Abstract
Dengue is a mosquito-borne disease caused by four related but distinct dengue viruses, DENV-1 to DENV-4. Dengue is endemic in most tropical countries, and over a third of the world's population is at risk of being infected. Although the global burden is high, no vaccine or antiviral is licensed to combat this disease. An obstacle complicating dengue research is the lack of animal challenge models that mimic human disease. Advances in immunocompromised murine infection models resulted in development of lethal DENV-2, DENV-3 and DENV-4 models in AG129 mice, which are deficient in both the IFN-α/β receptor (IFN-α/βR) and the IFN-γ receptor (IFN-γR). These models mimic features of dengue disease in humans. Here, we characterized lethal infection of AG129 mice by DENV-4 strain TVP-376 and found that AG129 mice developed clinical signs of illness and high viral loads in multiple tissues and succumbed 5 days after infection. Moreover, the splenic and hepatic histopathology of TVP-376-infected mice demonstrated the presence of cell activation and destruction of tissue architecture. Furthermore, infected mice had heightened levels of circulating cytokines. Comparison of the virulence phenotypes of DENV-4 strain TVP-376 and DENV-2 strain D2S10 revealed that TVP-376-induced mortality occurred in the absence of both IFN-α/βR and IFN-γR signalling, but not with intact signalling from the IFN-γR, whereas D2S10 required the absence of IFN-α/βR signalling only, indicating that it is more virulent than TVP-376. In conclusion, TVP-376 is lethal in AG129 mice, and this model provides a useful platform to investigate vaccine candidates and antivirals against DENV-4.
Introduction
The family Flaviviridae contains medically important mosquito-borne viruses, including West Nile, yellow fever, Japanese encephalitis and dengue virus types 1–4 (DENV-1 to -4). Dengue is endemic in most tropical countries, causing an estimated 400 million infections annually (Messina et al., 2014; Bhatt et al., 2013). Dengue fever is an acute illness with symptoms that include myalgia, headache, retro-orbital pain, maculopapular rash, vomiting and abdominal pain. Severe forms of the disease are characterized by thrombocytopenia and vascular leakage, leading to dengue haemorrhagic fever (DHF). Dengue shock syndrome (DSS) is characterized by hypovolaemic shock (Simmons et al., 2012). Although DHF/DSS can be fatal, no treatments are licensed to prevent or combat infections.
A limitation complicating dengue research is the lack of animal challenge models that mimic human disease. Recently, DENV-2 strains have been identified that produce a disease that contains features of the human illness, such as thrombocytopenia, viraemia, cytokine storm, and/or vascular leakage (Shresta et al., 2006; Tan et al., 2010; Mota & Rico-Hesse, 2009). Lethal DENV-2 infection models have been described in immunocompromised mouse strains, particularly in AG129 mice, deficient in IFN-α/β receptor (IFN-α/βR) and IFN-γ receptor (IFN-γR) signalling, and A129 and ifnar − / − mice, deficient in IFN-α/βR only (Johnson & Roehrig, 1999; Orozco et al., 2012; Prestwood et al., 2012; Zellweger et al., 2010; Balsitis et al., 2010). Other strains of immunocompromised mice are resistant to lethal infection by DENV-2, such as mice deficient in IFN regulatory factors 3 and 7 (IRF3 and IRF7), signal transducer and activator of transcription factor 1 or 2 (STAT1 or STAT2), and mitochondrial antiviral signalling protein (MAVS) (Chen et al., 2013; Perry et al., 2009, 2011; Ashour et al., 2010).
Lethal models for DENV-3 and DENV-4 in AG129 mice have also been reported. A non-adapted human isolate of DENV-3 (strain C0360/94) was found to cause severe systemic non-neurotropic infection, characterized by thrombocytopenia, vascular leakage, circulating cytokines, and TNF-α-mediated disease (Sarathy et al., 2015). Mouse-adapted DENV-4 strains derived from H241 and TVP-376 were used to study antibody protection and neutralization (Sukupolvi-Petty et al., 2013). These adapted viruses led to neurovirulent disease and for TVP-376 mortality increased with each of two passages, indicating that a single mouse passage can influence DENV-4 disease in AG129 mice. Finally, a new study described the virulence of non-adapted DENV-4 strain 703-4 in AG129 mice (Milligan et al., 2015). The animals succumbed to a lethal, non-neurovirulent systemic disease and developed elevated cytokine levels and vascular leakage. Recently, this 703-4 model was used to evaluate a candidate live attenuated dengue vaccine. The tetravalent dengue vaccine reduced viraemia and protected mice against lethal 703-4 challenge (Fuchs et al., 2014).
Subsequently, we determined that non-mouse-adapted DENV-4 strain TVP-376 causes systemic disease in AG129 mice similar to that caused by DENV-4 strain 703-4. In the current study, we present a characterization of this lethal TVP-376 infection in AG129 mice, and the sensitivity of WT, Mavs − / − , A129 and AGB6 mice to non-adapted TVP-376 is evaluated. Although DENV-2 has been examined previously in these mouse strains, there is variation in the viral strains and viral quantification methods (infectivity versus genome copies). Therefore, a side-by-side comparison between DENV-4 TVP-376 and DENV-2 D2S10 is included. The objectives of this study were to evaluate lethal TVP-376 infection and to provide a platform to compare the mechanisms of DENV-2 and DENV-4 infection in AG129 mice.
Results
Derivation of DENV-4 strain TVP-376
The DENV-4 reference strain TVP-376 was acquired from Dr Morag Ferguson of the National Institute of Biological Standards and Control (NIBSC), Potters Bar, UK, who received the virus from Dr Robert Putnak of the Walter Reed Army Institute for Research. This strain was isolated from a clinical acute serum sample of infection acquired in Puerto Rico in 1982 (R. Tesh, personal communication). Unfortunately, confusingly, TVP-376 has been mislabelled as TVP-360 and has also been mistakenly described as a Colombian isolate (Sukupolvi-Petty et al., 2013).
DENV-4 TVP-376 is lethal in AG129 mice
DENV-4 TVP-376 was tested in AG129 mice (6–8 weeks old) at doses comparable with those used for the other AG129 DENV models (Shresta et al., 2006; Sarathy et al., 2015; Milligan et al., 2015). With an inoculum of 107.0 p.f.u. or higher, 100 % of the mice succumbed within 1 week of infection (Fig. 1a). Animals lost weight starting at 3 days post-infection (days p.i.) (Fig. 1b) and exhibited physical signs of illness, such as limited mobility and hunched posture (data not shown). Only one case of neurological disease occurred – one mouse developed paralysis at 7 days p.i. Median survival times for 107.5 p.f.u.-infected and 107.0 p.f.u.-infected mice were 3.5 and 5.0 days, respectively. All AG129 mice infected with 106.0 p.f.u. TVP-376 survived, and did not lose weight or have detectable viraemia (Fig. 1b, c). An equivalent dose–response using DENV-2 strain D2S10 resulted in similar outcomes. Inoculation of 106.0, 107.0 and 107.5 p.f.u. D2S10 resulted in 14, 87 and 100 % mortality, respectively (Fig. 1a). Together, these results show that TVP-376 leads to lethal disease in AG129 mice with mortality similar to D2S10, and that both viruses have an LD50 of 106.5 p.f.u.
Fig. 1. DENV-4 TVP-376 is lethal in AG129 mice. (a) Kaplan–Meier survival curves of AG129-mice infected with 106.0, 107.0 or 107.5 p.f.u. D2S10 or TVP-376. #, Mouse developed paralysis. (b) Mean percentage weight lost ± se relative to animal weight of TVP-376-infected mice (107.0 p.f.u.: ANOVA P < 0.0001; Dunnett's post-test, significant at 3–6 days p.i.). (c) Viraemia titres (mean ± se) of mice infected with 106.0 and 107.0 p.f.u. TVP-376.
Lethal TVP-376 infection of AG129 mice is characterized by tissue viral loads
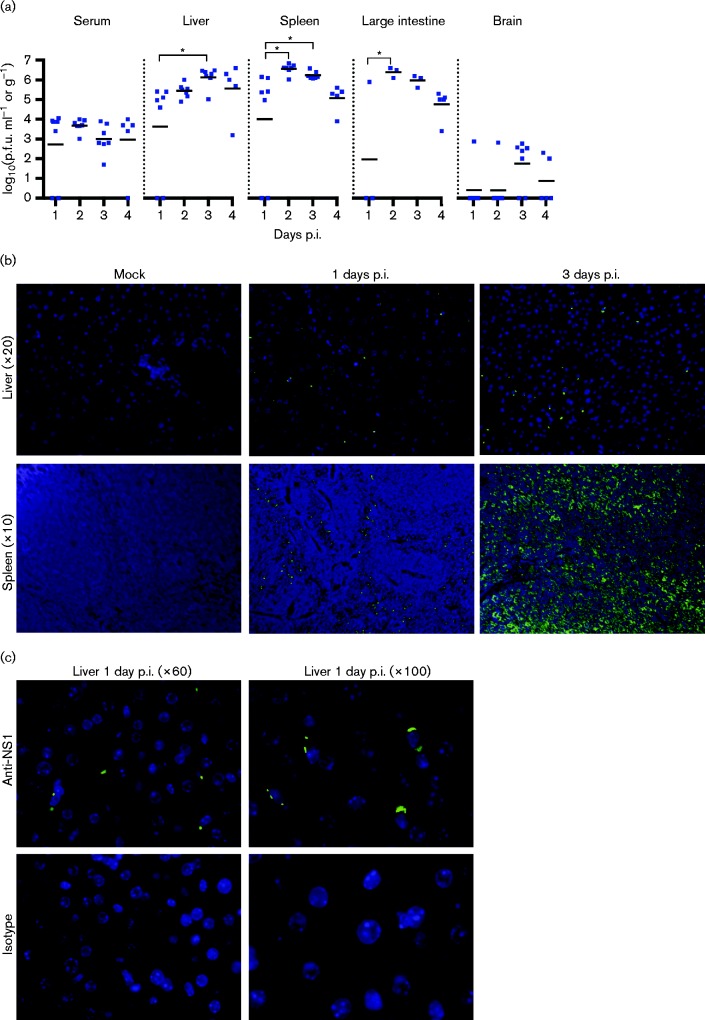
To characterize lethal infection by 107.0 p.f.u. DENV-4 strain TVP-376, groups of 3–5 animals were sacrificed on each day (1, 2, 3 and 4 days p.i.), and viral loads were assessed in serum, liver, spleen, large intestine and brain. At 1 day p.i., 71 % of the animals had infectious virus in the serum and visceral organs; this rose to 100 % of animals at 2, 3 and 4 days p.i. (Fig. 2a). The liver, spleen and large intestine contained high infectivity titres 2–4 days p.i. (mean 105–106.5 p.f.u. g− 1). In contrast, virus was detected in the brain of only 14 % of animals (1/7) at 1 and 2 days p.i., increasing to 71 % (5/7) on day 3, and falling to 40 % (2/5) on day 4. Brain infectivity was consistently lower than in other tissues.
Fig. 2. Lethal TVP-376 infection in AG129 mice is characterized by high viral loads in tissues. (a) Infectivity titres of serum and tissue samples from AG129 mice infected with 107.0 p.f.u. Data represent individual titres (symbols), mean daily titre (lines), and statistical significance (asterisks) analysed using ANOVA with Tukey's post-test. Results are combined from two separate studies: serum, liver, spleen and brain, n = 7 for days 1–3 and n = 5 for day 4; large intestine, n = 3 for days 1–3, and n = 5 for day 4. (b) Detection of DENV NS1 protein in liver (upper panels) and spleen (lower panels) sections of AG129 mice infected with 107.0 p.f.u. TVP-376; nuclei, blue; NS1, green; liver and spleen sections shown at × 20 and × 10 magnification, respectively. (c) Liver sections from 1 day p.i. immunostained with anti-NS1 (top panels) or isotype (bottom panels) at × 60 and × 100.
TVP-376 antigen was detected by fluorescence immunohistochemistry. Immunostaining of liver and spleen sections with anti-NS1 indicated that TVP-376 was present and replicating in infected tissues throughout the course of infection (days 1 and 3 shown; Fig. 2b, c). In the spleen, the viral antigen appeared in cells at the margins of the follicles at 1 day.p.i., and the immunoreactive cells were diffusely distributed by 3 days p.i. (i.e. they were not localized to a structurally delineated area of the spleen). No virus antigen was detected in mock-infected controls. Together, these data indicate that TVP-376-infected AG129 mice develop systemic infection.
AG129 mice infected with a lethal dose of TVP-376 develop leukopenia but not thrombocytopenia
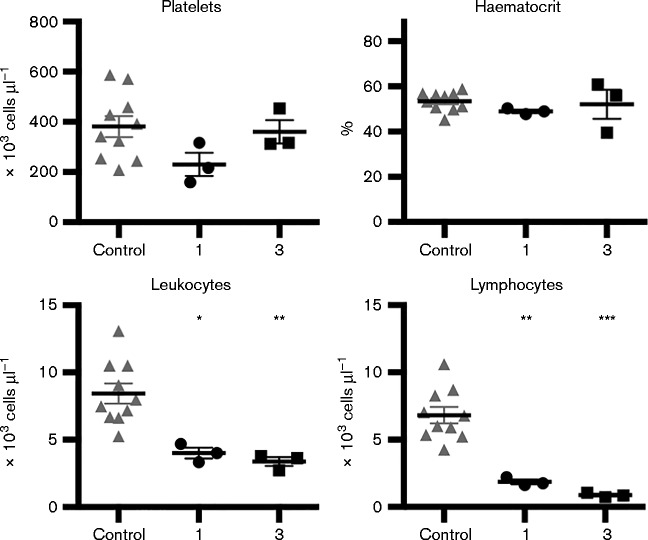
Blood samples from mock-infected (n = 10) or TVP-376-infected AG129 mice (107.0 p.f.u.) were collected at 1 (n = 3) and 3 days p.i. (n = 3). Compared with controls, TVP-376 infection resulted in leukopenia as early as 1 day p.i. (P < 0.01), including decreased lymphocyte counts (P < 0.001), which continued throughout infection (Fig. 3). Significantly, no changes were observed in platelet numbers or in the haematocrit percentage. These results indicate that lethal TVP-376 infection of AG129 mice includes some signs of human disease but no significant thrombocytopenia.
Fig. 3. TVP-376-infected AG129 mice exhibit leukopenia but not thrombocytopenia. Blood counts of naive (n = 10) or 107.0 p.f.u.-TVP-376-infected samples harvested at 1 and 3 days p.i. (n = 3 per group); platelet, leukocyte, and lymphocyte numbers and haematocrit percentage. Data represent individual values (symbols), mean ± se (lines), and statistical significance (asterisks) analysed using ANOVA with Dunnett's post-test.
Lethal TVP-376 infection causes histopathology in the spleen and liver of AG129 mice
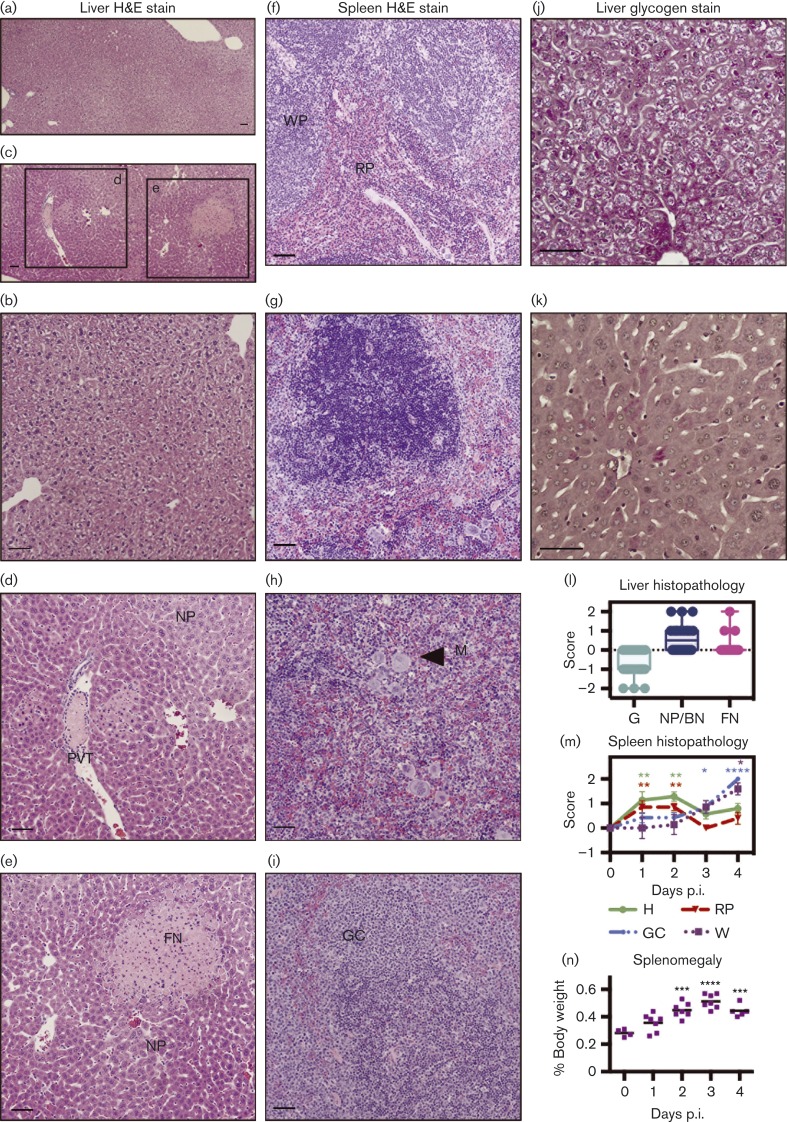
Histological examination was undertaken to further investigate the course of infection. Tissues were harvested after TVP-376 infection (at 1–4 days p.i.), and histopathological changes were observed in the liver and spleen (Fig. 4a–i), but not in large intestine or brain tissues (data not shown). Liver haematoxylin and eosin (H&E) sections were graded, and the plotted scores indicate the presence of tissue damage and cellular activation (Fig. 4l). Representative panels show increased focal necrosis, decreased glycogen content, and significant nuclear pleomorphism or binucleation of hepatocytes (Fig. 4c–e) (P < 0.05). Portal venule thrombosis was also observed (Fig. 4c, d). Additional liver sections were stained with periodic acid–Schiff stain (PAS) to confirm that glycogen stores were depleted in TVP-376-infected hepatocytes (Fig. 4j, k) (P < 0.0001) (scores not shown). Also, kinetic histological changes were detected in spleen H&E sections. Initially (1–2 days p.i.), the haematopoietic cell content and red pulp areas increased (P < 0.01), but they declined at 3 and 4 days p.i. (Fig. 4g–i, m). This change was countered with an increase in the white pulp and germinal centre cell transformation of the white pulp; both showed a steady, time-dependent increase and peaked at 4 days p.i. (P < 0.05 and P < 0.0001, respectively). Furthermore, infection led to a statistically significant (P < 0.001) increase in spleen weight by 2 days p.i., from 0.28 to 0.45 % of body weight, peaking at 3 days p.i. at 0.55 % (Fig. 4n), resulting in splenomegaly, which may be caused by the proliferative activation and cellular expansion occurring in the organ during TVP-376 infection.
Fig. 4. Histology of 107.0 p.f.u.-TVP-376-infected AG129 mice shows damage to liver and splenomegaly due to lymphoid hyperplasia. H&E stains of tissue sections harvested at 1–4 days p.i. from TVP-376-infected AG129 mice (n = 26) were compared and scored relative to those from naive and mock-infected mice (n = 8). Panels are representatives from two independent studies. Liver sections from mock-infected (a, b) and TVP-376-infected 1 day p.i. samples (c–e) were evaluated for glycogen content (G), nuclear pleomorphism (NP), and binucleation (BN), focal necrosis (FN) and portal venule thrombosis (PVT). Magnification: × 4 (a, c); × 10 (b, d, e). Spleen section H&E stains from mock-infected (f), 1 (g), 2 (h) or 3 days p.i. (i) Samples show white pulp (WP) and red pulp (RP) content of the spleen and megakaryocytes (M), indicators of haematopoietic cell (H) activity and germinal center cell transformation (GC). PAS-stained liver sections from a mock-infected (j) and TVP-376-infected (k) sample show depletion of glycogen content (absence of intense pink staining). (l, m) Graphical representations of semiquantitative scores of H&E-stained sections from a total of 26 infected mice. (l) Box and whisker plot of semiquantitative scores of liver sections. (m) Mean scores ± se of spleen sections. Significance was determined using ANOVA and Dunnett's post-test to compare scores from day 0 with days 1–4 and are denoted in green, red, blue and purple asterisks for H, RP, GC and WP, respectively. Spleen weights at necropsy were plotted, and horizontal lines represent the mean percentage of total body weight; significance analysed using ANOVA and Dunnett's post-test. Scale bars, 50 μm.
AG129 mice infected with TVP-376 develop increasing circulating cytokine levels until death
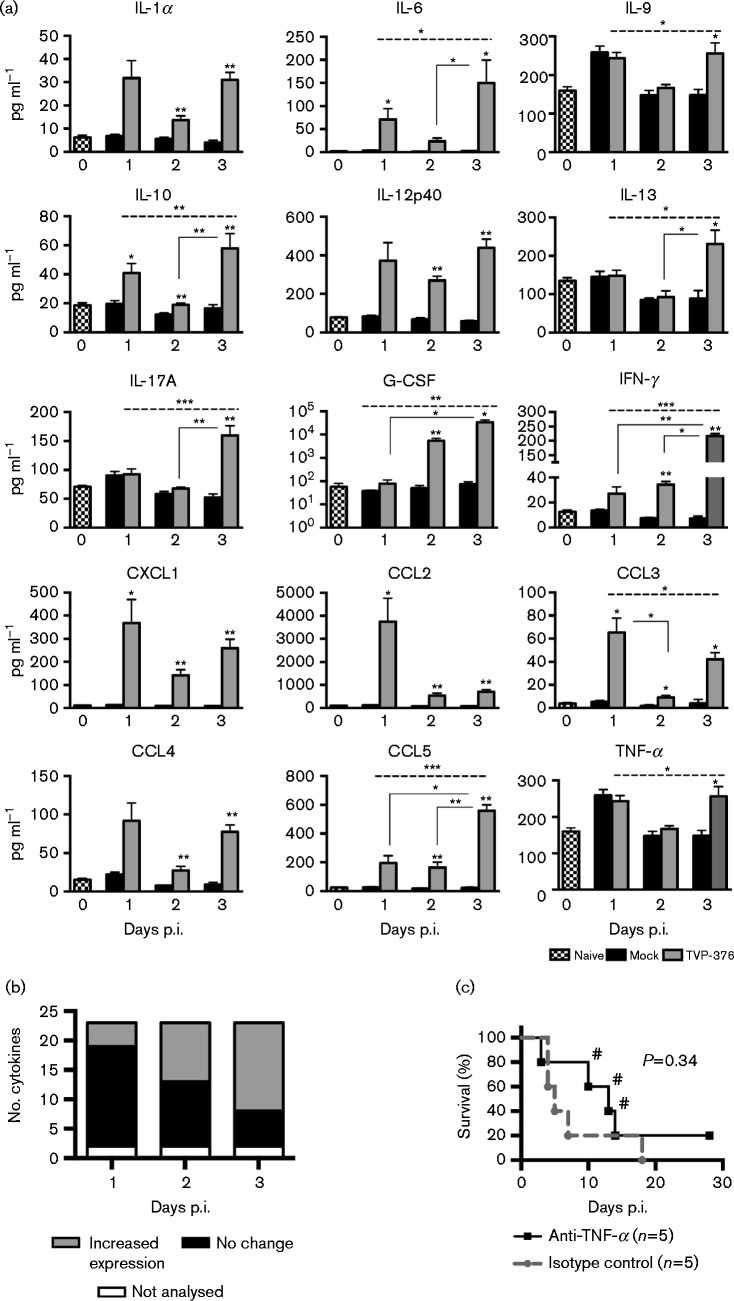
In order to evaluate the innate immune response to TVP-376 infection, sera from AG129 mice infected with 107.0 p.f.u. were analysed by Bioplex analysis (23-plex) (Fig. 5a). Compared with mock-infected controls, levels of 15 cytokines were significantly increased, while levels of six were comparable to controls (IL-1β, IL-2, IL-3, IL-4, IL-5 and IL-12p70; data not shown); Eotaxin and granulocyte–monocyte-colony-stimulating factor were excluded from the analysis because their values were outside the detectable range for the majority of the mock- and virus-infected animals. Chemokine (C-X-C motif) ligand 1 (CXCL1), chemokine (c-c motif) ligand 2 (CCL2), CCL3, CCL4 and CCL5 were all elevated during infection. Inflammatory cytokines IL-1α, IL-6, IL-12p40, IL-17A, IFN-γ and TNF-α were elevated, although the latter only showed significant elevation at 3 days p.i. Changes in serum levels of cytokines involved in cell growth, proliferation, and differentiation were also detected (IL-9 and granulocyte-colony-stimulating factor). Anti-inflammatory cytokines were also elevated: IL-10 on all days, and IL-13 later during infection. Thus, the lethal TVP-376 AG129 model leads to increased levels of cytokines that have been identified as markers for human dengue infection, including IL-6, CXCL1 and IFN-γ (Srikiatkhachorn & Green, 2010; Bozza et al., 2008; Rothman, 2011). The kinetics of the cytokine response to TVP-376 showed that at 1, 2 and 3 days p.i. the number of elevated cytokines increased from 5 to 10 to 15, respectively, indicating that the mechanism of TVP-376 infection involves overwhelming cytokine activation and release (Fig. 5b).
Fig. 5. TVP-376 infection in AG129 mice leads to kinetic cytokine responses but not to TNF-α-mediated lethality. (a) Bioplex was performed on sera harvested from naive (n = 8), mock-infected 1–3 days p.i. (n = 5), and 107 p.f.u. TVP-376-infected 1–3 days p.i. (n = 7) AG129 mice from two separate experiments. Bars represent means ± se. Cytokine levels from virus-infected animals were compared with same-day-matched mock-infected controls using the Mann–Whitney U-test (asterisks above individual bars). Values of infected samples for 1–3 days p.i. were compared using Kruskal–Wallis ANOVA (dashed line spanning the top of the graph) and multiple comparisons (solid lines connecting two bars). (b) Bioplex results were sorted by number of cytokines that were significantly elevated, unchanged, or not analysed, then plotted by day p.i. (c) Kaplan–Meier survival curves of AG129 mice infected with 107 p.f.u. TVP-376 and then administered either functional grade anti-TNF-α (n = 5) or isotype control (n = 5) for 3 days. #, Mouse developed paralysis.
TNF-α contributes to lethal infection in humans and in AG129 mouse models of DENV-2 (Srikiatkhachorn & Green, 2010; Atrasheuskaya et al., 2003; Shresta et al., 2006; Sarathy et al., 2015). Administration of anti-TNF-α antibody or isotype control (groups of five mice) into AG129 mice infected with TVP-376 resulted in 20 and 0 % survival, respectively; although the treatment increased the median survival time from 5 to 13 days, it was not statistically significant (P = 0.3383) (Fig. 5c). Furthermore, anti-TNF-α-treated animals that succumbed to infection after 10 days p.i. (n = 3) developed paralysis.
Mavs −/− mice are resistant to both D2S10 and TVP-376 infection
In order to determine if DENV-4 strain TVP-376 uses a similar mechanism to DENV-2 for mouse mortality, several comparisons were performed. The role of MAVS in DENV-2 infection has been examined using 1012 genomic equivalents of S221, a strain related to D2S10, but not in DENV-4 infection (Perry et al., 2009). Therefore, 107.5 p.f.u. D2S10 or TVP-376 was inoculated into 8-week-old WT and Mavs − / − mice. Neither virus induced mortality or weight loss in WT or Mavs − / − mice, and examination of viraemia indicated that WT mice were not infected at 3 days p.i. (Fig. 6a–c). A single D2S10-infected Mavs − / − mouse had detectable viraemia; whereas the majority of TVP-376-infected Mavs − / − mice had viraemia of approximately 102 p.f.u. ml− 1. Lastly, neutralization assays of sera harvested at 30 days p.i. indicate that both WT and Mavs − / − mice generate significantly higher neutralizing antibody titres to D2S10 [1272, 95 % confidence interval (CI) 1021–1585; 1255, 95 % CI 1154–1366, respectively] than to TVP-376 (321, 95 % CI 247–417; 898, 95 % CI 760–1063, respectively) (P < 0.0001). These results show that loss of MAVS signalling is not a major contributor to murine lethality by either D2S10 or TVP-376 because the animals showed no weight loss, had low viraemia at 3 days p.i. and survived infection.
Fig. 6. WT and Mavs − / − mice are resistant to D2S10 and TVP-376. (a) Kaplan–Meier survival curves of WT and Mavs − / − mice infected with 107.5 p.f.u. D2S10 or TVP-376. (b) Mean percentage weight lost ± se relative to animal weight. (c) Viraemia titres (mean ± se).
Mice deficient in IFN receptors have higher susceptibility to D2S10 than to TVP-376 infection
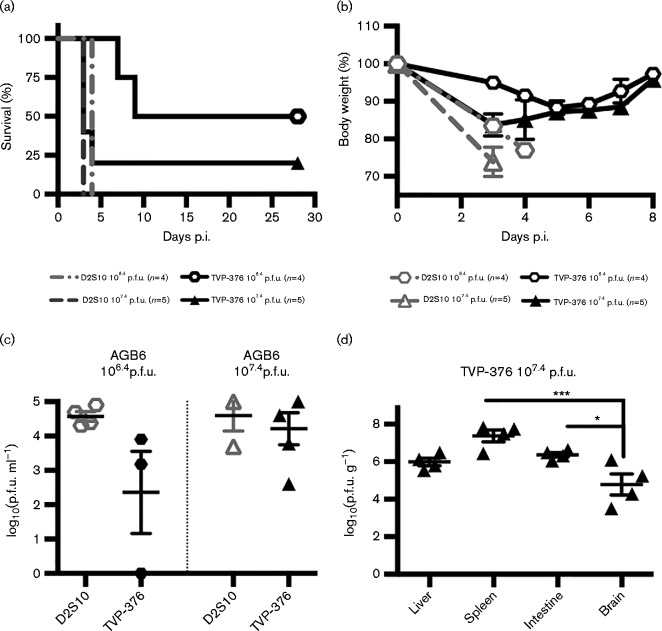
The lack of signalling from the IFN-α/βR and IFN-γR renders AG129 mice susceptible to lethal infection by DENV, including TVP-376 (Fig. 1a). Because both the 129 and B6 mouse backgrounds are frequently used in DENV-2 susceptibility studies, a comparison of AGB6 mice infected with D2S10 or TVP-376 was performed. AGB6 mice were highly susceptible to 107.4 p.f.u. of each virus, with a median survival of 3.0 days (Fig. 7a). Infection with a lower inoculum of 106.4 p.f.u. resulted in 100 and 50 % mortality and median survival times of 4.0 and 18.5 days (P = 0.0082, log-rank test) for D2S10 and TVP-376, respectively. All AGB6 mouse groups had significant weight loss starting at 3–4 days p.i. (Fig. 7b). In addition, the 107.4 p.f.u.-TVP-376-infected mice exhibited hunched posture and lethargy, while the 107.4 p.f.u.-D2S10-infected mice demonstrated more severe signs of disease, including hunched posture, diarrhoea and minimal mobility. The AGB6 mice that survived TVP-376 infection showed clinical signs of illness at 4–9 days p.i. but recovered, and had a mean terminal neutralizing antibody titre of 913 (95 % CI 727–1148; data not shown). All groups were viraemic at 3 days p.i., and the mean titres of D2S10-infected mice were slightly higher than those of TVP-376-infected: 106.4 p.f.u.-infected, 104.6 and 102.4 p.f.u. ml− 1, respectively; 107.4 p.f.u.-infected, 104.6 and 104.2 p.f.u. ml− 1, respectively (Fig. 7c). Lastly, the systemic TVP-376 viral burden of moribund AGB6 mice infected with 107.4 p.f.u. was evaluated (Fig. 7d). The liver, spleen, intestine and brain had viral loads of 106.0, 107.4, 106.4 and 104.8 p.f.u. g− 1, respectively. The titres in the intestine and spleen were significantly higher (P < 0.001, ANOVA) than in the brain, and no neurological clinical signs were observed. Taken together, these results show that AGB6 mice are highly sensitive to infection by both D2S10 and TVP-376, and this direct comparison shows that D2S10 kills 100 % of mice at a lower inoculum than TVP-376; therefore, it is more virulent than TVP-376, with respective LD50 values of 105.9 and 106.6 p.f.u.
Fig. 7. AGB6 mice are susceptible to D2S10 and TVP-376 infection. (a) Kaplan–Meier survival curves of AGB6 mice infected with 106.4 or 107.4 p.f.u. D2S10 or TVP-376. (b) Mean percentage weight lost ± se relative to animal weight. ANOVA and Dunnett's post-test: 106.4 p.f.u. D2S10, P < 0.0001 (significant at 3 and 4 days p.i.); 106.4 p.f.u. TVP-376, P < 0.0001 (significant at 4–6 days p.i.); 107.4 p.f.u. TVP-376, P < 0.01 (significant at 3 and 4 days p.i.); t-test of 107.4 p.f.u. D2S10 between 0 and 3 days p.i., P < 0.0001. (c) Viraemia titres of AGB6 mice assessed at 3 days p.i. (d) Infectivity titres of tissues harvested from AGB6 mice infected with 107.4 p.f.u. TVP-376 at moribund state (3–4 days p.i.) (n = 4). Data represent individual titres (symbols), mean daily titre (lines), and statistical significance (asterisks) analysed using ANOVA with Tukey's post-test.
IFN-γR signalling is sufficient for mouse resistance to TVP-376, but resistance to D2S10 requires both IFN-α/βR and IFN-γR
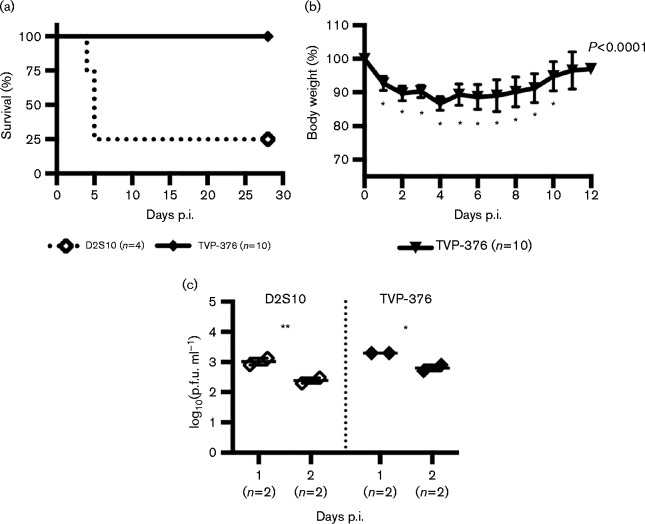
Studies with DENV-2 strains have shown that AG129 mice are more susceptible to infection than A129 mice (Orozco et al., 2012; Prestwood et al., 2012). However, no studies have been conducted with the other DENV serotypes. A129 mice were tested for susceptibility to 107.0 p.f.u. TVP-376, but none (n = 10) succumbed. In comparison, 75 % (n = 3) of A129 mice infected with D2S10 (107.4 p.f.u.) died. Thus, D2S10 but not TVP-376 is lethal in A129 mice (Fig. 8a) (log-rank test P = 0.0020). Notwithstanding, TVP-376-infected A129 mice lost significant weight by 1 day p.i. (P < 0.0001), dropping to 87 % of original weight on day 4, but regained weight to normal levels by 11 days p.i. (Fig. 8b). Also, A129 mice infected with D2S10 or TVP-376 had indistinguishable viraemia on days 1 and 2 p.i. (Fig. 8c). Furthermore, 2 days p.i. viraemia of AG129 (103.4 p.f.u.) and A129 (102.8 p.f.u.) infected with TVP-376 trended towards a difference but was not statistically significant (t-test P = 0.0794). Therefore, inoculation of D2S10 or TVP-376 into either A129 or AG129 mice results in virus infection, but only D2S10 leads to mortality in A129 mice.
Fig. 8. TVP-376 infects A129 mice but is not lethal. (a) Kaplan–Meier survival curves of A129 mice infected with 107.4 p.f.u. D2S10 or 107.0 p.f.u. TVP-376.(b) Mean percentage weight lost ± se relative to animal weight (ANOVA P < 0.0001; Dunnett's post-test, significant at 2–10 days p.i.). (c) Viraemia titres of mice infected with 107.4 p.f.u. D2S10 or 107.0 p.f.u. TVP-376; statistical significance (asterisks) between 1 and 2 days p.i. analysed using t-test).
Discussion
Anti-dengue therapies and vaccines are needed to address the growing global health threat. One obstacle has been the lack of suitable animal models to test DENV infection challenge (Zompi & Harris, 2012; Yauch & Shresta, 2008). Recent studies with DENV-2 models have made major contributions to this field (Mota & Rico-Hesse, 2009; Tan et al., 2010; Shresta et al., 2006). However, all four DENVs have been implicated in worldwide disease, and limited information is available that distinguishes murine models of different DENVs.
The lethal DENV-2 D2Y98P and D2S10, DENV-3 C0360/94 and DENV-4 703-4 mouse models have been established and characterized in AG129 mice; therefore, the AG129 model was chosen to further dissect the mechanism of TVP-376 virulence. Similarly to the above-mentioned DENV mouse models, TVP-376 infection leads to high viral loads and replicates in several tissues (Fig. 3). However, unlike D2S10 and C0360/94, TVP-376 infection did not cause thrombocytopenia (Fig. 3).
Histopathological changes occurred during TVP-376 infection. Liver sections contained hepatocytes with nuclear pleomorphism and areas of necrosis, similar to the D2Y98P, C0360/94 and 703-4 models (Tan et al., 2010; Sarathy et al., 2015; Milligan et al., 2015). Also, TVP-376 infection destroyed the splenic architecture and led to splenomegaly, highly proliferative cellular activation, and white pulp expansion (Fig. 4). To this end, the presence of NS1 in spleen sections at 1 day p.i. was restricted to the areas surrounding the follicles, but at 3 days p.i. the staining pattern was distributed throughout the organ (Fig. 2b). This splenic phenotype is similar to but more severe than that caused by C0360/94 and D2S10 infections, and resembles DENV-2 D2Y98P and 703-4 infection (Sarathy et al., 2015; Shresta et al., 2006; Tan et al., 2010; Milligan et al., 2015).
Several cytokines previously identified as markers of dengue infection in human patients and other models of infection were significantly elevated in TVP-376-infected AG129 sera, including IL-6, IL-8, IL-10, IFN-γ and TNF-α (Fig. 5a) (Srikiatkhachorn & Green, 2010; Bozza et al., 2008; Rothman, 2011). Also, the number of elevated cytokines increased throughout the course of infection (Fig. 5b). One difference among AG129 models is the kinetics of TNF-α induction. D2Y98P, D2S10, 703-4 and TVP-376 cause a time-dependent increase of TNF-α, but C0360/94 induces high levels of TNF-α beginning at 1 day p.i. (Tan et al., 2010; Shresta et al., 2006; Milligan et al., 2015; Sarathy et al., 2015). Neutralization of TNF-α in TVP-376-infected mice increased the median survival time from 5 to 13 days p.i. but the increase was not statistically significant (Fig. 6c). Similarly, anti-TNF-α delayed D2S10 mortality (from 4 to 10 days p.i., P < 0.0001) (Shresta et al., 2006), whereas it completely rescued mice from lethal C0360/94 infection (P < 0.0001) (Sarathy et al., 2015). These differences in survival and anti-TNF-α rescue could be attributed to the notion that TNF-α plays a different role in the disease mechanism depending on whether it is elevated early (1 day p.i.) or later (3–4 days p.i.) during the course of infection.
Mouse-passaged TVP-376 has been used at low doses of 104–105 p.f.u. to infect AG129 mice, but infections resulted in an extended mean time to death and neurological disease (Sukupolvi-Petty et al., 2013). The present study represents, to the best of our knowledge, the first detailed characterization of a non-mouse-adapted TVP-376 model in AG129 mice, as the infecting virus was only amplified three times in mosquito cells to obtain working stocks and to avoid mammalian adaptation. Notably, unlike in Sukupolvi-Petty et al. (2013), AG129 mice infected with a lower dose (106.0 p.f.u.) did not develop neurological disease up to 28 days p.i. Furthermore, none of the lethal-dose-infected AG129 mice sampled at 1–4 days p.i. exhibited any brain pathology. The few mice that had detectable infectivity in the brain had lower titres than detected in other organs, suggesting that virus detected in the brain may be from the viraemia. In the present study, lethal-dose-infected AG129 mice with delayed death or prolonged survival developed paralysis (Figs 1a and 5c). This phenomenon was restricted to AG129 mice; none of the surviving AGB6 mice developed paralysis. These differences suggest that delayed infection with non-adapted TVP-376 may ultimately lead to neurovirulence in AG129, an observation that has also been made in D2S10-infected AG129 mice, but not in AGB6. These results are similar to those observed previously for D2S10 but not for C0360/94.
Decades of experiments using immunocompetent mice to establish DENV infection have shown that these mice are generally resistant to lethal infection. This can be explained at least in part by differences between the mouse and the human immune response to DENV (Green et al., 2014; Zellweger & Shresta, 2014; Ashour et al., 2010; Aguirre et al., 2012). In the present study, WT mice inoculated with D2S10 or TVP-376 survived infection, showed no clinical signs of disease, and had no detectable viraemia.
MAVS has been shown to play a role in IFN induction in the hours after infection with DENV-2 S221; however, other factors control IFN signalling at later times, rendering Mavs − / − mice resistant to lethal infection (Perry et al., 2009). In the present study, DENV-2 D2S10 and DENV-4 TVP-376 were inoculated into Mavs − / − mice in order to compare their virulence. All animals survived infection, but more TVP-376-infected than D2S10-infected mice were viraemic at 3 days p.i. (Fig. 6a–c). This suggests that the kinetics of infection by the two viruses may differ in Mavs − / − mice; however, detailed studies would be required to determine whether this is a virus strain-specific or a virus serotype-specific phenomenon.
Infection of C57BL/6 mice deficient in both IFN-α/βR and IFN-γR (AGB6) by D2S10 and TVP-376 resulted in the expected results of viral infectivity and death. Notably, mice infected with D2S10 suffered more severe disease, accompanied by faster and higher mortality, leading to a slightly lower LD50 (105.9 p.f.u) compared with TVP-376 (106.6 p.f.u). Hence, D2S10 is fivefold more virulent than TVP-376 in AGB6 mice, whereas the LD50 for D2S10 and TVP-376 in AG129 mice was the same (106.5 p.f.u.). Therefore, these data suggest that there are genetic differences between the two mouse strains that affect DENV infection.
Detailed studies showed that IFN-γR-mediated responses protect mice from paralysis resulting from DENV-2 infection (Shresta et al., 2004; Prestwood et al., 2012) Previously, AG129-virulent DENV-3 and DENV-4 strains had not been evaluated in the absence of the IFN-α/βR; so in the current report, a comparison of D2S10 and TVP-376 in A129 mice was performed. As expected, D2S10 causes lethal disease in both AG129 and A129 mice but is more virulent in AG129 mice. TVP-376 is lethal in AG129 mice only (Fig. 1a, 8a). Despite the mortality differences, weight loss data show that TVP-376 infection affects A129 mice similarly to AG129 mice, but clinical infection in A129 is reversible because the lost weight is ultimately regained. No differences were detected between D2S10 and TVP-376 viraemia levels in A129 mice or between TVP-376-infected AG129 and A129 mice (Fig. 8c); thus, both viruses are capable of establishing infection at a similar rate in both A129 and AG129, but the clinical outcome varies.
In conclusion, our study examines the differences in susceptibility of WT and immunocompromised mice to two serologically and genetically distinct viruses, DENV-2 D2S10 and DENV-4 TVP-376. This study provided a platform to compare virulence and infectivity of two different viruses in the same experimental setting, including mouse strain, virus strain and dose. Understanding the similarities and differences of the four DENVs in the same lethality model will provide valuable information that is needed to better evaluate candidate vaccines and antivirals, and potentially provide information on the pathogenesis of the four DENVs.
Methods
Cell culture
Monkey kidney Vero cells were maintained at 37 °C in 5 % CO2 in minimum essential medium (MEM) supplemented with l-glutamine, non-essential amino acids, penicillin/streptomycin and 8 % bovine growth serum (BGS). C6/36 mosquito cells were maintained at 28 °C in MEM supplemented with l-glutamine, non-essential amino acids, penicillin/streptomycin, sodium pyruvate, tryptose phosphate broth and 10 % FBS.
Virus
DENV-4 strain TVP-376 was amplified three times in C6/36 cells with 2 % FBS, without mouse passaging or adaptation. Generation of DENV-2 D2S10 has been described previously (Shresta et al., 2006). Viruses were harvested and concentrated using a 50 kDa molecular weight cut-off Amicon filter at 1500 × g and 4 °C, for 20 min. Virus stocks were quantified by plaque titration assays in Vero cells. Briefly, cells were infected with 10-fold virus dilutions for 30 min before overlay with MEM containing 2 % BGS/1 % agar and incubated for 4 days at 37 °C. Plaques were counted 2 days after the second overlay with MEM agar containing 2 % neutral red, and titres are expressed as p.f.u. ml− 1. For some experimental titrations, focus formation assays were performed with overlay of 0.8 % carboxymethyl cellulose; immunostaining was performed with the pan-mosquito-borne flavivirus mAb 4G2. Extensive comparisons yielded equal results with plaque and focus assays.
Mouse infection
WT C57BL/6 mice (WT), C57BL/6 mice deficient in mitochondrial antiviral signalling protein (Mavs − / − ), C57BL/6 mice deficient in IFN-α/β and IFN-γ receptors (AGB6), 129/Sv mice deficient in IFN-α/β receptor (A129), or 129/Sv mice deficient in IFN-α/β and IFN-γ receptors (AG129) were bred and maintained at the University of Texas Medical Branch (UTMB). Experiments were approved by the UTMB Institutional Animal Care and Use Committee and performed according to institutional guidelines. Infections were performed in 6–8-week old mice via the intraperitoneal route. Mice were weighed and visually monitored; mice exhibiting signs of severe disease, weight loss below 80 % of original body weight, or neurological dysfunction were euthanized. Animals surviving infection were monitored for at least 4 weeks post-infection. Lethal TVP-376 infection of AG129 mice was characterized in mice inoculated with 107 p.f.u. in two separate experiments. At 1, 2, 3 and 4 days p.i., a group of three to five mice was sacrificed for downstream analysis, including determination of viral loads, tissue sectioning, blood counts, and cytokine analysis.
Mouse necropsy
Moribund AGB6 mice inoculated with 107.4 p.f.u. TVP-376 were sacrificed at 3–4 days p.i., and AG129 mice inoculated with 107.0 p.f.u. were sacrificed at 1–4 days p.i. Blood was collected by cardiac puncture; liver, spleen, intestine and brain samples were collected into pre-weighed tubes and homogenized; and virus was titrated. Mean limits of detection were as follows: serum 101.7 p.f.u., liver 102.2 p.f.u., spleen and large intestine 102.7 p.f.u, and brain 102.3 p.f.u.
Fluorescence immunohistochemistry
Paraffin-embedded sections from 107.0 p.f.u.-TVP-376-infected AG129 mice were immunostained for non-structural protein 1 (NS1) using rabbit polyclonal anti-DENV-2 NS1 (Genetex GTX124280) diluted 1 : 100, followed by detection with goat anti-rabbit IgG F(ab’)2–Alexa Fluor 488 (Molecular Probes) diluted 1 : 200, and mounting with Vectashield mounting medium containing DAPI (Vector Laboratories). As controls, sections from mock-infected animals were stained with anti-NS1, and infected sections were stained with rabbit IgG.
Complete blood count
Blood from AG129 mice inoculated with medium or infected with 107.0 p.f.u. TVP-376 was collected into EDTA anticoagulant tubes and analysed for blood counts using a Hemavet 950TS (Drew Scientific).
Histology
At the time of necropsy, liver, spleen, intestine and brain samples were harvested and immediately fixed in 10 % neutral-buffered formalin. Tissues were processed, and sections were stained with H&E at the UTMB Research Histopathology Core Laboratory. Slides from TVP-376-infected mouse tissues were analysed for histopathological changes, and spleen and liver slides were semi-quantitatively scored from − 2 to +2 relative to mock-infected controls, which were assigned a score of 0. The parameters examined and histological grading have been previously described and are listed in the legend of Fig. 4 (Sarathy et al., 2015). Liver sections were also stained with PAS to visualize the hepatocyte glycogen content (intense pink staining).
Cytokine analysis
Bio-Plex Pro Mouse Cytokine 23-plex (Bio-Rad) was used to test cytokine levels in 15 μl serum according to the manufacturer's instructions. Mice were infected with 107.0 p.f.u. TVP-376, and sera collected from AG129 mice 1–3 days p.i. were examined and compared with same-day-matched mock-infected samples.
In vivo TNF-α neutralization
AG129 mice were inoculated with 107.0 p.f.u. TVP-376 (n = 10). At 1, 2 and 3 days p.i., mice were administered 100 μg of either anti-TNF-α clone MP6-XT3 (eBioscience) (n = 5) or isotype control (n = 5), as described previously (Shresta et al., 2006). Mice were monitored daily, and those exhibiting severe disease were euthanized.
Virus neutralization
Focus reduction neutralization titration (FRNT50) assays were performed on terminal bleed serum harvested 4 weeks post-infection. Twofold serial serum dilutions were incubated with 50 p.f.u. infecting virus for 1 h and then used to infect Vero cells. Titres were analysed using the log(inhibitor) versus normalized response–variable slope non-linear regression model to acquire the FRNT50 and 95 % CI.
Statistical analyses
Neutralization curves were normalized using Microsoft Excel. LD50 was calculated using the Spearman–Karber method. All graphs and statistical analyses were determined using Graphpad Prism v.6.0. Statistical significance is depicted in the figures as follows: * * * *, P < 0.0001; * * *, P < 0.001; * *, P < 0.01; *, P < 0.05.
Acknowledgements
We thank Jeanon Smith for technical assistance. We thank Dr Michael Gale (University of Washington School of Medicine) for providing Mavs − / − mice. This work was supported in part by NIAID contract N01 AI 30065 to A. D. T. B. and N. B. V. V. S. was supported by NIAID T32 postdoctoral fellowship AI 7536-13.
References
- Aguirre S., Maestre A.M., Pagni S., Patel J.R., Savage T., Gutman D., Maringer K., Bernal-Rubio D., Shabman R.S., other authors (2012). DENV inhibits type I IFN production in infected cells by cleaving human STING PLoS Pathog 8 e1002934 10.1371/journal.ppat.1002934 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour J., Morrison J., Laurent-Rolle M., Belicha-Villanueva A., Plumlee C.R., Bernal-Rubio D., Williams K.L., Harris E., Fernandez-Sesma A., other authors (2010). Mouse STAT2 restricts early dengue virus replication Cell Host Microbe 8 410–421 10.1016/j.chom.2010.10.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atrasheuskaya A., Petzelbauer P., Fredeking T.M., Ignatyev G. (2003). Anti-TNF antibody treatment reduces mortality in experimental dengue virus infection FEMS Immunol Med Microbiol 35 33–42 10.1111/j.1574-695X.2003.tb00646.x . [DOI] [PubMed] [Google Scholar]
- Balsitis S.J., Williams K.L., Lachica R., Flores D., Kyle J.L., Mehlhop E., Johnson S., Diamond M.S., Beatty P.R., Harris E. (2010). Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification PLoS Pathog 6 e1000790 10.1371/journal.ppat.1000790 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Drake J.M., Brownstein J.S., Hoen A.G., other authors (2013). The global distribution and burden of dengue Nature 496 504–507 10.1038/nature12060 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza F.A., Cruz O.G., Zagne S.M.O., Azeredo E.L., Nogueira R.M.R., Assis E.F., Bozza P.T., Kubelka C.F. (2008). Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity BMC Infect Dis 8 86 10.1186/1471-2334-8-86 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.-W., King K., Tu J., Sanchez M., Luster A.D., Shresta S. (2013). The roles of IRF-3 and IRF-7 in innate antiviral immunity against dengue virus J Immunol 191 4194–4201 10.4049/jimmunol.1300799 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J., Chu H., O'Day P., Pyles R., Bourne N., Das S.C., Milligan G.N., Barrett A.D., Partidos C.D., Osorio J.E. (2014). Investigating the efficacy of monovalent and tetravalent dengue vaccine formulations against DENV-4 challenge in AG129 mice Vaccine 32 6537–6543 10.1016/j.vaccine.2014.08.087 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A.M., Beatty P.R., Hadjilaou A., Harris E. (2014). Innate immunity to dengue virus infection and subversion of antiviral responses J Mol Biol 426 1148–1160 10.1016/j.jmb.2013.11.023 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.J., Roehrig J.T. (1999). New mouse model for dengue virus vaccine testing J Virol 73 783–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina J.P., Brady O.J., Scott T.W., Zou C., Pigott D.M., Duda K.A., Bhatt S., Katzelnick L., Howes R.E., other authors (2014). Global spread of dengue virus types: mapping the 70 year history Trends Microbiol 22 138–146 10.1016/j.tim.2013.12.011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G.N., Sarathy V.V., Infante E., Li L., Campbell G.A., Beatty P.R., Harris E., Barrett A.D., Bourne N. (2015). A dengue virus type 4 model of disseminated lethal infection in AG129 mice PLoS One 10 e0125476 10.1371/journal.pone.0125476 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota J., Rico-Hesse R. (2009). Humanized mice show clinical signs of dengue fever according to infecting virus genotype J Virol 83 8638–8645 10.1128/JVI.00581-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco S., Schmid M.A., Parameswaran P., Lachica R., Henn M.R., Beatty R., Harris E. (2012). Characterization of a model of lethal dengue virus 2 infection in C57BL/6 mice deficient in the alpha/beta interferon receptor J Gen Virol 93 2152–2157 10.1099/vir.0.045088-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S.T., Prestwood T.R., Lada S.M., Benedict C.A., Shresta S. (2009). Cardif-mediated signaling controls the initial innate response to dengue virus in vivo J Virol 83 8276–8281 10.1128/JVI.00365-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S.T., Buck M.D., Lada S.M., Schindler C., Shresta S. (2011). STAT2 mediates innate immunity to dengue virus in the absence of STAT1 via the type I interferon receptor PLoS Pathog 7 e1001297 10.1371/journal.ppat.1001297 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwood T.R., Morar M.M., Zellweger R.M., Miller R., May M.M., Yauch L.E., Lada S.M., Shresta S. (2012). Gamma interferon (IFN-γ) receptor restricts systemic dengue virus replication and prevents paralysis in IFN-α/β receptor-deficient mice J Virol 86 12561–12570 10.1128/JVI.06743-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman A.L. (2011). Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms Nat Rev Immunol 11 532–543 10.1038/nri3014 . [DOI] [PubMed] [Google Scholar]
- Sarathy V.V., White M., Li L., Gorder S.R., Pyles R.B., Campbell G.A., Milligan G.N., Bourne N., Barrett A.D.T. (2015). A lethal murine infection model for dengue virus 3 in AG129 mice deficient in type I and II interferon receptors leads to systemic disease J Virol 89 1254–1266 10.1128/JVI.01320-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shresta S., Kyle J.L., Snider H.M., Basavapatna M., Beatty P.R., Harris E. (2004). Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical J Virol 78 2701–2710 10.1128/JVI.78.6.2701-2710.2004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shresta S., Sharar K.L., Prigozhin D.M., Beatty P.R., Harris E. (2006). Murine model for dengue virus-induced lethal disease with increased vascular permeability J Virol 80 10208–10217 10.1128/JVI.00062-06 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons C.P., Farrar J.J., van Vinh Chau N., Wills B. (2012). Dengue N Engl J Med 366 1423–1432 10.1056/NEJMra1110265 . [DOI] [PubMed] [Google Scholar]
- Srikiatkhachorn A., Green S. (2010). Markers of dengue disease severity Curr Top Microbiol Immunol 338 67–82 10.1007/978-3-642-02215-9_6. [DOI] [PubMed] [Google Scholar]
- Sukupolvi-Petty S., Brien J.D., Austin S.K., Shrestha B., Swayne S., Kahle K., Doranz B.J., Johnson S., Pierson T.C., other authors (2013). Functional analysis of antibodies against dengue virus type 4 reveals strain-dependent epitope exposure that impacts neutralization and protection J Virol 87 8826–8842 10.1128/JVI.01314-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G.K., Ng J.K.W., Trasti S.L., Schul W., Yip G., Alonso S. (2010). A non mouse-adapted dengue virus strain as a new model of severe dengue infection in AG129 mice PLoS Negl Trop Dis 4 e672 10.1371/journal.pntd.0000672 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch L.E., Shresta S. (2008). Mouse models of dengue virus infection and disease Antiviral Res 80 87–93 10.1016/j.antiviral.2008.06.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger R.M., Shresta S. (2014). Mouse models to study dengue virus immunology and pathogenesis Front Immunol 5 151 10.3389/fimmu.2014.00151 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger R.M., Prestwood T.R., Shresta S. (2010). Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease Cell Host Microbe 7 128–139 10.1016/j.chom.2010.01.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zompi S., Harris E. (2012). Animal models of dengue virus infection Viruses 4 62–82 10.3390/v4010062 . [DOI] [PMC free article] [PubMed] [Google Scholar]