Abstract
Crosslinking of membrane-bound immunoglobulins, which are B-cell antigen receptors, causes proliferation and differentiation of B cells or inhibition of their growth. The receptor-mediated signaling involves tyrosine phosphorylation of cellular proteins and rapid activation of Src-like kinases. The amino acid sequences of five proteolytic peptides of p75, a major substrate of protein-tyrosine(s) in the signaling, showed that p75 is the human HS1 gene product. The HS1 gene is expressed specifically in hematopoietic cells and encodes p75HS1, which carries both helix-turn-helix and Src homology 3 motifs. p75HS1 showed rapid tyrosine phosphorylation and association with a Src-like kinase, Lyn, after crosslinking of membrane-bound IgM. Thus, p75HS1 may be an important substrate of Lyn and possibly other protein-tyrosine kinases upon B-cell antigen receptor-mediated signaling.
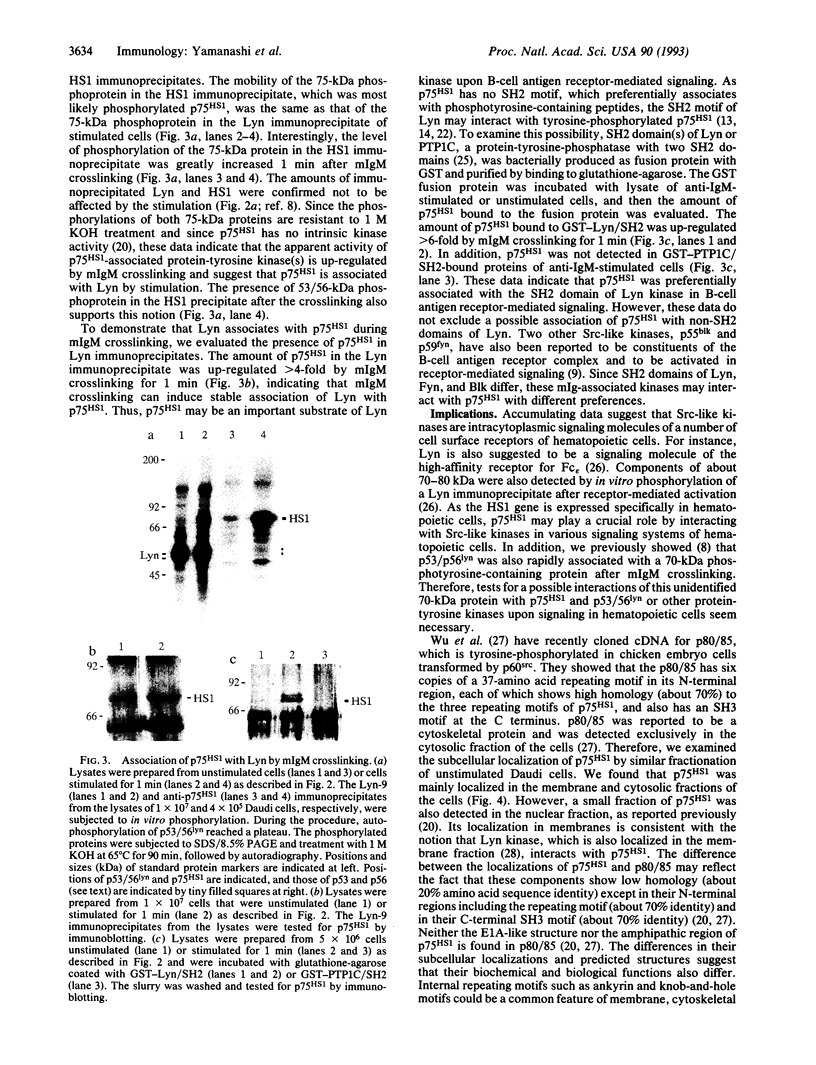
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alés-Martínez J. E., Cuende E., Martínez C., Parkhouse R. M., Pezzi L., Scott D. W. Signalling in B cells. Immunol Today. 1991 Jun;12(6):201–205. doi: 10.1016/0167-5699(91)90054-w. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt A. L., Brunswick M., Bolen J. B., Mond J. J. Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7410–7414. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. S., Hager E. J., Friedrich R. J., Cambier J. C. IgM antigen receptor complex contains phosphoprotein products of B29 and mb-1 genes. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3982–3986. doi: 10.1073/pnas.88.9.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Sefton B. M. Association between B-lymphocyte membrane immunoglobulin and multiple members of the Src family of protein tyrosine kinases. Mol Cell Biol. 1992 May;12(5):2315–2321. doi: 10.1128/mcb.12.5.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carter R. H., Park D. J., Rhee S. G., Fearon D. T. Tyrosine phosphorylation of phospholipase C induced by membrane immunoglobulin in B lymphocytes. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2745–2749. doi: 10.1073/pnas.88.7.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall K. M., McHugh J. C., Altman A. Predominant expression and activation-induced tyrosine phosphorylation of phospholipase C-gamma 2 in B lymphocytes. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5660–5664. doi: 10.1073/pnas.89.12.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiseman E., Bolen J. B. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992 Jan 2;355(6355):78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- Gold M. R., Chan V. W., Turck C. W., DeFranco A. L. Membrane Ig cross-linking regulates phosphatidylinositol 3-kinase in B lymphocytes. J Immunol. 1992 Apr 1;148(7):2012–2022. [PubMed] [Google Scholar]
- Hempel W. M., Schatzman R. C., DeFranco A. L. Tyrosine phosphorylation of phospholipase C-gamma 2 upon cross-linking of membrane Ig on murine B lymphocytes. J Immunol. 1992 May 15;148(10):3021–3027. [PubMed] [Google Scholar]
- Hirano T., Kinoshita N., Morikawa K., Yanagida M. Snap helix with knob and hole: essential repeats in S. pombe nuclear protein nuc2+. Cell. 1990 Jan 26;60(2):319–328. doi: 10.1016/0092-8674(90)90746-2. [DOI] [PubMed] [Google Scholar]
- Hutchcroft J. E., Harrison M. L., Geahlen R. L. Association of the 72-kDa protein-tyrosine kinase PTK72 with the B cell antigen receptor. J Biol Chem. 1992 Apr 25;267(12):8613–8619. [PubMed] [Google Scholar]
- Kitamura D., Kaneko H., Miyagoe Y., Ariyasu T., Watanabe T. Isolation and characterization of a novel human gene expressed specifically in the cells of hematopoietic lineage. Nucleic Acids Res. 1989 Nov 25;17(22):9367–9379. [PMC free article] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Bennett V. Analysis of cDNA for human erythrocyte ankyrin indicates a repeated structure with homology to tissue-differentiation and cell-cycle control proteins. Nature. 1990 Mar 1;344(6261):36–42. doi: 10.1038/344036a0. [DOI] [PubMed] [Google Scholar]
- Nada S., Okada M., MacAuley A., Cooper J. A., Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature. 1991 May 2;351(6321):69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- Reth M., Hombach J., Wienands J., Campbell K. S., Chien N., Justement L. B., Cambier J. C. The B-cell antigen receptor complex. Immunol Today. 1991 Jun;12(6):196–201. doi: 10.1016/0167-5699(91)90053-V. [DOI] [PubMed] [Google Scholar]
- Shen S. H., Bastien L., Posner B. I., Chrétien P. A protein-tyrosine phosphatase with sequence similarity to the SH2 domain of the protein-tyrosine kinases. Nature. 1991 Aug 22;352(6337):736–739. doi: 10.1038/352736a0. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Boguski M. S., Goebl M., Hieter P. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell. 1990 Jan 26;60(2):307–317. doi: 10.1016/0092-8674(90)90745-z. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Kobayashi T., Kondo J., Takahashi K., Nakamura H., Suzuki J., Nagai K., Yamada T., Nakamura S., Yamamura H. Molecular cloning of a porcine gene syk that encodes a 72-kDa protein-tyrosine kinase showing high susceptibility to proteolysis. J Biol Chem. 1991 Aug 25;266(24):15790–15796. [PubMed] [Google Scholar]
- Wu H., Reynolds A. B., Kanner S. B., Vines R. R., Parsons J. T. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol Cell Biol. 1991 Oct;11(10):5113–5124. doi: 10.1128/mcb.11.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi Y., Fukui Y., Wongsasant B., Kinoshita Y., Ichimori Y., Toyoshima K., Yamamoto T. Activation of Src-like protein-tyrosine kinase Lyn and its association with phosphatidylinositol 3-kinase upon B-cell antigen receptor-mediated signaling. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1118–1122. doi: 10.1073/pnas.89.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi Y., Fukushige S., Semba K., Sukegawa J., Miyajima N., Matsubara K., Yamamoto T., Toyoshima K. The yes-related cellular gene lyn encodes a possible tyrosine kinase similar to p56lck. Mol Cell Biol. 1987 Jan;7(1):237–243. doi: 10.1128/mcb.7.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi Y., Kakiuchi T., Mizuguchi J., Yamamoto T., Toyoshima K. Association of B cell antigen receptor with protein tyrosine kinase Lyn. Science. 1991 Jan 11;251(4990):192–194. doi: 10.1126/science.1702903. [DOI] [PubMed] [Google Scholar]
- Yamanashi Y., Miyasaka M., Takeuchi M., Ilic D., Mizuguchi J., Yamamoto T. Differential responses of p56lyn and p53lyn, products of alternatively spliced lyn mRNA, on stimulation of B-cell antigen receptor. Cell Regul. 1991 Dec;2(12):979–987. doi: 10.1091/mbc.2.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi Y., Mori S., Yoshida M., Kishimoto T., Inoue K., Yamamoto T., Toyoshima K. Selective expression of a protein-tyrosine kinase, p56lyn, in hematopoietic cells and association with production of human T-cell lymphotropic virus type I. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6538–6542. doi: 10.1073/pnas.86.17.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamori T., Iizuka Y., Takayama Y., Nishiya S., Iwashita S., Yamazaki A., Takatori T., Tsuruo T. Insulin-like growth factor I rapidly induces tyrosine phosphorylation of a Mr 150,000 and a Mr 160,000 protein in highly metastatic mouse colon carcinoma 26 NL-17 cells. Cancer Res. 1991 Nov 1;51(21):5859–5865. [PubMed] [Google Scholar]