Abstract
Purpose
Elderly people are thought to be more susceptible to periodontal disease due to reduced immune function associated with aging. However, little information is available on the nature of immune responses against putative periodontal pathogens in geriatric patients. The purpose of this study was to evaluate the serum IgG antibody responses to six periodontal pathogens in geriatric subjects.
Methods
The study population consisted of 85 geriatric patients and was divided into three groups: 29 mild (MCP), 27 moderate (MoCP) and 29 severe (SCP) chronic periodontitis patients. Serum levels of IgG antibody to Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum and Prevotella intermedia were measured by enzyme-linked immunosorbent assay (ELISA) and compared among the groups.
Results
All three groups showed levels of serum IgG in response to P. gingivalis, A. actinomycetemcomitans, and P. intermedia that were three to four times higher than levels of IgG to T. forsythia, T. denticola, and F. nucleatum. There were no significant differences among all three groups in IgG response to P. gingivalis (P=0.065), T. forsythia (P=0.057), T. denticola (P=0.1), and P. intermedia (P=0.167), although the IgG levels tended to be higher in patients with SCP than in those with MCP or MoCP (with the exception of those for P. intermedia). In contrast, there were significant differences among the groups in IgG levels in response to F. nucleatum (P=0.001) and A. actinomycetemcomitans (P=0.003). IgG levels to A. actinomycetemcomitans were higher in patients with MCP than in those with MoCP or SCP.
Conclusions
When IgG levels were compared among three periodontal disease groups, only IgG levels to F. nucleatum significantly increased with the severity of disease. On the contrary, IgG levels to A. actinomycetemcomitans decreased significantly in patients with SCP compared to those with MCP. There were no significant differences in the IgG levels for P. gingivalis, T. forsythia, T. denticola, and P. intermedia among geriatric patients with chronic periodontitis.
Keywords: Chronic periodontitis, Disease progression, Enzyme-Linked Immunosorbent Assay, Geriatrics, Immunoglobulin G
Graphical Abstract
INTRODUCTION
There are around 600 million people worldwide aged 60 years and over; this figure will double by 2025 and reach virtually two billion by 2050 to represent 22 percent of the world’s population [1]. Chronic diseases with multiple etiologies, such as cancer, cardiovascular disease, respiratory disease, diabetes, and oral infection are thought to be more prevalent in this age group, as the elderly become more susceptible to these diseases that may be potentiated by less competent immune systems. It is generally accepted that immune function is less effective in elderly people than in young people, probably due to decreased macrophage and T-cell activity, decreased amount of complement, and insufficient antibody production and response [6]. These changes are associated with increased susceptibility to infection, malignancy, and autoimmune disorders [7] .It has become clear that poor oral health can increase the risk of systemic disease, and vice versa [2,3,4,5]. However, the role of immune function in the multi-factorial natural history of chronic periodontitis in geriatric patients has not yet been elucidated.
It has been suggested that immune function in periodontal disease is like a “double-edged sword,” fighting pathogens as well as eliciting tissue damage in the host. Both cell-mediated and humoral immune responses against periodontal pathogens are well documented [8,9]. In fighting periodontal infections, pathogen-specific antibodies reduce or inhibit bacterial colonization, proliferation, and spread. However, antibody titers vary greatly between patients and vary before and after therapy [10,11]. When antibody-mediated protection is not sufficient to eliminate periodontal pathogens, periodontal destruction can occur [12]. In some cases, antibody titers may reflect previous exposure of the host to periodontal pathogens; a high titer could either indicate a positive immune response by the patient or reflect an inability of the immune system to destroy the pathogen [13]. Elevated serum IgG antibody to periodontal pathogens may indicate destructive periodontal disease, and may be a risk factor for disease progression [12,14]. On the other hand, animal and clinical studies have shown that serum antibody titers do not always correlate with the clinical stages of periodontitis [9].
Immuno-inflammatory responses to dental plaque biofilms may lead to periodontal destruction [8]. Although aging is associated with greater susceptibility to infectious disease [7], little information is available on the immune responses to periodontal pathogens involved in chronic periodontitis in geriatric patients. The purpose of this study was to evaluate humoral immune responses to important periodontal pathogens in geriatric patients.
MATERIALS AND METHODS
Subjects
The protocol of this study was approved by the Institutional Review Board (approval number: #1-2008-05-226), Chonnam National University Hospital, Gwangju, Korea. The study population included 85 geriatric subjects (37 males and 48 females) aged 60-82 years, who were residents of Gwangju and recruited for a public health survey performed jointly by Chonnam National University Colleges of Medicine and Dentistry, Gwangju, Korea. Subjects who had been exposed to antibiotics in the past four months or had a chronic inflammatory or immune disorder were excluded from participating in this survey. Each subject received a standard periodontal examination by a periodontist and was assigned to one of three groups based upon the severity of periodontitis according to previously published criteria [15]. These groups were (1) mild chronic periodontitis (MCP, n=29, mean age 64.1 years, range 60-70 years) with a clinical attachment loss (CAL) of less than 3 mm; (2) moderate chronic periodontitis (MoCP, n=27, mean age 67.6 years, range 60.8-83 years) with a CAL of 3-5 mm; and (3) severe chronic periodontitis (SCP, n=29, mean age 68.5 years, range 60.9-82 years) with a CAL of more than 5 mm.
Serum collection
Serum samples were provided by the Department of Pathology, Chonnam National University Medical School, Gwangju, Korea. Serum samples were collected from chronic periodontitis patients during a public health survey performed under an approved protocol (#1-2008-05-226). Blood was drawn by antecubital vein puncture and was allowed to coagulate for 30 minutes at 37°C. Serum was separated by centrifugation at 2000 g for 10 minutes at 4°C, then aliquoted in 1 mL and stored at -20°C until use.
Bacterial strains and growth
A. actinomycetemcomitans ATCC 33384 was cultured in 3% tryptic soy broth (BD Biosciences, San Jose, CA, USA) containing 1 mg/mL yeast extract (BD Biosciences) and 10% horse serum (Hyclone, Seoul, Korea). P. gingivalis ATCC 33277 was cultured in Brucella broth (BD Biosciences) containing 1 mg/mL yeast extract (Sigma, USA), 5 μg/mL hemin (Sigma-Aldrich, St. Louis, MO, USA) and 1 μg/mL menadione (Sigma-Aldrich, St. Louis, MO, USA). F. nucleatum ATCC 10953 and P. intermedia ATCC 25611 were cultured in Brucella broth containing yeast extract (1mg/mL), haemin (10 μg/mL), and menadione (5 μg/mL) (Sigma-Aldrich). T. denticola ATCC 35405 was cultured in TYGVS medium containing tryptone (10 mg/mL), brain heart infusion broth (5 mg/mL), yeast extract (10 mg/mL), gelatin (10 mg/mL), (NH4)2SO4 (0.5 mg/mL), MgSO4 (0.1 mg/mL), K2HPO4 (1.13 mg/mL), KH2PO4 (0.9 mg/mL), NaCl (1 mg/mL), glucose (1 mg/mL), cysteine hydrochloride (1 mg/mL), thiamine pyrophosphate (12.5 μg/mL), sodium pyruvate (0.25 mg/mL), 0.027% acetic acid, 0.01% propionic acid, 0.0064% n-butyric acid, 0.0016% n-valeric acid, 0.0016% isobutyric acid, 0.0016% isovaleric acid, 0.0016% DL-2-methylbutyric acid, and 10% heat-inactivated rabbit serum at 37°C in anaerobic conditions (85% N2, 10% H2, 5% CO2) for 48 h. T. forsythia 43037 (American Type Culture Collection, Manassas, VA) was grown anaerobically on 2% (w/v) tryptic soy agar (BD Sciences) supplemented with 0.4% (w/v) yeast extract (BD Biosciences), 0.4% (w/v) phytone (BD Biosciences), 0.001% (w/v) N-acetylmuramic acid (Sigma-Aldrich), 5 μg/mL hemin (Sigma-Aldrich), 1 μg/mL menadione (Sigma-Aldrich), and 5% (v/v) defibrinated sheep blood. Each strain was cultured twice prior to experiments.
Protein extraction from bacterial cells
SMARTTM bacterial protein extraction solution was used to prepare the bacterial cell lysate (iNtRON Biotechnology, Gyeonggi-do, Korea). Briefly, bacterial cells were grown and adjusted to concentration at OD600 = 1.5-3.0, then washed 3X with phosphate buffered saline (PBS), and subsequently harvested at 13,000 rpm for 5 minutes at 4°C. Bacterial cell pellets were vortexed vigorously and resuspended in 350 µL of SMARTTM solution. Evenly resuspended solutions were centrifuged at 13,000 rpm for 5 minutes in 4°C to separate insoluble proteins. The supernatant containing the soluble protein was transferred to a clean tube. A QuantiPro BCA assay kit was used to measure the protein concentration (Sigma-Aldrich).
Measurement of serum IgG antibody levels by ELISA
Bacterial cell lysates were added to each well (100 ng/well) in a polystyrene microtiter plate, and the plates were incubated overnight at room temperature. The plates were then washed 2X with Tris-buffered saline with 2% Tween (TBS-T), and then 250 µL of 2% skim milk solution was added and incubated for 1 hour at 37°C. After washing with TBS-T (2X), patient sera (1:100 dilution) were added to the plates and mixed for 2 hours at 37°C with gentle agitation. The plates were then washed with TBS-T 3X, and rabbit anti-human IgG with conjugated horseradish peroxidase (1:10000 dilution; Abcam, Cambridge, UK) was added. The plates were incubated for 1 h at 37°C then washed with TBST 5X. Subsequently, 50 µL of TMB substrate solution (GenDEPOT, TX, USA) was added for color development; this reaction was stopped after 2 minutes by adding 100 μL of stop solution, and followed by optical density reading at 450 nm using a Versamax ELISA Microplate Reader (Sunnyvale, CA, USA). All samples were analyzed in triplicate.
The mean absorbance values from the triplicates were used for analysis. Relative antibody levels were expressed as ELISA units (EU) measured from a standard curve created by assigning a value of 100 EU to a single sample of patient serum. Sample antibody levels were determined from the absorbance value plotted on the standard curve and expressed as EU.
Statistical analysis
All statistical analyses were performed using statistical software SPSS version 19.0 (IBM, Armonk, NY, USA). A one-way ANOVA was performed for the normally distributed data, and a non-parametric Kruskal-Wallis test was used to analyze the data that were not distributed normally. In addition, a Mann-Whitney U test with Bonferroni correction (α = 0.05/3 = 0.0167) and Tukey post-hoc tests were used for multiple comparisons among the groups.
RESULTS
A total of 85 serum samples were analyzed in this study. Twenty-nine subjects were diagnosed with MCP, 27 subjects with MoCP, and 29 subjects with SCP. Based upon the central limit theorem, it was determined through power analysis that if the sample size was greater than 30, the distribution could be treated as a normal distribution. Initially, 30 subjects were selected for each group. However, experimental data were not obtained from all the samples.
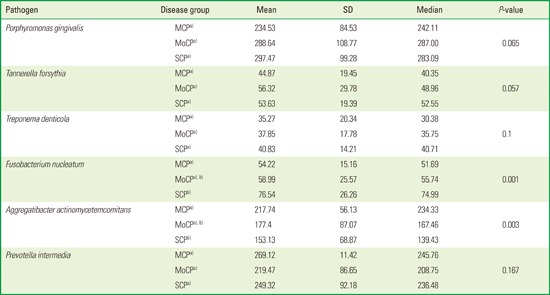
Table 1 shows the relative amounts of serum IgG to periodontal pathogens measured by ELISA. The IgG antibody responses to P. gingivalis, P. intermedia and A. actinomycetemcomitans were higher than those for T. denticola, F. nucleatum, and T. forsythia. Antibody reactivity to A. actinomycetemcomitans was less than that to P. gingivalis and P. intermedia, but three to four times higher than the antibody reactivity to T. denticola, F. nucleatum, or T. forsythia. Levels of IgG in response to T. forsythia and T. denticola appeared to be lowest among the six periodontal pathogens tested.
Table 1. Serum IgG titers to six periodontal pathogens, measured by enzyme-linked immunosorbent assay (ELISA).
| Pathogen | Disease group | Mean | SD | Median | P-value |
|---|---|---|---|---|---|
| Porphyromonas gingivalis | MCPa) | 234.53 | 84.53 | 242.11 | 0.065 |
| MoCPa) | 288.64 | 108.77 | 287.00 | ||
| SCPa) | 297.47 | 99.28 | 283.09 | ||
| Tannerella forsythia | MCPa) | 44.87 | 19.45 | 40.35 | 0.057 |
| MoCPa) | 56.32 | 29.78 | 48.96 | ||
| SCPa) | 53.63 | 19.39 | 52.55 | ||
| Treponema denticola | MCP a | 35.27 | 20.34 | 30.38 | 0.1 |
| MoCPa) | 37.85 | 17.78 | 35.75 | ||
| SCPa) | 40.83 | 14.21 | 40.71 | ||
| Fusobacterium nucleatum | MCPa) | 54.22 | 15.16 | 51.69 | 0.001 |
| MoCP a),b) | 58.99 | 25.57 | 55.74 | ||
| SCP b | 76.54 | 26.26 | 74.99 | ||
| Aggregatibacter actinomycetemcomitans | MCPa) | 217.74 | 56.13 | 234.33 | 0.003 |
| MoCP a),b) | 177.4 | 87.07 | 167.46 | ||
| SCP b | 153.13 | 68.87 | 139.43 | ||
| Prevotella intermedia | MCPa) | 269.12 | 11.42 | 245.76 | 0.167 |
| MoCPa) | 219.47 | 86.65 | 208.75 | ||
| SCPa) | 249.32 | 92.18 | 236.48 |
SD, standard deviation; MCP, mild chronic periodontitis (n=29); MoCP, moderate chronic periodontitis (n=27); SCP, severe chronic periodontitis (n=29)
Different letters (a, b) indicate a significant difference between two groups and identical letters (a, a) indicate that there is no difference between two groups.
When IgG levels were compared among three different disease groups, there were no significant differences among the groups with respect to P. gingivalis (P=0.065), T. forsythia (P= 0.057), T. denticola (P=0.1), and P. intermedia (P=0.167), although the values tended to be higher in the patients with SCP than in those with MCP or MoCP (with the exception of P. intermedia). The only significant differences among disease groups were for IgG to F. nucleatum (P=0.001) and A. actinomycetemcomitans (P=0.03). Interestingly, IgG levels for A. actinomycetemcomitans and P. intermedia were higher in patients with MCP than in those with MoCP or SCP. In addition, there were significant differences in IgG levels between MCP and SCP only in the responses to these two pathogens. IgG levels for F. nucleatum were increased in SCP compared to MCP, and IgG levels for A. actinomycetemcomitans were highest in patients with MCP, followed in decreasing order by MoCP and SCP.
DISCUSSION
The primary role of the immune response is to protect the host from attacks by pathogens; however, interaction between dental plaque biofilms and the host may result in the signs and symptoms of periodontal disease. The immune response is also thought to play a significant role in the initiation and progression of chronic periodontitis by causing tissue damage, yet data regarding this response in the geriatric population with chronic periodontitis have been lacking.
The purpose of this study was to assess humoral immune (IgG) responses to periodontal pathogens in elderly patients with chronic periodontitis in an effort to better understand immune function in this group. Serum samples were obtained from the same geriatric patients used in our previous microbiological study [16], and here we analyzed IgG antibody levels in response to six periodontal pathogens.
The results of the present study indicate that serum IgG antibody levels to P. gingivalis, T. forsythia, T. denticola, and F. nucleatum in patients with SCP were higher than in those with MCP. Our previous study showed that the amount of these bacteria increased as the severity of the disease increased from MCP to SCP, and that the presence of these bacteria in the saliva was associated with the severity of periodontal disease in geriatric patients [16]. Considering the fact that IgG levels may reflect a host’s previous exposure to periodontal pathogens [8], the present results suggest that increased IgG levels reflect the current disease status of chronic periodontitis.
Elderly subjects responded with a normal IgG immune response to P. gingivalis, and IgG levels were higher in the group with periodontal disease than in a control group of elderly subjects without periodontal disease [17]. Elderly subjects have been shown to exhibit the same effective immune response to P. gingivalis as younger subjects [18], and the present results support these findings, since the amount of bacteria increased to a greater extent, as did IgG levels, in the group with more advanced disease (SCP) than in those with MCP.
In contrast, serum IgG levels to T. forsythia, T. denticola and F. nucleatum were very low, suggesting that these three microorganisms are poorly immunogenic, which is consistent with other reports of decreased IgG antibody levels in adults with chronic periodontitis [19]. Another possibility is that immune activity in response to these pathogens is compromised, and that this contributes to the progression of periodontal disease in the elderly.
In the present study, the levels of serum IgG to P. gingivalis, A. actinomycetemcomitans and P. intermedia increased in periodontitis patients; however, these elevated levels did not coincide with the severity of periodontal disease. Other clinical and animal studies also showed that serum IgG titers might not correlate well with the clinical stages of periodontal disease [8,20,21].
The only significant increase in IgG levels in SCP compared to MCP was in the case of F. nucleatum. This might point to a role for anti-F. nucleatum IgG as an indicator of the status of periodontal disease in geriatric patients. However, levels of IgG to F. nucleatum were three- to four-fold lower than levels evoked by the other pathogens (Table 1), making them less sensitive as a marker of periodontal disease. Moreover, in our previous microbiological study using the same geriatric subjects [16], F. nucleatum was the most prevalent bacteria found in the saliva. Therefore, IgG levels to F. nucleatum might not truly represent the amount of bacteria present in geriatric patients. For these reasons, IgG levels to F. nucleatum may not be suitable as a marker of periodontal disease in geriatric patients.
IgG levels to A. actinomycetemcomitans were highest in patients with MCP, followed in decreasing order by levels in patients with MoCP or SCP. In our previous microbiological study, there was no difference in the levels of A. actinomycetemcomitans bacteria among the different disease groups [16]. Although the reason for this inverse relationship between IgG levels and disease severity is not clear, decreased IgG responses might lead to more severe disease if A. actinomycetemcomitans was associated with the progression of chronic periodontitis. A similar pattern was identified for P. intermedia, in which IgG levels were highest in the patients with MCP, followed by those with SCP and MoCP. Microbiological data indicated that there was no difference in P. intermedia among the different disease groups [16]. These data suggest that inadequate IgG responses lead to more periodontal destruction associated with A. actinomycetemcomitans and P. intermedia.
It is very difficult to predict the progression of periodontal disease, since it undergoes periods of quiescence and active exacerbation [22,23]. Although we do not have reliable disease markers that allow us to identify when significant periodontal destruction might occur, it has been suggested that antibodies against specific periodontal pathogens could be used as disease markers for periodontal disease, because they may reflect the presence of a larger quantity of the pathogens associated with periodontal disease.
The results of the present study suggest that IgG levels to the so-called ‘red complex bacteria’, a group that comprises P. gingivalis, T. forsythia and T. denticola, may not be used to evaluate the progression of periodontal disease in the elderly, because they were not significantly different among the disease groups. However, it has been suggested previously that elevated serum IgG antibody to P. gingivalis reflects destructive periodontal disease status and may be considered a risk factor for disease progression [14]. In addition, high P. gingivalis antibody titers were consistently associated with periodontal disease status in a study in US adults older than 40 years [24]. A recent study has shown that measurement of serum IgG antibodies to P. gingivalis may be a useful tool for the surveillance of periodontal disease in community-based Japanese elderly people [25].
In addition to its possible diagnostic role in periodontal disease, IgG may provide additional information regarding systemic diseases such as cardiovascular disease, respiratory disease, kidney disease, adverse pregnancy outcomes, and even certain types of cancer, because evidence of an epidemiological relationship between periodontal and systemic disease has strengthened in the past 10 to 15 years. Beck and colleagues [26] demonstrated that systemic antibody responses against multiple periodontal organisms including P. gingivalis were associated with coronary heart disease (CHD), while clinical signs of periodontal disease were not. This suggests that measuring IgG levels may provide more accurate information in predicting the risk of CHD. Iwasaki and colleagues [27] observed a significant association between elevated serum antibody titer to P. gingivalis and decreased kidney function in a community-based cohort of elderly Japanese. Recently, it was reported that high levels of antibodies against P. gingivalis conferred a twofold higher risk of pancreatic cancer than lower levels of these antibodies [28]. Collectively, these findings suggest that IgG levels to P. gingivalis might be a useful marker for several systemic diseases, as well as periodontal disease, especially in geriatric patients.
In summary, the levels of serum IgG to P. gingivalis, A. actinomycetemcomitans and P. intermedia were higher in all periodontal disease groups than those to T. forsythia, T. denticola and F. nucleatum. T. forsythia, T. denticola and F. nucleatum proved to be poorly immunogenic, suggesting that compromised serum IgG antibody activity against these pathogens may increase the risk for periodontal disease progression in geriatric patients. IgG antibody levels to F. nucleatum increased with the severity of periodontal disease, although the overall quantity of IgG was not as high as in the case of P. gingivalis, A. actimomycetemcomitans, and P. intermedia. IgG levels to A. actimomycetemcomitans were highest in patients with MCP, followed in decreasing order by those in MoCP and SCP.
ACKNOWLEDGEMENTS
This study was partially supported by Chonnam National University (2013, 2014) and Chonnam National University Hospital Research Institute of Clinical Medicine (Grant CRI 13015-1).
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Lee R. The outlook for population growth. Science. 2011;333:569–573. doi: 10.1126/science.1208859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davé S, Van Dyke T. The link between periodontal disease and cardiovascular disease is probably inflammation. Oral Dis. 2008;14:95–101. doi: 10.1111/j.1601-0825.2007.01438.x. [DOI] [PubMed] [Google Scholar]
- 3.Katz J, Wallet S, Cha S. Periodontal disease and the oral-systemic connection: "is it all the RAGE?". Quintessence Int. 2010;41:229–237. [PubMed] [Google Scholar]
- 4.Papapanou PN. Systemic effects of periodontitis: lessons learned from research on atherosclerotic vascular disease and adverse pregnancy outcomes. Int Dent J. doi: 10.1111/idj.12185. Forthcoming 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullinan MP, Seymour GJ. Periodontal disease and systemic illness: will the evidence ever be enough? Periodontol 2000. 2013;62:271–286. doi: 10.1111/prd.12007. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal S, Busse PJ. Innate and adaptive immunosenescence. Ann Allergy Asthma Immunol. 2010;104:183–190. doi: 10.1016/j.anai.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Boraschi D, Del Giudice G, Dutel C, Ivanoff B, Rappuoli R, Grubeck-Loebenstein B. Ageing and immunity: addressing immune senescence to ensure healthy ageing. Vaccine. 2010;28:3627–3631. doi: 10.1016/j.vaccine.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 8.Ebersole JL, Cappelli D, Holt SC. Periodontal diseases: to protect or not to protect is the question. Acta Odontol Scand. 2001;59:161–166. doi: 10.1080/000163501750266756. [DOI] [PubMed] [Google Scholar]
- 9.Ebersole JL, Taubman MA. The protective nature of host responses in periodontal diseases. Periodontol 2000. 1994;5:112–141. doi: 10.1111/j.1600-0757.1994.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 10.Darby IB, Mooney J, Kinane DF. Changes in subgingival microflora and humoral immune response following periodontal therapy. J Clin Periodontol. 2001;28:796–805. doi: 10.1034/j.1600-051x.2001.280812.x. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa I, Nakashima K, Koseki T, Nagasawa T, Watanabe H, Arakawa S, et al. Induction of the immune response to periodontopathic bacteria and its role in the pathogenesis of periodontitis. Periodontol 2000. 1997;14:79–111. doi: 10.1111/j.1600-0757.1997.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 12.Teng YT. The role of acquired immunity and periodontal disease progression. Crit Rev Oral Biol Med. 2003;14:237–252. doi: 10.1177/154411130301400402. [DOI] [PubMed] [Google Scholar]
- 13.Teng YT. Protective and destructive immunity in the periodontium: Part 1--innate and humoral immunity and the periodontium. J Dent Res. 2006;85:198–208. doi: 10.1177/154405910608500301. [DOI] [PubMed] [Google Scholar]
- 14.Craig RG, Boylan R, Yip J, Mijares D, Imam M, Socransky SS, et al. Serum IgG antibody response to periodontal pathogens in minority populations: relationship to periodontal disease status and progression. J Periodontal Res. 2002;37:132–146. doi: 10.1034/j.1600-0765.2002.00031.x. [DOI] [PubMed] [Google Scholar]
- 15.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Shet UK, Oh HK, Kim HJ, Chung HJ, Kim YJ, Kim OS, et al. Quantitative analysis of periodontal pathogens present in the saliva of geriatric subjects. J Periodontal Implant Sci. 2013;43:183–190. doi: 10.5051/jpis.2013.43.4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McArthur WP, Bloom C, Taylor M, Smith J, Wheeler T, Magnusson NI. Antibody responses to suspected periodontal pathogens in elderly subjects with periodontal disease. J Clin Periodontol. 1995;22:842–849. doi: 10.1111/j.1600-051x.1995.tb01782.x. [DOI] [PubMed] [Google Scholar]
- 18.De Nardin AM, Sojar HT, Grossi SG, Christersson LA, Genco RJ. Humoral immunity of older adults with periodontal disease to Porphyromonas gingivalis. Infect Immun. 1991;59:4363–4370. doi: 10.1128/iai.59.12.4363-4370.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Califano JV, Gunsolley JC, Schenkein HA, Tew JG. A comparison of IgG antibody reactive with Bacteroides forsythus and Porphyromonas gingivalis in adult and early-onset periodontitis. J Periodontol. 1997;68:734–738. doi: 10.1902/jop.1997.68.8.734. [DOI] [PubMed] [Google Scholar]
- 20.Baker PJ, Carter S, Dixon M, Evans RT, Roopenian DC. Serum antibody response to oral infection precedes but does not prevent Porphyromonas gingivalis-induced alveolar bone loss in mice. Oral Microbiol Immunol. 1999;14:194–196. doi: 10.1034/j.1399-302x.1999.140309.x. [DOI] [PubMed] [Google Scholar]
- 21.Albandar JM, DeNardin AM, Adesanya MR, Diehl SR, Winn DM. Associations between serum antibody levels to periodontal pathogens and early-onset periodontitis. J Periodontol. 2001;72:1463–1469. doi: 10.1902/jop.2001.72.11.1463. [DOI] [PubMed] [Google Scholar]
- 22.Nabers CL, Stalker WH, Esparza D, Naylor B, Canales S. Tooth loss in 1535 treated periodontal patients. J Periodontol. 1988;59:297–300. doi: 10.1902/jop.1988.59.5.297. [DOI] [PubMed] [Google Scholar]
- 23.Papapanou PN, Wennström JL, Gröndahl K. Periodontal status in relation to age and tooth type. A cross-sectional radiographic study. J Clin Periodontol. 1988;15:469–478. doi: 10.1111/j.1600-051x.1988.tb01602.x. [DOI] [PubMed] [Google Scholar]
- 24.Dye BA, Herrera-Abreu M, Lerche-Sehm J, Vlachojannis C, Pikdoken L, Pretzl B, et al. Serum antibodies to periodontal bacteria as diagnostic markers of periodontitis. J Periodontol. 2009;80:634–647. doi: 10.1902/jop.2009.080474. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki M, Minagawa K, Sato M, Kaneko N, Imai S, Yoshihara A, et al. Serum antibody to Porphyromonas gingivalis in metabolic syndrome among an older Japanese population. Gerodontology. doi: 10.1111/ger.12135. Forthcoming 2014. [DOI] [PubMed] [Google Scholar]
- 26.Beck JD, Eke P, Heiss G, Madianos P, Couper D, Lin D, et al. Periodontal disease and coronary heart disease: a reappraisal of the exposure. Circulation. 2005;112:19–24. doi: 10.1161/CIRCULATIONAHA.104.511998. [DOI] [PubMed] [Google Scholar]
- 27.Iwasaki M, Taylor GW, Manz MC, Kaneko N, Imai S, Yoshihara A, et al. Serum antibody to Porphyromonas gingivalis in chronic kidney disease. J Dent Res. 2012;91:828–833. doi: 10.1177/0022034512455063. [DOI] [PubMed] [Google Scholar]
- 28.Michaud DS, Izard J, Wilhelm-Benartzi CS, You DH, Grote VA, Tjønneland A, et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut. 2013;62:1764–1770. doi: 10.1136/gutjnl-2012-303006. [DOI] [PMC free article] [PubMed] [Google Scholar]


