Abstract
Background
The impact of percutaneous dilatational tracheostomy (PDT) on the development of post-median sternotomy wound infection (SWI) and mediastinitis is still controversial. We aimed to investigate the frequency of cross-infection and incidence of SWI after PDT.
Methods
In a retrospective design, out of a total of 4100 procedures, all patients who had undergone median sternotomy and postoperative PDT were included from January 2010 to May 2013. For comparison of the pathogens isolated from SWIs, data from all patients who developed an SWI without a PDT during the aforementioned period were also analyzed. Demographical, pre-, peri- and post-operative data were compared. Microbiologic analysis from cultures of sternal and tracheal wounds was performed. Day and duration of tracheostomy were correlated to SWI occurrence.
Results
Of the 265 patients who underwent a PDT, 25 (9.4 %) developed an SWI. In this cohort, identical pathogens were isolated from the tracheostomy and SWI in 36 % (9/25) of the patients. Of the pathogens isolated from the SWIs from the PDT + SWI group, 60 % were gram-positive bacteria, 20 % gram-negative bacteria and 20 % Candida spp. In the cross-infection group, the patients developed the following types of SWIs: 11.1 % CDC I, 55.6 % CDC II and 33.3 % mediastinitis (CDC III). The incidence of SWI in the group SWI + PDT was 9.4 % (9.4 % vs. 3.4 %, PDT + SWI and SWIw/oPDT, respectively, p = 0.0001). In group SWIw/oPDT, only 1.5 % (2/131 vs. 5/25; p = 0.001) Candida spp were isolated from SWI. The infection-related in-hospital mortality was high in groups PDT + SWI vs. SWIw/oPDT (20 % vs. 0 %, respectively; p = 0.0001). The statistical analysis did not demonstrate any correlation between time of performing PDT and occurrence of SWI.
Conclusions
There was a high incidence of microbial cross-infection from the PDTs to the sternal wounds in our study. We did not detect any correlation between the time of performing PDT and occurrence of SWI. According to our data, PDT seems to increase the incidence of SWI, especially caused by Candida spp., after cardiac surgery, which results in a prolonged hospital stay. Therefore, early antifungal prophylaxis after a PDT might be reasonable in high-risk patients on long-term mechanical ventilation if there is an impending SWI.
Keywords: Mediastinitis, Percutaneous Dilatational Tracheostomy (PDT), Cardiac surgery, Sternal Wound Infection (SWI), Cross-infection, Postoperative complication
Background
Sternal wound infection (SWI) and mediastinitis are devastating complications in cardiac surgery patients and are associated with high mortality rates between 10 and 50 % [1–4]. The incidence of deep sternal wound infection (DSWI) and mediastinitis have been reported to range between 0.16–3.20 % and 1–3 %, respectively [1, 3, 4]. The most common microbiological pathogens found in infected sternal wounds are gram-positive staphylococci (Staphylococcus aureus and coagulase-negative staphylococci) followed by gram-negative species [5–10]. Candida spp. were also isolated from SWIs and prevalent in patients on long-term mechanical ventilation.
In this retrospective study, we aimed to investigate the incidence of SWIs after percutaneous dilatational tracheostomies (PDTs), the frequency of cross-infection and if a PDT changed the microbial strains involved in an SWI.
Methods
From January 2010 to May 2013, 4100 cardiac surgery procedures through median sternotomy were performed at our institution. All cardiac surgery patients who had undergone cardiac surgery with a full median sternotomy and required a postoperative PDT were included in this retrospective study.
A PDT was performed within a monitored time window in 271 of 4100 patients undergoing full-sternotomy cardiac surgery in our unit. Of these 271 patients, six patients who developed SWI prior to PDT were excluded from the study. From the remaining 265 patients, 25 (9.4 %) developed an SWI or mediastinitis post-PDT (group PDT + SWI, n = 25). For comparison of the pathogens isolated from SWIs, data from all patients who developed an SWI without a PDT during the aforementioned period were also analyzed (group SWIw/oPDT, n = 131) (Fig. 1). EuroSCORE II and The Society of Thoracic Surgeons’ risk models (STS) scores were calculated for all patients. All patients with SWI were followed up routinely in our out patient clinic for 6 weeks after discharge from the hospital. The study protocol was cleared by the local ethical committee, Ethik-Kommission an der Medizinischen Fakultät der RWTH Aachen (KEK). Due to the retrospective character of this observational study, informed consent was waived by the local board at the Medical Faculty RWTH Aachen.
Fig. 1.
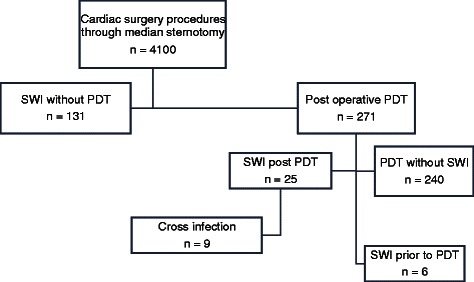
Classification of patients in groups
Procedural routine
All the patients underwent a similar pre-operative assessment and variety of cardiac procedures using standard median sternotomy. All the patients undergoing cardiac surgery in our department routinely receive Mupirocin nasal ointment on the day before operation and a single shot of peri-operative prophylactic antibiotics with either ampicillin/sulbactam (Unacid®) or, if a patient has a penicillin allergy, clindamycin.
According to our institutional standard operating procedures, tracheostomies were performed in patients who failed extubation twice or when patients were not expected to be extubated within 10 days. All PDTs were performed by an experienced team of anesthesiologists in the ICU using the Frova technique (RÜSCH Tracheostomy, Teleflex Medical GmbH, Kernen, Germany).
Data assessment
The following data were collected for all the patients: pre-operative, peri-operative and post-operative parameters, duration of intubation, duration of mechanical ventilation, microbiological findings from tracheal secretions, occurrence of SWI, all-cause in-hospital mortality and infection-related in-hospital-mortality, microbiological findings from sternal wound, common pathogens isolated from the sternal wound and tracheostoma, post-operative day on which PDT was performed, and the duration of ventilation through PDT.
Sternal wound infections and mediastinitis were classified according to the guidelines of the Centers for Disease Control and Prevention (CDC) [11] as follows: superficial sternal wound infection SSWI (CDC I), deep sternal wound infection DSWI (CDC II) and mediastinitis (CDC III). Aspirates from tracheal secretions were routinely collected and sent for microbiological analysis before or on the day a PDT was performed as well as the second or third day after the PDT procedure. In cases of infection, samples were taken at least two times a week.
An SWI was diagnosed by a clinical examination (signs of local infection, drainage of pus, fistulas and fever), computed tomography (CT) scans (retrosternal fluid collection and sternal dehiscence) and lab findings (leucocytosis and C-reactive protein). In all cases, the diagnosis was confirmed by microbiological findings. Wound swabs were taken before debridement and wound cleansing and sent for microbiological culture diagnostics. Once a mediastinal infection was evident, appropriate antibiotics were administered based on culture and sensitivity results.
Statistics
Data analysis was performed with SPSS 20 (IBM, Chicago, IL, USA). Continuous variables were compared with means ± SD using Student´s t-test. Categorical variables were analyzed with a Chi-Square test or, if appropriate, Fisher’s exact test. Mortality at defined time points and incidence of SWIs were compared using a Chi Square test. Incidence of SWIs in relation to a categorized time point after a PDT procedure and duration of respiratory therapy were analyzed using Pearson’s chi-squared test (χ2). All p-values were reported as three digit numbers or with at least one non-zero digit. A p-value < 0.05 was considered statistically significant.
Results
Demographics
In group PDT + SWI, both the EuroScore II and STS scores indicated that most of the patients were high risk.
The groups PDT + SWI and SWIw/oPDT differed significantly in many known risk factors (Table 1): mean body mass index (BMI), p = 0.034; peripheral arterial disease (PAD), p = 0.0005 and renal insufficiency, p = 0.029; pre-operative intra-aortic balloon pump (IABP), p = 0.024; and pre-operative inotrope therapy, p = 0.013. Group PDT + SWI had a higher mean EuroSCORE II score than group SWIw/oPDT (18.81 vs. 4.0, respectively, p = 0.0001), a higher mean STS score for risk of mortality (6.5 vs. 2.2, respectively, p = 0.0001), and no significant difference in mean STS score for risk of DSWI (0.6 vs. 0.5, respectively, p = 0.231). Group SWIw/oPDT included more patients who underwent CABG surgery than group PDT + SWI (71.8 % vs. 48.0 %, p = 0.033). There were no differences in perioperative variables between both groups. Only the mean cardiopulmonary bypass time (CPB) (p = 0.013) was significantly longer in group PDT + SWI.
Table 1.
Demographics and clinical data
| PDT + SWI (n = 25) | SWI w/o PDT (n = 131) | P-values | |
|---|---|---|---|
| Preoperative variables | |||
| Mean age (±) SD | 70 (±6.4) | 66 (±11) | 0.080 |
| Mean BMI (±) SD | 26.8 (±3.93) | 28.9 (±4.6) | 0.034 |
| Female % (n) | 36.0 (9) | 44.3 (58) | 0.511 |
| Preop. LVEF < 34 % (n) | 20.0 (5) | 4.6 (6) | 0.729 |
| NYHA IV % (n) | 36.0 (9) | 14.5 (19) | 0.420 |
| Hypertension % (n) | 88.0 (22) | 93.9 (123) | 0.385 |
| DM % (n) | 32.0 (8) | 42.0 (55) | 0.383 |
| COPD % (n) | 36.0 (9) | 24.4 (32) | 0.226 |
| PAD % (n) | 48.0 (12) | 14.5 (19) | 0.0005 |
| Smoking % (n) | 48.0 (12) | 47.3 (62) | 1.000 |
| Recent MI % (n) | 44.0 (11) | 42.7 (56) | 1.000 |
| Renal insufficiency % (n) | 68.0 (17) | 43.5 (57) | 0.029 |
| Preop. IABP % (n) | 8.0 (2) | 0 | 0.024 |
| Preop. Inotropes % (n) | 16.0 (4) | 2.2 (3) | 0.013 |
| Preop. Dialysis % (n) | 4.0 (1) | 0.7 (1) | 0.295 |
| Mean Euroscore II in % (±) SD | 18.8 (±15.6) | 4.0 (±4.6) | 0.0001 |
| Mean STS-score: Risk of Mortality % (±) SD | 6.5 (±4.3) | 2.2 (±2.1) | 0.0001 |
| Mean STS-score: Risk of DSWI % (±) SD | 0.6 (±0.26) | 0.5 (±0.4) | 0.231 |
| Operative procedures | |||
| CABG % (n) | 48.0 (12) | 71.8 (94) | 0.033 |
| Comb. CABG + Valve % (n) | 12.0 (3) | 16.0 (21) | 0.768 |
| Valve surgery % (n) | 8.0 (2) | 1.5 (2) | 0.120 |
| Thoracic aorta proc. % (n) | 16.0 (4) | 4.6 (6) | 0.055 |
| VAD-Implantation % (n) | 8.0 (2) | 0.8 (1) | 0.067 |
| Re-do % (n) | 8.0 (2) | 5.3 (7) | 0.637 |
| Perioperative variables | |||
| Mean Cross-clamp time (min) | 58.6 (±44.0) | 59.0 (±36.1) | 0.961 |
| Mean CPB time (min) | 133.8 (±59.5) | 103.8 (±54.3) | 0.013 |
| Postoperative Variables | |||
| Re-thoracotomy (%) | 20 (5) | 9.2 (12) | 0.153 |
| Stroke (%) | 12 (3) | 0 | 0.003 |
| Low cardiac output (%) | 20 (5) | 0 | 0.0001 |
| Pneumonia % (n) | 68 (17) | 13.7 (18) | 0.0001 |
| Respir. Insufficiency % (n) | 84 (21) | 19.0 (25) | 0.0001 |
| Delirium % (n) | 56 (14) | 22.9 (30) | 0.001 |
| Post-op MI % (n) | 0 | 1.5 (2) | 1.000 |
| Sepsis | 60 (15) | 13.7 (18) | 0.0001 |
| Mean vent. time in (h) (±) SD | 547 (±288) | 21 (±37) | 0.0001 |
| Mean number of packed RB cells (±) SD | 11 (±11) | 3 (±2) | 0.0001 |
| Mean days of ICU-stay (±) SD | 32 (±11) | 7 (±8) | 0.0001 |
| Mean LOS hospital (±) SD | 40 (±14) | 36 (±18) | 0.294 |
Description of pre-, peri-, post-operative clinical characteristics, surgical techniques and intensive care unit stays
Bold writing indicates significant values
BMI body mass index (kg/m2), LVEF left ventricle ejection fraction, NYHA New York Heart Association, COPD chronic obstructive pulmonary disease, DM diabetes mellitus, PAD peripheral arterial disease, Recent MI recent myocardial infarction, Preop pre-operative, IABP intra-aortic balloon pump, CABG coronary artery bypass graft, Other other open heart surgery procedures, CPB time total cardiopulmonary bypass time (in min), Valve heart valve surgery, VAD ventricular assist device implantation, Re-do second heart surgery, SD standard deviation, LOS length of stay, ICU intensive care unit; packed RB cells packed red blood cell
Infection rates and mortality
During the study period, the overall incidence of SWI was 3.8 % (156/4100 cardiac surgery procedures with a full median sternotomy) with a low incidence of mediastinitis (0.56 %; 23/4100). In group PDT + SWI, the incidence of SWI was significantly higher than in group SWIw/oPDT with 9.4 % versus 3.4 %, respectively; p = 0.0001 (Table 2). In both groups, most cases of SWI and mediastinitis were detected within the first 30 post-operative days (72 % in group PDT + SWI and 84 % in group SWIw/oPDT). In group PDT + SWI, five patients died because of mediastinitis-related septic multi-organ failure, compared to group SWIw/oPDT in which no infection-related in-hospital mortality was detected (20 % vs. 0 %, respectively; p = 0.0001). All-cause in-hospital mortality was significantly higher in group PDT + SWI than in group SWIw/oPDT (48 % vs. 3.8 %, respectively; p = 0.0001) (Fig. 2).
Table 2.
Incidence of SWI in group PDT + SWI vs. group SWI w/o PDT
| PDT + SWI (n = 25) | SWI w/o PDT (n = 131) | P-Values | |
|---|---|---|---|
| SWI % (n) | 9.4 % (25/265) | 3.4 % (131/3829) | 0.0001 |
| CDC I % (n) | 20 % (5) | 1.5 % (2) | |
| CDC II % (n) | 52 % (13) | 86.3 % (113) | |
| CDC III % (n) | 28 % (7) | 12.2 % (16) |
Bold writing indicates significant Values
CDC Centers for Disease Control and Prevention, SWI sternal wound infection
Fig. 2.
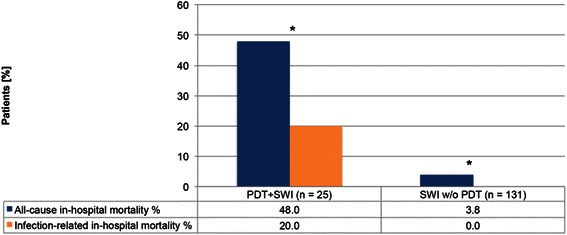
All-cause in-hospital mortality and infection-related in-hospital mortality. *p = 0.0001
Microbiological findings
Figure 3 demonstrates the microbiological pathogens isolated from tracheal secretions of the patients who received a PDT. In group PDT + SWI, identical pathogens were isolated from the tracheostomy and sternal wounds of nine (36 %) patients. The majority of patients with a post-operative SWI or mediastinitis had polymicrobial infections. The common pathogens isolated from the tracheal secretions and sternal wounds are shown in Fig. 4. In nine patients, the cross-infection SWIs were classified as follows: 11.1 % CDC I, 55.6 % CDC II and 33.3 % mediastinitis (CDC III). One patient with cross-infection died because of mediastinitis-related septic shock. The common cross-infection pathogen was Candida albicans.
Fig. 3.
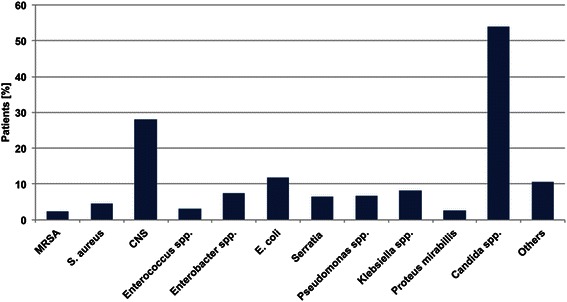
Bacteria isolated from tracheal secretions in group PDT + SWI. MRSA: Methicillin-resistant Staphylococcus aureus; CNS: Coagulase negative staphylococci; E. coli: Escherichia coli; others: described in Table 3
Fig. 4.
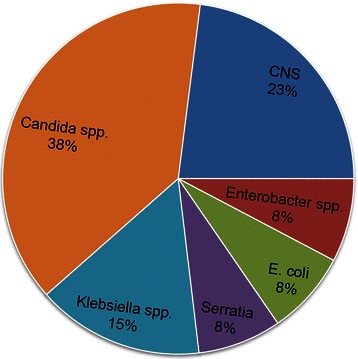
Common pathogens isolated from tracheostomas and sternal wounds. MRSA: Methicillin-resistant Staphylococcus aureus; CNS: Coagulase negative staphylococci; E. coli: Escherichia coli
Pathogens isolated from the SWI in groups PDT + SWI and SWIw/oPDT are outlined in Table 3. In our study, the incidence of Candida spp. infections was significantly higher (20 % vs. 1.5 %, p = 0.001) in group PDT + SWI than group SWIw/oPDT. However, the incidence of S. aureus infections (8 % vs. 29 %, p = 0.05) was significantly higher in group SWIw/oPDT than group PDT + SWI.
Table 3.
Pathogens isolated from SWIs
| PDT + SWI | SWI w/o PDT | P-values | |
|---|---|---|---|
| MRSA | 8 % (2) | 4.6 % (6) | 0.615 |
| Staphylococcus aureus | 8 % (2) | 29 % (38) | 0.026 |
| CNS | 32 % (8) | 40 % (52) | 0.516 |
| Enterococcus spp. | 12 % (3) | 3.1 % (4) | 0.082 |
| Enterobacter spp | 4 % (1) | 2.3 % (3) | 0.506 |
| Escherichia coli | 8 % (2) | 8.5 % (11) | 1.000 |
| Serratia marcescens | 4 % (1) | 3.1 % (4) | 0.587 |
| Pseudomonas spp. | 0 | 4.6 % (6) | 0.590 |
| Klebsiella spp. | 12 % (3) | 2.3 % (3) | 0.052 |
| Proteus mirabilis | 0 | 4.6 % (6) | 0.590 |
| Candida spp. | 20 % (5) | 1.5 % (2) | 0.001 |
| Others | 0 | 14.6 % (19) | 0.044 |
Bold writing indicates significant values
MRSA methicillin-resistant staphylococcus aureus, CNS coagulase negative staphylococci; others: Citrobacter youngae, Stenotrophomonas maltophilia, Aspergillus fumigatus, Streptococcus agalactiae, Morganella morganii, Haemophilus influenzae, Citrobacter braakii, Citrobacter koseri, Saccharomyces cerevisiae, Acinetobacter gyllenbergii and Lactobacillus curvatus
Incidence of SWI in relation to the time after PDT procedure and duration of respiratory therapy
During the first post-operative week, 207 patients received a PDT, and 20 patients in this group developed an SWI. During the second post-operative week, 49 patients underwent a PDT, and four developed an SWI. During the third week, a PDT was performed in nine patients; one developed an SWI (Fig. 5). After analyzing the relation of the incidence of SWIs to the categorized time point of PDTs and duration of respiratory therapy using Pearson’s Chi-squared test, no correlation was detected between the time of performing a PDT and SWI (p = 0.963, Fig. 6). However, there was a correlation (p = 0.0001) between the duration of ventilation through a PDT and occurrence of an SWI (Fig. 6).
Fig. 5.
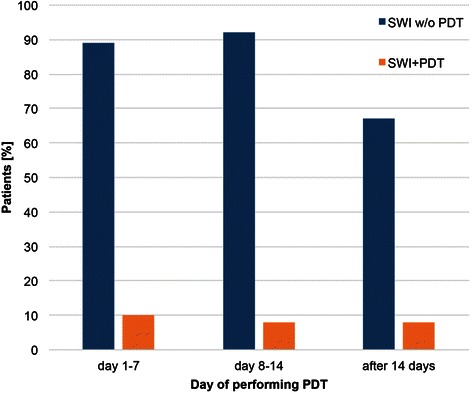
Correlation between time of performing PDT and SWI
Fig. 6.
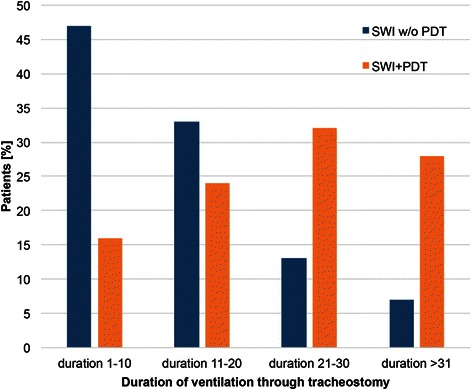
Correlation between duration of ventilation through PDT and SWI
Discussion
Clinical relevance of the bacterial strains
In group SWIw/oPDT, the most frequently isolated pathogens from SWIs were gram-positive staphylococci (CNS 40 %, followed by S. aureus 29 %). These findings are consistent with numerous studies [5–10]. In these studies, the incidence of fungi in SWI was reported to range between 1 and 5 %. In our group PDT + SWI, the incidence of S. aureus was significantly lower than group SWIw/oPDT. On the other hand, Candida spp. were isolated from 20 % of the SWIs after PDT. Candida spp. were also isolated from tracheal secretions in 53.6 % of the patients who had a PDT, as well as in 38 % of the patients who had cross-infection between the PDT and SWI. Five patients with an SWI due to Candida spp. contamination had the longest hospital stays (45 ± 15 days). One of them died during the first 30 POD from shock and multiorgan failure related to mediastinitis. The low incidence of S. aureus in SWI + PDT and subsequent high rate of candida spp. might be related to the fact that these patients already suffered a long term intensive care stay with multiple complications requiring long term antibiotics.
Our findings are consistent with the study from Modrau et al. [7]. They found that when cardiothoracic patients on mechanical ventilation are tracheally colonized with Candida spp., they have a high-risk for subsequently developing Candida DSWI. Additionally, patients who developed SWI with Candida spp. had a significantly higher mortality rates and longer ICU stays.
The patients who required a PDT were critically ill patients with postoperative complications such as pneumonia requiring long-term antibiotic therapy. Pittet et al. [12] identified the length of antibiotic therapy, severity of illness and degree of Candida spp. colonization as factors that predicted subsequent Candida infections. A Cochrane Review suggested that antifungal prophylaxis should be considered in non-neutropenic critically ill patients [13].
Cross-infection
We found cross-infection in nine (36 %) patients with an SWI after a PDT. These findings may be controversial because several previous studies did not find any correlation between early PDT and SWIs [14–16]. Compared with an open surgical tracheostomy, a PDT causes less microbial contamination at the tracheostomy site [17, 18]. Whether the PDT technique prevents microbiological cross-infection of an adjacent sternal wound remains a controversial matter.
Tracheostomy as a risk factor
Prolonged mechanical ventilation after cardiac surgery is an unfortunate adverse event occurring in patients suffering respiratory failure. This can be due to a variety of reasons, pneumonia being the most common.
Sun et al. [19] reported tracheostomy as an independent risk factor for SWI. On the other hand, cardiac surgery patients who require prolonged mechanical ventilation benefit from tracheostomy [20, 21] because of a reduced level of sedation and facilitated weaning from ventilator support, which results in a reduced incidence of ventilator-associated pneumonia [22, 23]. PDT has become a standard procedure in cardiac surgery patients who have a high risk for post-operative pulmonary complications and replaced open surgical tracheostomy [17, 24]. A PDT is easy to perform, safe cost-effective and can be performed at bedside [17, 24]. However, some authors state that a PDT does not increase the risk for an SWI following a median sternotomy, even when the PDT is carried out in the first post-operative week [16, 17, 25]. In accordance with Byhahn et al., Hubner et al. and Gaudino et al. [14, 15, 25], we found that there was no correlation between an early postoperative PDT and the development of SWI. From a clinical point of view, we strongly support Byhahn et al., Stamenkovic et al. and Gaudino et al. [14, 16, 25] who concluded that the indisputable advantages of early PDT far outweigh the potential risks of promoting an SWI. However, according to our findings, early antifungal prophylaxis when an SWI is imminent might be reasonable in high-risk patients with a PDT to avoid dire complications of a candida wound infection.
Limitations of study
One of the main limitations of the presented study is its retrospective design, although prospective designs can also be descriptive if there are ethical and practical limitations. Additionally, SWI registries would enhance data collection and aggregation and could be used to gain new insights on the topic.
Due to the small number of patients with simultaneous SWI and PDT, it was not possible to identify risk factors for SWIs caused by cross-infection.
As a matter of fact, PDT is limited to critically ill patients. Therefore a comparison of patients suffering SWI with or without PDT leads to a heterogeneous distribution of preoperative risk factors, limiting comparability of outcomes and postoperative characteristics. We emphasized this by highlighting the preoperative differences between groups in Table 1.
In the routine microbiological tests of tracheal secretions in our laboratory, not all bacteria of normal upper airways flora were directly identified. Therefore, they were not mentioned separately in the microbiological reports. Many pathogens belonging to normal upper airway flora are potential pathogens that can cause an SWI. Therefore, the rate of cross-infection could be higher than what we could detect.
Conclusion
The incidence of microbial cross-infection from a PDT to a sternal wound in our study was high. We could not detect any correlation between the time of performing a PDT and occurrence of an SWI. According to our data, PDT seems to increase the incidence of SWI, especially caused by Candida spp., after cardiac surgery, which results in a prolonged hospital stay. In high-risk patients on long-term mechanical ventilation early antifungal prophylaxis after a PDT might be reasonable as soon as an SWI is suspected in order to mitigate dire complications of a candida SWI.
Abbreviations
- BMI
mean body mass index
- CABG
coronary artery bypass grafting
- CDC
Centers for Disease Control and Prevention
- CNS
coagulase-negative staphylococci
- CPB
cardiopulmonary bypass time
- CT
computed tomography
- DSWI
deep sternal wound infection
- E. coli
escherichia coli
- IABP
intra-aortic balloon
- PAD
peripheral artery disease
- PDT
percutaneous dilatational tracheostomy
- spp.
species
- S. aureus
staphylococcus aureus
- SSWI
superficial sternal wound infection
- STS
The Society of Thoracic Surgeons’ risk models
- SD
standard deviation
- SWI
sternal wound infection
Footnotes
Lachmandath Tewarie and Rachad Zayat contributed equally to this work.
Competing interests
The author(s) declare that they have no competing interests.
Authors’ contributions
LT and RZ designed the study and developed the database. LT and RZ wrote the manuscript. LT and RZ performed the statistical analyses and drafted the manuscript. AG critically revised the statistical analysis, the study design and the manuscript. AM critically revised the manuscript in cooperation with the co-authors and interpreted the data. HH critically revised the microbiological findings. RA, as the department chair, supported this study and participated in designing the study. RA and JS critically revised the manuscript. RZ collected the patient data. All the authors have read and approved the submitted version of the manuscript.
Contributor Information
Lachmandath Tewarie, Email: ltewarie@ukaachen.de.
Rachad Zayat, Phone: +49 241 8089221, Email: rzayat@ukaachen.de.
Helga Haefner, Email: hhaefner@ukaachen.de.
Jan Spillner, Email: jspillner@ukaachen.de.
Andreas Goetzenich, Email: agoetzenich@ukaachen.de.
Rüdiger Autschbach, Email: rautschbach@ukaachen.de.
Ajay Moza, Email: amoza@ukaachen.de.
References
- 1.Diez C, Koch D, Kuss O, Silber RE, Friedrich I, Boergermann J. Risk factors for mediastinitis after cardiac surgery - a retrospective analysis of 1700 patients. J Cardiothorac Surg. 2007;2:23. doi: 10.1186/1749-8090-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollenbeak CS, Murphy DM, Koenig S, Woodward RS, Dunagan WC, Fraser VJ. The clinical and economic impact of deep chest surgical site infections following coronary artery bypass graft surgery. Chest. 2000;118(2):397–402. doi: 10.1378/chest.118.2.397. [DOI] [PubMed] [Google Scholar]
- 3.Ridderstolpe L, Gill H, Granfeldt H, Ahlfeldt H, Rutberg H. Superficial and deep sternal wound complications: incidence, risk factors and mortality. Eur J Cardiothorac Surg. 2001;20(6):1168–75. doi: 10.1016/S1010-7940(01)00991-5. [DOI] [PubMed] [Google Scholar]
- 4.Salehi Omran A, Karimi A, Ahmadi SH, Davoodi S, Marzban M, Movahedi N, et al. Superficial and deep sternal wound infection after more than 9000 coronary artery bypass graft (CABG): incidence, risk factors and mortality. BMC Infect Dis. 2007;7:112. doi: 10.1186/1471-2334-7-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardlund B, Bitkover CY, Vaage J. Postoperative mediastinitis in cardiac surgery - microbiology and pathogenesis. Eur J Cardiothorac Surg. 2002;21(5):825–30. doi: 10.1016/S1010-7940(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 6.Mekontso-Dessap A, Kirsch M, Brun-Buisson C, Loisance D. Poststernotomy mediastinitis due to Staphylococcus aureus: comparison of methicillin-resistant and methicillin-susceptible cases. Clin Infect Dis. 2001;32(6):877–83. doi: 10.1086/319355. [DOI] [PubMed] [Google Scholar]
- 7.Modrau IS, Ejlertsen T, Rasmussen BS. Emerging role of Candida in deep sternal wound infection. Ann Thorac Surg. 2009;88(6):1905–9. doi: 10.1016/j.athoracsur.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Mossad SB, Serkey JM, Longworth DL, Cosgrove DM, 3rd, Gordon SM. Coagulase-negative staphylococcal sternal wound infections after open heart operations. Ann Thorac Surg. 1997;63(2):395–401. doi: 10.1016/S0003-4975(96)00834-X. [DOI] [PubMed] [Google Scholar]
- 9.Munoz P, Menasalvas A. Bernaldo de Quiros JC, Desco M, Vallejo JL, Bouza E. Postsurgical mediastinitis: a case-control study. Clin Infect Dis. 1997;25(5):1060–4. doi: 10.1086/516068. [DOI] [PubMed] [Google Scholar]
- 10.San Juan R, Chaves F, Lopez Gude MJ, Diaz-Pedroche C, Otero J, Cortina Romero JM, et al. Staphylococcus aureus poststernotomy mediastinitis: description of two distinct acquisition pathways with different potential preventive approaches. J Thorac Cardiovasc Surg. 2007;134(3):670–6. doi: 10.1016/j.jtcvs.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16(3):128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 12.Pittet D, Monod M, Suter PM, Frenk E, Auckenthaler R. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg. 1994;220(6):751–8. doi: 10.1097/00000658-199412000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Playford EG, Webster AC, Sorrell TC, Craig JC. Antifungal agents for preventing fungal infections in non-neutropenic critically ill patients. Cochrane Database Syst Rev. 2006;(1):Cd004920. doi:10.1002/14651858.CD004920.pub2. [DOI] [PubMed]
- 14.Byhahn C, Rinne T, Halbig S, Albert S, Wilke HJ, Lischke V, et al. Early percutaneous tracheostomy after median sternotomy. J Thorac Cardiovasc Surg. 2000;120(2):329–34. doi: 10.1067/mtc.2000.108161. [DOI] [PubMed] [Google Scholar]
- 15.Hubner N, Rees W, Seufert K, Bockelmann M, Christmann U, Warnecke H. Percutaneous dilatational tracheostomy done early after cardiac surgery--outcome and incidence of mediastinitis. Thorac Cardiovasc Surg. 1998;46(2):89–92. doi: 10.1055/s-2007-1010196. [DOI] [PubMed] [Google Scholar]
- 16.Stamenkovic SA, Morgan IS, Pontefract DR, Campanella C. Is early tracheostomy safe in cardiac patients with median sternotomy incisions? Ann Thorac Surg. 2000;69(4):1152–4. doi: 10.1016/S0003-4975(99)01577-5. [DOI] [PubMed] [Google Scholar]
- 17.Westphal K, Byhahn C, Rinne T, Wilke HJ, Wimmer-Greinecker G, Lischke V. Tracheostomy in cardiosurgical patients: surgical tracheostomy versus ciaglia and fantoni methods. Ann Thorac Surg. 1999;68(2):486–92. doi: 10.1016/S0003-4975(99)00565-2. [DOI] [PubMed] [Google Scholar]
- 18.Park H, Kent J, Joshi M, Zhu S, Bochicchio GV, Henry S, et al. Percutaneous versus open tracheostomy: comparison of procedures and surgical site infections. Surg Infect (Larchmt) 2013;14(1):21–3. doi: 10.1089/sur.2011.059. [DOI] [PubMed] [Google Scholar]
- 19.Sun L, Boodhwani M, Baer H, McDonald B. The association between tracheostomy and sternal wound infection in postoperative cardiac surgery patients. Can J Anaesth. 2013;60(7):684–91. doi: 10.1007/s12630-013-9950-6. [DOI] [PubMed] [Google Scholar]
- 20.Provan JL, Austen WG. The role of elective tracheostomy after open-heart surgery. Ann Thorac Surg. 1966;2(3):358–67. doi: 10.1016/S0003-4975(10)66590-3. [DOI] [PubMed] [Google Scholar]
- 21.Marshall RD. A review of the management of 140 elective tracheostomies following open-heart surgery. Thorax. 1969;24(1):78–83. doi: 10.1136/thx.24.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diehl JL, El Atrous S, Touchard D, Lemaire F, Brochard L. Changes in the work of breathing induced by tracheotomy in ventilator-dependent patients. Am J Respir Crit Care Med. 1999;159(2):383–8. doi: 10.1164/ajrccm.159.2.9707046. [DOI] [PubMed] [Google Scholar]
- 23.Nieszkowska A, Combes A, Luyt CE, Ksibi H, Trouillet JL, Gibert C, et al. Impact of tracheotomy on sedative administration, sedation level, and comfort of mechanically ventilated intensive care unit patients. Crit Care Med. 2005;33(11):2527–33. doi: 10.1097/01.CCM.0000186898.58709.AA. [DOI] [PubMed] [Google Scholar]
- 24.Bacchetta MD, Girardi LN, Southard EJ, Mack CA, Ko W, Tortolani AJ, et al. Comparison of open versus bedside percutaneous dilatational tracheostomy in the cardiothoracic surgical patient: outcomes and financial analysis. Ann Thorac Surg. 2005;79(6):1879–85. doi: 10.1016/j.athoracsur.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 25.Gaudino M, Losasso G, Anselmi A, Zamparelli R, Schiavello R, Possati G. Is early tracheostomy a risk factor for mediastinitis after median sternotomy? J Card Surg. 2009;24(6):632–6. doi: 10.1111/j.1540-8191.2009.00907.x. [DOI] [PubMed] [Google Scholar]


