Abstract
Background
Delirium is a common complication after cardiac surgery and may be as a result of inadequate cerebral perfusion. We studied delirium after cardiac surgery in relation to intraoperative hypotension (IOH).
Methods
This observational single-centre, cohort study was nested in a randomized trial, on a single intraoperative dose of dexamethasone vs placebo during cardiac surgery. During the first four postoperative days, patients were screened for delirium based on the Confusion Assessment Method (CAM) for Intensive Care Unit on the intensive care unit, CAM on the ward, and by inspection of medical records. To combine depth and duration of IOH, we computed the area under the curve for four blood pressure thresholds. Logistic regression analyses were performed to investigate the association between IOH and the occurrence of postoperative delirium, adjusting for confounding and using a 99% confidence interval to correct for multiple testing.
Results
Of the 734 included patients, 99 patients (13%) developed postoperative delirium. The adjusted Odds Ratio for the Mean Arterial Pressure <60 mm Hg threshold was 1.04 (99% confidence interval: 0.99–1.10) for each 1000 mm Hg2 min2 AUC2 increase. IOH, as defined according to the other three definitions, was not associated with postoperative delirium either. Deep and prolonged IOH seemed to increase the risk of delirium, but this was not statistically significant.
Conclusions
Independent of the applied definition, IOH was not associated with the occurrence of delirium after cardiac surgery.
Keywords: cardiac surgical procedures; delirium dementia, amnestic cognitive disorders; hypotension
Editor's Key Points.
The pathophysiology of delirium is not well understood.
The authors investigated the association between hypotension during cardiac surgery and postoperative delirium.
No associations between intraoperative hypotension and delirium were found.
Delirium is characterized by an acute change in mental status1 and has been associated with adverse outcomes, such as prolonged hospital stay, long-term cognitive impairment and mortality.2–5 With an incidence between 3 and 52% during hospital admission,6–10 delirium frequently complicates cardiac surgery. Although the pathophysiology of postoperative delirium is incompletely understood, inadequate cerebral perfusion as a result of intraoperative hypotension (IOH) is one of the possible mechanisms.5,11,12
IOH is a common side-effect of general anaesthesia, but a widely accepted definition of IOH is not available.13 In previous studies, IOH has been associated with postoperative adverse events, such as mortality,14–16 acute kidney injury,17 and myocardial ischaemia.17,18 In studies on IOH and postoperative ischaemic stroke, no clear association was observed in different groups of patients.16,18–21 Nevertheless, it seems plausible that brain perfusion becomes compromised when a patient experiences a too low blood pressure for a too long period of time. Delirium may be a more sensitive manifestation of postoperative cerebral dysfunction as a result of temporarily insufficient cerebral perfusion than ischaemic stroke. However, the association of intraoperative blood pressure with delirium is currently unclear.
We hypothesized that severe and prolonged low blood pressure during anaesthesia and cardiac surgery increases the risk of postoperative delirium. The aim of the study was to investigate whether IOH is related to the occurrence of delirium after cardiac surgery.
Methods
Design and patients
This observational single-centre cohort study was nested within a large multicentre clinical trial, the Dexamethasone for Cardiac Surgery (DECS) trial. The design of the DECS trial has been published in detail elsewhere.22 Briefly, adult patients undergoing cardiac surgery requiring cardiopulmonary bypass (CPB), were randomized to a single injection of either 1 mg kg−1 dexamethasone or placebo at the induction of anaesthesia. Exclusion criteria were emergency procedures, or a preoperative life expectancy less than six months. Patients from the DECS trial who participated in a sub-study on postoperative delirium within one of the participating centres, the University Medical Center Utrecht, were eligible for the current study on IOH and postoperative delirium. Patients who had a stroke during the study period were excluded from postoperative delirium monitoring. This study was carried out according to Good Clinical Practice standards and national regulations. The Medical Ethics Committee of the University Medical Center Utrecht approved the DECS-trial (METC 05-301) and the sub-study on delirium (METC 12–423). All patients provided written informed consent before randomization. For the current study on IOH and delirium, the Medical Ethics Committee waived the need to obtain separate informed consent.
Data collection and anaesthesia protocol
Patient characteristic and postoperative data were obtained from the DECS trial database22 and the hospital information system. Intraoperative data from the patient monitor and anaesthesia machine were stored as the median for each min of collected data in the electronic anaesthesia information management system (AnStat®, CarePoint Nederland BV, Ede, the Netherlands). Intraoperative fluid management and anaesthesia were performed according to protocol. The patients did not receive premedication. Anaesthesia was initiated using 0.1 mg kg−1 midazolam, 1 µg kg−1 sufentanil. Pancuronium 0.1 mg kg−1 was given to facilitate orotracheal intubation. For maintenance, inhalation anaesthesia with sevoflurane or isoflurane was applied together with 0.5 µg−1 kg−1 h−1 sufentanil i.v. Patients received a restricted i.v. fluid regimen.
Intraoperative hypotension
IOH was defined as the cumulative exposure to mean arterial pressures (MAPs) below a predefined threshold during surgery. Intra-arterial blood pressure (IABP) measurements were used, or non-invasive bp (NIBP) measurements when IABP was unavailable at any time point. As inadequate brain perfusion was expected to depend on both the depth and the duration of hypotension, IOH was defined as the Area Under the Curve (AUC) for a certain MAP threshold.23,24 Therefore, the AUC was expressed in mm Hg min. Given the lack of a widely accepted definition for IOH,13 IOH was studied using four predefined, but exploratory thresholds: a MAP below 60 mm Hg, a MAP below 50 mm Hg, a MAP decrease >30% relative to baseline bp and a MAP decrease >40% relative to baseline bp. The baseline bp was defined as the mean of all measured bp measurements, in the operating theatre before induction of anaesthesia. Time of induction was defined as the moment of the administration of induction medication or three min before the first registration of expired carbon dioxide, whichever came first.13
Delirium assessment
The outcome of this study was the occurrence of delirium at any time point during the first four days after cardiac surgery. Trained research personnel assessed all patients for delirium daily, including weekend days.25 The following assessment scales were used: the Richmond Agitation Sedation Scale (RASS) to assess the level of consciousness, the Confusion Assessment Method adapted for the Intensive Care Unit (CAM-ICU) to detect delirium during Intensive Care Unit (ICU) admission, and the Confusion Assessment Method (CAM) to detect delirium during admission at the cardiothoracic surgery ward.26,27 In addition, medical records were screened for signs of delirium and treatment with haloperidol. Patients with uncertainty regarding the diagnosis of delirium were discussed with a neurologist-intensivist (AS), who made the final classification. Research personnel were unaware of the occurrence of IOH during their assessment of postoperative delirium.
Potential confounders
We selected a priori the following possible confounders, based on clinical experience and previously performed studies.28,29 These included the EuroSCORE,30 duration of surgery, duration of CPB, intraoperative use of vasopressors, intraoperative use of inotropes and administration of either dexamethasone or placebo. As the EuroSCORE includes age, sex, and various comorbidity and risk factors for surgical complexity, we did not adjust for these variables separately. Vasopressor use was expressed by the cumulative number of minutes in which a patient received phenylephrine, ephedrine, adrenaline or noradrenaline i.v. during surgery. Inotrope use was expressed by the cumulative number of minutes in which a patient received milrinone or dobutamine i.v. during surgery.
Statistical analysis
Continuous variables were visually assessed for a normal distribution using histograms and qq-plots. Normally distributed data were presented as means with standard deviations (sd) and studied using two-sample Student’s t-tests. Skewed continuous data were presented as medians with interquartile ranges (IQR) and evaluated using Wilcoxon signed rank tests. Categorical variables were expressed as numbers (percentage) and tested using χ2tests. The association between the AUC of each MAP threshold and occurrence of delirium was analysed using multivariable logistic regression analysis. Based on assessment for nonlinearity using restricted cubic splines, the AUCs for IOH were included into regression analysis after quadratic transformation. Odds ratios (ORs) were calculated per 1000 mm Hg2 min2 increase of intraoperative hypotension and presented as a scaled OR between the 75th and 25th percentile.
The analyses included the above described confounders. As four different MAP thresholds were analysed, Bonferroni correction was applied to adjust for multiple testing. The resulting two-sided α was rounded down to 0.01 and therefore 99% confidence intervals (99% CI) were used to present ORs. All analyses were performed using R (release 3.0.0; R foundation for Statistical Computing, Vienna, Austria).
Results
In total, 768 patients were included in the DECS trial at the University Medical Centre in Utrecht, between June 2009 and November 2011. Thirty patients could not be evaluated for occurrence of delirium, because of logistic reasons (i.e. unexpected rescheduling of the surgery, no available study nurse). One patient withdrew informed consent and was not analysed. Intraoperative data of three other patients could not be merged, because of an inability to link the unique surgery number to the DECS study number. Therefore, this study cohort included 734 patients (96% of the eligible 768 subjects), and most of them underwent combined cardiac surgery (Table 1). There were no missing values in patient-surgery-, or outcome-related variables. During the first four postoperative days, 99 patients (13%) were diagnosed with delirium. Patients who developed delirium were older, more often female, had a higher EuroSCORE, and more often had ischaemic stroke or peripheral artery disease in their medical history.
Table 1.
Patient characteristics. †EuroSCORE consists of the following weighted patient-, cardiac- and surgery-related variables: age, sex, chronic pulmonary disease, extracardiac arteriopathy, neurological dysfunction, previous cardiac surgery, serum creatinine, active endocarditis, critical preoperative state, presence of unstable angina, left ventricle dysfunction, recent myocardial infarction, pulmonary hypertension, emergency surgery, other than isolated coronary artery by-pass grafting, surgery on thoracic aorta, postinfarct septal rupture.30 ‡Definition of left ventricular function (LVF):31 good, ejection fraction of more than 50%; moderate, ejection fraction of 30% to 50%; and poor, ejection fraction of less than 30%. sd, standard deviation; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; LVF, left ventricular function
| All patients (n=734) | Non-delirious patients (n=635) | Delirious patients (n=99) | P-values | |
|---|---|---|---|---|
| Female, n (%) | 224 (31) | 179 (28) | 45 (45) | <0.01 |
| Age, mean (range), yr | 66 (20.5–88.8) | 65.1 (20.5–88.8) | 73.8 (30.2–87.5) | <0.01 |
| Essential hypertension, n (%) | 413 (56) | 350 (55) | 63 (64) | 0.11 |
| Diabetes, n (%) | 140 (19) | 117 (18) | 23 (23) | 0.26 |
| History of stroke, n (%) | 41 (6) | 35 (5) | 6 (6) | 0.02 |
| Peripheral artery disease, n (%) | 64 (9) | 49 (8) | 15 (15) | 0.02 |
| Preoperative creatinine concentration, median (IQR), µmol litre−1 | 92 (81–108) | 92 (81–107) | 93 (80–117) | 0.18 |
| EuroSCORE, median (IQR) | 5 (3–7) | 4 (3–7) | 7 (5–9) | <0.01 |
| Left ventricular function, n (%) | 0.51 | |||
| Good (>50%) | 528 (72) | 460 (72) | 68 (69) | |
| Moderate (30–50%) | 176 (24) | 151 (24) | 25 (25) | |
| Poor (<30%) | 30 (4) | 24 (4) | 6 (6) | |
| Type of surgery, n (%) | <0.01 | |||
| Mitral valve surgery | 73 (10) | 69 (11) | 4 (4) | |
| Aortic valve surgery | 104 (14) | 90 (14) | 14 (14) | |
| CABG | 266 (36) | 238 (37) | 28 (28) | |
| CABG with mitral valve surgery | 27 (4) | 26 (4) | 1 (1) | |
| CABG with aortic valve surgery | 83 (11) | 57 (9) | 26 (26) | |
| Other surgery | 181 (25) | 155 (25) | 26 (26) |
Table 2 provides an overview of the duration of surgery and use of vasopressors and inotropes. Overall, the mean duration of surgery was 284 min (sd 86) and the mean duration of CPB 91 min (sd 53). Delirious patients had longer cross-clamp durations and had more min of vasopressor infusion during surgery than non-delirious patients. On average, the baseline MAP of delirious patients and non-delirious patients were 90 (sd 17) and 92 (15) respectively.
Table 2.
Duration of surgery and use of vasopressors and inotropes. †Vasopressor use was expressed by the cumulative number of min in which a patient received phenylephrine, ephedrine, adrenaline, noradrenaline or dopamine i.v. during surgery. ‡Inotrope use was expressed by the cumulative number of min in which a patient received milrinone or dobutamine i.v. during surgery. AUC, area under the curve; MAP, mean arterial pressure; IQR, interquartile range; sd, standard deviation
| All patients (n=734) | Non-delirious patients (n=635) | Delirium patients (n=99) | P-values | |
|---|---|---|---|---|
| Duration of surgery, mean (sd), min | 284 (86) | 282 (83) | 299 (100) | 0.16 |
| Duration of cardiopulmonary bypass, mean (sd), min | 91 (53) | 91 (53) | 95 (51) | 0.21 |
| Duration of cross-clamp time, mean (sd), min | 31 (25) | 29 (23) | 38 (35) | <0.01 |
| Reoperation, n (%), yes | 45 (6) | 35 (5) | 10 (10) | 0.08 |
| Baseline bp, mean (sd), mm Hg | 92 (15) | 92 (15) | 90 (17) | 0.07 |
| Total min any vasopressor, median (IQR), min | 54 (0–165) | 46 (0–154) | 119 (0–226) | 0.01 |
| Total min any inotrope, median (IQR), min | 0 (0–0) | 0 (0–0) | 0 (0–52) | 0.09 |
Table 3 shows the median area under the curve of various thresholds for IOH. Delirious patients had higher median AUCs than non-delirious patients when MAP<60 mm Hg or MAP<50 mm Hg thresholds were applied. However, delirious patients had lower median AUCs based on both relative thresholds compared with non-delirious patients.
Table 3.
Intraoperative hypotension during cardiac surgery. Expressed by the area under the curve of various thresholds for hypotension in mm Hg min, by median (IQR); CPB, cardiopulmonary bypass; MAP, mean arterial pressure; IQR, interquartile range
| Area under the curve, median (IQR), mm Hg min | All patients (n=734) | Non-delirious patients (n=635) | Delirious patients (n=99) | P-values |
|---|---|---|---|---|
| MAP<60 mm Hg | 1272 (774–1861) | 1265 (768–1852) | 1399 (846–2088) | 0.09 |
| MAP<50 mm Hg | 251 (114–448) | 241 (110–444) | 297 (150–520) | 0.05 |
| MAP decrease >30% relative to baseline | 1901 (763–3653) | 1929 (811–3637) | 1796 (642–3964) | 0.41 |
| MAP decrease >40% relative to baseline | 631 (183–1570) | 649 (186–1542) | 559 (150–1795) | 0.59 |
In the crude analysis, an increase of the AUC of IOH based on MAP<60 mm Hg, was significantly associated with the occurrence of delirium (OR 1.05 per 1000 mm Hg2 min2 AUC increase, 99% CI 1.01–1.09) (Table 4). After adjusting for confounding and multiple testing, there were no significant associations between IOH based on any of the definitions, and delirium. Our findings did not change when we added age and aortic cross clamp time to the models. When comparing a patient with an AUC of the 75th percentile based on MAP<60 mm Hg threshold (1861 mm Hg min) to a patient with an AUC of 25th percentile based on MAP<60 mm Hg threshold (774 mm Hg min), the adjusted OR was 1.12 (99% CI 1.07–1.16). This can roughly be interpreted as a 12% increased risk for the occurrence of postoperative delirium.
Table 4.
Crude and adjusted odds ratios for the association between the area under the curve of intraoperative hypotension during cardiac surgery and occurrence of postoperative delirium. *Results were adjusted for EuroSCORE, duration of surgery and cardiopulmonary bypass, total intraoperative fluid, cumulative duration of vasopressors and inotropes. # Estimates per 1000 mm Hg2 min2 AUC2 increase of intraoperative hypotension depth and/or duration. ‡Considered statistically significant when P<0.01 and multiple testing was taken into account. †Interquartile ranges: MAP<60 mm Hg (774–1861 mm Hg min); MAP<50 mm Hg (114–448 mm Hg min); Decrease MAP decrease >30% relative to baseline (763–3653 mm Hg min); Decrease MAP decrease >40% relative to baseline (183–1570 mm Hg min). OR, odds ratio; CI, confidence interval; MAP, mean arterial pressure
| Intraoperative hypotension definition | Unadjusted analysis |
Adjusted analysis* |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR# | 99% CI | P-value‡ | 75th - 25th percentile |
OR# | 99% CI | P-value‡ | 75th - 25th percentile |
|||
| OR† | 99% CI | OR† | 99% CI | |||||||
| MAP<60 mm Hg | 1.05 | 1.01–1.09 | <0.01 | 1.14 | 1.10–1.18 | 1.04 | 0.99–1.10 | 0.04 | 1.12 | 1.07–1.16 |
| MAP<50 mm Hg | 1.22 | 1.02–1.66 | 0.04 | 1.04 | 0.81–1.33 | 1.14 | 0.98–1.53 | 0.09 | 1.03 | 0.80–1.31 |
| MAP decrease >30% relative to baseline | 1.00 | 0.996–1.01 | 0.23 | 1.03 | 1.03–1.04 | 1.00 | 0.99–1.01 | 0.45 | 1.02 | 1.02–1.03 |
| MAP decrease >40% relative to baseline | 1.00 | 0.990–1.02 | 0.32 | 1.01 | 1.00–1.02 | 1.00 | 0.99–1.02 | 0.55 | 1.01 | 1.00–1.02 |
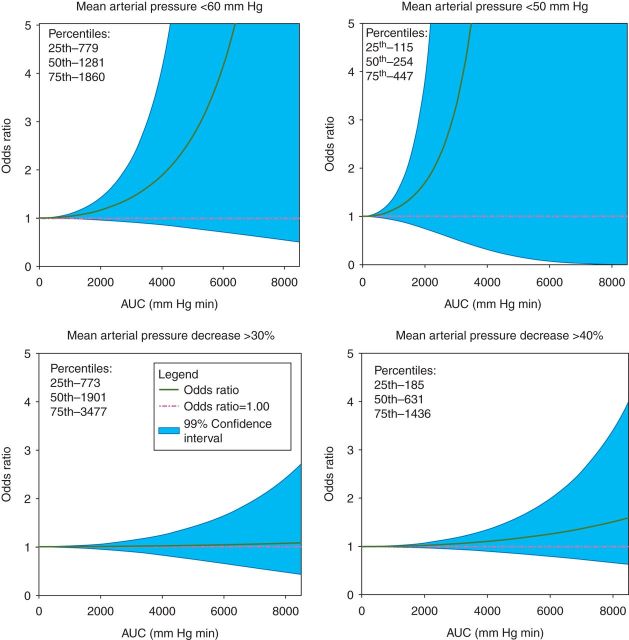
Figure 1 is a graphical representation of the results of the logistic regression analyses. In this figure, the AUCs based on the four MAP thresholds are plotted against the ORs from the multivariable regression analyses. In patients with a relatively low AUC, the OR remained close to 1, but the OR gradually increased with increasing AUCs for every definition. However, the risk of delirium associated with IOH did never reach statistical significance.
Fig 1.
The odds ratio (OR) for delirium as a function of intraoperative hypotension (IOH). The area under the curve (AUC) was plotted against the adjusted OR (solid green line) with its 99% confidence interval (solid pink lines). (a) Mean arterial pressure (MAP)<60 mm Hg threshold. The median and interquartile range was 1272 (774–1861). (b) MAP<50 mm Hg threshold. The median and interquartile range was 251 (114–448). (c) MAP decrease >30% relative to baseline. The median and interquartile range was 1901 (763–3653). (d) MAP decrease >40% relative to baseline. The median and interquartile range was 631 (183–1570).
Discussion
Several possible pathophysiologic mechanisms have been proposed for postoperative delirium and cognitive dysfunction after cardiac surgery. The cause of delirium appears to be multifactorial, where patient-related factors interact with perioperative events.32–34 Specifically, it has been suggested that alterations of neurotransmission, inflammation and an aberrant stress response play a role in the pathogenesis of delirium.34–36 These include dopaminergic hyperactivity and cholinergic deficiency.36 Levels of chemokines and cytokines have been associated with delirium as well. These inflammatory mediators may disrupt the blood-brain barrier and thereby induce neuro-inflammation influencing neurotransmission.37,38 Further, an aberrant stress response, as indicated by perioperative raised cortisol concentrations has been associated with postoperative delirium.39–41 Another suggested mechanism is inadequate cerebral perfusion.42 We therefore hypothesized that IOH is associated with the occurrence of postoperative delirium. With this observational cohort study using clinical trial data, we investigated whether IOH was related to the occurrence of delirium after cardiac surgery.
Our findings contrast with previous studies. Recently, a strategy was developed to prevent occurrence of postoperative delirium in patients undergoing cardiac surgery, by identifying patients with a high risk for poor cerebral haemodynamics. This strategy included preoperative transcranial doppler screening, perioperative cerebral oximetry monitoring, and intraoperative optimization of cerebral haemodynamics. In this retrospective cohort study, optimization of intraoperative haemodynamics was found to lower the incidence of postoperative delirium after cardiac surgery.43 However, there were some limitations, for example the observational design of this study, the fact that delirium assessment have not been performed by trained research personnel, and the fact that an important proportion of patients did not receive the complete work-up. These limitations could have resulted in confounding and selection bias.
In a recent randomized clinical trial, patients undergoing CABG were randomized to low (60–70 mm Hg) or high (80–90 mm Hg) systemic perfusion pressure during CPB. The incidence of postoperative delirium and cognitive dysfunction was significantly higher in the low pressure group (13%) compared with the high pressure group (0%, P=0.017).5 However, only the effects of low or high MAP during CPB were studied, and bps before and after CPB were not taken into account. In addition, delirium was assessed with the Mini-Mental-State examination, which has not been designed as a delirium scale. Occurrence of delirium has also been studied in elderly undergoing non-cardiac surgery. However, the results of these studies were ambiguous.44–46 In one study,44 no significant association was found between IOH and the occurrence of delirium, but in two other studies, IOH45 and the number of hypotensive episodes,46 were significantly associated with the occurrence of postoperative delirium.
The strengths of our study in comparison to the above studies on IOH and delirium, are the large sample size, and the active and continuous monitoring of delirium by trained research personnel during the first four postoperative days. Moreover, in the absence of a universally accepted definition for IOH, we studied four commonly used definitions.13 We further analysed IOH exposure as a continuous variable instead of a dichotomous variable. We did not dichotomize hypotension as this results in lack of detail and loss of information. Instead, we used the AUC of a certain blood pressure threshold, which is a function of both duration and severity of hypotension. As a result of the absent pulse pressure during CPB, we only chose definitions which included MAP thresholds and not systolic or diastolic thresholds.
Our study has some limitations. Firstly, although the results were adjusted for a priori defined confounders, residual confounding could still have influenced the results. Secondly, our predefined definitions were arbitrarily chosen non-individualized definitions. Thus, the bp thresholds were not based on patient's individualized cerebral autoregulatory curves. However, there are studies in which patients managed at bps during CPB below their cerebral autoregulatory range, may have an increased risk of postoperative major morbidity or operative mortality.47 Thirdly, we studied patients who received treatment for hypotension. Although we adjusted for intraoperative vasopressor and inotrope use, it is unknown in which direction and to what extent these drugs influenced the association of IOH and the occurrence of delirium. Fourthly, we assumed that the effect of hypotension was constant over time and that effect modification did not play a role. Finally, we did not register which form of delirium was present when delirium assessment was performed with the CAM(-ICU). Therefore, we might have patients in the immediate postoperative period, particularly with a hypoactive presentation. This might have led to an underestimation of delirium incidence and thus to a bias towards the null hypothesis and subsequent lack of statistical significant findings in this study. For example, the effect of hypotension during induction of anaesthesia was presumed to be similar to the consequences of hypotension later on during surgery. However, in this study we could not discriminate between different parts of surgery(i.e. before, during and after CPB or postoperatively).
The results of the present study have potential clinical implications for intraoperative bp management. Our results suggest that there is no absolute or relative cut-off point for low bp during cardiac surgery, below which the bp should definitely be treated to prevent postoperative delirium. Future studies should focus on autoregulation of cerebral perfusion during cardiac surgery, to increase our understanding of IOH and cerebral perfusion. In these studies a more heterogeneous study population could be analysed, for example patients undergoing non-cardiac surgery.
In conclusion, independent of the applied definition, IOH was not associated with the occurrence of delirium after cardiac surgery. A small number of patients with prolonged and/or deep hypotension had an increased risk to develop postoperative delirium, but this risk did not reach statistical significance.
Authors' contributions
Study design/planning: E.M.W., T.H.K., W.A.v.K., A.J.C.S.
Study conduct: E.M.W., T.H.K., J.M.D., D.v.D.
Data analysis: E.M.W., T.H.K., W.A.v.K., A.J.C.S.
Writing paper: E.M.W., T.H.K., W.A.v.K., J.M.D., D.v.D., A.J.C.S.
Revising paper: all authors
Declaration of interest
None declared.
Funding
This work was supported by departmental funding.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edn Text revision Washington, DC: American Psychiatric Association, 2000; 135–47 [Google Scholar]
- 2.Koster S, Hensens AG, Schuurmans MJ, van der Palen J. Consequences of delirium after cardiac operations. Ann Thorac Surg 2012; 93: 705–11 [DOI] [PubMed] [Google Scholar]
- 3.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med, 2012; 367: 30–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg 2009; 249: 173–8 [DOI] [PubMed] [Google Scholar]
- 5.Siepe M, Pfeiffer T, Gieringer A, et al. Increased systemic perfusion pressure during cardiopulmonary bypass is associated with less early postoperative cognitive dysfunction and delirium. Eur J Cardiothorac Surg 2011; 40: 200–7 [DOI] [PubMed] [Google Scholar]
- 6.Roach GW, Kanchuger M, Mangano CM, et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter study of perioperative ischemia research group and the ischemia research and education foundation investigators. N Engl J Med 1996; 335: 1857–63 [DOI] [PubMed] [Google Scholar]
- 7.Eriksson M, Samuelsson E, Gustafson Y, Aberg T, Engström KG. Delirium after coronary bypass surgery evaluated by the organic brain syndrome protocol. Scand Cardiovasc J 2002; 36: 250–5 [DOI] [PubMed] [Google Scholar]
- 8.Norkiene I, Ringaitiene D, Misiuriene I, et al. Incidence and precipitating factors of delirium after coronary artery bypass grafting. Scand Cardiovasc J 2007; 41: 180–5 [DOI] [PubMed] [Google Scholar]
- 9.Rudolph JL, Jones RN, Grande LJ, et al. Impaired executive function is associated with delirium after coronary artery bypass graft surgery. J Am Geriatr Soc 2006; 54: 937–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudolph JL, Inouye SK, Jones RN, et al. Delirium: an independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc 2010; 58: 643–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brady K, Hogue CW. Intraoperative hypotension and patient outcome - Does “One Size Fit All?” Anesthesiology 2013; 119: 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burkhart CS, Rossi A, Dell-Kuster S. Effect of age on intraoperative cerebrovascular autoregulation and near-infrared spectroscopy-derived cerebral oxygenation. Br J Anaesth 2011; 107: 742–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bijker JB, Van Klei WA, Kappen TH, Van Wolfswinkel L, Moons KGM, Kalkman CJ. Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology 2007; 107: 213–20 [DOI] [PubMed] [Google Scholar]
- 14.Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg 2005; 100: 4–10 [DOI] [PubMed] [Google Scholar]
- 15.Bijker JB, van Klei WA, Vergouwe Y, et al. Intraoperative hypotension and 1-year mortality after noncardiac surgery. Anesthesiology 2009; 111: 1217–26 [DOI] [PubMed] [Google Scholar]
- 16.Lienhart A, Auroy Y, Péquignot F, et al. Survey of anesthesiarelated mortality in France. Anesthesiology 2006; 105: 1087–97 [DOI] [PubMed] [Google Scholar]
- 17.Walsh M, Kurz A, Turan A, et al. Relationship between intraoperative mean. Anesthesiology 2013; 119: 507–15 [DOI] [PubMed] [Google Scholar]
- 18.Bijker JB, Persoon S, Peelen L, et al. Intraoperative hypotension and perioperative ischemic stroke after general surgery. Anesthesiology 2012; 116: 658–64 [DOI] [PubMed] [Google Scholar]
- 19.Leonardi-Bee J. Blood pressure and clinical outcomes in the international stroke trial. Stroke 2002; 33: 1315–20 [DOI] [PubMed] [Google Scholar]
- 20.Likosky DS, Marrin CAS, Caplan LR, et al. Determination of etiologic mechanisms of strokes secondary to coronary artery bypass graft surgery. Stroke 2003; 34: 2830–4 [DOI] [PubMed] [Google Scholar]
- 21.Limburg M, Wijdicks EFM, Li H. Ischemic stroke after surgical procedures: Clinical features, neuroimaging, and risk factors. Neurology 1998; 50: 895–901 [DOI] [PubMed] [Google Scholar]
- 22.Dieleman JM, Rosseel PM, Hofland J, et al. Intraoperative high-dose dexamethasone for cardiac surgery. JAMA 2012; 308: 1761–7 [DOI] [PubMed] [Google Scholar]
- 23.Van Haelst IMM, van Klei WA, Doodeman HJ, Kalkman CJ, Egberts TCG. Antidepressive treatment with monoamine oxidase inhibitors and the occurrence of intraoperative hemodynamic events: a retrospective observational cohort study. J Clin Psychiatry 2012; 73: 1103–9 [DOI] [PubMed] [Google Scholar]
- 24.Van Haelst IMM, Van Klei WA, Doodeman HJ, Kalkman CJ, Egberts TCG. Selective serotonin reuptake inhibitors and intraoperative blood pressure. Am J Hypertens 2012; 25: 223–8 [DOI] [PubMed] [Google Scholar]
- 25.Sauër AM, Slooter AJC, Veldhuijzen DS, van Eijk MM, Devlin JW, van Dijk D. Intraoperative dexamethasone and delirium after cardiac surgery: A randomized clinical trial. Anesth Analg 2014; 119: 1046–52 [DOI] [PubMed] [Google Scholar]
- 26.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004; 291: 1753–62 [DOI] [PubMed] [Google Scholar]
- 27.Inouye SK, Van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann of Intern Med 1990; 113: 941–8 [DOI] [PubMed] [Google Scholar]
- 28.Lin Y, Chen J, Wang Z. Meta-analysis of factors which influence delirium following cardiac surgery. J Card Surg 2012; 27: 481–92 [DOI] [PubMed] [Google Scholar]
- 29.Bakker RC, Osse RJ, Tulen JHM, Kappetein AP, Bogers AJJC. Preoperative and operative predictors of delirium after cardiac surgery in elderly patients. Eur J Cardiothorac Surg 2012; 41: 544–9 [DOI] [PubMed] [Google Scholar]
- 30.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999; 16: 9–13 [DOI] [PubMed] [Google Scholar]
- 31.Yared JP, Starr NJ, Torres FK, et al. Effects of single dose, postinduction dexamethasone on recovery after cardiac surgery. Ann Thorac Surg 2000; 69: 1420–4 [DOI] [PubMed] [Google Scholar]
- 32.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA 1996; 275: 852–7 [PubMed] [Google Scholar]
- 33.Chaput AJ, Bryson GL. Postoperative delirium: risk factors and management: continuing professional development. Can J Anaesth 2012; 59: 304–20 [DOI] [PubMed] [Google Scholar]
- 34.Koster S, Hensens AG, Schuurmans MJ, van der Palen J. Risk factors of delirium after cardiac surgery: a systematic review. Eur J Cardiovasc Nurs 2011; 10: 197–204 [DOI] [PubMed] [Google Scholar]
- 35.Inouye SK. Delirium in older persons. N Engl J Med 2006; 354: 1157–65 [DOI] [PubMed] [Google Scholar]
- 36.Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry 2000; 5: 132–48 [DOI] [PubMed] [Google Scholar]
- 37.Rudolph JL, Ramlawi B, Kuchel GA, et al. Chemokines are associated with delirium after cardiac surgery. J Gerontol A Biol Sci Med Sci 2008; 63: 184–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kazmierski J, Banys A, Latek J, Bourke J, Jaszewski R. Raised IL-2 and TNF-α concentrations are associated with postoperative delirium in patients undergoing coronary-artery bypass graft surgery. Int Psychogeriatr 2014; 26: 845–55 [DOI] [PubMed] [Google Scholar]
- 39.Kazmierski J, Banys A, Latek J, Bourke J, Jaszewski R. Cortisol levels and neuropsychiatric diagnosis as markers of postoperative delirium: a prospective cohort study. Crit Care 2013; 17: R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mu DL, Wang DX, Li LH, et al. High serum cortisol level is associated with increased risk of delirium after coronary artery bypass graft surgery: a prospective cohort study. Crit Care 2010; 14: R238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plaschke K, Fichtenkamm P, Schramm C, et al. Early postoperative delirium after open-heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin-6. Intensive Care Med 2010; 36: 2081–9 [DOI] [PubMed] [Google Scholar]
- 42.Fong TG, Bogardus ST, Daftary A, et al. Cerebral perfusion changes in older delirious patients using 99mTc HMPAO SPECT. J Gerontol Sci Med Sci 2006; 61: 1294–9 [DOI] [PubMed] [Google Scholar]
- 43.Palmbergen WAC, Van Sonderen A, Keyhan-Falsafi AM, Keunen RWM, Wolterbeek R. Improved perioperative neurological monitoring of coronary artery bypass graft patients reduces the incidence of postoperative delirium: the Haga Brain Care Strategy. Interact Cardiovasc Thorac Surg 2012; 15: 671–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marcantonio ER, Goldman L, Orav EJ, Cook EF, Lee TE. The association of intraoperative factors with the development of postoperative delirium. Am J Med 1998; 105: 380–4 [DOI] [PubMed] [Google Scholar]
- 45.Patti R, Saitta M, Cusumano G, Termine G, Di Vita G. Risk factors for postoperative delirium after colorectal surgery for carcinoma. Eur J Oncol Nurs 2011; 15: 519–23 [DOI] [PubMed] [Google Scholar]
- 46.Tognoni P, Simonato A, Robutti N, et al. Preoperative risk factors for postoperative delirium (POD) after urological surgery in the elderly. Arch Gerontol Geriatr, 2011; 52: 166–9 [DOI] [PubMed] [Google Scholar]
- 47.Ono M, Brady K, Easley RB, et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg 2014; 147: 483–9 [DOI] [PMC free article] [PubMed] [Google Scholar]