Abstract
Taste compounds detected by G protein-coupled receptors on the apical surface of Type 2 taste cells initiate an intracellular molecular cascade culminating in the release of ATP. It has been suggested that this ATP release is accomplished by pannexin 1 (PANX1). However, we report here that PANX1 knockout mice do not differ from wild-type controls in response to representative taste solutions, measured using 5-s brief-access tests or 48-h two-bottle choice tests. This implies that PANX1 is unnecessary for taste detection and consequently that ATP release from Type 2 taste cells does not require PANX1.
Key words: ATP release, CALHM1, gustometer, pannexin 1, two-bottle choice test
Introduction
Exposing the apical surface of Type 2 taste cells to various taste compounds initiates an intracellular molecular cascade that culminates in the release of ATP (e.g., Finger et al. 2005; Huang et al. 2007; Romanov et al. 2007; Chaudhari and Roper 2010). The ATP acts as a chemical transmitter to activate P2RX2 and P2RX3 receptors on nearby nerve fibers, which signal the brain (e.g., Finger et al. 2005). Many types of cell employ pannexin 1 (PANX1) channels to release ATP (reviews: MacVicar and Thompson 2010; Dahl and Muller 2014; Koval et al. 2014; Kurtenbach and Zoidl 2014), and PANX1 is expressed in taste cells (Huang et al. 2007; Romanov et al. 2007; Dando and Roper 2009), which raises the possibility that PANX1 is responsible for releasing ATP from taste cells. Indeed, a persuasive argument for the involvement of PANX1 in taste transduction has been made (e.g., Dando and Roper 2009; Chaudhari and Roper 2010; Huang and Roper 2010; Murata et al. 2010). However, there is evidence to the contrary. In particular, no difference was observed in the taste-stimulated release of ATP from taste cells of PANX1 KO and control mice (Romanov et al. 2012). Moreover, a novel voltage-gated ATP permeable channel, calcium homeostasis modulator 1 (CALHM1), satisfies the functions first ascribed to PANX1. CALHM1 is expressed in Type 2 taste cells (Moyer et al. 2009; Taruno et al. 2013b), transfecting naïve cells with Calhm1 renders them capable of releasing ATP (Taruno et al. 2013b), the Type 2 taste cells of CALHM1 KO mice do not release ATP in response to taste stimulation, and CALHM1 KO mice show few if any electrophysiological or behavioral responses to taste compounds that are transduced by G protein-coupled receptors (GPCRs; Taruno et al. 2013b).
Crucial evidence to support the contribution of CALHM1 and P2RX2 + P2RX3 receptors to taste perception derives from findings that knockout of these elements largely eliminates the behavioral responses to taste solutions (Eddy et al. 2009; Hallock et al. 2009; Taruno et al. 2013b). However, parallel studies with PANX1 KO mice have not been reported. Consequently, we compared the behavior of PANX1 KO and WT littermate control mice in response to several taste solutions presented in 5-s brief-access tests and in 48-h two-bottle choice tests.
Methods
Experiments followed the guidelines outlined in the National Research Council’s Guide for the Care and Use of Laboratory Animals, eighth edition. Protocols were approved by the Institutional Animal Care and Use Committee of the Monell Chemical Senses Center.
Mice
The PANX1 KO mice were generated as homozygous Panx1fl/fl (“floxed”) mutant mice, harboring 3 LoxP consensus sites inserted into a single-copy Panx1 gene (Dvoriantchikova et al. 2012). Founders with germline transmission of the embryonic stem cell-derived genome were heterozygous for the mutant Panx1 allele and were bred for homozygocity. The resulting mice were crossed with a CMV-Cre strain to create a global KO line. Cre-mediated recombination within the Panx1 gene resulted in a germline removal of the LoxP-flanked exons 3 and 4. This line was backcrossed to C57BL/6 mice for at least 7 generations and then bred to homozygocity. Descendants were transferred to C. H. Mitchell at the University of Pennsylvania who, in turn, provided breeding pairs to M. G. Tordoff at the Monell Chemical Senses Center. At the Monell Center, offspring of the breeding pairs were backcrossed to the C57BL/6J strain and their offspring were mated brother-to-sister to produce homozygous WT and KO mice for the experiments reported here. Males were used for the brief-access tests and females for the two-bottle choice tests. Heterozygous mice were not tested.
All mice were weaned when 21–23 days old, at which time a 1–2mm end-of-tail clip sample was collected for DNA analysis. Genomic DNA was extracted and each mouse’s status as a PANX1 WT or KO mouse was determined using the polymerase chain reaction with the following primers: 5′-CTTTGGCATTTTCCCAGTGT-3′ and 5′-CGCGGTTGTAGACTTTGTCA-3′ (585bp, WT allele) and 5′-CTTTGGCATTTTCCCAGTGT-3′ and 5′-GTCCCTACAGG AGGCACTGA-3′ (900bp, KO allele). In-house genotyping was independently confirmed by a commercial genotyping service (Transnetyx, Inc.).
The mice were maintained in a vivarium at 23 °C with a 12:12h light/dark cycle (lights off at 7 PM). They were housed in groups of up to 6 of the same sex until they were aged 5–8 weeks old, when they were transferred to individual cages. These were plastic tubs (26.5cm × 17cm × 12cm) with stainless steel wire lids, and wood shavings scattered on the floor. The mice were transferred to clean cages with fresh bedding once every 6–8 days. The cage lids included space for pelleted food and a water bottle [see Tordoff and Bachmanov (2001) for details]. The food was pelleted AIN-76A, a nutritionally complete semisynthetic diet [Dyets, Bethlehem, PA; no. 100000]. When mice were not being tested, deionized water was available from an inverted 300-mL glass bottle with a neoprene stopper and a stainless steel drinking spout. A detailed description of mouse husbandry, housing conditions, and other procedures is available online (Tordoff and Bachmanov 2001). Taste phenotyping tests began after the mice had adapted to individual housing for at least 7 days.
Brief-access tests
The methods were almost identical to those used to test the taste-licking behavior of CALHM1 KO and T1R3 KO mice (Taruno et al. 2013b; Tordoff et al. 2014a, 2014b). Licking responses during brief-exposure taste tests were measured with MS160-mouse gustometers. Each gustometer consists of a 14.5cm × 30cm × 15cm test chamber with a motorized shutter that controls access to a taste solution. Bottles of taste solution are mounted on a sliding rack that allows up to 8 different taste solutions to be presented to the mouse (one at a time). The drinking spout of each bottle is part of a contact circuit so that each lick the mouse makes is detected and recorded. To avoid any undue influence of subtle differences between individual gustometers in the suite of 10 we used, each mouse was always tested in the same gustometer and equal numbers of WT and KO mice were tested in each gustometer.
A total of 17 PANX1 WT and 18 PANX1 KO mice were tested. All were males. At the start of testing they ranged in age from 57 to 102 days (group means ± SEs; WT = 75±3 days, KO = 81±3 days). The PANX1 KO mice weighed slightly but significantly more than did the WT controls (WT = 24±1g; KO = 26±1g, t(33) = 2.26, P = 0.0309). Because of breeding and equipment constraints, the mice were tested in 2 batches, 1 of 8 WT and 9 KO mice and 1 of 9 WT and 9 KO mice.
In order to train each mouse to sample taste solutions, it was first water-deprived for 22.5h and then placed in a gustometer with the shutter open, allowing access to the water spout. During this first training session, the mouse had continuous access to water for 30min from the time it first licked the drinking spout. It was then returned to its home cage and given water for 1h. On the following 2 days, the mouse was placed in the gustometer for 20min, during which the shutter allowing access to water closed 5 s after each time the mouse began to lick, and reopened after a 7.5-s interval. At the end of each session, the mouse was returned to its home cage and given water for 1h. By the second test, all mice had learned to obtain water during the 5-s access periods.
The mice then began 5 test sessions, with each session involving one of the following taste compounds in the order listed: sucrose, quinine hydrochloride (QHCl), NaCl, HCl, and CaCl2. Before the test with sucrose, each mouse received free access to food and water for 24h. It then received 1g of food and 2mL of water, and the session began 24h later. The mouse first received a single exposure to 1000mM sucrose in order to kindle its interest in the drinking spout. After this, repeated series of 5 concentrations (0, 32, 100, 320, and 1000mM sucrose) were presented in a quasi-random order such that each concentration could appear only once in a series of 5 tests. For each exposure, the shutter was open for 5 s, during which licks of the drinking spout were counted. This was followed by 7.5 s with the shutter closed, during which a new taste solution was positioned ready for the next presentation. A total of 125 presentations (25 series of 5 tests) were given but the mouse was not required to respond; most licked during only the first 4 or 5 series then stopped behaving, presumably because they were satiated. After the session, the mouse had a recovery day with free access to food and water for 24h.
For the remaining 4 sessions, which involved taste solutions that are less preferred than sucrose, each mouse was water-deprived for 22.5h (longer than for the test with sucrose) but had food available. In the gustometer, it received repeated series of 5 concentrations (including water) presented in pseudorandom order. Additional 1-s “washout” trials with water were interposed between each trial with a taste solution. Thus, a mouse received access to a taste solution for 5 s followed by 7.5 s with the shutter closed, then access to water for 1 s followed by 7.5 s with the shutter closed, followed by the next taste solution for 5 s, and so on. We think the 1-s washout trials with water have the effect of cleansing the mouse’s palate and this helps prevent it from quitting because it expects only bad-tasting solutions. All test sessions consisted of 90 taste solution presentations and 90 wash-out tests although all mice stopped drinking well before the session ended. After the session, each mouse received water for 1h in its home cage and it was then water-deprived for ~22.5h in preparation for the next session.
For statistical analyses, we averaged the results from identical exposures to obtain the mean licks made by each mouse in response to each concentration of each taste compound. Several mice did not respond to a particular concentration of a taste compound and so they were not included in statistical analyses of that compound. Analyses were conducted using mixed-design 2-way analyses of variance (ANOVA) with factors of genotype group and concentration.
Two-bottle choice tests
Taste preferences were assessed using ascending concentration series of 48-h two-bottle choice tests with the following taste compounds, presented in the order listed: saccharin, QHCl, NaCl, HCl, CaCl2, Polycose, and monosodium glutamate (MSG). The main experiment involved 20 PANX1 WT and 19 KO naïve female mice tested in 3 batches (Batch 1, 8 WT and 6 KO; Batch 2, 3 WT and 4 KO; Batch 3, 9 WT and 9 KO). The mice ranged from 40 to 108 days old at the start of testing, and did not differ in body weight (WT = 19.3±0.3g, KO = 18.6±0.4g), which was measured at the beginning of the first test session.
In each test series, the mice first received 2 drinking tubes containing deionized water for 48h, and then a choice between deionized water and ascending concentrations of the taste compound, with each test lasting 48h. The positions of the 2 drinking tubes were switched every 24h. Intakes from each tube were obtained by recording the level of fluid (on a volumetric scale to the nearest 0.1mL) at the beginning and end of each 48-h test. Total fluid intakes were obtained by adding together the intakes from both drinking tubes. Preference scores were calculated as intake of the solution divided by total intake, and expressed as a percentage.
To identify statistically significant differences in intakes or preferences, we used ANOVA with factors of genotype group and concentration. Analyses were conducted using a criterion for significance of P < 0.05 (Statistica 10, Stat Soft, Inc., Tulsa, OK). Preliminary analyses showed that the intakes and preferences of each batch were similar with one exception: KO and WT mice in Batch 1 and 2 had similar NaCl intakes and preferences; however, KO mice in Batch 3 had significantly lower preferences for 0, 32, 75, and 150mM NaCl than did their littermate controls. To resolve this discrepancy, we (i) retested the Batch 3 mice with the NaCl concentration series after they had completed the other tests, and (ii) tested an additional 10 WT and 10 KO naïve mice with only the NaCl series. The results of both of these additional tests were similar to those obtained with the Batch 1 and 2 mice so, to simplify description, here we present NaCl results based on all 59 mice, using averages of the 2 series of NaCl tests for mice in Batch 3.
Verification of the PANX1 knockout
To confirm that PANX1 was present in WT mice and absent in PANX1 KO mice, total RNA from the whole tongue, the circumvallate region of the tongue, and 2 positive control tissues (nasal epithelium and cerebellum) was isolated using a NucleoSpin RNA kit (Machery-Nagle). First strand cDNA was synthesized from 160ng of total RNA using SuperScript III reverse transcriptase and oligo(dt)20 primers (Life Technologies). The cDNA template was PCR-amplified using HotMaster Taq DNA polymerase (5 PRIME) and the following primers: 5′-AGATCTCCATCGGTACCCAGA-3′ and 5′-GTGGGAGGTTTCCAGACTCG-3′ for Panx1 and 5′-ATCGTGGGCCGCTCTAGGCACC-3′ and 5′-CTCTTTGATGT CACGCACGATTTC-3′ for β-actin. The expected sizes of the PCR products are 971 and 543bp, respectively.
Results
Brief-access tests
There were no differences between the PANX1 WT and KO mice in response to any of the 5 taste solutions tested (Table 1 and Figure 1). Both groups showed concentration-dependent increases in licking for sucrose and concentration-dependent decreases in licking for QHCl, HCl, CaCl2 and high concentrations of NaCl.
Table 1.
Brief-access tests: group sizes and results of ANOVAs
| Group sizes | ANOVA results | ||||
|---|---|---|---|---|---|
| Taste solution | WT | KO | Group | Concentration | Group × Concentration |
| Sucrose | 15 | 12 | F(1,25) = 2.92, P = 0.0997 | F(4, 100) = 146.1, P < 0.0001 | F(4,100) = 0.61, P = 0.6577 |
| QHCl | 11 | 15 | F(1,24) = 1.18, P = 0.2888 | F(4, 96) = 49.8, P < 0.0001 | F(4,96) = 0.45, P = 0.7750 |
| NaCl | 14 | 14 | F(1,26) = 0.11, P = 0.7402 | F(4, 104) = 47.8, P < 0.0001 | F(4,104) = 0.26, P = 0.9028 |
| HCl | 16 | 14 | F(1,28) = 0.34, P = 0.5627 | F(4, 112) = 47.4, P < 0.0001 | F(4,112) = 0.43, P = 0.7835 |
| CaCl2 | 13 | 14 | F(1,25) = 0.12, P = 0.7314 | F(4, 100) = 82.3, P < 0.0001 | F(4,100) = 1.18, P = 0.3246 |
Group sizes: 17 WT and 18 KO mice were tested but group sizes were smaller because mice that did not respond to one or more concentration of a taste solution were excluded from analyses.
Figure 1.
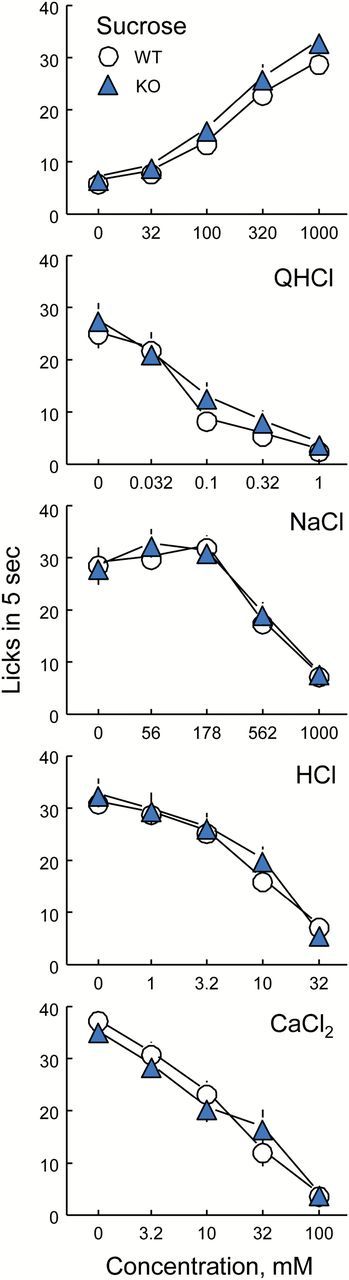
Licking by male PANX1 WT and KO mice to various concentrations of sucrose, quinine hydrochloride (QHCl), NaCl, HCl and CaCl2. Symbols are means (ns = 11–16); vertical lines are SEs. There were no significant differences between the PANX1 WT and KO groups for any concentration of any taste solution (Table 1).
Two-bottle choice tests
There were no differences between the PANX1 WT and KO mice in response to any of the 7 taste solutions tested, whether solution intakes or preferences were analyzed; nor were there differences in water intakes (Table 2 and Figure 2). Both groups showed concentration-dependent increases in intakes and preferences for saccharin and Polycose, concentration-dependent decreases in intakes and preferences for QHCl, HCl, and CaCl2 and inverted U-shaped concentration-response functions for NaCl and MSG.
Table 2.
Two-bottle choice tests preference scores: group sizes and results of ANOVAs
| Group sizes | ANOVA results | ||||
|---|---|---|---|---|---|
| Taste solution | WT | KO | Group | Concentration | Group × Concentration |
| Saccharin | 20 | 19 | F(1, 37) = 2.82, P = 0.1011 | F(2, 74) = 225.5, P < 0.0001 | F(2, 74) = 0.19, P = 0.8257 |
| QHCl | 20 | 19 | F(1, 37) = 1.30, P = 0.2614 | F(3, 111) = 43.4, P < 0.0001 | F(3, 111) = 1.21, P = 0.3086 |
| NaCl | 30 | 29 | F(1, 57) = 1.85, P = 0.1787 | F(5, 285) = 97.6, P < 0.0001 | F(5, 285) = 0.46, P = 0.8072 |
| HCl | 20 | 19 | F(1, 37) = 0.11, P = 0.7365 | F(3, 111) = 40.2, P < 0.0001 | F(3, 111) = 1.42, P = 0.2402 |
| CaCl2 | 20 | 19 | F(1, 37) = 0.35, P = 0.5565 | F(3, 111) = 48.1, P < 0.0001 | F(3, 111) = 0.08, P = 0.9730 |
| Polycose | 20 | 19 | F(1, 37) = 0.13, P = 0.7162 | F(3, 111) = 89.8, P < 0.0001 | F(3, 111) = 0.23, P = 0.8759 |
| MSG | 20 | 19 | F(1, 37) = 0.00, P = 0.9666 | F(5, 185) = 165.0, P < 0.0001 | F(5, 185) = 0.57, P = 0.7213 |
Figure 2.
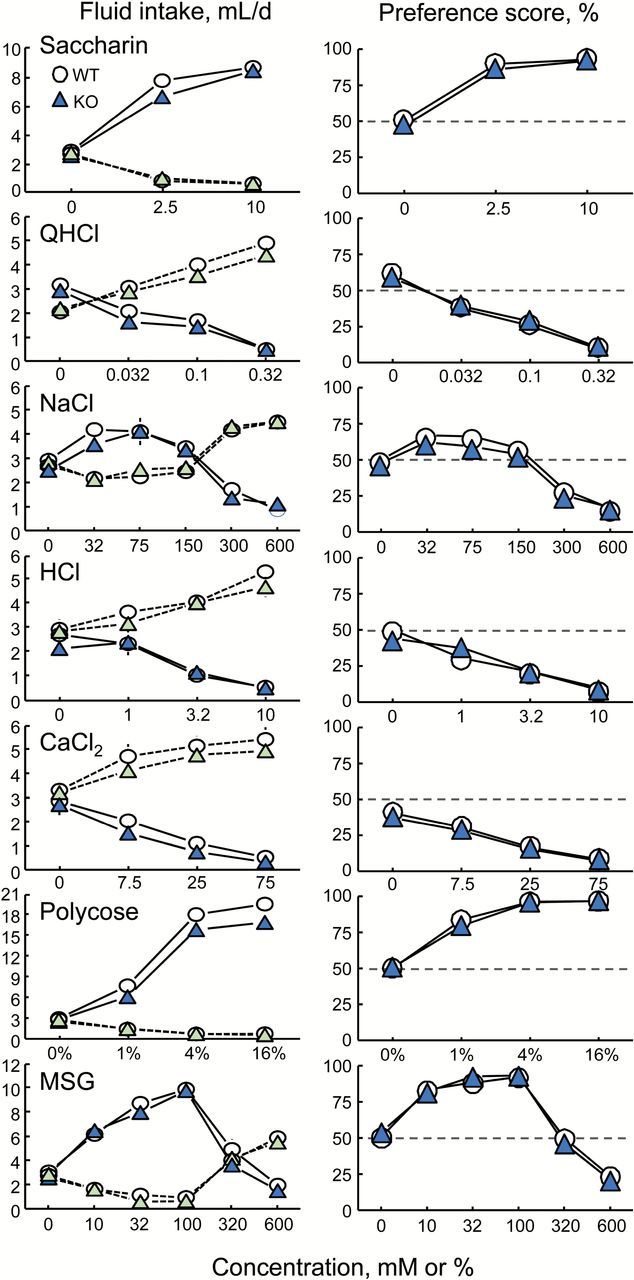
Two-bottle choice test daily intakes (left panels) and preference scores (right panels) of PANX1 WT and KO mice given ascending concentration series of 7 taste compounds. Symbols joined by solid lines show solution intakes or preferences; those joined by dotted lines show water intakes. Each symbol is a mean of 29–30 mice for tests with NaCl, 19–20 mice for all other tests. Most SEs are smaller than the symbols. There were no significant differences between the PANX1 WT and KO groups for any concentration of any taste solution (Table 2).
PANX1 expression
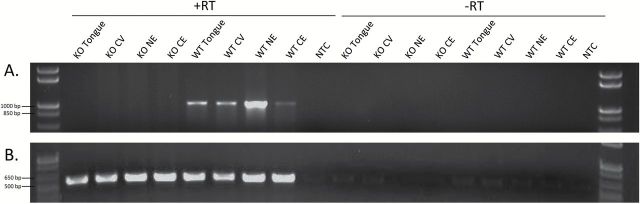
Panx1 mRNA was detected by reverse transcriptase PCR in PANX1 WT mice but was absent in PANX1 KO mice, in all 4 tissues sampled (circumvallate, whole tongue, nasal epithelium, and cerebellum; Figure 3). Figure 3 shows results obtained from a single WT and single KO mouse; 3 replications using DNA from other WT and KO mice gave identical results.
Figure 3.
RT-PCR products demonstrating that Panx1 mRNA is present in whole tongue, circumvallate taste tissue (CV), nasal epithelium (NE), and cerebellum (CE) of PANX1 wild-type (WT) but not knockout (KO) mice. (A) Panx1 (product = 971bp). (B) β-actin (product = 543bp). NTC = no template control. Right lanes (−RT) show control reactions without reverse transcriptase.
Discussion
There were no differences between PANX1 KO mice and their littermate controls in licking rates or preference scores for archetypal taste solutions, including several that are believed to involve activation of GPCRs in Type 2 taste cells (i.e., sucrose, saccharin, QHCl, CaCl2, Polycose, MSG, and high concentrations of NaCl). The implication is that PANX1 is unnecessary for the behavioral response to taste. This is consistent with biochemical evidence against a contribution of PANX1 to taste transduction (Romanov et al. 2012; see introduction) and the recent discovery that CALHM1 is an ATP channel in Type 2 taste cells that can fulfill the role previously ascribed to PANX1 (Taruno et al. 2013a, 2013b).
The conclusion that PANX1 KO has no effect on taste-related behavior involves accepting the null hypothesis, which is often perilous but is unlikely to be a problem here. Both experiments had more-than-adequate statistical power or, at least, group sizes were considerably larger than those used to demonstrate deficits in taste-related behavior of CALHM1 KO mice (Taruno et al. 2013b). Moreover, there were no concerted nonsignificant trends in the responses to any of the taste solutions, except for a genotype-related difference in NaCl preference scores present in 1 of 4 batches of mice tested. This was probably a statistical aberration because (i) the observed difference did not replicate when the same mice were retested, (ii) no trends were observed with NaCl intakes in brief-access tests, and (iii) one would expect the effects of PANX1 to be most clearly expressed in preferences for putative GPCR-mediated tastes (i.e., saccharin, QHCl, CaCl2, Polycose, and MSG) than in preferences for NaCl.
There is good reason to believe that the PANX1 knockout had its intended effect of eliminating PANX1 channels in taste cells. First, genotyping conducted independently in-house and by a commercial service confirmed that all the PANX1 KO mouse carried the expected deletion. Second, Panx1 mRNA was present in taste tissue of PANX1 WT but not PANX1 KO mice. Third, the line of PANX1 KO mice used here has been demonstrated to display reduced release of ATP from optic nerve head astrocytes (Beckel et al. 2014) and reduced post-ischemic retinal neurotoxicity (Dvoriantchikova et al. 2012), indicating that, at least for these cell types, the knockout is functional. It thus seems reasonable to infer that the knockout effectively ablated PANX1 from taste cells.
It remains feasible that PANX1 contributes to taste perception in intact mice but when PANX1 is knocked out other molecular mechanisms subsume this role. However, there are at least 2 arguments against this. First, there is no obvious substitute: The closest homologs for PANX1 are PANX2 and PANX3 but these are not expressed in taste cells (Romanov et al. 2012). Connexins have similar functions to pannexins (Koval et al. 2014) but connexins are only modestly expressed in Type 2 taste cells (Huang et al. 2007; Romanov et al. 2007) and connexin channels are normally open only when cytoplasmic calcium is low or absent, which is opposite to what happens during taste transduction (review; Chaudhari and Roper 2010). Second, as Romanov has argued (Romanov et al. 2012), it would be unsatisfying to have a redundant output in a nonredundant pathway. Other components of the Type 2 cell taste transduction cascade (e.g., PLCβ2, ITPR3, TRPM5, and CALHM1) are essential. In each case, knockout of the molecular element results in marked, often complete, diminution of GPCR-mediated taste responses (e.g., Zhang et al. 2003; Taruno et al. 2013b; Tordoff and Ellis 2013). When the rest of the transduction cascade is essential there is no benefit to employ multiple ATP release channels. This seems particularly true because CALHM1 appears to completely fulfill the role (Taruno et al. 2013b).
One line of evidence leading to the conclusion that PANX1 is involved in taste perception derives from findings that taste cell ATP release is inhibited by chemical or pharmacological blockade of PANX1 channels (Dando and Roper 2009). Inhibition was achieved by acidifying the cytosol, which blocks gap junctions, or with probenecid, which is considered a selective blocker of PANX1 channels (Silverman et al. 2008; Ma et al. 2009). However, acidification has many nonspecific effects and probenecid did not influence ATP release from Type 2 taste cells in our earlier work (Taruno et al. 2013b). The cause of this discrepancy is unclear.
If PANX1 does not influence taste solution acceptance then what is it doing in taste cells? One possibility is that it is involved in an aspect of taste perception not measured by brief-access or choice tests, such as the perception of taste quality or taste adaptation. In some cell types, PANX1 channels release ATP but are inhibited by extracellular ATP (Locovei et al. 2006). If this were to occur in taste cells it would allow for complex interplay between adjacent taste cells either without contributing to the taste signal or by contributing only under extreme conditions (e.g., during taste adaptation). More generally, the large pores of PANX1 channels may allow passage of ions or small molecules into or out of taste cells without contributing to the taste signal. PANX1 channels are activated by mechanical stress (Bao et al. 2004; Beckel et al. 2014), raising the possibility that they could help protect taste cells against physical onslaught, such as the tongue’s impact with ingested foods and drinks. Finally, in some cell types, PANX1 activation is involved in apoptosis (Chekeni et al. 2010), which is a distinctive feature of taste cells. The contribution of PANX1 in taste cells to these and other potential functions (reviews: MacVicar and Thompson 2010; Dahl and Muller 2014) remains to be explored. But whatever its function, our results indicate that PANX1 is not essential for determining the behavioral responses elicited by representative taste solutions.
Funding
This work was supported by National Institutes of Health grant DK-46791 to M.G.T., DC-12538 to J.K.F., and EY021517 to V.I.S. Mouse gustometry was performed using equipment belonging to the Monell Phenotyping Core, which is supported, in part, by funding from the National Institute on Deafness and Other Communication Disorders P30 Core Grant DC011735. R.L.P. was supported by National Institute on Deafness and Other Communication Disorders Training Grant T32 DC000014.
Acknowledgments
Adept technical assistance was provided by Chelsey Deal.
References
- Bao L, Locovei S, Dahl G. 2004. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 572(1–3):65–68. [DOI] [PubMed] [Google Scholar]
- Beckel JM, Argall AJ, Lim JC, Xia J, Lu W, Coffey EE, Macarak EJ, Shahidullah M, Delamere NA, Zode GS, et al. 2014. Mechanosensitive release of adenosine 5′-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: a mechanism for purinergic involvement in chronic strain. Glia. 62(9):1486–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Roper SD. 2010. The cell biology of taste. J Cell Biol. 190:285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, et al. 2010. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 467(7317):863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl G, Muller KJ. 2014. Innexin and pannexin channels and their signaling. FEBS Lett. 588(8):1396–1402. [DOI] [PubMed] [Google Scholar]
- Dando R, Roper SD. 2009. Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J Physiol. 587(Pt 24):5899–5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoriantchikova G, Ivanov D, Barakat D, Grinberg A, Wen R, Slepak VZ, Shestopalov VI. 2012. Genetic ablation of Pannexin1 protects retinal neurons from ischemic injury. PLoS One. 7(2):e31991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy MC, Eschle BK, Barrows J, Hallock RM, Finger TE, Delay ER. 2009. Double P2X2/P2X3 purinergic receptor knockout mice do not taste NaCl or the artificial sweetener SC45647. Chem Senses. 34(9):789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. 2005. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 310(5753):1495–1499. [DOI] [PubMed] [Google Scholar]
- Hallock RM, Tatangelo M, Barrows J, Finger TE. 2009. Residual chemosensory capabilities in double P2X2/P2X3 purinergic receptor null mice: intraoral or postingestive detection? Chem Senses. 34(9):799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Roper SD. 2010. Intracellular Ca(2+) and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. J Physiol. 588(Pt 13):2343–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. 2007. The role of pannexin 1 hemichannels in ATP release and cell–cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 104(15):6436–6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval M, Isakson BE, Gourdie RG. 2014. Connexins, pannexins and innexins: protein cousins with overlapping functions. FEBS Letters. 588:1185–1186. [DOI] [PubMed] [Google Scholar]
- Kurtenbach S, Zoidl G. 2014. Emerging functions of pannexin 1 in the eye. Front Cell Neurosci. 8:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Wang J, Dahl G. 2006. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 580(1):239–244. [DOI] [PubMed] [Google Scholar]
- Ma W, Hui H, Pelegrin P, Surprenant A. 2009. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther. 328(2):409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar BA, Thompson RJ. 2010. Non-junction functions of pannexin-1 channels. Trends Neurosci. 33(2):93–102. [DOI] [PubMed] [Google Scholar]
- Moyer BD, Hevezi P, Gao N, Lu M, Kalabat D, Soto H, Echeverri F, Laita B, Yeh SA, Zoller M, et al. 2009. Expression of genes encoding multi-transmembrane proteins in specific primate taste cell populations. PLoS One. 4(12):e7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Yasuo T, Yoshida R, Obata K, Yanagawa Y, Margolskee RF, Ninomiya Y. 2010. Action potential-enhanced ATP release from taste cells through hemichannels. J Neurophysiol. 104(2):896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Bystrova MF, Rogachevskaya OA, Sadovnikov VB, Shestopalov VI, Kolesnikov SS. 2012. The ATP permeability of pannexin 1 channels in a heterologous system and in mammalian taste cells is dispensable. J Cell Sci. 125(Pt 22):5514–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. 2007. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 26(3):657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman W, Locovei S, Dahl G. 2008. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 295(3):C761–C767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taruno A, Matsumoto I, Ma Z, Marambaud P, Foskett JK. 2013a. How do taste cells lacking synapses mediate neurotransmission? CALHM1, a voltage-gated ATP channel. Bioessays. 35(12):1111–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, et al. 2013b. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 495(7440):223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Aleman TR, McCaughey SA. 2014a. Heightened avidity for trisodium pyrophosphate in mice lacking Tas1r3. Chem Senses. 40(1):53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA. 2001. Monell mouse taste phenotyping project Available from: www.monell.org/MMTPP.
- Tordoff MG, Ellis HT. 2013. Taste dysfunction in BTBR mice due to a mutation of Itpr3, the inositol triphosphate receptor 3 gene. Physiol Genomics. 45(18):834–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Ellis HT, Aleman TR, Downing A, Marambaud P, Foskett JK, Dana RM, McCaughey SA. 2014b. Salty taste deficits in CALHM1 knockout mice. Chem Senses. 39(6):515–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. 2003. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 112(3):293–301. [DOI] [PubMed] [Google Scholar]