Abstract
Genetically highly divergent picornavirus (Newt/2013/HUN, KP770140) was detected using viral metagenomics in faecal samples of free-living smooth newts (Lissotriton vulgaris). Newt picornavirus was identified by reverse transcription-polymerase chain reaction (RT-PCR) in six (25 %) of the 24 samples originating from individuals caught in two out of the six investigated natural ponds in Hungary. The first picornavirus in amphibians expands the host range of members of the Picornaviridae, and opens a new research field in picornavirus evolution in lower vertebrates. Newt picornavirus represents a novel species in a novel genus within the family Picornaviridae, provisionally named genus Ampivirus (amphibian picornavirus).
The family Picornaviridae currently consists of 46 species grouped into 26 officially recognized genera (Knowles et al., 2012; ICTV Master Species List v3, 2014) and several candidate genera. Picornaviruses are small, non-enveloped viruses with positive-sense genomic ssRNA. In general, the 7.2–9.8 kb genomes have a common pattern of organization with a single ORF, which encodes the polyprotein flanked by 5′ and 3′ UTRs, although a canine picodicistrovirus (genus Dicipivirus) with two ORFs was recently discovered (Woo et al., 2012). The viral coding region is divided into three regions: the P1 region encodes the viral capsid proteins [VP0(VP4-VP2)-VP3-VP1], whereas the P2 and P3 regions encode proteins involved in protein processing (for example, 2Apro, 3Cpro and 3CDpro) and genome replication (2B, 2Chel, 3A, 3AB, 3BVPg, 3CDpro and 3Dpol) (Racaniello, 2007). In addition, some picornaviruses encode a leader (L) protein before the P1 region.
Picornaviruses include a wide range of pathogens (Knowles et al., 2012; http://www.picornaviridae.com) and have been found in various vertebrate host species including humans and other mammals (Kapoor et al., 2008b), birds (Boros et al., 2014) and recently in fish (Fichtner et al., 2013; Barbknecht et al., 2014; Lange et al.; 2014; Phelps et al., 2014) and reptiles (Heuser et al., 2010; Ng et al., 2015). All known picornaviruses in lower vertebrates (in fish and reptiles) represent a potentially novel but presently unassigned species or genus (http://www.picornaviridae.com). However, until now, to the best of our knowledge, picornaviruses have not been detected or reported in any amphibians within the superclass Tetrapoda. This lack of knowledge not only hinders our understanding of the evolution of picornaviruses and their phylogeny, but may also have veterinary (medical) and/or conservation biological implications.
The smooth newt (Lissotriton vulgaris; formerly Triturus vulgaris) is the most widespread caudate species in Europe; it can be found throughout the continent except the far north, areas of southern France and the Iberian Peninsula (Arntzen et al., 2009). Also, this species is one of the most successful salamandrids (family Salamandridae) as it maintains stable populations across much of its range despite the current population decline of many amphibians (Arntzen et al., 2009). Smooth newts breed in a wide variety of freshwater habitats; during the aquatic phase of their life cycle, their diet includes freshwater plankton, insect larvae and small molluscs (Nöllert & Nöllert, 1992).
This study reports the detection and complete genome characterization of a picornavirus in an amphibian, the smooth newt.
Clinically healthy male smooth newts (146 in total) were collected between 20 March and 26 April 2013 from six natural ponds located in or in the vicinity of the Pilis Mountains, Hungary (Békás-tó: 47° 34′ 35" N 18° 52′ 07" E; Mélymocsár: 47° 42′ 27" N 19° 02′ 24" E; Katlan-tó: 47° 42′ 42" N 19° 02′ 40" E; Ilona-tó: 47° 42′ 48" N 19° 02′ 25" E; Paprét-felsö: 47° 44′ 22" N 19° 00′ 42" E; Kerek-tó: 47° 38′ 41" N 18° 46′ 31" E). After capture, individuals were transported to the laboratory of the Experimental Station Júlianna major of the Plant Protection Institute, Centre for Agricultural Research, Hungarian Academy of Sciences, to participate in various experiments (not reported here). Prior to the collection of faecal samples, smooth newts were kept in groups (sizes ranging from two to thirteen) in plastic containers filled with approximately 2–5 l reconstituted soft water (APHA 1985) and fed ad libitum with sludge worms (Tubifex tubifex). Twenty-four faecal samples were collected between 22 April and 29 April from the containers (as a result, one sample could include faeces from more than one individual) and stored in 1.5 ml Eppendorf tubes at − 20 °C. Permissions to capture and conduct experiments on the animals were issued by the national authority of the Middle-Danube-Valley Inspectorate for Environmental Protection, Nature Conservation and Water Management (KTF: 5192-7/2013).
A specimen pool containing nine faecal samples (minimum one sample from each pond) was subjected to viral metagenomics. Phosphate-buffered saline-diluted specimens were passed through a 0.45 μm sterile filter and centrifuged at 6000 g for 5 min. The sample was subjected to a viral metagenomic analysis using sequence independent random RT-PCR amplification of viral-particle protected nucleic acids (Victoria et al., 2009). The viral cDNA library was constructed using a ScriptSeqTM v2 RNA-Seq Library Preparation kit (Epicentre) and sequenced by a Miseq Illumina platform, as described previously (Phan et al., 2013). The sequencing reads and assembled sequence contigs were compared to the GenBank nucleotide and protein databases using blastn/blastx. From the newt faecal pool, a total of 145 viral sequence reads (singletons and contigs) were obtained (blastx cut-off E score ≤ 10− 10) after de novo assembly from 23 472 viral reads (of the 2 988 560 total reads) from viruses of family Circoviridae (n = 18 146), Nanoviridae (n = 4253), Picornaviridae (n = 267), Parvoviridae (n = 119), Microviridae (n = 31), Secoviridae (n = 25), Partitiviridae (n = 17), Retroviridae (n = 15), Dicistroviridae (n = 14), Marnaviridae (n = 9) and other (n = 29) or unclassified (N = 547) virus families. The total of 267 picornavirus sequence reads covers a partial 2Chel (37 % amino acid identity to avian encephalomyelitis virus, GenBank accession no. NP_705601.1) and 3D (33 and 36 % amino acid identities to turkey gallivirus, GenBank accession no. YP_006576515.1, and rhinovirus, GenBank accession no. CADL32138.1) genome regions of picornaviruses, but none of them assembled to a long picornavirus contig (Fig. 1).
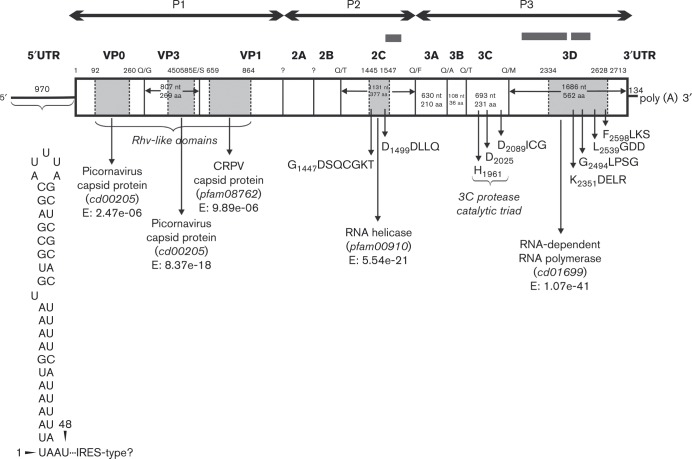
Fig. 1. Predicted genome map of the Newt/2013/HUN (KP770140) with the conserved peptide domains identified by the CDD search (light grey regions). The amino acid positions at the top of the map indicate the borders of the peptide domains. White areas show genome regions with no identifiable functions by CDD search. The first amino acid positions of the 2Chel, 3Cpro and 3Dpol conserved picornaviral amino acid motifs are indicated. P1 represents viral structural proteins and P2 and P3 represent non-structural proteins. The predicted nucleotide and amino acid length of each genome/protein region is indicated in each gene box. Above each gene box, sequence contigs acquired from next generation sequencing are indicated by dark grey bars. The secondary RNA structure of the 5′ UTR is shown below.
Specific primer-pairs were designed based on the sequence contigs from the sequencing reads, for verification of the metagenomic contigs and to determine the complete nucleotide sequence by primer walking methods using long-range and conventional RT-PCR and 5′/3′ RACE reactions through cDNA (Boros et al., 2011). PCR products were sequenced directly and run on an automated sequencer (ABI PRISM 310 Genetic Analyzer). Nucleotide and amino acid sequences of newt picornavirus (Newt/2013/HUN) were submitted to GenBank under accession number KP770140. The complete picornavirus genome, designated Newt/2013/HUN, is 9246 nt excluding the poly(A)-tail (Fig. 1). The G+C content of the entire genome is 45.1 %. The 8142 nt (2713 aa) single ORF was flanked by the 970 nt 5′ UTR and the 134 nt 3′ UTR. None of the UTRs showed significant nucleotide sequence similarities to the corresponding regions of the members of the order Picornavirales or other viruses using blastn search. The secondary RNA structure of the 5′ UTR was predicted, but with the exception of the first 46 nt (Fig. 1), there was no correspondence to any known type of 5′UTR and internal ribosomal entry site (IRES) (data not shown). The predicted first in-frame AUG initiation codon (AUAUCUA971UGA) is not found in an optimal Kozak-context (RNNAUGG).
The 8142 nt ORF encodes a 2713 aa polyprotein (Fig. 1). The sequence analysis of the N-terminal end of the predicted viral polyprotein did not support the presence of an L protein: neither the key amino acids of papain-like thiol protease (Gorbalenya et al., 1991) nor a putative zinc-binding motif (Chen et al., 1995) could be identified. Using the blastx Conserved Domain Database (CDD), conservative amino acid sequence and/or amino acid motifs were partially identifiable in Newt/2013/HUN in the capsid region (Rhv-like superfamily domains), 2Chel, 3Cpro and 3Dpol, although many of them contain unique amino acid substitutions like GDSQCGKT in 2Chel, GICG in 3Cpro, HGLPSG, LGDD and FLKS in 3Dpol (the unique amino acid is underlined) (Fig. 1). In the structural region (aa 659–864), Newt/2013/HUN has putative conserved domains with more similarity to cricket paralysis virus-like (CRPV, family Dicistroviridae) protein than to picornavirus capsid protein (Fig. 1). In addition, 28 % amino acid sequence identity was found between Newt/2013/HUN capsid (aa 415–564) and rice tungro spherical virus (GenBank accession no. NC_001632.1 aa 875–1020, family Secoviridae), which was the closest match in GenBank. In the non-structural region, up to 36 % and 29 % amino acid sequence identity were found between Newt/2013/HUN 2C (aa 1436–1587) and (3C)/3D (aa 2088–2674) and human cosavirus F (GenBank accession no. AFJ04539.1, aa 1124–1270, family Picornaviridae) and rice tungro spherical virus (GenBank accession no. CAJ81003, aa 2805–3398), respectively. The hypothetical cleavage map of the picornavirus polyprotein was derived from alignments with other picornaviruses sequences and by analogy. The polyprotein cleavage sites of Newt/2013/HUN could be predicted (but not confirmed experimentally) at the border of VP0/VP3 (AAAQ337/GIAH), VP3/VP1 (PEFE606/SDLP), 2B/2Chel (SGLQ1297/TPTA), 2Chel/3A (WDTQ1674/FQAP), 3A/3B (QGTQ1884/ASWY), 3B/3Cpro (APTQ1920/TQSD) and 3Cpro/3Dpol (VTFQ2151/MAVD); however, the DxExNPG ↓ P ‘ribosome skipping’ motif was not present in 2A protein or in any part of the polyprotein. The predicted 562 aa 3Dpol of Newt/2013/HUN has 30 % and 29 % amino acid identity to rice tungro spherical virus (GenBank accession no. NP_734463.1, coverage: 88 %, E-value: 1 × 10− 35, family Secoviridae) and turkey gallivirus (GenBank accession no. YP_006576515.1, coverage: 85 %, E-value: 1 × 10− 29, family Picornaviridae) respectively, as the closest matches in GenBank. The comparative amino acid identity based upon the predicted complete P1, 2Chel and 3Dpol proteins between Newt/2013/HUN and the prototype picornaviruses and some dicistro- and secoviruses are shown in Table 1. None of the predicted 2A, 2B, 3A and 3B proteins showed significant amino acid sequence similarities to viral sequences in GenBank.
Table 1. Pairwise amino acid sequence identities (%) between the complete P1, 2CHEL and 3DPOL proteins of Newt/2013/HUN (GenBank accession no. KP770140) compared with the representative members of the official and unassigned genera of family Picornaviridae and the most closely related viruses from the families Dicistroviridae and Secoviridae. Bold numbers indicate the highest amino acid identity values.
| Family | Genus | Type species/genotype | GenBank accession number | P1 | 2Chel | 3Dpol |
|---|---|---|---|---|---|---|
| Picornaviridae | Aphthovirus | Foot-and-mouth disease virus | NC_004004 | 13.5 | 22.0 | 18.1 |
| Aquamavirus | Aquamavirus A | EU142040 | 15.0 | 17.0 | 19.5 | |
| Avihepatovirus | Duck hepatitis A virus 1 | NC_008250 | 13.3 | 19.3 | 18.9 | |
| Avisivirus | Avisivirus A | KC465954 | 14.4 | 18.5 | 19.5 | |
| Cardiovirus | Encephalomyocarditis virus | NC_001479 | 14.8 | 18.3 | 22.4 | |
| Cosavirus | Cosavirus A | FJ438902 | 14.7 | 19.1 | 21.4 | |
| Dicipivirus | Cadicivirus A | JN819202 | 14.5 | 18.8 | 21.0 | |
| Enterovirus | Enterovirus C | NC_002058 | 14.2 | 15.2 | 22.8 | |
| Erbovirus | Equine rhinitis B virus | NC_003983 | 11.1 | 17.5 | 20.4 | |
| Gallivirus | Gallivirus A | JQ691613 | 13.7 | 17.4 | 24.4 | |
| Hepatovirus | Hepatitis A virus | NC_001489 | 14.5 | 18.0 | 19.5 | |
| Hunnivirus | Hunnivirus A | JQ941880 | 12.8 | 18.5 | 20.8 | |
| Kobuvirus | Aichivirus A | AB010145 | 13.9 | 20.2 | 21.5 | |
| Kunsagivirus | Kunsagivirus A | KC935379 | 14.8 | 15.9 | 17.0 | |
| Megrivirus | Melegrivirus A | HM751199 | 14.4 | 17.6 | 19.6 | |
| Mischivirus | Mischivirus A | JQ814851 | 12.1 | 18.8 | 20.3 | |
| Mosavirus | Mosavirus A | JF973687 | 13.3 | 17.9 | 21.3 | |
| Oscivirus | Oscivirus A | GU182408 | 14.7 | 21.2 | 21.2 | |
| Parechovirus | Human parechovirus | NC_001897 | 13.3 | 18.7 | 17.7 | |
| Pasivirus | Pasivirus A | JQ316470 | 12.4 | 17.2 | 18.6 | |
| Passerivirus | Passerivirus A | GU182406 | 13.6 | 19.6 | 21.8 | |
| Rosavirus | Rosavirus A | JF973686 | 13.7 | 20.3 | 18.5 | |
| Salivirus | Salivirus A | GQ179640 | 14.7 | 20.6 | 21.3 | |
| Sakobuvirus | Sakobuvirus A | KF387721 | 16.0 | 19.3 | 22.1 | |
| Sapelovirus | Porcine sapelovirus | NC_003987 | 12.5 | 18.8 | 19.9 | |
| Senecavirus | Seneca Valley virus | NC_011349 | 14.2 | 19.1 | 21.5 | |
| Sicinivirus | Sicinivirus A | KF741227 | 13.6 | 20.2 | 23.0 | |
| Teschovirus | Porcine teschovirus | NC_003985 | 12.5 | 17.5 | 20.5 | |
| Tremovirus | Avian encephalomyelitis virus | NC_003990 | 15.4 | 19.8 | 18.8 | |
| Unassigned | Eel picornavirus 1 | KC843627 | 14.8 | 19.0 | 20.2 | |
| Unassigned | Carp picornavirus 1 | KF306267 | 15.2 | 17.9 | 21.2 | |
| Unassigned | Fathead minnow PV 1 | KF874490 | 13.3 | 18.6 | 20.1 | |
| Unassigned | Tortoise picornavirus 1 | KM873611 | 13.2 | 20.8 | 19.6 | |
| Unassigned | Tortoise rafivirus A1 | KJ415177 | 14.8 | 17.4 | 21.1 | |
| Unassigned | Aalivirus A1 | KJ000696 | 15.0 | 17.0 | 18.7 | |
| Unassigned | Orivirus A1 | KM203656 | 14.9 | 20.1 | 19.0 | |
| Unassigned | Quail picornavirus 1 | JN674502 | 14.4 | 18.6 | 21.6 | |
| Unassigned | Pigeon picornavirus B | KC560801 | 12.7 | 17.8 | 21.7 | |
| Unassigned | Rabovirus A1 | KP233897 | 12.8 | 18.5 | 19.7 | |
| Unassigned | Crohivirus 1 | AB937989 | 15.3 | 19.8 | 20.9 | |
| Unassigned | Icavirus A1 | KP100644 | 11.4 | 19.8 | 20.4 | |
| Unassigned | Lesavirus 1 | KM396707 | 14.7 | 17.7 | 22.1 | |
| Unassigned | Chicken picornavirus 2 | KF979333 | 14.6 | 17.9 | 18.0 | |
| Dicistroviridae | Cripavirus | Plautia stali intestine virus | NC_003779 | 16.3 | 16.3 | 18.5 |
| Cripavirus | Triatoma virus | NC_003783 | 17.2 | 16.6 | 19.8 | |
| Secoviridae | Waikavirus | Rice tungro spherical virus | NP_042507 | – | – | 24.0 |
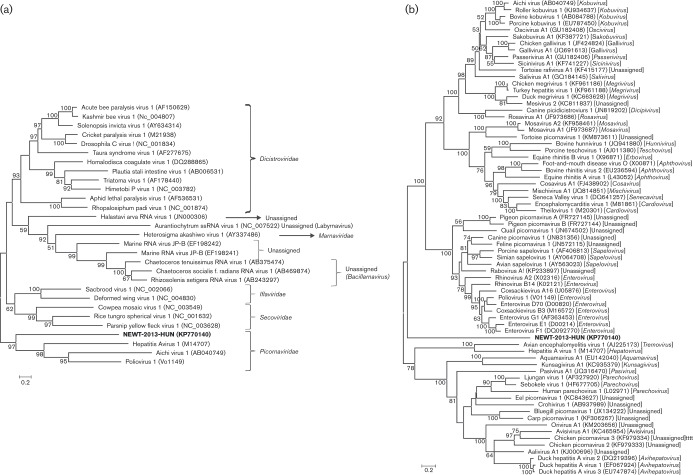
Representative picornavirus sequences were aligned using clustal_x (Thompson et al., 1997) and phylogenetic trees of the amino acid alignments of 3D proteins of the members of the order Picornavirales and the family Picornaviridae were recreated using the maximum-likelihood method, with the Le_Gascule_2008 substitution model (LG) and gamma distributed with invariant sites (G+I), using mega software (v. 6.06) (Tamura et al., 2013). The model was selected based on the results of the best model search of mega v.6.06. Bootstrap (BS) values (based on 1000 replicates) for each node are shown if BS >50 %. These phylogenetic trees indicate that Newt/2013/HUN is a member of the family Picornaviridae; however, it forms a highly divergent picornavirus lineage (Fig. 2).
Fig. 2. Phylogenetic analysis of newt picornavirus strain Newt/2013/HUN (KP770140) (indicated in bold) based on the complete amino acid sequence of the 3D protein among (a) the representative members of the order Picornavirales and (b) the representative members of the official and unassigned picornavirus genera of the family Picornaviridae, respectively. GenBank accession numbers are given in parentheses. Bars indicate amino acid substitutions per site. Bootstrap values (based on 1000 replicates) for each node are given if >50 %.
Faecal samples of smooth newts were screened for newt picornavirus by RT-PCR using screening primers (newtpico-F: 5′-AATTGGCCATACTCTCCATCT-3′ corresponding to nt 8398–8418 and newtpico-R: 5′-CATGAAGCCCGTCATCACCA corresponding to nt 8587–8606 of KP770140) designed for the nucleotide sequence of the 3Dpol sequence. Six (25 %) of the 24 newt faecal samples were PCR-positive for the novel newt picornavirus by conventional RT-PCR, which were collected from individuals caught in two out of the six studied ponds (Mélymocsár: n = 4; Paprét-felsö: n = 2).
Original faecal samples containing newt picornavirus were propagated for virus isolation. Vero (African green monkey kidney; ATCC CRL-1586) cells were used as a broad-purpose cell line for catching most cultivable viruses. Specimens (2.5 ml) were clarified by centrifugation and filtered using a 0.45 μm sterile filter (Millex-HV, Millipore). Virus cultures were inspected daily by inverted microscopy for cytopathic effect (CPE). After 12 days of incubation, subculturing to a fresh cell line was performed twice. At the end of the incubation, viral RNA was tested by RT-PCR. Neither CPE nor newt picornavirus RNA replication could be detected in Vero cells even after serial passages of newt picornavirus.
Until now, picornaviruses have been identified in four (mammals, birds, reptiles, fish) of the five classes of the subphylum Vertebrata. This study reports the first identification of picornavirus in an amphibian species, the smooth newt. Newt/2013/HUN showed no close sequence relation to any of the picornavirus genera or recently suggested picornavirus supergenus/subfamily (Lauber & Gorbalenya, 2012) and forms a basally rooted sequence lineage in family Picornaviridae by phylogenetic analysis. In addition, Newt/2013/HUN has some unique genome/protein features, including the presently unpredictable secondary RNA structure of a long 5′ UTR and IRES in comparison to the known picornavirus IRESes; the non-optimal Kozak-context of the predicted AUG initiation codon; and unique amino acids in highly conserved protein sequence motifs. Interestingly, while the sequence identity is low, some of the Newt/2013/HUN proteins are more closely related to other positive-sense ssRNA viruses in the order Picornavirales, like dicistrovirus (N-terminal part of the capsid protein to cricket paralysis virus capsid protein) and secovirus (3Dpol to rice tungro spherical virus), than the corresponding picornavirus proteins by blastx CDD search. Whether these features and the base of the tree phylogenetic position of Newt/2013/HUN represent significant results in (picorna)viral evolutionary aspects – e.g. early divergence timed to the diversification of the vertebrates – is remained unknown. However, the discovery of a genetically highly divergent picornavirus in a new class of ancient host species may help to better understand picornavirus evolution. Newt/2013/HUN represents the first member of a novel species in a novel genus within the family Picornaviridae, provisionally named as genus Ampivirus ( = amphibian picornavirus).
Ampivirus was detected in 25 % of the total faecal samples by conventional RT-PCR. These samples were collected from individuals captured in two ponds within the same geographical region, in Hungary, in year 2013. In addition, a further three faecal specimens tested positive by RT-PCR and sequencing for ampivirus, of a group of 16 collected in spring 2014 from additional free-living smooth newts (transitionally captured in the field, not kept in plastic containers and not fed ad libitum with sludge worms) living in a further three of four natural aquatic territories. These data indicate that smooth newts are possibly the natural hosts of this picornavirus. Further studies are needed to investigate the pathogenic potential, geographical distribution, genetic and possible host species diversity of this novel ampivirus. Nevertheless, the detection of picornavirus in smooth newts suggests that amphibians are susceptible to picornavirus infections and other amphibians (mostly salamanders, frogs and toads) are also potential hosts of picornaviruses. At present, the number of known amphibian species is approximately 7300, of which 88 % are frogs (Frost, 2014).
Although, on one hand, bushmeat consumption (hunting non-domesticated animals including amphibians for food) is increasing in the Americas, Asia and Africa, and on the other hand, keeping exotic animals as pets is becoming increasingly popular in the world, our knowledge about viruses of lower vertebrates is scarce. A better understanding of viral diversity (the virome) in amphibians and other lower vertebrates constitutes an important step to identify possible zoonotic and/or food-borne transmission risks. Although potential amphibian-infecting picornaviruses are not likely to pose a direct threat to humans and/or economically important livestock, the emergence of viral diseases may contribute to the decline of amphibian populations even in widely distributed species (Green et al., 2002; Collins et al., 2004).
The identification of the first picornavirus in amphibians expands the host range of Picornaviridae and opens a new research field in picornavirus evolution in lower vertebrates.
Acknowledgements
This work was financially supported by grants from the Hungarian Scientific Research Fund (OTKA/NKFIH K111615) and by NHLBI (R01-HL105770). Z. T. was supported by the ‘Lendület’ program of the Hungarian Academy of Sciences (MTA, LP2012-24/2012), a Hungarian Scientific Research Fund (OTKA, PD108938) and the MTA postdoctoral research program (SZ-029/2013). Á. B. was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences.
References
- APHA (1985). Standard Methods for the Examination of Wastewater 16th edn Washington, DC: American Public Health Association. [Google Scholar]
- Arntzen J.W., Kuzmin S., Beebee T., Papenfuss T., Sparreboom M., Ugurtas I.H., Anderson S., Anthony B., Andreone F., other authors (2009). Lissotriton vulgaris The IUCN Red List of Threatened Species. Version 2014.3 www.iucnredlist.org. Downloaded on 3 December 2014. [Google Scholar]
- Barbknecht M., Sepsenwol S., Leis E., Tuttle-Lau M., Gaikowski M., Knowles N.J., Lasee B., Hoffman M.A. (2014). Characterization of a new picornavirus isolated from the freshwater fish Lepomis macrochirus J Gen Virol 95 601–613 10.1099/vir.0.061960-0 . [DOI] [PubMed] [Google Scholar]
- Boros Á., Pankovics P., Simmonds P., Reuter G. (2011). Novel positive-sense, single-stranded RNA (+ssRNA) virus with di-cistronic genome from intestinal content of freshwater carp (Cyprinus carpio) PLoS One 6 e29145 10.1371/journal.pone.0029145 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros Á., Pankovics P., Reuter G. (2014). Avian picornaviruses: molecular evolution, genome diversity and unusual genome features of a rapidly expanding group of viruses in birds Infect Genet Evol 28 151–166 10.1016/j.meegid.2014.09.027 . [DOI] [PubMed] [Google Scholar]
- Chen H.H., Kong W.P., Roos R.P. (1995). The leader peptide of Theiler's murine encephalomyelitis virus is a zinc-binding protein J Virol 69 8076–8078 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J.P., Brunner J.L., Jancovich J.K., Schock D.M. (2004). A model host-pathogen system for studying infectious disease dynamics in amphibians: tiger salamanders (Ambystoma tigrinum) and Ambystoma tigrinum virus Herpetol J 14 195–200. [Google Scholar]
- Fichtner D., Philipps A., Groth M., Schmidt-Posthaus H., Granzow H., Dauber M., Platzer M., Bergmann S.M., Schrudde D., other authors (2013). Characterization of a novel picornavirus isolate from a diseased European eel (Anguilla anguilla) J Virol 87 10895–10899 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost D. (2014)., American Museum of Natural History: Amphibian species of the World 6.0.Online reference retrieved: 28 November 2014. http://research.amnh.org/vz/herpetology/amphibia/. [Google Scholar]
- Gorbalenya A.E., Koonin E.V., Lai M.M. (1991). Putative papain-related thiol proteases of positive-strand RNA viruses. Identification of rubi- and aphthovirus proteases and delineation of a novel conserved domain associated with proteases of rubi-, alpha- and coronaviruses FEBS Lett 288 201–205 10.1016/0014-5793(91)81034-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D.E., Converse K.A., Schrader A.K. (2002). Epizootiology of sixty-four amphibian morbidity and mortality events in the USA, 1996-2001 Ann N Y Acad Sci 969 323–339 10.1111/j.1749-6632.2002.tb04400.x . [DOI] [PubMed] [Google Scholar]
- Heuser W., Kaleta E., Giesow K., Keil G.M., Knowles N.J. (2010). Genome sequence of virus “X”, a picornavirus isolated from a spur-thighed tortoise (Testudo graeca). In EUROPIC 2010: XVI Meeting of the European Study Group on the Molecular Biology of Picornaviruses, pp. 147 St Andrews, Scotland, UK, 11-16 September 2010. Abstract H15. [Google Scholar]
- ICTV Master Species List v3 (2014). http://talk.ictvonline.org/files/ictv_documents/m/msl/5208.aspx . [Google Scholar]
- Kapoor A., Victoria J., Simmonds P., Wang C., Shafer R.W., Nims R., Nielsen O., Delwart E. (2008b). A highly divergent picornavirus in a marine mammal J Virol 82 311–320 10.1128/JVI.01240-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles N.J., Hovi T., Hyypiä T., King A.M.Q., Lindberg A.M., Pallansch M.A., Palmenberg A.C., Simmonds P., Skern T., other authors (2012). Picornaviridae. In Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses, pp. 855–880. Edited by King A. M. Q., Adams M. J., Carstens E. B., Lefkowitz E. J. San Diego: Elsevier. [Google Scholar]
- Lange J., Groth M., Fichtner D., Granzow H., Keller B., Walther M., Platzer M., Sauerbrei A., Zell R. (2014). Virus isolate from carp: genetic characterization reveals a novel picornavirus with two aphthovirus 2A-like sequences J Gen Virol 95 80–90 10.1099/vir.0.058172-0 . [DOI] [PubMed] [Google Scholar]
- Lauber C., Gorbalenya A.E. (2012). Toward genetics-based virus taxonomy: comparative analysis of a genetics-based classification and the taxonomy of picornaviruses J Virol 86 3905–3915 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T.F.F., Wellehan J.F., Coleman J.K., Kondov N.O., Deng X., Waltzek T.B., Reuter G., Knowles N.J., Delwart E. (2015). A tortoise-infecting picornavirus expands the host range of the family Picornaviridae Arch Virol 160 1319–1323 10.1007/s00705-015-2366-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nöllert A., Nöllert C. (1992). Die Amphibien Europas., Stuttgart: Bestimmung-Gefährdung-Schutz. Franckh-Kosmos Verlag. [Google Scholar]
- Phan T.G., Vo N.P., Boros Á., Pankovics P., Reuter G., Li O.T., Wang C., Deng X., Poon L.L., Delwart E. (2013). The viruses of wild pigeon droppings PLoS One 8 e72787 10.1371/journal.pone.0072787 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps N.B.D., Mor S.K., Armien A.G., Batts W., Goodwin A.E., Hopper L., McCann R., Ng T.F.F., Puzach C., other authors (2014). Isolation and molecular characterization of a novel picornavirus from baitfish in the USA PLoS One 9 e87593 10.1371/journal.pone.0087593 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V. (2007). Picornaviridae: the viruses and their replication. In Fields Virology 5th edn, pp. 795–838. Edited by Knipe D. M., Howley P. M. Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. (2013). mega6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30 2725–2729 10.1093/molbev/mst197 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. (1997). The clustal_x windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools Nucleic Acids Res 25 4876–4882 10.1093/nar/25.24.4876 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria J.G., Kapoor A., Li L., Blinkova O., Slikas B., Wang C., Naeem A., Zaidi S., Delwart E. (2009). Metagenomic analyses of viruses in stool samples from children with acute flaccid paralysis J Virol 83 4642–4651 10.1128/JVI.02301-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, P. C., Lau, S. K., Choi, G. K., Huang, Y., Teng, J. L., Tsoi, H. W., Tse, H., Yeung, M. L., Chan, K. H., other authors Woo P.C., Lau S.K., Choi G.K., Huang Y., Teng J.L., Tsoi H.W., Tse H., Yeung M.L., Chan K.H., other authors (2012). Natural occurrence and characterization of two internal ribosome entry site elements in a novel virus, canine picodicistrovirus, in the picornavirus-like superfamily J Virol 86 2797–2808 . [DOI] [PMC free article] [PubMed] [Google Scholar]