Abstract
Objective
To investigate incidence and timing, risk factors, prognostic significance, and electrophysiological mechanisms of atrial arrhythmia (AA) after lung transplantation.
Background
Although new-onset AA is common after thoracic surgery and is associated with poorer outcomes, prognostic and mechanistic data is sparse in lung transplant populations.
Method
A total of 293 consecutive isolated lung transplant recipients without known AA were retrospectively reviewed. Mean follow-up was 28±17 months. Electrophysiology studies (EPS) were performed in 25 patients with AA.
Results
The highest incidence of new-onset AA after lung transplantation occurred within 30 days postoperative AA, (25 % of all patients). In multivariable analysis, postoperative AA was associated with double lung transplantation (OR 2.79; p=0.005) and lower mean pulmonary artery pressure (OR 0.95; p=0.027). Patients with postoperative AA had longer hospital stays (21 days vs 12 days; p<0.001). Postoperative AA was independently associated with late AA (HR 13.52; p<0.001) but not mortality (HR 1.55; p=0.14). In EPS, there were 14 patients with atrial flutter alone and 11 with atrial flutter and fibrillation. Of all EPS patients, 20 (80%) had multiple AA mechanisms, including peritricuspid flutter (48%), perimitral flutter (36%), right atrial incisional reentry (24%), focal tachycardia from recipient pulmonary vein (PV) antrum (32 %), focal PV fibrillation (24%), and left atrial roof flutter (20%). Left atrial mechanisms were present in 80% (20/25) of EPS patients and originated from the anastomotic PV antrum.
Conclusions
Postoperative AA was independently associated with longer length of stay and late AA but not mortality. Pleomorphic PV antral arrhythmogenesis from native PV antrum is the main cause of AA after lung transplantation.
Keywords: Atrial arrhythmia, Atrial fibrillation, Atrial flutter, Lung transplant
Introduction
For the past decades, lung transplantation has been increasingly performed worldwide.1 Survival after lung transplantation has been reported in the U.S. Organ Procurement and Transplantation Network to be among the lowest survival rates of all adult solid organ transplantations.2 In addition to traditional risk factors for mortality, such as recipient history of diabetes mellitus or use of intravenous inotropes,1 the impact of atrial arrhythmia (AA) after lung transplantation on survival has recently been described.3–6 However, data from published literature have been inconsistent regarding an association between AA and post-lung transplant mortality.3–6
Although AA is common after thoracic surgery, the literature is sparse concerning AA after lung transplantation, specifically with regards to electrophysiological data. The currently accepted mechanistic paradigm of spontaneous atrial fibrillation (AF) in non-postoperative settings is that the pulmonary veins (PV) play a major role7, yet there is no specific evidence demonstrating an association between PV and postoperative AA. However, the occurrence of AA post lung transplantation has been reported to be higher than that of other thoracic surgeries, e.g. coronary artery bypass graft surgery,8 lung resection,9 or heart transplantation.10 During the lung transplantation surgical procedure, some or all of the recipient’s PV are surgically modified to create an anastomosis with the donor’s PV. Variable portions of donor’s atrial tissue remnants may be connected to variable portions of recipient’s PV and atrial tissue. Fibrosis at the surgical anastomosis between heterologous tissues theoretically should act as a barrier for the propagation of electrical impulses. The surgical instrumentation at or around the PV -where AF commonly originates- suggests a particular susceptibility of lung transplant recipients to AA.
In this study, we sought to investigate unclear aspects of AA after lung transplant, including: 1) incidence and timing, 2) risk factors, 3) prognostic significance, and 4) electrophysiological mechanisms.
Methods
Study design and patient selection
A retrospective observational study of consecutive patients who underwent isolated lung transplantation between June 2007 and February 2013 was conducted. A total of 324 cases of isolated lung transplantation were identified. Patients with preexisting history of AA prior to transplantation were excluded (n = 31), yielding a final cohort of 293 cases of isolated lung transplantation without prior history of AA. Institutional Review Board approval was obtained from Houston Methodist Hospital for this study.
Data collection and patients characteristics
Patient preoperative demographics, operative data, postoperative clinical features, and clinical events during the follow-up period were collected through review of medical record and lung transplantation registry databases. We categorized primary lung pathology according to the United Network for Organ Sharing classification of lung diseases.11 Group A is obstructive lung diseases (e.g., emphysema). Group B is pulmonary vascular diseases (e.g., primary pulmonary hypertension). Group C is cystic fibrosis or other immunodeficiency disorders. Group D is restrictive lung diseases (e.g., idiopathic pulmonary fibrosis).
The presence of coronary artery disease (CAD) was determined by coronary angiography. Pulmonary hypertension was defined by mean pulmonary artery pressure (mPAP) of more than 3.3 kPa (25 mmHg) obtained with a standard right heart catheterization.
Study outcomes
Clinical events were examined through systematic review of the medical record database, lung transplantation registry records and social security death index searches. The outcomes evaluated in this study were postoperative AA, late AA, all-cause mortality, new stroke, and postoperative length of stay.
Postoperative AA was defined as postoperative AF or atrial flutter (AFl) within 30 days during the index hospitalization for lung transplantation. Diagnosis of postoperative AA in our study required documentation of AA in twelve-lead electrocardiogram (EKG). Decision to acquire twelve-lead EKG was made by the patients’ medical team based on suspicion of cardiac arrhythmia on continuous telemetric EKG monitoring or other clinical indications. All patients were under twenty-four-hour EKG monitoring from hospital admission until discharge and only AA lasting at least 30 seconds duration was included. Late AA was defined by AF or AFl that occurred at any time during the follow-up period (≥ 30 days) after the index transplantation hospital discharge. These included: routine follow up clinic visits at 1, 3, 6, 9, 12 months and then annually, emergency room visit records and hospital admission records. AA rhythm was required to be documented in twelve-lead EKG to meet our criteria for late AA. All-cause mortality included any death after lung transplantation. Time to death was calculated from the date of lung transplantation to the date of death. Postoperative length of stay was calculated from the date of lung transplantation to the date of hospital discharge.
Electrophysiology studies and ablation
Patients with postoperative AA were treated medically with ventricular-rate-control strategy (n=40), and transient administration of anti-arrhythmic medications (n=33). Those with AA persisting or occurring beyond one month postoperatively were considered for invasive electrophysiology studies and ablation (n=25). Briefly, vascular access was obtained through the femoral and jugular veins. Multipolar catheters were positioned in the coronary sinus or tricuspid annulus as needed. Intracardiac echocardiography was used to guide trans-septal puncture and 3-dimensional maps of propagation patterns were constructed using NavX (St Jude Medical, St Paul, MN, USA) or CARTO (Biosense-Webster, Diamond Bar, CA, USA) mapping systems. Irrigated ablation catheters (Thermocool, Biosense-Webster) were used for radiofrequency ablation.
Statistical Analysis
Independent Student’s t-test was used to compare normally-distributed continuous variables. Wilcoxon-Mann-Whitney U test was utilized to compare non-normally-distributed continuous variables. For comparison between categorical variables, chi square analysis (and Fisher’s Exact when necessary) was performed.
To identify possible risk factors for postoperative AA, univariable and multivariable analyses using logistic regression models were performed. All study variables with p-values of less than 0.25 in univariable analyses were included in multivariable modeling procedures.12 The risk for developing late AA, new stroke, and death associated with postoperative AA was examined using Cox regression modeling. The assumption of proportional hazard was met by using graphical methods. All univariable predictors with p-values < 0.25 were included into multivariable Cox regression models. The logistic and Cox proportional regression analysis results are presented as odd ratio (OR) and hazard ratio (HR) with 95% confidence interval (CI) respectively.
All statistical analyses were performed with IBM SPSS/PASW Statistics 20 (SPSS Inc., Chicago, IL). A two-tailed p-value of < 0.05 was considered statistically significant.
Results
Patient characteristics
The final cohort comprised of 293 patients with a mean age of 57±13 years. Fifty-eight percent of patients were male. Primary lung pathologies were obstructive lung diseases (Group A, 26%), pulmonary vascular diseases (Group B, 2%), cystic fibrosis or immunodeficiency disorders (Group C, 7%), and restrictive lung diseases (Group D, 65%). The median lung allocation score prior to lung transplantation was 38 (IQR 34–44). Double lung transplantation was performed in 63% of the patients. Mean ischemic time was 205±66 minutes.
New-onset AA after lung transplantation: Incidence and Timing
New-onset AA occurred in 31% (90/293) of patients after lung transplantation. AA incidence exhibited a bimodal distribution with the highest incidence in the immediate postoperative period (postoperative AA) accounting for 81% (73/90) of all AA. The second smaller peak in AA incidence occurred 3–4 years post transplant (19%;17/90), as demonstrated in Figure 1. Incidence of postoperative AA peaked at the 5th postoperative day (median = 5 days, IQR = 3–9 days) and reached 95% by day 15 post lung transplant, as shown in Figure 1. Incidence of AF was higher than AFl in the immediate postoperative period; however, incidence of AFl was increasing as time passed (Figure 1).
Figure 1.
Incidence of postoperative atrial arrhythmia and time to onset after isolated lung transplantation in patients without known history of atrial arrhythmia
Postoperative AA: Risk factors
Associations between patient characteristics and postoperative AA are summarized in Table 1. Compared to patients without postoperative AA, those with postoperative AA had a significant higher mean age (p=0.006), higher proportion of male gender (p=0.001), and higher mean body mass index (BMI; p=0.038), but lower rate of pulmonary hypertension (p=0.034). Analysis of preoperative workups revealed that patients with postoperative AA had a significantly larger left ventricular end diastolic diameter (LVEDD) detected by preoperative echocardiography (p=0.01) and lower invasive mPAP (p=0.040) than those without postoperative AA. Patients with postoperative AA underwent double lung transplantation at a higher rate (p=0.043) and required vasopressors more frequently in the postoperative period (p=0.001) than those without postoperative AA. Other patient characteristics and their OR were not significantly different between the two groups (Table 1).
Table 1.
Postoperative atrial arrhythmia
| Characteristics | No postoperative AA n = 220 |
With postoperative AA n = 73 |
Univariable OR (95%CI) |
p-value | Multivariable OR(95%CI) |
p-value |
|---|---|---|---|---|---|---|
| Age(year) | 56±14 | 60±10 | 1.03 (1.01,1.06) | 0.007 | 1.03 (1.00,1.07) | 0.061 |
| Male gender | 114 (51.8) | 54 (74.0) | 2.64 (1.47,4.75) | 0.001 | 1.97 (0.95,4.09) | 0.069 |
| BMI(kg/m2) | 25.1±5.6 | 26.7±5.2 | 1.05 (1.00,1.10) | 0.040 | 1.05 (0.98,1.13) | 0.174 |
| Pulmonary pathology | ||||||
| Group A; obstructive | 60 (27.3) | 17 (23.3) | 1.00 (Reference) | - | - | - |
| Group B; vascular | 5 (2.3) | 1 (1.4) | 0.71 (0.08,6.46) | 0.758 | - | - |
| Group C; immunologic | 17 (7.7) | 4 (5.5) | 0.83 (0.25,2.80) | 0.764 | - | - |
| Group D; restrictive | 138 (62.7) | 51 (69.9) | 1.30 (0.70,2.44) | 0.406 | - | - |
| Preoperative O2 support (lpm) | 4 (3–6) | 4 (3–6) | 0.98 (0.93,1.04) | 0.507 | - | - |
| Ventilator/ECMO | 6 (2.7) | 1 (1.4) | 0.50 (0.06,4.18) | 0.519 | - | - |
| History of CAD | 88 (40.0) | 33 (45.2) | 1.20 (0.68,2.11) | 0.534 | - | - |
| History of hypertension | 116 (52.7) | 42 (57.9) | 1.22 (0.71,2.07) | 0.476 | - | - |
| History of diabetes mellitus | 69 (31.4) | 17 (23.3) | 0.66 (0.36,1.23) | 0.191 | 0.48 (0.22,1.07) | 0.074 |
| History of dyslipidemia | 86 (39.1) | 37 (50.7) | 1.60 (0.94,2.73) | 0.083 | 1.58 (0.43,5.79) | 0.491 |
| History of smoking | 88 (40.0) | 33 (45.2) | 1.24 (0.73,2.11) | 0.434 | - | - |
| History of stroke | 4 (1.8) | 1 (1.4) | 0.75 (0.08,6.82) | 0.798 | - | - |
| CHADS2 score | 1 (0–1) | 1 (0–1) | 0.80 (0.47,1.38) | 0.429 | - | - |
| Serum creatinine (mg/dl) | 0.8±0.3 | 0.8±0.3 | 1.20 (0.43,3.35) | 0.726 | - | - |
| Cardiovascular medications | ||||||
| Statins | 76 (34.5) | 31 (42.5) | 1.40 (0.81,2.40) | 0.224 | 0.83 (0.24,3.31) | 0.866 |
| Aspirin | 50 (22.7) | 20 (27.4) | 1.28 (0.70,2.35) | 0.418 | - | - |
| Beta blockers | 40 (18.2) | 13 (17.8) | 0.98 (0.49,1.95) | 0.943 | - | - |
| ACEI | 28 (12.7) | 11 (15.1) | 1.22 (0.57,2.59) | 0.610 | - | - |
| ARB | 21 (9.5) | 7 (9.6) | 1.01 (0.41,2.47) | 0.991 | - | - |
| Calcium channel blockers | 36 (16.4) | 13 (17.8) | 1.11 (0.55,2.23) | 0.774 | - | - |
| Diuretics | 68 (30.9) | 18 (24.7) | 0.73 (0.40,1.34) | 0.311 | - | - |
| Echocardiographic data | ||||||
| LVEF (%) | 60±6 | 60±5 | 1.00 (0.96,1.05) | 0.873 | ||
| LVEDD (cm) | 4.1±0.6 | 4.4±0.5 | 1.91 (1.16,3.15) | 0.011 | 1.37 (0.75,2.50) | 0.307 |
| IVSD (cm) | 1.00±0.89 | 0.92±0.36 | 0.83 (0.50,1.39) | 0.479 | - | - |
| LAV (cm) | 40±19 | 43±21 | 1.01 (0.99,1.03) | 0.315 | - | - |
| LAA (cm2) | 16±10 | 16±5 | 1.00 (0.97,1.04) | 0.929 | - | - |
| Right heart catheterization data | ||||||
| mPAP (kPa) | 3.47±1.33 (26±10 mmHg) | 3.20±1.07 (24±8 mmHg) | 0.97 (0.93,1.00) | 0.042 | 0.95 (0.91,0.99) | 0.027 |
| Pulmonary hypertension | 88 (43.6) | 18 (28.6) | 0.52 (0.28,0.96) | 0.036 | - | - |
| PCWP (kPa) | 1.33±0.67 (10±5 mmHg) | 1.33±0.67 (10±5 mmHg) | 1.00 (0.94,1.06) | 0.900 | - | - |
| Double lung transplant | 130 (59.4) | 53 (72.6) | 1.81 (1.02,3.24) | 0.044 | 2.79 (1.36,5.75) | 0.005 |
| Lung allocation score | 38.2 (33.6–45.0) | 37.5 (34.5–43.8) | 1.00 (0.99,1.02) | 0.765 | ||
| Ischemic time(minute) | 202±68 | 217±61 | 1.00 (1.00,1.01) | 0.096 | 1.00 (0.99,1.01) | 0.732 |
| Peak troponin (mg/dl) | 6.2 (3.5–12.5) | 8.6 (3.6–15.1) | 1.00 (1.00,1.01) | 0.509 | - | - |
| Vasopressor use | 179 (81.4) | 68 (93.2) | 3.12 (1.18,8.21) | 0.022 | 1.81 (0.55,5.98) | 0.329 |
In multivariable analysis, mPAP (OR 0.95;95%CI 0.91–0.99; p=0.027) and double lung transplant (OR 2.79;95%CI 1.36–5.75; p=0.005) were significantly associated with postoperative AA, as shown in Table 1.
Postoperative AA: Prognosis
Mean follow up after lung transplant was 28±17 months. During the follow up period 64 deaths, 42 late AA, and 3 strokes occurred.
Postoperative length of stay
Median postoperative length of stay of all patients was 14 days (IQR 9–26 days). Patients with postoperative AA had significantly higher postoperative length of stays than patients without postoperative AA (21 days, IQR 13–32 days vs 12 days, IQR 8–21 days; p<0.001).
Occurrence of late AA
After discharge from index hospitalization for lung transplantation, late AA occurred in 14% (42/293) of the cohort. The median interval from lung transplant to late AA was 13.9 months (IQR 2.4–30.8 months). Development of late AA was significantly higher in lung transplant recipients who had postoperative AA than recipients without postoperative AA (34% vs 8%; p<0.001). Differences in patient characteristics of patients with and without late AA and their associations with late AA are shown in Table 2. In the multivariable model, postoperative AA (HR 13.52;95%CI 3.90–46.93; p<0.001), Group B primary lung pathology (HR 80.83;95%CI 4.07–1604.32; p=0.004), history of CAD (HR 3.89;95%CI 1.03–14.68; p=0.045), and pre-transplant use of statins (HR 0.17;95% CI 0.05–0.65; p=0.009) were independently associated with late AA, as shown in Table 2. There was no significant association between specific type of postoperative AA (AF vs AFl) and type of late AA (AF vs AFl) (p>0.05). Mortality impact of late AA was analyzed using Kaplan-Meier statistics. There was no significant difference in median survival between patients with and without late AA (p=0.749; log-rank test).
Table 2.
Late atrial arrhythmia
| Characteristics | No late AA n = 251 |
With late AA n = 42 |
Univariable HR(95%CI) |
p-value | Multivariable HR(95%CI) |
p-value |
|---|---|---|---|---|---|---|
| Age(year) | 56±14 | 61±11 | 1.04 (1.01,1.07) | 0.015 | 1.03 (0.97,1.09) | 0.364 |
| Male gender | 140 (55.8) | 28 (66.7) | 1.59 (0.83,3.02) | 0.159 | 0.89 (0.27,2.93) | 0.852 |
| BMI(kg/m2) | 25.3±5.6 | 26.7±5.2 | 1.05 (0.99,1.11) | 0.103 | 1.00 (0.90,1.10) | 0.955 |
| Pulmonary pathology | ||||||
| Group A; obstructive | 66 (26.3) | 11 (26.2) | 1.00 (Reference) | - | 1.00 (Reference) | - |
| Group B; vascular | 3 (1.2) | 3 (7.1) | 4.21 (1.16,15.26) | 0.028 | 80.83 (4.07,1604.32) | 0.004 |
| Group C; immunologic | 20 (8.0) | 1 (2.4) | 0.33 (0.04,2.57) | 0.291 | - | - |
| Group D; restrictive | 162 (64.5) | 27 (64.3) | 1.32 (0.65,2.67) | 0.438 | - | - |
| Preoperative O2 support(lpm) | 4 (3–6) | 2 (2–5) | 0.97 (0.90,1.04) | 0.369 | - | - |
| Ventilator/ECMO | 7 (2.8) | 0 (0) | 0.05 (0.01,>100) | 0.586 | - | - |
| History of CAD | 99 (46.5) | 22 (57.9) | 1.54 (0.81,2.95) | 0.188 | 3.89 (1.03,14.68) | 0.045 |
| History of hypertension | 131 (52.2) | 27 (64.3) | 1.63 (0.87,3.06) | 0.131 | 1.40 (0.42,4.65) | 0.586 |
| History of diabetes mellitus | 71 (28.3) | 15 (35.7) | 1.57 (0.83,2.95) | 0.165 | 2.41 (0.73,7.96) | 0.149 |
| History of dyslipidemia | 104 (41.4) | 19 (45.2) | 1.30 (0.71,2.39) | 0.404 | - | - |
| History of smoking | 103 (41.0) | 18 (42.9) | 1.04 (0.57,1.93) | 0.891 | - | - |
| History of stroke | 5 (2.0) | 0 (0) | 0.05 (0.01,>100) | 0.543 | - | - |
| CHADS2 score | 1 (0–1) | 1 (1–2) | 1.08 (0.59,1.99) | 0.808 | - | - |
| Serum creatinine (mg/dl) | 0.8±0.2 | 0.8±0.3 | 2.11 (0.67,6.70) | 0.204 | 0.78 (0.12,5.10) | 0.799 |
| Cardiovascular medications | ||||||
| Statins | 96 (38.2) | 11 (26.2) | 0.65 (0.32,1.28) | 0.212 | 0.17 (0.05,0.65) | 0.009 |
| Aspirin | 61 (24.3) | 9 (21.4) | 0.86 (0.41,1.81) | 0.699 | - | - |
| Beta blockers | 41 (16.3) | 12 (28.6) | 2.04 (1.04,3.99) | 0.037 | 1.06 (0.28,4.01) | 0.933 |
| ACEI | 33 (13.1) | 6 (14.3) | 1.26 (0.53,2.98) | 0.606 | - | - |
| ARB | 25 (10.0) | 3 (7.1) | 0.62 (0.19,2.00) | 0.423 | - | - |
| Calcium channel blockers | 40 (15.9) | 9 (21.4) | 1.50 (0.72,3.14) | 0.280 | - | - |
| Diuretics | 72 (28.7) | 14 (33.3) | 1.44 (0.75,2.73) | 0.271 | - | - |
| Echocardiographic data | ||||||
| LVEF (%) | 60±6 | 60±4 | 1.01 (0.95,1.06) | 0.851 | - | - |
| LVEDD (cm) | 4.2±0.6 | 4.3±0.6 | 1.25 (0.71,2.21) | 0.447 | - | - |
| IVSD (cm) | 1.00±0.83 | 0.89±0.41 | 0.73 (0.33,1.62) | 0.443 | - | - |
| LAV (cm3) | 40±19 | 44±18 | 1.01 (0.99,1.04) | 0.176 | 0.99 (0.96,1.02) | 0.543 |
| LAA (cm2) | 16±9 | 17±8 | 1.01 (0.98,1.05) | 0.360 | - | - |
| Right heart catheterization data | ||||||
| mPAP (kPa) | 3.47±1.20 (26±9 mmHg) | 3.60±1.60 (27±12 mmHg) | 1.01 (0.98,1.04) | 0.508 | - | - |
| Pulmonary hypertension | 89 (39.2) | 17 (44.7) | 1.18 (0.62,2.23) | 0.620 | - | - |
| PCWP (kPa) | 1.33±0.53 (10±4 mmHg) | 1.33±0.80 (10±6 mmHg) | 1.01 (0.94,1.08) | 0.874 | - | - |
| Double lung transplant | 157 (62.8) | 26 (61.9) | 0.98 (0.53,1.84) | 0.959 | - | - |
| Lung allocation score | 37.8 (33.8–45.0) | 38.2 (33.9–43.6) | 1.00 (0.98,1.02) | 0.795 | - | - |
| Ischemic time (minute) | 204±67 | 213±63 | 1.00 (1.00,1.01) | 0.361 | - | - |
| Peak troponin (mg/dl) | 7.0 (3.7–12.5) | 8.0 (4.1–17.8) | 1.00 (0.99,1.01) | 0.728 | - | - |
| Vasopressor use | 212 (84.5) | 35 (83.3) | 1.26 (0.56,2.87) | 0.575 | - | - |
| Postoperative AA | 48 (19.1) | 25 (59.5) | 6.08 (3.25,11.38) | <0.001 | 13.52 (3.90,46.93) | <0.001 |
New stroke
New ischemic stroke occurred in 3 cases during the follow-up period. Two cases were in the postoperative AA group (1.4 years and 3.0 years post transplant) and another case was in the no postoperative AA group (2.0 years post transplant). All of these patients did not have late AA documented and their CHADS2 scores were 1, therefore they were not on anticoagulation therapy. CHADS2 score was not statistically different between the postoperative AA group and no postoperative AA group (1 IQR 0–1 vs 1 IQR 0–1; p=0.574). Having a postoperative AA was not associated with new stroke after lung transplantation during follow-up (Univariable HR 6.17; p=0.140).
All-cause mortality
Overall, death occurred in 22% (64/293) of the cohort (median time to death = 3.1 months; IQR 0.9–13.3): 31% (22/73) in the postoperative AA group versus 19% (42/220) in the without postoperative AA group. Kaplan-Meier statistics showed overall median survival was 49 months. Significant univariable predictors for death were history of smoking (HR 1.71;95%CI 1.05–2.79; p=0.032), postoperative AA (HR 1.71;95%CI 1.02–2.86; p=0.043), and peak troponin postoperatively (HR 1.01;95%CI 1.00–1.01; p=0.036). In multivariable analysis, postoperative AA did not have a statistically significant association with death (HR 1.55;95%CI 0.87–2.77; p=0.139). Only history of smoking (HR 2.02;95%CI 1.17–3.49; p=0.012) and Group D primary lung pathology (HR 2.50;95%CI 1.17–5.33; p=0.018 compared to Group A pathology) were independently associated with death. There was no significant association between mortality and early AF, early AFl, late AA, late AF or late AFl (p>0.05 compared to no AA).
AA management and electrophysiology studies
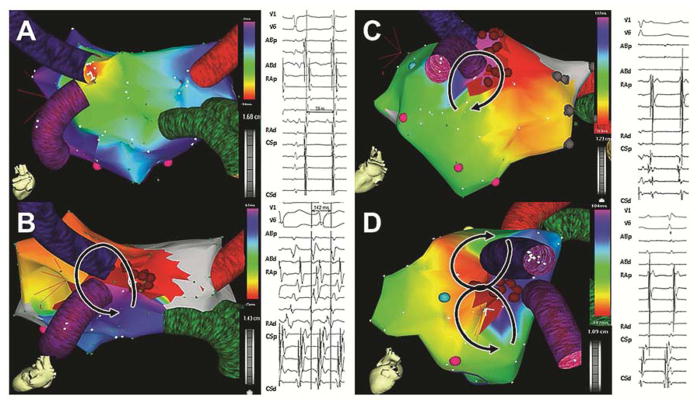
A total of 25 patients underwent invasive EPS due to symptomatic AFl (56%;14/25) or coexisting AFl and AF (44 %;11/25). Table 4 shows a summary of the AA mechanisms. All PV antral arrhythmias could be linked to the native, recipient PV antrum at the anastomotic side. Complex, multicomponent fractionated local electrograms were recorded from the PV antrum during PV antral focal tachycardias (Figure 2AB) or PV antral reentry (Figure 2E). Electrical activity from the donor’s PV antrum was either absent or dissociated from the atrial activations (Figure 2C). In 3 patients, 2:1 activation patterns were demonstrable from the PV antrum to the rest of the left atrium, but activations were all within the native recipient’s tissue (Figure 2D). We did not find evidence of electrical connections between the donor and recipient tissue through the surgical anastomosis. Even after ablation of focal PV tachycardia, reentrant left atrial rhythms were commonly inducible (Figure 2F). Multiple mechanisms were inducible in most patients (80%), including multiple mechanisms of anastomotic PV antral arrhythmia (Figure 3). Overall, arrhythmias arising from the left atrium only were present in 80% of the patients, and arrhythmias arising exclusively from the right atrium were present in 20%.
Figure 2.
Pulmonary vein (PV) antral arrhythmogenesis. PV antral tachycardia arose from the inferior aspect of the native and anastomotic left PV atrum (A, B). The site of origin had complex, prolonged fractionated signals consistent with local conduction slowing as marked by the red arrow (B). The donor PV was not part of the arrhythmia and had dissociated signals (C). Focal activations arose from the native, anastomotic right inferior PV antrum with a 2:1 conduction pattern over the neighboring left atrium, as shown by the red arrow (D). The site of origin was the native PV antrum, as shown by the close proximity of the recording sites, acquired by the circular multipolar catheter in the same position. Coexisting roof-dependent (E) and perimitral flutter (F) in the same patient, with an area of slow conduction (ablation site indicated by the red arrow and asterisk in E).
Figure 3.
Multiple expressions of pulmonary vein (PV) antral arrhythmogenesis in one patient. The anastomotic left PV was the origin of focal atrial tachycardia (A), counterclockwise PV reentry (B), clockwise PV antral reentry (C), and figure-eight (D).
Discussion
The primary results in our study were the bimodal distribution of time to occurrence of new-onset AA after lung transplantation, analysis of risk factors for new-onset AA, and analysis of its prognostic implications. In addition, our study also included the largest series in the literature of electrophysiological findings in lung transplant recipients who developed postoperative AA, giving important insight to electrophysiological mechanisms.
AA incidence, risk factors and prognostic significance
We showed that the incidence of new-onset AA following lung transplantation displayed a bimodal distribution with the highest incidence in the early postoperative period, within 30 days, followed by the second lower peak at 3–4 years post-transplant. In contrast to postoperative AA, which was comprised mainly of AF, late AA was comprised of AF and AFl equally. This distribution is consistent with a previous study that revealed the second rise in occurrence of AA was due to AFl rather than AF.13 Our cumulative incidence of postoperative AA was also similar to other prior studies that reported cumulative incidence of postoperative AA at 19%–28% before hospital discharge,4, 13–16 39% within 14 days,3 and 34% by 4 weeks.5, 16 In our study, the peak incidence of postoperative AA was at 5 days post-transplant, which was consistent with the previous studies that reported the peak incidences of combined AA between 2–5 days after the transplantation 3, 4, 15 but earlier than the peak incidence of pure AFl which was between 10–12 days 17.
In this study, we found invasive mPAP to be inversely associated with postoperative AA. Previous literature reporting relationship between PAP and post lung transplant AA are inconsistent including positive5, no significant4 or even negative18 relationship between invasive PAP and postoperative AA. The explanation of this finding discrepancy are unclear. Several theories for the inverse relationship between PAP and postoperative AA have been proposed, which include the protective effect of higher right heart pressures for development of postoperative AA secondary to dilatation of left atrium.18
With regard to prognosis, our study detected the impact of postoperative AA on mortality, postoperative length of stay, and the occurrence of late AA after being discharged from the lung transplant hospital stay; however, the association of postoperative AA and higher mortality became insignificant after adjustment in the multivariable analysis. These findings suggested that postoperative AA was a marker of a higher risk patient. The current evidence showing risk of death associated with postoperative AA following lung transplantation is still inconsistent.3–6 Differences in patient demographics and management might have contributed to this inconsistency.
We also showed that postoperative AA predicted occurrence of AA after the index hospital discharge (late AA), as previously reported.14 This finding may have important clinical implications since anticoagulation therapy in those with high risk for stroke (e.g. CHADS2 score ≥ 2) and continuous ambulatory EKG monitoring could be considered in patients with postoperative AA upon hospital discharge. History of CAD and statin therapy has also been identified as independent predictors for late AA. These results were consistent with previous literature, which demonstrated an association between AF and CAD;19, 20 however, the impact of preoperative statin use on new-onset AF after non-cardiac surgery remains unsettled.21 In term of prognosis related to late AA, we did not detect the difference in survival between those with and without late AA.
AA mechanisms: iatrogenic PV antral arrhythmogenesis
Our study provides notable mechanistic understanding of postoperative AA. Multiple mechanisms of AA were present, often in the same patient. Typical peritricuspid AFl was the most common arrhythmia, but usually it was present in combination with other mechanisms, or induced during EPS. Roughly, electrophysiological mechanisms of AA can be divided into right atrial mechanisms (peritricuspid or right atrial incisional reentry) and left atrial mechanisms (anastomotic PV antral, roof, or perimitral reentry). See and colleagues have determined that anastomotic regions are common sites of focal activation.13 However, some AA have also been reported to originate from the donor’s side with conduction across the anastomosis as previously suggested in both lung13 and heart transplantation.22, 23 In our study, we could not demonstrate such connectivity between heterologous tissues over the surgical anastomosis. In our series, the origin of focal PV antral arrhythmogenesis was consistently the native PV antrum, an otherwise well-documented origin of atrial arrhythmias.7 We propose that the surgical anastomosis creates an inflammatory process and atrial stretching in the native PV antrum that leads to its arrhythmogenesis. This is further supported by our observation that double lung transplant, as compared to single lung transplant, was associated with increased risk of postoperative AA. Surgically, double lung transplant involves more extensive area of cut-and-sew than single lung transplant, which theoretically would lead to higher inflammatory response and atrial stretching than single lung transplantation.24 The specific propagation patterns are pleomorphic and range from focal atrial tachycardias (often with 2:1 propagation) to secondary macroreentrant patterns in the left atrium (perimitral, peritricuspid), and often coexist in individual patients. These findings may have important clinical implications in consideration of alternative lung transplant surgical techniques or intraoperative prophylactic interventions for AA; however, further research is much needed.
Limitations
There were several limitations to our study. First, our study was a retrospective observational cohort study, therefore causal relationship could not be assumed and there might still be confounding effect despite our best attempt to adjust for it by statistical means. Second, to satisfy the definition of AA in our study, AF and AFl had to be documented in twelve-lead EKG. We decided on this definition because we observed that numerous artifacts on telemetric EKG could look similar to AA rhythm but were not confirmed on a twelve-lead EKG. This could have led to underestimation of AA incidence, especially during the acute post-transplant period. AA is usually paroxysmal and short-lived in nature. However, whether these short-lived AA occurrences affect outcomes is not clear. In order to have the most accurate detection of AA, precise, continuous cardiac rhythm monitoring has to be employed in all study participants, regardless of symptoms or degree of clinical suspicion of AA, which is not practical clinically. Additionally, late AA in our study was mostly symptomatic late AA, as no ambulatory, continuous EKG monitoring was employed in our patients. The patients were evaluated with twelve-lead EKG based on clinical suspicion of AA. Third, despite our relatively long-term follow up, some of the more organized AA can certainly have much more delayed onset and this may potentially explain the differences in findings between our study and previous literature. Last, electrophysiological studies were only performed on a small proportion of selected patients based on their clinical presentations, therefore; the findings may limit generalizability.
Conclusion
New-onset AA is common after adult lung transplantation. Its incidence exhibited bimodal distribution over time from transplant, with the highest occurrence during the postoperative period. Development of postoperative AA has prognostic implication on length of stay and occurrence of late AA after the hospital discharge, but not survival. From our clinical and electrophysiological findings, we propose that the surgical anastomosis creates an inflammatory process and anatomical distortion in the native PV antrum that leads to its arrhythmogenesis.
Table 3.
Mechanisms of postoperative atrial arrhythmia by electrophysiology studies
| Findings | n | % |
|---|---|---|
| Atrial fibrillation | 11 | 44 |
| Atrioventricular node reentrant tachycardia | 3 | 12 |
| Cavo-tricuspid isthmus-dependent atrial flutter | 12 | 48 |
| Incisional right atrial flutter | 6 | 24 |
| PV focal atrial tachycardia (juxta-anastomotic) | 8 | 32 |
| PV antral reentry (juxta-anastomotic) | 5 | 20 |
| PV antral fibrillation (juxta-anastomotic) | 6 | 24 |
| Roof-dependent atrial flutter | 5 | 20 |
| Perimitral atrial flutter | 9 | 36 |
| Mechanism categories | n | % |
|---|---|---|
| Single mechanism | 5 | 20 |
| Multiple mechanisms | 20 | 80 |
| Exclusively right atrial mechanisms | 5 | 20 |
| Left atrial mechanisms | 20 | 80 |
Perspectives.
Competency in Medical Knowledge
Postoperative atrial arrhythmia is common after lung transplantation. Development of postoperative atrial arrhythmia has prognostic implication on occurrence of late atrial arrhythmia after the hospital discharge but not survival. The arrhythmogenesis of postoperative atrial arrhythmia is from inflammatory processes at the surgical anastomosis sites and anatomical distortion in the native pulmonary vein antrum.
Translational Outlook 1
Although continuous electrocardiogram monitoring was employed for all patients during the entire hospitalization, underestimation the arrhythmia incidence could still have occurred especially during acute post-transplant period since atrial arrhythmia is usually paroxysmal and short-lived in nature.
Acknowledgments
The authors appreciate the help of Jennifer P. Connell, PhD, for the critical reading and editing of the manuscript.
Abbreviations list
- AA
atrial arrhythmia
- ACEI
angiotensin-converting enzyme inhibitors
- AF
atrial fibrillation
- AFl
atrial flutter
- ARB
angiotensin II receptor blockers
- BMI
body mass index
- CAD
coronary artery disease
- CI
confidence interval
- ECMO
extracorporeal membrane oxygenation
- EKG
electrocardiogram
- EPS
electrophysiology study
- HR
hazard ratio
- IQR
interquartile range
- IVSD
interventricular septal diameter
- LAA
left atrial area
- LAV
left atrial volume
- lpm
liter per minute
- LVEDD
left ventricular end diastolic diameter
- LVEF
left ventricular ejection fraction
- mPAP
mean pulmonary artery pressure
- PAP
pulmonary artery pressure
- PCWP
pulmonary capillary wedge pressure
- PV
pulmonary vein
- SD
standard deviation
Footnotes
Conflict of interest: None
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, Taylor DO, Kucheryavaya AY, Hertz MI. Registry of the international society for heart and lung transplantation: Twenty-fifth official adult lung and heart/lung transplantation report--2008. J Heart Lung Transplant. 2008;27:957–969. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 2.2004 Annual Report of the US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1994–2003. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; Rockville, MD: United Network for Organ Sharing; Richmond, VA: University Renal Research and Education Association; Ann Arbor, MI: [Google Scholar]
- 3.Nielsen TD, Bahnson T, Davis RD, Palmer SM. Atrial fibrillation after pulmonary transplant. Chest. 2004;126:496–500. doi: 10.1378/chest.126.2.496. [DOI] [PubMed] [Google Scholar]
- 4.Mason DP, Marsh DH, Alster JM, Murthy SC, McNeill AM, Budev MM, Mehta AC, Pettersson GB, Blackstone EH. Atrial fibrillation after lung transplantation: Timing, risk factors, and treatment. Ann Thorac Surg. 2007;84:1878–1884. doi: 10.1016/j.athoracsur.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Isiadinso I, Meshkov AB, Gaughan J, Sandhu P, Lim S, Cordova F, Criner G. Atrial arrhythmias after lung and heart-lung transplant: Effects on short-term mortality and the influence of amiodarone. J Heart Lung Transplant. 2011;30:37–44. doi: 10.1016/j.healun.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Orrego CM, Cordero-Reyes AM, Estep JD, Seethamraju H, Scheinin S, Loebe M, Torre-Amione G. Atrial arrhythmias after lung transplant: Underlying mechanisms, risk factors, and prognosis. J Heart Lung Transplant. 2014;33:734–740. doi: 10.1016/j.healun.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 8.Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, Collins JJ, Jr, Cohn LH, Burstin HR. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996;94:390–397. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 9.Roselli EE, Murthy SC, Rice TW, Houghtaling PL, Pierce CD, Karchmer DP, Blackstone EH. Atrial fibrillation complicating lung cancer resection. J Thorac Cardiovasc Surg. 2005;130:438–444. doi: 10.1016/j.jtcvs.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Ommen SR, Odell JA, Stanton MS. Atrial arrhythmias after cardiothoracic surgery. N Engl J Med. 1997;336:1429–1434. doi: 10.1056/NEJM199705153362006. [DOI] [PubMed] [Google Scholar]
- 11.United network for organ sharing (unos) A guide to calculating the lung allocation score. available from https://www.Unos.Org/docs/lung_allocation_score.Pdf.
- 12.Lemeshow HA. Applied logistic regression. New York, NY: Wiley; 1989. [Google Scholar]
- 13.See VY, Roberts-Thomson KC, Stevenson WG, Camp PC, Koplan BA. Atrial arrhythmias after lung transplantation: Epidemiology, mechanisms at electrophysiology study, and outcomes. Circ Arrhythm Electrophysiol. 2009;2:504–510. doi: 10.1161/CIRCEP.109.867978. [DOI] [PubMed] [Google Scholar]
- 14.Lee G, Wu H, Kalman JM, Esmore D, Williams T, Snell G, Kistler PM. Atrial fibrillation following lung transplantation: double but not single lung transplant is associated with long-term freedom from paroxysmal atrial fibrillation. Eur Heart J. 2010;31:2774–2782. doi: 10.1093/eurheartj/ehq224. [DOI] [PubMed] [Google Scholar]
- 15.Kogan A, Ilgaev N, Sahar G, Kramer M, Saute M, Aravot D, Berman M, Vidne BA. Atrial fibrillation after adult lung transplantation. Transplant Proc. 2003;35:679. doi: 10.1016/s0041-1345(03)00096-4. [DOI] [PubMed] [Google Scholar]
- 16.Dizon JM, Chen K, Bacchetta M, Argenziano M, Mancini D, Biviano A, Sonett J, Garan H. A comparison of atrial arrhythmias after heart or double-lung transplantation at a single center: Insights into the mechanism of post-operative atrial fibrillation. J Am Coll Cardiol. 2009;54:2043–2048. doi: 10.1016/j.jacc.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 17.Azadani PN, Kumar UN, Yang Y, Scheinman MM, Hoopes CW, Marcus GM, Rifkin C, Olgin JE, Lee BK. Frequency of atrial flutter after adult lung transplantation. Am J Cardiol. 2011;107:922–6. doi: 10.1016/j.amjcard.2010.10.076. [DOI] [PubMed] [Google Scholar]
- 18.Malik A, Hsu JC, Hoopes C, Itinarelli G, Marcus GM. Elevated pulmonary artery systolic pressures are associated with a lower risk of atrial fibrillation following lung transplantation. J Electrocardiol. 2013;46:38–42. doi: 10.1016/j.jelectrocard.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: The Framingham study. N Engl J Med. 1982;306:1018–1022. doi: 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- 20.AFFIRM Investigators. Atrial Fibrillation Follow-up Investigation of Rhythm Management. Baseline characteristics of patients with atrial fibrillation: The AFFIRM study. Am Heart J. 2002;143:991–1001. doi: 10.1067/mhj.2002.122875. [DOI] [PubMed] [Google Scholar]
- 21.Bang CN, Greve AM, Abdulla J, Kober L, Gislason GH, Wachtell K. The preventive effect of statin therapy on new-onset and recurrent atrial fibrillation in patients not undergoing invasive cardiac interventions: A systematic review and meta-analysis. Int J Cardiol. 2013;167:624–630. doi: 10.1016/j.ijcard.2012.08.056. [DOI] [PubMed] [Google Scholar]
- 22.Lefroy DC, Fang JC, Stevenson LW, Hartley LH, Friedman PL, Stevenson WG. Recipient-to-donor atrioatrial conduction after orthotopic heart transplantation: Surface electrocardiographic features and estimated prevalence. Am J Cardiol. 1998;82:444–450. doi: 10.1016/s0002-9149(98)00359-2. [DOI] [PubMed] [Google Scholar]
- 23.Saoudi N, Redonnet M, Anselme F, Poty H, Cribier A. Catheter ablation of atrioatrial conduction as a cure for atrial arrhythmia after orthotopic heart transplantation. J Am Coll Cardiol. 1998;32:1048–1055. doi: 10.1016/s0735-1097(98)00360-x. [DOI] [PubMed] [Google Scholar]
- 24.Kuijpers NH, Potse M, van Dam PM, ten Eikelder HM, Verheule S, Prinzen FW, Schotten U. Mechanoelectrical coupling enhances initiation and affects perpetuation of atrial fibrillation during acute atrial dilation. Heart rhythm. 2011;8:429–436. doi: 10.1016/j.hrthm.2010.11.020. [DOI] [PubMed] [Google Scholar]