Abstract
Degeneration and loss of retinal pigment epithelium (RPE) is the cause of a number of degenerative retinal diseases, including age-related macular degeneration (AMD), retinitis pigmentosa, and diabetic retinopathy, leading to blindness that affects three million Americans as of now. Transplantation of RPE aims to restore retinal structure and the interaction between the RPE and photoreceptors, which is fundamental to sight. Although a significant amount of progress has been made in the past 20 years in autologous RPE transplantation, sources for RPE cells are limited. Recent advances in stem cell culture and differentiation techniques have allowed the generation of RPE cells from pluripotent stem cells. In this review, we discuss strategies for generating functional RPE cells from human embryonic stem cells and induced pluripotent stem cells, and summarize transplantation studies of these derived RPEs. We conclude with challenges in cell-replacement therapies using human embryonic and induced pluripotent stem cell-derived RPEs.
Keywords: embryonic stem cells, adult retinal stem cells, retinal progenitor cells, retinal degeneration
I. RETINAL PIGMENT EPITHELIUM PHYSIOLOGY AND FUNCTION
I.A. Retinal Pigment Epithelium
Retinal pigment epithelium (RPE) cells form the outermost layer of the retina, and are composed of a single layer of terminally differentiated cells lining photoreceptor cells that perform complex functions such as maintenance of photoreceptor physiology, absorption of light, formation of the blood-retinal barrier, and phagocytosis of shed outer segments of photoreceptors. To maintain the phototransduction machinery, RPE cells constantly phagocytose photoreceptor outer segments at a rate of 25,000 to 30,000 disks/cell/d.1 In addition, RPE transports metabolic wastes from the retina into the choriocapillaries. Intracellular RPE melanin absorbs extra light not captured by photoreceptors and thus prevents retinal damage.2 Light causes isomerization of 11-cis-retinal to trans-retinal in the outer segments of the photoreceptors. RPE is involved in the re-isomerization of all-trans-retinal to 11-cis-retinal,3 in the maintenance of structural integrity of the retina, and in the uptake of vitamin A from the choroidal capillaries and photoreceptors.4 All of these functions are dependent on the two-cell system of photoreceptors and RPE cells. This two-cell system probably developed due to the enzymatic isomerization mechanism and/or the high packing density of the visual pigments in the rod and cone outer segments to achieve maximum optical density of structures. Overall, RPE cells are implicated in light absorption, epithelial transport, maintenance of visual cycle, phagocytosis of photoreceptor outer segments, and secretion of growth factors,5-8 as extensively described in the literature.9
I.B. RPE Development
The phenotype of RPE development is characterized by changes in morphology, gene and protein expression, and protein localization, including the formation of tight junctions.10 Neuroepithelial cells destined to form the RPE or neural retinal cells oppose each other with a slight lumen between them after invagination of the optic cup. The RPE is generated as a result of expression of myocardin-related transcription factor (Mrtf) and inhibition of Chx10 in the developing vertebrate eye via epithelial-mesenchymal interactions.11 A unique interrelationship between the RPE and photoreceptor differentiation is essential for proper development of the retina.
Expression of the tyrosinase promoter that marks the onset of melanogenesis promotes the maturation of RPE from neuroectodermal cells. The RPE and endothelial basement membrane form the Bruch’s membrane, which is comprised of inner collagenous layers, the basement membrane of the RPE, an elastin layer, an outer collagenous layer, and the basement membrane of choriocapillaris. Ezrin, a cyclic AMP-dependent protein kinase-anchoring protein, is also shown to be essential for the formation of the microvilli of the RPE. Another important feature is the formation of tight junctions between the RPE cells. In the second phase of interaction between the RPE and photoreceptors, the photoreceptor cells extend outer segments, to which the RPE responds by elongating its apical microvilli into the subretinal space. The microvilli then start to surround the growing outer segments of the photoreceptors. In addition, a number of research articles have been published on protein expression of RPE during development. Amongst these proteins, alpha-5 integrin has been shown to be absent in the newborn rat but is detected at postnatal day 7 and increases to a steady-state level at postnatal day 11, a process that is correlated with the ability of RPE to phagocytose photoreceptors.12 Extracellular matrix metalloproteinase inducer (EMMPRIN) undergoes a reversal in polarity from basolateral to only apical expression during the postnatal to P14 stage of development.13
I.C. Retinal Degeneration
1. Age-related Macular Degeneration
Age-related macular degeneration (AMD) occurs in about 30% of the elderly population of the United States.14,15 Environmental, genetic, and nutritional factors contribute to the pathogenesis of AMD. Cigarette smoking, exposure to sunlight leading to generation of reactive oxygen species, and low dietary intake of antioxidant compounds are the leading factors for disease onset.16 There are two clinical subtypes of AMD, the dry form, corresponding to 90% of cases with geographic atrophy, and the wet form, with choroidal neovascularization.17 Between the RPE and the Bruch’s membrane, deposits of lipids, glycosaminoglycans, and esterified/non-esterified cholesterol molecules are formed during the early stages of AMD.18,19 Iron overload has also been shown to be associated with AMD20; however, it is unknown if iron accumulation is a cause or effect of AMD. Primarily, mitochondrial damage and oxidative stress have been shown to be responsible for AMD.21 The RPE is highly susceptible to oxidative stress, especially in relation to phagocytosing outer segments of the photoreceptors and the presence of multiple photosensitizers that induce reactive oxygen species on exposure to intense visible light.16 The imbalance between VEGF and PEDF leads to chronic neovascularization in AMD patients, probably due to amyloid protein accumulation.22 Indocyanine green angiography can be utilized to visualize the choroidal vasculature and choroidal neovascularization related to AMD23 that indicate deposits in Brusch’s membrane. Further supporting evidence for mitochondrial dysfunction with the generation of reactive oxygen species in human RPE cells has been shown to be implicated in AMD.16 Transplantation of fetal RPEs is limited by donor availability for treatment of RPE dysfunction in diseased states such as AMD and retinitis pigmentosa.
2. Retinitis Pigmentosa
Retinitis pigmentosa is the most common form of inherited retinal degeneration. It begins with night blindness and reduced or delayed electroretinograms, and is primarily caused by mutations in at least 180 genes,24 notably aryl hydrocarbon receptor-interacting protein-like 1 (AIPL1). Mutations in RPE65 lead to autosomal recessive retinitis pigmentosa.25 There are three forms of retinitis pigmentosa: non-syndromic, syndromic, and systemic. The disease is characterized by the progression of pigment deposits initially in the peripheral retina, tunnel vision, attenuation of blood vessels, and retinal atrophy.26 Mutations in the Mertk gene have been shown to induce retinitis pigmentosa in rat models using the RCS (Royal College of Surgeons) mutant strain of rats.27 In addition, there could be a relationship between RPE-based endothelin-1 (ET-1) secretion and the pathogenesis of retinitis pigmentosa.28 There is also evidence for alterations in the deposition of cell surface proteoglycans from RPE in the retinitis pigmentosa pathological condition.29,30 Retinitis pigmentosa is also associated with progressive apoptosis of photoreceptors.31 Transcription factors, proteins involved in retinal metabolism, splicing factors, and intracellular proteins have also been implicated in the onset of retinitis pigmentosa pathogenesis.32 Further, alterations in retinal metabolism are associated with rod and cone involvement and deposition in the RPE.33 Electroretinogram measurements show reduction in retinal photoreceptor function before symptotic night blindness or decreased visual acuity arise.34 Gene therapy, retinal prosthesis, sunlight exposure, and vitamin therapy are some of the therapeutic options for retinitis pigmentosa disorders.
3. Diabetic Retinopathy
The early state of diabetic retinopathy is a nonproliferative stage with the formation of retinal vascular aneurysms and blot hemorrhages. The middle stage involves retinal capillary loss and ischemia, followed by neovascularization, fibrovascular proliferation, and retinal detachment in the advanced hyperproliferative stage.35 The onset of diabetic retinopathy is most likely associated with neuroglial abnormalities that are related to vascular and neuronal pathological effects. Diabetic complications are multifactorial, and include hyperglycemic effects on retinal degeneration leading to the generation of reactive oxygen species; overexpression of vascular endothelial growth factors leading to leakiness, neovascularization, and collagen I and IV thickening in the basement membranes. One of the main deleterious products of diabetic retinopathy is advanced glycation end products (AGE) that stimulate the receptor for advanced glycation end products (RAGE) signaling cascade with downstream generation of tumor necrosis factor (TNF)-alpha, interleukin (IL)-6, and vascular endothelial growth factor (VEGF). Excellent research articles highlighting the significance of AGE-RAGE signaling in retinal diabetic complications are available in the literature.36-39 From a metabolic perspective, there is also information on the polyol pathway, the two-step conversion of glucose to sorbitol catalyzed by aldose reductase, followed by conversion to fructose that leads to downstream AGE product synthesis via fructose-3-phosphate and 3-deoxyglucosone precursors, with vascular complications implicated in pathogenesis of diabetic retinopathy.40,41 More recently, there has been supporting evidence for hyperglycemic-mediated endoplasmic reticulum stress and amino acid deprivation that leads to the synthesis of VEGF implicated in the pathogenesis of diabetic retinopathy.42,43 There is also increasing evidence for immunological mechanisms implicated in the pathogenesis of diabetic retinopathy. Infiltration of macrophages and leukocytes into the choriocapillaries with subsequent losses in endothelial cells has been reported in the literature.44 It has been demonstrated that inflammatory cytokines and molecules contributing to early stages of diabetic retinopathy include iNOS, COX-2, ICAM-1, caspase1, and nuclear factor kappa beta (NF-κB), increased production of nitric oxide, and prostaglandin E2.45
I.D. Therapeutic Approaches to Retinal Degenerative Disease
1. Pharmaceutical Intervention
An excellent review discussing pharmacological drugs and naturally occurring molecules that have been developed and tested in clinical trials for inhibition of retinal neovascularization and thickening of capillary basement membranes is available.46 Prominent among these drugs is Lucentis (ranibizumab), a drug synthesized by Genentech that prevents leakiness of blood and neovascularization in AMD patients by blocking multiple forms of VEGF.47 In vitro studies confirm inhibition of human umbilical vein endothelial cells (HUVEC) proliferation. This is the first therapy for AMD in vision defect patients. Ranibizumab and bevacizumab48 are both potent anti-angiogenic molecules; however, these molecules partially overlap with the receptor-binding site of VEGF and act mainly via steric hindrance and therefore do not block VEGF activity completely.49 More recently, it has been shown that ranibizumab binds to VEGF-165, VEGF-110, and VEGF-121, and inhibits pharmacological activity. Novel anti-VEGF antibodies synthesized from synthetic phage display libraries possess the quality to bind to both mouse and human VEGF, and therefore have more applicability in animal model testing for various retinal diseases. The bevacizumab to ranibizumab cost ratio is 1:39; however, the latter is more efficacious in treating AMD. Further considerations in animal model testing and improving the efficacy of bevacizumab might improve AMD testing for approximately 25,000 cases in the UK annually.50 A variety of therapeutic approaches have been identified for the treatment of diabetic retinopathy, including aldose reductase, carbonic anhydrase, and protein kinase-C inhibitors. These interventions, however, do not have a dramatic effect on retarding the development of retinopathy.51
2. Gene Therapy
Short-term studies of gene therapy have been found them to be safe and favorable for adeno-associated virus (AAV) carrier for human RPE65 DNA delivery to dogs for 8 years after treatment. This treatment is for Leber’s congenital amaurosis wherein seven genes are preferentially expressed in photoreceptor and RPE cells52; however, major issues with this technology include reproducibility of data, persistence of stable function, and systemic or ocular complications arising from high-dose vectors. Further, in recessive retinal pigmentosa Prph2Rd2/Rd2 knockout mice, injection of AAV carrying the gene has shown limited success in both young and older animals, with fewer numbers of outer segment induction, although with stabilization of structure due to delayed and inappropriate onset of transgene expression.53 It has been shown that the Rhodopsin kinase (RK) promoter enhances phosphodiesterase synthesis, a stabilizing molecule for rod and cone photoreceptors.54
Overall, although surgical, pharmacological, and genetic interventions have been utilized to treat retinal degenerative diseases, there has been limited success in clinical trials for inhibiting disease progression. Thus, there is a need to utilize a suitable cell source that has the potential to generate fully functional RPEs for transplantation in retinal disease conditions.
II. CELL TRANSPLANTATION FOR RETINAL REPAIR
Transplantation of RPE in the subretinal space has proven successful in many animal models and human trials of AMD and retinitis pigmentosa in which the degeneration of RPE leads to photoreceptor and subsequent vision loss. The goal of transplantation for retinal repair is to restore the subretinal anatomy and to re-establish the connection between the RPE and the photoreceptors that is critical to sight. Transplantation studies started about 20 years ago through transplantation of RPE cells into the dystrophic RCS rat, in which a defect in the RPE cells leads to death of the photoreceptors and makes the model suitable for RPE transplantation studies. It was shown that transplantation of freshly prepared cells within the first month of life rescued the photoreceptors and maintained visual function. Animal studies with the transplantation ARPE-19 cells (an immortalized human RPE cell line) demonstrated the efficacy of the technique by preventing the secondary damage to photoreceptors that resulted in an improvement of visual acuity without transplantation of photoreceptors.55,56 Clinical applications so far have focused on using fetal retinal sheets (neural retina and the underlying RPE) or autologous RPE/choroid from the peripheral retina to the macula as the donor tissue. A recent clinical trial for transplantation of fetal retinal sheets in AMD and retinitis pigmentosa patients demonstrated the efficacy of the technique and an improvement in visual acuity in 70% of the patients.57 However, because neither autologous or fetal tissues are abundantly available, the research focus has shifted toward exploring alternative sources of cells for retinal transplantation studies.
II.A. Cell Sources
There are two fundamentally different approaches to retinal transplantation. One is to limit the progress of photoreceptor loss by introducing cells before such loss occurs and thus maintaining surviving photoreceptors. The second approach is to replace the photoreceptors once they are gone. The strategy that is chosen depends on the primary reason for photoreceptor loss. If the RPE cells are lost or degenerating as in the case of AMD and retinitis pigmentosa, the defective cells can be replaced with freshly harvested adult RPE cells. However, clinical studies have shown very little improvement in vision because the patients included in the study had end-stage disease in which secondary damage to the retina prevented functional recovery. In addition, adult RPE cells are known to poorly attach to the Bruch’s membrane upon transplantation, and this is necessary for their survival.58 Moreover, there are the risks of immune rejection and transfer of infection with the transplantation of allograft RPE cells. To overcome these issues, autologous RPE harvested from the peripheral retina of the same eye has been used.59 Other researchers have explored the use of iris pigmented epithelial cells from the same eye, which have been found to have the capacity to expand in culture and then be transplanted.60,61 Limited human studies have shown some indication of functional rescue.62 Immortalized RPE has also been used as an alternative cell source in animal models,63 but this has not been attempted in human studies yet.
II.B. Embryonic Stem Cells
An attractive cell source for replacing RPE is to use embryonic stem cells. Human embryonic stem cells (hESCs) are pluripotent and have the capacity for indefinite self-renewal when maintained under appropriate cell culture conditions. Given appropriate cues, they can form all three germ layers,64-66 so they are an invaluable cell source for therapies involving cell transplantation. Embryonic stem cell lines are derived from the inner cell mass of blastocyst stage embryos,64,67,68 and are cultured on various types of feeder cells or a suitable extracellular matrix under feeder-free conditions.69,70 The pluripotency of hESCs is maintained in culture with knockout serum replacement and supplemental basic fibroblast growth factor.71,72 The ethical issues related to the derivation and use of embryonic stem cells led to the generation of induced pluripotent stem (iPS) cells,73 which are made by introducing pluripotency factors into adult somatic cells. Initial studies used four transcription factors, Oct4, Sox2, c-Myc, and Klf4, which were introduced into adult mouse fibroblasts through retroviral transfection.73 Through unknown mechanisms, these transcription factors reprogram somatic cells into the pluripotent stage. Like hESCs, iPS cells have compact colony morphology and can be maintained in culture for prolonged periods. They express pluripotency markers such as Oct4, Nanog, SSEA-1, and can differentiate in vitro and in vivo into the three germ layers. One of the concerns about the use of iPS cells in cell therapy has been the use of oncogenes, c-Myc and Klf4, to induce pluripotency. However, later studies showed that replacing c-Myc and Klf4 with Nanog and Lin-28 was also successful.74 Small molecules such as valproic acid, a histone deacetylase inhibitor, have been used instead of c-Myc and Klf4, and have also been successful in inducing pluripotency in human fibroblasts.75 Another concern about the use of iPS cells involved the use of viral transfection, which integrates a viral genome into the recipient genome to deliver the pluripotency genes.73 Recent studies showed that gene delivery could be achieved by using the non-integrating adenoviruses76 or episomal vectors.77 Given that the directed differentiation of iPS cells into the cell type of interest can be efficiently achieved, iPS cells are considered a safe, promising alternative to hESCs to generate patient-specific cells.
II.C. Differentiation of hESCs and iPS Cells Into RPEs
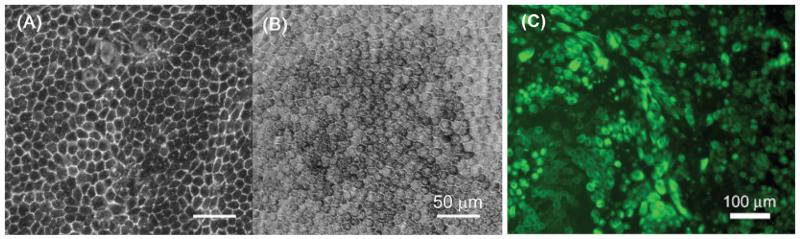
The differentiation of ESCs into RPE cells was first shown for primate embryonic stem cells.78 Primate ESCs were cultured on PA6 feeders and induced to differentiate into neuronal lineage. Unexpectedly, 3 weeks after the induction of differentiation, large patches of pigmented cells were found in about 8% of the primate ESC colonies. These cells had polygonal epithelial morphology resembling the RPE of the eye. Further characterization of these cells revealed that they were positive for tight junction protein ZO-1, RPE65, and cellular retinaldehyde-binding protein, all markers of RPE cells.79 Later studies showed that pigmented cells also appear in overgrown hESC cultures on mouse embryonic fibroblast (MEF) feeders for 2 to 6 weeks, which can easily be manually picked out due to the pigment. We also observed pigmented cell colonies in H1 hESC cultures within 3 to 4 weeks. These colonies were picked up manually and expanded on laminin resembling a morphology similar to human fetal RPE cells (Fig. 1). The cells were positive for adult RPE marker proteins and were capable of phagocytosis, an important RPE function.80 A comprehensive transcriptome analysis of RPE cell lines derived from several hESC lines revealed that the gene expression of the ESC-derived RPE cells were more similar to adult RPE cells than the immortalized RPE cell lines available.81 Further, the ESC-derived RPE cells were transplanted in developing chick embryos and were shown to integrate into chick retina, confirming the potential of these cells to generate retinal cells.82 When transplanted in dystrophic rat retina, the hESC-derived RPE cells were shown to improve visual performance by 100% over untreated controls.83,84 The spontaneous generation of functional RPE cells under MEF co-culture conditions suggest that the environment created by MEFs contains factors that may predispose cells to RPE differentiation.85
FIGURE 1.
Bright-field micrographs of (A) human fetal RPE and (B) hESC-derived RPE cells. (C) Cytokeratin staining of hESC-derived RPE cells.
A recent study reported directed differentiation of hESCs into RPE cells under defined conditions.86 The rate generation of pigmented colonies was greatly enhanced after treatment with nicotinamide for 8 weeks: 72% of the colonies were pigmented compared with 13% observed without the nicotinamide treatment at 8 weeks. Dynamic gene expression analysis revealed that the RPE differentiation from hESCs followed the gene expression pattern observed during development. Further, it was reported that the efficiency of RPE differentiation was greatly improved through TGF-β signaling. Recently, differentiation of iPS cells into RPE cells similar to hESC differentiation has also been reported.87-89 These preliminary but promising results suggest that not only are human ESCs a valuable source for generating functional RPE cells for therapeutic purposes, but also can generate patient-specific cells using iPS cells for the treatment of retinal degenerative diseases.
II.D. Transplantation of hESC/iPS-derived RPEs
Early transplantation studies using non-human primate and hESC-derived RPE cells in dystrophic RCS rats indicated the survival of pigmented cells in vivo with an absence of tumor formation.79,83 There was also functional outcome in vivo. Haruta et al. showed improvement in vision through measurement of the optokinetic head-tracking response.79 They also reported evidence for phagocytosis of rod outer segments by grafted primate-derived RPE. The findings by Haruta et al. were confirmed in a later study using hESC-derived RPE cells in which the visual function was assessed more comprehensively.83,84 RPE cells derived under defined conditions were also tested in dystrophic RCS rats.86 Upon transplantation into the subretinal space, there was no teratoma or tumor formation after 19 weeks of observation. Eighty-nine percent of the transplanted cells were also RPE65 positive, but only 50% of them were pigmented, suggesting the presence of non-RPE-like cells within the transplanted population. Photoreceptors near grafted cells were salvaged, and it was suggested that the effect might be due to trophic factors released, as well as the phagocytosis function provided by the grafted cells. More recently, similar results were reported for iPS-derived RPE cells.90 A long-term study to determine the safety and efficacy of hES-derived RPE cells in small animal models has also been published. 91 In accordance with previous reports, hESC-derived RPE cells did not form teratomas or tumors during the lifetime of NIH-III-immunodeficient mice, and after transplantation into the subretinal space of RCS rats, the transplanted cells survived for 8 months. The hESC-derived RPE cells rescued visual function in a dose-dependent manner in terms of measured visual acuity and luminescence threshold response. Rescue of visual function was also observed in Elov14 mice, a genetic model of Stargardt’s macular dystrophy, which is the most common cause of juvenile macular degeneration, suggesting the efficacy of the hESC-derived RPE cells in a clinically relevant human disease.
III. CHALLENGES IN CELL TRANSPLANTATION FOR RETINAL REPAIR
III.A. Extracellular Matrix
A major issue in RPE transplantation is the failure of transplanted RPE cells to attach to the Bruch’s membrane, which must occur because if it doesn’t, anchorage-dependent apoptosis might result. In addition, a damaged Bruch’s membrane is ineffective as a substrate for RPE attachment,58 probably due to the accumulation of inhibitory molecules within the membrane that interfere with interactions between integrins and extracellular matrix ligands.92,93 Therefore, it may be necessary to chemically or mechanically treat the Bruch’s membrane or the cells to enhance attachment prior to cell transplantation.94-96 Such techniques include debridement of the Bruch’s membrane prior to transplantation97,98 or overexpression of integrins in donor RPE cells.99 Another approach is to use tissue-engineering techniques to allow RPE cell monolayer formation on collagen or amniotic membrane in vitro prior to transplantation.100,101
III.B. Inflammation and Immune Responses
The immune status of the subretinal space is atypical in the sense that gross infiltration by lymphocytes is rarely seen after allograft transplantation. Therefore the subretinal space has been considered to be a partially immunologically privileged site.102,103 Even though prolonged survival has been described, foreign tissue in the subretinal space is not necessarily protected from immune attack, and the allogeneic grafts can still be lost despite immunosuppressive therapy. The mismatch in MHC haplotypes can lead to graft rejection.104 However, because ESCs fail to express MHC type II,105 they may have a lower potential for activating the immune system, especially if grafted in immune privileged sites.106 Moreover, the poor outcome in RPE transplantations in humans with AMD may be due to the loss of vascular integrity in advanced stages of the diseases, and these are the patients in whom most transplantation trials have been performed.107,108 Therefore, the reformation of the blood-retina barrier is essential to supporting graft survival.
IV. CONCLUSIONS
RPE transplantation is the ultimate therapy for the survival of photoreceptors, the loss of which leads to blindness. The lack of functional and readily available cell sources is one obstacle to RPE transplantation studies. Recent advances in the RPE differentiation of pluripotent stem cells, either embryonic or induced, make them an attractive and promising cell source for generating a large number of functional RPE cells for use in cell-based therapies.
ABBREVIATIONS
- AAV
adeno-associated virus
- AGE
advanced glycation end products
- AIPL1
aryl hydrocarbon receptor-interacting protein-like 1
- AMD
age-related macular degeneration
- EMMPRIN
extracellular matrix metalloproteinase inducer
- ET-1
endothelin-1
- hESC
human embryonic stem cells
- IL
interleukin
- iPS
induced pluripotent stem cells
- MEF
mouse embryonic fibroblast
- MHC
major histocompatibility complex
- Mrtf
myocardin-related transcription factor
- NF-κB
nuclear factor kappa beta
- RAGE
receptor for advanced glycation end products
- RCS
Royal College of Surgeons
- RK
Rhodopsin kinase
- RPE
retinal pigment epithelium
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
REFERENCES
- 1.Besharse J, Defoe D. In: The role of retinal pigment epithelium in photoreceptor membrane turnover. Marmor M, Wolfensberger T, editors. Oxford University Press; New York: 1998. [Google Scholar]
- 2.Arevalo JF, Fernandez CF, Mendosa AJ. Normal anatomy of the macula. In: Alfaro DV, Liggett PE, Mieler WF, Quiroz-Mercado H, Jager RD, Tano Y, editors. Age-related macular degeneration. Lippincott Williams and Wilkins; Philadelphia: 2005. pp. 1–13. [Google Scholar]
- 3.McBee JK, Van Hooser JP, Jang GF, Palczewski K. Isomerization of 11-cis-retinoids to all-trans-retinoids in vitro and in vivo. J Biol Chem. 2001;276(51):48483–93. doi: 10.1074/jbc.M105840200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retin Eye Res. 2001;20(4):469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 5.Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Basic fibroblast growth factor and local injury protect photoreceptors from light damage in the rat. J Neurosci. 1992;12(9):3554–67. doi: 10.1523/JNEUROSCI.12-09-03554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hewitt AT, Lindsey JD, Carbott D, Adler R. Photoreceptor survival-promoting activity in interphotoreceptor matrix preparations: characterization and partial purification. Exp Eye Res. 1990;50(1):79–88. doi: 10.1016/0014-4835(90)90013-k. [DOI] [PubMed] [Google Scholar]
- 7.Park CM, Hollenberg MJ. Growth factor-induced retinal regeneration in vivo. Int Rev Cytol. 1993;146:49–74. doi: 10.1016/s0074-7696(08)60379-4. [DOI] [PubMed] [Google Scholar]
- 8.Zhao S, Rizzolo LJ, Barnstable CJ. Differentiation and transdifferentiation of the retinal pigment epithelium. Int Rev Cytol. 1997;171:225–66. doi: 10.1016/s0074-7696(08)62589-9. [DOI] [PubMed] [Google Scholar]
- 9.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85(3):845–81. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 10.Marmorstein AD, Finnemann SC, Bonilha VL, Rodriguez-Boulan E. Morphogenesis of the retinal pigment epithelium: toward understanding retinal degenerative diseases. Ann N Y Acad Sci. 1998;857:1–12. doi: 10.1111/j.1749-6632.1998.tb10102.x. [DOI] [PubMed] [Google Scholar]
- 11.Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–96. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- 12.Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc Natl Acad Sci U S A. 1997;94(24):12932–7. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marmorstein AD, Bonilha VL, Chiflet S, Neill JM, Rodriguez-Boulan E. The polarity of the plasma membrane protein RET-PE2 in retinal pigment epithelium is developmentally regulated. J Cell Sci. 1996;109(Pt 13):3025–34. doi: 10.1242/jcs.109.13.3025. [DOI] [PubMed] [Google Scholar]
- 14.Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100(10):1519–35. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- 15.Seddon J. Age related macular degeneration. In: Ryan S, editor. Retina. 3rd ed. St. Louis: Mosby; 2001. pp. 1039–50. [Google Scholar]
- 16.Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76(4):397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 17.Shu X, Tulloch B, Lennon A, Hayward C, O’Connell M, Cideciyan AV, Jacobson SG, Wright AF. Biochemical characterization of the C1QTNF5 gene associated with late-onset macular degeneration. A genetic model of age-related macular degeneration. In: Hollyfield JG, Anderson RE, LaVail MM, editors. Retinal degenerative diseases. Springer; New York: 2006. pp. 41–8. [DOI] [PubMed] [Google Scholar]
- 18.Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99(23):14682–7. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malek G, Li CM, Guidry C, Medeiros NE, Curcio CA. Apolipoprotein B in cholesterol-containing drusen and basal deposits of human eyes with age-related maculopathy. Am J Pathol. 2003;162(2):413–25. doi: 10.1016/S0002-9440(10)63836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blasiak J, Sklodowska A, Ulinska M, Szaflik JP. Iron and age-related macular degeneration. Klin Oczna. 2009;111(4-6):174–7. [PubMed] [Google Scholar]
- 21.Jarrett SG, Lin H, Godley BF, Boulton ME. Mitochondrial DNA damage and its potential role in retinal degeneration. Prog Retin Eye Res. 2008;27(6):596–607. doi: 10.1016/j.preteyeres.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida T. [Molecular mechanism of choroidal neovascularization in age-related macular degeneration] Nippon Ganka Gakkai Zasshi. 2007;111(11):881–91. [PubMed] [Google Scholar]
- 23.Lutty G, Grunwald J, Majji AB, Uyama M, Yoneya S. Changes in choriocapillaris and retinal pigment epithelium in age-related macular degeneration. Mol Vis. 1999;5:35. [PubMed] [Google Scholar]
- 24.Daiger SP, Bowne SJ, Sullivan LS. Perspective on genes and mutations causing retinitis pigmentosa. Arch Ophthalmol. 2007;125(2):151–8. doi: 10.1001/archopht.125.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai X, Conley SM, Naash MI. RPE65: role in the visual cycle, human retinal disease, and gene therapy. Ophthalmic Genet. 2009;30(2):57–62. doi: 10.1080/13816810802626399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamel C. Retinitis pigmentosa. Orphanet J Rare Dis. 2006;1:40. doi: 10.1186/1750-1172-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, Apfelstedt-Sylla E, Vollrath D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet. 2000;26(3):270–1. doi: 10.1038/81555. [DOI] [PubMed] [Google Scholar]
- 28.Vingolo EM, Lupo S, Grenga PL, Salvatore S, Zinnamosca L, Cotesta D, Petramala L, Letizia C. Endothelin-1 plasma concentrations in patients with retinitis pigmentosa. Regul Pept. 2010 Feb 25;160(1-3):64–7. doi: 10.1016/j.regpep.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Hewitt AT, Newsome DA. Altered proteoglycans in cultured human retinitis pigmentosa retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1988;29(5):720–6. [PubMed] [Google Scholar]
- 30.Yue BY, Fishman GA. Synthetic activities of cultured retinal pigment epithelial cells from a patient with retinitis pigmentosa. Arch Ophthalmol. 1985;103(10):1563–6. doi: 10.1001/archopht.1985.01050100139036. [DOI] [PubMed] [Google Scholar]
- 31.Shintani K, Shechtman DL, Gurwood AS. Review and update: current treatment trends for patients with retinitis pigmentosa. Optometry. 2009;80(7):384–401. doi: 10.1016/j.optm.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 32.Hims MM, Diager SP, Inglehearn CF. Retinitis pigmentosa: genes, proteins and prospects. Dev Ophthalmol. 2003;37:109–25. doi: 10.1159/000072042. [DOI] [PubMed] [Google Scholar]
- 33.van SS, Westerveld A, de Jong PT, Bleeker-Wagemakers EM, Bergen AA. Retinitis pigmentosa: defined from a molecular point of view. Surv Ophthalmol. 1999;43(4):321–34. doi: 10.1016/s0039-6257(98)00046-0. [DOI] [PubMed] [Google Scholar]
- 34.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368(9549):1795–809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 35.Ali TK, El-Remessy AB. Diabetic retinopathy: current management and experimental therapeutic targets. Pharmacotherapy. 2009;29(2):182–92. doi: 10.1592/phco.29.2.182. [DOI] [PubMed] [Google Scholar]
- 36.Goh SY, Cooper ME. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93(4):1143–52. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- 37.Murata T, Nagai R, Ishibashi T, Inomuta H, Ikeda K, Horiuchi S. The relationship between accumulation of advanced glycation end products and expression of vascular endothelial growth factor in human diabetic retinas. Diabetologia. 1997;40(7):764–9. doi: 10.1007/s001250050747. [DOI] [PubMed] [Google Scholar]
- 38.Oldfield MD, Bach LA, Forbes JM, Nikolic-Paterson D, McRobert A, Thallas V, Atkins RC, Osicka T, Jerums G, Cooper ME. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE) J Clin Invest. 2001;108(12):1853–63. doi: 10.1172/JCI11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakurai S, Yonekura H, Yamamoto Y, Watanabe T, Tanaka N, Li H, Rahman AK, Myint KM, Kim CH, Yamamoto H. The AGE-RAGE system and diabetic nephropathy. J Am Soc Nephrol. 2003;14(8 Suppl 3):S259–63. doi: 10.1097/01.asn.0000077414.59717.74. [DOI] [PubMed] [Google Scholar]
- 40.Chung SS, Chung SK. Aldose reductase in diabetic microvascular complications. Curr Drug Targets. 2005;6(4):475–86. doi: 10.2174/1389450054021891. [DOI] [PubMed] [Google Scholar]
- 41.Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res. 2007;2007:61038. doi: 10.1155/2007/61038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abcouwer SF, Marjon PL, Loper RK, Vander Jagt DL. Response of VEGF expression to amino acid deprivation and inducers of endoplasmic reticulum stress. Invest Ophthalmol Vis Sci. 2002;43(8):2791–8. [PubMed] [Google Scholar]
- 43.Roybal CN, Yang S, Sun CW, Hurtado D, Vander Jagt DL, Townes TM, Abcouwer SF. Homocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4. J Biol Chem. 2004;279(15):14844–52. doi: 10.1074/jbc.M312948200. [DOI] [PubMed] [Google Scholar]
- 44.Adamis AP, Berman AJ. Immunological mechanisms in the pathogenesis of diabetic retinopathy. Semin Immunopathol. 2008;30(2):65–84. doi: 10.1007/s00281-008-0111-x. [DOI] [PubMed] [Google Scholar]
- 45.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clark AF, Yorio T. Ophthalmic drug discovery. Nat Rev Drug Discov. 2003;2(6):448–59. doi: 10.1038/nrd1106. [DOI] [PubMed] [Google Scholar]
- 47.Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26(8):859–70. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- 48.Lien S, Lowman HB. Therapeutic anti-VEGF antibodies. Handb Exp Pharmacol. 2008;(181):131–50. doi: 10.1007/978-3-540-73259-4_6. [DOI] [PubMed] [Google Scholar]
- 49.Muller YA, Chen Y, Christinger HW, Li B, Cunningham BC, Lowman HB, de Vos AM. VEGF and the Fab fragment of a humanized neutralizing antibody: crystal structure of the complex at 2.4 A resolution and mutational analysis of the interface. Structure. 1998;6(9):1153–67. doi: 10.1016/s0969-2126(98)00116-6. [DOI] [PubMed] [Google Scholar]
- 50.Raftery J, Clegg A, Jones J, Tan SC, Lotery A. Ranibizumab (Lucentis) versus bevacizumab (Avastin): modelling cost effectiveness. Br J Ophthalmol. 2007;91(9):1244–6. doi: 10.1136/bjo.2007.116616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Einarsdottir AB, Stefansson E. Prevention of diabetic retinopathy. Lancet. 2009;373(9672):1316–8. doi: 10.1016/S0140-6736(09)60750-9. [DOI] [PubMed] [Google Scholar]
- 52.Miller JW. Preliminary results of gene therapy for retinal degeneration. N Engl J Med. 2008;358(21):2282–4. doi: 10.1056/NEJMe0803081. [DOI] [PubMed] [Google Scholar]
- 53.Sarra GM, Stephens C, de Alwis M, Bainbridge JW, Smith AJ, Thrasher AJ, Ali RR. Gene replacement therapy in the retinal degeneration slow (rds) mouse: the effect on retinal degeneration following partial transduction of the retina. Hum Mol Genet. 2001;10(21):2353–61. doi: 10.1093/hmg/10.21.2353. [DOI] [PubMed] [Google Scholar]
- 54.Sun X, Pawlyk B, Xu X, Liu X, Bulgakov OV, Adamian M, Sandberg MA, Khani SC, Tan MH, Smith AJ, Ali RR, Li T. Gene therapy with a promoter targeting both rods and cones rescues retinal degeneration caused by AIPL1 mutations. Gene Ther. 2010 Jan;17(1):117–31. doi: 10.1038/gt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Lund RD, Adamson P, Sauvé Y, Keegan DJ, Girman SV, Wang S, Winton H, Kanuga N, Kwan AS, Beauchène L, Zerbib A, Hetherington L, Couraud PO, Coffey P, Greenwood J. Subretinal transplantation of genetically modified human cell lines attenuates loss of visual function in dystrophic rats. Proc Natl Acad Sci U S A. 2001 Aug 14;98(17):9942–7. doi: 10.1073/pnas.171266298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sauve Y, Pinilla I, Lund RD. Partial preservation of rod and cone ERG function following subretinal injection of ARPE-19 cells in RCS rats. Vision Res. 2006 Apr;46(8-9):1459–72. doi: 10.1016/j.visres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 57.Radtke ND, Aramant RB, Petry HM, Green PT, Pidwell DJ, Seiler MJ. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am J Ophthalmol. 2008 Aug;146(2):172–82. doi: 10.1016/j.ajo.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 58.Gullapalli VK, Sugino IK, Van Patten Y, Shah S, Zarbin MA. Impaired RPE survival on aged submacular human Bruch’s membrane. Exp Eye Res. 2005 Feb;80(2):235–48. doi: 10.1016/j.exer.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Binder S, Stolba U, Krebs I, Kellner L, Jahn C, Feichtinger H, Povelka M, Frohner U, Kruger A, Hilgers RD, Krugluger W. Transplantation of autologous retinal pigment epithelium in eyes with foveal neovascularization resulting from age-related macular degeneration: a pilot study. Am J Ophthalmol. 2002 Feb;133(2):215–25. doi: 10.1016/s0002-9394(01)01373-3. [DOI] [PubMed] [Google Scholar]
- 60.Rezai KA, Kohen L, Wiedemann P, Heimann K. Iris pigment epithelium transplantation. Graefes Arch Clin Exp Ophthalmol. 1997 Sep;235(9):558–62. doi: 10.1007/BF00947084. [DOI] [PubMed] [Google Scholar]
- 61.Abe T, Yoshida M, Tomita H, Kano T, Nakagawa Y, Sato M, Wada Y, Fuse N, Yamada T, Tamai M. Functional analysis after auto iris pigment epithelial cell transplantation in patients with age-related macular degeneration. Tohoku J Exp Med. 1999 Dec;189(4):295–305. doi: 10.1620/tjem.189.295. [DOI] [PubMed] [Google Scholar]
- 62.Abe T, Yoshida M, Tomita H, Kano T, Sato M, Wada Y, Fuse N, Yamada T, Tamai M. Auto iris pigment epithelial cell transplantation in patients with age-related macular degeneration: short-term results. Tohoku J Exp Med. 2000 May;191(1):7–20. doi: 10.1620/tjem.191.7. [DOI] [PubMed] [Google Scholar]
- 63.Coffey PJ, Girman S, Wang SM, Hetherington L, Keegan DJ, Adamson P, Greenwood J, Lund RD. Long-term preservation of cortically dependent visual function in RCS rats by transplantation. Nat Neurosci. 2002 Jan;5(1):53–6. doi: 10.1038/nn782. [DOI] [PubMed] [Google Scholar]
- 64.Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000 Nov 15;227(2):271–8. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 65.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998 Nov 6;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 66.Zwaka TP, Thomson JA. Differentiation of human embryonic stem cells occurs through symmetric cell division. Stem Cells. 2005 Feb;23(2):146–9. doi: 10.1634/stemcells.2004-0248. [DOI] [PubMed] [Google Scholar]
- 67.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981 Jul 9;292(5819):154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 68.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klimanskaya I, Chung Y, Meisner L, Johnson J, West MD, Lanza R. Human embryonic stem cells derived without feeder cells. Lancet. 2005 May 7-13;365(9471):1636–41. doi: 10.1016/S0140-6736(05)66473-2. [DOI] [PubMed] [Google Scholar]
- 70.Skottman H, Dilber MS, Hovatta O. The derivation of clinical-grade human embryonic stem cell lines. FEBS Lett. 2006 May 22;580(12):2875–8. doi: 10.1016/j.febslet.2006.03.083. [DOI] [PubMed] [Google Scholar]
- 71.Enver T, Soneji S, Joshi C, Brown J, Iborra F, Orntoft T, Thykjaer T, Maltby E, Smith K, Dawud RA, Jones M, Matin M, Gokhale P, Draper J, Andrews PW. Cellular differentiation hierarchies in normal and culture-adapted human embryonic stem cells. Hum Mol Genet. 2005 Nov 1;14(21):3129–40. doi: 10.1093/hmg/ddi345. [DOI] [PubMed] [Google Scholar]
- 72.Schatten G, Smith J, Navara C, Park JH, Pedersen R. Culture of human embryonic stem cells. Nat Methods. 2005 Jun;2(6):455–63. doi: 10.1038/nmeth0605-455. [DOI] [PubMed] [Google Scholar]
- 73.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 74.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007 Dec 21;318(5858):1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 75.Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, Melton DA. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008 Jul;26(7):795–7. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008 Nov 7;322(5903):945–9. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009 May 8;324(5928):797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kawasaki H, Suemori H, Mizuseki K, Watanabe K, Urano F, Ichinose H, Haruta M, Takahashi M, Yoshikawa K, Nishikawa S, Nakatsuji N, Sasai Y. Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc Natl Acad Sci U S A. 2002 Feb 5;99(3):1580–5. doi: 10.1073/pnas.032662199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haruta M, Sasai Y, Kawasaki H, Amemiya K, Ooto S, Kitada M, Suemori H, Nakatsuji N, Ide C, Honda Y, Takahashi M. In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Invest Ophthalmol Vis Sci. 2004 Mar;45(3):1020–5. doi: 10.1167/iovs.03-1034. [DOI] [PubMed] [Google Scholar]
- 80.Carr AJ, Vugler A, Lawrence J, Chen LL, Ahmado A, Chen FK, Semo M, Gias C, da Cruz L, Moore HD, Walsh J, Coffey PJ. Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Mol Vis. 2009;15:283–95. [PMC free article] [PubMed] [Google Scholar]
- 81.Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 2004;6(3):217–45. doi: 10.1089/clo.2004.6.217. [DOI] [PubMed] [Google Scholar]
- 82.Aoki H, Hara A, Nakagawa S, Motohashi T, Hirano M, Takahashi Y, Kunisada T. Embryonic stem cells that differentiate into RPE cell precursors in vitro develop into RPE cell monolayers in vivo. Exp Eye Res. 2006 Feb;82(2):265–74. doi: 10.1016/j.exer.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 83.Lund RD, Wang S, Klimanskaya I, Holmes T, Ramos-Kelsey R, Lu B, Girman S, Bischoff N, Sauvé Y, Lanza R. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 2006 Fall;8(3):189–99. doi: 10.1089/clo.2006.8.189. [DOI] [PubMed] [Google Scholar]
- 84.Vugler A, Carr AJ, Lawrence J, Chen LL, Burrell K, Wright A, Lundh P, Semo M, Ahmado A, Gias C, da Cruz L, Moore H, Andrews P, Walsh J, Coffey P. Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Exp Neurol. 2008 Dec;214(2):347–61. doi: 10.1016/j.expneurol.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 85.Prowse AB, McQuade LR, Bryant KJ, Marcal H, Gray PP. Identification of potential pluripotency determinants for human embryonic stem cells following proteomic analysis of human and mouse fibroblast conditioned media. J Proteome Res. 2007 Sep;6(9):3796–807. doi: 10.1021/pr0702262. [DOI] [PubMed] [Google Scholar]
- 86.Idelson M, Alper R, Obolensky A, Ben-Shushan E, Hemo I, Yachimovich-Cohen N, Khaner H, Smith Y, Wiser O, Gropp M, Cohen MA, Even-Ram S, Berman-Zaken Y, Matzrafi L, Rechavi G, Banin E, Reubinoff B. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009 Oct 2;5(4):396–408. doi: 10.1016/j.stem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 87.Buchholz DE, Hikita ST, Rowland TJ, Friedrich AM, Hinman CR, Johnson LV, Clegg DO. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009 Oct;27(10):2427–34. doi: 10.1002/stem.189. [DOI] [PubMed] [Google Scholar]
- 88.Hirami Y, Osakada F, Takahashi K, Okita K, Yamanaka S, Ikeda H, Yoshimura N, Takahashi M. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009 Jul 24;458(3):126–31. doi: 10.1016/j.neulet.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 89.Osakada F, Ikeda H, Sasai Y, Takahashi M. Stepwise differentiation of pluripotent stem cells into retinal cells. Nat Protoc. 2009;4(6):811–24. doi: 10.1038/nprot.2009.51. [DOI] [PubMed] [Google Scholar]
- 90.Carr AJ, Vugler AA, Hikita ST, Lawrence JM, Gias C, Chen LL, Buchholz DE, Ahmado A, Semo M, Smart MJ, Hasan S, da Cruz L, Johnson LV, Clegg DO, Coffey PJ. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 2009;4(12):e8152. doi: 10.1371/journal.pone.0008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lu B, Malcuit C, Wang S, Girman S, Francis P, Lemieux L, Lanza R, Lund R. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009 Sep;27(9):2126–35. doi: 10.1002/stem.149. [DOI] [PubMed] [Google Scholar]
- 92.Bourgin C, Murai KK, Richter M, Pasquale EB. The EphA4 receptor regulates dendritic spine remodeling by affecting beta1-integrin signaling pathways. J Cell Biol. 2007 Sep 24;178(7):1295–307. doi: 10.1083/jcb.200610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol. 2000 Feb;2(2):62–9. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- 94.Afshari FT, Fawcett JW. Improving RPE adhesion to Bruch’s membrane. Eye (Lond) 2009 Oct;23(10):1890–3. doi: 10.1038/eye.2008.411. [DOI] [PubMed] [Google Scholar]
- 95.Hu Y, Zhang T, Wu J, Li Y, Lu X, Qian F, Yin Z, Ma Z. Autologous transplantation of RPE with partial-thickness choroid after mechanical debridement of Bruch membrane in the rabbit. Invest Ophthalmol Vis Sci. 2008 Jul;49(7):3185–92. doi: 10.1167/iovs.07-1299. [DOI] [PubMed] [Google Scholar]
- 96.Lee CJ, Fishman HA, Bent SF. Spatial cues for the enhancement of retinal pigment epithelial cell function in potential transplants. Biomaterials. 2007 Apr;28(13):2192–201. doi: 10.1016/j.biomaterials.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 97.Gullapalli VK, Sugino IK, Van Patten Y, Shah S, Zarbin MA. Retinal pigment epithelium resurfacing of aged submacular human Bruch’s membrane. Trans Am Ophthalmol Soc. 2004;102:123–37. [PMC free article] [PubMed] [Google Scholar]
- 98.Phillips SJ, Sadda SR, Tso MO, Humayan MS, de Juan E, Jr., Binder S. Autologous transplantation of retinal pigment epithelium after mechanical debridement of Bruch’s membrane. Curr Eye Res. 2003 Feb;26(2):81–8. doi: 10.1076/ceyr.26.2.81.14508. [DOI] [PubMed] [Google Scholar]
- 99.Fang IM, Yang CH, Yang CM, Chen MS. Overexpression of integrin alpha6 and beta4 enhances adhesion and proliferation of human retinal pigment epithelial cells on layers of porcine Bruch’s membrane. Exp Eye Res. 2009 Jan;88(1):12–21. doi: 10.1016/j.exer.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 100.Thumann G, Viethen A, Gaebler A, Walter P, Kaempf S, Johnen S, Salz AK. The in vitro and in vivo behaviour of retinal pigment epithelial cells cultured on ultrathin collagen membranes. Biomaterials. 2009 Jan;30(3):287–94. doi: 10.1016/j.biomaterials.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 101.Stanzel BV, Espana EM, Grueterich M, Kawakita T, Parel JM, Tseng SC, Binder S. Amniotic membrane maintains the phenotype of rabbit retinal pigment epithelial cells in culture. Exp Eye Res. 2005 Jan;80(1):103–12. doi: 10.1016/j.exer.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 102.Jiang LQ, Streilein JW. Immune responses elicited by transplantation and tissue-restricted antigens expressed on retinal tissues implanted subconjunctivally. Transplantation. 1991 Sep;52(3):513–9. doi: 10.1097/00007890-199109000-00025. [DOI] [PubMed] [Google Scholar]
- 103.Grisanti S, Ishioka M, Kosiewicz M, Jiang LQ. Immunity and immune privilege elicited by cultured retinal pigment epithelial cell transplants. Invest Ophthalmol Vis Sci. 1997 Jul;38(8):1619–26. [PubMed] [Google Scholar]
- 104.Zhang X, Bok D. Transplantation of retinal pigment epithelial cells and immune response in the subretinal space. Invest Ophthalmol Vis Sci. 1998 May;39(6):1021–7. [PubMed] [Google Scholar]
- 105.VanBuskirk AM, Pidwell DJ, Adams PW, Orosz CG. Transplantation immunology. JAMA. 1997 Dec 10;278(22):1993–9. [PubMed] [Google Scholar]
- 106.Drukker M. Immunogenicity of human embryonic stem cells: can we achieve tolerance? Springer Semin Immunopathol. 2004 Nov;26(1-2):201–13. doi: 10.1007/s00281-004-0163-5. [DOI] [PubMed] [Google Scholar]
- 107.Wenkel H, Streilein JW. Analysis of immune deviation elicited by antigens injected into the subretinal space. Invest Ophthalmol Vis Sci. 1998 Sep;39(10):1823–34. [PubMed] [Google Scholar]
- 108.Wekerle H, Sun D, Oropeza-Wekerle RL, Meyermann R. Immune reactivity in the nervous system: modulation of T-lymphocyte activation by glial cells. J Exp Biol. 1987 Sep;132:43–57. doi: 10.1242/jeb.132.1.43. [DOI] [PubMed] [Google Scholar]