Abstract
Background:
Students of medicine are prone to contact with various infectious agents such as hepatitis A virus (HAV). Infection with HAV may lead to morbidity and in rare cases, mortality. We evaluated the seroprevalence of HAV among 1st-year medical students to assess the necessity of vaccination/preventive immunoglobulin in this at-risk population.
Methods:
This cross-sectional study was carried out in 2007 among 403 1st-year medical students in Isfahan, Iran. Participants filled out a questionnaire including items on demographic characteristics, medical history, and hygiene. Then, the anti-HAV IgG antibody was assessed using the ELISA method (Diagnostic Bioprobes, Dia-Pro, Milan, Italy).
Results:
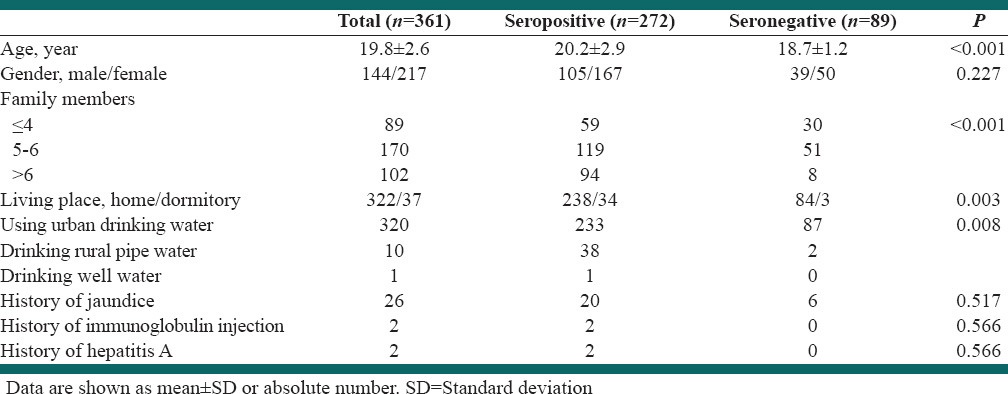
Among the 403 students invited to attend the study, 361 ones (89.5%) agreed to participate (61.1% female), with a mean age of 19.8 ± 2.6 years. Of the participants, 272 (75.3%) students were seropositive. Seropositivity was not associated with gender (P = 0.222), but was associated with the number of family members (P < 0.001), residence place (P = 0.003), age (P < 0.001), and the type of drinking water (P = 0.008).
Conclusions:
One of the four medical college students of our society is prone to hepatitis A infection. Accordingly, vaccination/preventive immunoglobulin is suggested for this population; however, whether a prior serological screening is cost-effective needs further evaluation by epidemiologic data from our society.
Keywords: Epidemiology, health occupations students, Hepatitis A, Iran, prevention, vaccination
INTRODUCTION
Viral hepatitis infections are among the most important public health problems, and hepatitis A virus (HAV) is the most common cause of acute viral hepatitis worldwide. Each year, almost 1.5 million cases of acute hepatitis A infection are reported throughout the world; however, the results of serological studies indicate a higher number of infected cases.[1] Age is one of the important prognostic factors in a clinical course of hepatitis A infection. In communities with low to middle socioeconomic state, the age of infection occurrence is within the childhood period.[2] In many parts of the world including Latin America, the age of acquisition of the disease has shifted from childhood to adulthood, which is due to improved health level.[3] Although the age of infection occurrence has increased in different communities with the economic advancements and improved level of health, being affected with the disease in higher ages is accompanied with more severe clinical symptoms and even hepatic failure.[2] In such countries, the increased work absence, hospitalization, and even increased mortality have increased the burden of the disease.[1] Therefore, prevention of the disease is of great importance.
Considering the high costs of vaccination programs, following the development of safe and very effective vaccine and immunoglobulin against HAV, a fundamental issue is the determination of target individuals or groups for receiving the vaccine/preventive immunoglobulin. Healthcare workers and medical students are among the at-risk groups of the disease. Because of the lack of sufficient experience in performing many clinical procedures, and also exposure to blood and body fluids of the patients, medical students are at the risk of the infection and thus its complications.[4,5,6]
There is a lack of data on the seroprevalence of HAV among healthcare team in our society. In one study from North Iran, Saffar et al. found the seroprevalence of hepatitis A as 90.36% among the healthcare workers.[7] However, to the best of our knowledge, no study, so far, has been carried out to determine the seroprevalence of HAV and thus the need for vaccination/preventive immunoglobulin among the medical students in our society. Thus, the current study was performed to evaluate the serological prevalence of hepatitis A in 1st-year medical students in Isfahan, central Iran.
METHODS
This cross-sectional study was carried out in 2007 in Isfahan, central Iran. After explaining the project, all 1st-year students of different schools of the Isfahan University of Medical Sciences (n = 403) were invited to participate in the study at the registration day. After obtaining written consent, participants filled out a questionnaire, which included items on demographic characteristics, number of family members, residence place (dormitory or home), type of drinking water (urban drinking water, rural pipe water, well water), history of jaundice and hepatitis, and history of immunoglobulin injection.
A blood sample (10 ml) was obtained from each participant under aseptic condition. The samples were kept in the cold box and were sent to the laboratory of Isfahan Research Center of Infectious and Tropical Diseases in <3 h. After centrifugation of the samples, blood sera were extracted and separately kept in microtubes (aliquots) at -70°C. To evaluate the IgG antibody against HAV (HAV Ab), ELISA kit (Diagnostic Bioprobes, Dia-Pro, Milan, Italy) was used. The kit is competitive- and qualitative-based, and the results obtained were interpreted according to Co/S of the samples in OD = 450 nm, and with the calibration mentioned in the guidelines of the kit. The cut-off calculation was carried out using the formula: Cut-off = (NP + NC) 3, and the results were interpreted as follows: <0.9 was considered negative, 0.9–1.1: equivocal, and >1.1: Positive.
Finally, the data were analyzed using Chi-square and independent t-test, by the SPSS software, version 16.0 (SPSS Inc., Chicago IL., USA). P < 0.05 were considered significant in all analyses.
RESULTS
Of the 403 students, 361 ones (89.5%) agreed to participate including 61.1% females, with the mean age of 19.8 ± 2.6 years. Those who did not agree to participate were not different from the participants regarding age (19.9 ± 2.8), but they were different from the participants with regard to the gender (83.3% female). Positive anti-HAV IgG antibody was found in 272 students (75.3%). Univariate analysis showed higher age in seropositive students (P < 0.001), but no relationship between seropositivity and gender (P = 0.227). Number of family members was significantly higher in seropositive cases (P < 0.001). Furthermore, seropositivity was more frequent in those who used to live in a dormitory (P = 0.003), and in those with a history of drinking rural pipe water (P = 0.008), Table 1.
Table 1.
Frequency distribution of risk factors of hepatitis A
Considering different factors associated with seropositivity, a binary logistic regression analysis was performed. The results showed that age (odd ratio [OR] = 1.49, confidence interval [CI]: 95%: 1.22–1.82) and number of family members (OR = 1.45, CI: 95%: 1.18–1.78) were independently associated with seropositivity.
DISCUSSION
Many cases of hospital infections of hepatitis A have been reported, and the number of occupational infections with HAV is increasing.[8,9,10] Close contact with patients, not observing hygiene points, not wearing gloves when contacting body fluids, eating and drinking in hospital wards, and children fecal contamination may pose healthcare workers at the risk of these infections.[11,12,13] The results of our study demonstrated that 75.3% of the medical students in our society had IgG antibody against HAV at entering the medical college. Indeed, one from four students was not immune against HAV. To the best of our knowledge, no study in Iran has been carried out to determine the prevalence of immunity against hepatitis A in healthcare students. In a similar study in Turkey, the seropositivity of medical students was reported to be 64%[14] and another study in Delhi found seropositivity in 63% of medical students,[15] which were almost similar to our findings. However, in Korea, seropositivity is reported as 11.4% among 1st-to 3rd-year medical school students,[16] and in nursing students in Thailand, prevalence of hepatitis A antibody was 8.9% in one study[17] and 17.5% in another study.[18] In another survey from Greece, 14.6% of healthcare students were immune to HAV.[19] These differences between studies are attributed to the geographic differences of hepatitis A seroepidemiology affected by health behaviors and environmental factors, and differences between studies in age of the studied population, as even in the same population, there is a considerable geographic seroepidemiologic difference.
We did not find a statistically significant relationship between acquisition of hepatitis A infection and students’ gender, which is in agreement with the observations of the study performed in Turkey and India.[14,15] Moreover, in the study carried out in 2007 in Isfahan province, no relationship was found between gender and hepatitis prevalence in the general population.[20] The type of drinking water is another factor that is important in the acquisition of hepatitis A. As expected, we found a relationship between hepatitis A and the type of drinking water. Moreover, we found a relationship between the residence place (home or dormitory), and acquisition of hepatitis A. As we included students at the 1st year of their entrance to the college, factors belonging to living in dormitory cannot justify this association. Indeed, the association can be explained by the observation that medical students used to live in dormitory are from the towns near Isfahan, and the difference in prevalence of hepatitis A among inhabitants of Isfahan and its neighboring towns has been found in the study on Isfahan province general population.[20] Perhaps, the residency during childhood is more important but we did not gather such data. The size of the family is another item, which was shown to be related to the prevalence of hepatitis A in our study. Our findings in this respect are consistent with those of the previous studies, which indicated that as the size of family increases, the risk of acquisition of the disease increases.[14]
The prevalence of HAV infection in the developed countries is almost 1%/year of life, and if a case of infection is indentified, all individuals with close contact with the patient in the past 2 weeks must receive gamma globulin. However, in the developing countries, different statistics have been reported with regard to the HAV prevalence. A high portion of the population has acquired the infection in the first decade of their life. Gamma globulin injection will prevent the acquisition of the infection in the childhood, which has fewer complications. Thus, the risk of infection in higher ages, which is accompanied with more severe complications, is increased. In countries like Iran, in which most people acquire HAV infection in childhood, gamma globulin administration in the second decade of life and especially after the third decade is of no use. Furthermore, administration of gamma globulin has high costs and is accompanied with some side-effects.
From the economic point of view, a course of vaccination costs three times of performing the screening test; therefore, the vaccination target group should be selected carefully. According to the recommendations of Centers for Disease Control and Prevention, if the frequency of positive cases and presence of immunity in a particular age range is above 33%, and the cost of screening test and its related medical visit is less than one-third of the vaccine price, carrying out the screening test is suggested. A study on cost-effectiveness of vaccination for hepatitis A in healthcare workers in the USA estimated that with vaccination, the incidence of hepatitis A will be reduced by 94%, death from infection by 92.5%, and lost years of life by 94.3%.[21] Because the seropositivity of hepatitis A was very high in the population studied, serological screening prior to vaccination might be less cost-effective than the universal vaccination. Of course, a cost-effectiveness study by data from our own society could provide a more accurate response to this issue. Such study needs data on yearly incidence rate and mortality/morbidity of hepatitis A from our society.
CONCLUSIONS
One out of four medical students in our society is prone to hepatitis A infection and at its age, it could be very serious. Therefore, vaccination is suggested for medical students in our society. Whether serological screening prior to vaccination is cost-effective needs further evaluation in our society. Furthermore, it should be considered that observation of health measures, access to healthy drinking water, and hygiene of residence place, as well as other points of personal and social health can prevent the acquisition of infection. A cost-effectiveness study by data from our own society is warranted.
ACKNOWLEDGMENTS
This study was supported by the Vice Chancellor for Research at the Isfahan University of Medical Sciences. We are thankful to staff from Isfahan Infectious Diseases and Tropical Medicine Research Center who helped us in conducting the study. Authors are thankful to Dr. Ali Gholamrezaei for data analysis and editing this report.
Footnotes
Source of Support: Vice Chancellor for Research at the Isfahan University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28:6653–7. doi: 10.1016/j.vaccine.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 2.Jeong SH, Lee HS. Hepatitis A: Clinical manifestations and management. Intervirology. 2010;53:15–9. doi: 10.1159/000252779. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka J. Hepatitis A shifting epidemiology in Latin America. Vaccine. 2000;18(Suppl 1):S57–60. doi: 10.1016/s0264-410x(99)00466-1. [DOI] [PubMed] [Google Scholar]
- 4.Tarantola A, Abiteboul D, Rachline A. Infection risks following accidental exposure to blood or body fluids in health care workers: A review of pathogens transmitted in published cases. Am J Infect Control. 2006;34:367–75. doi: 10.1016/j.ajic.2004.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler CS, McGuinn M, Spec A, Christensen J, Baragi R, Hershow RC. Underreporting of blood and body fluid exposures among health care students and trainees in the acute care setting: A 2007 survey. Am J Infect Control. 2011;39:129–34. doi: 10.1016/j.ajic.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Denic LM, Ostric I, Pavlovic A, Dimitra KO. Knowledge and occupational exposure to blood and body fluids among health care workers and medical students. Acta Chir Iugosl. 2012;59:71–5. [PubMed] [Google Scholar]
- 7.Saffar M, Jooyan A, Mahdavi M, Khalilian A. Seroprevalence of hepatitis A, B, and C and hepatitis B vaccination status among health care workers in Sari-Iran, 2003. J Mazandaran Univ Med Sci. 2003;15:67–77. [Google Scholar]
- 8.Franco E, Giambi C, Ialacci R, Coppola RC, Zanetti AR. Risk groups for hepatitis A virus infection. Vaccine. 2003;21:2224–33. doi: 10.1016/s0264-410x(03)00137-3. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs RJ, Gibson GA, Meyerhoff AS. Cost-effectiveness of hepatitis A-B vaccine versus hepatitis B vaccine for healthcare and public safety workers in the western United States. Infect Control Hosp Epidemiol. 2004;25:563–9. doi: 10.1086/502440. [DOI] [PubMed] [Google Scholar]
- 10.Jung SI, Lee CS, Park KH, Kim ES, Kim YJ, Kim GS, et al. Sero-epidemiology of hepatitis A virus infection among healthcare workers in Korean hospitals. J Hosp Infect. 2009;72:251–7. doi: 10.1016/j.jhin.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Chodick G, Ashkenazi S, Lerman Y. The risk of hepatitis A infection among healthcare workers: A review of reported outbreaks and sero-epidemiologic studies. J Hosp Infect. 2006;62:414–20. doi: 10.1016/j.jhin.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Shariati B, Shahidzadeh-Mahani A, Oveysi T, Akhlaghi H. Accidental exposure to blood in medical interns of Tehran University of Medical Sciences. J Occup Health. 2007;49:317–21. doi: 10.1539/joh.49.317. [DOI] [PubMed] [Google Scholar]
- 13.Talas MS. Occupational exposure to blood and body fluids among Turkish nursing students during clinical practice training: Frequency of needlestick/sharp injuries and hepatitis B immunisation. J Clin Nurs. 2009;18:1394–403. doi: 10.1111/j.1365-2702.2008.02523.x. [DOI] [PubMed] [Google Scholar]
- 14.Oncu S, Oncu S, Sakarya S. Hepatitis A and B seropositivity among medical students. Health Policy. 2005;74:39–45. doi: 10.1016/j.healthpol.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Jindal M, Rana SS, Gupta RK, Das K, Kar P. Serological study of hepatitis A virus infection amongst the students of a medical college in Delhi and evaluation of the need of vaccination. Indian J Med Res. 2002;115:1–4. [PubMed] [Google Scholar]
- 16.Kim S, Lee JH, Hwang JH, Lee CS. Hepatitis A antibody seroprevalence among medical school students. Am J Infect Control. 2011;39:889–90. doi: 10.1016/j.ajic.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Pilakasiri C, Gibbons RV, Jarman RG, Supyapoung S, Myint KS. Hepatitis antibody profile of Royal Thai Army nursing students. Trop Med Int Health. 2009;14:609–11. doi: 10.1111/j.1365-3156.2009.02264.x. [DOI] [PubMed] [Google Scholar]
- 18.Samakoses R, Myint KS, Rangsin R, Areekul W, Kerdpanich A, Watanaveeradej V, et al. Seroprevalence of hepatitis A in Thai army medical cadets and nursing students: A reflection of regional risk differences. Mil Med. 2007;172:1275–8. doi: 10.7205/milmed.172.12.1275. [DOI] [PubMed] [Google Scholar]
- 19.Pavlopoulou ID, Daikos GL, Tzivaras A, Bozas E, Kosmidis C, Tsoumakas C, et al. Medical and nursing students with suboptimal protective immunity against vaccine-preventable diseases. Infect Control Hosp Epidemiol. 2009;30:1006–11. doi: 10.1086/605923. [DOI] [PubMed] [Google Scholar]
- 20.Ataei B, Javadi AA, Nokhodian Z, Kassaeian N, Shoaei P, Farajzadegan Z, et al. HAV in Isfahan province: A population-based study. Trop Gastroenterol. 2008;29:160–2. [PubMed] [Google Scholar]
- 21.Smith S, Weber S, Wiblin T, Nettleman M. Cost-effectiveness of hepatitis A vaccination in healthcare workers. Infect Control Hosp Epidemiol. 1997;18:688–91. doi: 10.1086/647513. [DOI] [PubMed] [Google Scholar]


