Abstract
Background
Escherichia coli has emerged as a promising platform microorganism to produce biofuels and fine chemicals of industrial interests. Certain obstacles however remain to be overcome, among which organic-solvent tolerance is a crucial one.
Results
We used global transcription machinery engineering (gTME) to improve the organic-solvent tolerance (OST) of E. coli JM109. A mutant library of σ70 encoded by rpoD was screened under cyclohexane pressure. E. coli JM109 strain harboring σ70 mutant C9 was identified with capability of tolerating 69 % cyclohexane. The rpoD mutant contains three amino-acid substitutes and a stop-codon mutation, resulting a truncated sequence containing regions σ1.1 and σ1.2. Total protein difference produced by E. coli JM109 strain harboring C9 was examined with 2D-PAGE, and 204 high-abundant proteins showed over twofold variation under different solvent stress.
Conclusions
Our results show that several genes (gapA, sdhB, pepB and dppA) play critical roles in enhanced solvent tolerance of E. coli, mainly involving in maintaining higher intracellular energy level under solvent stress. Global transcription machinery engineering is therefore a feasible and efficient approach for engineering strain with enhanced OST-phenotype.
Electronic supplementary material
The online version of this article (doi:10.1186/s12934-015-0368-4) contains supplementary material, which is available to authorized users.
Keywords: Global transcription machinery engineering, Sigma factor 70, Organic solvent tolerance, Escherichia coli, gapA, sdhB, pepB
Background
The increasing attention to green chemistry has prompted the production of non-renewable fuels, materials, pharmaceuticals, and fine chemicals by microbial factories [1]. Escherichia coli as one of the most important platform microorganisms, could be applied as a whole-cell biocatalyst, which provides safe intracellular environment for enzymes [2]. In whole-cell biocatalysis, nonaqueous system (such as organic solvents) is often adopted to facilitate the solubility of hydrophobic substrates and (or) products [3]. Organic solvents are toxic to most microorganisms. E. coli was reported to barely tolerate organic solvents with LogP values lower than 3.4–3.8 [4]. For example, toluene is toxic to E. coli cells even at concentrations as low as 0.1 % [5]. Hence, it is of great importance to develop organic-solvent tolerant (OST) E. coli strains for industrial applications.
Since the first toluene tolerant strain Pseudomonas putida IH-2000 identified in 1989, extensively work had been focused on P. putida and Clostridium species etc. [6, 7]. Various OST mechanisms have been proposed, including cell membrane adaptations [8], cell morphology [9], and efflux pumps etc. [10, 11]. Traditional strain engineering methods, such as adaptation [12], enrichment cultivation [13], chemical and physical mutagenesis [14], have been widely used for developing OST strains. Global transcription machinery engineering (gTME) is a novel directed evolution strategy to assist in unlocking complex phenotypes by disturbing the transcription at genome level. Alper and co-workers obtained yeast strains that tolerated ethanol up to 20 % (v/v) [15]. In the past few years, a number of gTME-aided studies have outperformed those of traditional methods, resulting desired phenotypes more effectively. Several transcription factors, such as sigma factor in bacteria [16], zinc finger-containing artificial transcription factor [17], Spt15 in yeast [18] were widely used as a potential tool to improve strain tolerance and increase biofilm formation. Sigma factor 70 (σ70) is the most common transcription factor in E. coli. In addition to binding to RNA polymerase and recognizing DNA template strand, it can also alter the affinity of RNA polymerase to the promoter. Most importantly, the transcriptional efficiency can be regulated by mutation of σ factor [19, 20]. Alper and Stephanopoulos successfully constructed an E. coli strain that could tolerate as high as 60 g/L ethanol by mutating rpoD [21]. By random mutagenesis of rpoD and rpoS, Yu and coworkers obtained an E. coli mutant that could produce 561.4 mg/L hyaluronic acid [22].
In our previous study, an OST P. putida JUCT1 that tolerated 60 % cyclohexane was obtained by gradient adaptation. Based on two-dimensional electrophoresis (2-DE), two 3-hydroxyacid dehydrogenase family genes, mmsB (from P. putida) and zwf (from E. coli), were identified and proved to be critical for the enhanced solvent tolerance in both P. putida and E. coli [23, 24].
In this study, we evaluated the efficacy of gTME in E. coli by screening rpoD mutant library under cyclohexane pressure. We aimed at isolating σ70 mutants to improve the solvent tolerance of E. coli, which could potentially be applied in non-aqueous biocatalysis and biofuel production.
Results
Screening of solvent tolerance σ70 mutants
To improve the solvent tolerance of E. coli, a rpoD mutant library was constructed and screened under cyclohexane pressure. After preliminary screening, 9 strains resulted in OD660 of over 1.1 were subjected to secondary screening, where mutants were enriched through repeated subcultures supplemented with escalating cyclohexane concentration. Finally, E. coli strain carrying σ70 mutant C9 showed the highest cyclohexane tolerance was selected. When grew in 38 % cyclohexane, its OD660 could reach 0.83, while the parent strain JM109/pHACM-rpoDWT could not even grow under this condition (Fig. 1a). Then solvent tolerance of σ70 mutant C9 was determined under higher cyclohexane concentrations. The result showed that E. coli harboring C9 could tolerate 69 % cyclohexane (Fig. 1b). In the absence of cyclohexane, there was no significant difference between the cell growth of WT and C9 mutant strains (Fig. 1c), suggesting rpoD mutagenesis would not affect the normal growth of the E. coli strain.
Fig. 1.
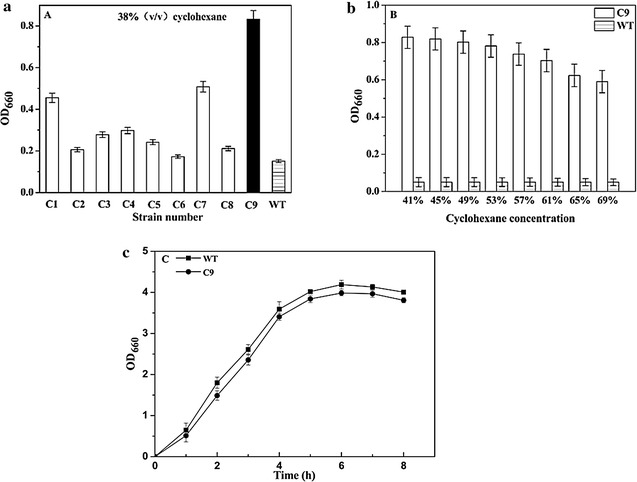
a Screening of solvent tolerance σ70 mutants C1–C9 at 38 % cyclohexane; b cyclohexane tolerance of E. coli JM109 harboring σ70 mutants C9 and WT; c cell growth of E. coli JM109 harboring C9 mutant and WT in absence of organic solvent. Strains were cultured at 37°C. For a and b, different concentration of cyclohexane was added when OD660 reached 0.2. Cell density was determined after 8 h of growth
Solvent tolerance towards other solvents was also investigated. E. coli carrying C9 showed increasing in cell density when cultivated in presence of 1.0 % butanol, 13 % hexane, 0.4 % toluene, and 0.5 % butyl acetate, whereas WT σ70 could merely survive under 0.1 % butanol, 5 % hexane, 0.1 % toluene and 0.2 % butyl acetate (Fig. 2).
Fig. 2.
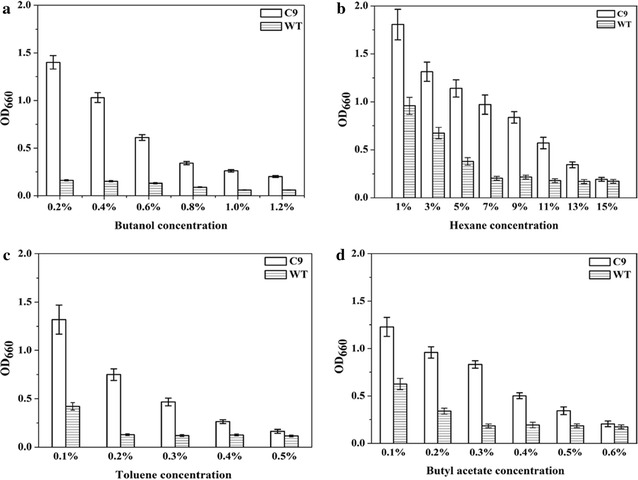
Solvent tolerance of E. coli JM109 harboring σ70 mutant C9 and WT towards different concentrations of butanol (a), hexane (b), toluene (c), and butyl acetate (d) Strains JM109/pHACM-rpoD C9 and JM109/pHACM-rpoD WT were cultured at 37 °C. Different concentrations of butanol, hexane, toluene and butyl acetate were added when OD660 reached 0.2. Cell density was determined after 8 h of growth
Among various organic solvents, C9 mutant strain showed higher tolerance to cyclohexane. Since cyclohexane pressure was used in the library screening, isolated mutants often show higher preference to cyclohexane. Additionally, it has been proved that cyclohexane could be oxidized into cyclohexanol with less toxicity by microorganisms in our previous study [23].
The sequence alignment of σ70 WT and C9 revealed that rpoD mutant gene C9 contains two amino-acid mutations in region 1.1 (D39E, A72V) and two other mutations in region 1.2 (T94M, and a stop codon mutation at residue 123).
2-DE analysis and protein identification by MALDI-TOF/TOF
Two-DE, a powerful protein separation technique to illustrate proteins associated with certain phenotype, was used to investigate the proteomics of E. coli strains harboring C9 mutant when grown with or without cyclohexane. 2-DE analysis of WT strain (without cyclohexane) was also conducted as control. Our results show that there was no obvious difference between WT and C9 strain in the absence of cyclohexane (Additional file 1: Figure S1). Compared with control (C9 without solvent), 204 high-abundant proteins in C9 strain showed over twofold difference in the presence of 38 % cyclohexane (Fig. 3).
Fig. 3.
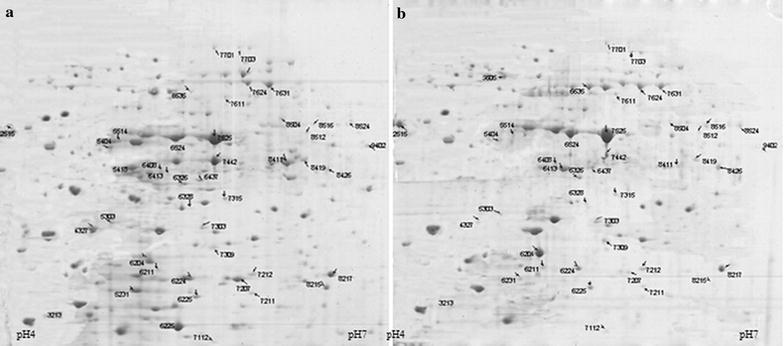
2D-PAGE of total proteins of E. coli JM109/pHACM-rpoD C9 under different solvent treatments. a Without solvent; b with 38 % (v/v) cyclohexane. For each treatment condition, 2-DE experiment was conducted in triplicates
Among 204 high-abundant proteins, 43 independent protein spots were cut off and analyzed by MALDI-TOF/TOF. Finally, 22 proteins including 19 up-regulated and 3 down-regulated proteins were successfully identified (Table 1). These up-regulated proteins are involved in nucleotide synthesis, amino acid and glucose metabolism, transporter and porin proteins synthesis, etc.
Table 1.
Proteins identification by MALDI-TOF/TOF
| Spot no. | Homologous protein annotation | Coding gene | Mass (Da) | pI | Up/down regulation | Function |
|---|---|---|---|---|---|---|
| 2512 | Phosphotransferase enzyme family protein | UMNK88_2189 | 32,682 | 4.98 | ↑ | Unknown |
| 5231 | Universal stress global response regulator | uspA | 16,028 | 5.08 | ↑ | Modulate and reorganize the carbon flow |
| 5303 | Conserved protein, UPF0070 family | yfgM | 22,162 | 5.07 | ↑ | Unknown |
| 5514 | Galactose-binding transport protein | mglB | 35,720 | 5.68 | ↑ | Galactose-binding transport |
| 6204 | Chain A, outer membrane protein | ompX | 16,350 | 5.04 | ↑ | Promote bacterial adhesion |
| 6224 | Global DNA-binding transcriptional dual regulator H-NS | Hns | 15,331 | 5.24 | ↑ | Global DNA-binding transcriptional dual regulator H-NS |
| 6326 | Chaperones protein HchA | hchA | 31,190 | 5.63 | ↑ | HSP31 molecular chaperone |
| 6328 | Chain A, outer membrane protein Ompw | ompW | 21,661 | 6.03 | ↑ | Acts as a receptor for colicin S4 |
| 6413 | pspA Protein | pspA | 25,562 | 5.51 | ↑ | Regulatory protein for phage-shock-protein operon |
| 6437 | Adenylate kinase | Adk | 23,712 | 6.01 | ↑ | Energy metabolism |
| 6524 | Outer membrane protein OmpA | ompA | 17,186 | 5.99 | ↑ | Serves as a receptor for a number of T-even like phage |
| 6635 | Tryptophanase | tnaA | 53,098 | 5.88 | ↑ | Tryptophanase/l-cysteine desulfhydrase |
| 7212 | 50S Ribosomal protein L9 | Prll | 15,759 | 6.15 | ↑ | RNA-binding protein |
| 7303 | Superoxide dismutase | soda | 15,974 | 5.95 | ↑ | Destroys radicals in the cells |
| 7315 | ATP-dependent Clp protease, proteolytic subunit ClpP | Clp | 23,286 | 5.52 | ↑ | Acts to disaggregate proteins |
| 8419 | Two-component system phosphate regulon response regulator OmpR | ompR | 26,757 | 6.01 | ↑ | DNA-binding protein |
| 7611 | Aminopeptidase B | pepB | 46,483 | 5.60 | ↑ | Probably acts in intracellular peptide degradation |
| 8425 | Succinate dehydrogenase iron-sulfur subunit | sdhB | 27,394 | 6.31 | ↑ | Iron-sulfur protein subunit of succinate dehydrogenase |
| 8524 | Glyceraldehyde-3-phosphate dehydrogenase A | gapA | 35,550 | 6.58 | ↑ | Encodes one of the two subunits of GapA |
| 5404 | Outer membrane porin protein C | ompC | 40,343 | 4.58 | ↓ | Forms passive diffusion pores |
| 4327 | Thiol peroxidase | Bcp | 17,995 | 5.03 | ↓ | Bacterioferritin comigratory protein |
| 7703 | Chain A, dipeptide transporter | dppA | 57,599 | 5.74 | ↓ | Dipeptide-binding protein of a transport system that can be subject to osmotic shock |
↑ Represents up-regulated genes; ↓ represents down-regulated genes
Among them, up-regulated genes gapA (glyceraldehyde-3-phosphate dehydrogenase A) and sdhB (FeS subunit of succinate dehydrogenase) are involved in the glycolysis process and TCA cycle, respectively [25, 26]. Both of them could produce intracellular ATP and provide high energy storage to overcome solvent stress. The expression levels of pepB (aminopeptidase B) and yfgM (a hypothetical protein) were remarkably enhanced in C9 mutant under solvent treatment, whereas their functions have barely been reported. Both bcp (thiol peroxidase) and dppA (dipeptide transporter) genes were significantly down-regulated. It has been reported that bcp is related to the organic solvent (such as phenol) and oxidative stress tolerance [27]. Gene dppA could function as dipeptide transporter, which might responsible for the organic solvent transportation into cytoplasm [28]. Therefore, these six genes (gapA, sdhB, pepB, yfgM, dppA and bcp) were selected for further characterization. Real-time RT-qPCR analysis confirmed that the transcription levels of 4 up-regulated genes (yfgM, gapA, sdhB, pepB) in C9 strain were enhanced for 15.6, 2.9, 4.2, and 12.4-fold under solvent treatment, and the transcription levels of 2 down-regulated genes (bcp and dppA) were decreased by 14.1 and 10 folds (Additional file 2: Figure S2).
OST-related properties of candidate genes
SDS-PAGE analysis of retro-complementation strains
We constructed four gene deletion E. coli strains, JM109(ΔyfgM), JM109(ΔsdhB), JM109(ΔgapA) and JM109(ΔpepB), and their corresponding retro-complementary strains. The successful expression of these retro-complementary genes was confirmed by SDS-PAGE (Additional file 3: Figure S3). The molecular weights of YFGM, FeS subunit of succinate dehydrogenase, glyceraldehyde-3-phosphate dehydrogenase A, and Aminopeptidase B were estimated to be 22, 26, 35 and 46 kDa, respectively, in agreement with their theoretical values.
OST assay of up- and down-regulated genes
Colony-formation efficiency method was employed for OST assay. E. coli knockout strains were cultured in the presence of 1 % (v/v) cyclohexane. As shown in Fig. 4a, all four single-gene deletion strains, as well as retro-complementary strain JM109(ΔyfgM)/yfgM showed no tolerance to cyclohexane. For the other 3 retro-complementary strains, JM109(ΔsdhB)/sdhB, JM109(ΔgapA)/gapA and JM109(ΔpepB)/pepB, the colony-formation efficiency was over 103 magnitude higher than that of the control (E. coli JM109), exhibiting remarkably higher cyclohexane tolerance than the control.
Fig. 4.
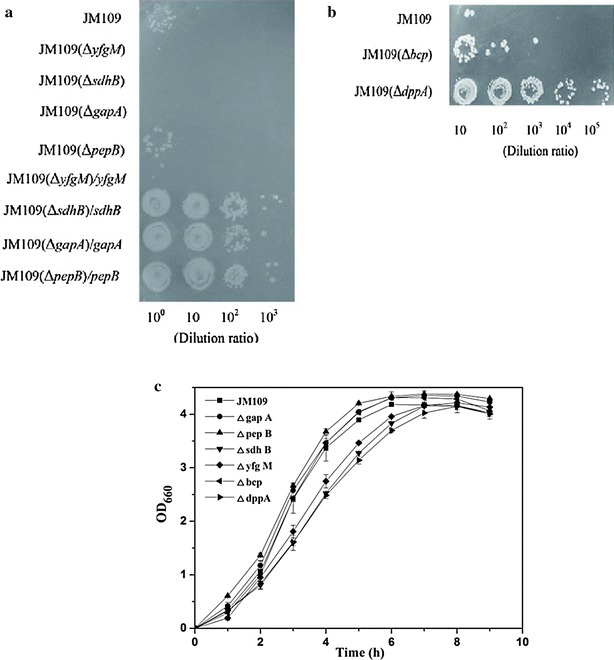
Colony formation efficiency of various E. coli knockout and retro-complementary strains of a up-regulated genes and b down-regulated genes under 1 % (v/v) cyclohexane; c cell growth of knockout strains in absence of cyclohexane. The cultures were spotted in 10-fold dilutions and incubated at 37 °C for 24 h
OST assay of down-regulated genes (bcp and dppA) was also performed. After overnight incubation, the colony-formation efficiency of JM109(ΔdppA) was over 104 magnitude higher than that of the control (Fig. 4b). Strain JM109(Δbcp) also showed slightly increased solvent tolerance.
To further evaluate the effect of gene deletion on cell growth under standard condition, six knockout strains were cultured in LB liquid medium without solvent. Figure 4c shows there was no obvious difference between the cell growth of JM109 and the knockout strains in the absence of cyclohexane. Similar cell density (OD660 around 4.2) was reached by all the strains after 8 h of growth, indicating these gene deletions would not affect the normal cell growth of E. coli.
Effect of organic solvents on the intracellular ATP
It has been reported that the presence of organic solvent could lower intracellular ATP level [29]. When JM109 harboring σ70 C9 and WT were cultured without cyclohexane, the intracellular ATP concentrations were determined to be 2.65 ± 0.58 μmol/g and 2.98 ± 0.65 μmol/g. The presence of 1 % cyclohexane caused a decrease in ATP to 1.45 ± 0.46 μmol/g and 0.78 ± 0.34 μmol/g for C9 and WT, respectively (Additional file 4: Table S1). And while C9 was grown in LB with 38 % cyclohexane, the content of ATP was 0.56 μmol/g (data not shown). These results indicate that σ70 mutant C9 could help to maintain higher intracellular ATP level than its WT.
Application of pHACM-rpoDC9 in whole-cell biotransformation
Using whole-cell biocatalyst E. coli BL21(DE3)/BmGDH-CgCR/pHACMC9, biocatalytic preparation of ethyl (R)-4-chloro-3-hydroxybutanoate [(R)-CHBE] catalyzed in aqueous/butyl acetate and aqueous/toluene biphasic reaction systems was attempted. After 2 h of reaction at 30 °C, (R)-CHBE was achieved in higher yields in both biphasic systems compared with control strain (without pHACMC9). At substrate (COBE) concentration of 100 g/L, optical pure (R)-CHBE was produced in 100 % yield in aqueous/butyl acetate biphasic system, and a bit lower yield of 94.6 % in aqueous/toluene system. Whereas lower yields of 79.5 and 85.5 % were obtained by the control strain in the same biphasic systems (Table 2).
Table 2.
Asymmetric synthesis of (R)-CHBE in aqueous/organic solvent (1:1) biphasic systems
| Strains | Enzyme activity (U/mg) | Yield (%) | ee (%) | ||
|---|---|---|---|---|---|
| GDH | CgCR | Aqueous/butyl acetate | Aqueous/toluene | ||
| E. coli/BmGDH-CgCR | 9.5 | 0.9 | 79.5 | 85.5 | >99 |
| E. coli/BmGDH-CgCR/pHACMC9 | 8.9 | 0.8 | 100 | 94.6 | >99 |
Reaction conditions: 0.5 g wet cells, 250 g/L glucose, 100 g/L of ethyl 4-chloro-3-oxobutanoate (COBE) in 5 mL potassium phosphate buffer (0.1 mol/L, pH 6.5) and 5 mL organic solvent, in 30 °C shaker for 2 h
Discussion
The organic-solvent resistance of microorganisms is important for their applications in non-aqueous whole-cell biocatalysis and biofuel (such as alcohol) fermentation processes. In our previous study, an OST strain (P. putida JUCT1), capable of growing in 60 % (v/v) cyclohexane, was isolated following gradient solvent adaptation [23]. Singh and co-workers developed an OST-adapted strain P. putida which could grow in the presence of 30 % (v/v) cyclohexane [30]. In recent years, global transcriptional engineering (gTME) approach has been successfully utilized to improve the stress-resistance performance of microbial cells [15]. Zhang and co-workers also reported the isolation of E. coli strain tolerating 1.2 % (v/v) butanol using this approach [9]. Here, gTME was adopted to enhance the solvent tolerance of E. coli. After two rounds of screening, an OST E. coli strain harboring σ70 could survive in the presence of 69 % cyclohexane. Furthermore, C9 mutant did not affect the normal growth of E. coli strains.
OST assay indicates that four genes (gapA, sdhB,pepB and dppA) play critical roles in OST of E. coli. Glyceraldehyde-3-phosphate dehydrogenase A (gapA) is involved in glycolysis process that could produce pyruvic acid and ATP [25]. FeS subunit of succinate dehydrogenase (sdhB) is involved in TCA cycle and could provide coenzyme for succinate dehydrogenase that catalyzes the synthesis of fumarate and ATP [26]. Consequently, up-regulated expression of gapA and sdhB can increase the intracellular ATP level, providing high energy storage. Organic solvents usually have negative effect on the intracellular ATP level. On one hand, most of organic solvents could partially inhibit the membrane ATPase and lowered the intracellular ATP level [29]. On the other hand, cells need to consume more ATP to provide energy to pump organic solvents out of the cells and decrease its toxicity [31]. Therefore, mutant C9 with high intracellular ATP level will result in improved OST for cells.
The solvent-tolerant mechanism of pepB is still unknown and needs to be further studied. The dppA knockout could improve solvent tolerance of E. coli, since dppA could function as dipeptide transporter that was reported to be responsible for organic solvent transportation into cytoplasm [28]. However, retro-complementation of yfgM, an up-regulated gene, did not improve the solvent tolerance of E. coli significantly. It is speculated that synergistic effect also plays critical roles in the OST-related functions of a number of genes, such as yfgM. In other studies, a number of critical genes involved in solvent tolerance phenotype of microorganisms had been identified. For example, Honda’s group reported that the solvent tolerance of E. coli was markedly enhanced by overexpression of manXYZ [32] and purR [33]. Some OST-related genes were listed (Additional file 5: Table S2).
There are 7 σ factors (σ70, σ54, σ32, σS, σE, σF and σFecI) in E. coli, and σ70 is the main control factor that is responsible for the transcription of over 1000 genes. σ70 consists of 4 parts, namely σ1 (region 1.1), σ2 (region 1.2 and region 2), σ3 (region 3) and σ4 (region 4). In 2007, Alper and Stephanopoulos generated σ70 mutant gene which retained only σ4 region to enhance the ethanol tolerance of E. coli [21]. In 2008, Yu and coworkers isolated σ70 mutant comprised of only σ1 and σ2 for enhanced hyaluronic acid accumulation [22]. In this study, a truncated mutation also occurred in rpoD mutant C9, remaining regions σ1.1and σ1.2 (Fig. 5). Our results indicate that a truncated mutation in rpoD may markedly change cell phenotype such as OST by regulating the transcription of a number of related genes.
Fig. 5.

Genetic composition of σ70 WT and C9
Zhang and coworkers reported that cell morphological may change to adapt to the harsh condition, such as solvent environment [9]. In this study, both E. coli strains harboring WT and C9 σ70 exhibited a much elongated and narrower shape in the presence of 4.0 % (v/v) cyclohexane, compared with those without solvent (data not shown). It is presumed that a lower specific surface area of E. coli harboring C9 could conduce to its higher solvent tolerance.
In this study, the potential of σ70 mutant in whole-cell biocatalysis in aqueous/solvent biphasic systems was also validated. Using a recombinant E. coli strain transformed with pHACMC9, higher yield was achieved in different biphasic reaction systems containing extremely toxic solvents, i.e. butyl acetate (LogP = 1.7) and toluene (LogP = 2.5). It suggests that σ70 mutant could conduce to enhanced whole-cell biocatalytic efficiency in non-aqueous system by enhancing solvent tolerance of microbial cells.
Conclusions
Microbial OST mechanisms are complicated and regulated by multi-mechanisms. In this study, random mutagenesis of σ70 is a feasible and efficient approach for engineering strain with OST-phenotype. Based on proteomic analysis, several genes (gapA, sdhB, pepB and dppA) contributed to the enhanced solvent tolerance of E. coli. Additionally, our results provide molecular basis to construct OST strains for industrial applications such as biofuel production and non-aqueous biocatalysis.
Methods
Bacterial strains and chemicals
Organic-solvent sensitive E. coli JM109 was used as the starting strain and was cultured as previously described [24]. Strains and plasmids used in this study are shown in Table 3. Primers used in this study are listed (Additional file 6: Table S3). PrimeSTAR®HS DNA Polymerase and restriction enzymes were purchased from Takara (Tokyo, Japan). GeneMorph II Random Mutagenesis Kit was purchased from Stratagene (La Jolla, CA, USA). The dam-methylated DNA specific restriction enzyme DpnI was purchased from New England Biolabs (Ipswich, MA, USA). Cyclohexane, toluene, and other solvents were obtained from Sinopharm Chemical Reagent (Shanghai, China).
Table 3.
Strains and plasmids used in this study
| Strains and plasmids | Genotype | Reference |
|---|---|---|
| Escherichia coli strains | ||
| JM109 | F’(traD36, proAB+, lacIq, Δ(lacZ)M15) endA1 recA1 hsdR17 (r− K, m+ K) mcrA supE44 λ− gyrA96 relA1 Δ(lac-proAB) thi −1 | Takara |
| JM109(ΔyfgM) | Same as JM109, but with ΔyfgM | This study |
| JM109(ΔsdhB) | Same as JM109, but with ΔsdhB | This study |
| JM109(ΔgapA) | Same as JM109, but with ΔgapA | This study |
| JM109(ΔpepB) | Same as JM109, but with ΔpepB | This study |
| JM109(Δbcp) | Same as JM109, but with Δbcp | This study |
| JM109(ΔdppA) | Same as JM109, but with ΔdppA | This study |
| JM109(ΔyfgM)/yfgM | Same as JM109, but with ΔyfgM/pQE-yfgM | This study |
| JM109(ΔsdhB)/sdhB | Same as JM109, but with ΔsdhB/pQE-sdhB | This study |
| JM109(ΔgapA)/gapA | Same as JM109, but with ΔgapA/gapA | This study |
| JM109(ΔpepB)/pepB | Same as JM109, but with ΔpepB/pQE-pepB | This study |
| Plasmids | ||
| pKD13, pKD46, and pCP20 | Expression vector | 34 |
| pQE80L | Expression vector | QIAGEN |
| pHACM-rpoD WT | Plasmid harboring WT rpoD | 22 |
| pHACM-rpoD C9 | Plasmid harboring rpoD mutant C9 | This study |
Construction of random mutagenesis library
Random mutagenesis of rpoD was performed with GeneMorph® II Random Mutagenesis Kit using a low-copy number plasmid pHACM-rpoDWT (a kind gift from Dr. Huimin Yu, Tsinghua University) as template (around 120 ng, aiming at 5–9 mutations/kb) [22]. Whole plasmid PCR was performed to accomplish library construction, and the amplified recombinant plasmids were transformed into E. coli JM109 after digestion by DpnI. E. coli transformants were spread on LB agar plate containing 34 μg/mL of chloramphenicol, and the colonies were scraped off to form rpoD mutant library for further OST phenotype selection. The total library size of rpoD mutants was approximately 106.
Phenotype selection
Primary screening
The rpoD mutant library was inoculated into LB/Cm liquid medium. When OD660 reached 0.2, 4 % (v/v) cyclohexane was added into the culture. After 8 h of growth at 200 rpm and 37 °C, cells were spread onto LB/Cm agar plates. Colonies from agar plate were picked and inoculated into LB/Cm liquid medium supplemented with 4 % (v/v) cyclohexane. JM109/pHACM-rpoDWT was used as the control. After 8 h of incubation at 200 rpm and 37 °C, cell density was measured and 9 mutants with OD660 above 1.1 were chosen for re-screening.
Secondary screening
Better mutants selected were further cultured under higher cyclohexane (4–70 %) concentrations by 2 % (v/v) gradient. The cell density was measured after 8 h of growth. Finally, the best mutant named C9 was chosen.
The solvents were supplemented in volume percentage unless otherwise stated.
Resistance to various organic solvents
Escherichia coli strain harboring σ70 mutant C9 was incubated at 37 °C and 220 rpm for overnight, and the overnight culture was inoculated (1.0 %, v/v) into fresh medium. Different concentrations of butanol, hexane, toluene and butyl acetate were added when OD660 reached 0.2. Cell density was analyzed by measuring OD660 after incubation for 8 h.
Extraction of total cellular protein
Escherichia coli strain harboring σ70 mutant C9 was cultured overnight without (as control) or with 38 % cyclohexane. Cells were harvested by centrifugation at 4000×g and 4 °C for 10 min, and washed three times with cold deionized water. Then cell pellets were resuspended in lysis buffer (2 M thiourea, 8 M urea, 65 mM dithiothreitol, 40 mM Tris-base, 4 % (w/v) CHAPS, and 0.001 % (w/v) bromophenol blue), and treated with ultrasonication (300 W, pulse 1 s, pause 3 s for 15 min) in ice water bath. The total cellular protein was obtained by centrifugation at 15,000×g and 4 °C for 15 min to remove the cell debris. Protein concentration was measured using Sangon Biotech Non-Interference Protein Assay Kit (Shanghai, China). All protein samples were stored at −80 °C for further 2-D electrophoresis analysis.
2-D electrophoresis and protein identification by MALDI-TOF/TOF
2-D electrophoresis was performed as previous described [22]. Briefly, the total cellular protein samples (800 μg per sample) were subjected to 2-DE on IPG pH 4–7 strips (24 cm, GE Healthcare). In order to obtain wide distribution of all proteins spots, IPG 3–10 strips (7 cm, GE Healthcare, Pittsburgh, PA, USA) was initially used in isoelectric focusing electrophoresis (IEF). The result indicates that most proteins were located over pH range of 4–7. Therefore, IPG 4–7 strips (24 cm, GE Healthcare) were chosen. The isoelectric focusing was performed at 20 °C using the following program: 150 V for 1 h, gradient to 250 V for 1 h, gradient to 500 V for 1.5 h, gradient to 1000 V for 2.5 h, gradient to 5000 V for 4 h, gradient to 10,000 V for 2.5 h, holding at 10,000 V, 90,000 V/h, and for the total of 65,000 V/h. Then, each strip was equilibrated, washed twice and transferred onto 12 % SDS-polyacrylamide gels. After 2-DE, the destained gel images obtained by ImageScanner III (GE Healthcare, PA, USA) was analyzed by PDQuest™2-D Analysis Software (Bio-Rad, CA, USA). For each treatment condition, 2-DE experiment was conducted in triplicate.
Protein spots were identified by MALDI-TOF/TOF using the method described previously [22].
RNA isolation and real-time quantitative reverse transcription PCR
Total cellular RNA was extracted from E. coli strain harboring σ70 mutant C9 grown overnight with or without 38.0 % (v/v) cyclohexane using the Simply P Total RNA Extraction Kit (BioFlux, Japan). Reverse transcription step was carried out using RevertAid First Strand cDNA Synthesis Kit (Thermo, USA) with random primer mix following the manufacturer’s manual. Real-time quantitative reverse transcription PCR (RT-qPCR) was performed with RealMasterMix (SYBR Green) (TIANGEN, China) using Bio-Rad iQ5 real-time PCR detection system (Bio-Rad, USA). The bacterial 16S rRNA gene sequence was used as a reference gene in real-time PCR (Additional file 6: Table S3). The real-time PCR conditions were as follows: 1 min at 94 °C, 35 cycles at 94 °C for 10 s, followed by 55 °C for 30 s and 68 °C for 15 s. To analyze the gene expression level, ΔΔCt method was chosen and the standard curves of each primer were plotted to ensure similar amplification efficiency compared with the reference gene.
Gene knockout and retro-complementation
Using Red-mediated recombination [34], six genes including four up-regulated (gapA, sdhB, pepB, yfgM) and two down-regulated (dppA and bcp) genes were knocked out from genome of E. coli JM109 (Table 3). Meanwhile, retro-complementation strains of four up-regulated genes (gapA, sdhB, pepB and yfgM) were also generated using pQE80L as expression vector. The expression of these genes was analyzed by SDS-PAGE after induction with 0.2 mM IPTG.
Colony formation efficiency assay
For four up-regulated genes (gapA, sdhB, pepB and yfgM) and two down-regulated genes (dppA and bcp), six knockout strains and four corresponding retro-complementation strains of up-regulated genes were cultured in LB medium. Strains JM109 was used as control. For four retro-complementation strains, 0.2 mM IPTG were added when OD660 reached 0.2 to initiate induction. For all strains, 1 % cyclohexane was added when OD660 reached 1.0. The cells were further cultivated for 90 min. Then the cultures were diluted for 105, 104, 103 and 102, 10 folds with aseptic water. Then 10 μL of the diluted culture was spread onto LB ager plate, and further incubated at 37 °C for 20 h.
Quantification of intracellular ATP
For E. coli strains harboring σ70 WT or C9, cyclohexane [1 % (v/v)] was added at exponential phase of. Cells were harvested at stationary phases by centrifugation at 4000×g and 4 °C for 10 min. The cells were treated with lysis solution, and ATP concentrations were determined with ATP bioluminescence assay kit from Beyotime (Haimen, China) based on fluorescence intensity. The intracellular ATP content was calculated according to the standard curve.
Whole-cell biocatalysis in biphasic systems
Plasmid pHACM-rpoDC9 was transformed into E. coli BL21(DE3)/BmGDH-CgCR harboring a carbonyl reductase and glucose dehydrogenase, which had been previously constructed for the asymmetric synthesis of ethyl (R)-4-chloro-3-hydroxybutanoate [(R)-CHBE], a chiral intermediate for the synthesis of l-carnitine [35]. The resulted strain E. coli BL21(DE3)/BmGDH-CgCR/pHACMC9 was applied as whole-cell biocatalyst in aqueous/solvent biphasic systems. And E. coli BL21(DE3)/BmGDH-CgCR was used as control. Reaction mixture consists of 0.5 g wet cells, 250 g/L glucose, 100 g/L of ethyl 4-chloro-3-oxobutanoate (COBE) in 5 mL potassium phosphate buffer (0.1 mol/L, pH 6.5) and 5 mL organic solvent, and was incubated in a 30 °C shaker for 2 h. The reaction mixture was extracted by ethyl acetate for further GC analysis.
Authors’ contributions
FZ, XQ, and HS performed all the experiments and analyzed the data. FZ drafted the manuscript. GX and RH revised the manuscript. YN designed the study, and critically revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to the Natural Science Foundation of China (21276112, 31401634), Natural Science Foundation of Jiangsu Province (BK20150003), Fundamental Research Funds for the Central Universities (JUSRP51409B), the Program of Introducing Talents of Discipline to Universities (111-2-06), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions for the financial support of this research.
Competing interests
The authors declare that they have no competing interests.
Additional files
10.1186/s12934-015-0368-4 2D-PAGE of total proteins of E. coli JM109/pHACM-rpoD WT without solvent.
10.1186/s12934-015-0368-4 Real-time qRT-PCR of up- and down-regulated genes.
10.1186/s12934-015-0368-4 Recombinant expression of pepB, gapA, sdhB and yfgM in E. coli JM109 and their corresponding knockout strains.
10.1186/s12934-015-0368-4 Intracellular ATP concentration in E. coli harboring WT and C9 mutant rpoD.
10.1186/s12934-015-0368-4 OST-related genes identified in microorganisms.
10.1186/s12934-015-0368-4 Primers used in this study.
Contributor Information
Fa Zhang, Email: zf23.32@163.com.
Xiaohong Qian, Email: xiaohongqian2011@hotmail.com.
Haiming Si, Email: 601610917@qq.com.
Guochao Xu, Email: guochaoxu@jiangnan.edu.cn.
Ruizhi Han, Email: hanrz@jiangnan.edu.cn.
Ye Ni, Email: yni@jiangnan.edu.cn.
References
- 1.Luque R, Herrero-Davila L, Campelo JM, Clark JH, Hidalgo JM, Luna D, Marinas JM, Romero AA. Biofuels: a technological perspective. Energy Environ Sci. 2008;1:542–564. doi: 10.1039/b807094f. [DOI] [Google Scholar]
- 2.de Carvalho CC. Enzymatic and whole cell catalysis: finding new strategies for old processes. Biotechnol Adv. 2011;29:75–83. doi: 10.1016/j.biotechadv.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Heipieper HJ, Neumann G, Cornelissen S, Meinhardt F. Solvent-tolerant bacteria for biotransformations in two-phase fermentation systems. Appl Microbiol Biotechnol. 2007;74:961–973. doi: 10.1007/s00253-006-0833-4. [DOI] [PubMed] [Google Scholar]
- 4.Inoue A, Horikoshi K. Estimation of solvent-tolerance of bacteria by the solvent parameter log P. J Ferment Bioeng. 1991;71:194–196. doi: 10.1016/0922-338X(91)90109-T. [DOI] [Google Scholar]
- 5.Sardessai Y, Bhosle S. Tolerance of bacteria to organic solvents. Res Microbiol. 2002;153:263–268. doi: 10.1016/S0923-2508(02)01319-0. [DOI] [PubMed] [Google Scholar]
- 6.Inoue A, Horikoshi K. A Pseudomonas thrives in high concentrations of toluene. Nature. 1989;227:264–265. doi: 10.1038/338264a0. [DOI] [Google Scholar]
- 7.Mi J, Becher D, Lubuta P, Dany S, Tusch K, Schewe H, Buchhaupt M, Schrader J. De novo production of the monoterpenoid geranic acid by metabolically engineered Pseudomonas putida. Microb Cell Fact. 2014;13:170. doi: 10.1186/s12934-014-0170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alsaker KV, Paredes C, Papoutsakis ET. Metabolite stress and tolerance in the production of biofuels and chemicals: gene-expression-based systems analysis of butanol, butyrate, and acetate stresses in the anaerobe Clostridium acetobutylicum. Biotechnol Bioeng. 2010;105:1131–1147. doi: 10.1002/bit.22628. [DOI] [PubMed] [Google Scholar]
- 9.Zhang HF, Chong HQ, Ching CB, Song H, Jiang RR. Engineering global transcription factor cyclic AMP receptor protein of Escherichia coli for improved 1-butanol tolerance. Appl Microbiol Biotechnol. 2012;94:1107–1117. doi: 10.1007/s00253-012-4012-5. [DOI] [PubMed] [Google Scholar]
- 10.Tsukagoshi N, Aono R. Entry into and release of solvents by Escherichia coli in an organic-aqueous two-liquid-phase system and substrate specificity of the AcrAB-TolC solvent-extruding pump. J Bacteriol. 2000;182:4803–4810. doi: 10.1128/JB.182.17.4803-4810.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rojas A, Segura A, Guazzaroni ME, Teran W, Hurtado A, Gallegos MT, Ramos JL. In vivo and in vitro evidence that TtgV is the specific regulator of the TtgGHI multidrug and solvent efflux pump of Pseudomonas putida. J Bacteriol. 2003;185:4755–4763. doi: 10.1128/JB.185.16.4755-4763.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minty JJ, Lesnefsky AA, Lin FM, Chen Y, Zaroff TA, Veloso AB, Xie B, McConnell CA, Ward RJ, Schwartz DR, Rouillard JM, Gao Y, Gulari E, Lin XN. Evolution combined with genomic study elucidates genetic bases of isobutanol tolerance in Escherichia coli. Microb Cell Fact. 2011;10:18. doi: 10.1186/1475-2859-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin YL, Blaschek HP. Butanol production by a butanol-tolerant strain of Clostridium acetobutylicum in extruded corn broth. Appl Environ Microbiol. 1983;45:966–973. doi: 10.1128/aem.45.3.966-973.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermann M, Fayolle F, Marchal R, Podvin L, Sebald M, Vandecasteele JP. Isolation and characterization of butanol-resistant mutants of Clostridium acetobutylicum. Appl Environ Microbiol. 1985;50:1238–1243. doi: 10.1128/aem.50.5.1238-1243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alper H, Moxley J, Nevoigt E, Fink GR, Stephanopoulos G. Engineering yeast transcription machinery for improved ethanol tolerance and production. Science. 2006;314:1565–1568. doi: 10.1126/science.1131969. [DOI] [PubMed] [Google Scholar]
- 16.Klein-Marcuschamer D, Stephanopoulos G. Assessing the potential of mutational strategies to elicit new phenotypes in industrial strains. Proc Natl Acad Sci USA. 2008;105:2319–2324. doi: 10.1073/pnas.0712177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park KS, Lee DK, Lee H, Lee Y, Jang YS, Kim YH, Yang HY, Lee SI, Seol W, Kim JS. Phenotypic alteration of eukaryotic cells using randomized libraries of artificial transcription factors. Nat Biotechnol. 2003;21:1208–1214. doi: 10.1038/nbt868. [DOI] [PubMed] [Google Scholar]
- 18.Liu HM, Yan M, Lai CG, Xu L, Ouyang PK. gTME for improved xylose fermentation of Saccharomyces cerevisiae. Appl Biochem Biotechnol. 2010;160:574–582. doi: 10.1007/s12010-008-8431-9. [DOI] [PubMed] [Google Scholar]
- 19.Siegele DA, Hu JC, Walter WA, Gross CA. Altered promoter recognition by mutant forms of the σ 70 subunit of Escherichia coli RNA polymerase. J Mol Biol. 1989;206:591–603. doi: 10.1016/0022-2836(89)90568-8. [DOI] [PubMed] [Google Scholar]
- 20.Gardella T, Moyle H, Susskind MM. A mutant Escherichia coli sigma 70 subunit of RNA polymerase with altered promoter specificity. J Mol Biol. 1989;206:579–590. doi: 10.1016/0022-2836(89)90567-6. [DOI] [PubMed] [Google Scholar]
- 21.Alper H, Stephanopoulos G. Global transcription machinery engineering: a new approach for improving cellular phenotype. Metabolic Eng. 2007;9:258–267. doi: 10.1016/j.ymben.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Yu H, Tyo K, Stephanopoulos G. A high-throughput screen for hyaluronic acid accumulation in recombinant Escherichia coli transformed by libraries of engineered sigma factors. Biotechnol Bioeng. 2008;101:788–796. doi: 10.1002/bit.21947. [DOI] [PubMed] [Google Scholar]
- 23.Ni Y, Song L, Qian XH, Sun ZH. Proteomic analysis of Pseudomonas putida reveals an organic solvent tolerance-related gene mmsB. PLoS One. 2013;8:e55858. doi: 10.1371/journal.pone.0055858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian XH, Song L, Ni Y. Enhanced organic solvent tolerance of Escherichia coli by 3-hydroxyacid dehydrogenase family genes. Appl Biochem Biotechnol. 2014;172:3106–3115. doi: 10.1007/s12010-014-0726-4. [DOI] [PubMed] [Google Scholar]
- 25.Charpentier B, Branlant C. The Escherichia coli gapA gene is transcribed by the vegetative RNA polymerase holoenzyme E sigma 70 and by the heat shock RNA polymerase E sigma 32. J Bacteriol. 1994;176:830–839. doi: 10.1128/jb.176.3.830-839.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooley JW, Howitt CA, Vermaas WFJ. Succinate: quinol oxidoreductases in the cyanobacterium Synechocystis sp. strain PCC 6803: presence and function in metabolism and electron transport. J Bacteriol. 2000;182:714–722. doi: 10.1128/JB.182.3.714-722.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos PM, Benndorf D, Sá-Correia I. Insights into Pseudomonas putida KT2440 response to phenol-induced stress by quantitative proteomics. Proteomics. 2004;4:2604–2652. doi: 10.1002/pmic.200300793. [DOI] [PubMed] [Google Scholar]
- 28.Kang A, Chang MW. Identification and reconstitution of genetic regulatory networks for improved microbial tolerance to isooctane. Mol Biosyst. 2012;8:1350–1358. doi: 10.1039/c2mb05441h. [DOI] [PubMed] [Google Scholar]
- 29.Bowles LK, Ellefson WL. Effects of butanol on Clostridium acetobutylicum. Appl Environ Microbiol. 1985;50:1165–1170. doi: 10.1128/aem.50.5.1165-1170.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh SK, Singh SK, Tripathi VR, Khare SK, Garg SK. A novel psychrotrophic, solvent tolerant Pseudomonas putida SKG-1 and solvent stability of its psychro-thermoalkalistable protease. Process Biochem. 2011;46:1430–1435. doi: 10.1016/j.procbio.2011.03.012. [DOI] [Google Scholar]
- 31.Reyes LH, Almario MP, Kao KC. Genomic library screens for genes involved in n-butanol tolerance in Escherichia coli. PLoS One. 2011;6:e17678. doi: 10.1371/journal.pone.0017678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okochi M, Kurimoto M, Shimizu K, Honda H. Increase of organic solvent tolerance by overexpression of manXYZ in Escherichia coli. J Appl Microbiol Biotechnol. 2007;73:1394–1399. doi: 10.1007/s00253-006-0624-y. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu K, Hayashi S, Doukyu N, Kobayashi T, Honda H. Time-course data analysis of gene expression profiles reveals purR regulon concerns in organic solvent tolerance in Escherichia coli. J Biosci Bioeng. 2005;99:72–74. doi: 10.1263/jbb.99.72. [DOI] [PubMed] [Google Scholar]
- 34.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu GC, Yu HL, Zhang ZJ, Xu JH. Stereocomplementary bioreduction of β-ketonitrile without ethylated byproduct. Org Lett. 2013;15:5408–5411. doi: 10.1021/ol402733y. [DOI] [PubMed] [Google Scholar]


