Abstract
Objective
Cytokeratin 5 (CK5) is an epithelial cell marker implicated in stem and progenitor cell activity in glandular reproductive tissues and endocrine and chemotherapy resistance in estrogen receptor (ER)+ breast cancer. The goal of this study was to determine the prevalence of CK5 expression in ovarian cancer and the response of CK5+ cell populations to cisplatin therapy.
Materials and Methods
CK5 expression was evaluated in two ovarian tissue microarrays, representing 137 neoplasms, and six ovarian cancer cell lines. Cell lines were treated with IC50 cisplatin and the prevalence of CK5+ cells pre- and post-treatment determined. Proliferation of CK5+ vs. CK5− cell populations was determined using bromodeoxyuridine (BrdU) incorporation. Chemotherapy induced apoptosis in CK5+ vs. CK5− cells was measured using immunohistochemical staining for cleaved caspase-3.
Results
CK5 was expressed in 39.3% (42/107) of epithelial ovarian cancers with a range of 1-80% positive cells. Serous and endometrioid histologic subtypes had the highest percentage of CK5+ specimens. CK5 expression correlated with ER positivity (38/42 CK5+ tumors were also ER+). CK5 was expressed in 5/6 overall and 4/4 ER+ epithelial ovarian cancer cell lines ranging from 2.4-52.7% positive cells. CK5+ compared to CK5− cells were slower proliferating. The prevalence of CK5+ cells increased following 48 hour cisplatin treatment in 4/5 cell lines tested. CK5+ compared to CK5− ovarian cancer cells were more resistant to cisplatin induced apoptosis.
Conclusions
CK5 is expressed in a significant proportion of epithelial ovarian cancers and represents a slower proliferating, chemoresistant subpopulation that may warrant co-targeting in combination therapy.
Keywords: epithelial ovarian cancer, cytokeratin 5, cisplatin, drug resistance, estrogen receptor.
Introduction
Ovarian cancer is the most lethal gynecologic malignancy in the United States with an overall 5-year survival rate of 44% (1). Although overall survival is low, a strong correlation exists between survival and stage of disease at diagnosis. Unfortunately, most women are diagnosed with late stage disease. Seventy-five percent of patients with advanced stage disease will respond to first line therapy (1). However, nearly 60% of treated patients will eventually relapse with a median progression free survival of 18 months (2). Most patients with recurrent ovarian cancer will eventually develop platinum-resistant disease. A host of genetic, epigenetic, and cytologic changes are postulated to contribute to the resistant phenotype (3). Individual patient and tumor characteristics are also increasingly recognized as playing a role in ovarian disease progression (4). These factors each contribute to tumor heterogeneity, a leading cause of drug resistance and recurrence (5). There is an urgent need to identify the causes of disease recurrence and to develop targeted treatment strategies to improve outcomes for ovarian cancer patients.
One explanation for drug resistance related to tumor heterogeneity that has recently been explored is the cancer stem cell (CSC) theory, which posits that tumors contain a small population of cells that share properties of normal stem cells including self-renewal, tumor initiation, and the ability to generate differentiated progeny (6, 7). CSCs are less proliferative and have the propensity to extrude drugs, enabling them to escape current cytotoxic therapies. CSCs were first identified for epithelial malignancies in breast cancer by the signature CD44(+)CD24(−/low) (8), and have since been detected in most solid tumors, frequently by cell surface markers CD44+ and CD133+, and aldehyde dehydrogenase 1 activity (ALDH1)+ (9). Studies extended to ovarian cancer have also implicated CD44, CD133, and ALDH1, as marking putative CSC populations, in addition to CD117 and the multidrug resistant pump ABCG2 (reviewed in 10, 11). CD133, CD44, and ALDH1 ovarian cancer cell populations have been demonstrated to be enriched after first line chemotherapy (12). However, one study found widespread variation in CD133 populations among primary ovarian cancers, and substantial fluctuation in levels between passaged xenograft tumors, suggesting ovarian CSC markers are not uniformly or consistently expressed. (13). Ovarian cancer in particular is a heterogeneous entity comprised of multiple histologic subtypes. This likely confounds the existence of unique ovarian CSCs and infers tumors may have multiple therapy-resistant cell subpopulations.
Ovarian CSCs share some similarities to those found in breast cancer such as expression of CD44 and ALDH1 (8, 14). These markers tend to be more prevalent in triple negative breast tumors that lack expression of sex steroid receptors and have non-amplified HER2. Estrogen receptor (alpha, ER)+ breast cancers are absent for, or contain a low abundance of CD44+ and ALDH1+ cells (15-17). We have previously identified that half of ER+ breast tumors contain a subpopulation of cells that express the epithelial-specific intermediate filament cytokeratin 5 (CK5) (18, 19), a marker of luminal progenitor and stem cells in the normal breast (20, 21). CK5+ compared to intratumoral CK5− cells are relatively quiescent, tumor initiating, and have enhanced endocrine and chemotherapy resistance (18, 19). These cells usually lack expression of ER and are thus insensitive to endocrine targeting agents. Furthermore, they are postulated to be precursors to ER+ cells and thus spawn breast cancer recurrences which remain clinically ER+, but are endocrine resistant. Around 60% of ovarian cancers are ER+ (22), a similar proportion to breast cancer (21). There is some evidence that estrogens are tumor promotional in pre-clinical models of ovarian cancer (23, 24). However, targeted endocrine therapies are mostly ineffective in patients (25-27). We hypothesized that epithelial ovarian cancers, particularly those that are ER+, may also contain subpopulations of CK5+ cells that could confer resistance and spark tumor recurrence. Herein, we describe the pattern of expression of CK5 in ovarian cancer patient tumors and cell lines, and the relative response of CK5 populations to monotherapy with cisplatin. Our studies suggest that CK5+ ovarian cancer cells are present in a subset of epithelial ovarian cancers that express ER, and that these cells are relatively resistant to cisplatin treatment.
Materials and Methods
Cell lines and reagents
The ovarian cancer cell lines used for this study were originally obtained from the following sources: SKOV3 (ATCC, Manassas, VA), OV432 (Dr. Susan Murphy, Duke University, Durham, NC), PEO1 and PEO4 (Texas A&M, College Station, TX) (28), OVCAR3 (ATCC, Manassas, VA), and 2008 (Dr. Michael Mathis, University of Texas Southwestern, Dallas, TX). Cell lines were authenticated by polymorphic short tandem repeat analysis at the University of Colorado DNA Sequencing and Analysis Core. Cells were routinely cultured at 37°C and 5% CO2 in RPMI 1640 with 10% fetal bovine serum. All cell lines were routinely tested for the absence of Mycoplasma. Reagents utilized included cisplatin (Sigma-Aldrich, St. Louis, MO), and 5-bromo-2’-deoxyuridine (BrdU) (Invitrogen, Camarillo, CA).
Half maximal Inhibitory concentration (IC50) of cisplatin
For all cell lines, cells were plated at 5 × 103 cells per well in 96 well plates for 24 hours prior to testing. Cells were then treated with serial dilutions of cisplatin ranging from 0.8-200 μM for 48 hours. MTS assay was utilized for measurement of cell viability and Graphpad Prism 6.0 used to plot data and calculate IC50 using non-linear regression fits (Supplemental Figure 1).
Immunohistochemistry (IHC)
Ovarian cancer tissue arrays were purchased from Biochain (Hayward, CA). Paraffin embedded cell line pellets were created by collecting and fixing live cells in 4% paraformaldehyde, with embedding performed by the University of Colorado Tissue Biobanking & Processing Core. Sections were stained by IHC with an ImmPRESS™ polymer doublestain system (Vector Laboratories) with antibodies to ER (DAB, brown) and CK5 (Alkaline phosphatase (AP), fast red). Antibodies used were rabbit mAb to ER (alpha, SP1, Thermo Scientific, Waltham, MA) and mouse mAb CK5 (XM26, Leica Biosystems, Buffalo Grove, IL). Antibodies were validated on known ER+/ER− and CK5+/CK5− histological specimens. The specificity of the CK5 antibody was further confirmed by a study in which positively or negatively immunostained cells were isolated using laser capture microdissection, and RNA profiling revealed the gene for CK5 as the highest differentially expressed transcript (18). Slides were counterstained with hematoxylin. Images were obtained with an Olympus BH-2 microscope and cellSens software (Olympus). IHC staining on arrays was scored by a pathologist (M.P.) defined as >1% CK5 or ER immunoreactive cells in the tumor specimen.
Immunocytochemistry (ICC)
ICC experiments were performed by fixing cells to glass cover slips in ice cold 70% acetone/30%methanol for 5 minutes followed by immunostaining with the indicated antibodies. Antibodies used were rabbit mAb to ERα (SP1); mouse mAb to CK5 (XM26); rabbit mAb to CK5 (ab75869, Abcam, Cambridge, MA); rabbit mAb to BrdU (ab152095, abcam, Cambridge, MA); and rabbit pAB to cleaved caspase-3 (G748A, Promega, Madison, WI). Secondary antibodies utilized included anti-mouse Alexa Fluors 594 (red) and 488 (green), as well as anti-rabbit Alexa Fluors 594 (red) and 488 (green) (Thermo Scientific). For BrdU experiments, cells were treated with 0.25 mg/mL BrdU labeling reagent for 1 hour prior to fixation. Cell nuclei were counterstained with DAPI. All experiments were performed in biological triplicate averaging 5 high power field images per slide. An Olympus CKX41 microscope was used for photography and image analysis. Images were taken in black and white using cellSens software (Olympus) and merged in Adobe Photoshop (Adobe).
Cell death ELISA
Cell Death Detection ELISA Plus (Roche) was utilized for cell death analysis. Ten thousand cells per well were plated in 96 well plates in biological triplicate and treated with vehicle or IC50 cisplatin for 48 hours as described. Light analysis was then performed according to the protocol provided by Roche.
Biostatistics
GraphPad Prism v6.0 was used to analyze and graph all data. For ICC, all experiments were performed in biological triplicate with 5 fields per biological sample counted. Statistical significance was determined using Student's t test or one-way ANOVA.
Results
CK5+ cell populations exist in many epithelial ovarian cancers and ovarian cancer cell lines
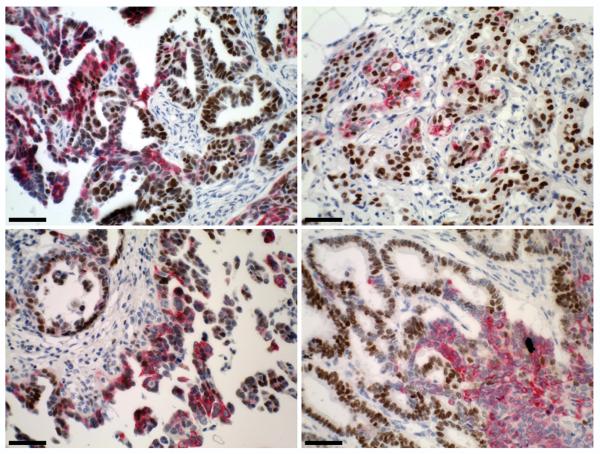
To determine the prevalence of CK5+ cell populations in ovarian cancers and whether they correlate with ER positivity, two ovarian cancer tissue arrays representing 137 individual cases were stained by dual IHC for CK5 and ER (alpha). Representative immunostains are depicted in Figure 1 and results summarized in Table 1. Of the 137 specimens, 120 had readable tumor tissue following immunostaining. Among these, epithelial ovarian cancer represented 107 cases (89.2% of the cohort). Seventy two percent (73/107) of the epithelial ovarian cancers were ER+, ranging from 10-90% positive cells. CK5+ cells were present in the tumor tissue (at 1% or greater) in 39.3% (42/107) of epithelial ovarian cancers and were not detected in any of the 13 non-epithelial tumor specimens. CK5+ cell populations are categorized as low (1-10%), medium (10-80%), and high (>80%) representing percentage of immunostaining cells. As seen in Table 1, CK5+ cell populations most commonly immunostain between 1-10% of tumor sample. Among ER+ tumors, half (52.1%, 38/73) expressed CK5. Notably, nearly all CK5+ tumors were also ER+ (90.5%, 38/42). In some tumors, CK5 and ER were co-expressed in the same cells, whereas in other tumors CK5+ and ER+ cells were mutually exclusive. Of the histologic subtypes highly represented on the array (≥20 cases), serous and endometrioid adenocarcinomas had the highest prevalence of CK5+ tumors, with fewer noted in mucinous tumors. Serous tumors demonstrated 50% CK5 positivity (19/38), endometrioid had 48% (13/25), and mucinous had 15% (3/20).
Figure 1.
Cytokeratin 5 (CK5) is expressed in over half of epithelial ovarian cancers and correlates with estrogen receptor (ER) expression. Paraffin sections of primary ovarian cancers were stained by dual IHC for nuclear ER (brown) and cytoplasmic CK5 (pink). Sections were counterstained with hematoxylin. Representative sections are shown for two ovarian serous carcinomas (top panels) and two ovarian adenocarcinomas (bottom panels). Scale bars, 50 microns.
Table 1.
Cytokeratin 5 (CK5) and estrogen receptor (ER) expression in ovarian malignancies
| Tumor type | number of cases | ER–a | ER+ | CK5–b | CK5+c | CK5+/ER+d |
|---|---|---|---|---|---|---|
| Epithelial | ||||||
| Serous | 38 | 6 (16%) | 32 (84%) | 19 (50%) | 19 (50%) Low: 12 (63%) Med: 5 (26%) High: 2 (11%) |
17 (45%) |
| Mucinous | 20 | 13 (65%) | 7 (35%) | 17 (85%) | 3 (15%) Low: 2 (67%) Med: 1 (33%) High: 0 |
3 (15%) |
| Endometrioid | 25 | 4 (16%) | 21 (84%) | 12 (48%) | 13 (52%) Low: 8 (62%) Med: 5 (38%) High: 0 |
13 (52%) |
| Clear cell | 1 | 1 (100%) | 1 (100%) | |||
| Transitional cell | 1 | 1 (100%) | 1 (100%) | |||
| Squamous | 1 | 1 (100%) | 1 (100%) Low: 0 Med: 0 High: 1 |
|||
| Adenocarcinoma, unspecified | 21 | 9 (43%) | 12 (57%) | 15 (71%) | 6 (29%) Low: 2 (33%) Med: 4 (67%) High: 0 |
5 (24%) |
| Total | 107 | 34 (32%) | 73 (68%) | 65 (61%) | 42 (39%) Low: 24 (57%) Med: 15 (36%) High: 3 (7%) |
38 (36%) |
| Non-epithelial | ||||||
| Granulosa cell | 3 | 1 | 2 | 3 | ||
| Thecoma | 5 | 5 | 5 | |||
| Yolk sac tumor | 2 | 2 | 2 | |||
| Dysgerminoma | 2 | 2 | 2 | |||
| Sarcoma | 1 | 1 | 1 | |||
| Total | 13 | 11 | 2 | 13 |
Number of cases determined by IHC using a mAB to ER(alpha), positive scored as >1% immunoreactive cells.
Number of cases determined by IHC using a mAB to CK5, positive scored as >1% immunoreactive cells.
Low, medium, & high scored as percent immunoreactive cells (1-10%, 10-80%, >80%)
Scored as having both CK5+ and ER+ cells within the same tumor, regardless of co-expression in the same cells.
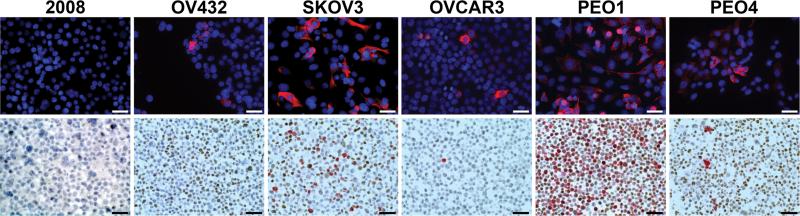
We next determined CK5 expression in six epithelial ovarian cancer cell lines (2008, OV432, SKOV3, OVCAR3, PEO1, and PEO4) using ICC (Figure 2 and Supplementary Table 1). Five cell lines contained a CK5+ cell population ranging from 2.4-52.7% positive cells, with 2008 cells as the exception. We performed dual IHC for CK5/ER on paraffin embedded cell pellets to confirm CK5 and assess ER expression. 2008 cells were negative for both CK5 and ER. OV432, SKOV3, PEO1, and PEO4 cells all contained both ER+ and CK5+ cells. OVCAR3 contained CK5+ cells but no ER immunoreactivity (Supplemental Figure 2). Previous reports describe that 2008 are ER− (23), and that SKOV3, PEO1, and PEO4 are ER+ (24, 29). OVCAR3 were originally reported as ER+ using radioligand binding assay (30) but were negative for ER (α) by immunoblot (24). Out results indicate that all four ER+ ovarian cancer cell lines co-expressed with CK5 positivity, while one/two ER− cell lines expressed CK5.
Figure 2.
Cytokeratin 5 (CK5) is expressed in the majority of epithelial ovarian cancer cell lines. Six ovarian cancer cell lines (2008, OV432, SKOV3, OVCAR3, PEO1, and PEO4) were stained by fluorescent immunocytochemistry (ICC) for CK5 (red) (top). Sections were counterstained for DAPI (blue). Paraffin embedded cell pellets of ovarian cancer cell lines were stained by dual immunohistochemistry (IHC) for nuclear estrogen receptor (ER) (brown) and cytoplasmic CK5 (pink) (bottom). Scale bars, 50 microns.
Cisplatin therapy enriches for CK5+ cells in ovarian cancer cell lines
To determine the response of CK5+ cell populations to cisplatin treatment, we first calculated the relative IC50 for the six ovarian cancer cell lines by treatment with serial dilutions of cisplatin ranging from 0.8-200 μM and computation using nonlinear regression curves (Supplemental Figure 1). All calculated IC50s were consistent with the existing literature (Supplemental Table 1). Cell lines were then treated with vehicle or their calculated IC50 cisplatin dose for 48 hours prior to fixation. ICC was performed for CK5 and the relative percent of CK5+ cells pre- and post- treatment measured (Figure 3). The prevalence of CK5+ cells significantly increased with cisplatin therapy in 4 out of 5 cell lines tested. In vehicle vs. cisplatin respectively the CK5+ percent increased from 2.4 to 5.2% in OV432, 3.9 to 14.8% in OVCAR3, 48.7 to 75.8% in PEO1, and 22.7 to 34.0% in PEO4. SKOV3 showed no significant change in CK5+ cells following cisplatin treatment.
Figure 3.
The Cytokeratin 5 (CK5)+ population is enriched following cisplatin therapy in multiple ovarian cancer cell lines. Ovarian cancer cell lines (OV432, SKOV3, OVCAR3, PEO1, and PEO4) were treated with calculated IC50 cisplatin doses (Table 2, Supplementary Figure 1) for 48 h and stained by fluorescent immunocytochemistry (ICC) for CK5. The percent of CK5+ cells was calculated for control vs. cisplatin treated samples in biological triplicate and plotted as mean CK5+ percent ± SEM. ** P<0.001, *** P<0.0001; t-test comparing CK5+ to CK5− in each cell line.
CK5+ cells are slower proliferating in ovarian cancer cell lines
Because cisplatin and other cytotoxic chemotherapies have maximum efficacy on actively replicating cells, we determined the relative proliferation of CK5+ compared to CK5− cells. BrdU (0.25 mg/mL) was added to subconfluent PEO1 and PEO4 cells for one hour prior to fixation, and cells then stained by dual fluorescent ICC for BrdU/CK5. Representative immunostains are depicted in Figure 4A (BrdU, green nuclear; CK5, red cytoplasmic). In both PEO1 and PEO4 cultures, CK5+ cells were significantly less proliferative than CK5− cells (7.4 compared to 23.5%, and 2.5 compared to 25.5%, respectively) (Figure 4B).
Figure 4.
Cytokeratin 5 (CK5)+ compared to CK5− ovarian cancer cells are slower proliferating. A. PEO1 and PEO4 cultures were spiked with 0.25 mg/mL BrdU for 1 h prior to fixation. Representative fields of PEO1 and PEO4 cells stained by dual immunocytochemistry (ICC) for CK5 (red) and BrdU (green) (right). Sections were counterstained for DAPI (blue) (left). Scale bars, 50 microns. B. Cells were counted and averaged from five high powered fields each of biological triplicates and the percent of BrdU positive CK5+ and CK5− cells/field ± SEM plotted. *** P<0.0001; t-test comparing CK5+ to CK5− in each cell line.
CK5+ cells are less apoptotic following cisplatin treatment
To determine if CK5+ cells have differences in chemotherapy-induced apoptosis, we treated PEO1 and PEO4 cell lines with IC50 cisplatin for 48 hours and measured total cell death, and the proportion of CK5+ and CK5− cells that were apoptotic. Total cell death was confirmed by cell death ELISA for the PEO1 and PEO4 cell lines (Figure 5A). Dual ICC for CK5 and cleaved caspase-3 as a measure of apoptosis was performed for vehicle and cisplatin treated cells (Figure 5B). The proportion of CK5 increased in both cell lines (36.0 to 62.9% in PEO1, and 17.9 to 28.1% in PEO4), confirming results described in Figure 3. There were few apoptotic cells in vehicle treated samples and no difference between the CK5+ and CK5− populations (Figure 5C). Following cisplatin treatment, the CK5+ were significantly less apoptotic compared to CK5− cells in both cell lines (11.0 compared to 24.3% in PEO1, and 9.8 compared to 23.2% in PEO4).
Figure 5.
Cytokeratin 5 (CK5)+ cells resist chemotherapy induced apoptosis cells in ovarian cancer cell lines. Triplicate samples of PEO1 and PEO4 cells were treated with vehicle (veh) or 12.1 and 48 μM cisplatin (cis), respectively, for 48 h. A. Relative cell death of control vs. cisplatin treated cells detected by apoptosis ELISA, measuring cytoplasmic nucleosomes. Mean absorbance ± SEM is plotted. *** P<0.0001; t-test comparing control to treatment. B. Representative images of vehicle or cisplatin treated PEO1 and PEO4 cells stained by dual fluorescent immunocytochemistry (ICC) for CK5 (green) and cleaved caspase-3 (red). Sections were counterstained for DAPI (blue) (left). Scale bars, 50 microns. C. Relative apoptosis (cleaved caspase-3 staining) of CK5+ vs. CK5− cells determined by counting multiple fields for a minimum of 500 total cells and plotted as mean ± SEM. **P<0.01; t-test comparing CK5+ to CK5− in each cell line.
Discussion
Recurrent platinum resistant ovarian cancer remains a critical obstacle for improving overall survival (OS) and cure rates for this lethal disease. Studies such as ICON4 and CALYPSO have demonstrated chemotherapy regimens with modest short term improvement in progression free survival (PFS) for recurrent platinum-sensitive recurrence (31, 32). However, no treatment modality has demonstrated any superiority for recurrent platinum-resistant ovarian cancer, which most patients will eventually manifest. The natural course of ovarian cancer aligns with the CSC hypothesis, suggesting specific subpopulations of cells drive treatment relapse (11). Several cell surface and enzymatic CSC markers identified in other malignancies also prospectively mark ovarian CSCs, albeit with no clear consensus. Here we define that a significant proportion of epithelial ovarian cancers contain subpopulations of CK5+ cells, and that CK5+ compared to CK5− ovarian cancer cells are relatively resistant to cisplatin therapy. We speculate that CK5+ cells represent a distinct dedifferentiated epithelial cancer cell subpopulation in a subset of ER+ ovarian neoplasms that could be pursued for therapeutic intervention.
CK5 is an early epithelial marker found in many tissues and is usually associated with the myoepithelial/basal layer, although this is controversial in the breast (33). In glandular reproductive tissues such as the breast and prostate, CK5+ cells give rise to hormone receptor positive cells (21, 34). CK5 expression is prevalent in multiple epithelial neoplasms. It is ubiquitously expressed in squamous cell carcinomas, particularly those of the skin, head and neck, and more sporadically expressed in many other malignancies (35, 36). There is little existing literature on CK5 expression in ovarian cancer. One report by Chu et al. found CK5/6 expressed in 6/24 (25%) ovarian cancers of unspecified histology (35). Here we report that ~40% of all epithelial ovarian cancers express CK5, ranging from 1% to >80% positive cells (Table 1), and 90% of those cancers are ER+. Half of ER+ epithelial ovarian cancers (38/73) express CK5. We observed a histologic preference with half of serous and endometrioid carcinomas containing CK5+ cells, while in mucinous and non epithelial subtypes CK5 expression was infrequent. In breast cancer, ~50% of luminal ER+ tumors contain subpopulations of CK5+ cells where they show heightened endocrine and chemotherapy resistance (19). CK5 is expressed ubiquitously in basal-like breast cancer and is a signature marker of this subtype. In fact, CK5+ luminal progenitor cells are postulated as the cell of origin of BRCA1 deficient breast cancers (21, 37). We speculate that ovarian malignancies that contain CK5+ cells may share a similar cell of origin.
We demonstrate that five of the six epithelial ovarian cancer cell lines tested contained a CK5+ positive subpopulation (Figure 2), and that the prevalence of CK5 increased following cisplatin treatment in 4/5 of these cell lines (Figure 3). Platinum covalently bonds to DNA and inhibits DNA synthesis, causing cell death. As CK5+ cells are relatively slower proliferating compared to coculture CK5− cells (Figure 4), it is reasonable to infer that they are less sensitive to such therapy. Indeed, cisplatin-induced apoptosis is relatively less in CK5+ compared to CK5− cells (Figure 5).
We observed a significant positive correlation of CK5 expression with ER (alpha isoform) status in ovarian cancers. Although ~60% of ovarian cancers express ER, a role for estrogens and ERs in ovarian cancer remains an enigma. Although estrogens increase ovarian cancer cell growth in pre-clinical models (23, 24), targeted hormonal therapy has minimal efficacy in treating ovarian cancers (38). ERs, however, may have prognostic significance. ER-alpha status is associated with improved disease free survival in endometrioid carcinoma (39). ER-beta is present at lower levels in cancer vs. normal ovarian tissue and potentially exerts a growth inhibitory protective effect (40). In the normal breast and in breast cancer there is a clear separation of CK5+ and ER(alpha)+ cell populations, with very few (<5%) dual expressing cells (CK5+ER+). We observed no uniform separation of these populations in ovarian cancer tissues and cell lines, as some displayed distinct separation and others showed a predominance of dual expression (CK5+ER+ cells). Thus, the hierarchical relationship between CK5+/ER+ cell populations in ovarian cancers, and prospectively in normal ovarian tissue, remains unknown.
In conclusion, CK5 is demonstrated to be a marker for a cisplatin resistant subpopulation of cells in specific subset of ER+ epithelial ovarian cancers. Further research to determine the plasticity of this population and their expression profile is needed to pursue interventions. Identification of existing or novel therapeutics aimed at thwarting this population of cells has the potential to reduce ovarian cancer recurrences.
Supplementary Material
Funding & acknowledgments
This work was supported by a University of Colorado AMC Department of Obstetrics and Gynecology fellowship (BRC) and NIH RO1 CA140985 (CAS). JFS was supported by NIH 1F32CA177081. We thank the University of Colorado AMC Tissue Biobanking & Processing Shared Resource supported by P30CA046934 for their technical assistance and services.
Footnotes
Conflict of Interest Statement: The authors of this manuscript have no conflicts of interest to report.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014 Jan-Feb;64(1):9–29. doi: 10.3322/caac.21208. PubMed PMID: 24399786. Epub 2014/01/09. eng. [DOI] [PubMed] [Google Scholar]
- 2.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004 Dec 9;351(24):2519–29. doi: 10.1056/NEJMra041842. PubMed PMID: 15590954. [DOI] [PubMed] [Google Scholar]
- 3.Merlo LM, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006 Dec;6(12):924–35. doi: 10.1038/nrc2013. PubMed PMID: 17109012. Epub 2006/11/17. eng. [DOI] [PubMed] [Google Scholar]
- 4.Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta. 2010 Jan;1805(1):105–17. doi: 10.1016/j.bbcan.2009.11.002. PubMed PMID: 19931353. Epub 2009/11/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012 Mar 20;21(3):283–96. doi: 10.1016/j.ccr.2012.03.003. PubMed PMID: 22439924. Epub 2012/03/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001 Nov 1;414(6859):105–11. doi: 10.1038/35102167. PubMed PMID: 11689955. [DOI] [PubMed] [Google Scholar]
- 8.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):3983–8. doi: 10.1073/pnas.0530291100. PubMed PMID: 12629218. Pubmed Central PMCID: 153034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012 Feb;12(2):133–43. doi: 10.1038/nrc3184. PubMed PMID: 22237392. Epub 2012/01/13. eng. [DOI] [PubMed] [Google Scholar]
- 10.Tomao F, Papa A, Rossi L, Strudel M, Vici P, Lo Russo G, et al. Emerging role of cancer stem cells in the biology and treatment of ovarian cancer: basic knowledge and therapeutic possibilities for an innovative approach. J Exp Clin Cancer Res. 2013;32:48. doi: 10.1186/1756-9966-32-48. PubMed PMID: 23902592. Pubmed Central PMCID: 3734167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah MM, Landen CN. Ovarian cancer stem cells: are they real and why are they important? Gynecol Oncol. 2014 Feb;132(2):483–9. doi: 10.1016/j.ygyno.2013.12.001. PubMed PMID: 24321398. Epub 2013/12/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steg AD, Bevis KS, Katre AA, Ziebarth A, Dobbin ZC, Alvarez RD, et al. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin Cancer Res. 2012 Feb 1;18(3):869–81. doi: 10.1158/1078-0432.CCR-11-2188. PubMed PMID: 22142828. Pubmed Central PMCID: 3271164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart JM, Shaw PA, Gedye C, Bernardini MQ, Neel BG, Ailles LE. Phenotypic heterogeneity and instability of human ovarian tumor-initiating cells. Proc Natl Acad Sci U S A. 2011 Apr 19;108(16):6468–73. doi: 10.1073/pnas.1005529108. PubMed PMID: 21451132. Epub 2011/04/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell stem cell. 2007 Nov;1(5):555–67. doi: 10.1016/j.stem.2007.08.014. PubMed PMID: 18371393. Pubmed Central PMCID: 2423808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009 Feb 15;69(4):1302–13. doi: 10.1158/0008-5472.CAN-08-2741. PubMed PMID: 19190339. Epub 2009/02/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10(2):R25. doi: 10.1186/bcr1982. PubMed PMID: 18366788. Epub 2008/03/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K, et al. The CD44+/CD24-phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10(3):R53. doi: 10.1186/bcr2108. PubMed PMID: 18559090. Epub 2008/06/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horwitz KB, Dye WW, Harrell JC, Kabos P, Sartorius CA. Rare steroid receptor-negative basal-like tumorigenic cells in luminal subtype human breast cancer xenografts. Proc Natl Acad Sci U S A. 2008 Apr 15;105(15):5774–9. doi: 10.1073/pnas.0706216105. PubMed PMID: 18391223. Pubmed Central PMCID: 2311360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabos P, Haughian JM, Wang X, Dye WW, Finlayson C, Elias A, et al. Cytokeratin 5 positive cells represent a steroid receptor negative and therapy resistant subpopulation in luminal breast cancers. Breast Cancer Res Treat. 2011 Jul;128(1):45–55. doi: 10.1007/s10549-010-1078-6. PubMed PMID: 20665103. Epub 2010/07/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bocker W, Moll R, Poremba C, Holland R, Van Diest PJ, Dervan P, et al. Common adult stem cells in the human breast give rise to glandular and myoepithelial cell lineages: a new cell biological concept. Lab Invest. 2002 Jun;82(6):737–46. doi: 10.1097/01.lab.0000017371.72714.c5. PubMed PMID: 12065684. Epub 2002/06/18. eng. [DOI] [PubMed] [Google Scholar]
- 21.Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009 Aug;15(8):907–13. doi: 10.1038/nm.2000. PubMed PMID: 19648928. Epub 2009/08/04. eng. [DOI] [PubMed] [Google Scholar]
- 22.Rao BR, Slotman BJ. Endocrine factors in common epithelial ovarian cancer. Endocr Rev. 1991 Feb;12(1):14–26. doi: 10.1210/edrv-12-1-14. PubMed PMID: 1851084. Epub 1991/02/01. eng. [DOI] [PubMed] [Google Scholar]
- 23.Spillman MA, Manning NG, Dye WW, Sartorius CA, Post MD, Harrell JC, et al. Tissue-specific pathways for estrogen regulation of ovarian cancer growth and metastasis. Cancer Res. 2010 Nov 1;70(21):8927–36. doi: 10.1158/0008-5472.CAN-10-1238. PubMed PMID: 20959477. Pubmed Central PMCID: 3804411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Donnell AJ, Macleod KG, Burns DJ, Smyth JF, Langdon SP. Estrogen receptor-alpha mediates gene expression changes and growth response in ovarian cancer cells exposed to estrogen. Endocr Relat Cancer. 2005 Dec;12(4):851–66. doi: 10.1677/erc.1.01039. PubMed PMID: 16322326. [DOI] [PubMed] [Google Scholar]
- 25.Argenta PA, Thomas SG, Judson PL, Downs LS, Jr., Geller MA, Carson LF, et al. A phase II study of fulvestrant in the treatment of multiply-recurrent epithelial ovarian cancer. Gynecol Oncol. 2009 May;113(2):205–9. doi: 10.1016/j.ygyno.2009.01.012. PubMed PMID: 19239974. Epub 2009/02/26. eng. [DOI] [PubMed] [Google Scholar]
- 26.Bowman A, Gabra H, Langdon SP, Lessells A, Stewart M, Young A, et al. CA125 response is associated with estrogen receptor expression in a phase II trial of letrozole in ovarian cancer: identification of an endocrine-sensitive subgroup. Clin Cancer Res. 2002 Jul;8(7):2233–9. PubMed PMID: 12114425. Epub 2002/07/13. eng. [PubMed] [Google Scholar]
- 27.Williams C, Simera I, Bryant A. Tamoxifen for relapse of ovarian cancer. Cochrane Database Syst Rev. 2010;(3):CD001034. doi: 10.1002/14651858.CD001034.pub2. PubMed PMID: 20238312. Epub 2010/03/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langdon SP, Lawrie SS, Hay FG, Hawkes MM, McDonald A, Hayward IP, et al. Characterization and properties of nine human ovarian adenocarcinoma cell lines. Cancer Res. 1988 Nov 1;48(21):6166–72. PubMed PMID: 3167863. [PubMed] [Google Scholar]
- 29.Hua W, Christianson T, Rougeot C, Rochefort H, Clinton GM. SKOV3 ovarian carcinoma cells have functional estrogen receptor but are growth-resistant to estrogen and antiestrogens. J Steroid Biochem Mol Biol. 1995 Dec;55(3-4):279–89. doi: 10.1016/0960-0760(95)00187-5. PubMed PMID: 8541224. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton TC, Young RC, McKoy WM, Grotzinger KR, Green JA, Chu EW, et al. Characterization of a human ovarian carcinoma cell line (NIH:OVCAR-3) with androgen and estrogen receptors. Cancer Res. 1983 Nov;43(11):5379–89. PubMed PMID: 6604576. [PubMed] [Google Scholar]
- 31.Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003 Jun 21;361(9375):2099–106. doi: 10.1016/s0140-6736(03)13718-x. PubMed PMID: 12826431. [DOI] [PubMed] [Google Scholar]
- 32.Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, Gebski V, Heywood M, Vasey PA, et al. Pegylated liposomal Doxorubicin and Carboplatin compared with Paclitaxel and Carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol. 2010 Jul 10;28(20):3323–9. doi: 10.1200/JCO.2009.25.7519. PubMed PMID: 20498395. Epub 2010/05/26. eng. [DOI] [PubMed] [Google Scholar]
- 33.Gusterson BA, Ross DT, Heath VJ, Stein T. Basal cytokeratins and their relationship to the cellular origin and functional classification of breast cancer. Breast Cancer Res. 2005;7(4):143–8. doi: 10.1186/bcr1041. PubMed PMID: 15987465. Pubmed Central PMCID: 1175069. Epub 2005/07/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uzgare AR, Xu Y, Isaacs JT. In vitro culturing and characteristics of transit amplifying epithelial cells from human prostate tissue. J Cell Biochem. 2004 Jan 1;91(1):196–205. doi: 10.1002/jcb.10764. PubMed PMID: 14689591. [DOI] [PubMed] [Google Scholar]
- 35.Chu PG, Weiss LM. Expression of cytokeratin 5/6 in epithelial neoplasms: an immunohistochemical study of 509 cases. Mod Pathol. 2002 Jan;15(1):6–10. doi: 10.1038/modpathol.3880483. PubMed PMID: 11796835. [DOI] [PubMed] [Google Scholar]
- 36.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. PubMed PMID: 6186379. [DOI] [PubMed] [Google Scholar]
- 37.Molyneux G, Geyer FC, Magnay FA, McCarthy A, Kendrick H, Natrajan R, et al. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell. 2010 Sep 3;7(3):403–17. doi: 10.1016/j.stem.2010.07.010. PubMed PMID: 20804975. Epub 2010/09/02. eng. [DOI] [PubMed] [Google Scholar]
- 38.Sommeijer DW, Sjoquist KM, Friedlander M. Hormonal treatment in recurrent and metastatic gynaecological cancers: a review of the current literature. Curr Oncol Rep. 2013 Dec;15(6):541–8. doi: 10.1007/s11912-013-0343-3. PubMed PMID: 24097282. Epub 2013/10/08. eng. [DOI] [PubMed] [Google Scholar]
- 39.Sieh W, Kobel M, Longacre TA, Bowtell DD, deFazio A, Goodman MT, et al. Hormone-receptor expression and ovarian cancer survival: an Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013 Aug;14(9):853–62. doi: 10.1016/S1470-2045(13)70253-5. PubMed PMID: 23845225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bossard C, Busson M, Vindrieux D, Gaudin F, Machelon V, Brigitte M, et al. Potential role of estrogen receptor beta as a tumor suppressor of epithelial ovarian cancer. PLoS One. 2012;7(9):e44787. doi: 10.1371/journal.pone.0044787. PubMed PMID: 22970307. Epub 2012/09/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.