Abstract
Diverse clinical factors, including intestinal ischemia, contribute to acute lung injury (ALI), which has up to a 40% mortality rate. During the development of lung injury an immune response is elicited that exacerbates the lung insult. Neutrophils have been well studied in mediating the pulmonary insults through an assortment of mechanisms, such as release of granule contents and production of proinflammatory cytokines due to the overactivation of complement and cytokines. In this study, we found that enhanced endoplasmic reticulum (ER) stress was observed in infiltrated neutrophils in the early stage of an ALI mice model. In neutrophils, complement 5a (C5a) inspires strong ER stress through inositol-requiring kinase 1a and, to a less extent, the protein kinase R–like ER kinase signaling pathway. The granule release induced by C5a was ER stress mediated. Knowkdown of X-box–binding protein 1, a downstream signaling molecule of inositol-requiring kinase 1a, impaired granule release, based on myeloperoxidase production. Further analysis revealed that C5a induced ER stress by binding to C5a receptor in neutrophils. Using xbpf/f MRP8-cre mice in which X-box–binding protein 1 is deficient specifically in neutrophils and ER stress is deprived, we confirmed that ER stress in neutrophils was required for granule release in vivo and led to ALI, whereas dampening ER stress in neutrophils substantially alleviated ALI. Taken together, our results demonstrated that C5a receptor–mediated ER stress induced granule release in neutrophils, contributing to the development of ALI. This novel mechanism suggests a new potential therapeutic target in autophagy regulation for ALI.
Introduction
During diverse clinical procedures, transient ischemia/reperfusion (IR) inflicts on organs or tissues and causes intense inflammation, both locally and systemically (1, 2), which in turn leads to various injuries or even multiple organ failure that contributes to high mortality. Acute lung injury (ALI) is a common outcome of IR, which usually happens in intestinal ischemia and leads to a higher mortality of 60–80% (3). Additionally, ALI is a life-threatening complication associated with sepsis, pneumonia, trauma, and many other clinical conditions. Although the management of critically ill patients is improving, the mortality rate remains at ∼40%, and survivors often do not return to a normal life (4). During the IR process, ischemia initiates a local inflammatory response by releasing proinflammatory factors and activating or attracting inflammatory cells, such as neutrophils, macrophages, and lymphocytes (5). For example, intestinal IR induced ALI-like injury in mice is characterized by decreased blood oxygenation, altered breathing patterns, neutrophilic alveolitis, and formation of microthrombi (6). Oxidative stress from ischemia also contributes to the IR injury. Owing to the unique anatomic and physiological features, the lung is susceptible to infliction by IR through proinflammatory cytokine storms (7). Presently, only a few options for pharmacologic treatments are available for IR-induced ALI by the inhibition of inflammation or anti-oxidative effects (8). More efforts are urgently needed to clarify the underlying pathophysiological mechanisms of ALI and to find more efficient therapeutic methods.
Studies have shown that neutrophils have been extensively accumulated in the lung during the initial stage of ALI, which substantially contributes to the severity of this disorder in human and animal models in several mechanisms: 1) release of granule components, such as serine proteases, matrix metalloproteinases, and lactoferrin; 2) generation of reactive oxygen species; and 3) formation of neutrophil extracellular traps, which are networks of extracellular fibers primarily composed of DNA from neutrophils, which function in infection and as danger signals, thus making neutrophils a potential target for ALI attenuation (9–12). Different therapeutic approaches have been developed against neutrophil infiltration, content release, or apoptosis induction.
Endoplasmic reticulum (ER) is an active site for protein biosynthesis and assemblage. During these processes, the presence of misfolded and unfolded proteins in the ER, especially for secretory complex and transmembrane proteins, instigates an evolutionary response called the unfolded protein response (UPR), which leads to accumulation of unfolded and misfolded proteins in the ER and disrupts energy/nutrient homeostasis, that is, ER stress (13, 14). Loss of protein folding homeostasis is critical to various diseases. More evidence has suggested that ER stress is a major contributor in the development or pathology of a diseased state besides other cellular stress (15, 16). The proximal “sensors” of ER stress are the chaperone grp78 (also known as BiP) along with protein kinase R–like ER kinase (PERK), activating transcription factor (ATF)6, and inositol-requiring kinase 1 (IRE1), as well as their downstream transcriptional effectors ATF4, ATF6, and X-box–binding protein 1 (XBP1)s, respectively (17, 18). As part of normal cellular housekeeping, a complex molecular network has evolved to promote the proper folding of proteins and to identify and degrade misfolded proteins. However, mutations that predispose to misfolding in both substrate and chaperones altered cellular metabolism, local factors, and environmental factors. These factors, including infection, can all promote the protein misfolding (18, 19), leading to ER stress and inflammatory signaling via the stressed cells.
ER stress potentially affects the survival and apoptosis of neutrophils in ALI. ER stress is essential for leukocyte generation, and deficiency of C/EBP homologous protein, a target of the PERK signaling pathway, leads to increased total myeloid subpopulations such as neutrophils and F4/80+ macrophages (20). There is no basal IRE1a activity for resting neutrophils (21). However, the neutrophils were displayed as hyperactive in response to inflammatory stress when ER stress was enhanced (22). The UPR modifier, p97/VCP, a valosin-containing protein, contributes to ER stress, inflammation, and NF-κB activation. The modifier also augments pathogenesis of sepsis and ALI, potentially through neutrophil infiltration (23). However, the role of ER stress in neutrophils remains to be elucidated in the pathogenesis of ALI.
This study aimed to clarify the role of ER stress for neutrophil biology during ALI development. The results indicated that ER stress evoked in ALI contributed to infiltration of neutrophils, thus exacerbating the ALI symptoms. This novel mechanism provides a potential role of the regulation of ER stress, which may help for future therapeutic applications in ALI.
Materials and Methods
Reagents and Abs
All Abs for flow cytometry analysis were obtained from BioLegend (San Diego, CA) unless specified otherwise. The conjugated Ab FITC-Ly6b (7/4) was purchased from Abcam (Cambridge, MA). PE-Ly6G (1A8) and allophycocyanin-Gr1 (RB6-8C5) were purchased from BioLegend. Glucose-regulated protein of 78 kDa (GRP78), IRE1a, PERK, and ATF6 Abs were obtained from Cell Signaling Technology (Boston, MA). The anti-mouse complement 5a (C5a) receptor (C5aR) Ab was obtained from OriGene (Rockville, MD). ELISA kits for C5a, TNF-α, IL-6, and myeloperoxidase (MPO) were purchased from R&D Systems (Minneapolis, MN).
Experimental model
Eight-week-old C57BL/6 male mice were used for intestinal IR, as described previously (6). Briefly, animals were weighed and anesthetized i.p. with 0.1 mg ketamine and 0.01 mg xylazine per gram of mouse weight. IR gut injury resulted in severe inflammation, apoptosis, and remote organ damage, including ALI. A medial laparotomy was performed, and intestines were carefully moved, allowing access to the superior mesenteric artery. The superior mesenteric artery was clamped using a vascular clamp (Fine Surgical Instruments). The vascular clamp was released after 90 min to allow reperfusion. For the sham control group, operations were performed as in the ALI group except for the application of vascular clamp.
XBP1flox/flox mice were established and bred by Usun Biotech (Shanghai, China), and mice that expressed Cre recombinase, under the control of the neutrophil-specific MRP8 promoter (MRP8-Cre), were obtained from The Jackson Laboratory (Bar Harbor, ME). XBP1flox/flox mice were bred with MRP8-Cre mice to achieve XBP1 deficiency in neutrophils (xbpf/f MRP8-cre) mice. XBP1flox/flox mice were used as controls in parallel with xbpf/f MRP8-cre mice. All experiments were conducted in accordance with the established guidelines and under approval of Animal Care and Use Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine.
Histopathology
Histopathology was perfermed as described previously (6). Briefly, lung samples were fixed and sectioned and stained with H&E. Morphologic examinations were performed using light microscopy and then scored.
Cell culture and transfection
The murine myeloid cell line 32Dcl3 (CRL-11346) was obtained from the American Type Culture Collection (Manassas, VA). Neutrophils from peripheral blood and 32Dcl3 cells were cultured in RPMI 1640 medium supplemented with 10% FBS. 32Dcl3 cells were transfected with Lipofectamine (Invitrogen), according to the manufacturer’s instructions.
Determination of C5a in murine bronchoalveolar lavage fluid by ELISA
Determination of C5a in murine bronchoalveolar lavage fluid (BALF) was by ELISA assay as described previously (6).
Immunoblotting
Immunoblotting was performed as described previously (6). Briefly, cell lysates were prepared and separated by SDS-PAGE before transferring to polyvinylidene difluoride membranes. Bands were detected with ECL plus detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ).
Fluorescence confocal microscopy
Fluorescence staining was performed as described previously (6). Briefly, cells grown on coverslips were fixed in 4% paraformaldehyde and incubated with indicated primary and conjugated secondary Abs. Cells were visualized on an Olympus FluoView confocal microscope with proper emission filters (Olympus, Tokyo, Japan).
Pulmonary leukocyte isolation
Pulmonary leukocyte isolation was performed as described previously (6). Briefly, BALF was collected and cells were dispersed by repetitive suction through a 10 ml syringe and centrifuged at 1100 rpm for 10 min. Pellets were resuspended in 5 ml complete medium or PBS.
Flow cytometric analysis
Flow cytometric analysis was performed as described previously (6). Briefly, cells (50,000 cells) in 100 μl flow assay buffer were incubated with FITC-conjugated goat anti-rabbit IgG or stained with other indicated Abs (BioLegend). Cells were washed, resuspended in 3% paraformaldehyde, and then analyzed using a FACSCalibur flow cytometer (BD Biosciences, Mountain View, CA).
Statistical analysis
A two-tailed Student t test or one-way ANOVA was used for statistical analyses. A p value <0.05 was denoted as statistically significant.
Results
Accumulated neutrophils in BALF from intestinal IR-induced ALI in mice
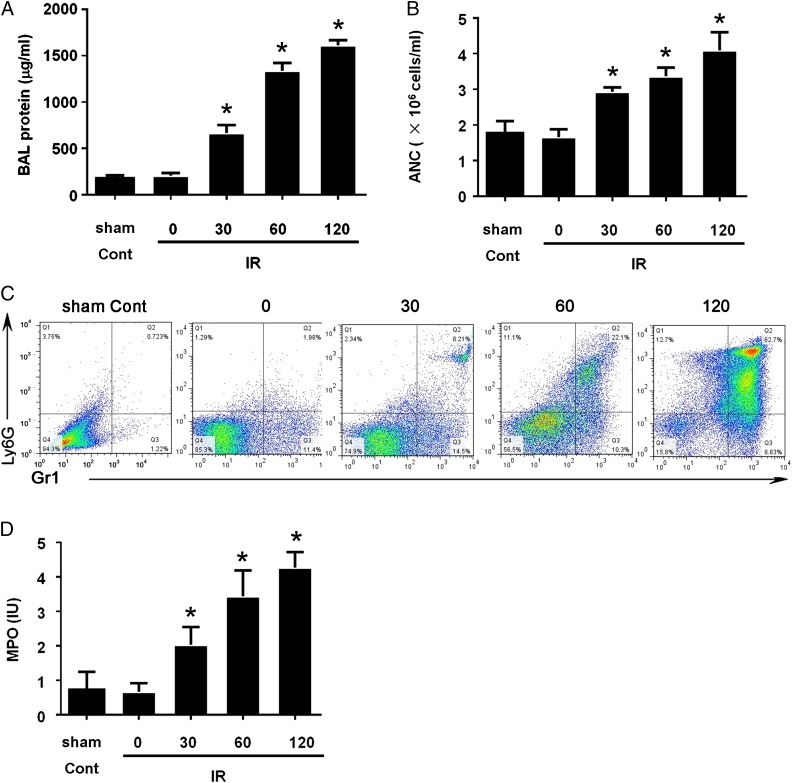
During the development of ALI, endothelial cells rapidly separate from the underlying basal membrane, as soon as 5 min after ischemia, suggesting the dysfunction of capillary walls. Owing to the dysfunction of capillary walls, the presence of fluid and protein leakage into the lung parenchyma was observed (Fig. 1A), as well as more blood granulocytes present in BALF (Fig. 1B). Both events were observed as early as 5 min after ischemia, and they correlated with increased lung fluid and the presence of shock and hemoconcentration, which are typical characteristics in ALI. To characterize the neutrophil more accurately, we performed flow cytometry analysis of the cells present in BALF. Result indicated considerably more Gr1+Ly6G+ cells (neutrophils) in BALF (Fig. 1C). We also validated our labeling for neutrophils, and the results provided similar profiles (Supplemental Fig. 1). The activated neutrophils release an assortment of proteins, such as MPO, lactoferrin, serine protease, collagenase, and gelatinase, which contribute to ALI (24). Based on the literature, we sought to detect the presence of MPO in BALF, and our data showed much higher levels of MPO in BALF than those in sham control mice (Fig. 1D). In our ALI model, we observed high protein infiltration and presence of neutrophils in BALF, which are characteristic features of ALI.
FIGURE 1.
Accumulated neutrophils in BALF from intestinal IR-induced ALI in a mouse model. Eight-week-old C57BL/6 male mice (six mice per group) were subjected to intestinal IR at the indicated times. BALF was collected, protein contents were measured (A), and absolute neutrophil count (ANC) was measured (B). The cells collected from BALF were stained with Gr1 and Ly6G+ Ab and were then subjected to flow cytometry analysis (C). Data are representative of three experiments. MPO levels in BALF were detected with an ELISA assay (D). Quantification is expressed as mean ± SD of three independent experiments. *p < 0.05.
ER stress was enhanced in neutrophils in an ALI mice model
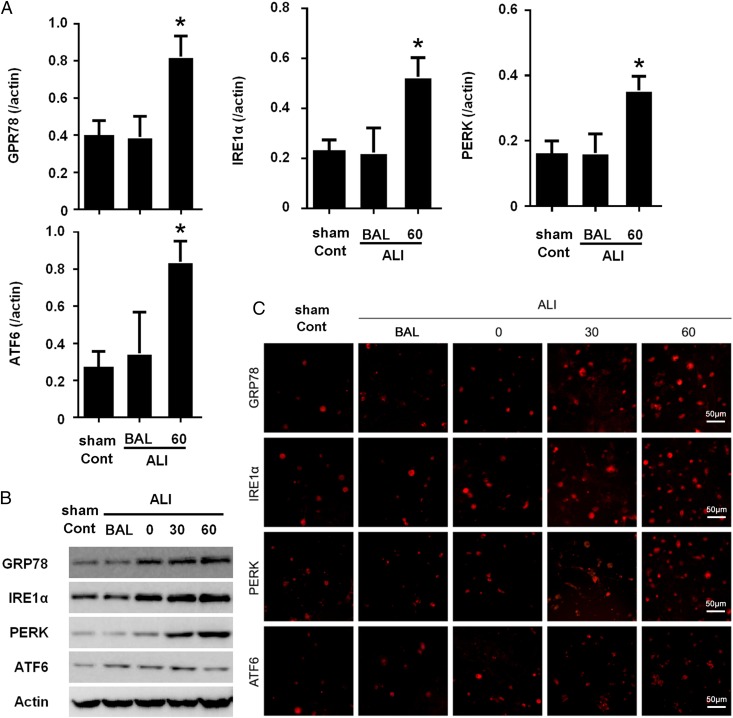
Neutrophils represent the first defense system, and acute neutrophil infiltration is obvious in ALI. To characterize the ER stress in the infiltrated neutrophils, we first collected the neutrophils from BALF of ALI mice. The key factor in the initiation of the UPR is a heat shock protein, GRP78, which is the most abundant ER chaperon (25). In mammals, there are three major ER membrane–associated proteins acting as ER stress sensors, IRE1a, PERK, and ATF6. The total RNA was prepared with neutrophils from peripheral blood or BALF. The expression of ER stress–related molecules was determined with quantitative RT-PCR. Our data showed that expression of GRP78, IRE1a, PERK, and ATF6 was significantly increased in a time-dependent manner (Fig. 2A). Consistently, increased expression of these proteins was observed in neutrophils by Western blotting and immunofluorescence staining (Fig. 2B, 2C). Collectively, our results clearly indicated enhanced ER stress in neutrophils from ALI mice.
FIGURE 2.
ER stress was significantly enhanced in neutrophils in ALI. (A) Eight-week-old C57BL/6 male mice (eight mice per group) were subjected to intestinal IR. BALF and peripheral blood were collected 60 min after the procedures. Neutrophils were prepared from BALF and peripheral blood, and total RNA was extracted from neutrophils, followed by reverse transcription and quantitative PCR. Data were from three independent experiments and are presented as mean ± SD. (B) Neutrophils from normal mice and IR-induced ALI mice were isolated and lysed. Lysates were analyzed by immunoblotting with indicated Abs. Images shown are representatives of three experiments. (C) Neutrophils were prepared from normal or ALI mice BALF and were subjected to immunofluorescence staining with indicated Abs. Images shown are representatives of three experiments. Quantification is expressed as mean ± SD of three independent experiments. *p < 0.05.
C5a induces ER stress in neutrophils
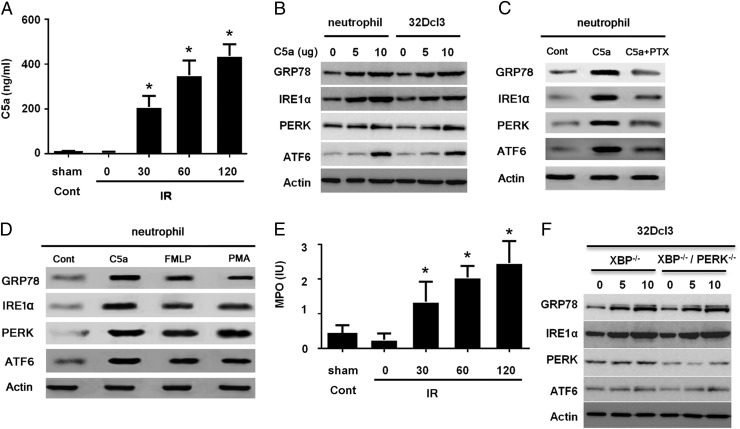
During IR-induced ALI, multiple inflammatory factors are released in local lesions. Multiple cytokines and chemokines, such as TNF-α, IL-6, and MCP-1, were significantly increased in BALF from ALI mice compared with the levels in normal organs. Additionally, C5a was also considerably enhanced in BALF from ALI (Fig. 3A). C5a played a key role in chemotaxis and the activation of neutrophils, consistent with other studies. C5a-induced activation was pronounced in ALI, which could be a hallmark of the disease process. Therefore, we assumed that C5a contributed to ER stress in neutrophils. We tested our hypothesis in neutrophils isolated from peripheral blood and also in murine myeloid cell line, 32Dcl3 (26). First, the neutrophils were stimulated with C5a and GRP78. The ER stress sensors IRE1a, PERK, and AATF6 were analyzed by Western blotting. Our studies demonstrated that C5a prompted the ER stress in neutrophils based on the expression of GRP78, IRE1a, PERK, and ARF6 (Fig. 3B). We also prepared neutrophils from mouse peripheral blood cells and pretreated with pertussis toxin (200 ng/ml) overnight before treating the cells with C5a, and we found that pertussis toxin obviated the effects on ER stress (Fig. 3C), implying the engagement of the C5a receptor (C5aR). Additionally, we treated the cells with fMLF or PMA for 60 min before preparing the cell lysate for detecting ER stress protein synthesis, and our data showed that treatment with fMLF or PMA increased synthesis of ER stess proteins, but to a less extent compared with C5a treatment (Fig. 3D). Thus, our results did not exclude the effects from other pathways, but it seems as if the C5aR-mediated pathway is important for ER stress. We also checked the granule release of MPO with C5a stimulation. The results showed a significant increase in MPO (Fig. 3E), which is detrimental for pulmonary parenchyma. To elucidate the role of ER stress in granule release, we eliminated the ER stress signaling by knocking down XBP1, a downstream signaling molecule of IRE1a, or silencing PERK with small interfering RNA (siRNA) in 32Dcl3 cells (Fig. 3F), followed by stimulation of C5a. We found that the knockdown of XBP1 can partially impair the effects of C5a on granule release by 40% (Supplemental Fig. 2). The effect is synergic with the double knockdown of both XBP1 and PERK (Fig. 3F), suggesting the collaboration between these two signaling pathways and the critical role of ER stress in C5a-induced granule release. Taken together, our data suggested that C5a contributed to neutrophil activation, based on granule release of MPO, mainly through inducing ER stress.
FIGURE 3.
C5a induces ER stress of neutrophil. (A) Eight-week-old C57BL/6 male mice (six mice per group) were subjected to intestinal IR. BALF was collected 60 min after the procedures. C5a was measured using ELISA. Data are from three independent experiments. (B) Neutrophils prepared from mice peripheral blood or murine myeloid cell line 32Dcl3 were treated with C5a for 4 h and then lysed. Lysates were analyzed by immunoblotting with indicated Abs. Images shown are representatives of three experiments. (C) Neutrophils prepared from mice peripheral blood were treated with C5a alone or combined with pertussis toxin (PTX) for 4 h and then lysed. Lysates were analyzed by immunoblotting with indicated Abs. Images shown are representatives of three experiments. (D) Neutrophils prepared from mouse peripheral blood were treated with fMLF (200 nM) or PMA (100 nM) for 60 min and then lysed. Lysates were analyzed by immunoblotting with indicated Abs. Images shown are representatives of three experiments. (E) Supernatants from cultured neutrophils described as in (B) were collected, and MPO was measured using ELISA. (F) Murine myeloid cells, 32Dcl3, were transiently transfected with siXBP1 overnight, followed by 4 h of C5a treatment or 4 h of starvation and then lysed. Lysates were analyzed by immunoblotting with indicated Abs. Images shown are representatives of three experiments. Quantification is expressed as mean ± SD of three independent experiments. *p < 0.05.
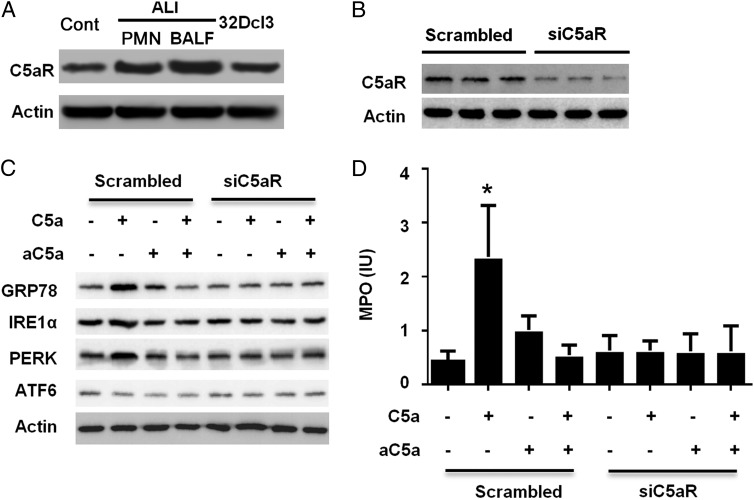
C5a interacting with C5aR induced ER stress
C5aR is expressed in multiple cell types, such as neutrophils and alveolar macrophages. The binding of C5aR with its ligand, C5a, initiates the downstream signaling cascades. We confirmed the expression of C5aR in neutrophils from peripheral blood, BALF, and also in the 32Dcl3 cell line (Fig. 4A). The neutrophils from ALI BALF expressed arguably higher C5a (Supplemental Fig. 3), whereas there was no significant difference from the neutrophils from peripheral blood. To determine whether the induction of ER stress in neutrophils is through C5aR signaling, we knocked down C5aR in 32Dcl3 cells with siRNA (Fig. 4B) and then stimulated the cells with C5a. As shown in Fig. 4C, the knockdown of C5aR abolished the effect of C5a on ER stress in the cells, and it also nullified the granule release of MPO (Fig. 4D). These data confirmed the essential role of C5aR in C5a-induced ER stress and granule release in neutrophils. Our results indicated that C5a-induced ER stress was through the binding of C5aR.
FIGURE 4.
C5a interacting with C5aR induces ER stress in neutrophils. (A) Neutrophils prepared from mouse peripheral blood or murine myeloid cell line 32Dcl3 were lysed for immunoblotting with C5aR Ab. Images shown are representative of three experiments. Band intensities were determined with ImageJ. (B) Murine myeloid cells (32Dcl3) were transiently transfected with siC5aR overnight and then lysed. Lysates were analyzed by immunoblotting with C5aR Ab. Images shown are representatives of three experiments. (C) Murine myeloid cells (32Dcl3) were transiently transfected with siC5aR overnight, followed by treatment with C5a and/or C5a neutralizing Ab for 4 h and then lysed. Lysates were analyzed by immunoblotting with indicated Abs. Images shown are representatives of three experiments. (D) Supernatants from cultured neutrophils described as in (C) were collected, and MPO was measured using ELISA. Data are expressed as mean ± SD of three independent experiments. *p < 0.05.
Inhibition of ER stress in vivo decreases ALI
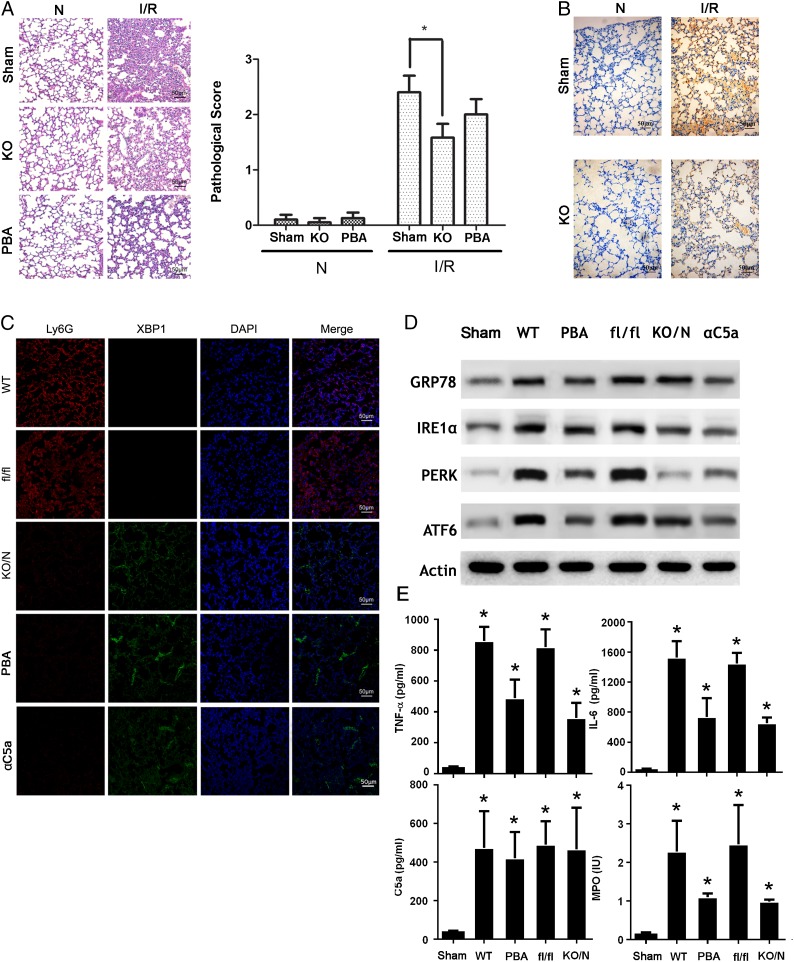
As previously mentioned, C5a-mediated ER stress leads to activation of neutrophils and granule release in neutrophils, which potentially contributes to the development of ALI. To clarify this phenomenon in vivo, we generated mice with XBP1 deficiency in neutrophils (xbpf/f MRP8-cre) by crossing XBP1fl/fl mice (27) with MRP8-cre mice (28). The newly generated mice lacked the UPR response specifically in neutrophils. XBP1 is a downstream signaling molecule of the UPR that is essential for restoring the protein folding homeostasis through the reduction in protein translation and the increase in folding capacity and ER-associated degration (18). We established the ALI model with xbpf/f MRP8-cre mice crossed with xbpf/f mice. The lung injury is significantly attenuated in xbpf/f MRP8-cre mice, based on the inflammatory cell infiltration detected in H&E staining (Fig. 5A). Pathological scores given by an experienced pathologist also confirmed the successful construction of the ALI model, but there was no significant difference on neutrophil accumulation for negative control groups (Fig. 5A). To further confirm the phenomenon, we stained the slide with Ly6G (1A8) Ab, and we observed no significant difference (Fig. 5B). We used xbpf/f MRP8-cre mice to further investigate the role of ER stress of neutrophil in ALI. We used an alternative method, immunofluorescence staining, to quantitatively test the expression of XBP1 in lung (Fig. 5C). The expression of XBP1 was completely deficient in Ly6G+ cells (neutrophils). Additionally, Western blotting analysis consolidated the absence of XBP1 in neutrophils from the lung in mice (Fig. 5D). C5a blocking with neutralizing Ab (aC5a) in xbpf/f mice has similar effects. The extensive measurement of diverse inflammatory factors, including TNF-α, IL-6, C5a, and MPO, also indicated differential changes. Inflammatory cytokines, such as TNF-α and IL-6, showed an increased level, but no significant change in C5a was observed (Fig. 5E). As expected, we observed a marked decrease of MPO (Fig. 5E), confirming the essential role of ER stress in granule release in vivo. As expected, inhibition of ER stress with ER inhibitor PBA has similar effects to XBP1 knockdown (Fig. 5C–E). Collectively, our studies supported the indispensable role of ER stress in neutrophil activation and granule release, thus contributing to ALI.
FIGURE 5.
Inhibition of ER stress in neutrophils decreases ALI in vivo. (A) xbpf/f MRP8-cre mice (KO) and XBP1f/f mice were used to establish IR-induced ALI model. Lungs samples were fixed and embedded in paraffin. Tissue blocks were sectioned at 5 μm and stained with H&E. Morphology was examined using light microscopy. Pathological scores were given by an experienced pathologist. (B) Sections were stained with Ly6G (1A8) Ab. (C) Neutrophils were prepared from the BALF of normal or ALI xbpf/f MRP8-cre mice and XBP1fl/fl mice, and they were subjected to immunofluorescence staining with indicated Abs. Images shown are representatives of three experiments. (D) Neutrophils were prepared from the BALF of normal or ALI xbpf/f MRP8-cre mice and XBP1fl/fl mice and then lysed. Lysates were analyzed by immunoblotting with indicated Abs. Images shown are representatives of three experiments. (E) BALF was collected from xbpf/f MRP8-cre mice (KO/N) and XBP1fl/fl mice (fl/fl). TNF-α, IL-6, C5a, and MPO were measured using ELISA. Quantification is expressed as mean ± SD of three independent experiments. Scale bars, 50 μm. *p < 0.05.
Discussion
The role for neutrophils in ALI has been well established, including releasing of granule components and generation of reactive oxygen species due to the excessive activation and formation of neutrophil extracellular traps. The evidence showed biological alteration of neutrophils involved in the initiation, development, and resolution of ALI. As a part of normal cellular physiology, the UPR potentially affects neutrophil behaviors. In this study, we found that ER stress contributed to the overactivation of neutrophils, thus leading to more severe pulmonary insults in ALI. During the disease progress, complement is activated to produce complement components, such as C3a and C5a (29–31). Complement cascades are necessary for the initiation and amplification of tissue injury (8, 32, 33). Our study showed that C5aR expression was upregulated in neutrophils in ALI induction via intestinal IR. Binding of C5a induced ER stress and subsequent granule release in neutrophils, contributing to the pulmonary insult in ALI.
The complement system is a key step in innate immunity, however, an overwhelming activation of complement can lead to severe tissue injury (1). Complement has been shown to be involved in diverse processes, such as angiogenesis, inflammation, tissue injury, and tissue regeneration (33). Three distinct pathways are known to initiate the complement cascades, and all of them lead to the activation of C3. C3 is essential for the production of C5a in animal models with reperfusion injury. C5a is required for the infiltration of neutrophils and for the full development of IR-induced injury in animal models with lung injury. C5a directly activates neutrophils and macrophages to produce proinflammatory cytokines and chemokines (29, 34, 35). However, the underlying mechanisms of C5a on neutrophil granule release remains to be clarified. In this study, we found that C5a bound C5aR in neutrophils and induced ER stress, which is required for granule release, one hallmark event in ALI development.
ER stress has been shown to intertwine with inflammation and involve diverse biological events in leukocytes, such as monocytes, macrophages, and lymphocytes (13, 25). Physiologically, XBP1 is necessary for differentiation of B cells, NK cells, and macrophages, although there is no detected basal IRE-1 activity for naive T cells and monocytes from spleen (17). There are only sporadic data on neutrophils (21), and in our study we found that in neutrophils presented in injured lung, the ER stress was strongly induced, based on GRP78, IRE-1a, and ATF6. Then we further identified C5a as one of ER stress inducers, as presence of C5a neutralizing Ab obviated the ER stress in neutrophils. Usually, C5a binds to C5aR, which expresses in multiple cell types, including granulocytes and monocytes, and it initiates downstream signaling. The C5aR is required for C5a-induced ER stress in neutrophils in ALI, and C5aR knockdown deprives the induction of ER stress. Besides C5aR, C5a also interacts with C5aR–like 2 (36), as we detected no expression in neutrophils (data not shown), and thus it is probable that C5aR–like 2 has no essential role in C5a-induced ER stress. Additionally, C5aR should be more important in this biological event, as C5aR knockdown with specific siRNA almost deprived the C5a activities completely. Indeed, we also observed that fMLF or PMA treatment of neutrophils contributed to ER stress, but to a less extent compared with C5a treatment (Fig. 3D). Thus, it is likely that there is more than one pathway to induce ER stress of neutrophils. However, the C5aR-mediated pathway seems to be an indispensable one.
The ALI animal model with mice with XBP1 deficiency in neutrophils (xbpf/f MRP8-cre) showed alleviated symptoms of ALI, such as cytokine production, MPO levels in BALF, and tissue injury, and even the C5a level is still high, emphasizing the essential contribution of ER stress in ALI. Inhibition of ER stress in neutrophils has a considerable effect on production of multiple cytokines, providing more evidence on the complicated interaction between ER stress and inflammation, which needs more studies to elucidate the regulation pathways between them. Additionally, how C5aR signaling crosstalks with ER stress signaling cascades is also an interesting subject for further exploration.
In summary, our results shed light on the role of C5a-mediated ER stress in neutrophil granule release, thus contributing to ALI development, and they identify potential therapeutic targets for ALI.
Supplementary Material
This work is supported by National Natural Science Foundation of China Grants 81272083 and 81301655.
The online version of this article contains supplemental material.
- ALI
- acute lung injury
- ATF
- activating transcription factor
- BALF
- bronchoalveolar lavage fluid
- C5a
- complement 5a
- C5aR
- C5a receptor
- ER
- endoplasmic reticulum
- GRP78
- glucose-regulated protein of 78 kDa
- IR
- ischemia/reperfusion
- IRE1
- inositol-requiring kinase 1
- MPO
- myeloperoxidase
- PERK
- protein kinase R–like ER kinase
- siRNA
- small interfering RNA
- UPR
- unfolded protein response
- XBP1
- X-box–binding protein 1.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Fleming S. D., Tsokos G. C. 2006. Complement, natural antibodies, autoantibodies and tissue injury. Autoimmun. Rev. 5: 89–92. [DOI] [PubMed] [Google Scholar]
- 2.Vercellotti G. M., Moldow C. F., Jacob H. S. 2012. Complement, oxidants, and endothelial injury: how a bedside observation opened a door to vascular biology. J. Clin. Invest. 122: 3044–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui T., Miksa M., Wu R., Komura H., Zhou M., Dong W., Wang Z., Higuchi S., Chaung W., Blau S. A., et al. 2010. Milk fat globule epidermal growth factor 8 attenuates acute lung injury in mice after intestinal ischemia and reperfusion. Am. J. Respir. Crit. Care Med. 181: 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraham E., Carmody A., Shenkar R., Arcaroli J. 2000. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 279: L1137–L1145. [DOI] [PubMed] [Google Scholar]
- 5.Eltzschig H. K., Eckle T. 2011. Ischemia and reperfusion—from mechanism to translation. Nat. Med. 17: 1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu R., Chen Z. F., Yan J., Li Q. F., Huang Y., Xu H., Zhang X., Jiang H. 2014. Complement C5a exacerbates acute lung injury induced through autophagy-mediated alveolar macrophage apoptosis. Cell Death Dis. 5: e1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Perrot M., Liu M., Waddell T. K., Keshavjee S. 2003. Ischemia-reperfusion-induced lung injury. Am. J. Respir. Crit. Care Med. 167: 490–511. [DOI] [PubMed] [Google Scholar]
- 8.Gorsuch W. B., Chrysanthou E., Schwaeble W. J., Stahl G. L. 2012. The complement system in ischemia-reperfusion injuries. Immunobiology 217: 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matute-Bello G., Frevert C. W., Martin T. R. 2008. Animal models of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 295: L379–L399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthay M. A., Howard J. P. 2012. Progress in modelling acute lung injury in a pre-clinical mouse model. Eur. Respir. J. 39: 1062–1063. [DOI] [PubMed] [Google Scholar]
- 11.Chopra M., Reuben J. S., Sharma A. C. 2009. Acute lung injury:apoptosis and signaling mechanisms. Exp. Biol. Med. (Maywood) 234: 361–371. [DOI] [PubMed] [Google Scholar]
- 12.Brinkmann V., Zychlinsky A. 2012. Neutrophil extracellular traps: is immunity the second function of chromatin? J. Cell Biol. 198: 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaudhari N., Talwar P., Parimisetty A., Lefebvre d’Hellencourt C., Ravanan P. 2014. A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell. Neurosci. 8: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hetz C. 2012. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13: 89–102. [DOI] [PubMed] [Google Scholar]
- 15.Guerriero C. J., Brodsky J. L. 2012. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiol. Rev. 92: 537–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S., Kaufman R. J. 2012. The impact of the unfolded protein response on human disease. J. Cell Biol. 197: 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssens S., Pulendran B., Lambrecht B. N. 2014. Emerging functions of the unfolded protein response in immunity. Nat. Immunol. 15: 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hetz C., Martinon F., Rodriguez D., Glimcher L. H. 2011. The unfolded protein response: integrating stress signals through the stress sensor IRE1α. Physiol. Rev. 91: 1219–1243. [DOI] [PubMed] [Google Scholar]
- 19.Groenendyk J., Agellon L. B., Michalak M. 2013. Coping with endoplasmic reticulum stress in the cardiovascular system. Annu. Rev. Physiol. 75: 49–67. [DOI] [PubMed] [Google Scholar]
- 20.Grant R., Nguyen K. Y., Ravussin A., Albarado D., Youm Y. H., Dixit V. D. 2014. Inactivation of C/ebp homologous protein-driven immune-metabolic interactions exacerbate obesity and adipose tissue leukocytosis. J. Biol. Chem. 289: 14045–14055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osorio F., Tavernier S. J., Hoffmann E., Saeys Y., Martens L., Vetters J., Delrue I., De Rycke R., Parthoens E., Pouliot P., et al. 2014. The unfolded-protein-response sensor IRE-1α regulates the function of CD8α+ dendritic cells. Nat. Immunol. 15: 248–257. [DOI] [PubMed] [Google Scholar]
- 22.Esposito V., Grosjean F., Tan J., Huang L., Zhu L., Chen J., Xiong H., Striker G. E., Zheng F. 2013. CHOP deficiency results in elevated lipopolysaccharide-induced inflammation and kidney injury. Am. J. Physiol. Renal Physiol. 304: F440–F450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodas M., Min T., Vij N. 2010. Early-age-related changes in proteostasis augment immunopathogenesis of sepsis and acute lung injury. PLoS One 5: e15480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aimone J. B., Li Y., Lee S. W., Clemenson G. D., Deng W., Gage F. H. 2014. Regulation and function of adult neurogenesis: from genes to cognition. Physiol. Rev. 94: 991–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasnain S. Z., Lourie R., Das I., Chen A. C., McGuckin M. A. 2012. The interplay between endoplasmic reticulum stress and inflammation. Immunol. Cell Biol. 90: 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henley J. M., Craig T. J., Wilkinson K. A. 2014. Neuronal SUMOylation: mechanisms, physiology, and roles in neuronal dysfunction. Physiol. Rev. 94: 1249–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee A. H., Scapa E. F., Cohen D. E., Glimcher L. H. 2008. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320: 1492–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott E. R., Van Ziffle J. A., Scapini P., Sullivan B. M., Locksley R. M., Lowell C. A. 2011. Deletion of Syk in neutrophils prevents immune complex arthritis. J. Immunol. 187: 4319–4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosmann M., Ward P. A. 2012. Role of C3, C5 and anaphylatoxin receptors in acute lung injury and in sepsis. Adv. Exp. Med. Biol. 946: 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kambas K., Markiewski M. M., Pneumatikos I. A., Rafail S. S., Theodorou V., Konstantonis D., Kourtzelis I., Doumas M. N., Magotti P., Deangelis R. A., et al. 2008. C5a and TNF-α up-regulate the expression of tissue factor in intra-alveolar neutrophils of patients with the acute respiratory distress syndrome. J. Immunol. 180: 7368–7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shushakova N., Skokowa J., Schulman J., Baumann U., Zwirner J., Schmidt R. E., Gessner J. E. 2002. C5a anaphylatoxin is a major regulator of activating versus inhibitory FcγRs in immune complex-induced lung disease. J. Clin. Invest. 110: 1823–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrar C. A., Asgari E., Schwaeble W. J., Sacks S. H. 2012. Which pathways trigger the role of complement in ischaemia/reperfusion injury? Front. Immunol. 3: 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ricklin D., Hajishengallis G., Yang K., Lambris J. D. 2010. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11: 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flierl M. A., Rittirsch D., Sarma J. V., Huber-Lang M., Ward P. A. 2008. Adrenergic regulation of complement-induced acute lung injury. Adv. Exp. Med. Biol. 632: 93–103. [PubMed] [Google Scholar]
- 35.Sun S., Wang H., Zhao G., An Y., Guo Y., Du L., Song H., Qiao F., Yu H., Wu X., et al. 2011. Complement inhibition alleviates paraquat-induced acute lung injury. Am. J. Respir. Cell Mol. Biol. 45: 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li R., Coulthard L. G., Wu M. C., Taylor S. M., Woodruff T. M. 2013. C5L2: a controversial receptor of complement anaphylatoxin, C5a. FASEB J. 27: 855–864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.