the United States’ two most common conventional radiology studies: a frontal and lateral chest x-ray series (effective dose of 0.065 mSv)18 and a screening mammography series (including 2 views of each breast, effective dose of 0.42 mSv).18
Although effective dose best reflects a patient’s overall exposure to radiation, organ-specific dose may be more appropriate for estimating lifetime cancer risk for non-uniform exposures such as CT. For example, if a patient undergoes an imaging study that radiates only the breast, her risk of cancer from that examination will primarily reflect her increase in breast cancer. As an example of how organ-specific dose varies between CT and conventional radiography, we show for CT coronary angiography (which primarily imparts radiation to the lungs and to the breasts), a comparison between its organ-specific absorbed doses with those of a chest series (lung dose=0.06 milliGray [mGy]18) and a mammography series (breast dose=3.5 mGy).18
Estimating Lifetime Attributable Cancer Risk
The BEIR VII (2006) report provides a method to estimate lifetime attributable risk of cancer based on the magnitude of a single radiation exposure and a patient’s age at the time of that exposure.7 The lifetime attributable risk is defined as additional cancer risk above and beyond baseline cancer risk. This can be calculated for specific cancers as well as for all cancers combined. The age- and sex-specific lifetime attributable risk of all cancer incidence for the median and interquartile range of effective doses, for each type of study, was calculated using the BEIR VII risk estimates. We used all cancer as the outcome to compare all types of CT studies included in this report. For comparison purposes, we also estimated the lifetime attributable risk of cancer using a second approach for a subset of patients for whom we have more detailed dose information (see Appendix 3 and Appendix Figure 1) and we used these results to develop an adjustment. We estimated the number of patients undergoing CT that would lead to the development of one radiation-induced cancer, by type of CT examination, age at the time of exposure and sex. For each type of study, we also ranked the patients from those who received the lowest to highest effective dose, and calculated the adjusted lifetime attributable risk of cancer corresponding to each effective dose, had those doses been received by patients aged 20, 40 or 60.
RESULTS
Table 1 shows the types of CT studies we examined and the clinical indications that led to them. Across all study types, the mean patient age was 59 years and 535 of the 1,119 patients (48 percent) were female. These 11 study types comprise approximately 80% of all CTs performed. The remaining types of CT studies not included reflect a large number of additional study types, none of which contributed more than 1% to the total number of CTs.
Variation in Dose Between Study Types
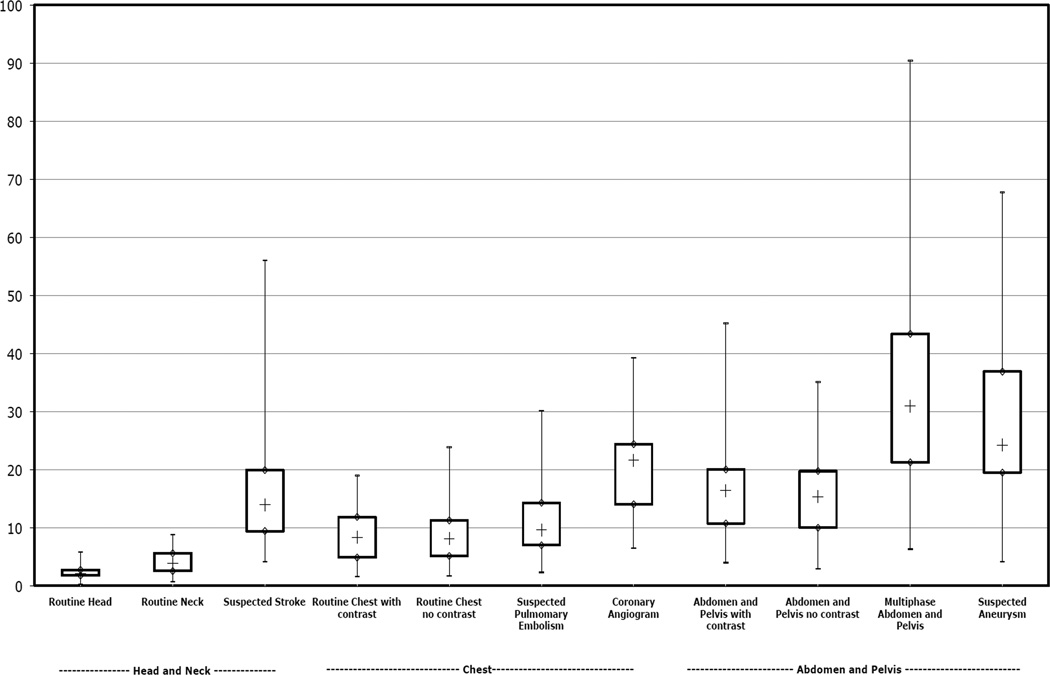
Within each anatomic area, the median effective dose varied widely between study types (Table 2). For scans of the head and neck, the median effective dose varied from 2.1 mSv for a routine head (interquartile range [IQR] 1.8 to 2.8 mSv) to 14 mSv (IQR 9.4 to 20 mSv) for a suspected stroke CT. For chest scans, the median effective dose varied from 8.2 mSv (IQR 5.1 to 11 mSv) for a routine chest to 22 mSv (IQR 14 to 24 mSv) for coronary angiography (IQR 14 to 24 mSv). For abdomen and pelvis scans, a routine CT without contrast had the lowest median effective dose (15 mSv [IQR 10 to 20 mSv]), whereas a multiphase abdominal and pelvis CT had the highest median effective dose (31 mSv [IQR 21 to 43 mSv]). For each anatomic area, studies that included an assessment of arteries (i.e., suspected stroke, coronary angiography and suspected aneurysm or dissection) and the multiphase studies had higher exposures, resulting from the use of repeated series with these study types. Table 2 also shows the comparable number of conventional projection radiographs that result in a similar effective dose. The median effective dose delivered through a single CT scan was as high as 74 mammography series and 442 chest x-ray series. Our comparison of organ specific doses demonstrated that a CT coronary angiogram delivers a dose to the breast equivalent to approximately 15 mammography studies (51 mGy breast dose for CT coronary angiogram versus 3.5 mGy breast dose for a mammography series) and delivers a dose to the lung equivalent to 711 chest x-ray series (64 mGy lung dose for CT coronary angiogram versus 0.09 mGy lung dose for a PA and lateral chest x-ray).
Table 2.
Median effective radiation dose for each type of CT study
| Anatomic Area | Type of CT Study | CT Effective dose (mSv) |
Conventional Radiographs Resulting in Equivalent Dose |
|||
|---|---|---|---|---|---|---|
| Median | Interquartile Range |
Absolute Range [Min, Max] |
Chest X-ray Series |
Mammography Series |
||
| Head and Neck | Routine Head | 2.1 | 1.8 2.8 | [0.27,5.8] | 30 | 5 |
| Routine Neck | 3.9 | 2.6 5.6 | [0.72, 8.8] | 55 | 9 | |
| Suspected Stroke | 14 | 9.4 20 | [4.1, 56] | 199 | 33 | |
| Chest | Routine Chest, no contrast | 8.2 | 5.1 11 | [1.7, 24] | 117 | 20 |
| Routine Chest, with contrast | 8.3 | 4.9 12 | [1.6, 19] | 119 | 20 | |
| Suspected Pulmonary Embolism | 9.6 | 7.0 14 | [2.3, 30] | 137 | 23 | |
| Coronary Angiogram | 22 | 14 24 | [6.5, 39] | 309 | 51 | |
| Abdomen - Pelvis | Routine Abdomen-Pelvis, no contrast | 15 | 10 20 | [2.9, 43] | 220 | 37 |
| Routine Abdomen-Pelvis, with contrast | 16 | 11 20 | [4.0, 45] | 234 | 39 | |
| Multiphase Abdomen-Pelvis | 31 | 21 43 | [6.4, 90] | 442 | 74 | |
| Suspected Aneurysm or Dissection | 24 | 20 37 | [4.1, 68] | 347 | 58 | |
Variation in Dose within Study Types
Even within study type, radiation dose varied substantially (Figure 1). There was a mean 13-fold variation between the highest and lowest dose for each CT study type included (range 6- to 22-fold difference across the different study types). The effective doses tended to be higher and more variable in the abdomen and pelvis, where the widest range in dose was documented for multiphase abdomen and pelvis CT (range 6.4 mSv to 90.4 mSv). The variation in doses occurred both within and across institutions (Table 3). The mean doses differed two-fold across institutions; and for several of the study types, the mean dose across institutions differed by three-fold or more. For example, the mean effective dose for a suspected stroke CT was 7.6 mSv (SD 2 mSv) at site 3 compared to 28.5 mSv (SD 8.0 mSv) at site 4. We observed no consistent pattern for which institution had the highest radiation dose; rather, each site had the highest dose for at least one of the included study types.
Figure 1.
Box-plot demonstrating distribution of estimated effective dose for each CT study type, grouped by anatomic area. The “whiskers” show the minimum and maximum observed values.
- Minimum, Maximum;
◊ First Quartile, Third Quartile;
+ Median
Table 3.
Mean (SD) effective dose for each type of CT study at each of the four sites
| Anatomic Area | Type of CT Study | Site 1 | Site 2 | Site 3 | Site 4 | P value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | |||
| Head and Neck | Routine Head | 2.8 | (1.0) | 1.8 | (0.3) | 2.8 | (1.2) | 1.9 | (0.4) | p<.00001 |
| Routine Neck | 2.8 | (1.0) | 5.9 | (1.5) | 5.1 | (1.4) | 2.3 | (0.6) | p<.00001 | |
| Suspected Stroke | 18 | (13) | 15 | (3.3) | 7.6 | (2.0) | 29 | (8.0) | p<.00001 | |
| Chest | Routine Chest, no contras | 5.2 | (2.6) | 12 | (6.6) | 11 | (3.9) | 7.4 | (3.4) | p<.00001 |
| Routine Chest, with contrast | 6.6 | (5.4) | 11 | (4.8) | 11 | (4.0) | 7.9 | (3.8) | p<.00001 | |
| Suspected Pulmonary Embolism | 8.1 | (3.4) | 21 | (6.8) | 9.0 | (2.0) | 9.0 | (3.3) | p<.00001 | |
| Coronary Angiogram | 21.4 | (8.9) | 19.7 | (6.2) | p=.75 | |||||
| Abdomen and Pelvis | Routine Abdomen-Pelvis, no contrast | 12 | (7.0) | 19 | (7.4) | 20 | (7.2) | 12 | (5.2) | p<.00001 |
| Routine Abdomen-Pelvis, with contrast | 12 | (6.1) | 16 | (6.7) | 20 | (7.4) | 15 | (6.2) | p=.00027 | |
| Multiphase Abdomen-Pelvis | 24 | (13) | 35 | (8.0) | 45 | (14) | 34 | (17) | p= .00001 | |
| Suspected Aneurysm or Dissection | 49 | (14) | 25 | (18) | 22 | (8.0) | 25 | (10) | p<.00001 | |
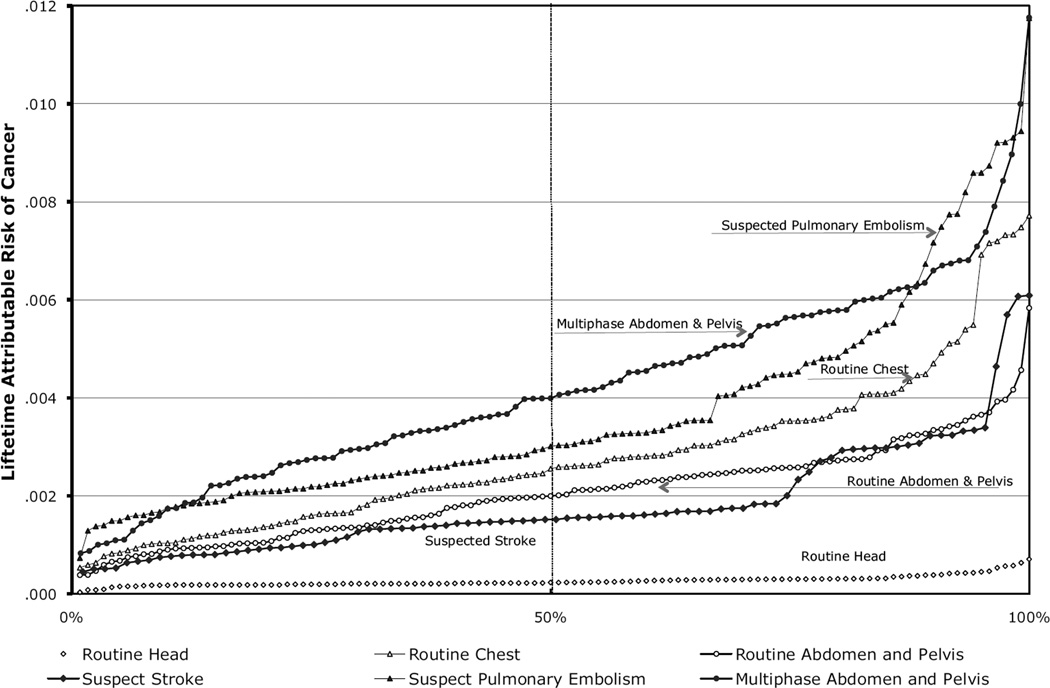
Adjusted Lifetime Attributable Risks of Cancer
For six of the study types, the estimated effective doses for each study type, sorted from the lowest (1%) to the highest (100%) across patients and the corresponding adjusted lifetime attributable risk of cancer assuming all exams were received by a 20-year-old woman, are shown in Figure 2. For a routine head CT, the median effective dose was 2.1 mSv, and the corresponding median adjusted lifetime attributable risk of cancer was 0.23 cancers per 1,000 patients (range=0.03–0.70 cancers per 1,000 patients). For a multiphase abdomen and pelvis CT, the median effective dose was 31 mSv, and the corresponding median adjusted lifetime attributable risk of cancer was 4 cancers per 1,000 patients (range=0.83–11.1 cancers per 1,000 patients). For some study types, the range in the associated effective dose was narrow, with a correspondingly narrow range in the adjusted lifetime attributable risks of cancer (e.g., routine head CT). In contrast, the effective dose for most studies had a much wider range with a correspondingly broad range in the adjusted lifetime attributable risks of cancer.
Figure 2.
Estimated range in the lifetime attributable risk of cancer if a 20-year-old woman underwent one of several types of CT studies using the distribution in radiation dose exposure from our report.
The Estimated Number of CTs that Would Lead to Cancer by Study Type
For each study type, Table 4 shows the estimated number of patients undergoing CT that would lead to the development of one radiation-induced cancer, by age at exposure and sex. As expected, the number of CTs that would result in a cancer varies widely by sex, age, and study type. Reflecting a higher cancer risk of radiation among women, it would take far fewer CTs to result in a cancer among women compared with men. Coronary angiography had the lowest number of CTs that would result in a single cancer. Among 40-year-old women who underwent a coronary angiography CT, we estimate that one cancer would be expected to occur among approximately 270 women as a result of the radiation exposure of the examination (IQR 1 in 250 to 1 in 420.) In contrast, it would take the largest numbers of routine head CTs to result in a single cancer. Among 40-year-old females, one cancer would occur among 8,105 patients who underwent a routine head CT (IQ 1 in 6110 to 1 in 9500.) For a 60-year old woman, the risks were substantially lower and varied from approximately 1 in 420 exams for coronary angiography CT (IQR 1/370 to 1/640) to 1 in 12,250 examinations for a routine head CT (IQR 1/9,230 to 1/14,360). For a 20-year old woman, the risks were substantially higher and varied from approximately 1 in 150 exams for coronary angiography CT (IQ range 1/130 to 1/230) to 1 in 4,360 examinations for a routine head CT (IQ 1/3290 to 1/5110).
Table 4.
Estimated number of patients under going CT that would lead to the development of one radiation-induced cancer, by type of CT examination and age at the time of exposure, based on the median and interquartile radiation dose observed
| Anatomic Area | Type of CT Study | 20 Year Old | 40 Year Old | 60 Year Old | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female |
Male |
Female |
Male |
Female |
Male |
||||||||
| Median | (25% 75%) | Median | (25% 75%) | Median | (25% 75%) | Median | (25% 75%) | Median | (25% 75%) | Median | (25% 75%) | ||
| Head and Neck | Routine Head | 4360 | (3290, 5110) | 7350 | (5540, 8620) | 8100 | (6110, 9500) | 11080 | (8350, 12990) | 12250 | (9230, 14360) | 14680 | (11070, 14680) |
| Routine Neck | 2390 | (1640, 3540) | 4020 | (2770, 5970) | 4430 | (3050, 6580) | 6058 | (4170, 8990) | 6700 | (4620, 9940) | 8030 | (5530, 8030) | |
| Suspected Stroke | 660 | (460, 980) | 1120 | (770, 1650) | 1230 | (850, 1820) | 1682 | (1170, 2490) | 1860 | (1290, 2750) | 2230 | (1550, 2230) | |
| Chest | Routine Chest, no contrast | 390 | (290, 630) | 1040 | (770, 1670) | 720 | (540, 1160) | 1566 | (1170, 2520) | 1090 | (820, 1760) | 2080 | (1550, 2080) |
| Routine Chest, with contrast | 380 | (270, 650) | 1020 | (710, 1740) | 720 | (500, 1210) | 1538 | (1070, 2620) | 1070 | (750, 1830) | 2040 | (1420, 2040) | |
| Suspected Pulmonary Embolism | 330 | (230, 460) | 880 | (610, 1220) | 620 | (420, 850) | 1333 | (920, 1840) | 930 | (640, 1280) | 1770 | (1220, 1770) | |
| Coronary Angiogram | 150 | (130, 230) | 390 | (350, 610) | 270 | (250, 420) | 595 | (540, 920) | 420 | (370, 640) | 790 | (710, 790) | |
| Abdomen and Pelvis | Routine Abdomen-Pelvis, no contrast | 500 | (380, 770) | 660 | (510, 1024) | 930 | (710, 1430) | 1002 | (770, 1540) | 1400 | (1080, 2160) | 1330 | (1020, 1330) |
| Routine Abdomen-Pelvis, with contrast | 470 | (380, 700) | 620 | (510, 930) | 870 | (710, 1300) | 942 | (770, 1400) | 1320 | (1080, 1960) | 1250 | (1020, 1250) | |
| Multiphase Abdomen-Pelvis | 250 | (180, 370) | 330 | (240, 490) | 460 | (330, 680) | 498 | (360, 730) | 700 | (500, 1030) | 660 | (480, 660) | |
| Suspected Aneurysm or Dissection | 320 | (210, 390) | 420 | (280, 510) | 590 | (390, 710) | 636 | (420, 770) | 890 | (580, 1080) | 840 | (550, 840) | |
DISCUSSION
We documented higher and more variable doses than typically quoted from the most common types of diagnostic CT studies performed in clinical practice. For example, the median effective dose of an abdomen and pelvis CT (the most common type of CT examination performed in the United States 12) is often quoted as 8–10 mSv. 6, 16, 19 Yet we found the median dose of a routine abdomen and pelvis was 66% higher, and the median dose of a multiphase abdomen and pelvis was nearly four-fold higher. Further, we found substantial variation in doses within and across institutions, with a mean 13-fold variation between the highest and lowest dose for each CT study type included. Thus, depending on where an individual patient received imaging and the specific technical parameters used, the effective dose received could substantially exceed the median. While some of this variation may be clinically indicated to accommodate patients of different size or the specifics of the clinical question that was being addressed, the variation in effective dose was dramatic, and of greater magnitude than widely considered acceptable, particularly considering that the patients were already stratified within relatively well-defined clinical groups. The variation in dose across the four clinical sites reflects site-specific methods of choosing different technical parameters to answer the same clinical question.
The corresponding lifetime attributable risks of cancer were also higher than typically reported and markedly variable by study type, patient, and hospital. For example, it is commonly reported that a CT scan may be associated with an increase in the risk of cancer of approximately 1 in 2,000. 2, 19 Based on the highest effective dose we observed, a 20-year-old female who underwent suspected pulmonary embolism CT, coronary angiography CT, or a multiphase abdomen and pelvis CT could have an associated increased risk of developing cancer of as high as 1 in 80 (Figure 2). The risks declined substantially with age and were lower for males, so radiation-associated cancer risks are of particular concern for younger, female patients. It is precisely because the risks of cancer are so high among younger patients that we chose to illustrate the risk of cancer when CT is used in a 20-year-old female patient. Although it is generally assumed that very little CT imaging occurs in children and young adults, approximately 5% of all CT examinations occur in children, 10% of all CT examinations occur in those age 20–30, and 5% of 20-year-old patients undergo CT imaging per year. 20 And the frequency of CT imaging in children and young adults is increasing.
The doses we documented may be higher than typically reported for three main reasons. First, we estimated radiation doses received by patients in clinical practice, whereas many previous studies have assessed the dose received in idealized settings on phantoms—sophisticated plastic models created to measure dose when put in a real scanners. Study parameters applied in phantoms may differ substantially from those used in actual clinical settings.21, 22
Second, most prior work described experience at a single institution or a single type of CT examination study, where the specific instructions for conducting studies may be standardized. We studied patients in clinical practice, imaged for a range of symptoms and clinical indications and across different institutions, where there was no standardization related to our study. For example, a common clinical indication for a multiphase abdomen and pelvis CT is suspicion of renal cancer in patients with hematuria (blood in the urine). This type of study may start out as a single phase, non-contrast examination (a low dose study to assess for renal calculi), but may be expanded to include contrast and multiple phases of imaging to evaluate for renal or bladder cancer (a resulting high dose study). In fact, the variation in the evaluation of this symptom was dramatic, with large differences in the number of series that were obtained, both within and across institutions, contributing to the large difference in means and standard deviations for this study type.
Third, most prior work grouped all studies within the same anatomic area together; however, even within one anatomic area, not all CTs involve similar doses. Protocols requiring more images by increasing the scan length or repeatedly scanning through the same area result in higher radiation exposure. For example, an increasingly common indication for CT is to assess a patient for the possibility of pulmonary embolism. The mean effective doses for three of the hospitals for the suspected pulmonary embolism CT were 8.1, 9.0, and 9.0 mSv, whereas the mean effective dose for the fourth site was 21.4 mSv. The fourth was the only site where, in addition to images through the chest to directly assess for pulmonary embolism, they also increased the scan length and scanned through the patient’s pelvis and proximal thighs to assess for the presence of deep vein thromboses (DVTs). While it is not uncommon, nor necessarily unreasonable, to include lower-extremity venography when a patient is referred for suspected pulmonary embolism CT—pulmonary embolisms and DVTs are considered two manifestations of one pathologic process and share the same treatment 23—this difference in CT protocol leads to a substantial increase in radiation exposure and, thus, cancer risk. We found radiation exposure was more than two-fold greater for this study type when the extra images were included. A two-fold difference in average radiation exposure is not insignificant and needs to be considered when specific protocols are set, and needs to be understood by referring clinicians when they weigh the risks and benefits of this study.
The possibility that CT may cause more cancers than it prevents has been raised with respect to full body screening CT examinations conducted in asymptomatic persons. 24 In contrast, CT is generally considered to have a very favorable risk: benefit profile among symptomatic patients. However, the threshold for using CT has declined, so that it is no longer used only in very sick patients, but rather, it is used in those with mild, self-limited illness who are otherwise healthy. In these patients, the value of CT needs to be balanced against this small, but real risk of carcinogenesis resulting from its use. Neither physicians 25, 26 nor patients 27 are generally aware of the radiation associated with CT, its risk of carcinogenesis, nor the importance of limiting exposure among younger patients. It is important to make both physicians and patients aware that this risk exists.
Consensus is growing that patients’ exposure to radiation through medical imaging needs to be reduced, and we believe three general approaches should be taken. First, CT examination protocols and techniques should be optimized and standardized to limit the radiation associated with individual scans. This would include standardizing protocols across sites, reducing multiple series within each examination, implementing dose reduction strategies, and encouraging participation in accreditation programs such as that offered by the American College of Radiology. Practically, these guidelines have not been widely embraced, perhaps because no regulatory component is associated with their use. While the Food and Drug Administration (FDA) requires manufacturers to record dosing information when phantoms are scanned using set imaging parameters, they do not set upper bounds for these doses and the FDA does not monitor or regulate dose associated with clinical CT applications. In contrast, the FDA monitors and regulates the dose associated with mammography examinations and has successfully standardized the associated doses. Creating specific standards for CT examinations and requiring adoption would likely lead to a reduction in mean and outlier doses. For example, for some CT study types, dose reduction techniques can reduce the dose by 50%. 28 Great Britain and several European countries have been more aggressive in trying to limit radiation exposure from CT with some success. 29 Interestingly, a recent report of the doses associated with coronary angiography CT documented substantial variation in dose across facilities, but the mean effective dose they report was approximately half the effective dose we found for the same type of CT study.30 Many of the study sites were in the UK and Europe where efforts to minimize radiation dose have been ongoing for several years. Among pediatric patients, efforts have been more common and successful to reduce the radiation dose, 31 in part resulting from articles highlighting that when standard adult settings are used in children, the resulting cancer risks are much higher. 32
A second approach to minimize medical radiation exposure should focus on reducing the number of CT examinations. Although difficult to fully assess, it has been reported that 30% or more of the CT examinations currently performed may be unnecessary. The European Commission Office of Radiation Protection and the Canadian Association of Radiologists developed guidelines highlighting where CT imaging should be curtailed, 33, 34 including repeating investigations that have already been done; investigations unlikely to affect patient management because a positive finding is irrelevant, such as assessment and surveillance of incidental findings; investigating too often—before the disease could have progressed or before the results could influence treatment; performing the wrong investigation; and over-investigating. Many CT examinations in the United States almost certainly fall into these categories, for example, the repeated use of CT for patients with documented renal stones, and more explicit discussion and guidelines are needed on how to reduce these unnecessary CT studies.
The third approach to reducing exposure may be to track and collect dose information at the patient level, as patients may undergo repeated imaging over time. 13 Tracking detailed dose information at the patient level, and in a system-wide fashion such as within a searchable, electronic medical record, would help educate patients and providers about radiation exposure and could facilitate activities to minimize dose where possible. The impact of this could be particularly dramatic among the subset of patients who have repeated imaging and who are thus at greatest risk of radiation-associated cancer.
Our study has several strengths. We collected data from four large institutions, including about 100 patients for each type of study, and results were collected on consecutive patients for each study type at each institution. We also included the most frequent types of CT examinations patients undergo, making the results highly relevant. Further, we collected data from actual clinical practice, not a when a protocol was used, and no one knew the study was being conducted so they couldn’t change practice. Our study also has several weaknesses. Our cohort was insufficiently large to understand the reasons for variation of dose associated with each type of study, including the technologist’s experience, the availability of physicians to check studies in real time that might lead them to add or subtract additional series, geographic variation, type and specific dose-reduction or -modulation algorithms available or used, and patient level factors (such as weight) that may have led to differences in dose. Our work highlights the pressing need for large national studies to understand how these factors contribute to variation in dose.
Similarly, we did not assess the relationship between image quality and radiation dose; there is a pressing need to determine optimum dose for each type of study that balances image quality, with keeping the dose as low are possible. We grouped studies by the clinical indications that led to the studies, but there may have been imprecision in our characterizing the indications that led to these studies. All scans were performed using a single manufacturer’s scanners, but doses depend on manufacturer and model. Limiting the results to a single manufacturer will have underestimated the true variation in dose. The methods we used to assess dose and estimate lifetime risks of cancer are imprecise. We presented effective dose calculated using the scanner-provided DLP measurement, because this is simple to calculate, straightforward, and reliable, and thus can be used as an easy starting point to begin to record patient-level exposure; but, different metrics will yield slightly different estimates, and these methods are based on assumptions of patient size that may not be applicable to all patients. However, this method is highly concordant with other methods of estimating dose. 21, 35 Similarly, we used a simple method to estimate lifetime attributable risk of cancer, but found high agreement with a more detailed method that relies on organ doses calculated with computer simulation models. Furthermore, several other uncertainties exist in the methods used to project lifetime risk from radiation exposure,7 so the lifetime attributable risks should not be viewed as exact patient risks. Lastly, the lifetime attributable risk of cancer needs to be put into context of the patients’ remaining life expectancy, and our calculations were based on the assumption of normal life expectancy. For individuals with lower life expectancy, these estimates will overestimate their lifetime risk; if mortality rates were increased by 20%, the risks of carcinogenesis would have been overestimated by 5%. 36
The radiation exposure associated with CT has increased substantially over the past two decades, and efforts need to be undertaken to minimize radiation exposure from CT, including reducing unnecessary studies, reducing the dose per study, and reducing the variation in dose across patients and facilities. Patient outcomes studies are needed to help define when CT leads to the greatest benefit, and when these studies may have no impact, so that the radiation may be a greater risk than the benefit expected from the examinations. Understanding exposures to medical radiation delivered through actual clinical studies is a crucial first step toward developing reasonable strategies to minimize unnecessary exposures.
Supplementary Material
Acknowledgment
The authors acknowledge Edward Baker, MD (California Pacific Medical Center, San Francisco, CA), Dennis Orwig, MD (Marin General Hospital, Greenbrae, CA), and Erik Gaensler, MD (Alta Bates Medical Center, Berkeley, CA) for their generous help in completing this work. The UCSF School of Medicine Bridge Funding Program, NIH NIBIB Training Grant T32 EB001631 and NIH NCI Grants CA125036 and CA 131698 provided funding for this work. None of the funders were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. The PI had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix 1: Dosimetry Quantities and Glossary of Terms
When radiation interacts with biological matter, the resulting biological effect depends on the amount of radiation energy absorbed into the material and the type of radiation. Organ dose or organ specific absorbed dose is defined as the energy imparted per unit mass of an organ or tissue. The absorbed dose is measured in Gray (Gy), which equals 1 joule per kilogram. The Gy replaced the rad (radiation absorbed dose), the traditional unit of absorbed dose, which is equal to 0.01 Gy.
Different types of radiation (e.g., alpha, beta, neutron, photon, etc) result in different amounts of biological damage, even when the absorbed doses are the same. The greater the rate at which the radiation transfers energy to tissue, the greater the biological damage. The term equivalent dose was introduced to reflect the different biological effects of different radiation types. The equivalent dose is calculated by multiplying absorbed dose by radiation weighting factor. The radiation-weighting factor is unity for the type of radiation that comes from conventional x-rays and CT (photons) and therefore, equivalent dose and absorbed dose are the same for radiation exposure from CT scans. The equivalent dose is measured in Sievert (Sv). The traditional unit of equivalent dose was the rem (1 rem = 0.01 Sv).
When a part of the body is exposed (a common situation from medical radiation exposure), the biological damage depends on the exposed organ’s sensitivity to that radiation. The International Commission on Radiation Protection (ICRP) introduced the concept of a tissue-weighting factor, which represents the relative contribution of each tissue or organ to the total effects resulting from uniform irradiation of the whole body. Therefore, effective dose is defined as the tissue-weighted sum of the equivalent doses to specified organs and tissues of the body. The effective dose reflects the radiation effects of a non-uniform exposure in terms of an equivalent whole body exposure. Therefore, effective dose can be used to compare radiation exposure across the different types of CT studies and across the different medical study modalities.
Absorbed dose: the amount of energy absorbed by ionizing radiation in a unit mass of tissue. The unit is the Gray (Gy), defined as joules per kilogram. The unit of Gray can be used for any type of radiation, but it does not describe the biological effects of different types of radiation.
Radiation weighting factor: a dimensionless factor by which the organ absorbed dose (rad or gray) is multiplied to obtain a quantity that expresses, on a common scale for all ionizing radiation, the biological damage (rem, Sievert) to an exposed person. It is used because some types of radiation, such as alpha particles, are more biologically damaging internally than others. It is used to derive the equivalent dose from the absorbed dose averaged over a tissue or organ.
Equivalent dose: a quantity used in radiation protection to place all radiation on a common scale for calculating tissue damage. This relates the absorbed dose in human tissue to the effective biological damage of the radiation. Equivalent dose is the absorbed dose in grays times the radiation-weighting factor. The radiation-weighting factor accounts for differences in radiation effects caused by different types of ionizing radiation. Some radiation, including alpha particles, causes a greater amount of damage per unit of absorbed dose than other radiation. The Sievert (Sv) is the unit used to measure equivalent dose.
Tissue weighting factor (wR):
The factor by which the equivalent dose in a tissue or organ is weighted to represent the relative contribution of that tissue or organ to the total health detriment resulting from uniform irradiation of the body.
Effective dose: a Dosimetry quantity useful for comparing the overall health effects of non-uniform exposure in terms of an equivalent to whole body exposure. It takes into account the absorbed doses received by various organs and tissues and weighs them according to present knowledge of the sensitivity of each organ to radiation. It also accounts for the type of radiation and the potential for each type to inflict biologic damage. The unit of effective dose is the Sievert.
Appendix 2 Estimating Effective Radiation Dose
Because dose modulation techniques (automatic programs that adjust settings throughout the CT examination to minimize radiation dose) were used on many of the patient scans in our study, technical parameters needed for more detailed dose estimation could not always be easily extracted. Our method that relies on DLP automatically accounts for the variation in dose due to modulation software. However, for a subset of 18 patients who had undergone a head, chest, or abdominal CT examination, we compared the dose estimates from our approach to a more detailed approach based on a commercially available computer software program (ImPACT CT Patient Dosimetry Calculator version 0.99×).17 This involved abstracting dose data within each patient’s scan for each set of images that used a similar mAS and was thus very time consuming, given the widespread use of dose modulation techniques. Similar to a prior report 21, we found high levels of agreement in the two methods for estimating effective dose (concordance correlation coefficient rc=0.94; 95% confidence interval 0.84, 0.98).
Appendix 3 Estimating Lifetime Attributable Cancer Risk
We compared two methods of estimating the lifetime attributable risks (LAR) of cancer, one based on using total effective dose and the second using organ-specific dose. These two methods of assessing the lifetime attributable risk of cancer were compared in a subset of 18 patients described above who had undergone a head, chest, or abdominal CT examination, and we estimated the lifetime attributable risk of cancer based on sex and three ages at the time of exposure (ages 20, 40, and 60). For each examination, we used the organ-specific radiation doses generated using the ImPACT CT software, combined with the age and organ-specific BEIR VII risk estimates, to generate a lifetime attributable risk for each type of cancer. We then summed the lifetime attributable risks for each individual cancer to calculate the lifetime attributable risks for all cancers. We found moderate levels of concordance between these two methods of estimating the lifetime attributable risk of cancer: head (rc=0.77, 95% CI=0.65, 0.85), chest (rc=0.65, 95% CI=0.43, 0.80); and abdomen (rc=0.79, 95% CI=0.62, 0.89). In the head, our method of estimating lifetime attributable risk based on using total effective dose slightly overestimated risk in both men and women; in the chest, our method underestimated risk, especially in women; and in the abdomen, our method overestimated risk in women only (Appendix Figure 1A). To improve agreement, we developed an adjustment to our method of estimating the lifetime attributable risk. To adjust the lifetime attributable risks, we multiplied the sex-specific mean of the ratio of the organ-specific method to our method (head: 0.66 for women and men; chest: 1.9 for women and 1.2 for men; abdomen: 0.79 for women and no adjustment for men). The adjusted lifetime attributable risk of cancer showed an even higher degree of agreement with the organ-specific approach: in the head (rc=0.95, 95% CI=0.91, 0.98), chest (rc=0.98, 95% CI=0.96, 0.99), and abdomen (rc=0.91, 95% CI=0.84, 0.95) and was used throughout the paper.
References
- 1.Medicare Payment Advisory Commission. [accessed 15 November 2008];A Data Book: Healthcare Spending and the Medicare Program. 2007 Jun; http://www.medpac.gov/documents/Jun07DataBook_Entire_report.pdf.
- 2.Amis ES, Jr, Butler PF, Applegate KE, et al. American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol. 2007 May;4(5):272–284. doi: 10.1016/j.jacr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 3.IMV Medical Information Division. CT census database and market summary report. 2008. [Google Scholar]
- 4.Linet M, Kim K, Rajaraman P. Children’s Exposure to Diagnostic Medical Radiation and Cancer Risk: Epidemiologic and Dosimetric Considerations. Pediatr Radiol. 2009;39(S1):S4–S26. doi: 10.1007/s00247-008-1026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mettler FA, Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008 Jul;248(1):254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 6.National Council on Radiation Protection and Measurements. NCRP Report No 160, Ionizing Radiation Exposure of the Population of the United States, press release available, full report soon to be released. 2009 http://www.ncrponline.org/. Published Last Modified Date|. Accessed Dated Accessed|.
- 7.Board of Radiation Effects Research Division on Earth and Life Sciences National Research Council of the National Academies. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, D.C.: The National Academies Press; 2006. [PubMed] [Google Scholar]
- 8.Pierce DA, Preston DL. Radiation-related cancer risks at low doses among atomic bomb survivors. Radiat Res. 2000 Aug;154(2):178–186. doi: 10.1667/0033-7587(2000)154[0178:rrcral]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007 Jul;168(1):1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 10.Preston DL, Pierce DA, Shimizu Y, Ron E, Mabuchi K. Dose response and temporal patterns of radiation-associated solid cancer risks. Health Phys. 2003 Jul;85(1):43–46. doi: 10.1097/00004032-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Brenner DJ, Doll R, Goodhead DT, et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci U S A. 2003 Nov 25;100(24):13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mettler FA, Jr, Thomadsen BR, Bhargavan M, et al. Medical radiation exposure in the U.S. in 2006: preliminary results. Health Phys. 2008 Nov;95(5):502–507. doi: 10.1097/01.HP.0000326333.42287.a2. [DOI] [PubMed] [Google Scholar]
- 13.Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009 Apr;251(1):175–184. doi: 10.1148/radiol.2511081296. [DOI] [PubMed] [Google Scholar]
- 14.International Commission on Radiological Protection. Managing patient dose in multi-detector computed tomography (MDCT) 2007. [Google Scholar]
- 15.Shrimpton PC, Hillier MC, Lewis MA, Dunn M. National survey of doses from CT in the UK: 2003. Br J Radiol. 2006 Dec;79(948):968–980. doi: 10.1259/bjr/93277434. [DOI] [PubMed] [Google Scholar]
- 16.American Association of Physicists in Medicine. AAPM Report No 96. The Measurement, reporting and management of radiation dose in CT, Report of AAPM Task group 23 of the Diagnostic Imaging Council CT Committee. College Park, MD: American Association of Physicists in Medicine; 2008. [Google Scholar]
- 17.Impact Group is the UK's CT scanner evaluation centre. They provide a wide range of CT scanner related services to the UK's National Health Service. http://www.impactscan.org/.
- 18.National Cancer Institute. Radiation risks and pediatric computed tomography (CT): A guide for health care providers. 2009 http://www.cancer.gov/cancertopics/causes/radiation-risks-pediatric-CT. Published Last Modified Date|. Accessed Dated Accessed|.
- 19.What are the Radiation Risks from CT? 2008 http://www.fda.gov/cdrh/CT/risks.html.
- 20.Smith-Bindman R, Miglioretti D, Larson E. Rising Use of Diagnostic Medical Imaging in an Integrated Health Plan. Health Affairs. 2008;26(6):1491–1502. doi: 10.1377/hlthaff.27.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007 Jul 18;298(3):317–323. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 22.Conference of Radiation Control Program Directors. Nationwide evaluation of x-ray trends (NEXT): Tabulation and graphical summary of 2000 survey of computed tomography. Frankfort, KY: 2007. [Google Scholar]
- 23.Hunsaker AR, Zou KH, Poh AC, et al. Routine Pelvic and Lower Extremity CT VEnography in Patients Undergoing Pulmonary CT Angiography. AJR. 2008;190(2):322–326. doi: 10.2214/AJR.07.2568. [DOI] [PubMed] [Google Scholar]
- 24.Twombly R. Full body CT screening: Preventing or Producing Cancer. J Natl Cancer Inst. 2004;96(22):1650–1651. doi: 10.1093/jnci/96.22.1650. [DOI] [PubMed] [Google Scholar]
- 25.Griffey RT, Sodickson A. Cumulative radiation exposure and cancer risk estimates in emergency department patients undergoing repeat or multiple CT. AJR Am J Roentgenol. 2009 Apr;192(4):887–892. doi: 10.2214/AJR.08.1351. [DOI] [PubMed] [Google Scholar]
- 26.McBride Paxton B, Warddrop R. Majority of Ordering Physicians Lack Knowledge of Radiation Exposure Risks from CT. American Roentgen Ray Society, 2009, Boston MA. 2009 [Google Scholar]
- 27.Caolili EM, H Cohan R, Ellis JH. Medical Decision Making Regarding Computed Tomographic Radiation Dose and Associated Risk: The Patient’s Perspective. Archives of Internal Medicine. 2009;169(11):1069–1071. doi: 10.1001/archinternmed.2009.139. [DOI] [PubMed] [Google Scholar]
- 28.Greess H, Wolf H, Baum U, et al. Dose reduction in computed tomography by attenuation-based on-line modulation of tube current: evaluation of six anatomical regions. Eur Radiol. 2000;10(2):391–394. doi: 10.1007/s003300050062. [DOI] [PubMed] [Google Scholar]
- 29.Nagel HD, Blobel J, Brix G, et al. 5 years of "concerted action dose reduction in CT" -- what has been achieved and what remains to be done? Rofo. 2004 Nov;176(11):1683–1694. doi: 10.1055/s-2004-813532. [DOI] [PubMed] [Google Scholar]
- 30.Hausleiter J, Meyer T, Hermann F. Estimated Radiation dose associated with cardiac CT angiography. JAMA. 2009;301(5):500–507. doi: 10.1001/jama.2009.54. [DOI] [PubMed] [Google Scholar]
- 31.Arch M, Frush D. Pediatric body MDCT: a 5-year follow-up survey of scanning parameters used by pediatric radiologists. Am J Roentgenol. 2008;191:611–617. doi: 10.2214/AJR.07.2989. [DOI] [PubMed] [Google Scholar]
- 32.Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001 Feb;176(2):289–296. doi: 10.2214/ajr.176.2.1760289. [DOI] [PubMed] [Google Scholar]
- 33.Radiation protection 118 : referral guidelines for imaging. Luxembourg European Commission, Directorate-General for the Environment; 2001. [Google Scholar]
- 34. [Accessed Feb 13, 2009];Diagnostic Imaging Referral Guidelines. 2005 http://www.car.ca/content.aspx?pg=Guidelines&spg=Stds_Guidelns&lang=E&lID=. Updated Last Updated Date.
- 35.Hurwitz LM, Reiman RE, Yoshizumi TT, et al. Radiation dose from contemporary cardiothoracic multidetector CT protocols with an anthropomorphic female phantom: implications for cancer induction. Radiology. 2007 Dec;245(3):742–750. doi: 10.1148/radiol.2453062046. [DOI] [PubMed] [Google Scholar]
- 36.Berrington de Gonzalez A, Darby SC. Risk of cancer from diagnostic x-rays: estiamtes for the UK and 14 other countries. The Lancet. 2004;363(9406):345–351. doi: 10.1016/S0140-6736(04)15433-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.