Abstract
Staphylococcus aureus possesses a lone extracytoplasmic function (ECF) sigma factor, σS. In Bacillus subtilis, the ECF sigma factor, σW, is activated through a proteolytic cascade that begins with cleavage of the RsiW anti-sigma factor by a site-1 protease (S1P), PrsW. We have identified a PrsW homologue in S. aureus (termed PrsS) and explored its role in σS regulation. Herein, we demonstrate that although a cognate σS anti-sigma factor currently remains elusive, prsS phenocopies sigS in a wealth of regards. Specifically, prsS expression mimics the upregulation observed for sigS in response to DNA-damaging agents, cell wall-targeting antibiotics and during ex vivo growth in human serum and murine macrophages. prsS mutants also display the same sensitivities of sigS mutants to the DNA-damaging agents methyl methane sulfonate (MMS) and hydrogen peroxide, and the cell wall-targeting antibiotics ampicillin, bacitracin and penicillin-G. These phenotypes appear to be explained by alterations in abundance of proteins involved in drug resistance (Pbp2a, FemB, HmrA) and the response to DNA damage (BmrA, Hpt, Tag). Our findings seem to be mediated by putative proteolytic activity of PrsS, as site-directed mutagenesis of predicted catalytic residues fails to rescue the sensitivity of the mutant to H2O2 and MMS. Finally, a role for PrsS in S. aureus virulence was identified using human and murine models of infection. Collectively, our data indicate that PrsS and σS function in a similar manner, and perhaps mediate virulence and resistance to DNA damage and cell wall-targeting antibiotics, via a common pathway.
Introduction
Staphylococcus aureus is an opportunistic pathogen that colonizes up to 50 % of the human population (Diekema et al., 2001). In addition to its lifestyle as a commensal, S. aureus is able to exist in almost every niche of the human body, causing diseases ranging from mild skin infections to life-threatening conditions. As such, S. aureus causes more morbidity and mortality than any other infectious agent and leads to more annual deaths than HIV/AIDS in the United States (Tonks, 2007; Klevens et al., 2006, 2007; Moran et al., 2006).
S. aureus is such a successful pathogen due to its many virulence determinants and the way they are regulated in order to establish and maintain infection. These virulence determinants include alternative sigma factors, of which S. aureus possesses three: σB, σH and σS (Shaw et al., 2008; Tao et al., 2010; Wu et al., 1996). This latter component was discovered by our group, and is a member of the extracytoplasmic function (ECF) sigma factor family, which has been implicated in the response to cell envelope stress, DNA damage resistance and the progression of infection (Miller et al., 2012; Shaw et al., 2008).
ECF sigma factors are typically activated in response to numerous environmental conditions via regulated intramembrane proteolysis (RIP), which enables the dissociation of a sigma factor from its cognate anti-sigma factor (Brown & Hughes, 1995; Brown et al., 2000; Flynn et al., 2004; Helmann, 2002). RIP is particularly well characterized for σW of B. subtilis, which is involved in the response to cell envelope damage (Ellermeier & Losick, 2006; Heinrich & Wiegert, 2006; Helmann, 2006; Kunst et al., 1997; Pietiäinen et al., 2005). Upon sensing antimicrobial peptide stress or alkaline shock, σW is released from its cognate anti-sigma factor, RsiW, by sequential cleavage; first by the site-1 protease (S1P), PrsW, followed by RasP-mediated site-2 proteolysis (Ellermeier & Losick, 2006; Heinrich & Wiegert, 2006; Schöbel et al., 2004). Newly released σW subsequently impacts the transcription of more than 60 genes involved in maintaining cell envelope integrity (Cao et al., 2002).
The role of PrsW has also recently been studied in Clostridium difficile (Ho & Ellermeier, 2011). This Gram-positive opportunistic pathogen has three ECF sigma factors, CsfT, CsfU and CsfV, along with cognate anti-sigma factors RsiT, RsiU and RsiV, respectively. CsfT is upregulated by the cell envelope-targeting agents bacitracin and lysozyme. The activation of CsfT is induced by RIP, whereby PrsW cleaves RsiT. This process was shown to be important in C. difficile virulence, as prsW mutants have a decreased ability to colonize the gastrointestinal tract of hamsters.
For S. aureus, a clear RasP/RseP homologue is present in the MEROPS protease database (http://merops.sanger.ac.uk/); however, no counterpart for PrsW has been annotated. In this work, we have investigated the function of a PrsW homologue in S. aureus (SAUSA300_0230), designated ‘PrsS’ for ‘putative regulator of SigmaS’.
Methods
Bacterial strains, plasmids and growth conditions.
S. aureus and Escherichia coli strains used in this study are listed in Table 1. Strains were grown as previously described, unless otherwise indicated (Shaw et al., 2008).
Table 1. Strains and plasmids used in this study.
| Strain or plasmid | Genotype or description | Reference or source |
| E. coli | ||
| DH5α | Cloning strain | Salisbury et al. (1972) |
| DC10B | Cloning strain | Monk et al. (2012) |
| S. aureus | ||
| RN4220 | Restriction-deficient transformation recipient | Lab stocks |
| 8325-4 | WT laboratory strain, rsbU − | Lab stocks |
| SH1000 | WT laboratory strain, rsbU + | Horsburgh et al. (2002) |
| Newman | WT laboratory strain, human clinical isolate | Lab stocks |
| USA300 | USA300-HOU MRSA isolate cured of pUSA300-HOU-MRSA | Kolar et al. (2011) |
| CNK622 | RN4220 pAZ106 : : prsS-lacZ, prsS + | This study |
| CNK955 | 8325-4 pAZ106 : : prsS-lacZ, prsS + | This study |
| CNK661 | SH1000 pAZ106 : : prsS-lacZ, prsS + | This study |
| CNK876 | Newman pAZ106 : : prsS-lacZ, prsS + | This study |
| CNK875 | USA300 pAZ106 : : prsS-lacZ, prsS + | This study |
| NE166 | USA300 JE2 prsS : : bursa aurealis, prsS− | NARSA |
| CNK1460 | USA300 HOU prsS : : bursa aurealis, prsS − | This study |
| CNK1462 | RN4220 pMK4 : : prsS, prsS + | This study |
| CNK1467 | USA300 HOU prsS : : bursa aurealis pMK4 : : prsS, prsS + | This study |
| CNK1872 | RN4220 pMK4 : : prsS E215A,E216A | This study |
| CNK1873 | USA300 HOU prsS : : bursa aurealis pMK4 : : prsS E215A,E216A | This study |
| HKM852 | USA300 HOU sigS : : tet, sigS− | This study |
| HKM779 | RN4220 pSC-A : : tet : : sigS-lacZ, sigS + | Burda et al. (2014) |
| CNK957 | USA300 HOU pSC-A : : tet : : sigS-lacZ, sigS + | This study |
| CNK1870 | USA300 HOU prsS : : bursa aurealis sigS : : tet, prsS −, sigS- | This study |
| NE1203 | USA300 JE2 SAUSA300_1495 : : bursa aurealis, SAUSA300_1495− | NARSA |
| NE1644 | USA300 JE2 SAUSA300_1788 : : bursa aurealis, SAUSA300_1788− | NARSA |
| NE1783 | USA300 JE2 SAUSA300_1684 : : bursa aurealis, SAUSA300_1684− | NARSA |
| NE1942 | USA300 JE2 SAUSA300_0014 : : bursa aurealis, SAUSA300_0014− | NARSA |
| CNK1784 | USA300 HOU pSC-A : : tet : : sigS-lacZ, sigS +, SAUSA300_1495 : : bursa aurealis, SAUSA300_1495− | This study |
| CNK1785 | USA300 HOU pSC-A : : tet : : sigS-lacZ, sigS +, SAUSA300_1788 : : bursa aurealis, SAUSA300_1788− | This study |
| CNK1786 | USA300 HOU pSC-A : : tet : : sigS-lacZ, sigS +, SAUSA300_1684 : : bursa aurealis, SAUSA300_1684− | This study |
| CNK1787 | USA300 HOU pSC-A : : tet : : sigS-lacZ, sigS +, SAUSA300_0014 : : bursa aurealis, SAUSA300_0014− | This study |
| LNS1788 | USA300 pAZ106 : : ctpA-lacZ, ctpA + | Carroll et al. (2014) |
| JAI1287 | USA300 HOU rpoE : : bursa aurealis, rpoE − | Weiss et al. (2014) |
| Plasmid | ||
| pAZ106 | Promoterless lacZ suicide vector | Kemp et al. (1991) |
| pMK4 | Gram-positive shuttle vector | Sullivan et al. (1984) |
| pCNK622 | pAZ106 containing a 1.1 kb fragment of the prsS promoter | This study |
| pCNK1461 | pMK4 containing a 1.7 kb fragment with the prsS promoter and coding region | This study |
| pCNK1871 | pMK4 containing a 1.7 kb fragment with the prsS promoter and prsS E215A,E216A mutations | This study |
| pSC-A | TA clone suicide vector | StrataClone |
Construction of lacZ reporter fusion strains.
In order to construct a prsS-lacZ reporter fusion strain, the prsS promoter region was amplified using primers OL888/OL887 (Table S1, available in the online Supplementary Material, for all oligonucleotides). This PCR product was cloned into the Gram-positive suicide vector pAZ106 (Kemp et al., 1991; Salisbury et al., 1972), creating pCNK622. S. aureus RN4220 was electroporated with pCNK622 as described previously (Shaw et al., 2008), and the recombination event confirmed using gene-specific primer OL888 and plasmid-specific primer OL761, creating strain CNK622. This verified clone was used to generate a phage lysate to transduce strains 8325-4, SH1000 (Horsburgh et al., 2002), Newman and USA300 (Kolar et al., 2011) via Φ11-mediated transduction (Shaw et al., 2008). The resulting strains CNK955, CNK661, CNK876 and CNK875, respectively, were confirmed by PCR using primers OL761/OL887. sigS-lacZ and ctpA-lacZ fusion strains were previously reported (Burda et al., 2014; Carroll et al., 2014).
Construction of mutant sigS-lacZ reporter fusion strains.
To create sigS-lacZ reporter-fusions containing individual mutations of uncharacterized membrane-bound proteins, Φ11 lysates were generated using Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) transposon mutants NE1203 (SAUSA300_1495), NE1644 (SAUSA300_1788), NE1783 (SAUSA300_1684) and NE1942 (SAUSA300_0014) (Fey et al., 2013). Strain CNK957 was transduced with individual Φ11 lysates, creating strains CNK1784, CNK1785, CNK1786 and CNK1787, respectively. Clones were confirmed as previously described (Bae et al., 2004).
Construction of mutant strains.
A prsS mutant (NE166) obtained from the NARSA transposon mutant library (Fey et al., 2013) was used to generate a Φ11 lysate to transduce USA300. The resulting strain CNK1460 was confirmed by PCR using OL888, located upstream of prsS, and transposon-specific primer OL1472. A USA300 sigS mutant strain was constructed via Φ11-mediated transduction using a lysate from a previously constructed SH1000 sigS mutant (Shaw et al., 2008). The resulting strain (HKM852) was confirmed by PCR using OL281, located upstream of sigS, and OL429, located downstream of sigS. A USA300 HOU prsS sigS double mutant was constructed via Φ11-transduction of CNK1460 using a lysate generated from the previously constructed SH1000 sigS mutant (Shaw et al., 2008). The resulting strain (CNK1870) was confirmed as described above by PCR. An rpoE mutant was previously constructed (Weiss et al., 2014).
Construction of prsS complement strains.
A prsS complement strain was constructed by amplifying the prsS promoter and coding region using primers OL888/OL1469, and cloning this product into the Gram-positive shuttle vector pMK4 via transformation of DC10B (Monk et al., 2012; Sullivan et al., 1984), creating pCNK1461. S. aureus RN4220 was electroporated with pCNK1461, with clones verified using gene-specific primer OL888 and plasmid-specific primer OL1057, creating strain CNK1462. Clones were used to generate a Φ11 lysate for the transduction of a USA300 HOU prsS mutant. The resulting strain (CNK1467) was confirmed by PCR using primers OL888/OL1057. A prsS site-directed-mutant complement was constructed by performing targeting E215A/E216A mutagenesis in the prsS coding region. This was achieved by splicing by overhang extension (SOEing) PCR using primers OL888/OL2138 and OL2137/OL1469, which contained mutated nucleotide sequences. These products were cloned into pMK4, and verified by sequencing (MWG Operon), creating pCNK1871. S. aureus RN4220 was electroporated with this construct, with clones verified by PCR, creating strain CNK1872. Clones were used to generate a lysate for the transduction of a USA300 prsS mutant via Φ11, creating strain CNK1873.
5′-rapid amplification of cDNA ends (5′-RACE).
5′-rapid amplification of cDNA ends (5′-RACE) was performed as described previously (Carroll et al., 2012) using RNA extracted during exponential growth (3 h) of USA300 WT. RNA extraction was performed using a Qiagen RNeasy kit, and 5′-RACE was performed using a Takara 5′-RACE kit, and primers OL968-72, according to the manufacturer’s protocol. Amplified cDNA products were cloned into the pSC-A TA cloning vector, and 11 constructs were analysed by sequencing (MWG Operon).
β-Galactosidase assays.
β-Galactosidase assays on tryptic soy agar (TSA) plates and in tryptic soy broth (TSB), RAW 264.7 murine macrophage-like cells, human serum and porcine serum were performed as previously described (Carroll et al., 2012; Miller et al., 2012; Shaw et al., 2008). Minimal levels of native β-galactosidase activity were subtracted when calculating β-galactosidase activity in all assays.
MICs of cell wall-targeting antibiotics.
The MICs of cell wall-targeting antibiotics were determined as previously described (Burda et al., 2012).
Methyl methane sulfonate (MMS) and hydrogen peroxide (H2O2) survival assays.
Survival assays were performed as previously described (Miller et al., 2012). Briefly, exponentially growing USA300 WT, prsS mutant and prsS complement strains were washed three times with PBS. Cells were resuspended in PBS and MMS or H2O2 was added to a final concentration of 50 mM or 1.3 M, respectively. Cultures were incubated shaking at 37 °C, and the percent recovery determined by comparing pre-exposure c.f.u. ml−1 to final c.f.u. ml−1 after 30 min incubation with MMS, or 5 min incubation with H2O2. Data are presented from three independent experiments.
Hybrigenics ULTImate yeast two-hybrid (Y2H) screen.
A Y2H screen was performed by Hybrigenics as described previously to identify an anti-sigma factor in Helicobacter pylori (Colland et al., 2001). We used a bait plasmid containing the coding region of sigS and an S. aureus prey library with over two million clones. Putative interacting proteins were assigned a ‘predicted biological score’ as an indicator of bait–prey interaction specificity, which was generated as an e-value dependent on the number of hits for a specific protein domain and data accumulated from previously performed Hybrigenics screens (Rain et al., 2001). The predicted biological score ranges from A, indicating the highest probability of specificity, to E, indicating the lowest specificity between two proteins.
Proteomic analyses.
Triplicate USA300 WT or prsS mutant strains were grown to post-exponential (5 h) or stationary phase (15 h) in TSB. To isolate membrane proteins, cultures were washed three times with PBS, and protoplasts generated by incubating cells with 100 µg of lysostaphin at 37 °C for 30 min. Membrane proteins were isolated from protoplasts as described previously (Eymann et al., 2004). Cytoplasmic proteins were obtained by mechanical disruption of cells using 0.1 mm glass beads and a Biospec Mini BeadBeater-16. Membrane proteins were standardized to 200 µg, and cytoplasmic proteins were standardized to 70 µg. Samples were then subjected to filter aided sample preparation and trypsin digestion, as described previously (Rivera et al., 2012). The resulting peptides were de-salted and analysed by linear trap quadrupole tandem mass spectrometry (LTQ-MS/MS); and Mascot data files were interrogated and normalized using Scaffold software with parameters to allow a false protein discovery rate of <1 %. Ratios and fold changes of protein abundance were calculated as prsS mutant/WT spectral counts and significance was determined using Fisher’s exact test (P<0.05).
Transmission electron microscopy (TEM).
USA300 WT and prsS mutant cultures were grown to exponential phase, and prepared and photographed at the University of South Florida Microscopy Core Facility as previously described (Kolar et al., 2011).
Quantitative real-time PCR (qPCR).
qPCR was performed on USA300 WT, prsS mutant, and sigS mutant strains in triplicate as previously described (Kolar et al., 2011) to analyse expression of prsS, sigS, bmrA, ezrA, femB, hmrA, hpt and tag. Control primers for 16S rRNA were used as previously described (Koprivnjak et al., 2006).
Whole human blood survival assay.
Human blood survival assays were performed as previously described (Kolar et al., 2011). Briefly, exponentially growing USA300 WT, prsS mutant and complement strains were washed three times with PBS and resuspended in 1 ml of pooled whole human blood (Bioreclamation). Blood cultures were incubated with shaking at 37 °C for 3 h, and percent recovery determined by comparing initial c.f.u. ml−1 to final c.f.u. ml−1. Data presented are from blood from five different people and three biological replicates.
Murine sepsis infection model.
Murine sepsis infection models were performed using ten female 6-week-old CD-1 mice (Charles River) per strain, as described previously (Kolar et al., 2013). Mice were infected via tail vein injection with 1×108 c.f.u. of USA300 WT or prsS mutant strains. After 7 days, mice were euthanized and brain, heart, lungs, liver, spleen and kidneys were harvested. C.f.u./organ loads were determined by homogenizing organs in PBS, serially diluting and plating on TSA. This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of the University of South Florida (Permit Number: A-4100-01).
Statistical analyses.
All statistical analyses in this study were performed using SAS software (version 9.2; SAS Institute). The distribution of data was determined in SAS through tests for normality (SAS proc univariate) and equality of variance (SAS proc ttest). For parametrically distributed data, Student’s t-test was used. The statistical significance of bacterial recovery from organs in the murine model of sepsis was evaluated using a Mann–Whitney Test. For all statistical analyses, the significance level was set at α = 0.05.
Results
Identification of a PrsW homologue in S. aureus
A protein blast analysis of the S. aureus genome using the B. subtilis PrsW amino acid sequence identified SAUSA300_0230 as having 25 % identities and 47 % positives (e = 8×10−8). The SAUSA300_0230 protein in S. aureus is 380 amino acids in length, which is longer than its homologue in B. subtilis at 218 amino acids and in C. difficile at 238 amino acids (Fig. S1). Alignment analysis reveals conservation of the proposed catalytic residues: E75, E76 and H175 in B. subtilis; E78, E79 and H184 in C. difficile; E215, E216 and H325 in S. aureus (Ellermeier & Losick, 2006; Heinrich & Wiegert, 2006). Interestingly, it appears that PrsW-like elements are also broadly conserved across a number of other bacterial species (Figs S2 and S3). For the purposes of this study, we termed the SAUSA300_0230 gene ‘prsS’, for ‘putative regulator of SigmaS’.
Yeast two-hybrid (Y2H) analysis to identify a σS anti-sigma factor
Given that S1P and S2P homologues are conserved in S. aureus, we sought to investigate whether a σS anti-sigma factor could be uncovered using a Y2H screen. Accordingly, we identified 13 proteins that putatively interact with σS including components of RNA polymerase and transcription factors, all of which are expected for a sigma factor (Table S2). Unexpectedly, however, no candidate anti-sigma factor was identified in this screen. As such, we set out to characterize the role and regulation of prsS in S. aureus, to assess whether it phenocopied our findings from studies with sigS.
Identification of the prsS transcriptional start site by 5′-RACE
In order to characterize the expression of prsS in S. aureus, we began by identifying its transcriptional start site. In total, 11 different 5′-RACE fragments were subject to Sanger sequencing, leading to the identification of one transcriptional start site at an adenine residue 115 bases 5′ of the prsS start codon. This putative promoter region comprises a −35 sequence of TgGAaA, followed by a 17 bp spacer, and −10 sequence of TAaAAT (lower case bases vary from σA consensus sequences). A putative Shine–Dalgarno sequence (aGAGG) is located 2 bp upstream of the ATG start codon. Collectively, these elements are potentially ideal for strong recognition by both σA and the ribosome.
prsS expression mirrors that of sigS and is upregulated by DNA damage and cell wall stress
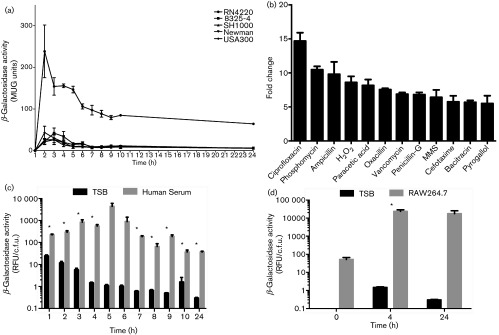
We next sought to understand if prsS expression mirrored that of sigS. Accordingly, a prsS-lacZ reporter fusion in strains RN4220, 8325-4, SH1000, Newman and USA300 was analysed at hourly intervals. These experiments revealed that prsS, much like sigS (Miller et al., 2012), is transcribed at low levels under standard laboratory conditions in all strains apart from RN4220, with an approximately 11.2-fold increase in RN4220, and peak expression occurring during exponential growth (Fig. 1a). To assess whether prsS expression, like sigS, is inducible in response to external stress, we employed a modified disk diffusion assay in conjunction with the USA300 prsS-lacZ reporter fusion strain and a library of stress chemicals. A variety of agents induced prsS expression, including those that elicit oxidative/DNA damage stress (ciprofloxacin, H2O2, MMS, peracetic acid and pyrogallol), as well as cell wall-targeting antibiotics (ampicillin, bacitracin, cefotaxime, oxacillin, penicillin-G, phosphomycin and vancomycin). Most of these agents, including H2O2, MMS, ciprofloxacin, cefotaxime, ampicillin, oxacillin and phosphomycin, also elicit the same effects on sigS expression (Miller et al., 2012). These findings were validated by β-galactosidase assays performed on USA300 prsS-lacZ cultures grown in TSB containing subinhibitory concentrations of these agents (Figs 1b, S4 and S5). As a control, to demonstrate that the effects observed are unique to prsS and sigS, we analysed expression of the membrane-associated protease, CtpA, using a ctpA-lacZ reporter fusion (Carroll et al., 2014). We determined that no increases in expression were observed when this construct was tested in a similar fashion (data not shown). Collectively, these results mirror closely what we have previously shown for sigS expression (Miller et al., 2012), and suggest that PrsS and σS may serve common functions in the S. aureus cell.
Fig. 1. Analysis of prsS transcription. β-galactosidase activity was measured as relative fluorescence units (RFU) of 4-methylumbelliferyl-β-D-galactopyranoside (MUG) activity for prsS-lacZ fusion strains in RN4220, 8325-4, SH1000, Newman and USA300 under standard conditions in TSB (a), in the presence of subinhibitory concentrations of the agents shown (b), in human serum (c) or in murine RAW 264.7 cells (d). Assays were performed on duplicate samples, and the values averaged (mean) from at least three independent experiments. Error bars are shown±sem; *P<0.05 using Student’s t-test.
prsS expression is increased in human serum and murine macrophages
Previous studies have demonstrated that sigS expression is increased in both porcine serum and RAW 264.7 murine macrophage-like cells (Miller et al., 2012). To examine if we observe similar alterations, the USA300 prsS-lacZ reporter fusion strain was incubated first in human serum, with expression measured every hour for 10 h and again at 24 h (Fig. 1c). We determined that prsS expression is increased in human serum compared to TSB at all hours, with the most significant increase (386-fold) occurring at 4 h. Similar increases in prsS expression were observed when this experiment was performed using prsS-lacZ fusions in RN4220, 8325-4 and SH1000 backgrounds (Fig. S6). Next, murine macrophages were infected with a USA300 prsS-lacZ reporter fusion strain, and prsS expression was measured at 4 h and 24 h post-infection (Fig. 1d). We observed increased prsS expression in RAW 264.7 cells compared to TSB at both time points post-infection, with a 16,180-fold increase at 4 h, and a 58,875-fold increase at 24 h. Similar increases in prsS expression were observed when these experiments were performed with fusions in RN4220, 8325-4 and SH1000 backgrounds (Fig. S7). Collectively, these data again closely mirror that which we have previously demonstrated for sigS (Miller et al., 2012).
PrsS, like σS, protects S. aureus against killing by DNA-damaging and cell wall-targeting agents
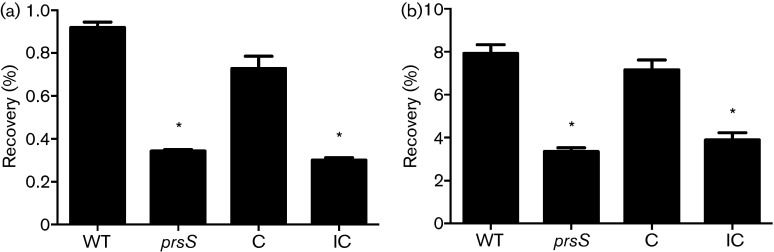
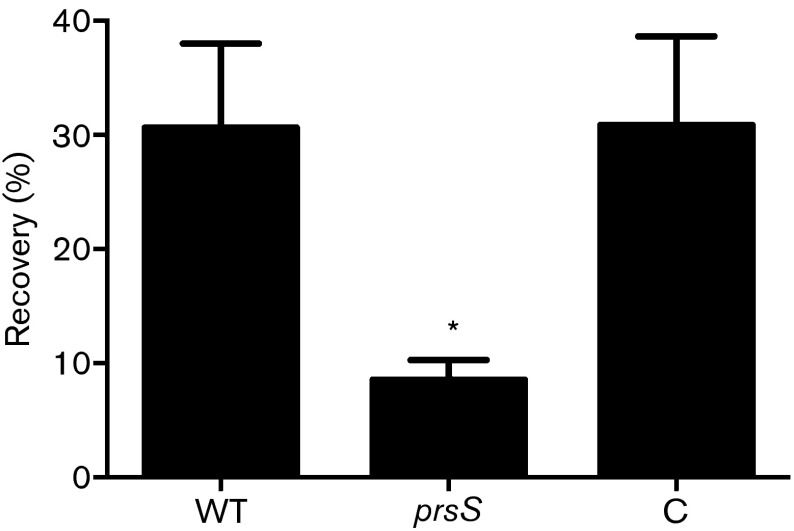
Herein we show that prsS expression is induced by DNA-damaging inducing agents and cell wall-targeting antibiotics. Additionally, we have previously shown that sigS mutants are more sensitive to killing by these types of stress (Miller et al., 2012). To investigate if, as with transcription, similar responses were observed between sigS and prsS mutants, we first exposed the prsS knockout to lethal concentrations of MMS (50 mM) and hydrogen peroxide (1.3 M). The mutant strain was found to be significantly more sensitive to killing by these types of stress (Fig. 2a). After incubation for 30 min with MMS, percent recovery of the WT strain was 2.7-fold greater than the prsS mutant. Likewise, prsS mutants show similar sensitivity to exposure to hydrogen peroxide; after 5 min, post-incubation percent recovery of the WT (7.9 %) is 2.4-fold higher than that of the prsS mutant (3.4 %) (Fig. 2b). Importantly, complementation of these phenotypes was achieved in both assays.
Fig. 2. PrsS, like σS, protects S. aureus against killing by DNA-damaging agents. Viability of the USA300 WT, prsS mutant (prsS), prsS complement (C), and prsS E215A/E216A inactive complement (IC) strains was assessed in the presence of 50 mM MMS (a) or 1.3 M hydrogen peroxide (b). C.f.u. counts were determined for strains pre- and 30 min (a) or 5 min (b) post exposure to these agents, and are averaged (mean) from three independent experiments. Error bars are shown±sem; *P<0.05 using Student’s t-test.
Previous studies on PrsW in B. subtilis have employed site-directed mutagenesis to confirm the proteolytic activity of this protein (Ellermeier & Losick, 2006; Heinrich & Wiegert, 2006). As such, we hypothesized that site-directed mutagenesis of the putative conserved catalytic amino acids (E215A, E216A; Fig. S1), would fail to rescue S. aureus from killing by MMS and H2O2 if PrsS also possesses protease activity. Accordingly, we complemented our prsS mutant with a prsS E215A/E216A variant carried on a plasmid. We then assessed the sensitivity of this strain to killing by both agents. Importantly, this complementation construct was unable to rescue sensitivity of the prsS mutant to either compound (Fig. 2a, b), suggesting that these conserved putatively proteolytic residues are necessary for the function of PrsS.
We next sought to determine if prsS mutants mirror those of sigS in their sensitivity to cell wall-damaging agents. We determined that the prsS mutant strain, just like sigS mutants (Miller et al., 2012), had elevated sensitivity to β-lactam agents, as well as the peptide antibiotic bacitracin. Specifically, the ampicillin MIC for the WT was 102 µg ml−1, which is 10.2-fold higher than that of the prsS mutant (10 µg ml−1). More profoundly, the penicillin-G MIC for the WT is 617 µg ml−1, which is 132.1-fold higher than that of the mutant (4.67 µg ml−1). In the context of bacitracin, the MIC for the WT is 158 µg ml−1, which is 4.8-fold higher than that of the prsS mutant (33.33 µg ml−1).
To assess whether there were any additive effects during loss of both prsS and sigS, we constructed a double mutant, and repeated each of these assays. We determined that the prsS sigS double mutant behaved exactly like that of the individual mutants in these assays, revealing no additional effects (data not shown). These results indicate that, akin to σS, PrsS is involved in the response of S. aureus to DNA damage and cell wall-targeting antimicrobial agents.
Proteomic analysis to identify a candidate σS anti-sigma factor
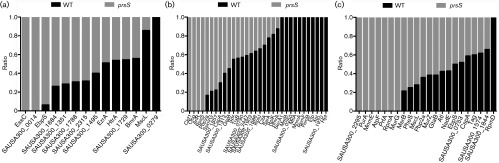
Despite the fact that our Y2H screen failed to identify a σS anti-sigma factor, our transcription and phenotypic assays show that prsS and sigS function in similar fashions. As such, we used proteomic techniques in an attempt to identify an anti-sigma factor. Proteomic analyses of cells from post-exponential growth reveal a significant alteration in the stability of 14 membrane proteins in the mutant strain compared to the parent (Fig. 3a, Table S3). Of note, five of the eight proteins with increased abundance in the mutant strain are uncharacterized hypothetical proteins (SAUSA300_1684, SAUSA300_1351, SAUSA300_1788, SAUSA300_1495 and SAUSA300_0014). To assess if any of these proteins represented a putative anti-sigma factor, we individually introduced NARSA transposon mutations for four of these genes (no SAUSA300_1351 mutant was available) into a USA300 sigS-lacZ reporter fusion strain. If any of these genes encoded an anti-sigma factor, then one would observe a blue colouration in these strains, resulting from increased free σS, and upregulation of the sigS gene via its auto-regulatory activity (Shaw et al., 2008). In each case, disruption of these uncharacterized proteins did not induce sigS expression, suggesting that they are not σS anti-sigma factors. As a SAUSA300_1351 mutant is not present in the NARSA transposon mutant library, we performed a bioinformatic analysis on this protein using the sequences of known anti-sigma factors. Such comparison revealed that it does not share any homology with such proteins, suggesting that it is also not an anti-sigma factor.
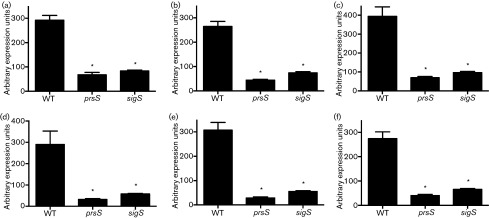
Fig. 3. Proteomic analyses of prsS mutant proteomes. Proteomic analyses were performed on WT and mutant strains to determine differences in membrane protein abundance during post-exponential growth (a) and cytoplasmic membrane protein abundance during post-exponential (b) and stationary (c) growth. Shown are the mean ratios of spectral counts for the prsS mutant / WT, and these are averaged (mean) from three independent replicates.
prsS mutants have alterations in cell size and abundance of proteins involved in meticillin resistance
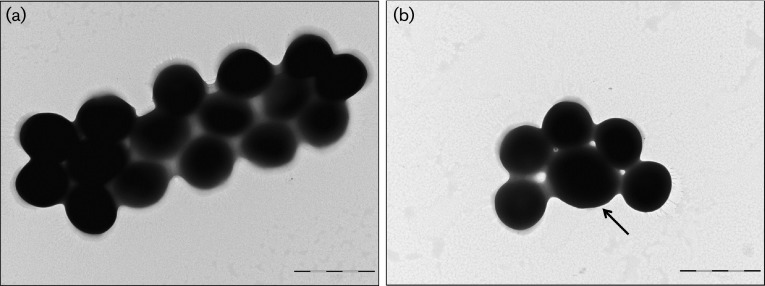
When examining other proteins altered in the prsS mutant membrane proteome, we observed a 2.04-fold reduction in the septation ring formation regulator EzrA. To determine if this alteration in EzrA had a similar effect to that previously reported for ezrA mutants (Jorge et al., 2011), we performed TEM of prsS mutant cells. Upon deletion of prsS, we observed similar heterogeneity in cell size to that of ezrA mutants when compared to WT strains (Fig. 4), in which very large cells accounted for approximately 10 % of the total population.
Fig. 4. prsS mutants display a larger cell size than WT strains. Transmission electron micrographs are shown for exponentially growing WT (a) and prsS mutant (b) cells. Images are representative of at least ten different frames, and are typical for each cell population. The black arrow highlights the increased cell size observed for prsS mutants. Bars, 1 µm.
In addition to decreased levels of EzrA, there was also increased abundance of penicillin-binding protein 2a (Pbp2a) in the cytoplasmic fractions of prsS mutant strains from both post-exponential (5 h) (Fig. 3b, Table S4) and stationary (15 h) (Fig. 3c, Table S5) cultures. Moreover, we also observed decreased accumulation of the meticillin resistance protein FemB in prsS mutants, as well as the HmrA protease, which is also required for resistance to β-lactam antibiotics (Botelho et al., 2011; Henze et al., 1993). Collectively, these data demonstrate that the decreased sensitivity of prsS mutants to β-lactam antibiotics is perhaps mediated by altered levels of key meticillin resistance proteins.
Proteome analyses demonstrate a decreased abundance of DNA damage repair proteins in prsS mutants
We also observed decreased abundance of several proteins in the prsS mutant that are involved in DNA damage repair, including a 2.26-fold decrease in the putative multi-drug resistant transport protein BmrA, which has been shown to export a number of agents that induce DNA damage (Orelle et al., 2003; Steinfels et al., 2004). Additionally, there were significant decreases in the mutant strain for xanthine phosphoribosyltransferase (Xpt, fourfold), and hypoxanthine phosphoribosyltransferase (Hpt, 4.67-fold). Both of these proteins are involved in nucleotide salvage pathways, which become highly important to cells during DNA damage repair (Christiansen et al., 1997; Kilstrup et al., 2005; Nilsson & Lauridsen, 1992). We also observed a 2.50-fold decrease in DNA-3-methyladenine glycosidase in the mutant, which is involved in the base excision repair pathway (Rain et al., 2001), and a 2.92-fold decrease in a bacterioferritin comigratory protein SAUSA300_1844, which is involved in the response to oxidative stress (Jeong et al., 2000). Similar to this latter protein, there was a decreased abundance in the mutant of three enzymes that have either a known or predicted role in oxidative stress resistance, including Spx, the Spx homologue SAUSA300_0790, and TrxB (Nakano et al., 2003, 2005; Pamp et al., 2006). Given that cells undergoing oxidative stress typically die from DNA damage, it appears that key components that facilitate the cellular response to DNA destabilization and reactive oxygen species have decreased accumulation in prsS mutant cells. Collectively, these proteomic findings seem to provide an explanation for the observed sensitivity of prsS mutants to this kind of stress.
The altered accumulation of stress resistance proteins in prsS mutants is mediated at the transcriptional level
PrsS, as with its counterparts from B. subtilis and C. difficile, appears to function as a protease in the cell, or at least conserved catalytic residues are required for its role in resistance to DNA damage and oxidative stress. To determine if the observed differences in protein accumulation in prsS mutants are mediated by transcriptional or post-translation regulation (e.g. proteolysis), we performed qPCR on bmrA, ezrA, femB, hmrA, hpt and tag using the WT strain, alongside prsS and sigS mutants (Fig. 5). Notably, we observed significantly decreased expression of all genes in both mutant strains. As a control, we also analysed the expression of these same six genes in an unrelated mutant (rpoE, the δ-subunit of RNAP; Weiss et al., 2014) to ensure that the effects were specific to sigS and prsS, and found no significant changes in expression for any gene (data not shown). Taken together, these results suggest that the decreased abundance of proteins involved in resistance to cell wall-targeting antibiotics and DNA damage in prsS mutants results from transcriptional regulation, rather than direct PrsS proteolysis. Whilst these data do not directly implicate PrsS in controlling σS activity (putatively by cleaving an anti-sigma factor), they do suggest that both elements function in a similar manner within the cell.
Fig. 5. The altered accumulation of stress resistance proteins in prsS mutants is mediated at the transcriptional level. Expression levels of bmrA (a), ezrA (b), femB (c), hmrA (d), hpt (e) and tag (f) were measured in WT, prsS and sigS mutant strains. Expression units are averaged (mean) from three independent replicates. Error bars are shown±sem; *P<0.05 using Student’s t-test.
PrsS is required for both ex vivo and in vivo infection
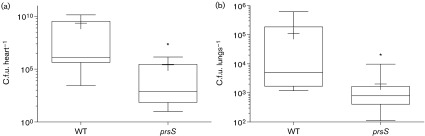
As prsS expression is increased in serum and murine macrophage-like cells, we assessed survival of prsS mutants using ex vivo and in vivo infection models. Firstly, exponentially growing WT USA300, prsS mutant and complement strains were incubated in whole human blood, and their percent recovery determined. After 3 h, the WT returned 30.6 % of the inoculum, whilst the prsS mutant returned only 8.5 %, representing a >3.5-fold change (Fig. 6). Importantly, prsS complementation restored survival to WT levels, with a recovery of 30.9 %. Next, we sought to determine if these ex vivo findings were recapitulated in vivo in a murine model of sepsis. Whilst we did not observe statistically significant dissemination to the brain, liver, spleen or kidneys, we did note a major impairment in the ability to infect the heart (8885-fold decrease, Fig. 7a) and lungs (55-fold decrease, Fig. 7b) by the prsS mutant strain. Collectively, these findings strongly implicate PrsS in the ability of S. aureus strains to cause disease in both human and murine systems, and mimic our findings with sigS mutants (Miller et al., 2012; Shaw et al., 2008).
Fig. 6. PrsS provides a survival advantage in whole human blood. The viability of the USA300 WT, prsS mutant (prsS), and its complement (C) strain in whole human blood (pooled from five samples) was analysed. C.f.u. counts were determined for strains pre- and 3 h post-inoculation. Error bars are shown±sem; *P<0.05 using Student’s t-test.
Fig. 7. PrsS is required for full virulence in murine models of infection. Bacterial loads in the heart (a) and lungs (b) are shown for mice infected with the WT or prsS mutant (prsS) strain. Box and whisker plots represent the minimum and maximum values (whiskers), as well as the 25th–75th percentile (boxes). The median c.f.u. organ−1 for each group is indicated as a solid horizontal black line, whilst the mean is indicated by +. Statistical significance was determined using a Mann–Whitney test; *P<0.05.
Discussion
In this study, we have identified a protein (PrsS) in S. aureus with homology to PrsW from B. subtilis. Interestingly, no anti-sigma has yet been identified for σS in S. aureus. Herein, we perform Y2H screens and proteomic studies, but do not identify an inhibitory protein that interacts with σS. As such, one might predict that this regulator falls into the small class of ECF sigma factors that do not possess an inhibitory counterpart, such as HrpL in Pantoea stewartii and Erwinia herbicola (Merighi et al., 2003; Nizan-Koren et al., 2003). However, we show in this study that the role and regulation of prsS phenocopy sigS in S. aureus. Whilst this does not definitively link PrsS to a role in directly controlling σS activity (putatively via proteolysis of an anti-sigma factor), it suggests that both elements function in a similar manner within the cell, perhaps via a common pathway. Interestingly, expression of sigS in prsS mutants is not altered in either standard conditions or sigS-inducting conditions, suggesting that PrsS mediated-control of σS occurs at the protein level (data not shown). Furthermore, we demonstrate that under standard conditions, prsS is lowly expressed in laboratory and clinical strains, with a strong increase in expression in the mutagenic strain RN4220, which mirrors that of sigS (Miller et al., 2012).
Although low levels of expression are observed for prsS during standard conditions, expression is elevated when exposed to stressors that elicit cell wall perturbations and DNA damage. In addition to increased expression for both of these conditions, we demonstrate that prsS mutants are more sensitive to DNA damage and certain cell wall-targeting antibiotics, which is again identical to sigS mutants (Miller et al., 2012). Collectively, the mirroring of sigS/prsS expression patterns, and the phenotypes of their respective mutants, suggests that they may be involved in the same functional pathway. As such, we suggest that, despite not yet being identified, it is possible that PrsS may modulate σS activity. Indeed, such an observation is the mirror image of a study in B. subtilis, where Hastie et al. (2013) demonstrate RIP mediated degradation of the σV anti-sigma factor RsiV, yet the responsible S1P that initiates cleavage remains to be identified.
In efforts to understand the mechanism by which PrsS contributes to protection of the S. aureus cell against stress, we performed proteomic studies with the prsS mutant. Importantly, we observed altered accumulation of several proteins involved in the response to DNA damage and oxidative stress. Specifically, there is decreased abundance of the putative multi-drug resistance transport protein BmrA in the mutant strain, which has been shown to export DNA-damaging compounds ethidium bromide, Hoechst 33342, doxorubicin and 7-aminoactinomycin D in B. subtilis (Orelle et al., 2003; Steinfels et al., 2004). We also noted decreases in both Xpt and Hpt, each of which functions in nucleotide salvage pathways. Following conditions that elicit DNA damage, there is a major increase in demand for nucleotides within bacterial cells (Malachowa et al., 2011); thus, an impaired ability to salvage such building blocks results in enhanced sensitivity to this type of stress. Finally, the base excision repair protein DNA-3-methyladenine glycosidase (Rain et al., 2001) was also decreased in prsS mutants. The base excision repair pathway is a key mediator of the response to DNA damage, and is necessary to remove damaged or wrongly incorporated bases (Barnes et al., 1993; Karran et al., 1980). Importantly, E. coli mutants of this protein demonstrate increased susceptibility to alkylating agents (Karran et al., 1980). Such sensitivity is mimicked by prsS mutants when exposed to the alkylating agent MMS. There was also decreased presence of a HtrA protease in the membrane, a protein with a demonstrated role in the oxidative and DNA damage response in bacteria (Hansen & Hilgenfeld, 2013), although its precise role in S. aureus remains to be elucidated. Collectively, these alterations in proteome profile would appear to explain the sensitivity of prsS mutants to DNA-damaging stressors.
We also found that, as for sigS mutants, prsS mutants are more sensitive to ampicillin, bacitracin and penicillin-G. This also correlates with findings from the literature, which reveal prsW mutants of C. difficile are also sensitive to bacitracin (Ho & Ellermeier, 2011). Proteomic analyses performed herein revealed alterations in numerous proteins which contribute to antibiotic resistance in the mutant strain. Specifically, we noted an accumulation in the meticillin resistance protein Pbp2a in prsS mutant cytoplasm, coupled with a decrease in EzrA in the membrane of mutant strains. The alteration in abundance of these two proteins is of particular interest as ezrA mutants are incapable of properly localizing penicillin-binding proteins (Jorge et al., 2011; Steele et al., 2011). Further to this, we observed a decrease in the HmrA protease in prsS mutant strains, which has been shown to contribute to meticillin resistance by facilitating the activity of Pbp2a in S. aureus (Botelho et al., 2011). Finally, we observed decreased abundance of the aminoacyltransferase FemB in the mutant strain. FemB is involved in the formation of pentaglycine interpeptide bridges, and cells lacking this enzyme display irregular septation patterns, and have increased susceptibility to meticillin (Henze et al., 1993). Collectively, each of these observations begins to explain our findings that disruption of PrsS activity in S. aureus cells leads to increased sensitivity to β-lactam antibiotics and other cell wall-targeting agents.
Despite this information, it was not clear at what level these changes in protein abundance in prsS mutants were mediated, e.g. transcriptional or post-translation (i.e. putative proteolysis by PrsS). Using qPCR, we demonstrate that the alteration in abundance of key stress proteins actually occurs at the level of gene expression. Interestingly, transcription of these same genes is also decreased to similar levels in sigS mutant strains. Thus, the observed decrease in expression of these genes in both strains further demonstrates that prsS mutants behave like sigS mutants. Again, whilst this does not draw a direct connection between PrsS and σS activity, it does tend to suggest that these two elements function in a like manner to protect the cell from stress, putatively via a common pathway.
In the context of pathogenesis, we demonstrate that prsS expression is drastically increased ex vivo in human serum and RAW 264.7 murine macrophage-like cells. This would suggest that prsS is necessary to protect against the effects of the immune system during infection. We propose that this observation corroborates the observed upregulation of prsS in response to cell envelope and DNA damage stressors, as components of the immune system cause both of these kinds of stress (Cooke et al., 2003; Hancock & Rozek, 2002; MacMicking et al., 1997; O’Rourke et al., 2003). In addition to these transcriptional effects, we also found that PrsS is required for survival in human blood and for dissemination to hearts and lungs during murine systemic infections. Of interest, PrsW proteins have previously been implicated in infection, as is the case in C. difficile, where this element is necessary for colonization of the caecum in a hamster model of infection (Ho & Ellermeier, 2011).
A possible explanation for the decrease in infectious capability of prsS mutants may be alterations in several proteins involved in type-VII secretion of virulence determinants. A previous study describes a regulatory network in which repression of the type-VII secretion system (T7SS) is mediated by SarA, whilst activation is controlled by ArlR and SpoVG (Burts et al., 2005, 2008; Guinn et al., 2004; Schulthess et al., 2012). Interestingly, prsS mutants display alterations in all three of these proteins; specifically, we observed increases in both SpoVG and ArlR, and a decrease in the abundance of SarA. Additionally, prsS mutants display a decrease in EssC and an increase in EsaA, key components of this secretion system (Anderson et al., 2011; Burts et al., 2005). Collectively, these alterations indicate that PrsS may have some role in the regulation of T7SS, which may explain its impaired virulence in both human and murine models of disease.
In summary, we present the characterization of the PrsS protein in S. aureus. Despite the absence of a candidate anti-sigma factor, there are key similarities between sigS and prsS. Specifically, the expression of prsS mirrors that of sigS in the context of strain variation, response to external stress and interaction with the innate immune system. In addition, prsS mutants phenocopy their sigS counterparts for sensitivity to DNA-damaging agents and cell wall-targeting antibiotics; and their role in ex vivo and in vivo survival. These findings appear to be, at least in part, the result of alterations in proteins involved in DNA damage repair and cell wall integrity upon prsS disruption, and are putatively mediated at the level of transcription, findings that are also mirrored in sigS mutants. Collectively, whilst our studies do not establish a direct link between PrsS function and σS activity (as would be predicted if RIP were occurring), they demonstrate a strong series of commonalities between these two factors. A further exploration of the relationship between these two proteins is currently underway in our laboratory.
Acknowledgements
We would like to thank Ronan Carroll, Whittney Burda and Gary Camper for their help with this study and critical reading of this manuscript. We would also like to thank Edward Haller, from the Microscopy Core Facility in the Department of Integrative Biology at the University of South Florida, for his help with this study. Strains NE166, NE1203, NE1644, NE1783 and NE1942 were obtained through the NARSA programme, supported under NIAID, NIH contract no. HHSN272200700055C. This study was supported in part by the National Institute of Allergies and Infectious Diseases, grant AI080626 to L. N. S.
Footnotes
Edited by: T. Msadek
References
- Anderson M., Chen Y. H., Butler E. K., Missiakas D. M. ( 2011. ). EsaD, a secretion factor for the Ess pathway in Staphylococcus aureus . J Bacteriol 193, 1583–1589. 10.1128/JB.01096-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae T., Banger A. K., Wallace A., Glass E. M., Aslund F., Schneewind O., Missiakas D. M. ( 2004. ). Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc Natl Acad Sci U S A 101, 12312–12317. 10.1073/pnas.0404728101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D. E., Lindahl T., Sedgwick B. ( 1993. ). DNA repair. Curr Opin Cell Biol 5, 424–433. 10.1016/0955-0674(93)90007-D [DOI] [PubMed] [Google Scholar]
- Botelho T. O., Guevara T., Marrero A., Arêde P., Fluxà V. S., Reymond J. L., Oliveira D. C., Gomis-Rüth F. X. ( 2011. ). Structural and functional analyses reveal that Staphylococcus aureus antibiotic resistance factor HmrA is a zinc-dependent endopeptidase. J Biol Chem 286, 25697–25709. 10.1074/jbc.M111.247437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. L., Hughes K. T. ( 1995. ). The role of anti-sigma factors in gene regulation. Mol Microbiol 16, 397–404. 10.1111/j.1365-2958.1995.tb02405.x [DOI] [PubMed] [Google Scholar]
- Brown M. S., Ye J., Rawson R. B., Goldstein J. L. ( 2000. ). Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell 100, 391–398. 10.1016/S0092-8674(00)80675-3 [DOI] [PubMed] [Google Scholar]
- Burda W. N., Fields K. B., Gill J. B., Burt R., Shepherd M., Zhang X. P., Shaw L. N. ( 2012. ). Neutral metallated and meso-substituted porphyrins as antimicrobial agents against gram-positive pathogens. Eur J Clin Microbiol Infect Dis 31, 327–335. 10.1007/s10096-011-1314-y [DOI] [PubMed] [Google Scholar]
- Burda W. N., Miller H. K., Krute C. N., Leighton S. L., Carroll R. K., Shaw L. N. ( 2014. ). Investigating the genetic regulation of the ECF sigma factor σS in Staphylococcus aureus . BMC Microbiol 14, 280. 10.1186/s12866-014-0280-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burts M. L., Williams W. A., DeBord K., Missiakas D. M. ( 2005. ). EsxA and EsxB are secreted by an ESAT-6-like system that is required for the pathogenesis of Staphylococcus aureus infections. Proc Natl Acad Sci U S A 102, 1169–1174. 10.1073/pnas.0405620102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burts M. L., DeDent A. C., Missiakas D. M. ( 2008. ). EsaC substrate for the ESAT-6 secretion pathway and its role in persistent infections of Staphylococcus aureus . Mol Microbiol 69, 736–746. 10.1111/j.1365-2958.2008.06324.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M., Kobel P. A., Morshedi M. M., Wu M. F., Paddon C., Helmann J. D. ( 2002. ). Defining the Bacillus subtilis sigma(W) regulon: a comparative analysis of promoter consensus search, run-off transcription/macroarray analysis (ROMA), and transcriptional profiling approaches. J Mol Biol 316, 443–457. 10.1006/jmbi.2001.5372 [DOI] [PubMed] [Google Scholar]
- Carroll R. K., Robison T. M., Rivera F. E., Davenport J. E., Jonsson I. M., Florczyk D., Tarkowski A., Potempa J., Koziel J., Shaw L. N. ( 2012. ). Identification of an intracellular M17 family leucine aminopeptidase that is required for virulence in Staphylococcus aureus . Microbes Infect 14, 989–999. 10.1016/j.micinf.2012.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R. K., Rivera F. E., Cavaco C. K., Johnson G. M., Martin D., Shaw L. N. ( 2014. ). The lone S41 family C-terminal processing protease in Staphylococcus aureus is localized to the cell wall and contributes to virulence. Microbiology 160, 1737–1748. 10.1099/mic.0.079798-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen L. C., Schou S., Nygaard P., Saxild H. H. ( 1997. ). Xanthine metabolism in Bacillus subtilis: characterization of the xpt-pbuX operon and evidence for purine- and nitrogen-controlled expression of genes involved in xanthine salvage and catabolism. J Bacteriol 179, 2540–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colland F., Rain J.-C., Gounon P., Labigne A., Legrain P., De Reuse H. ( 2001. ). Identification of the Helicobacter pylori anti-σ28 factor. Mol Microbiol 41, 477–487. 10.1046/j.1365-2958.2001.02537.x [DOI] [PubMed] [Google Scholar]
- Cooke M. S., Evans M. D., Dizdaroglu M., Lunec J. ( 2003. ). Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J 17, 1195–1214. 10.1096/fj.02-0752rev [DOI] [PubMed] [Google Scholar]
- Diekema D. J., Pfaller M. A., Schmitz F. J., Smayevsky J., Bell J., Jones R. N., Beach M., SENTRY Partcipants Group ( 2001. ). Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis 32 (Suppl 2), S114–S132. 10.1086/320184 [DOI] [PubMed] [Google Scholar]
- Ellermeier C. D., Losick R. ( 2006. ). Evidence for a novel protease governing regulated intramembrane proteolysis and resistance to antimicrobial peptides in Bacillus subtilis . Genes Dev 20, 1911–1922. 10.1101/gad.1440606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eymann C., Dreisbach A., Albrecht D., Bernhardt J., Becher D., Gentner S., Tam T., Büttner K., Buurman G., et al. ( 2004. ). A comprehensive proteome map of growing Bacillus subtilis cells. Proteomics 4, 2849–2876. 10.1002/pmic.200400907 [DOI] [PubMed] [Google Scholar]
- Fey P. D., Endres J. L., Yajjala V. K., Widhelm T. J., Boissy R. J., Bose J. L., Bayles K. W. ( 2013. ). A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. MBio 4, e00537-12. 10.1128/mBio.00537-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J. M., Levchenko I., Sauer R. T., Baker T. A. ( 2004. ). Modulating substrate choice: the SspB adaptor delivers a regulator of the extracytoplasmic-stress response to the AAA+ protease ClpXP for degradation. Genes Dev 18, 2292–2301. 10.1101/gad.1240104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinn K. M., Hickey M. J., Mathur S. K., Zakel K. L., Grotzke J. E., Lewinsohn D. M., Smith S., Sherman D. R. ( 2004. ). Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis . Mol Microbiol 51, 359–370. 10.1046/j.1365-2958.2003.03844.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Rozek A. ( 2002. ). Role of membranes in the activities of antimicrobial cationic peptides. FEMS Microbiol Lett 206, 143–149. 10.1111/j.1574-6968.2002.tb11000.x [DOI] [PubMed] [Google Scholar]
- Hansen G., Hilgenfeld R. ( 2013. ). Architecture and regulation of HtrA-family proteins involved in protein quality control and stress response. Cell Mol Life Sci 70, 761–775. 10.1007/s00018-012-1076-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie J. L., Williams K. B., Ellermeier C. D. ( 2013. ). The activity of σV, an extracytoplasmic function σ factor of Bacillus subtilis, is controlled by regulated proteolysis of the anti-σ factor RsiV. J Bacteriol 195, 3135–3144. 10.1128/JB.00292-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich J., Wiegert T. ( 2006. ). YpdC determines site-1 degradation in regulated intramembrane proteolysis of the RsiW anti-sigma factor of Bacillus subtilis . Mol Microbiol 62, 566–579. 10.1111/j.1365-2958.2006.05391.x [DOI] [PubMed] [Google Scholar]
- Helmann J. D. ( 2002. ). The extracytoplasmic function (ECF) sigma factors. Adv Microb Physiol 46, 47–110. 10.1016/S0065-2911(02)46002-X [DOI] [PubMed] [Google Scholar]
- Helmann J. D. ( 2006. ). Deciphering a complex genetic regulatory network: the Bacillus subtilis sigmaW protein and intrinsic resistance to antimicrobial compounds. Sci Prog 89, 243–266. 10.3184/003685006783238290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze U., Sidow T., Wecke J., Labischinski H., Berger-Bächi B. ( 1993. ). Influence of femB on methicillin resistance and peptidoglycan metabolism in Staphylococcus aureus . J Bacteriol 175, 1612–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T. D., Ellermeier C. D. ( 2011. ). PrsW is required for colonization, resistance to antimicrobial peptides, and expression of extracytoplasmic function σ factors in Clostridium difficile . Infect Immun 79, 3229–3238. 10.1128/IAI.00019-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh M. J., Aish J. L., White I. J., Shaw L., Lithgow J. K., Foster S. J. ( 2002. ). sigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J Bacteriol 184, 5457–5467. 10.1128/JB.184.19.5457-5467.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W., Cha M.-K., Kim I.-H. ( 2000. ). Thioredoxin-dependent hydroperoxide peroxidase activity of bacterioferritin comigratory protein (BCP) as a new member of the thiol-specific antioxidant protein (TSA)/Alkyl hydroperoxide peroxidase C (AhpC) family. J Biol Chem 275, 2924–2930. 10.1074/jbc.275.4.2924 [DOI] [PubMed] [Google Scholar]
- Jorge A. M., Hoiczyk E., Gomes J. P., Pinho M. G. ( 2011. ). EzrA contributes to the regulation of cell size in Staphylococcus aureus . PLoS ONE 6, e27542. 10.1371/journal.pone.0027542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran P., Lindahl T., Ofsteng I., Evensen G. B., Seeberg E. ( 1980. ). Escherichia coli mutants deficient in 3-methyladenine-DNA glycosylase. J Mol Biol 140, 101–127. 10.1016/0022-2836(80)90358-7 [DOI] [PubMed] [Google Scholar]
- Kemp E. H., Sammons R. L., Moir A., Sun D., Setlow P. ( 1991. ). Analysis of transcriptional control of the gerD spore germination gene of Bacillus subtilis 168. J Bacteriol 173, 4646–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilstrup M., Hammer K., Ruhdal Jensen P., Martinussen J. ( 2005. ). Nucleotide metabolism and its control in lactic acid bacteria. FEMS Microbiol Rev 29, 555–590. 10.1016/j.fmrre.2005.04.006 [DOI] [PubMed] [Google Scholar]
- Klevens R. M., Edwards J. R., Tenover F. C., McDonald L. C., Horan T., Gaynes R., National Nosocomial Infections Surveillance System ( 2006. ). Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992-2003. Clin Infect Dis 42, 389–391. 10.1086/499367 [DOI] [PubMed] [Google Scholar]
- Klevens R. M., Morrison M. A., Nadle J., Petit S., Gershman K., Ray S., Harrison L. H., Lynfield R., Dumyati G., et al. ( 2007. ). Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298, 1763–1771. 10.1001/jama.298.15.1763 [DOI] [PubMed] [Google Scholar]
- Kolar S. L., Nagarajan V., Oszmiana A., Rivera F. E., Miller H. K., Davenport J. E., Riordan J. T., Potempa J., Barber D. S., et al. ( 2011. ). NsaRS is a cell-envelope-stress-sensing two-component system of Staphylococcus aureus . Microbiology 157, 2206–2219. 10.1099/mic.0.049692-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar S. L., Ibarra J. A., Rivera F. E., Mootz J. M., Davenport J. E., Stevens S. M., Horswill A. R., Shaw L. N. ( 2013. ). Extracellular proteases are key mediators of Staphylococcus aureus virulence via the global modulation of virulence-determinant stability. Microbiologyopen 2, 18–34. 10.1002/mbo3.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivnjak T., Mlakar V., Swanson L., Fournier B., Peschel A., Weiss J. P. ( 2006. ). Cation-induced transcriptional regulation of the dlt operon of Staphylococcus aureus . J Bacteriol 188, 3622–3630. 10.1128/JB.188.10.3622-3630.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst F., Ogasawara N., Moszer I., Albertini A. M., Alloni G., Azevedo V., Bertero M. G., Bessières P., Bolotin A., et al. ( 1997. ). The complete genome sequence of the gram-positive bacterium Bacillus subtilis . Nature 390, 249–256. 10.1038/36786 [DOI] [PubMed] [Google Scholar]
- MacMicking J., Xie Q. W., Nathan C. ( 1997. ). Nitric oxide and macrophage function. Annu Rev Immunol 15, 323–350. 10.1146/annurev.immunol.15.1.323 [DOI] [PubMed] [Google Scholar]
- Malachowa N., Whitney A. R., Kobayashi S. D., Sturdevant D. E., Kennedy A. D., Braughton K. R., Shabb D. W., Diep B. A., Chambers H. F., et al. ( 2011. ). Global changes in Staphylococcus aureus gene expression in human blood. PLoS ONE 6, e18617. 10.1371/journal.pone.0018617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi M., Majerczak D. R., Stover E. H., Coplin D. L. ( 2003. ). The HrpX/HrpY two-component system activates hrpS expression, the first step in the regulatory cascade controlling the Hrp regulon in Pantoea stewartii subsp. stewartii . Mol Plant Microbe Interact 16, 238–248. 10.1094/MPMI.2003.16.3.238 [DOI] [PubMed] [Google Scholar]
- Miller H. K., Carroll R. K., Burda W. N., Krute C. N., Davenport J. E., Shaw L. N. ( 2012. ). The extracytoplasmic function sigma factor σS protects against both intracellular and extracytoplasmic stresses in Staphylococcus aureus . J Bacteriol 194, 4342–4354. 10.1128/JB.00484-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk I. R., Shah I. M., Xu M., Tan M. W., Foster T. J. ( 2012. ). Transforming the untransformable: application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. MBio 3, e00277-11. 10.1128/mBio.00277-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran G. J., Krishnadasan A., Gorwitz R. J., Fosheim G. E., McDougal L. K., Carey R. B., Talan D. A., EMERGEncy ID Net Study Group ( 2006. ). Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 355, 666–674. 10.1056/NEJMoa055356 [DOI] [PubMed] [Google Scholar]
- Nakano S., Küster-Schöck E., Grossman A. D., Zuber P. ( 2003. ). Spx-dependent global transcriptional control is induced by thiol-specific oxidative stress in Bacillus subtilis . Proc Natl Acad Sci U S A 100, 13603–13608. 10.1073/pnas.2235180100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano S., Erwin K. N., Ralle M., Zuber P. ( 2005. ). Redox-sensitive transcriptional control by a thiol/disulphide switch in the global regulator, Spx. Mol Microbiol 55, 498–510. 10.1111/j.1365-2958.2004.04395.x [DOI] [PubMed] [Google Scholar]
- Nilsson D., Lauridsen A. A. ( 1992. ). Isolation of purine auxotrophic mutants of Lactococcus lactis and characterization of the gene hpt encoding hypoxanthine guanine phosphoribosyltransferase. Mol Gen Genet 235, 359–364. 10.1007/BF00279381 [DOI] [PubMed] [Google Scholar]
- Nizan-Koren R., Manulis S., Mor H., Iraki N. M., Barash I. ( 2003. ). The regulatory cascade that activates the Hrp regulon in Erwinia herbicola pv. gypsophilae . Mol Plant Microbe Interact 16, 249–260. 10.1094/MPMI.2003.16.3.249 [DOI] [PubMed] [Google Scholar]
- O’Rourke E. J., Chevalier C., Pinto A. V., Thiberge J. M., Ielpi L., Labigne A., Radicella J. P. ( 2003. ). Pathogen DNA as target for host-generated oxidative stress: role for repair of bacterial DNA damage in Helicobacter pylori colonization. Proc Natl Acad Sci U S A 100, 2789–2794. 10.1073/pnas.0337641100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orelle C., Dalmas O., Gros P., Di Pietro A., Jault J. M. ( 2003. ). The conserved glutamate residue adjacent to the Walker-B motif is the catalytic base for ATP hydrolysis in the ATP-binding cassette transporter BmrA. J Biol Chem 278, 47002–47008. 10.1074/jbc.M308268200 [DOI] [PubMed] [Google Scholar]
- Pamp S. J., Frees D., Engelmann S., Hecker M., Ingmer H. ( 2006. ). Spx is a global effector impacting stress tolerance and biofilm formation in Staphylococcus aureus . J Bacteriol 188, 4861–4870. 10.1128/JB.00194-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietiäinen M., Gardemeister M., Mecklin M., Leskelä S., Sarvas M., Kontinen V. P. ( 2005. ). Cationic antimicrobial peptides elicit a complex stress response in Bacillus subtilis that involves ECF-type sigma factors and two-component signal transduction systems. Microbiology 151, 1577–1592. 10.1099/mic.0.27761-0 [DOI] [PubMed] [Google Scholar]
- Rain J.-C., Selig L., De Reuse H., Battaglia V., Reverdy C., Simon S., Lenzen G., Petel F., Wojcik J., et al. ( 2001. ). The protein-protein interaction map of Helicobacter pylori . Nature 409, 211–215. 10.1038/35051615 [DOI] [PubMed] [Google Scholar]
- Rivera F. E., Miller H. K., Kolar S. L., Stevens S. M., Jr, Shaw L. N. ( 2012. ). The impact of CodY on virulence determinant production in community-associated methicillin-resistant Staphylococcus aureus . Proteomics 12, 263–268. 10.1002/pmic.201100298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury V., Hedges R. W., Datta N. ( 1972. ). Two modes of “curing” transmissible bacterial plasmids. J Gen Microbiol 70, 443–452. 10.1099/00221287-70-3-443 [DOI] [PubMed] [Google Scholar]
- Schöbel S., Zellmeier S., Schumann W., Wiegert T. ( 2004. ). The Bacillus subtilis sigmaW anti-sigma factor RsiW is degraded by intramembrane proteolysis through YluC. Mol Microbiol 52, 1091–1105. 10.1111/j.1365-2958.2004.04031.x [DOI] [PubMed] [Google Scholar]
- Schulthess B., Bloes D. A., Berger-Bächi B. ( 2012. ). Opposing roles of σB and σB-controlled SpoVG in the global regulation of esxA in Staphylococcus aureus . BMC Microbiol 12, 17. 10.1186/1471-2180-12-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L. N., Lindholm C., Prajsnar T. K., Miller H. K., Brown M. C., Golonka E., Stewart G. C., Tarkowski A., Potempa J. ( 2008. ). Identification and characterization of sigma, a novel component of the Staphylococcus aureus stress and virulence responses. PLoS ONE 3, e3844. 10.1371/journal.pone.0003844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele V. R., Bottomley A. L., Garcia-Lara J., Kasturiarachchi J., Foster S. J. ( 2011. ). Multiple essential roles for EzrA in cell division of Staphylococcus aureus . Mol Microbiol 80, 542–555. 10.1111/j.1365-2958.2011.07591.x [DOI] [PubMed] [Google Scholar]
- Steinfels E., Orelle C., Fantino J.-R., Dalmas O., Rigaud J.-L., Denizot F., Di Pietro A., Jault J.-M. ( 2004. ). Characterization of YvcC (BmrA), a multidrug ABC transporter constitutively expressed in Bacillus subtilis . Biochemistry 43, 7491–7502. 10.1021/bi0362018 [DOI] [PubMed] [Google Scholar]
- Sullivan M. A., Yasbin R. E., Young F. E. ( 1984. ). New shuttle vectors for Bacillus subtilis and Escherichia coli which allow rapid detection of inserted fragments. Gene 29, 21–26. 10.1016/0378-1119(84)90161-6 [DOI] [PubMed] [Google Scholar]
- Tao L., Wu X., Sun B. ( 2010. ). Alternative sigma factor sigmaH modulates prophage integration and excision in Staphylococcus aureus . PLoS Pathog 6, e1000888. 10.1371/journal.ppat.1000888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks A. ( 2007. ). MRSA may kill more US citizens than HIV BMJ 335, 850 10.1136/bmj.335.7625.850-b [DOI] [Google Scholar]
- Weiss A., Ibarra J. A., Paoletti J., Carroll R. K., Shaw L. N. ( 2014. ). The δ subunit of RNA polymerase guides promoter selectivity and virulence in Staphylococcus aureus . Infect Immun 82, 1424–1435. 10.1128/IAI.01508-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., de Lencastre H., Tomasz A. ( 1996. ). Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J Bacteriol 178, 6036–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]