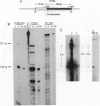
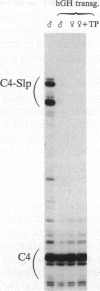
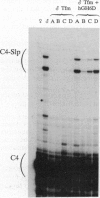
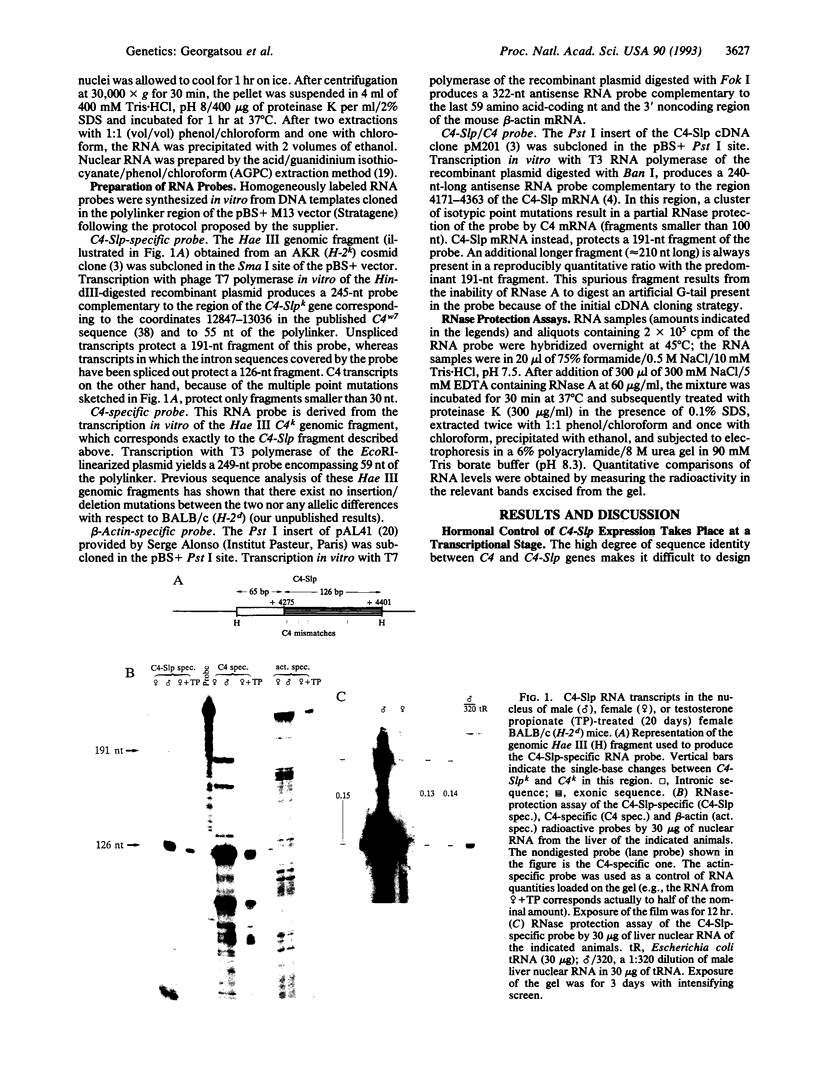
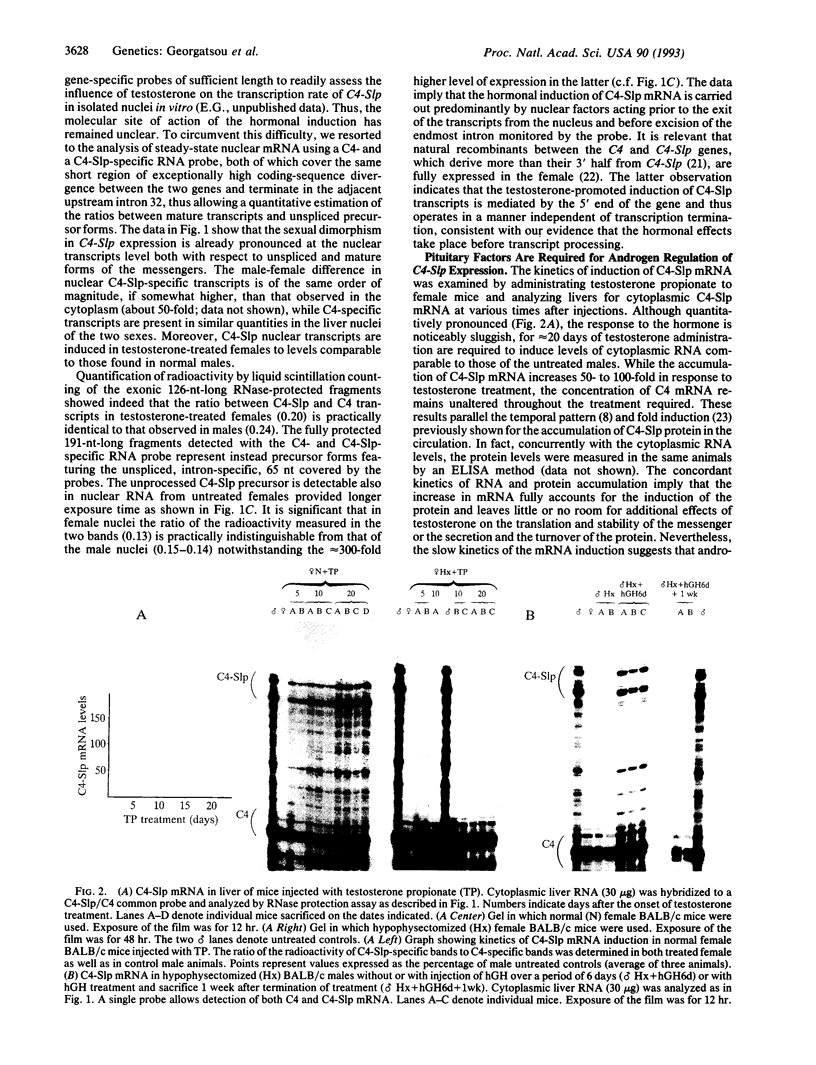
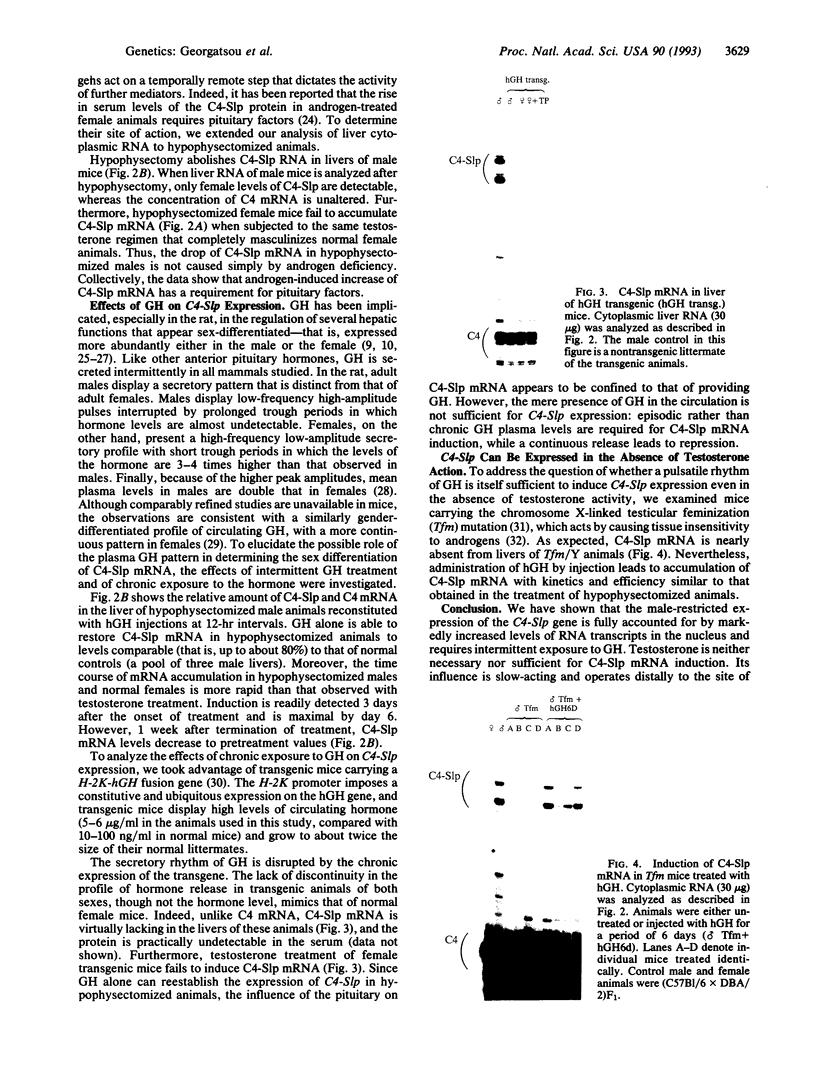
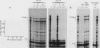Abstract
Sex-limited protein (Slp), an isoform of mouse complement component C4, is expressed predominantly in liver and nearly exclusively in sexually mature males or testosterone-treated females. It is encoded by a gene (C4-Slp) whose hormonal dependence has been attributed to an androgen-responsive transcriptional enhancer introduced accidentally, alongside the C4-Slp promoter, in the guise of the 5' long terminal repeat of an ancient retrovirus. We demonstrate that the pronounced rise of C4-Slp mRNA promoted by androgens in the liver is due to nuclear factors acting at a transcriptional stage. Curiously, hypophysectomized animals of either sex fail to express the gene and are refractory to testosterone. However, gene expression at male levels is restored even more promptly by injections of growth hormone alone. Additionally, animals carrying an ubiquitously expressed human growth hormone transgene lack C4-Slp mRNA and are insensitive to testosterone treatment. That growth hormone is sufficient to induce expression in a manner independent of androgen-receptor activity is shown by the hormonal treatment of Tfm mice. These androgen receptor-defective animals lack C4-Slp mRNA, which however can be fully induced by growth hormone injections. We conclude that the sexual dimorphism of C4-Slp expression employs liver nuclear mediators distinct from those directly instructed by androgens and is brought about by the intermittent rise of growth hormone, dictated by testosterone.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso S., Minty A., Bourlet Y., Buckingham M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23(1):11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- Atkinson J. P., Karp D. R., Seeskin E. P., Killion C. C., Rosa P. A., Newell S. L., Shreffler D. C. H-2 S region determined polymorphic variants of the C4, Slp, C2, and B complement proteins: a compilation. Immunogenetics. 1982;16(6):617–623. doi: 10.1007/BF00372032. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Clark A. J., Clissold P. M., Hainey S., Francke U. Two main groups of mouse major urinary protein genes, both largely located on chromosome 4. EMBO J. 1982;1(5):615–620. doi: 10.1002/j.1460-2075.1982.tb01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. J., Shreffler D. C. Female expression of the H-2-linked sex-limited protein (Slp) due to non-H-2 genes. Immunogenetics. 1980;10(1):19–29. doi: 10.1007/BF01561549. [DOI] [PubMed] [Google Scholar]
- Bruisten S. M., Demant P., Robins D. M. Trans-regulatory genes affect Slpa and Slpo expression and act in a tissue-specific manner. Immunogenetics. 1989;29(5):340–345. doi: 10.1007/BF00352844. [DOI] [PubMed] [Google Scholar]
- Bullock L. P., Bardin C. W., Ohno S. The androgen insensitive mouse: absence of intranuclear androgen retention in the kidney. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1537–1543. doi: 10.1016/s0006-291x(71)80261-9. [DOI] [PubMed] [Google Scholar]
- Chaplin D. D., Woods D. E., Whitehead A. S., Goldberger G., Colten H. R., Seidman J. G. Molecular map of the murine S region. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6947–6951. doi: 10.1073/pnas.80.22.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Danoff T. M., Goldman M. B., Goldman J. N. Murine sex-limited protein expression requires androgens and pituitary hormones. Immunogenetics. 1986;23(1):7–10. doi: 10.1007/BF00376515. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Eichinger D., Nussenzweig V. The murine sex-limited protein (Slp): reassessment of its sex limitation. J Immunol. 1982 Oct;129(4):1506–1508. [PubMed] [Google Scholar]
- Gustafsson J. A., Mode A., Norstedt G., Skett P. Sex steroid induced changes in hepatic enzymes. Annu Rev Physiol. 1983;45:51–60. doi: 10.1146/annurev.ph.45.030183.000411. [DOI] [PubMed] [Google Scholar]
- Hansen T. H., Shreffler D. C. Characterization of a constitutive variant of the murine serum protein allotype, Slp. J Immunol. 1976 Nov;117(5 Pt 1):1507–1513. [PubMed] [Google Scholar]
- Jansson J. O., Edén S., Isaksson O. Sexual dimorphism in the control of growth hormone secretion. Endocr Rev. 1985 Spring;6(2):128–150. doi: 10.1210/edrv-6-2-128. [DOI] [PubMed] [Google Scholar]
- Loreni F., Stavenhagen J., Kalff M., Robins D. M. A complex androgen-responsive enhancer resides 2 kilobases upstream of the mouse Slp gene. Mol Cell Biol. 1988 Jun;8(6):2350–2360. doi: 10.1128/mcb.8.6.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J., Zaphiropoulos P. G., Mode A., Warner M., Gustafsson J. A. Hormonal regulation of cytochrome P-450 gene expression. Adv Pharmacol. 1991;22:325–354. doi: 10.1016/s1054-3589(08)60040-x. [DOI] [PubMed] [Google Scholar]
- Lyon M. F., Hawkes S. G. X-linked gene for testicular feminization in the mouse. Nature. 1970 Sep 19;227(5264):1217–1219. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- McIntosh I., Bishop J. O. Differential expression in male and female mouse liver of very similar mRNAs specified by two group 1 major urinary protein genes. Mol Cell Biol. 1989 May;9(5):2202–2207. doi: 10.1128/mcb.9.5.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mode A., Wiersma-Larsson E., Gustafsson J. A. Transcriptional and posttranscriptional regulation of sexually differentiated rat liver cytochrome P-450 by growth hormone. Mol Endocrinol. 1989 Jul;3(7):1142–1147. doi: 10.1210/mend-3-7-1142. [DOI] [PubMed] [Google Scholar]
- Morello D., Moore G., Salmon A. M., Yaniv M., Babinet C. Studies on the expression of an H-2K/human growth hormone fusion gene in giant transgenic mice. EMBO J. 1986 Aug;5(8):1877–1883. doi: 10.1002/j.1460-2075.1986.tb04439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K., Nonaka M., Yokoyama S., Yeul Y. D., Pattanakitsakul S. N., Takahashi M. Recombination of two homologous MHC class III genes of the mouse (C4 and Slp) that accounts for the loss of testosterone dependence of sex-limited protein expression. J Immunol. 1987 Jan 15;138(2):620–627. [PubMed] [Google Scholar]
- Nonaka M., Kimura H., Yeul Y. D., Yokoyama S., Nakayama K., Takahashi M. Identification of the 5'-flanking regulatory region responsible for the difference in transcriptional control between mouse complement C4 and Slp genes. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7883–7887. doi: 10.1073/pnas.83.20.7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norstedt G., Palmiter R. Secretory rhythm of growth hormone regulates sexual differentiation of mouse liver. Cell. 1984 Apr;36(4):805–812. doi: 10.1016/0092-8674(84)90030-8. [DOI] [PubMed] [Google Scholar]
- Ogata R. T., Rosa P. A., Zepf N. E. Sequence of the gene for murine complement component C4. J Biol Chem. 1989 Oct 5;264(28):16565–16572. [PubMed] [Google Scholar]
- Ogata R. T., Sepich D. S. Murine sex-limited protein: complete cDNA sequence and comparison with murine fourth complement component. J Immunol. 1985 Dec;135(6):4239–4244. [PubMed] [Google Scholar]
- Ogata R. T., Zepf N. E. The murine Slp gene. Additional evidence that sex-limited protein has no biologic function. J Immunol. 1991 Oct 15;147(8):2756–2763. [PubMed] [Google Scholar]
- Passmore H. C., Shreffler D. C. A sex-limited serum protein variant in the mouse: hormonal control of phenotypic expression. Biochem Genet. 1971 Apr;5(2):201–209. doi: 10.1007/BF00485645. [DOI] [PubMed] [Google Scholar]
- Passmore H. C., Shreffler D. C. A sex-limited serum protein variant in the mouse: inheritance and association with the H-2 region. Biochem Genet. 1970 Jun;4(3):351–365. doi: 10.1007/BF00485752. [DOI] [PubMed] [Google Scholar]
- Rosa P. A., Shreffler D. C. Cultured hepatocytes from mouse strains expressing high and low levels of the fourth component of complement differ in rate of synthesis of the protein. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2332–2336. doi: 10.1073/pnas.80.8.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A. K., Chatterjee B. Sexual dimorphism in the liver. Annu Rev Physiol. 1983;45:37–50. doi: 10.1146/annurev.ph.45.030183.000345. [DOI] [PubMed] [Google Scholar]
- Sinha Y. N., Salocks C. B., Wickes M. A., Vanderlaan W. P. Serum and pituitary concentrations of prolactin and growth hormone in mice during a twenty-four hour period. Endocrinology. 1977 Mar;100(3):786–791. doi: 10.1210/endo-100-3-786. [DOI] [PubMed] [Google Scholar]
- Stavenhagen J. B., Robins D. M. An ancient provirus has imposed androgen regulation on the adjacent mouse sex-limited protein gene. Cell. 1988 Oct 21;55(2):247–254. doi: 10.1016/0092-8674(88)90047-5. [DOI] [PubMed] [Google Scholar]
- Tosi M., Lévi-Strauss M., Georgatsou E., Amor M., Meo T. Duplications of complement and non-complement genes of the H-2S region: evolutionary aspects of the C4 isotypes and molecular analysis of their expression variants. Immunol Rev. 1985 Oct;87:151–183. doi: 10.1111/j.1600-065x.1985.tb01149.x. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Pampori N. A., Ram P. A., Agrawal A. K., Shapiro B. H. Interpulse interval in circulating growth hormone patterns regulates sexually dimorphic expression of hepatic cytochrome P450. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6868–6872. doi: 10.1073/pnas.88.15.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G., Kawajiri K., Negishi M. Gene family of male-specific testosterone 16 alpha-hydroxylase (C-P-450(16) alpha) in mouse liver: cDNA sequences, neonatal imprinting, and reversible regulation by androgen. Biochemistry. 1987 Dec 29;26(26):8683–8690. doi: 10.1021/bi00400a029. [DOI] [PubMed] [Google Scholar]
- Yu D. Y., Nonaka M., Takahashi M. Mapping of the transcriptional regulatory domains responsible for the difference in the promoter activity between mouse C4 and Slp (sex-limited protein) genes. J Immunol. 1988 Dec 15;141(12):4381–4387. [PubMed] [Google Scholar]