Abstract
Here, the influence of metabolizable sugars on the susceptibility of Escherichia coli to β-lactam antibiotics was investigated. Notably, monitoring growth and survival of mono- and combination-treated planktonic cultures showed a 1000- to 10 000-fold higher antibacterial efficacy of carbenicillin and cefuroxime in the presence of certain sugars, whereas other metabolites had no effect on β-lactam sensitivity. This effect was unrelated to changes in growth rate. Light microscopy and flow cytometry profiling revealed that bacterial filaments, formed due to β-lactam-mediated inhibition of cell division, rapidly appeared upon β-lactam mono-treatment and remained stable for up to 18 h. The presence of metabolizable sugars in the medium did not change the rate of filamentation, but led to lysis of the filaments within a few hours. No lysis occurred in E. coli mutants unable to metabolize the sugars, thus establishing sugar metabolism as an important factor influencing the bactericidal outcome of β-lactam treatment. Interestingly, the effect of sugar on β-lactam susceptibility was suppressed in a strain unable to synthesize the nutrient stress alarmone (p)ppGpp. Here, to the best of our knowledge, we demonstrate for the first time a specific and significant increase in β-lactam sensitivity due to sugar metabolism in planktonic, exponentially growing bacteria, unrelated to general nutrient availability or growth rate. Understanding the mechanisms underlying the nutritional influences on antibiotic sensitivity is likely to reveal new proteins or pathways that can be targeted by novel compounds, adding to the list of pharmacodynamic adjuvants that increase the efficiency and lifespan of conventional antibiotics.
Introduction
The emergence and dissemination of antibiotic resistance is a significant threat to global public health (WHO, 2014). As new antibiotics are rarely discovered (Ling et al., 2015), alternative approaches such as combination therapies constitute a promising strategy to extend the lifespan of existing antibiotics (Ejim et al., 2011). Several decades of antibiotic research have provided insight into the molecular interactions between antimicrobials and their targets, and their consequences for the bacterial cell; however, much remains to be learned about exogenous and endogenous factors affecting the outcome of antibiotic therapy. Understanding the link(s) between environmental changes and the consequent changes in cell physiology and antibiotic tolerance can provide important insight into the bactericidal activity of antimicrobials as well as mechanisms contributing to antibiotic tolerance, which may be targeted by adjuvants to conventional antibiotics. The penicillin-binding proteins (PBPs) are major determinants of Escherichia coli cell morphology and targets of a clinically important class of antibiotics: the β-lactams. The essential PBP3 (FtsI) is required for septum formation during cell division (Chung et al., 2009) and inhibition of PBP3 via β-lactam-mediated blockage leads to the formation of polynucleoid bacterial filaments, which eventually lyse through an unknown mechanism (Spratt, 1975). Bacterial cell division is regulated according to nutrient availability, but the manner in which nutritional status is relayed to the control of the cell division machinery is still poorly understood, although several metabolites have been suggested to play a role in cell size regulation and cell division (Hill et al., 2013; Monahan et al., 2014). A main regulator of bacterial growth rate and cell division is the nucleotide (p)ppGpp (Potrykus et al., 2011). Two homologous enzymes control the level of (p)ppGpp: RelA, which synthesizes (p)ppGpp under amino acid starvation (Haseltine et al., 1972), and the dual-function synthetase/hydrolase SpoT, which is necessary for (p)ppGpp accumulation during various stresses, including carbon and fatty acid starvation (Seyfzadeh et al., 1993; Xiao et al., 1991). It is well known that high levels of (p)ppGpp give rise to increased β-lactam tolerance due to an indirect inhibitory effect on peptidoglycan biosynthesis (Rodionov & Ishiguro, 1995).
Here, we examined the effect of available carbon sources on in vitro β-lactam susceptibility of E. coli. We demonstrated that the presence of metabolizable sugars promote lysis of filaments formed following β-lactam exposure, leading to significantly increased bacterial killing, whereas other tested carbon sources had no impact on survival. Furthermore, our results implicated the nutritional alarmone (p)ppGpp in this response, as the advanced lysis was abolished in a (p)ppGpp0 strain.
Methods
Bacterial strains and growth conditions
WT E. coli K-12 MG1655 or derivatives of MG1655 were used throughout this study [ΔaraC (Desai & Rao, 2010), Δglc (ΔptsG, ΔptsM, Δglk and Δgcd) (Zuroff et al., 2010), MG1655 (CF1648), ΔrelA (CF12510) (Brown et al., 2002) and ΔrelAΔspoT (CF10237) (Magnusson et al., 2007)] along with two selected clinical E. coli strains, CFT073 (Welch et al., 2002) and IBD p7 (Petersen et al., 2009). All cultures were grown in low-salt Luria–Bertani (LB) broth adjusted to a starting pH of 7.5 at 37 °C with shaking unless otherwise stated. Casamino acids (BD Bacto) were autoclaved separately and added as described in Results. Sterile filtered supplements were added to the medium after autoclaving when required. Ciprofloxacin, gentamicin, cefuroxime and the tested carbon sources were purchased from Sigma-Aldrich, and carbenicillin disodium salt was purchased from Invitrogen.
Antibiotic susceptibility testing
For planktonic antibiotic tolerance testing, an overnight culture was diluted 1 : 100 into fresh LB broth and grown until OD595 0.48–0.52. Treatment was initiated by aliquoting the culture into a 96-well polystyrene plate containing sugar and antibiotic (240 μl cell culture, 10 μl antibiotic and 2.5 μl sugar). Sugar concentrations were adjusted to deliver 60 mM carbon. Incubation and measurements were carried out in a TECAN Sunrise or F200 microtitre plate reader on living cells at 37 °C every 20 min for a period of 18 h with intermediate shake cycles in linear mode. Cell cultures were allowed to settle for 200 s after each shake cycle before measurement. OD595 was then measured. Cell survival was estimated after 18 h of treatment by plating appropriate 10-fold serial dilutions of the cultures on LB agar plates followed by determination of the c.f.u. count after overnight growth at 37 °C. RNA polymerase suppressor mutants occur spontaneously at a relatively high frequency in the ΔrelAΔspoT background (Murphy & Cashel, 2003). To measure the frequency of suppressor mutants, ΔrelAΔspoT cultures were plated on M9 (BD Difco)+0.2 % glucose agar plates supplemented with 1 μg thiamine ml− 1 after treatment and the percentage revertants was determined as the ratio of c.f.u. ml− 1 on minimal and LB plates. For the data presented here, < 5 and < 1 % of the population reverted after growth without or with carbenicillin, respectively. In the figures, error bars indicate sd of n biological replicates, where n ≥ 3, as indicated in the legends. P values were determined by Student's t-test using Prism version 5.0.
Minimum bactericidal concentrations (MBCs) for carbenicillin were determined by the broth microdilution method using an overnight culture grown in LB medium without additives. The culture was diluted in fresh LB medium and inocula with cell densities ranging from the standard inoculum of 5 × 105 to 5 × 108 c.f.u. ml− 1 were grown for 24 h at 37 °C with a twofold serial dilution of carbenicillin ranging from 4 to 1024 mg l− 1 in the presence or absence of a fixed concentration of sugar (corresponding to 60 mM carbon). Two aliquots of 10 μl from wells with no visible growth were spotted on LB agar and incubated overnight at 37 °C. MBC was scored as the lowest concentration of carbenicillin from which no viable cells were recovered.
Flow cytometry
To characterize the morphology of β-lactam-treated cells, E. coli cells were collected at different time points during β-lactam treatment. Approximately 106 cells were harvested, resuspended in PBS and profiled on an Accuri C6 flow cytometer counting 50 000 cells per profile. To eliminate non-bacterial particulate material, we used 0.22 μm filtered PBS and gated the instrument using an FSC-H threshold of 10 000.
Microscopy
E. coli cells were collected by centrifugation at 3000 g after 18 h of treatment, washed in 0.9 % NaCl and resuspended in a smaller volume of saline. Cells were observed by bright-field microscopy on a thin layer of 1 % (v/w) agarose in 0.9 % NaCl using a Zeiss LSM 710 microscope (Carl Zeiss) equipped with a × 100/1.4 oil objective lens.
Results
Sugar metabolites potentiate β-lactam action
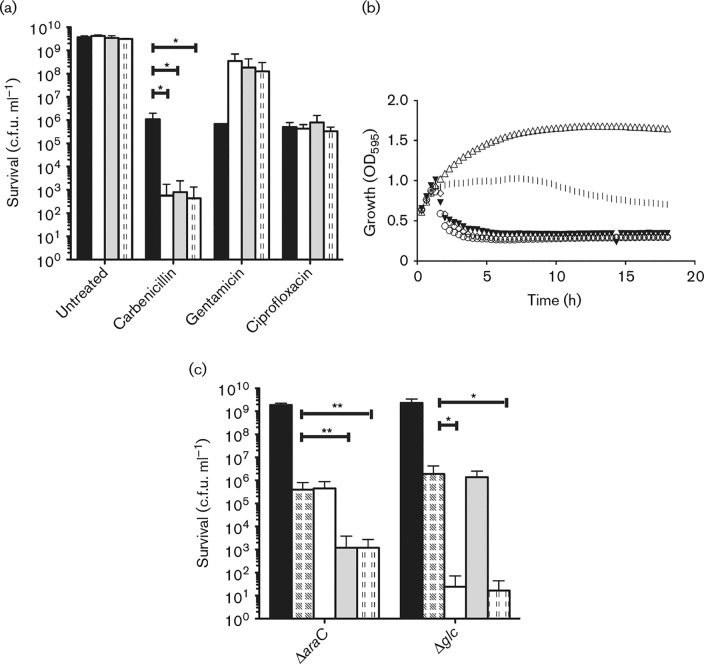
A prior observation from our lab demonstrating the effect of arabinose on E. coli gentamicin susceptibility (Goltermann et al., 2013) led us to test how sugar metabolites impact on E. coli susceptibility to antibiotics belonging to the β-lactam, aminoglycoside, and fluoroquinolone classes. Planktonic cultures of E. coli in the late exponential growth phase (∼108 c.f.u. ml− 1) were treated with 30 μg carbenicillin ml− 1, 3 μg gentamicin ml− 1 or 0.125 μg ciprofloxacin ml− 1, giving rise to a 100-fold reduction in viable cell count. Interestingly, the presence of l-arabinose, D-glucose or D-mannitol sensitized E. coli to carbenicillin and further reduced survival three to four orders of magnitude following 18 h of treatment (Fig. 1a); from the growth curves it was evident that the effect occurred within the first few hours of treatment, with a sudden drop in culture turbidity indicative of cell lysis (Fig. 1b). Under these in vitro conditions, the bactericidal effect of 30 μg carbenicillin ml− 1 and 10 mM d-mannitol was similar to that of 60 μg carbenicillin ml− 1 in mono-treatment (Fig. S1a, available in the online Supplementary Material). The potentiating effect of d-mannitol was also observed when applied in combination with the cephalosporin cefuroxime (Fig. S1b), indicating that the observed effect on survival applies to different subgroups within the β-lactam class of antibiotics. In the remaining text, we have used the acronym SPB (sugar potentiation of β-lactam antibiotics) to describe the cooperative action between sugar metabolites and β-lactam antibiotics. In contrast to what was found for the β-lactam antibiotics, exponential-phase E. coli was less sensitive to the bactericidal activity of gentamicin in the presence of sugar, whereas the ciprofloxacin susceptibility of E. coli was unaffected (Fig. 1a). E. coli cultures treated with different sugars alone showed no sugar-dependent reduction in cell survival (Fig. 1a) and, importantly, addition of sugars did not significantly change the growth rate as judged by OD595 measurements (Fig. S2). Any changes of growth rate did not correlate with the ability of the sugar to potentiate β-lactam action (Fig. S2).
Fig. 1. Impact of sugar metabolites on E. coli antibiotic sensitivity. (a) MG1655 WT was treated with 30 μg carbenicillin ml− 1, 3 μg gentamicin ml− 1 or 0.125 μg ciprofloxacin ml− 1 alone (black bars) or in combination with 12 mM l-arabinose (white bars), 10 mM d-glucose (grey bars) or 10 mM d-mannitol (dotted bars). Survival was monitored after 18 h of treatment. (b) OD595 of MG1655 cultures during 18 h of treatment without (open triangles) or with 30 μg carbenicillin ml− 1 alone (vertical lines) or in combination with sugars (arabinose, open circles; glucose, closed triangles; mannitol, open diamonds). (c) Survival of MG1655 mutants deficient in arabinose metabolism (ΔaraC) and glucose metabolism (Δglc) after 18 h of no treatment (black bars), carbenicillin treatment alone (chequered bars) or in combination with sugars (arabinose, white bars; glucose, grey bars; mannitol, dotted bars). Concentrations as in (a). All c.f.u. ml− 1 values are shown as mean ± sd (n>3). *p < 0.05, **p < 0.01.
We then asked if the SPB effects observed using carbenicillin and cefuroxime were general for cell-wall-targeting antibiotics (Fig. S3). An SPB effect was observed for 20 μg d-cycloserine ml− 1 (Fig. S3a, b), which works upstream of carbenicillin by inhibiting d-Ala–d-Ala formation (Neuhaus & Lynch, 1964). No SPB effect on growth was observed for the penicillin derivative mecillinam (Fig. S3c, d), which targets PBP2 (Spratt, 1975) or for cephalexin (Fig. S3e), a first-generation cephalosporin known to be less efficient against Gram-negatives than second-generation cephalosporins such as cefuroxime.
In addition, we investigated whether the SPB effect also manifests in clinical isolates of E. coli, i.e. the uropathogenic CFT073 strain (Welch et al., 2002) and the inflammatory bowel disease isolate IBD p7 (Petersen et al., 2009) (Fig. S4). Similar to MG1655, the CFT073 strain and the IBD p7 strain both displayed a pronounced SPB effect with two to three orders of magnitude reduction in cell survival upon combination treatment using carbenicillin and sugar (Fig. S4).
To examine whether β-lactam sensitivity in E. coli MG1655 extends to other metabolites, we tested carbenicillin in combination with a selection of carbon sources entering central metabolism via glycolysis, the pentose phosphate pathway, the Entner–Doudoroff pathway, or the tricarboxylic acid cycle. l-Arabinose, glucose, mannitol, xylose, ribose, lactose and galacturonate all increased β-lactam sensitivity. In contrast, glycerol and succinate, which are relatively poor energy/carbon sources, and d-arabinose and sucrose, which cannot be metabolized by E. coli MG1655, had no effect on carbenicillin tolerance (Fig. S1c). These results furthermore rule out a change in medium osmolarity as the underlying cause of SPB.
The sensitivity of E. coli MG1655 to carbenicillin displayed a pronounced inoculum effect (Table 1), i.e. the antimicrobial efficacy of carbenicillin decreased with increasing bacterial density. Notably, the carbenicillin MBC of a standard inoculum (5 × 105 c.f.u. ml− 1) was unaffected by the presence of sugar in the growth medium, whereas the MBC was lowered two- to fourfold at cell densities ≥ 5 × 107 c.f.u. ml− 1 (Table 1). Thus, the SPB effect strongly depended on bacterial cell density, with increasing activity at elevated bacterial density.
Table 1. Dependency of carbenicillin MBC on inoculum density and presence of sugars.
| MBC (μg ml− 1) at inoculum density (c.f.u. ml− 1) | ||||
|---|---|---|---|---|
| Sugar | 5 × 105 | 5 × 106 | 5 × 107 | 5 × 108 |
| No sugar | 16 | 16 | 32 | 256 |
| 12 mM l-arabinose | 16 | 16 | 16 | 64 |
| 10 mM d-glucose | 16 | 16 | 16 | 64 |
| 10 mM d-mannitol | 16 | 16 | 16 | 64 |
Active metabolism is required for β-lactam potentiation
We next investigated whether functional sugar metabolism was required for SPB by using an E. coli strain deficient in arabinose metabolism through mutation of the transcriptional activator encoded by araC. Similar to the WT, the ΔaraC strain was sensitized to carbenicillin by D-glucose and D-mannitol; however, the survival was unaffected by addition of l-arabinose (Fig. 1c). Correspondingly, a Δglc strain deficient in glucose metabolism was sensitized by the presence of l-arabinose or D-mannitol, but showed no SPB effect upon addition of D-glucose in combination with carbenicillin. These results confirmed that sugar metabolism is essential and, as such, only sugars that were metabolized by the respective strains were able to potentiate the effect of β-lactams (Fig. 1).
Sugar metabolites induce morphological changes in β-lactam-treated cells
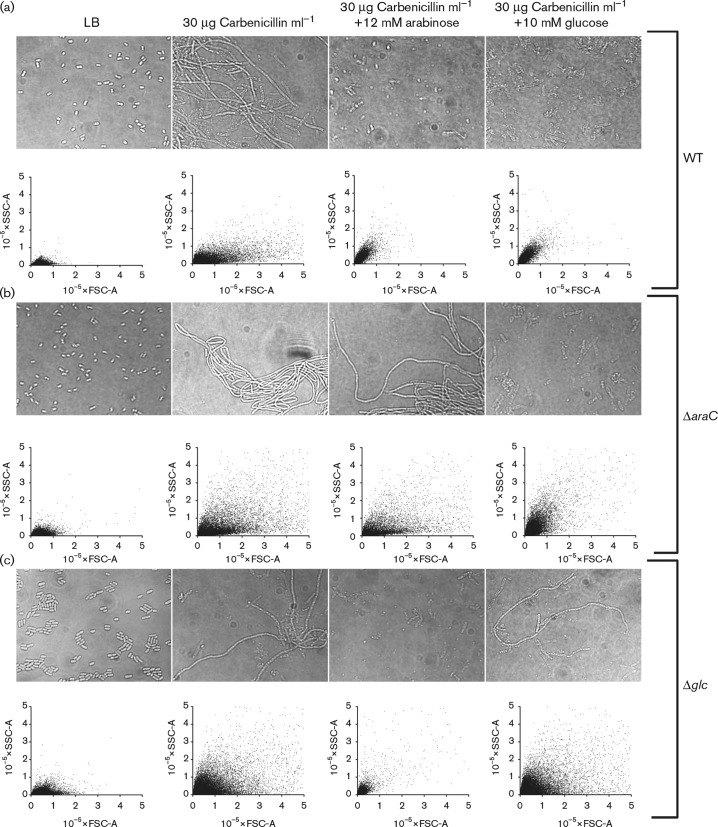
Morphological characterization of carbenicillin-treated cells showed the formation of filaments caused by β-lactam-mediated inhibition of cell division as expected (Fig. 2) (Spratt, 1975). These filaments were, however, absent from samples treated for 18 h with carbenicillin and sugar in combination (Fig. 2), and only semi-transparent cell debris remained. Flow cytometry confirmed the microscopy results and showed a wide distribution of filament sizes (as measured by forward scatter (FSC)] and granularity [as measured by side scatter (SSC)] in the carbenicillin-treated population whereas only small granular debris remained after 18 h combination treatment with carbenicillin and sugar (Fig. 2). By comparison, untreated bacteria consisted of a population of small, smooth cells characterized by low FSC and SSC values grouping to the lower left corner of the flow cytometry diagram. Following treatment with carbenicillin, filaments formed, as detected by an increase in the FSC values stretching along the horizontal axis. As the filaments lysed, when carbenicillin was combined with sugar, the FSC vales decreased. Time-course flow cytometry showed that bacterial filaments rapidly formed upon β-lactam mono-treatment and accumulated throughout the treatment period. The presence of metabolizable sugars in the medium did not change the initial rate of filamentation, but led to lysis of the filaments, as indicated by a significant change in the flow cytometry profile between 2 and 4 h of treatment (Fig. S5). The morphological changes in the combination-treated cultures were dependent on active sugar metabolism. The metabolism-deficient ΔaraC and Δglc strains were morphologically unaffected by addition of arabinose and glucose, respectively, whilst filaments were still eradicated upon addition of metabolizable sugar (Fig. 2).
Fig. 2. Filament formation and lysis of E. coli MG1655 WT (a) and mutants deficient in arabinose metabolism (ΔaraC) (b) or glucose metabolism (Δglc) (c) were treated with carbenicillin alone or in combination with 12 mM l-arabinose or 10 mM d-glucose. Untreated controls were included. Cells were analysed by light microscopy (upper panel) and flow cytometry (lower panel) after 18 h of treatment. Flow cytometry results are shown as FSC-A, indicating cell size, versus SSC-A, indicating surface granularity. Scale bar 10 microns.
Bacterial stringent response modulates the SPB effect
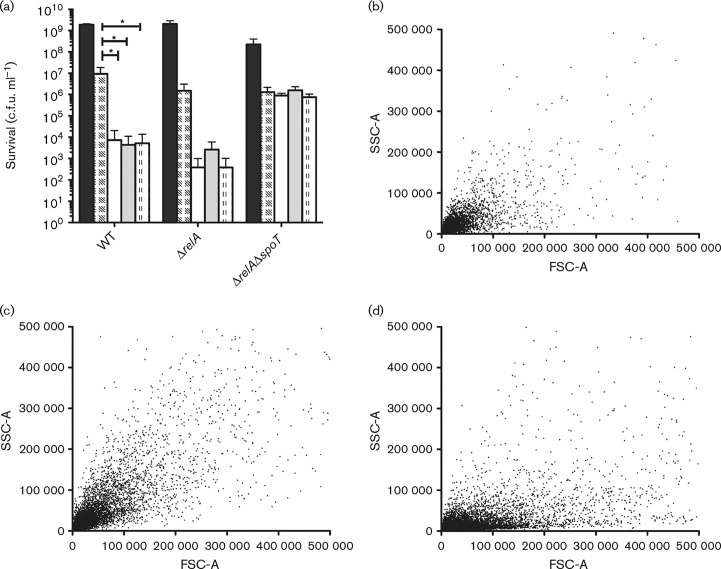
At high cell densities, when nutrients are limited, the level of intrabacterial (p)ppGpp increases, thereby aiding the adaptation of bacteria to suboptimal growth conditions (Potrykus et al., 2011). High levels of (p)ppGpp can cause β-lactam tolerance due to an indirect inhibitory effect on peptidoglycan synthesis (Rodionov & Ishiguro, 1995). We therefore speculated that dense bacterial cultures might accumulate (p)ppGpp due to exhaustion of the preferred carbon sources (Sezonov et al., 2007), and that addition of metabolizable sugars would decrease the (p)ppGpp level and consequently abolish β-lactam tolerance. To test this hypothesis, ΔrelA and ΔrelAΔspoT mutants and their isogenic parent were treated with carbenicillin in combination with sugar as described above. Following combination treatment, survival of the ΔrelA strain was reduced approximately three orders of magnitude (Fig. 3), which is comparable with the isogenic WT. However, the potentiation was completely lost in the ΔrelAΔspoT background (Fig. 3), suggesting that the (p)ppGpp level or the SpoT protein itself is important for the SPB effect. In concordance, flow cytometry data indicated that bacterial filaments formed by the WT and ΔrelA strains were eradicated (Fig. 3b, c), whereas the ΔrelAΔspoT strain remained filamentous after treatment with carbenicillin in combination with glucose (Fig. 3c).
Fig. 3. β-Lactam sensitivity of a (p)ppGpp0 strain is unaffected by sugar. (a) Bacterial cell survival was determined after 18 h of treatment without (black bars) or with 30 μg carbenicillin ml− 1 alone (chequered bars) or in combination with 12 mM l-arabinose (white bars), 10 mM d-glucose (grey bars) or 10 mM d-mannitol (dotted bars) for WT, ΔrelA and ΔrelAΔspoT strains producing decreasing amounts of the (p)ppGpp alarmone. Data shown as mean ± sd (n ≥ 4). *p < 0.05. (b–d) Flow cytometry FSC versus SSC profiles of WT (b), ΔrelA (c) and ΔrelAΔspoT (d) strains after 18 h of treatment with 30 μg carbenicillin ml− 1 in combination with 10 mM d-glucose.
As a control, we treated WT cultures in the late exponential growth phase with carbenicillin in combination with individual amino acids (glycine, l-serine, l-alanine or l-threonine), which are among the amino acids depleted first from complex media (Prüss et al., 1994; Sezonov et al., 2007), or with 0.2 % Casamino acids and determined survival following 18 h of incubation. None of these supplements affected survival during carbenicillin treatment (Fig. S1d), confirming that the SPB effect on β-lactam antibiotics was specific to the metabolizable sugars and not a general effect of delaying nutrient depletion of the growth medium.
Discussion
Here, we report the novel observation that planktonic E. coli was sensitized to β-lactam antibiotics when a metabolizable sugar (e.g. l-arabinose, d-glucose or d-mannitol) was administered along with the antibiotic – an effect which we have dubbed ‘sugar potentiation of β-lactam antibiotics (SPB)’. The cooperative action of sugar and β-lactam led to a 1000- to 10 000-fold reduction in bacterial survival compared with the β-lactam alone in a manner specific to this combination. Sugar was unable to potentiate antimicrobials targeting other cellular functions (DNA replication and translation) and, conversely, β-lactam susceptibility was unaffected by non-carbohydrate carbon sources. If the observed effect was simply a result of increased growth rate, we would expect a similar potentiation of aminoglycoside and quinolone antibiotics as susceptibility to both increases with growth rate (Eng et al., 1991). Moreover, other carbon sources such as Casamino acids did not potentiate the effect of carbenicillin, although these would also be expected to increase general metabolism in late exponential growth phase. The addition of excess glucose or other sugars could furthermore be expected to result in increased acetate production (Rice et al., 2005), which in Staphylococcus aureus has been linked to increased β-lactam susceptibility (Rice et al., 2005). However, a similar link has not been demonstrated for Gram-negative bacteria and as our results could be reproduced in E. coli BL21(DE3) (data not shown), a strain which is known to have low acetic acid production (Bäcklund et al., 2011), we do not expect overflow metabolism to be part of the underlying mechanism. Rather, we believe that β-lactam potentiation relies on interplay between specific metabolites, their effect on cell division and the inhibitory effect of β-lactams on PBP function.
The β-lactams used in this study (carbenicillin and cefuroxime) have relatively high affinities for PBP3 and PBP1a (Curtis et al., 1979), and β-lactam treatment leads to formation of polynucleoid filaments due to PBP3 inhibition at sub-MIC concentrations. Similar filamentation was observed when sugar and carbenicillin were combined; however, as indicated by culture turbidity and flow cytometry (FSC), the filaments rapidly lysed after ∼2–4 h of treatment. Using metabolism-deficient mutants, we confirmed that active sugar metabolism was necessary for the SPB effect. Rapid β-lactam-induced lysis is often preceded by the formation of membrane protrusions emanating from the mid-cell (Staugaard et al., 1976; Yao et al., 2012), which indicates that bacteria are particularly prone to β-lactams during septation. The cell division is governed by a protein complex, the divisome, which forms at the mid-cell beginning with polymerization of FtsZ into a contractile ring. In E. coli, β-lactam antibiotics activate the SOS response leading to controlled inhibition of FtsZ polymerization and temporary β-lactam tolerance (Miller et al., 2004). Cell division is furthermore regulated according to nutrient availability in a manner that is not fully understood, although several modulators of FtsZ polymerization have been found to link cell division with central carbon metabolism in E. coli and Bacillus subtilis (Hill et al., 2013; Monahan et al., 2014; Weart et al., 2007). The metabolizable sugars tested in the present study quickly promote β-lactam-induced lysis of E. coli; thus, we speculate that the nutritional up-shift leads to changes in the divisome that either advance cell division leading to recruitment of murein hydrolases or impede the implementation of protective measurements such as controlled arrest of cell division that could otherwise avert lysis. This notion is supported by the observation that neither mecillinam nor cephalexin was potentiated by sugar metabolites under our experimental conditions. Although both of these β-lactams give rise to morphological alterations (round cells and filaments, respectively), they are non-lytic and non-lethal at high cell densities (Aono et al., 1979; Chung et al., 2009), suggesting that sugar metabolites do not destabilize bacterial filaments per se, but rather reinforce the existing lytic effect of β-lactam antibiotics.
Notably, the effect of sugar on β-lactam sensitivity was completely lost in a ΔrelAΔspoT mutant, which was unable to synthesize (p)ppGpp, as opposed to the WT and ΔrelA strains. High levels of (p)ppGpp have been linked to antibiotic tolerance in E. coli and several other organisms (Abranches et al., 2009; Geiger et al., 2014; Pomares et al., 2008; Rodionov & Ishiguro, 1995), and SpoT is responsible for (p)ppGpp accumulation in response to various environmental cues, including carbon limitation (Xiao et al., 1991). However, because β-lactam sensitivity was unaffected when amino acids were added as a carbon source, we find it unlikely that the SPB effect is caused solely by a SpoT-mediated reduction in (p)ppGpp levels in response to the availability of carbon. However, we cannot exclude that sugars specifically regulate the enzymic activities of SpoT in a manner that is unrelated to total carbon accessibility. In this regard, it will be interesting to know whether carbohydrate and non-carbohydrate carbon sources exert different regulatory effects on SpoT activity. Contrary to the inhibitory effect of high (p)ppGpp concentrations on cell division, a basal level of (p)ppGpp is required for cell division to proceed as seen by septation deficiency in the ΔrelAΔspoT mutant leading to filamentation due to unknown mechanisms (Xiao et al., 1991). As an alternative, we speculate that loss of SPB in the ΔrelAΔspoT background can be explained by the inherent septation deficiency of the strain, which possibly masks a nutrient-dependent change in the divisome that could form the basis of potentiation as discussed above.
Early observations have furthermore suggested a link between the second messenger, cAMP, and the regulation of cell cycle progression and envelope biogenesis (Jaffé et al., 1986; Kumar, 1976; Utsumi et al., 1989). The intracellular cAMP level is regulated in response to carbon availability and, based on the observations of Epstein et al. (1975), carbon sources supporting SPB under our conditions are expected to give rise to low cAMP levels, whereas succinate, glycerol or Casamino acids lead to higher cAMP levels. Mutants devoid of cAMP (cya− ) are highly resistant to mecillinam (Aono et al., 1979; D'Ari et al., 1988), which can be reversed by addition of cAMP to the growth medium, but only a modest decrease in susceptibility to other β-lactams has been observed (Aono et al., 1979; Jaffé et al., 1983). Any involvement of cAMP as the underlying cause of SPB would imply that low cAMP levels under our conditions sensitize the bacteria to β-lactam antibiotics, which is contrary to previous observations. Furthermore, the addition of 2 mM cAMP to the growth medium did not prevent the SPB effect (data not shown) and, consequently, we find the involvement of cAMP unlikely.
Our findings that survival during gentamicin exposure was improved by the presence of sugar metabolites is in sharp contrast to recent reports demonstrating the ability of specific carbohydrates to restore the aminoglycoside susceptibility of non- or slowly growing persister cells in planktonic cultures and in biofilms (Allison et al., 2011; Barraud et al., 2013). The authors attributed the effect to the generation of a proton motive force across the cytoplasmic membrane, which facilitates the uptake of aminoglycoside antibiotics in bacteria with otherwise low metabolic activity. In our experimental setup, we examined actively growing bacteria, which have a fundamentally different metabolism and physiology from persisters (Prax & Bertram, 2014). As persisters typically comprise < 0.01 % of the total cell count in growing cultures (Keren et al., 2004), it is of utmost importance to understand how metabolites affect the antibiotic tolerance of the entire bacterial population. Recent work has linked (p)ppGpp levels with toxin/antitoxin systems and the Lon protease in the context of persister formation (Maisonneuve et al., 2013). It would be interesting to explore these links in our exponential cultures.
In a previous report, Zuroff et al. (2010) demonstrated that glucose impacts the β-lactam tolerance of E. coli growing in colony biofilms, but failed to detect an effect on planktonic cells as we report here. We believe that this discrepancy is due to differences in β-lactam concentration and/or differences in bacterial growth phase at the onset of antibiotic treatment. As bacteria living in biofilms can be substantially more tolerant to antibiotics than their planktonic counterparts (Stewart & Costerton, 2001), the possibility that metabolites can also sensitize sessile bacteria to β-lactam antibiotics is very intriguing and warrants further investigation.
The SPB effect is an example of altering the phenotypic tolerance of a bacterial population and uncovering the molecular mechanisms underlying SPB will help to provide important insight into this elusive phenomenon as well as the bactericidal effect of β-lactams in general. Our observations, which extend to two different clinical E. coli isolates, emphasize the need for a more comprehensive understanding of the link between central carbon metabolism and antibiotic susceptibility, which may add further targets for novel pharmacodynamic adjuvants to our conventional antibiotics.
Acknowledgements
We are grateful to Christopher V. Rao, Ross P. Carlson and Michael Cashel for providing bacterial strains. We thank Flemming Bjerrum for discussions on the manuscript. This work was supported by the Danish Research Council (1333-00113 to L. G. and 1323-00177 to T. T. N.) and the Carlsberg Foundation (CF14-0144 to T. T. N.).
Supplementary Data
Supplementary Data
Abbreviations:
- FSC
forward scatter
- MBC
minimum bactericidal concentration
- PBP
penicillin-binding protein
- SPB
sugar potentiation of β-lactam antibiotics
- SSC
side scatter
References
- Abranches J., Martinez A.R., Kajfasz J.K., Chávez V., Garsin D.A., Lemos J.A. (2009). The molecular alarmone (p)ppGpp mediates stress responses, vancomycin tolerance, and virulence in Enterococcus faecalis J Bacteriol 191 2248–2256 10.1128/JB.01726-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison K.R., Brynildsen M.P., Collins J.J. (2011). Metabolite-enabled eradication of bacterial persisters by aminoglycosides Nature 473 216–220 10.1038/nature10069 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono R., Yamasaki M., Tamura G. (1979). High and selective resistance to mecillinam in adenylate cyclase-deficient or cyclic adenosine 3′,5′-monophosphate receptor protein-deficient mutants of Escherichia coli J Bacteriol 137 839–845 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäcklund E., Ignatushchenko M., Larsson G. (2011). Suppressing glucose uptake and acetic acid production increases membrane protein overexpression in Escherichia coli Microb Cell Fact 10 35 10.1186/1475-2859-10-35 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraud N., Buson A., Jarolimek W., Rice S.A. (2013). Mannitol enhances antibiotic sensitivity of persister bacteria in Pseudomonas aeruginosa biofilms PLoS One 8 e84220 10.1371/journal.pone.0084220 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L., Gentry D., Elliott T., Cashel M. (2002). DksA affects ppGpp induction of RpoS at a translational level J Bacteriol 184 4455–4465 10.1128/JB.184.16.4455-4465.2002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H.S., Yao Z., Goehring N.W., Kishony R., Beckwith J., Kahne D. (2009). Rapid β-lactam-induced lysis requires successful assembly of the cell division machinery Proc Natl Acad Sci U S A 106 21872–21877 10.1073/pnas.0911674106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N.A., Orr D., Ross G.W., Boulton M.G. (1979). Affinities of penicillins and cephalosporins for the penicillin-binding proteins of Escherichia coli K-12 and their antibacterial activity Antimicrob Agents Chemother 16 533–539 10.1128/AAC.16.5.533 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ari R., Jaffé A., Bouloc P., Robin A. (1988). Cyclic AMP and cell division in Escherichia coli J Bacteriol 170 65–70 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai T.A., Rao C.V. (2010). Regulation of arabinose and xylose metabolism in Escherichia coli Appl Environ Microbiol 76 1524–1532 10.1128/AEM.01970-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejim L., Farha M.A., Falconer S.B., Wildenhain J., Coombes B.K., Tyers M., Brown E.D., Wright G.D. (2011). Combinations of antibiotics and nonantibiotic drugs enhance antimicrobial efficacy Nat Chem Biol 7 348–350 10.1038/nchembio.559 . [DOI] [PubMed] [Google Scholar]
- Eng R.H., Padberg F.T., Smith S.M., Tan E.N., Cherubin C.E. (1991). Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria Antimicrob Agents Chemother 35 1824–1828 10.1128/AAC.35.9.1824 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Rothman-Denes L.B., Hesse J. (1975). Adenosine 3′ : 5′-cyclic monophosphate as mediator of catabolite repression in Escherichia coli Proc Natl Acad Sci U S A 72 2300–2304 10.1073/pnas.72.6.2300 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T., Kästle B., Gratani F.L., Goerke C., Wolz C. (2014). Two small (p)ppGpp synthases in Staphylococcus aureus mediate tolerance against cell envelope stress conditions J Bacteriol 196 894–902 10.1128/JB.01201-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goltermann L., Good L., Bentin T. (2013). Chaperonins fight aminoglycoside-induced protein misfolding and promote short-term tolerance in Escherichia coli J Biol Chem 288 10483–10489 10.1074/jbc.M112.420380 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W.A., Block R., Gilbert W., Weber K. (1972). MSI and MSII made on ribosome in idling step of protein synthesis Nature 238 381–384 10.1038/238381a0 . [DOI] [PubMed] [Google Scholar]
- Hill N.S., Buske P.J., Shi Y., Levin P.A. (2013). A moonlighting enzyme links Escherichia coli cell size with central metabolism PLoS Genet 9 e1003663 10.1371/journal.pgen.1003663 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffé A., Chabbert Y.A., Derlot E. (1983). Selection and characterization of beta-lactam-resistant Escherichia coli K-12 mutants Antimicrob Agents Chemother 23 622–625 10.1128/AAC.23.4.622 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffé A., D'Ari R., Norris V. (1986). SOS-independent coupling between DNA replication and cell division in Escherichia coli J Bacteriol 165 66–71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren I., Kaldalu N., Spoering A., Wang Y., Lewis K. (2004). Persister cells and tolerance to antimicrobials FEMS Microbiol Lett 230 13–18 10.1016/S0378-1097(03)00856-5 . [DOI] [PubMed] [Google Scholar]
- Kumar S. (1976). Properties of adenyl cyclase and cyclic adenosine 3′,5′-monophosphate receptor protein-deficient mutants of Escherichia coli J Bacteriol 125 545–555 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L.L., Schneider T., Peoples A.J., Spoering A.L., Engels I., Conlon B.P., Mueller A., Schäberle T.F., Hughes D.E., other authors (2015). A new antibiotic kills pathogens without detectable resistance Nature 517 455–459 10.1038/nature14098 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson L.U., Gummesson B., Joksimović P., Farewell A., Nyström T. (2007). Identical, independent, and opposing roles of ppGpp and DksA in Escherichia coli J Bacteriol 189 5193–5202 10.1128/JB.00330-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve E., Castro-Camargo M., Gerdes K. (2013). (p)ppGpp controls bacterial persistence by stochastic induction of toxin-antitoxin activity Cell 154 1140–1150 10.1016/j.cell.2013.07.048 . [DOI] [PubMed] [Google Scholar]
- Miller C., Thomsen L.E., Gaggero C., Mosseri R., Ingmer H., Cohen S.N. (2004). SOS response induction by β-lactams and bacterial defense against antibiotic lethality Science 305 1629–1631 10.1126/science.1101630 . [DOI] [PubMed] [Google Scholar]
- Monahan L.G., Hajduk I.V., Blaber S.P., Charles I.G., Harry E.J. (2014). Coordinating bacterial cell division with nutrient availability: a role for glycolysis MBio 5 e00935-14 10.1128/mBio.00935-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy H., Cashel M. (2003). Isolation of RNA polymerase suppressors of a (p)ppGpp deficiency Methods Enzymol 371 596–601 10.1016/S0076-6879(03)71044-1 . [DOI] [PubMed] [Google Scholar]
- Neuhaus F.C., Lynch J.L. (1964). The enzymatic synthesis of d-alanyl-d-alanine. 3. On the inhibition of d-alanyl-d-alanine synthetase by the antibiotic d-cycloserine Biochemistry 3 471–480 10.1021/bi00892a001 . [DOI] [PubMed] [Google Scholar]
- Petersen A.M., Nielsen E.M., Litrup E., Brynskov J., Mirsepasi H., Krogfelt K.A. (2009). A phylogenetic group of Escherichia coli associated with active left-sided inflammatory bowel disease BMC Microbiol 9 171 10.1186/1471-2180-9-171 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomares M.F., Vincent P.A., Farías R.N., Salomón R.A. (2008). Protective action of ppGpp in microcin J25-sensitive strains J Bacteriol 190 4328–4334 10.1128/JB.00183-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrykus K., Murphy H., Philippe N., Cashel M. (2011). ppGpp is the major source of growth rate control in E. coli Environ Microbiol 13 563–575 10.1111/j.1462-2920.2010.02357.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prax M., Bertram R. (2014). Metabolic aspects of bacterial persisters Front Cell Infect Microbiol 4 148 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüss B.M., Nelms J.M., Park C., Wolfe A.J. (1994). Mutations in NADH : ubiquinone oxidoreductase of Escherichia coli affect growth on mixed amino acids J Bacteriol 176 2143–2150 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice K.C., Nelson J.B., Patton T.G., Yang S.-J., Bayles K.W. (2005). Acetic acid induces expression of the Staphylococcus aureus cidABC and lrgAB murein hydrolase regulator operons J Bacteriol 187 813–821 10.1128/JB.187.3.813-821.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov D.G., Ishiguro E.E. (1995). Direct correlation between overproduction of guanosine 3′,5′-bispyrophosphate (ppGpp) and penicillin tolerance in Escherichia coli J Bacteriol 177 4224–4229 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfzadeh M., Keener J., Nomura M. (1993). spoT-dependent accumulation of guanosine tetraphosphate in response to fatty acid starvation in Escherichia coli Proc Natl Acad Sci U S A 90 11004–11008 10.1073/pnas.90.23.11004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezonov G., Joseleau-Petit D., D'Ari R. (2007). Escherichia coli physiology in Luria-Bertani broth J Bacteriol 189 8746–8749 10.1128/JB.01368-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B.G. (1975). Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12 Proc Natl Acad Sci U S A 72 2999–3003 10.1073/pnas.72.8.2999 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staugaard P., van den Berg F.M., Woldringh C.L., Nanninga N. (1976). Localization of ampicillin-sensitive sites in Escherichia coli by electron microscopy J Bacteriol 127 1376–1381 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P.S., Costerton J.W. (2001). Antibiotic resistance of bacteria in biofilms Lancet 358 135–138 10.1016/S0140-6736(01)05321-1 . [DOI] [PubMed] [Google Scholar]
- Utsumi R., Noda M., Kawamukai M., Komano T. (1989). Control mechanism of the Escherichia coli K-12 cell cycle is triggered by the cyclic AMP-cyclic AMP receptor protein complex J Bacteriol 171 2909–2912 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weart R.B., Lee A.H., Chien A.-C., Haeusser D.P., Hill N.S., Levin P.A. (2007). A metabolic sensor governing cell size in bacteria Cell 130 335–347 10.1016/j.cell.2007.05.043 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R.A., Burland V., Plunkett G., III, Redford P., Roesch P., Rasko D., Buckles E.L., Liou S.-R., Boutin A., other authors (2002). Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli Proc Natl Acad Sci U S A 99 17020–17024 10.1073/pnas.252529799 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2014). Antimicrobial Resistance: Global Report on Surveillance Geneva: World Health Organization. [Google Scholar]
- Xiao H., Kalman M., Ikehara K., Zemel S., Glaser G., Cashel M. (1991). Residual guanosine 3′,5′-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations J Biol Chem 266 5980–5990 . [PubMed] [Google Scholar]
- Yao Z., Kahne D., Kishony R. (2012). Distinct single-cell morphological dynamics under beta-lactam antibiotics Mol Cell 48 705–712 10.1016/j.molcel.2012.09.016 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuroff T.R., Bernstein H., Lloyd-Randolfi J., Jimenez-Taracido L., Stewart P.S., Carlson R.P. (2010). Robustness analysis of culturing perturbations on Escherichia coli colony biofilm beta-lactam and aminoglycoside antibiotic tolerance BMC Microbiol 10 185 10.1186/1471-2180-10-185 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data