Abstract
Equine sarcoids are highly recurrent bovine papillomavirus (BPV)-induced fibroblastic neoplasms that are the most common skin tumours in horses. In order to facilitate the study of potential equine sarcoid prophylactics or therapeutics, which can be a slow and costly process in equines, a murine model for BPV-1 protein-expressing equine sarcoid-like tumours was developed in mice through stable transfection of BPV-1 E5 and E6 in a murine fibroblast tumour cell line (K-BALB). Like equine sarcoids, these murine tumour cells (BPV-KB) were of fibroblast origin, were tumorigenic and expressed BPV-1 proteins. As an initial investigation of the preclinical potential of this tumour model for equine sarcoids prophylactics, mice were immunized with BPV-1 E5E6 Venezuelan equine encephalitis virus replicon particles, prior to BPV-KB challenge, which resulted in an increased tumour-free period compared with controls, indicating that the BPV-KB murine model may be a valuable preclinical alternative to equine clinical trials.
Equine sarcoids are fibroblastic tumours that are the most common dermo-epidermal neoplasias in equids worldwide (Bogaert et al., 2008b; Nasir & Campo, 2008). The prevalence of these tumours varies by study and has been stated to be 2–12 % in different populations, and tumours develop primarily in horses between 3 and 6 years old (Bogaert et al., 2008a). These sarcoids do not metastasize, but they are often highly invasive at multiple sites and untreatable, which leads to the loss of valuable animals, with morbidity rates reported as high as 14 per 1000 horses (Campo, 2002; Mohammed et al., 1992). Bovine papillomavirus (BPV) type 1 and less commonly type 2 and 13 DNA have been found in virtually all equine lesions (Lunardi et al., 2013; Otten et al., 1993), making BPV the prime aetiological agent in equine sarcoids. Unlike BPV-1 infections in cattle that can cause non-invasive skin warts and benign fibropapillomas (Campo, 2002), the invasive nature of equine sarcoid lesions makes the treatment of equine sarcoids challenging. Additionally, BPV infection of equines does not result in viral particle production, making the mode of transmission unclear and the use of virus particle-based vaccines unfeasible (Chambers et al., 2003). As such, treatments have limited success, with conventional surgical excisions having 50–64 % recurrence rates (McConaghy et al., 1994). Current treatment limitations in equine sarcoids, loss of animals and cost of equine studies indicate that an alternative model to evaluate preclinical therapies for equine sarcoids would be of great value.
Whilst treatments such as chemotherapies may halt sarcoid growth, they do not target the underlying viral infection. Hence, latent and/or persistent BPV infection can result in recurrences or lead to the development of new tumours. Therefore, vaccinations and immunotherapies have been explored that may stimulate the immune system to combat the causative viral infection. For instance, the Bacille de Calmette et Guérin (BCG) vaccination, is already used as an off-label treatment for peri-ocular sarcoids with a success rate of nearly 100 % (Klein et al., 1986). However, BCG is less effective on other parts of the body, including the ventral abdomen, which is most common locale for sarcoids (Bogaert et al., 2008a), and may induce severe side effects, including prolonged lymphangitis. A pilot study using the immunostimulant 5 % imiquimod also showed a positive influence on sarcoid regression (Nogueira et al., 2006). However, topical treatment directly on tumours can be painful and require sedation. Whilst these immunotherapeutics are promising, prophylactic options could be more effective and easier to implement.
No preclinical mouse model exists in equine sarcoid research. Whilst early studies showed that BPV-1 could transform mouse cells (Laatikainen et al., 1990; Mäntyjärvi et al., 1988), the antigens expressed or the use of vaccines to enhance immune responses to them was not evaluated. Thus, equine sarcoid research requires the use of horses or other equids to test treatments. Although vaccination experiments in horses are more predictive, they come with high costs, lack of standardization and high inter-animal variation, which can make results hard to interpret. Therefore, a murine model would be highly beneficial, providing an affordable, standardized and fast model to allow evaluation of treatments prior to confirmation in horses. Therefore, the first aim of this study was to develop a tumour model in mice that mimics BPV-1-induced equine sarcoids, the second was to create a BPV-1 vaccine that was immunogenic in mice and the third was to evaluate the utility of our BPV-1 vaccine in this novel equine sarcoid-like murine tumour model.
In order to create an equine sarcoid-like mouse tumour model, K-BALB cells, murine fibroblastic sarcoma cells (Aaronson & Weaver, 1971), were transfected with BPV-1 E5 and E6, which were chosen as they are the transforming proteins of BPV (Chambers et al., 2003) and their expression can lead to sarcoid development. K-BALB cells were used because of their high tumorigenicity and fibroblastic origin, and therefore BALB/c mice were used in tumour challenge experiments. BPV-1 E5 and E6 ORFs were amplified via PCR (Stratagene) with added restriction sites from sarcoid-derived BPV-1 DNA. Primers with restriction sites are presented in Table S1 (available in the online Supplementary Material) and gene products are shown in Table S2. E5 and E6 were blunt-end cloned into pCR4 Blunt TOPO (Invitrogen). Next, E5 DNA was subcloned into pCEP4 (Invitrogen), under control of a cytomegalovirus promoter. E6 DNA was subcloned into pZeoSV2(+) (Invitrogen), under control of a simian virus 40 promoter. All plasmids were sequenced to confirm correct insert orientation. The BPV-KB cell line was generated by stably transfecting cultures of K-BALB cells sequentially with E5-pCEP4 and E6-pZeoSV2(+) using Lipofectamine 2000 (Invitrogen) and selection with hygromycin and/or zeocin. BPV-KB cells were clonally expanded in vitro from BPV-KB tumours in mice. All protocols were in accordance with institutional guidelines and approved by the University of Southern California Institutional Animal Care and Use Committee and Ghent University Ethical Committee (EC2010/166).
Specific-pathogen-free female BALB/c mice (6–8 weeks old; Harlan) were injected subcutaneously with 0.25 × 105–1 × 106 BPV-KB cells (five mice per group) and tumour growth was recorded bi-weekly. One month after challenge, tumours were analysed for BPV-1 E5 and E6 expression. RNA was isolated using an RNeasy Minikit (Qiagen) and cDNA synthesis was performed using a SuperScript kit (Invitrogen). Finally, reverse transcription (RT)-PCR was performed with ImmoMix master mix (Bioline). A ‘no reverse transcriptase’ control for glyceraldehyde 3-phosphate dehydrogenase was used to check for successful removal of all DNA. Our results confirmed BPV-1 E5 and E6 expression in the resulting BPV-KB tumours in mice (Fig. 1a).
Fig. 1. Characteristics of the BPV-KB equine sarcoid-like tumour cell line. (a) BPV E5/E6 expression by RT-PCR on BPV-KB tumour cells using E5 primers (lanes 1–3) or E6 primers (lanes 5–7). Lanes 1 and 5, BPV-1 plasmid DNA; lanes 2 and 6, ‘no reverse transcriptase’ control; lanes 3 and 7, BPV-KB cDNA. Lane 4, 100bp DNA ladder. (b) MHC I expression on tumour cells. Black curves represent FITC-labelled anti-MHC I antibody and grey curves are isotype controls (IC). Shown is a representative example of an experiment performed in triplicate. MHC I expression in BPV-KB cells [mean fluorescence intensity (MFI)51656] is equivalent to that in WT K-BALB cells (MFI51619). (c) BPV-KB cells induce progressively growing tumours in 100 % of BALB/c mice (n = 55 per group). Mean tumour volume is plotted versus days after challenge.
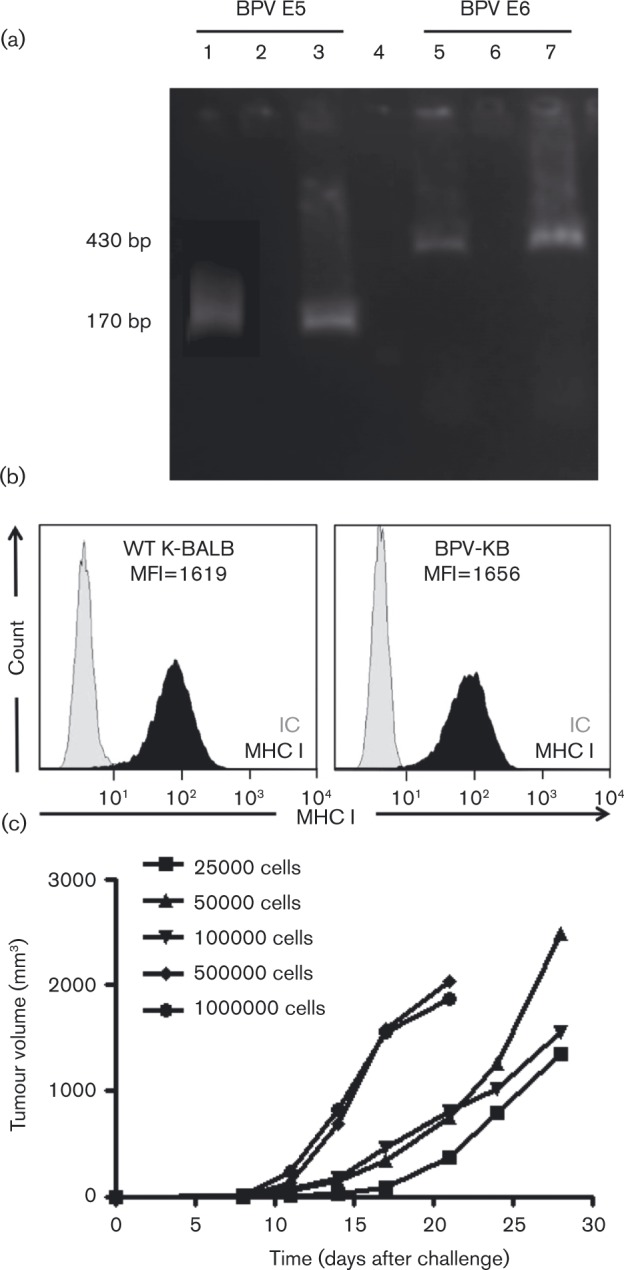
As results have demonstrated that BPV-1 E5 can decrease antigen presentation through MHC class I (MHC I) downregulation in BPV-infected bovine cells (Ashrafi et al., 2002; Chambers et al., 2003; Marchetti et al., 2002), the expression of MHC I on BPV-KB cells was evaluated. BPV-KB cells were stained with FITC-labelled anti-H2-Dd (BioLegend) and analysed by flow cytometry. MHC I expression on BPV-KB cells was not reduced compared with WT K-BALB cells (Fig. 1b). Importantly, this infers that BPV-KB cells are not impaired in their peptide presentation capabilities. Additionally, whilst equine fibroblast expression of MHC I is attenuated by E5, it is not abrogated (Marchetti et al., 2009), allowing equine cells to similarly present peptides via MHC I. After subcutaneous injection, BPV-KB cells were tumorigenic in 100 % of mice and their growth varied with cell number: 0.5–1 × 106 cells induced rapid growth and required euthanasia within 14 days, whereas 0.25–1 × 105 cells induced delayed tumour onset (14 days after challenge) (Fig. 1c). The MHC I expression and growth characteristics indicate that BPV-KB cells are not immunogenic enough to induce tumour regression despite the expression of BPV-1 genes and the ability to present antigen.
Next we sought to design a vaccine that could induce BPV-1 immune responses in mice. Possible targets of a vaccine against equine sarcoids are BPV-1 E5 and E6 because of their constitutive expression in all sarcoids with high mRNA expression (Bogaert et al., 2007; Chambers et al., 2003). Furthermore, we have previously shown that Venezuelan equine encephalitis virus replicon particles (VEEV-VRPs) can eradicate human papillomavirus type 16-expressing tumours in mice (Velders et al., 2001). Therefore, BPV-1 E5E6 replication-incompetent VEEV-VRPs were constructed by subcloning a fusion gene product comprising BPV-1 E5 and E6 into the replicon plasmid, pVR200 (Alphavax). Before subcloning, point mutations were inserted at the C terminus of E5 (Horwitz et al., 1988) and the four CxxC motifs of E6 (Thomas et al., 2006), regions critical for proper gene function, in order to avoid transforming effects in the host and to enhance safety.
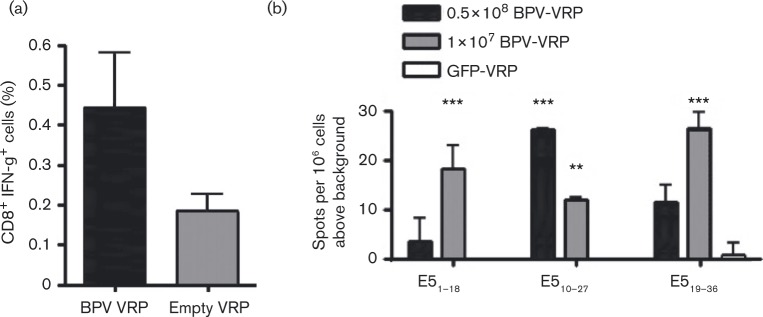
To test the immunogenicity of the BPV-1 E5E6 VRPs in mice, female C57BL/6 mice (Taconic Farms) were vaccinated with 0.5 × 108 IU BPV-1 E5E6 VRPs or empty VRPs (n = 5 per group). After 5 days, splenocytes were harvested and stained with antibodies for cell surface CD8 and intracellular IFN-γ after stimulation with lipopolysaccharide (LPS) blasts pulsed with a BPV-1 peptide pool. Specifically, LPS blasts were prepared by culturing splenocytes in the presence of LPS and dextran sulfate, followed by incubation with either a BPV-1 peptide pool or prostate stem cell antigen (PSCA83–91) that we demonstrated previously to elicit immune responses in mice (de la Luz Garcia-Hernandez et al., 2008). BPV-1 peptides (E51–18, E54–12, E510–19, E510–27, E519–36, E528–44, E519–26, E652–60, E6105–113) were chosen from MHC I peptide binding prediction programs (http://tools.immuneepitope.org, http://www-bimas.cit.nih.gov/molbio/hla_bind/, http://faostat.fao.org/site/339/default.aspx). Splenocytes from BPV-1 E5E6 VRP- or empty VRP-vaccinated animals were co-cultured with peptide-stimulated LPS blasts (3 : 1). Cells were then treated with a Cytofix/Cytoperm Plus Fixation/Permeabilization kit (BD Biosciences). Next, cells were stained with allophycocyanin–anti-CD8a (BD Biosciences) followed by staining with phycoerythrin–anti-IFN-γ (BioLegend) and analysed by flow cytometry. Our results demonstrate that the BPV-1 E5E6 VRP vaccination resulted in a higher proportion of CD8+ IFN-γ+ splenocytes than empty VRPs (Fig. 2a).
Fig. 2. BPV VRP are immunogenic in mice. (a) Intracellular IFN-γ staining revealed a higher proportion of CD8+ IFN-γ+ cells in BPV-VRP (n = 5)-vaccinated mice compared with controls (n = 5) (P = 0.05; t-test). (b) BPV-VRP vaccination induced BPV-specific T-cell responses in C57Bl/6 mice. IFN-γ ELISPOT assay was used to detect functional BPV-specific T-cells after stimulation with various peptides. Only peptides inducing IFN-γ production significantly higher than GFP-VRP-vaccinated mice are shown. **P < 0.01, ***P < 0.001 (t-test).
Additionally, mice were vaccinated intramuscularly with BPV-1 E5E6 VRPs or GFP-VRPs and boosted 7 days later. Functional IFN-γ-producing antigen-specific cells were detected 9 days after final BPV-1 E5E6 VRP vaccination via an IFN-γ ELISPOT assay (BD Pharmingen). Freshly isolated splenocytes from BPV-1 E5E6 VRP-vaccinated animals (n = 5 per group) were stimulated with various BPV-1 peptides and cells were added to IFN-γ-coated plates. Next, plates were washed and incubated with biotinylated anti-IFN-γ antibody followed by incubation with streptavidin–HRP (Sigma-Aldrich), and wells were developed and counted. The E51–18, E510–27, and E519–36 peptides induced significantly higher IFN-γ production in mice immunized with BPV-1 E5E6 VRPs compared with control VRPs (Fig. 2b), demonstrating the ability of the BPV-1 E5E6 VRP vaccine to induce a BPV-specific T-cell response in mice.
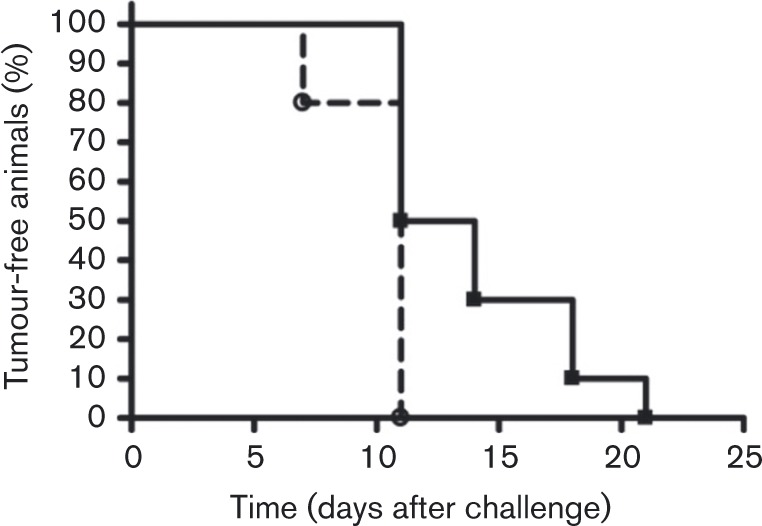
To determine the value of the BPV-KB tumour model in evaluating equine sarcoid prophylactics, BALB/c mice were vaccinated twice with a 14 day interval with BPV-1 E5E6 VRPs (n = 10) or GFP-VRPs (n = 5). Ten days after vaccination, mice were challenged subcutaneously in the right flank with 0.25 × 105 BPV-KB tumour cells. Throughout the duration of the experiments, tumour growth was monitored bi-weekly for 6 weeks. All mice developed tumours irrespective of the vaccine. Whilst no difference in survival was observed (data not shown), our results demonstrated that the tumour-free period was significantly longer in BPV-1 E5E6 VRP-vaccinated mice compared with GFP-VRP-vaccinated animals (P < 0.05; log-rank test) (Fig. 3).
Fig. 3. Evaluation of BPV-VRP vaccine with the BPV-KB tumour model. The per cent tumour-free BPV-VRP-vaccinated BALB/c mice (n = 10; ▪) or GFP-VRP-vaccinated BALB/c mice (n = 5; ○) against days after challenge with BPV-KB tumour cells is presented. (P < 0.05, log-rank test.).
The BPV-1 protein-expressing equine sarcoid-like murine cell line described herein is of fibroblastic origin, and constitutively expresses BPV-1 E5 and E6, inducing progressively growing tumours in the flanks of mice. With this model, prophylactic and therapeutic evaluation of candidate vaccines against equine sarcoids can be performed in a standardized, fast and convenient manner. In vivo studies may thus only be required in horses, the target species, in the second phase of testing. The observed tumour growth in mice suggests that the BPV-1 antigens in BPV-KB cells are not immunogenic enough to induce spontaneous tumour regression, warranting testing of BPV vaccines in this model. One explanation for the poor recognition of these tumour cells by the immune system is that there may be relatively low expression of tumour-associated antigens. In the current study, E5 and E6 transfection resulted in high mRNA expression, which suggests that low antigen expression may not explain the lack of anti-BPV-KB immune responses.
BPV-KB challenge experiments in BALB/c mice showed that a longer tumour-free interval was achieved when mice were vaccinated with BPV-1 E5E6 VRPs compared with GFP-VRPs. Nevertheless, all animals developed tumours. These results point to a limited effect of this particular vaccine. This could possibly be explained by the insertion of point mutations in the BPV-1 E5E6 fusion protein prior to the production of VRPs to enhance safety for use in horses as the locations of the mutations in the E5 C terminus (aa 36 and 38) were in two predicted epitopes (E519–36 and E528–44), and one of the CxxC motifs in E6 (106-CxxxC-109) was in a predicted epitope (E6105–113).
In conclusion, the novel BPV-KB murine equine sarcoid-like tumour model that expresses BPV-1 E5 and E6 may be used as an in vivo screening tool to obtain proof-of-concept of candidate vaccine/immunotherapy treatments prior to evaluation in horses. In the future, the evaluation of vaccinations post-challenge in a therapeutic setting could further improve this promising preclinical model. Although our VRP-based vaccine did not result in survival benefits, the results highlight the potential utility of the BPV-KB model in the preclinical evaluation of such equine sarcoid prophylactics and therapeutics.
Acknowledgements
Excellent technical assistance from Cindy De Baere and Kristel Demeyere is greatly appreciated. We thank AlphaVax (Durham, NC, USA) for the production of VRPs. L. B. is a Fulbright Scholar supported by grants from the National Research Foundation – Flanders and the Ghent University Special Research Fund. W. M. K. holds the Walter A. Richter Cancer Research Chair and his papillomavirus research is supported by the National Institutes of Health (RO1 CA 74397-15). A. W. is supported by a TL1 Scholar award from the Southern California Clinical and Translational Science Institute (TL1TR000132) and the ARCS Foundation John and Edith Leonis award.
Supplementary Data
Supplementary Data
References
- Aaronson S.A., Weaver C.A. (1971). Characterization of murine sarcoma virus (Kirsten) transformation of mouse and human cells J Gen Virol 13 245–252 10.1099/0022-1317-13-2-245 . [DOI] [PubMed] [Google Scholar]
- Ashrafi G.H., Tsirimonaki E., Marchetti B., O'Brien P.M., Sibbet G.J., Andrew L., Campo M.S. (2002). Down-regulation of MHC class I by bovine papillomavirus E5 oncoproteins Oncogene 21 248–259 10.1038/sj.onc.1205008 . [DOI] [PubMed] [Google Scholar]
- Bogaert L., Van Poucke M., De Baere C., Dewulf J., Peelman L., Ducatelle R., Gasthuys F., Martens A. (2007). Bovine papillomavirus load and mRNA expression, cell proliferation and p53 expression in four clinical types of equine sarcoid J Gen Virol 88 2155–2161 10.1099/vir.0.82876-0 . [DOI] [PubMed] [Google Scholar]
- Bogaert L., Martens A., Depoorter P., Gasthuys F. (2008a). Equine sarcoids–part 1: clinical presentation and epidemiology Vlaams Diergen Tijds 77 2–9. [Google Scholar]
- Bogaert L., Martens A., Van Poucke M., Ducatelle R., De Cock H., Dewulf J., De Baere C., Peelman L., Gasthuys F. (2008b). High prevalence of bovine papillomaviral DNA in the normal skin of equine sarcoid-affected and healthy horses Vet Microbiol 129 58–68 10.1016/j.vetmic.2007.11.008 . [DOI] [PubMed] [Google Scholar]
- Campo M.S. (2002). Animal models of papillomavirus pathogenesis Virus Res 89 249–261 10.1016/S0168-1702(02)00193-4 . [DOI] [PubMed] [Google Scholar]
- Chambers G., Ellsmore V.A., O'Brien P.M., Reid S.W., Love S., Campo M.S., Nasir L. (2003). Association of bovine papillomavirus with the equine sarcoid J Gen Virol 84 1055–1062 10.1099/vir.0.18947-0 . [DOI] [PubMed] [Google Scholar]
- de la Luz Garcia-Hernandez M., Gray A., Hubby B., Klinger O.J., Kast W.M. (2008). Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity Cancer Res 68 861–869 10.1158/0008-5472.CAN-07-0445 . [DOI] [PubMed] [Google Scholar]
- Horwitz B.H., Burkhardt A.L., Schlegel R., DiMaio D. (1988). 44-amino-acid E5 transforming protein of bovine papillomavirus requires a hydrophobic core and specific carboxyl-terminal amino acids Mol Cell Biol 8 4071–4078 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein W.R., Bras G.E., Misdorp W., Steerenberg P.A., de Jong W.H., Tiesjema R.H., Kersjes A.W., Ruitenberg E.J. (1986). Equine sarcoid: BCG immunotherapy compared to cryosurgery in a prospective randomised clinical trial Cancer Immunol Immunother 21 133–140 10.1007/BF00199861 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laatikainen A., Sarkkinen H., Syrjänen K., Mäntyjärvi R. (1990). Local immune reaction in syngeneic mice against tumorigenic and nontumorigenic BPV-transformed mouse cell lines APMIS 98 909–915 10.1111/j.1699-0463.1990.tb05014.x . [DOI] [PubMed] [Google Scholar]
- Lunardi M., de Alcântara B.K., Otonel R.A., Rodrigues W.B., Alfieri A.F., Alfieri A.A. (2013). Bovine papillomavirus type 13 DNA in equine sarcoids J Clin Microbiol 51 2167–2171 10.1128/JCM.00371-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäntyjärvi R.A., Sarkkinen H., Karajalainen H., Parkkinen S., Ryhänen A., Syrjänen K., Jägerroos H., Nurmi T. (1988). Tumour-associated antigens in primary mouse fibroblasts induced by transformation with bovine papillomavirus type 1 Exp Pathol 35 25–33 10.1016/S0232-1513(88)80115-4 . [DOI] [PubMed] [Google Scholar]
- Marchetti B., Ashrafi G.H., Tsirimonaki E., O'Brien P.M., Campo M.S. (2002). The bovine papillomavirus oncoprotein E5 retains MHC class I molecules in the Golgi apparatus and prevents their transport to the cell surface Oncogene 21 7808–7816 10.1038/sj.onc.1205885 . [DOI] [PubMed] [Google Scholar]
- Marchetti B., Gault E.A., Cortese M.S., Yuan Z., Ellis S.A., Nasir L., Campo M.S. (2009). Bovine papillomavirus type 1 oncoprotein E5 inhibits equine MHC class I and interacts with equine MHC I heavy chain J Gen Virol 90 2865–2870 10.1099/vir.0.014746-0 . [DOI] [PubMed] [Google Scholar]
- McConaghy F.F., Davis R.E., Hodgson D.R. (1994). Equine sarcoid: a persistent therapeutic challenge Compend Contin Educ Pract Vet 16 1022–1029. [Google Scholar]
- Mohammed H.O., Rebhun W.C., Antczak D.F. (1992). Factors associated with the risk of developing sarcoid tumours in horses Equine Vet J 24 165–168 10.1111/j.2042-3306.1992.tb02808.x . [DOI] [PubMed] [Google Scholar]
- Nasir L., Campo M.S. (2008). Bovine papillomaviruses: their role in the aetiology of cutaneous tumours of bovids and equids Vet Dermatol 19 243–254 10.1111/j.1365-3164.2008.00683.x . [DOI] [PubMed] [Google Scholar]
- Nogueira S.A., Torres S.M., Malone E.D., Diaz S.F., Jessen C., Gilbert S. (2006). Efficacy of imiquimod 5% cream in the treatment of equine sarcoids: a pilot study Vet Dermatol 17 259–265 10.1111/j.1365-3164.2006.00526.x . [DOI] [PubMed] [Google Scholar]
- Otten N., von Tscharner C., Lazary S., Antczak D.F., Gerber H. (1993). DNA of bovine papillomavirus type 1 and 2 in equine sarcoids: PCR detection and direct sequencing Arch Virol 132 121–131 10.1007/BF01309847 . [DOI] [PubMed] [Google Scholar]
- Thomas M., Pim D., Banks L. (2006). The role of the HPV E6 oncoprotein in malignant progression. In Papillomavirus Research: From Natural History to Vaccines and Beyond, pp. 115–131. Edited by Saveria Campo M. Wymondham: Caister Academic Press. [Google Scholar]
- Velders M.P., McElhiney S., Cassetti M.C., Eiben G.L., Higgins T., Kovacs G.R., Elmishad A.G., Kast W.M., Smith L.R. (2001). Eradication of established tumors by vaccination with Venezuelan equine encephalitis virus replicon particles delivering human papillomavirus 16 E7 RNA Cancer Res 61 7861–7867 . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data