Abstract
Oxidative stress injury is an important pathogenesis of influenza virus in critically ill patients. The present study investigated the efficacy of carnosine, an antioxidant and free radical scavenger, on a model of acute lung injury (ALI) induced by H9N2 swine influenza virus. Female specific-pathogen-free BALB/c mice were randomized into four groups and treated as follows: (1) H9N2 group, (2) mock control group, (3) H9N2+carnosine group and (4) carnosine control group. The H9N2 group mice were inoculated intranasally with A/Swine/Hebei/012/2008/ (H9N2) virus (100 μl) in allantoic fluid (AF), whilst mock-infected animals were intranasally inoculated with non-infectious AF. Carnosine [10 mg (kg body mass)− 1] was administered orally (100 μl) for 7 days consecutively. The survival rate, lung water content, TNF-α and IL-1β levels, lung histopathology, myeloperoxidase (MPO) activity, and Toll-like receptor (TLR)-4 levels were determined at 2, 4, 6, 8 and 14 days after inoculation. Carnosine treatment effectively decreased the mortality (43 versus 75 %, P < 0.05), significantly ameliorated pathological lesions in lungs and decreased the lung wet/dry mass ratio (P < 0.05). It also inhibited MPO activity, suppressed TNF-α and IL-1β release, decreased the H9N2 viral titre, and markedly inhibited levels of TLR-4 mRNA and protein in the lungs of infected mice (P < 0.05), which supported the use of carnosine for managing severe influenza cases.
Introduction
Since the first case of human infection with H5N1 avian influenza virus was reported in 1997, a few other subtype animal influenza viruses, such as H9N2, H7N9 and new H1N1, have been confirmed to cause human infection or result in epidemics (CDC, 1997; Peiris et al., 1999, 2009; Wiwanitkit, 2013). Although vaccination is thought to be an effective method for preventing such epidemics, the genetic recombination of different viral genotypes makes it difficult to predict which vaccine strain will be suitable to prevent a new influenza pandemic. Consequently, utilizing drug prevention or therapy in critical illness is still an optimal strategy. Neuraminidase inhibitor drugs have been demonstrated to effectively inhibit influenza A virus replication. However, the efficacy of the approved anti-influenza drugs is limited because of the emergence of resistant strains. Moreover, studies investigating the pathogenesis of H5N1 or H1N1 viruses confirm that uncontrolled excessive lung and systemic inflammation or oxidative stress inducing acute lung injury (ALI) are the main reasons for the death of critical patients, rather than uncontrolled viral infection (Matthay & Zemans, 2011). In patients with influenza, elevated proinflammatory cytokines appeared to play a key role in the conformation of acute respiratory distress syndrome (ARDS) (Hien et al., 2009). However, some studies demonstrated that immunosuppressive inhibitors could neither ameliorate the severity of ALI or ARDS nor decrease the mortality in patients or experimental mice (Tran et al., 2004; Salomon et al., 2007; Xu et al., 2009), indicating that some other important factors might be involved in the development of ALI. To date, a growing body of evidence indicates that oxidative stress plays an important role in the pathogenesis of lung diseases (Ryrfeldt et al., 1993; Akaike et al., 1996). Interestingly, the involvement of free radicals in ALI induced by influenza virus in mice has been reported (Narasaraju et al., 2011; He et al., 2013). Hence the rapid increase of interest in the role of ‘oxidative stress’ in ALI induced by influenza virus has mainly focused attention on antioxidant agents that prevent the generation of reactive oxygen species (ROS) or enhance their metabolism. The generation of ROS, including anions (O2− ), hydrogen peroxide (H2O2) and hydroxyl radicals (OH·), results in an imbalance between the pro-oxidant/antioxidant systems, which causes damage to cellular macromolecules such as nucleic acids, proteins and lipids. In particular, these free radicals more easily attack the polyunsaturated fatty acids in biomembranes and induce lipid peroxidation, and then cause lung injury. Some antioxidant agents, such as N-acetyl-l-cysteine (NAC) and glutathione (GSH), were chosen to interfere in the course of ALI induced by different influenza viral strains (Zhang et al., 2014; Amatore et al., 2015).
The biological importance of carnosine, an endogenous dipeptide composed of the amino acids β-alanine and l-histidine, is attributed to its chelating effect against metal ions, superoxide dismutase (SOD)-like activity, and ROS and free radical scavenging properties (Dahl et al., 1988; Babizhayev et al., 1994; Chan et al., 1994). Several studies have indicated that carnosine has beneficial effects against diabetes, kidney insult and bleomycin-induced lung injury. In the ALI mouse model, Cuzzocrea et al. (2007) demonstrated that carnosine treatment abolished immunostaining for inducible nitric oxide synthase in the lungs of animals treated with bleomycin, whereas Noori & Mahboob (2010) showed the curative effect of carnosine on cisplatin-induced kidney insult. Moreover, studies have indicated that carnosine has antioxidative and free radical scavenging functions (Guney et al., 2006), and carnosine supplementation has been shown to effectively decrease the level of TNF-α and IL-6 which may consequently diminish inflammatory-oriented endothelial dysfunction and coagulation risk (Lee et al., 2005). Oral carnosine attenuated proinflammatory cytokine-induced nitric oxide production during the management of virulent H1N1 influenza virus infection, suggesting carnosine should have a promising effect on influenza A virus infection for disease control and prevention (Babizhayev & Deyev, 2012). Although the antioxidative effect of carnosine has been known in some diseases, little is known about its effect on ALI induced by H9N2 swine influenza virus (SIV). Therefore, this study aimed to investigate the effects of carnosine on ALI induced by H9N2 SIV infection in the mouse model, which might benefit further therapeutic interventions for human ALI induced by zoonotic influenza viruses.
Results
Carnosine treatment effectively ameliorated clinical symptoms and prolonged the survival time of H9N2-infected mice
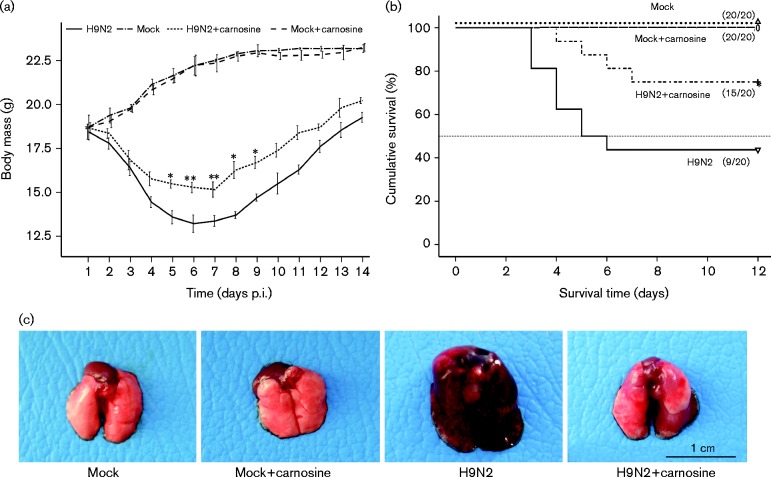
At day 2 post-inoculation (p.i.), inappetence (loss of appetite), inactivity and ruffled fur appeared on H9N2-infected mice, and by day 4 p.i., clinical signs of respiratory disease were observed, including visual signs of laboured respiration. Also, more severe inappetence and emaciation appeared on some of the mice with a body mass decrease to ∼75 % of the original mass by 6 day p.i. (Fig. 1a). In the carnosine-treated group, the H9N2-infected mice also exhibited depression, inappetence and clinical signs of respiratory disease. The severity was significantly alleviated compared with the no-treatment H9N2-infected mice. Meanwhile, carnosine treatment significantly decreased the loss of body mass and remarkably prolonged the survival time of H9N2-infected mice (10.3 ± 1.2 versus 7.6 ± 3.4 days; carnosine treatment versus no-treatment H9N2-infected mice). During the experiment, 75 % of the mice died in the H9N2 group compared with 43.75 % in the carnosine-treated group (Fig. 1b). Gross pathological observation of the lungs demonstrated more serious oedema with profuse areas of haemorrhage and congestion in no-treatment mice. However, in the carnosine-treated group, the gross pathological lesion of lungs was not as serious as that of no-treatment H9N2-infected mice, whilst mock-infected group mice treated with carnosine or normal saline did not show any clinical signs or gross pathological changes in the lungs (Fig. 1c).
Fig. 1. Effect of carnosine treatment on body mass, surviveal rate and gross pathology of BALB/c mice infected with H9N2 SIV. SPF 6- to 8-week-old BALB/c mice (n = 20) were infected intranasally with a dose of H9N2 SIV [1 × 102 50 % mouse infectious dose (MID50)], and monitored daily for morbidity as measured by mass and mortality for 14 days consecutively p.i. (a) Changes of body mass of H9N2 SIV-infected mice after treated with carnosine. *P < 0.05; **P < 0.01 was considered significant. (b) Kaplan–Meier survival curve of carnosine-treated or no-treatment mice following H9N2 SIV infection. The values represent mean ± sd. *P < 0.05 was considered significant. (c) Gross pathology of H9N2 SIV-infected lung at 6 days p.i. H9N2 virus-infected lung treated with carnosine, displaying mild oedematous, and congestive and haemorrhagic changes compared with no-treatment H9N2-infected mice.
Carnosine treatment attenuated lung pathological lesions
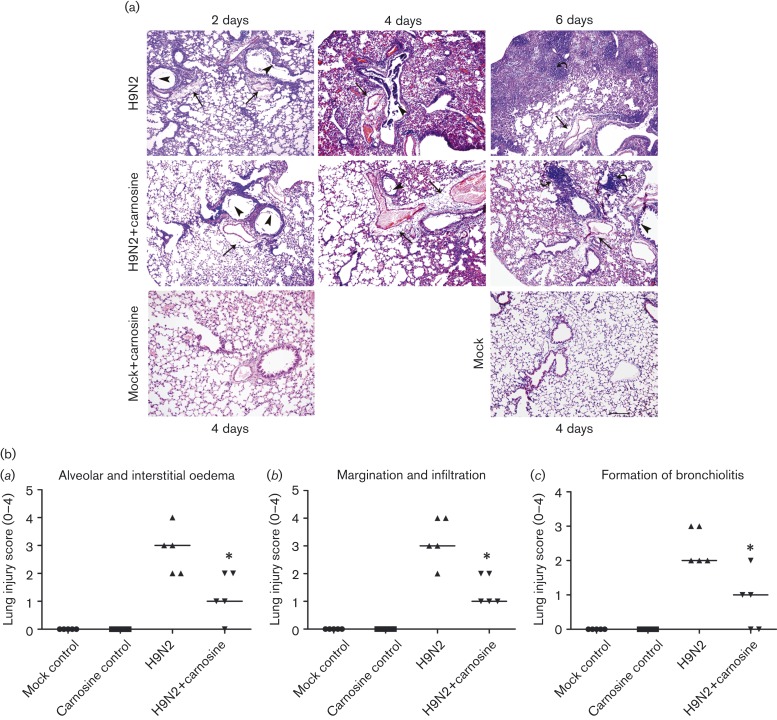
To assess the pathological changes, haematoxylin/eosin (HE) staining and a lung injury score system were used in our study. As shown in Fig. 2(a), both no-treatment and carnosine-treated mice infected with H9N2 displayed a similar histopathological pattern, including initial peribronchiolar patchy pneumonia and fully developed bronchiolitis and bronchopneumonia. However, carnosine treatment alleviated the severity of lung histopathological lesions significantly, which was confirmed by the score of histopathological changes (Fig. 2b). The degree of formation of bronchiolitis, alveolar oedema and interstitial oedema, and margination and infiltration were markedly decreased in the no-treatment H9N2-infected group on day 6 p.i. (P < 0.05) (Fig. 2b).
Fig. 2. Effect of carnosine treatment on lung damage of BALB/c mice infected with H9N2 SIV. (a) Lung histopathology as shown by HE staining after therapy with carnosine in H9N2-infected mice. The similar histopathological pattern in carnosine-treated mice showed symptoms not as serious as that of no-treatment H9N2-infected mice. On days 2–6 p.i., the lung histopathology of H9N2 mice showed interstitial oedema around small blood vessels and bronchioles (straight arrows), dropout of mucous epithelium (triangle arrowhead) and fully developed bronchiolitis (curved arrows), and bronchopneumonia. However, after carnosine treatment, the pathological changes in the lung tissues were relieved. Mock control and carnosine control groups showed no pathological lesions. Representative HE-stained sections were derived from mice treated as described in the text (five mice in each group at different points). Bar, 200 μm. (b) Histopathological scores of five mice at day 6 p.i. were analysed by the Kruskal–Wallis test; P < 0.05 was considered significant.
Carnosine treatment decreased lung oedema
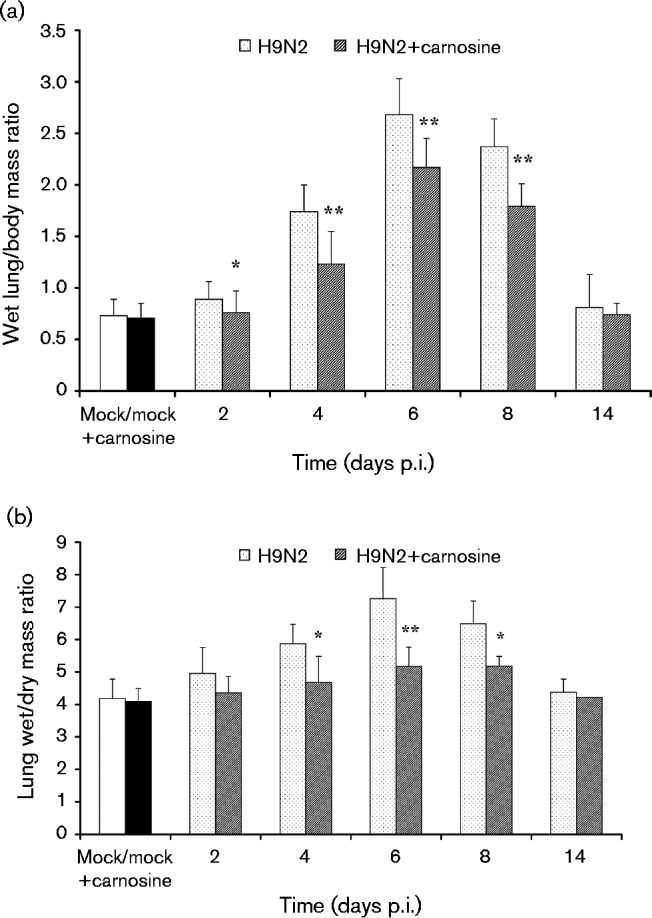
As shown in Fig. 3(a), the wet lung/body mass ratio increased significantly in H9N2-infected mice on days 2–8 p.i. compared with the mock-infected or carnosine control group (P < 0.01), whereas the ratio in carnosine-treated H9N2-infected mice significantly decreased compared with that in no-treatment H9N2-infected mice (P < 0.01). The results of the lung wet/dry mass ratio in Fig. 3(b) also showed similar changes. On 4–8 days p.i., the lung wet/dry mass ratio in the carnosine-treated group decreased significantly compared with no-treatment H9N2-infected mice (P < 0.01).
Fig. 3. (a) Wet lung mass/body mass ratios and (b) lung wet/dry mass ratios after therapy with carnosine. The mean ± sd (n = 5) at each time point is shown. *P < 0.05; **P < 0.01 compared with H9N2.
Carnosine treatment alleviated blood endothelial lesions and decreased pulmonary capillary permeability
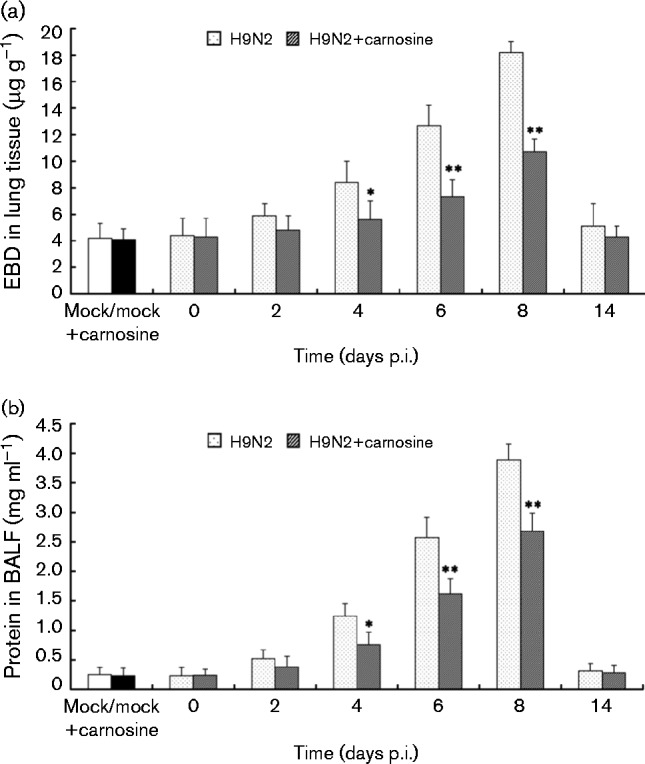
In order to determine the role of carnosine in pulmonary capillary permeability, capillary leakage was assessed by pulmonary extravasation of Evans blue dye (EBD) and total protein in pure bronchoalveolar lavage fluid (BALF) as detailed in Methods. As shown in Fig. 4, there was a significant increase in EBD extravasation (Fig. 4a) and protein in BALF (Fig. 4b) of H9N2 SIV-infected mice compared with non-infected mice. However, EBD extravasation and protein in BALF in the carnosine-treated H9N2-infected group decreased significantly compared with no-treatment H9N2-infected mice (P < 0.05).
Fig. 4. Effect of carnosine on pulmonary capillary permeability of BALB/c mice infected with H9N2 SIV. BALB/c mice were inoculated intranasally with a dose of H9N2 SIV (1 × 102 MID50) and treated with carnosine (10 mg kg− 1). At the indicated time, (a) EBD in lung lysates and (b) protein in BALF was evaluated for pulmonary capillary permeability. The mean ± sd (n = 5) at each time point is shown. *P < 0.05; **P < 0.01 compared with H9N2.
Carnosine treatment disturbed total SOD (T-SOD) and OH· scavenging activity, and decreased malondialdehyde (MDA) content in the lung tissues
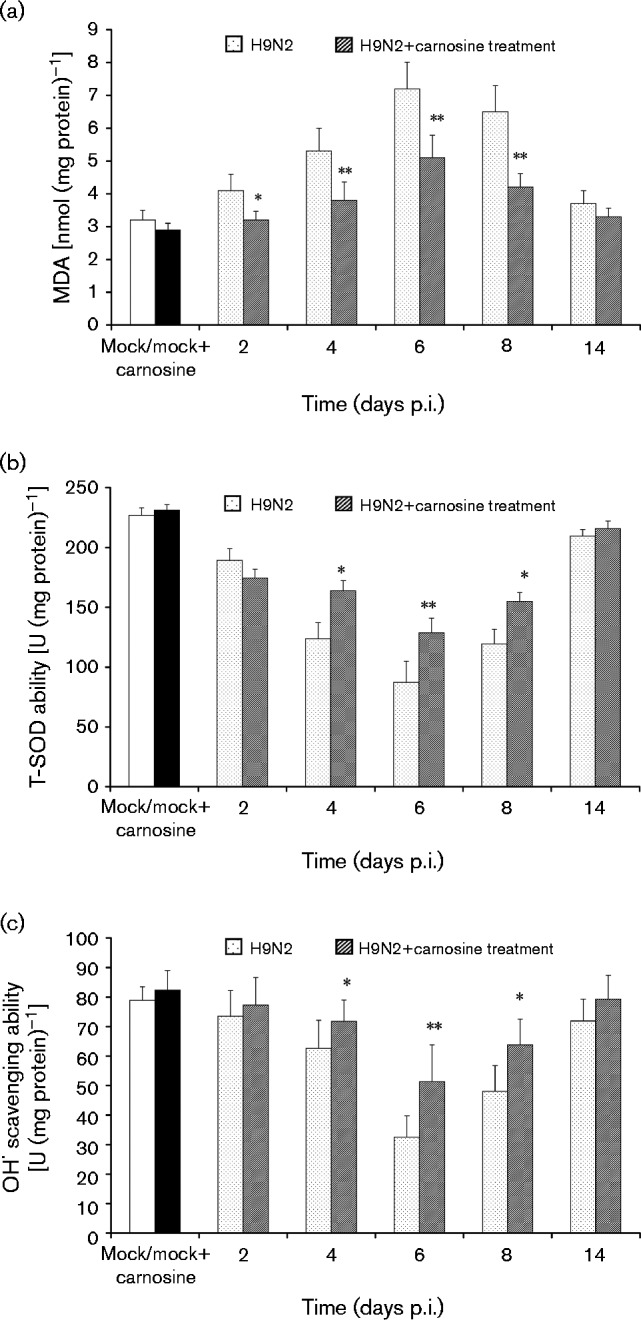
MDA is the direct product of lipid peroxidation and is an indicator of oxidative damage (He et al., 2013). As shown in Fig. 5(a), the MDA content in no-treatment H9N2-infected mice increased markedly from days 2 to 8 p.i. compared with mock-infected control group mice (P < 0.01). However, the MDA content significantly decreased in carnosine-treated H9N2-infected mice compared with no-treatment H9N2-infected mice (P < 0.01). Nevertheless, as a specific superoxide radical scavenger, SOD is an indicator of the free radical scavenging ability of carnotine (He et al., 2013). In this study, T-SOD activity was significantly higher in the carnosine-treated H9N2-infected group compared with no-treatment H9N2-infected mice on days 2–6 p.i. (P < 0.01) (Fig. 5b). Similarly, OH· scavenging activity was also markedly higher in carnosine-treated H9N2-infected mice compared with no-treatment H9N2-infected mice (P < 0.01) (Fig. 5c).
Fig. 5. (a) MDA content, (b) T-SOD activity and (c) OH· scavenging activity in the lung tissue homogenates. The mean ± sd (n = 5) at each time point is shown. *P < 0.05; **P < 0.01 compared with H9N2.
Carnosine treatment decreased inflammatory cell infiltration, myeloperoxidase (MPO) activity and cytokine content in BALF
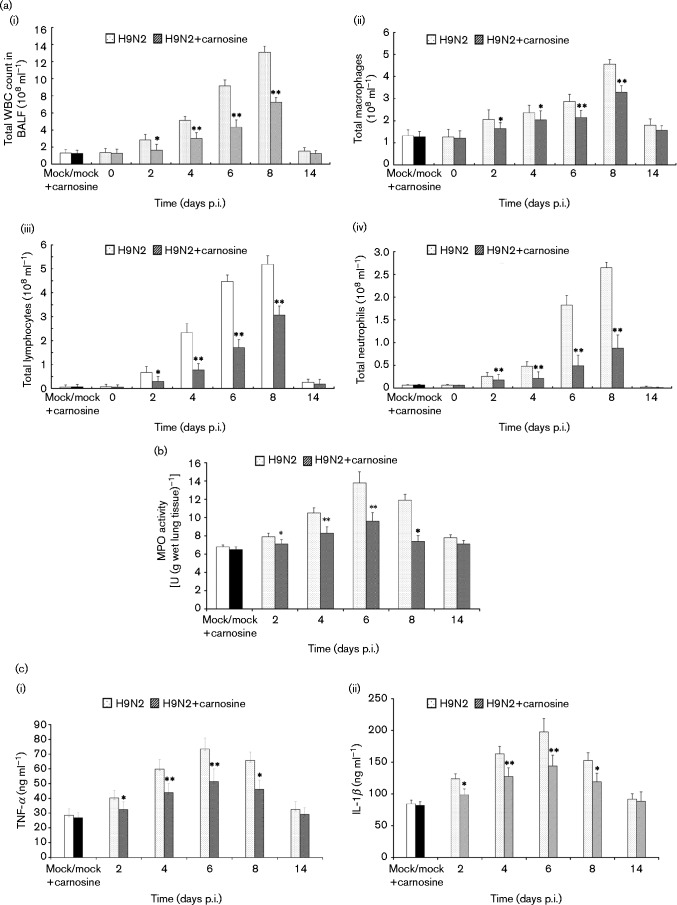
The total white blood cell (WBC) count in BALF of H9N2 SIV-infected mice increased dramatically compared with no-infected mice (P < 0.05) [Fig. 6a(i)]. The differential counts showed that all kinds of leukocytes (macrophages, lymphocytes and neutrophils) increased significantly during the course of H9N2 SIV infection. Among the three kinds of leukocytes mentioned above, the ratio of neutrophils in total WBCs increased more significantly than the other cells. However, the total WBC count and the differential counts decreased obviously after carnosine treatment [Fig. 6a(i–iv)] (P < 0.05). At the same time, MPO activity, reflecting the activation of neutrophils, also decreased significantly in the carnosine-treated group compared with the no-treatment group (Fig. 6b) (P < 0.05). As shown in Fig. 6(c), The TNF-α and IL-1β levels in BALF of no-treatment H9N2-infected mice dramatically increased from days 2 to 8 p.i. compared with BALF of mock-infected group mice (P < 0.05), but these indices in BALF of carnosine-treated H9N2-infected mice were lower than those in BALF of no-treatment H9N2-infected mice. Statistically significant differences were observed in the levels of the aforementioned cytokines in BALF on days 4–8 p.i. between carnosine-treated and no-treatment H9N2-infected mice (P < 0.01).
Fig. 6. Changes in total WBC counts, differential counts, MPO activity and cytokine content in BALF. After infection with H9N2 virus with or without carnosine treatment, mice were sacrificed and their lungs were lavaged. Cells in the BALF were collected and cytospin preparations were made. (a) Total WBCs (i), macrophages (ii), lymphocytes (iii) and neutrophils (iv) in BALF were analysed. (b) MPO activity, and [c(i)] TNF-α and [c(ii)] IL-1β in BALF were determined at the indicated time points. The mean ± sd (n = 5) at each time point is shown. *P < 0.05; **P < 0.01 compared with H9N2.
Effect of carnosine treatment on viral infection of lungs
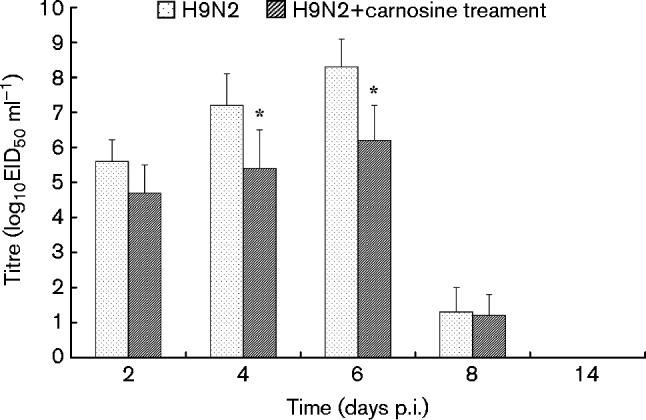
The effectiveness of carnosine on H9N2 virus replication in the lungs of mice was also investigated, and the results showed that viral titres in the lungs of H9N2-infected mice increased on days 2–8 p.i. with the peak viral titre reaching 8.2 log10 50 % egg infectious dose (EID50). It was also found that carnosine treatment affected the amount of virus proliferation from the lungs of carnosine-treated H9N2-infected mice and their viral titres were significantly lower than those of no-treatment H9N2-infected mice on days 4–6 p.i. (P < 0.05) (Fig. 7).
Fig. 7. Replication of H9N2 virus in the lungs of mice after therapy with carnosine. Mice were infected with 1 × 102 MID50, tissues were collected on different days post-inoculation and the virus was titrated in embryonated eggs; the mean ± sd virus titres from five mice per group are expressed as log10EID50 ml–1. The limit of virus detection was 101.2 EID50 ml–1 for lungs. *P < 0.05; **P < 0.01 compared with H9N2.
Carnosine treatment attenuated mRNA levels and decreased protein production of Toll-like receptor (TLR)-4
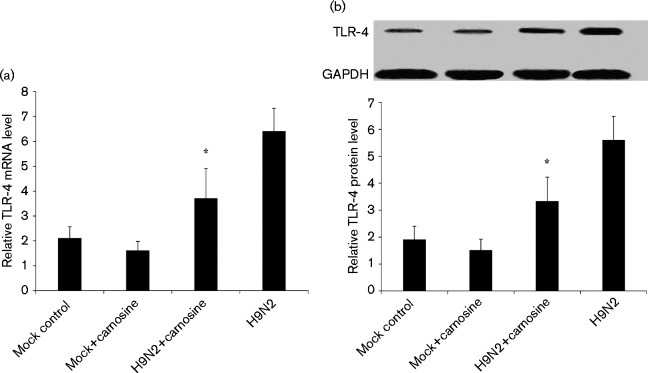
TLR-4 mRNA expression in carnosine-treated H9N2-infected mice decreased significantly compared with no-treatment H9N2-infected mice from days 2 to 6 p.i. (P < 0.05). Fig. 8(a) shows a significant decrease of TLR-4 mRNA expression in carnosine-treated H9N2-infected mice compared with no-treatment H9N2-infected mice on day 6 p.i. (P < 0.01). At the same time, the Western blot analysis also demonstrated that TLR-4 protein levels in the lung tissue dramatically decreased in carnosine-treated H9N2-infected mice compared with no-treatment H9N2-infected mice from days 2 to 6 p.i. (P < 0.05). Fig. 8(b) shows the TLR-4 protein levels in each group on day 6 p.i.
Fig. 8. Effect of carnosine on the levels of mRNA and protein of TLR-4 in H9N2-infected mice. (a) qRT-PCR showing the levels of TLR-4 mRNA in mock control, carnosine control, H9N2 and H9N2+carnosine mice on day 6 p.i. (b) Western blot showing the effect of carnosine on the levels of TLR-4 protein in each group of mice on day 6 p.i. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as loading control. *P < 0.05 compared with H9N2.
Discussion
ALI or its most severe form, ARDS, is a clinical syndrome characterized by rapid-onset respiratory failure following a variety of direct and indirect insults to the parenchyma or vasculature of the lungs (Raghavendran & Napolitano, 2011). At present, ALI/ARDS is confirmed as one of the most important clinical signs in H5N1 or new H1N1 patients (Yu et al., 2008; Estenssoro et al., 2010; Kumar, 2011). Furthermore, oxidative stress injury was demonstrated to be an important pathogenesis of influenza virus in critically ill patients (Narasaraju et al., 2011; He et al., 2013). Therefore, more and more therapies for ALI induced by influenza virus have focused on antioxidants.
In the present study, after orally administering carnosine to H9N2-infected mice at a dose of 10 mg kg− 1 daily for 7 days consecutively, clinical signs including decreased food intake and loss of body mass were alleviated, mean survival time was prolonged, and mortality was decreased. Meanwhile, lung histopathological observations indicated less inflammatory cell infiltration, interstitial and alveolar oedema, and haemorrhage in carnosine-treated mice than in H9N2-infected mice.
A few studies have demonstrated that the adaptive immune component of respiratory viral infection results in increasing lung vascular permeability that occurs primarily at the level of lung microcirculation, which in turn leads to the accumulation of protein-rich pulmonary oedema fluid (Matthay & Zimmerman, 2005). Therefore, lung vascular injury is the most important initial cause of ALI/ARDS (Matthay & Zemans, 2011). To investigate the effect of carnosine on lung vascular permeability, the extravasated EBD concentration, the content of protein in BALF as well as the lung wet/dry mass ratio were observed dynamically after carnosine treatment, and it was found that carnosine treatment significantly reduced the vascular permeability of EDB and protein content in BALF, and decreased the lung wet/dry mass ratio of H9N2-infected mice in this study. This suggested that carnosine effectively alleviated lung vascular lesions, and the exudation of water and protein in the lungs. In response to an inciting event of infection in the course of ALI, pulmonary macrophages and endothelium were activated and expressed adhesion molecules, which resulted in a large amount of inflammatory cells infiltrating the alveolar space and interstitial tissue, and release of proinflammatory and cytotoxic compounds including TNF-α, IL-1β and ROS. These compounds ultimately lead to profound injuries to the alveolocapillary membrane and respiratory failure (Lee & Downey, 2001; Khadaroo & Marshall, 2002; Chow et al., 2003). Furthermore, some investigations also indicated that inflammatory cytokines or ROS increased dramatically in H1N1 and H5N1 patients or some animal models (Akaike et al., 1996; He et al., 2013; Babizhayev et al., 2014). Interestingly, we found that the total WBC count in BALF decreased dramatically in the carnosine-treated H9N2-infected group compared with no-treatment H9N2-infected mice. The differential WBC counts in BALF, especially the neutrophil counts, significantly decreased with carnosine treatment. Meanwhile, our data showed that MPO activity, reflecting the activation of neutrophils, also decreased significantly in the carnosine-treated H9N2-infected group. It was also found that the TNF-α, IL-1β and MDA content and MPO activity of H9N2-infected mice increased dramatically in this investigation. However, the proinflammatory and cytotoxic compounds mentioned above decreased significantly after carnosine treatment. Furthermore, as indicators of superoxide radical scavenging ability, T-SOD activity and OH· scavenging activity increased dramatically in carnosine-treated mice compared with no-treatment mice. Hence, our data showed that carnosine effectively ameliorated H9N2 SIV-induced ALI in mice, reducing cytotoxic compounds and cytokine production.
TLRs were the first family of pattern recognition receptors to be discovered in mammals and are widely expressed on a range of immune or non-immune cells, including neutrophils, macrophages, dendritic cells, lung endothelial cells and mucosal epithelial cells (Lee et al., 2000; Lafferty et al., 2010; Xiang et al., 2010). Previous studies demonstrated that activation of TLRs resulted in acute inflammation and controlled the adaptive immune response at various levels (Lafferty et al., 2010). In this study, the mRNA and protein levels of TLR-4 expression were analysed by quantitative real-time (qRT)-PCR and Western blotting to investigate their role in ALI induced by H9N2 virus. The results of qRT-PCR and Western blot analysis indicated that TLR-4 mRNA levels and protein expression in the lungs of H9N2-infected mice were much higher than those in the lungs of carnosine-treated H9N2-infected mice, suggesting that TLR-4 was a key pathway involved in ALI induced by H9N2 infection. Imai et al. (2008) reported oxidative stress and TLR-4 as key pathways controlling the severity of ALI induced by H5N1 avian influenza virus (Imai et al., 2008). In a piglet model challenged by lipopolysaccharide, Hou et al. (2013) found that NAC supplementation markedly alleviated intestinal inflammation and reduced the levels of TLR-4 mRNA, which indicated that ROS appear to participate in the regulation of TLR-4 gene expression. Furthermore, some researchers also suggested that the ROS-induced injury was considered to occur because of a direct toxic effect of ROS on cellular components or an indirect effect mediated via activation of cell signalling pathways culminating in the generation of a number of proinflammatory molecules (Ziegler-Heitbrock et al., 1993; Fan et al., 2002). Therefore, one potential strategy for alleviating the effects of oxidative stress is to employ antioxidant strategies aimed at neutralizing oxidants or enhancing endogenous antioxidant mechanisms.
As mentioned in the preceding paragraphs, the effective protection offered by carnosine against H9N2 SIV-induced ALI is mainly because of its antioxidant and free radical scavenging functions. However, whether it could influence the replication of H9N2 SIV was still unknown. Therefore, the effectiveness of carnosine on H9N2 viral replication was investigated in this study and it was found that carnosine treatment also significantly inhibited H9N2 virus replication in mice. At the same time, previous studies demonstrated that another antioxidant, NAC, significantly inhibits human respiratory syncytial virus and H5N1 avian influenza virus infections in vitro (Geiler et al., 2010; Mata et al., 2012). Cai et al. (2003) also reported that inclusion of GSH in drinking water decreased the viral titre of influenza strain A/X-31 in both lung and trachea homogenates of BALB/c mice. Babizhayev et al. (2014) found that oral carnosine decreased H1N1 viral replication by modulating respiratory burst and ROS production in neutrophils. So far, growing evidence indicates that viral replication is regulated by the redox state of the host cell, although the mechanisms underlying this virus-related oxidative stress are still a matter of great debate (Vlahos et al., 2011, 2012). Nencioni et al. (2003) demonstrated that GSH inhibited expression of viral matrix protein, and inhibited virally induced caspase activation and Fas upregulation during influenza virus infection. Zhang et al. (2015) also found that resveratrol, which has anti-inflammatory, antioxidant and antitumour functions, inhibited EV71 replication and cytokine secretion in EV71-infected RD cells through blocking the IκB kinase/NFκB signalling pathway. Other authors thought that biochanin A affected H5N1 viral replication by inhibiting the activation of signalling molecules involved in virus-induced signalling, including AKT, extracellular regulated kinase 1/2 and NFκB (Pinto et al., 2011; Sithisarn et al., 2013). Moreover, Amatore et al. (2015) demonstrated that inhibition of NADPH oxidase 4 activity through RNA silencing blocks ROS increase, prevents mitogen-activated protein kinase phosphorylation, and inhibits viral ribonucleoprotein nuclear export and viral release. In this paper, we confirmed that carnosine dramatically increased OH· scavenging activity and decreased depletion of T-SOD, and thus affected the redox state of the lung tissue. Furthermore, we also found that carnosine significantly affected the expression of TLR-4 mRNA and protein. Imai et al. (2008) demonstrated that the TLR-4/NFκB signalling pathway resulted in acute inflammation and controlled the adaptive immune response at various levels, and was involved in ALI induced by H5N1 avian influenza virus. Therefore, our data suggested that carnosine could also affect viral replication mainly by influencing the redox state of the host cell and the activation of TLR-4 signalling molecules. However, the detailed mechanisms remain to be investigated.
Our data demonstrate that antioxidants such as carnosine not only significantly decrease the production of ROS and inflammatory cytokines, but also slightly inhibit H9N2 viral replication, representing a potential additional treatment option that should be considered in the severe case of influenza A virus pandemics. However, whilst the results of this work are interesting, a detailed protective mechanism of carnosine affecting H9N2 SIV replication still remains to be investigated.
Conclusion
We conclude that carnosine can protect against H9N2 SIV-induced ALI mainly by scavenging ROS, downregulating the expression of TLR-4 and inhibiting inflammatory cytokine production during viral infection. Our result supports the beneficial effects of carnosine treatment for the management of severe influenza cases during future influenza virus epidemics.
Methods
Virus
A/Swine/Hebei/012/2008/ (H9N2) virus was inoculated into 10-day-old specific-pathogen-free (SPF) chicken embryos (Beijing Laboratory Animal Research Centre, Beijing, China) and consecutively blind passaged for three generations. The 50 % mouse infectious dose (MID50) was determined according to the methods described by Lu et al. (1999). The allantoic fluid (AF) was collected and stored at − 80 °C.
Mice and ethics statement
SPF 6- to 8-week-old BALB/c female mice (Beijing Laboratory Animal Research Centre) were raised in negative-pressure micro-isolator cages ventilated with high-efficiency particulate air filters, and provided with standard rodent chow and water ad libitum. The study was approved by the Animal Care and Use Committee of Hebei North University (approval IDS 2013-1-0-06). All animal procedures followed the ethics guidelines of the Animal Care and Use Committee; we also referred to the National Research Council Guide for Care and Use of Laboratory Animals (1996). In this study, a humane end-point was used when the animals showed >30 % body mass loss and laboured breathing. Once animals started to display the above-mentioned clinical signs, they were considered to have reached the experiment end-point and were euthanized immediately according to the protocol of the Animal Care and Use Committee.
ALI mouse model
The ALI mouse model was established as described by Dong et al. (2012). In brief, the mice were slightly anaesthetized with diethyl ether and inoculated intranasally (100 μl) with 1 × 102 MID50 H9N2 virus diluted in sterile normal saline or an equivalent dilution of non-infectious AF.
Determination of the optimal treatment dose of carnosine in H9N2 SIV-infected mice
The efficacy of three different therapeutic doses of carnosine (5, 10 and 30 mg kg− 1) was tested in order to determine the optimal treatment dose of carnosine. Each group (16 mice) was orally administered different doses of carnosine beginning on day 0 or 2 post-H9N2 virus infection (day 0 was the day immediately administered with carnosine when infected with H9N2 virus, day 2 was the day after infection with H9N2 virus and the infected mice presented clinical signs). Observations indicated that the effect of carnosine treatment was dose- and time-dependent (data not shown); a dose of 10 mg kg− 1 and a time of day 0 were the most effective. According to other researchers (Noori & Mahboob, 2010), a dose of 10 mg kg− 1 and a treatment time of day 0 were chosen in our experiment.
Experimental group and carnosine administration
SPF 6- to 8-week-old BALB/c female mice were randomized into four groups as follows: (1) H9N2 group mice, which were subjected to H9N2 SIV-induced ALI with infectious AF; (2) mock control group mice, which were given treatment identical to that of H9N2 group mice, except that the non-infectious AF was administered instead of H9N2 SIV; (3) H9N2+carnosine group mice, which were given identical treatment to that of H9N2 group mice, whilst carnosine (Solon) was dissolved in sterile normal saline and administered orally to mice at a dose of 10 mg kg− 1 body mass daily for 7 days consecutively; and (4) carnosine control group mice, which were given treatment identical to that of mock-infected group mice and carnosine was administered as in the H9N2+carnosine group.
Observations of clinical signs and assessment of lung injury
Mass, food intake and mortality were measured daily for morbidity for 14 days consecutively p.i. in 20 mice of each group. In another experiment, another five mice of each group were weighed and euthanized on days 2, 4, 6, 8 10 and 14 p.i. The lung mass/body mass ratio and lung wet/dry mass ratio, which would be taken as indicators of lung oedema, were measured according to the methods described by Lang et al. (2005). Meanwhile, parts of the left lobes of lungs were fixed in 4 % paraformaldehyde and embedded in paraffin, and 5 μm sections were stained with HE for light microscopy. Tissue sections were photographed and histopathology was evaluated by a veterinary pathologist on a blind basis according to our previously described methods (Xu et al., 2009). Lung injury score was measured by a blinded pathologist using a 0–4 point scale according to combined assessments of formation of bronchiolitis, alveolar oedema and interstitial oedema, and margination and infiltration. A score of 0 represented no damage, l represented mild damage, 2 represented moderate damage, 3 represented severe damage and 4 represented very severe histological changes (Parsey et al., 1998).
Quantification of total protein and WBCs in BALF
BALF samples were collected from animals as described by Majeski et al. (2003) and Xu et al. (2006). Briefly, another five mice from each group were euthanized at the indicated time points and the lungs were lavaged twice with a total of 1.0 ml saline (4 °C) through the endotracheal tube with the recovery rate of BALF >90 % for all tested animals. After that, the BALF was centrifuged for 10 min (300 g, 15 min, 4 °C). The supernatant was then centrifuged again (16 500 g, 10 min, 4 °C). Total protein in pure BALF was measured using a BCA (bicinchoninic acid) Protein Assay kit (Thermo Scientific). The number of leukocytes in BALF was counted as described by Xu et al. (2006). The supernatant was stored at − 80 °C for the measurement of cytokines using ELISA kits (Sigma).
Assessment of pulmonary capillary permeability
The pulmonary capillary permeability was determined using the EBD extravasation technique as described previously (Choi et al., 2013). Briefly, EBD (20 mg kg− 1; Sigma) was injected into the tail vein 1 h before termination of the experiment, and extravasated EBD concentration in lung homogenates was calculated using a standard curve and reported as μg EBD (g lung)–1.
Measurement of MPO, T-SOD and OH· scavenging activity, and MDA content
MPO activity, T-SOD activity, OH· scavenging activity and MDA content, were determined in the lung tissues using detection kits (Nanjing Jiancheng Institute of Biotechnology, Nanjing, China). Sample protein concentrations were determined by BCA assay, and the other results were presented as U (mg lung tissue protein)–1, except for MPO activity that was expressed as U (g wet lung tissue)–1.
Cytokine analysis
The concentrations of TNF-α and IL-1β in mouse BALF were determined with ELISA kits (Sigma) following the manufacturer's instructions.
Virus titration
Virus titration was performed as per our previously described methods (Xu et al., 2006). Parts of the left lobes of lungs were collected, weighed and homogenized using a mortar and pestle in cold PBS and antibiotics at the indicated time points. At the same time, clarified homogenates were titrated for viral infectivity in embryonated chicken eggs from initial dilutions of 1 : 10 and viral titres were expressed as mean log10EID50 g–1.
TLR-4 qRT-PCR
Total RNA was extracted from the lung tissues using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. Reverse transcription reactions were performed using a RT Reagent kit with a gDNA Eraser kit (TaKaRa Bio). Meanwhile, the qRT-PCR mixture was prepared using a SYBR Premix Ex Taq II kit (TaKaRa) with the primers: TLR-4 (forward 5′-CTGTATTCCCTCAGCACTCTTG-3′, reverse 5′-CCCACTGCAGGAATTTCTGATG-3′) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (forward 5′-TTCACCACCATGGAGAAGGC-3′, reverse 5′-GGCATGGACTGTGGTCATGA-3′). All PCR amplifications were performed using an ABI 9700 (Perkin-Elmer Applied Biosystems).
Western blot analysis of TLR-4
Proteins were extracted from the lung tissues a using protein extraction kit (BOSTER) and separated by SDS-PAGE. Proteins were then transferred to nitrocellulose membranes. The blots were incubated for 2 h with primary antibodies for TLR-4 (Abcam) and GAPDH (Abcam). The secondary antibody (HRP-conjugated goat anti-rabbit immunoglobulin) was added and incubated at room temperature for 1 h. Peroxidase labelling was detected by diaminobenzidine using commercially available kits (BOSTER) and the protein level was analysed by a densitometry system. The relative protein level was normalized to GAPDH.
Statistical methods
All data were expressed as mean ± sd of n observations, where n represented the total number of animals investigated. In the experiments involving histology, results shown are representative of the results of five experiments performed on different experimental days and analysed by the Kruskal–Wallis test. The statistical analysis of survival data was performed using the Kaplan–Meier test. However, other results were analysed by one-way ANOVA followed by Fisher's protected least significant difference test for multiple comparisons using spss for Windows, version 18.0 (SPSS). P < 0.05 was considered significant.
Acknowledgements
This work was supported by the Natural Science Foundation of Hebei Province, China (C2011405002 and C2015405016), the Natural Science Research Key Program of Educational Department of Hebei Province (ZD20131045) and the Natural Science Research Key Program of Hebei North University (ZD201306).
References
- Akaike T., Noguchi Y., Ijiri S., Setoguchi K., Suga M., Zheng Y.M., Dietzschold B., Maeda H. (1996). Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals Proc Natl Acad Sci U S A 93 2448–2453 10.1073/pnas.93.6.2448 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatore D., Sgarbanti R., Aquilano K., Baldelli S., Limongi D., Civitelli L., Nencioni L., Garaci E., Ciriolo M.R., Palamara A.T. (2015). Influenza virus replication in lung epithelial cells depends on redox-sensitive pathways activated by NOX4-derived ROS Cell Microbiol 17 131–145 10.1111/cmi.12343 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babizhayev M.A., Deyev A.I. (2012). Management of the virulent influenza virus infection by oral formulation of nonhydrolyzed carnosine and isopeptide of carnosine attenuating proinflammatory cytokine-induced nitric oxide production Am J Ther 19 e25–e47 10.1097/MJT.0b013e3181dcf589 . [DOI] [PubMed] [Google Scholar]
- Babizhayev M.A., Seguin M.C., Gueyne J., Evstigneeva R.P., Ageyeva E.A., Zheltukhina G.A. (1994). l-Carnosine (beta-alanyl-l-histidine) and carcinine (beta-alanylhistamine) act as natural antioxidants with hydroxyl-radical-scavenging and lipid-peroxidase activities Biochem J 304 509–516 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babizhayev M.A., Deyev A.I., Yegorov Y.E.l-C. (2014). l-Carnosine modulates respiratory burst and reactive oxygen species production in neutrophil biochemistry and function: may oral dosage form of non-hydrolyzed dipeptide l-carnosine complement anti-infective anti-influenza flu treatment, prevention and self-care as an alternative to the conventional vaccination? Curr Clin Pharmacol 9 93–115 10.2174/1574884709999140311125601 . [DOI] [PubMed] [Google Scholar]
- Cai J., Chen Y., Seth S., Furukawa S., Compans R.W., Jones D.P. (2003). Inhibition of influenza infection by glutathione Free Radic Biol Med 34 928–936 10.1016/S0891-5849(03)00023-6 . [DOI] [PubMed] [Google Scholar]
- CDC (1997). Isolation of avian influenza A(H5N1) of viruses from humans: Hong Kong May–December 1997 MMWR Morb Mortal Wkly Rep 46 1204–1207 . [PubMed] [Google Scholar]
- Chan W.K.M., Decker E.A., Lee J.B., Butterfield D. (1994). EPR spin-trapping studies of the hydroxy radical scavenging activity of carnosine and related dipeptides J Agric Food Chem 42 1407–1410 10.1021/jf00043a003. [DOI] [Google Scholar]
- Choi Y.H., Jin G.Y., Li L.C., Yan G.H. (2013). Inhibition of protein kinase C delta attenuates allergic airway inflammation through suppression of PI3K/Akt/mTOR/HIF-1 alpha/VEGF pathway PLoS One 8 e81773 10.1371/journal.pone.0081773 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C.W., Herrera Abreu M.T., Suzuki T., Downey G.P. (2003). Oxidative stress and acute lung injury Am J Respir Cell Mol Biol 29 427–431 10.1165/rcmb.F278 . [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S., Genovese T., Failla M., Vecchio G., Fruciano M., Mazzon E., Di Paola R., Muià C., La Rosa C., other authors (2007). Protective effect of orally administered carnosine on bleomycin-induced lung injury Am J Physiol Lung Cell Mol Physiol 292 L1095–L1104 10.1152/ajplung.00283.2006 . [DOI] [PubMed] [Google Scholar]
- Dahl T.A., Midden W.R., Hartman P.E. (1988). Some prevalent biomolecules as defenses against singlet oxygen damage Photochem Photobiol 47 357–362 10.1111/j.1751-1097.1988.tb02737.x . [DOI] [PubMed] [Google Scholar]
- Dong W., Li-Feng X., Cun-Lian W., Ming-Ju X., Rui-Hua Z., Ying L., Tong X. (2012). A mouse model of swine influenza virus H9N2 infection with acute lung injury Acta Virol 56 227–233 10.4149/av_2012_03_227 . [DOI] [PubMed] [Google Scholar]
- Estenssoro E., Ríos F.G., Apezteguía C., Reina R., Neira J., Ceraso D.H., Orlandi C., Valentini R., Tiribelli N., other authors (2010). Pandemic 2009 influenza A in Argentina: a study of 337 patients on mechanical ventilation Am J Respir Crit Care Med 182 41–48 10.1164/201001-0037OC . [DOI] [PubMed] [Google Scholar]
- Fan J., Kapus A., Marsden P.A., Li Y.H., Oreopoulos G., Marshall J.C., Frantz S., Kelly R.A., Medzhitov R., Rotstein O.D. (2002). Regulation of Toll-like receptor 4 expression in the lung following hemorrhagic shock and lipopolysaccharide J Immunol 168 5252–5259 10.4049/jimmunol.168.10.5252 . [DOI] [PubMed] [Google Scholar]
- Geiler J., Michaelis M., Naczk P., Leutz A., Langer K., Doerr H.W., Cinatl J., Jr (2010). N-Acetyl-l-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus Biochem Pharmacol 79 413–420 10.1016/j.bcp.2009.08.025 . [DOI] [PubMed] [Google Scholar]
- Guney Y., Turkcu U.O., Hicsonmez A., Andrieu M.N., Guney H.Z., Bilgihan A., Kurtman C. (2006). Carnosine may reduce lung injury caused by radiation therapy Med Hypotheses 66 957–959 10.1016/j.mehy.2005.11.023 . [DOI] [PubMed] [Google Scholar]
- He G., Dong C., Luan Z., McAllan B.M., Xu T., Zhao L., Qiao J. (2013). Oxygen free radical involvement in acute lung injury induced by H5N1 virus in mice Influenza Other Respi Viruses 7 945–953 10.1111/irv.12067 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien N.D., Ha N.H., Van N.T., Ha N.T., Lien T.T., Thai N.Q., Trang V.D., Shimbo T., Takahashi Y., other authors (2009). Human infection with highly pathogenic avian influenza virus (H5N1) in northern Vietnam, 2004-2005 Emerg Infect Dis 15 19–23 10.3201/eid1501.080073 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Wang L., Yi D., Ding B., Yang Z., Li J., Chen X., Qiu Y., Wu G. (2013). N-Acetylcysteine reduces inflammation in the small intestine by regulating redox, EGF and TLR4 signaling Amino Acids 45 513–522 10.1007/s00726-012-1295-x . [DOI] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Neely G.G., Yaghubian-Malhami R., Perkmann T., van Loo G., Ermolaeva M., Veldhuizen R., Leung Y.H., other authors (2008). Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury Cell 133 235–249 10.1016/j.cell.2008.02.043 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadaroo R.G., Marshall J.C. (2002). ARDS and the multiple organ dysfunction syndrome: common mechanisms of a common systemic process Crit Care Clin 18 127–141 10.1016/S0749-0704(03)00069-1 . [DOI] [PubMed] [Google Scholar]
- Kumar A. (2011). Pandemic H1N1 influenza J Thorac Dis 3 262–270 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty E.I., Qureshi S.T., Schnare M. (2010). The role of Toll-like receptors in acute and chronic lung inflammation J Inflamm (Lond) 7 57 10.1186/1476-9255-7-57 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J.D., Figueroa M., Sanders K.D., Aslan M., Liu Y., Chumley P., Freeman B.A. (2005). Hypercapnia via reduced rate and tidal volume contributes to lipopolysaccharide-induced lung injury Am J Respir Crit Care Med 171 147–157 10.1164/rccm.200302-305OC . [DOI] [PubMed] [Google Scholar]
- Lee W.L., Downey G.P. (2001). Neutrophil activation and acute lung injury Curr Opin Crit Care 7 1–7 10.1097/00075198-200102000-00001 . [DOI] [PubMed] [Google Scholar]
- Lee Y.S., Kang Y.S., Lee S.H., Kim J.A. (2000). Role of NAD(P)H oxidase in the tamoxifen-induced generation of reactive oxygen species and apoptosis in HepG2 human hepatoblastoma cells Cell Death Differ 7 925–932 10.1038/sj.cdd.4400717 . [DOI] [PubMed] [Google Scholar]
- Lee Y.T., Hsu C.C., Lin M.H., Liu K.S., Yin M.C. (2005). Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation Eur J Pharmacol 513 145–150 10.1016/j.ejphar.2005.02.010 . [DOI] [PubMed] [Google Scholar]
- Lu X., Tumpey T.M., Morken T., Zaki S.R., Cox N.J., Katz J.M. (1999). A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans J Virol 73 5903–5911 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeski E.I., Paintlia M.K., Lopez A.D., Harley R.A., London S.D., London L. (2003). Respiratory reovirus 1/L induction of intraluminal fibrosis, a model of bronchiolitis obliterans organizing pneumonia, is dependent on T lymphocytes Am J Pathol 163 1467–1479 10.1016/S0002-9440(10)63504-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata M., Sarrion I., Armengot M., Carda C., Martinez I., Melero J.A., Cortijo J. (2012). Respiratory syncytial virus inhibits ciliagenesis in differentiated normal human bronchial epithelial cells: effectiveness of N-acetylcysteine PLoS One 7 e48037 10.1371/journal.pone.0048037 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay M.A., Zemans R.L. (2011). The acute respiratory distress syndrome: pathogenesis and treatment Annu Rev Pathol 6 147–163 10.1146/annurev-pathol-011110-130158 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay M.A., Zimmerman G.A. (2005). Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management Am J Respir Cell Mol Biol 33 319–327 10.1165/rcmb.F305 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasaraju T., Yang E., Samy R.P., Ng H.H., Poh W.P., Liew A.A., Phoon M.C., van Rooijen N., Chow V.T. (2011). Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis Am J Pathol 179 199–210 10.1016/j.ajpath.2011.03.013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nencioni L., Iuvara A., Aquilano K., Ciriolo M.R., Cozzolino F., Rotilio G., Garaci E., Palamara A.T. (2003). Influenza A virus replication is dependent on an antioxidant pathway that involves GSH and Bcl-2 FASEB J 17 758–760 . [DOI] [PubMed] [Google Scholar]
- Noori S., Mahboob T. (2010). Antioxidant effect of carnosine pretreatment on cisplatin-induced renal oxidative stress in rats Indian J Clin Biochem 25 86–91 10.1007/s12291-010-0018-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey M.V., Tuder R.M., Abraham E. (1998). Neutrophils are major contributors to intraparenchymal lung IL-1β expression after hemorrhage and endotoxemia J Immunol 160 1007–1013 . [PubMed] [Google Scholar]
- Peiris M., Yuen K.Y., Leung C.W., Chan K.H., Ip P.L., Lai R.W., Orr W.K., Shortridge K.F. (1999). Human infection with influenza H9N2 Lancet 354 916–917 10.1016/S0140-6736(99)03311-5 . [DOI] [PubMed] [Google Scholar]
- Peiris J.S., Poon L.L., Guan Y. (2009). Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans J Clin Virol 45 169–173 10.1016/j.jcv.2009.06.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto R., Herold S., Cakarova L., Hoegner K., Lohmeyer J., Planz O., Pleschka S. (2011). Inhibition of influenza virus-induced NF-kappaB and Raf/MEK/ERK activation can reduce both virus titers and cytokine expression simultaneously in vitro and in vivo Antiviral Res 92 45–56 10.1016/j.antiviral.2011.05.009 . [DOI] [PubMed] [Google Scholar]
- Raghavendran K., Napolitano L.M. (2011). ALI and ARDS: challenges and advances Crit Care Clin 27 xiii–xxiv 10.1016/j.ccc.2011.05.012 . [DOI] [PubMed] [Google Scholar]
- Ryrfeldt A., Bannenberg G., Moldéus P. (1993). Free radicals and lung disease Br Med Bull 49 588–603 . [DOI] [PubMed] [Google Scholar]
- Salomon R., Hoffmann E., Webster R.G. (2007). Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection Proc Natl Acad Sci U S A 104 12479–12481 10.1073/pnas.0705289104 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithisarn P., Michaelis M., Schubert-Zsilavecz M., Cinatl J., Jr, (2013). Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells Antiviral Res 97 41–48 10.1016/j.antiviral.2012.10.004 . [DOI] [PubMed] [Google Scholar]
- Tran T.H., Nguyen T.L., Nguyen T.D., Luong T.S., Pham P.M., Nguyen V., Pham T.S., Vo C.D., Le T.Q., other authors (2004). Avian influenza A (H5N1) in 10 patients in Vietnam N Engl J Med 350 1179–1188 10.1056/NEJMoa040419 . [DOI] [PubMed] [Google Scholar]
- Vlahos R., Stambas J., Bozinovski S., Broughton B.R., Drummond G.R., Selemidis S. (2011). Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation PLoS Pathog 7 e1001271 10.1371/journal.ppat.1001271 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahos R., Stambas J., Selemidis S. (2012). Suppressing production of reactive oxygen species (ROS) for influenza A virus therapy Trends Pharmacol Sci 33 3–8 10.1016/j.tips.2011.09.001 . [DOI] [PubMed] [Google Scholar]
- Wiwanitkit V. (2013). H7N9 influenza: the emerging infectious disease N Am J Med Sci 5 395–398 10.4103/1947-2714.115764 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M., Fan J., Fan J. (2010). Association of Toll-like receptor signaling and reactive oxygen species: a potential therapeutic target for posttrauma acute lung injury Mediators Inflamm 2010 1–8 10.1155/2010/916425 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Qiao J., Zhao L., Wang G., He G., Li K., Tian Y., Gao M., Wang J., other authors (2006). Acute respiratory distress syndrome induced by avian influenza A (H5N1) virus in mice Am J Respir Crit Care Med 174 1011–1017 10.1164/rccm.200511-1751OC . [DOI] [PubMed] [Google Scholar]
- Xu T., Qiao J., Zhao L., He G., Li K., Wang J., Tian Y., Wang H. (2009). Effect of dexamethasone on acute respiratory distress syndrome induced by the H5N1 virus in mice Eur Respir J 33 852–860 10.1183/09031936.00130507 . [DOI] [PubMed] [Google Scholar]
- Yu H., Gao Z., Feng Z., Shu Y., Xiang N., Zhou L., Huai Y., Feng L., Peng Z., other authors (2008). Clinical characteristics of 26 human cases of highly pathogenic avian influenza A (H5N1) virus infection in China PLoS One 3 e2985 10.1371/journal.pone.0002985 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R.H., Li C.H., Wang C.L., Xu M.J., Xu T., Wei D., Liu B.J., Wang G.H., Tian S.F. (2014). N-Acetyl-l-cystine (NAC) protects against H9N2 swine influenza virus-induced acute lung injury Int Immunopharmacol 22 1–8 10.1016/j.intimp.2014.06.013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Li Y., Gu Z., Wang Y., Shi M., Ji Y., Sun J., Xu X., Zhang L., other authors (2015). Resveratrol inhibits enterovirus 71 replication and pro-inflammatory cytokine secretion in rhabdosarcoma cells through blocking IKKs/NF-κB signaling pathway PLoS One 10 e0116879 10.1371/journal.pone.0116879 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H.W., Sternsdorf T., Liese J., Belohradsky B., Weber C., Wedel A., Schreck R., Bäuerle P., Ströbel M. (1993). Pyrrolidine dithiocarbamate inhibits NF-kappa B mobilization and TNF production in human monocytes J Immunol 151 6986–6993 . [PubMed] [Google Scholar]