Abstract
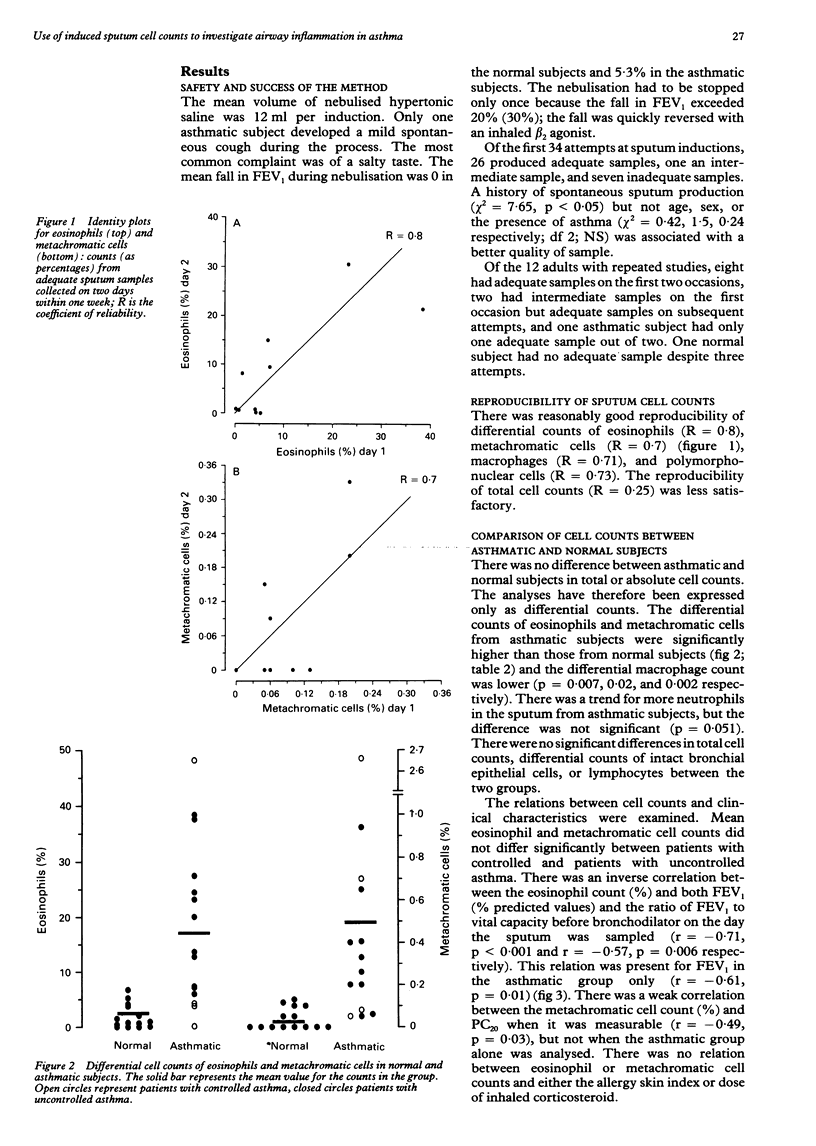
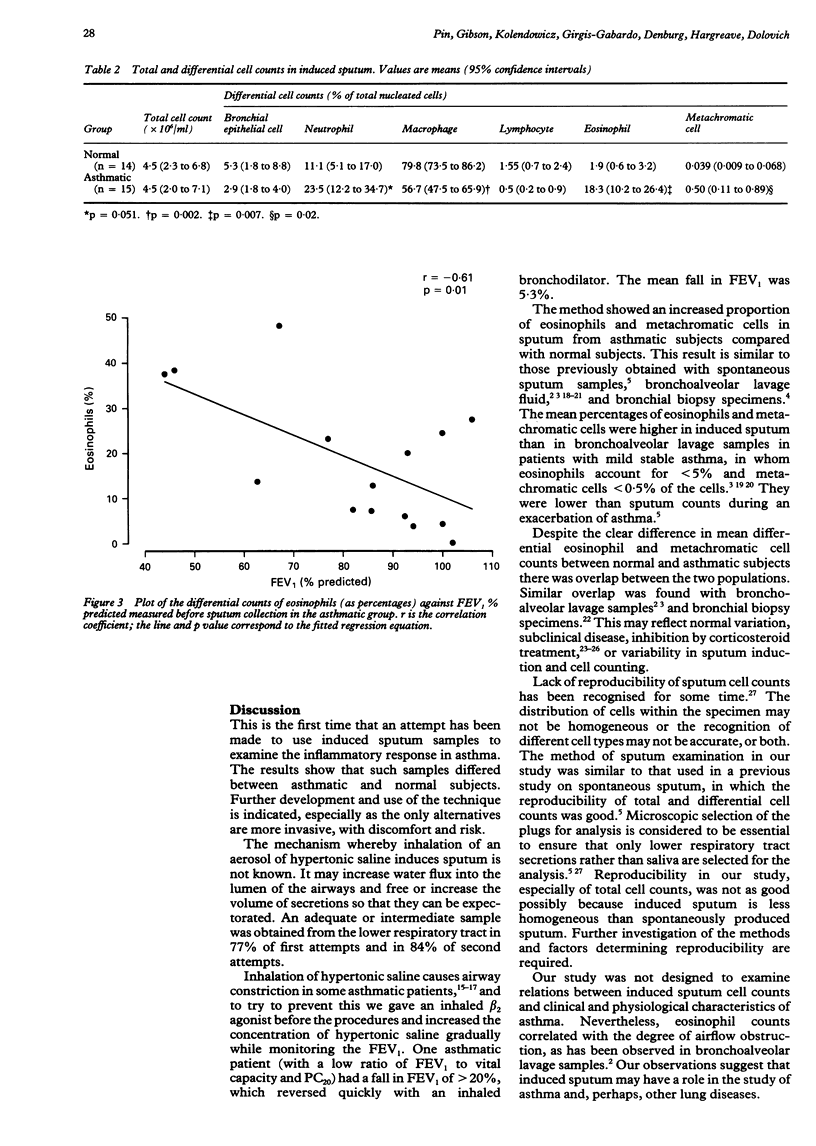
BACKGROUND: Airway inflammation is considered to be important in asthma but is relatively inaccessible to study. Less invasive methods of obtaining sputum from patients unable to produce it spontaneously should provide a useful investigational tool in asthma. METHODS: A method to induce sputum with inhaled hypertonic saline was modified for use in 17 asthmatic patients and 17 normal subjects who could not produce sputum spontaneously. The success rate and safety of the method, the reproducibility of cell counts, and differences in cell counts between the asthmatic and normal groups were examined. Hypertonic saline solution 3-5% was inhaled for up to 30 minutes after inhalation of salbutamol. Subjects were asked to expectorate sputum every five minutes. The quality of the sample was scored on the volume of plugs and the extent of salivary contamination. Plugs from the lower respiratory tract were selected for a total cell count and for differential cell counts of eosinophils and metachromatic cells (mast cells and basophils) in direct smears. RESULTS: Adequate samples from the lower respiratory tract were obtained in 76% of first attempts. The mean fall in the forced expiratory volume in one second (FEV1) during inhalation of saline was 5.3% and the maximum fall 20%. Eosinophil and metachromatic cell counts were reproducible (reliability coefficient 0.8 and 0.7 respectively). When compared with sputum from normal subjects sputum from asthmatic patients contained a significantly higher proportion of eosinophils (mean 18.5% (SE 3.8%) v 1.9% (0.6%)) and metachromatic cells (0.50% (0.18%) v 0.039% (0.014%)). In the asthmatic group the differential eosinophil count correlated with the baseline FEV1. CONCLUSION: Induced sputum is capable of detecting differences in cell counts between normal and asthmatic subjects and merits further development as a potential means of assessing airway inflammation in asthma.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. D., Schoeffel R. E., Finney M. Evaluation of ultrasonically nebulised solutions for provocation testing in patients with asthma. Thorax. 1983 Apr;38(4):284–291. doi: 10.1136/thx.38.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki H., Sly P. D. Inhalation of hypertonic saline as a bronchial challenge in children with mild asthma and normal children. J Allergy Clin Immunol. 1989 Jul;84(1):99–107. doi: 10.1016/0091-6749(89)90183-8. [DOI] [PubMed] [Google Scholar]
- Beasley R., Roche W. R., Roberts J. A., Holgate S. T. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am Rev Respir Dis. 1989 Mar;139(3):806–817. doi: 10.1164/ajrccm/139.3.806. [DOI] [PubMed] [Google Scholar]
- Bigby T. D., Margolskee D., Curtis J. L., Michael P. F., Sheppard D., Hadley W. K., Hopewell P. C. The usefulness of induced sputum in the diagnosis of Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome. Am Rev Respir Dis. 1986 Apr;133(4):515–518. doi: 10.1164/arrd.1986.133.4.515. [DOI] [PubMed] [Google Scholar]
- Boulet L. P., Legris C., Thibault L., Turcotte H. Comparative bronchial responses to hyperosmolar saline and methacholine in asthma. Thorax. 1987 Dec;42(12):953–958. doi: 10.1136/thx.42.12.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHODOSH S., ZACCHEO C. W., SEGAL M. S. The cytology and histochemistry of sputum cells. I. Preliminary differential counts in chronic bronchitis. Am Rev Respir Dis. 1962 May;85:635–648. doi: 10.1164/arrd.1962.85.5.635. [DOI] [PubMed] [Google Scholar]
- Crapo R. O., Morris A. H., Gardner R. M. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981 Jun;123(6):659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- Dark J., Corris P. A. The current state of lung transplantation. Thorax. 1989 Sep;44(9):689–692. doi: 10.1136/thx.44.9.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint K. C., Leung K. B., Hudspith B. N., Brostoff J., Pearce F. L., Johnson N. M. Bronchoalveolar mast cells in extrinsic asthma: a mechanism for the initiation of antigen specific bronchoconstriction. Br Med J (Clin Res Ed) 1985 Oct 5;291(6500):923–926. doi: 10.1136/bmj.291.6500.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godard P., Aubas P., Calvayrac P., Taib J., Michel F. B. Endoscopie et lavage bronchiolo-alvéolaire chez l'asthmatique allergique. Nouv Presse Med. 1981 Oct 24;10(38):3141–3148. [PubMed] [Google Scholar]
- Hargreave F. E., Dolovich J., Newhouse M. T. The assessment and treatment of asthma: a conference report. J Allergy Clin Immunol. 1990 Jun;85(6):1098–1111. doi: 10.1016/0091-6749(90)90056-a. [DOI] [PubMed] [Google Scholar]
- Kelly C., Ward C., Stenton C. S., Bird G., Hendrick D. J., Walters E. H. Number and activity of inflammatory cells in bronchoalveolar lavage fluid in asthma and their relation to airway responsiveness. Thorax. 1988 Sep;43(9):684–692. doi: 10.1136/thx.43.9.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby J. G., Hargreave F. E., Gleich G. J., O'Byrne P. M. Bronchoalveolar cell profiles of asthmatic and nonasthmatic subjects. Am Rev Respir Dis. 1987 Aug;136(2):379–383. doi: 10.1164/ajrccm/136.2.379. [DOI] [PubMed] [Google Scholar]
- Kramer M. S., Feinstein A. R. Clinical biostatistics. LIV. The biostatistics of concordance. Clin Pharmacol Ther. 1981 Jan;29(1):111–123. doi: 10.1038/clpt.1981.18. [DOI] [PubMed] [Google Scholar]
- Leigh T. R., Parsons P., Hume C., Husain O. A., Gazzard B., Collins J. V. Sputum induction for diagnosis of Pneumocystis carinii pneumonia. Lancet. 1989 Jul 22;2(8656):205–206. doi: 10.1016/s0140-6736(89)90382-6. [DOI] [PubMed] [Google Scholar]
- Lundgren R., Söderberg M., Hörstedt P., Stenling R. Morphological studies of bronchial mucosal biopsies from asthmatics before and after ten years of treatment with inhaled steroids. Eur Respir J. 1988 Dec;1(10):883–889. [PubMed] [Google Scholar]
- Pitchenik A. E., Ganjei P., Torres A., Evans D. A., Rubin E., Baier H. Sputum examination for the diagnosis of Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. Am Rev Respir Dis. 1986 Feb;133(2):226–229. doi: 10.1164/arrd.1986.133.2.226. [DOI] [PubMed] [Google Scholar]
- Wardlaw A. J., Cromwell O., Celestino D., Fitzharris P., Geddes D. M., Collins J. V., Kay A. B. Morphological and secretory properties of bronchoalveolar lavage mast cells in respiratory diseases. Clin Allergy. 1986 Mar;16(2):163–173. doi: 10.1111/j.1365-2222.1986.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Wardlaw A. J., Dunnette S., Gleich G. J., Collins J. V., Kay A. B. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. Am Rev Respir Dis. 1988 Jan;137(1):62–69. doi: 10.1164/ajrccm/137.1.62. [DOI] [PubMed] [Google Scholar]



