Abstract
STUDY QUESTION
What are the effects of fatty acids on placental inflammatory cytokine with respect to toll-like receptor-4/nuclear factor-kappa B (TLR4/NF-kB)?
SUMMARY ANSWER
Exogenous fatty acids induce a pro-inflammatory cytokine response in human placental cells in vitro via activation of TLR4 signaling pathways.
WHAT IS KNOWN ALREADY
The placenta is exposed to changes in circulating maternal fatty acid concentrations throughout pregnancy. Fatty acids are master regulators of innate immune pathways through recruitment of toll-like receptors and activation of cytokine synthesis.
STUDY DESIGN, SIZE, DURATION
Trophoblast cells isolated from 14 normal term human placentas were incubated with long chain fatty acids (FA) of different carbon length and degree of saturation. The expression and secretion of interleukin-6 (IL-6), IL-8 and tumor necrosis factor-alpha (TNF-α) were measured by reverse transcription-polymerase chain reaction and enzyme-linked immunosorbent assay. Antibodies against TLR4 ligand binding domain, downstream signaling and anti-p65 NFkB-inhibitor were used to characterize the pathways of FA action.
PARTICIPANTS/MATERIALS, SETTING, METHODS
General approach used primary human term trophoblast cell culture. Methods and end-points used real-time quantitative PCR, cytokine measurements, immunohistochemistry, western blots.
MAIN RESULTS AND THE ROLE OF CHANCE
The long chain saturated fatty acids, stearic and palmitic (PA), stimulated the synthesis as well as the release of TNF-α, IL-6 and IL-8 by trophoblast cells (2- to 6-fold, P < 0.001). In contrast, the unsaturated (palmitoleic, oleic, linoleic) acids did not modify cytokine expression significantly. Palmitate-induced inflammatory effects were mediated via TLR4 activation, NF-kB phosphorylation and nuclear translocation.
LIMITATIONS, REASONS FOR CAUTION
TNF-α protein level was close to the limit of detection in the culture medium even when cells were cultured with PA.
WIDER IMPLICATIONS OF THE FINDINGS
These mechanisms open the way to a better understanding of how changes in maternal lipid homeostasis may regulate placental inflammatory status.
STUDY FUNDING/COMPETING INTEREST(S)
X.Y. was recipient of fellowship award from West China Second University Hospital, Sichuan University (NIH HD 22965-19). The authors have nothing else to disclose.
TRIAL REGISTRATION NUMBER
None.
Keywords: fatty acids, trophoblasts, toll-like receptor 4, NF-kB, cytokine, human, placenta, inflammation
Introduction
Fatty acids (FA) exert multiple regulatory roles in the distribution of energy during the course of pregnancy (Herrera, 2002). At early developmental stages, long chain fatty acids impact oocyte maturation (McKeegan and Sturmey, 2011) and, later in gestation, they contribute to the establishment of physiological insulin resistance (Sivan et al., 1998). Fatty acids adversely impact pregnancy outcomes (Chen and Scholl, 2008; Larqué et al., 2012). Modifications in the amount or in the composition of FA availability may contribute to metabolic programming of the fetus (Innis, 2011). At the end of pregnancy, the long chain oleic and palmitic acids constitute ∼60% of the non-esterified fatty acids present in the maternal circulation (Villa et al., 2009). The placenta transports as much as 50% of the daily fatty acid requirement and all of the essential fatty acids required for fetal development (Kalkhoff, 1991; Haggarty et al., 1997; Larqué et al., 2012). The mechanisms of FA transport and metabolism by the human placenta have started to be characterized (Gil-Sánchez et al., 2012). However, the relative contribution of maternal circulating FA versus placental derived FA to fetal supply has yet to be elucidated (Szabo et al., 1973; Coleman, 1986).
At the cellular level, FAs act as key messengers and modulator molecules of several signaling transduction pathways (Shi et al., 2006). A large body of literature has documented cross-talk between dietary fatty acids and the innate immune system (Lee and Hwang, 2006). For example, FAs activate the toll-like receptor-4 (TLR4) signaling in monocytes and adipocytes (Schaeffler et al., 2009). Mice lacking TLR4 do not develop insulin resistance on a high-fat diet (Shi et al., 2006). At the molecular level, the activation of TLR4 leads to the recruitment of NF-kB, and increased synthesis of several chemokines and inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-8 (IL-8) (Medzhitov et al., 1997). The induction of TLR4 pathways by FAs varies with the length of their carbon chain and degree of saturation (Lee et al., 2001). The placenta is exposed to acute and chronic changes in systemic concentrations of a variety of saturated and unsaturated FAs in relation to maternal diet and lifestyle (Hwang and Rhee, 1999). The high level of expression of TLR4 on human placental membranes (Beijar et al., 2006) prompted us to investigate whether FAs promote placental inflammation. Toward this aim, the effects of FAs on cytokine synthesis and secretion were investigated with respect to toll-like receptor-4/nuclear factor-kappa B (NF-kB) pathways in trophoblast cells isolated from human term placenta.
Materials and Methods
Human subjects
Fourteen pregnant women with uncomplicated pregnancies were recruited at term scheduled Cesarean delivery. Women with multiple gestation, fetal anomalies, intrauterine growth restriction and diabetes (pre-existing and gestational) were excluded. Informed consent was obtained from the pregnant women according to the Institutional Research Board protocol approved by MetroHealth Medical Center (IRB05-288).
Human trophoblast cell isolation and cell culture
Placenta were collected within 5 min of delivery and immediately processed for cell isolation. Human trophoblast cells were isolated by sequential trypsin and DNase digestion followed by gradient centrifugation (Kliman et al., 1986). Cells were plated into 12-well plates at a density of 1.5 × 106 cells/well and cultured overnight in Iscoves's modified Dulbecco's Modified Eagle's Medium (DMEM) culture medium supplemented with 10%(v/v) fetal bovine serum (FBS) and 1%(v/v) penicillin/streptomycin at 37°C under 5% CO2. After 16 h plating, the medium was replaced with 1 ml DMEM with 1% FBS 2 h before 500 μM fatty acids (FAs) or 100 ng/ml lipopolysaccharide (LPS) were added for 24 h. FAs (Sigma, St. Louis, USA) were dissolved in 2% fatty acids free bovine serum albumin (BSA) (Sigma) with the molar ratio of 1:1. Inhibition of TLR4 signaling was achieved by adding antagonists of TLR4 pathways to the culture medium 1 h prior to FA stimulation. Antibodies against TLR4 extracellular binding domain (H-80, Santa Cruz, Dallas, USA), intracellular binding domain (CLi-095, Invitrogen, Grand Island, USA), and NF-kB translocation (EMD Millipore Corporation, Billerica, USA) were added at final concentrations of 100 mM, 200 µg/ml and 5 µg/µl, respectively. Medium with PBS alone or PBS with 2% BSA was used as controls. LPS, FAs and BSA were from Sigma. BSA was fatty acid free. Cell viability was estimated by lactate dehydrogenase (LDH) cytotoxicity assay (Cayman, Ann Arbor, USA).
Immunofluorescence
Freshly isolated trophoblast cells were plated on culture chamber slides and grown with 500 µM palmitic acid (PA) or 100 ng/ml LPS as a positive control. At designated time points, the cells were rinsed with PBS and fixed with ice-cold methanol for 10 min. The slides were incubated overnight at 4°C with antibodies to TLR4 (1:50, rabbit polyclonal antibody, Santa Cruz, H-80) and Cytokeratin 7 (1:2000, mouse monoclonal antibody, Dako, M7018, Carpinteria, USA). Alexa Fluor 488 and 594 secondary antibodies (1:1000, Jackson Immunoresearch, West Grove, USA) were used for visualization. The slides were imaged with Leica Microsystem SP2 Confocal Microscope. For NF-kb translocation experiments, cells were fixed after 1 h treatment, blocked with phosphate buffered saline (PBS)/1% BSA/0.3% Triton X-100/5% normal donkey serum and incubated overnight with anti-NF-kB antibody (1:200, Cell Signaling, #8242, Danvers, USA), followed by Alexa Fluor 488 secondary antibody. Nuclei were stained with To-Pro 3 (Sigma).
RNA preparation and real-time PCR
Total RNA was extracted from 1.5 × 106 primary cultured cells using TRIzol (Invitrogen). RNA was reversed-transcribed using a Superscript II RNase H-Reverse Transcriptase system (Invitrogen). Gene expression was measured by real-time PCR (RT–PCR) using a Roche thermal cycler (Roche Applied Science, Indianapolis, USA) with Lightcycler Fast–start DNA Sybr Green 1 master mix and primers from Integrated DNA Technologies (Coralville, IA, USA). Specific primers were designed as: TLR4 (NG-011475) forward: 5′-CCCACCACTCACCAGCTAAT-3′, reverse: 5′-GCCCTGTGGTTCAGAGAAAG-3′; IL-6 (NM_000600) forward: 5′-TACCCCCAGGAGAAGATTCC-3′, reverse: 5′-TTTTCTGCCAGTGCCTCTTT-3′; IL-8 (NM_000584) forward: 5′-GTGCAGTTTTGCCAAGGAGT-3′, reverse: 5′-CTCTGCACCCAGTTTTCCTT-3′; TNF-alpha (NM_000594) forward: 5′-TCCTTCAGACACCCTCAACC-3′, reverse: 5′-AGGCCCCAGTTTGAATTCTT-3′; Caspase 3 (NM_004346) forward: 5′-TTTTTCAGAGGGGATCGTTG-3′, reverse: 5′-CGGCCTCCACTGGTATTTTA-3′; Caspase 9 (NM_001229) forward: 5′-CATGTTTGCCCACACCCAGT-3′, reverse: 5′-GCATTAGCGACCCTAAGCAG-3′; β-actin (NM_001101) forward: 5′-GGACTTCGAGCAAGAGATGG-3′, reverse: 5′-AGCACTGTGTTGGCGTACAG-3′. Results obtained with comparative Ct method were normalized for actin and expressed as fold changes between treated and control groups.
Protein detection by western blot and enzyme-linked immunosorbent assay
Total protein lysates were prepared by homogenization of cultured trophoblasts 1.5 × 106 cells/50 µl buffer (10 mM Tris pH 8, 130 mM NaCl, 1% Triton X-100, 10 mM NaF, 10 mM Sodium phosphate (NaPi), 10 mM Sodium pyrophosphate (NaPPi)) with protease inhibitors (Sigma, P8340), and centrifuged at 13 000g for 10 min at 4°C. Protein concentrations were measured with a bicinchoninic acid protein assay kit (Pierce). 100 µg total protein per well were loaded on 7.5% sodium dodecyl sulphate (SDS)-running gel (Bio-Rad) and transferred to nitrocellulose filter (Invitrogen). The membrane was blocked with 5% nonfat milk for 1 h, incubated with rabbit polyclonal TLR4 (1:200, Santa Cruz, H-80), NF-kB p65 (1:2000, Cell Signaling, #8242), phospho-NF-κB p65(Ser536)(93H1) (1:1000, Cell Signaling, #3033) and β-actin (1:2000, Abcam, Cambridge, USA) overnight then secondary antibodies 1:2000 and 1:6000 for 1 h. Amersham ECL Plus Western blotting Reagents (GE Healthcare, Pittsburgh, PA, USA) was used for detection. Densitometric data of autoradiograms were quantified by Image J. IL-6, IL-8 and TNF-α concentration in cell lysates and culture medium were measured by enzyme-linked immunosorbent assay (ELISA) (R&D System, HS600B, HS800, and HSTA00D, S6050, S8000C, and STA00C, Minneapolis, USA).
Statistical analysis
Means ± standard errors of the mean (SEM) were used in figures. Differences among dependent variables were analyzed by one-way ANOVA. Statistical mean differences for RT–PCR analysis were calculated by Kruskal–Wallis for non-parametric data. Statistical significance was set at P < 0.05.
Results
Maternal anthropometric parameters
The pregnant women recruited in this study were 27.6 ± 5.0 years old and their pre-gravid body mass index was 27.6 ± 5.9 kg/m2. The neonatal anthropometrics were as follows, gestational age: 38.9 ± 0.3 weeks, placental weight: 615 ± 86 g and birthweight: 3.5 ± 0.2 kg.
Effect of palmitic and oleic acid on inflammatory cytokine expression
The optimum conditions for FA stimulation of human primary trophoblasts were investigated using the long chain saturated palmitic (C16:0) and unsaturated oleic acid (C18:1), the most abundant fatty acids in placental phospholipid membranes (Kien et al., 2014). Palmitic acid induced a dose- and time-dependent stimulation of IL-6, IL-8 and TNF-α mRNA expression in trophoblast cells which reached a maximum (25 fold versus control) with 500 μM for 24 h (P < 0.001). (Fig. 1A and B). In contrast, oleic acid did not induce a significant stimulation of pro-inflammatory cytokine expression. Cells cultured with 500 μM palmitic acid for 24 h did not exhibit changes in caspase 3, caspase 9 expression nor LDH concentration in culture medium (Fig. 2) suggesting that these experimental conditions do not induce apoptosis or loss of trophoblast cell viability.
Figure 1.
Effect of palmitic (PA) and oleic (OA) acids on cytokine expression in cultured human trophoblast cells. (A) Trophoblast cells were cultured in the presence of increasing concentrations of PA or OA for 24 h. (B) Trophoblast cells were cultured in the presence of 500 µM PA or OA for 6, 12, 24 or 72 h. Lipopolysaccharide (LPS) 100 ng/ml was used as a positive control. Cytokine mRNA was measured by RT–PCR of the cell extracts. Results were normalized with beta-actin and expressed as fold changes PA versus control (CTL; cells treated with 2% bovine serum albumin). Shown are mean ± SEM, n = 3 independent experiments from three placentas. 1.5 × 106/well primary cultured cells were plated in 12-well plate for each condition, *P < 0.05.
Figure 2.
Effect of palmitic (PA) and oleic (OA) acids on markers of apoptosis. Trophoblast cells were treated for 24 h with 500 µM PA or OA. (A) Caspase3 and caspase 9 mRNA were measured by RT–PCR of the cell extracts. Results were normalized for beta-actin and expressed as fold changes versus control (CTL; cells treated with 2% BSA). Shown are means ± SEM, n = 3 placenta with triplicate wells/condition. 1.5 × 106/well primary cultured cells were plated in 12-well plate for each condition. There were no significant differences between control and fatty acid (FA) treated groups. (B) Lactate dehydrogenase (LDH) activities were measured in the culture medium to test the cytotoxicity resulting from 500 µM PA and OA by Cayman's LDH Cytotoxicity Assay Kit. Shown are means ± SEM, n = 6 placenta with duplicate wells/condition. There were no significant difference between CTL and FA treated groups.
Effect of saturated and unsaturated FAs on pro-inflammatory cytokine expression
The inflammatory effects of fatty acids were analyzed as a function of the carbon chain length and degree of saturation. The saturated palmitic (C16:0) and stearic (C18:0) acids significantly increased IL-6, IL-8, TNF-α and TLR4 mRNA expression by 4- to 10-fold over control levels (P < 0.05) (Fig. 3). At sharp contrast, FAs with the same carbon chain length but with one or two degrees of saturation, i.e. palmitoleic acid (POA; C16:1), oleic acid (OA; C18:1), linoleic acid (LA; C18:2) induced only a modest cytokine mRNA response which did not reach statistical significance (Fig. 3). The intensity of cytokine response was not statistically different when cells were isolated from placenta of lean and overweight women (data not shown). PA and SA significantly increased the protein concentration of all three cytokines in trophoblast cell lysates (Fig. 4A). PA treatment also enhanced the release of IL-8 and IL-6 in the culture medium (Fig. 4B). When expressed in absolute concentration the release of IL-8 protein from the PA treated cells (5000 pg/ml) was 10-fold higher than that of IL-6 (700 pg/ml). TNF-α protein level was close to the limit of detection in the culture medium even when cells were cultured with PA (Fig. 4A and B). TLR4 protein expression visualized by western blot and immunocytochemistry exhibited a significant activation upon PA stimulation of primary trophoblasts (Supplementary Fig. S1).
Figure 3.
Effect of fatty acids on toll-like receptor 4 (TLR4) and cytokine mRNA expression. Trophoblast cells were cultured with myristic acid (MA), palmitic acid (PA), stearic acid (SA), palmitoleic acid (POA), oleic acid (OA) or linoleic acid (LA), respectively, for 24 h at a concentration of 500 µM for MA, PA, SA, POA, OA and LA. Lipopolysaccharide (LPS) 100 ng/ml was used as positive control. Abundance of mRNA for toll-like receptor-4 (TLR4) (A), tumor necrosis factor-alpha (TNF-α) (B), interleukin-6 (IL-6) (C) and interleukin-8 (IL-8) (D). mRNA in trophoblast cells was measured by RT–PCR. Data were normalized for actin and calculated as fold changes versus Control (CTL; cells treated with 2% BSA for 24 h). Shown are means ± SEM, n = 14 independent experiments performed in triplicate, and 1.5 × 106/well primary cultured cells were plated in 12-well plate for each condition, *P < 0.05.
Figure 4.
Effect of fatty acids on cytokine synthesis and release. Trophoblast cells were cultured with lipopolysaccharide (LPS), palmitic acid (PA), stearic acid (SA), palmitoleic acid (POA) and oleic acid (OA) for 24 h. The final concentrations in the medium were 100 ng/ml for LPS, 500 µM for PA, SA, POA and OA. Cells treated without or with 2% BSA for 24 h were used as negative controls (CTL). Levels of IL-6, IL-8 and TNF-α in the medium (B) and cell lysates (A) were measured by enzyme-linked immunosorbent assay . Data are shown as means ± SEM, n = 6 (A) and 3 (B), and 1.5 × 106/well primary cultured cells were plated in 12-well plate for each condition, *P < 0.05 versus CTL.
Signaling pathways of FA-induced inflammation
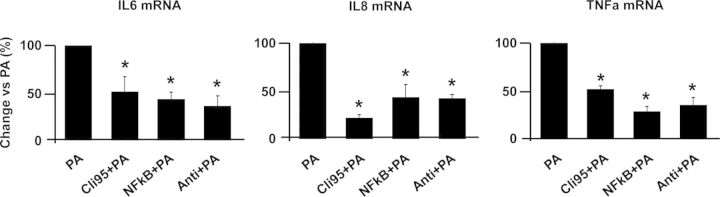
NF-κB phosphorylation was significantly increased after 24 h stimulation with PA (Fig. 5B). Immunostaining analysis confirmed that PA stimulated the translocation of NF-kb from the cytoplasm to the nucleus (Fig. 5A). To further investigate the signaling pathways activated by PA, trophoblast cells were incubated with a panel of specific inhibitors of TLR4 signaling and NF-kB translocation. Trophoblasts cultured with inhibitors of TLR4 signal transduction (CLi-095, and anti-TLR4 H-80) inhibited PA-induced IL-6, IL-8 and TNF-α mRNA levels by 25–50% (P < 0.05). The NF-kB translocation inhibitor inhibited PA induced cytokine expression by 40% (Fig. 6).
Figure 5.
Effect of fatty acids on phosphorylation and nuclear translocation of nuclear factor kappa Beta (NF-kB). Primary trophoblasts were cultured with 2% bovine serum albumin (BSA; CTL), 100 ng/ml lipopolysaccharide (LPS) or 500 µM palmitic acid (PA) for 60 min. (A) Immunohistochemistry shows CTL cells have no NF-kB in the nucleus but abundant staining in the cytoplasm. Arrows point to translocation of NF-kB from the cytoplasm to the nucleus after LPS and PA treatment, n = 3 independent experiments, 0.5 × 106/well primary cultured cells were plated in 4-well chamber slides for each condition; (B) Representative western blot of NF-kB (p65) and phospho-NF-κB p65 (p65p); (C) p65p protein phosphorylation was normalized per non phosphorylated NF-kB (p65), n = 3 triplicate experiments from three individual placentas. 3.0 × 106/well primary cultured cells were plated in 6-well plate for each condition.
Figure 6.
Effects of inhibitors of the toll-like receptor-4 (TLR4) signaling cascade. Trophoblast cells were cultured with 500 µM palmitic acid (PA) for 24 h after adding inhibitors of TLR4 signaling (100 mM CLi-095 or 200 µg/ml anti-TLR4 antibody) and nuclear factor kappa Beta (NFkB) signaling pathways (5 µg/µl anti p65) for 1 h. Interleukin-6 (IL-6), interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α) mRNA were measured by RT–PCR and data were normalized for beta actin. Results are shown as means ± SEM, n = 14, and 1.5 × 106/well primary cultured cells were plated in 12-well plate for each condition, *P < 0.05. PA: palmitic acid; CLi: CLi-095; anti NF: NF-kB inhibitor; anti: anti-TLR4 antibody.
Discussion
The primary findings of this study are that saturated FAs induced an innate immune response in human placental cells. We demonstrated that in vitro treatment of trophoblast cells with palmitic and stearic acid stimulated the synthesis and release of three major inflammatory cytokines IL-6, IL-8 and TNF-α. These data show that the placenta exhibits intrinsic mechanisms to sense changes in circulating fatty acid composition and reacts by generating an inflammatory reaction cascade. These data suggest that the functional pathways linking lipid homeostasis to inflammation in term human placenta are similar to the adipose tissue and the pancreatic beta cell (Schaeffler et al., 2009; Eguchi et al., 2012). The magnitude of FA induced inflammation is in line with the chronic low grade (sterile) inflammation we have described in vivo in pregnancy (Challier et al., 2008; Basu et al., 2011) rather than with an acute septic reaction to foreign pathogens like LPS (Fig. 4). Our findings support previous data that FA induced placental lipid accumulation correlates with cytokine production (Pathmaperuma et al., 2010). The different inflammatory properties of long chain saturated versus unsaturated FA have been previously documented in other systems (De Jong et al., 2014). Unsaturated FAs do not classically generate inflammation, and can even display potent anti-inflammatory properties (Calder, 2013; Delmastro-Greenwood et al., 2014). Several mechanisms have been proposed to explain the opposite properties of long chain saturated and unsaturated FA. Compared with their saturated counterparts, the unsaturated FAs are readily oxidized in the mitochondria and promote release of anti-inflammatory mediators (Eaton, 2002). Additionally, unsaturated FA are ligands for peroxisome proliferator-activated receptors (PPARs), which have the potential to bind to promoters of anti-inflammatory genes (Sessler and Ntambi, 1998). Along this line, oleate can provide protection from palmitate-induced inflammation by promoting genes regulating mitochondrial β-oxidation as well as by shunting excess palmitate through inhibition of the PKCθ-NFκB cascade (Forman et al., 1997).
TLR4 is a pattern recognition receptor that plays a key role in initializing innate immune responses. The coordinated induction of cytokine expression and other immune-related genes through activation of TLR4 has been described in several models and cell lines in response to LPS (Poltorak et al., 1998; Shimazu et al., 1999; Pietsch et al., 2006). This study highlights primary mechanisms of FA action in the placenta through TLR4-dependent phosphorylation and nuclear translocation of NF-kB p65. The longer time course for FA compared with LPS effect (Fig. 1) suggests that FAs may act in the cell via intermediary modulator or second messenger (Haus et al., 2009; Schilling et al., 2013).
In normal pregnancy, maternal metabolism is characterized by lower glycemia phosphorylation of to pregravid and physiological hyperlipidemia in the third trimester (Potter and Nestel, 1979). We have shown that these metabolic traits are exacerbated in obese pregnant women and associated with increased levels of placental inflammation (Challier et al., 2008; Basu et al., 2011). Furthermore, elevated FA have been shown to be associated with adverse pregnancy outcomes including pre-term delivery (Chen and Scholl, 2008), intrauterine growth retardation, pre-eclampsia (Alvino et al., 2008) and excess fetal adiposity. The current study brings to light novel mechanisms of physiological inflammatory sensing at the maternal-fetal interface. Diet fluctuations in humans are reflected in the concentration of FA in plasma erythrocytes and adipose tissue (Field et al., 1985; Skeaff et al., 2006; Kien et al., 2014). However, few studies have described the effect of maternal dietary intake on the FA composition of the placenta (Lakin et al., 1998). Many placental FA carriers or transporters have been identified (Gil-Sánchez et al., 2012). Unfortunately, to date there is little data relating the specificity of these proteins to a given FA or even class of FA. Only 3–4% of total FA-uptake in the placenta is derived from free-FAs. Majority of FAs are taken up after hydrolysis of triglycerides associated with lipoproteins. Our hypothesis is that the hydrolysis of TG releases a variety of FA (carbon length, degree of saturation, etc.) which upon uptake by the trophoblast cells may initiate intracellular cascade of events. The 500 μM FA concentration used in our in vitro cultured trophoblasts is in the order of magnitude of maternal plasma FA concentration, i.e. 400–600 μM after an overnight fast in pregnant women (Phelps et al., 1981). Hence, our data suggest that fluctuations in maternal lipid homeostasis may influence inflammatory placental status also within physiological conditions.
In conclusion, saturated FAs, primarily palmitic acid and stearic acid play a dynamic role in initiating innate immune responses in the placenta. Based on our findings, further in vivo studies may open the way toward practical dietary management in pregnancy.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
X.Y. contributed to experiments design, data generating, analysis and manuscript writing. M.H. contributed to data generating. P.G. contributed to immunostaining and imaging. J.M. contributed to patients recruiting and trophoblasts isolation. P.M.C. contributed to manuscript revision. S.H.-d.M. contributed to manuscript writing and revision as corresponding author.
Funding
X.Y. was recipient of fellowship award from West China Second University Hospital, Sichuan University (NIH HD 22965-19).
Conflict of interest
None declared.
Supplementary Material
References
- Alvino G, Cozzi V, Radaelli T, Ortega H, Herrera E, Cetin I. Maternal and fetal fatty acid profile in normal and intrauterine growth restriction pregnancies with and without preeclampsia. Pediatr Res 2008;64:615–620. [DOI] [PubMed] [Google Scholar]
- Basu S, Haghiac M, Surace P, Challier JC, Guerre-Millo M, Singh K, Waters T, Minium J, Presley L, Catalano PM, et al. Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity (Silver Spring) 2011;19:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijar EC, Mallard C, Powell TL. Expression and subcellular localization of TLR-4 in term and first trimester human placenta . Placenta 2006;27:322–326. [DOI] [PubMed] [Google Scholar]
- Calder PC. n-3 fatty acids, inflammation and immunity: new mechanisms to explain old actions. Proc Nutr Soc 2013;72:326–336. [DOI] [PubMed] [Google Scholar]
- Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, Hauguel-de Mouzon S. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 2008;29:274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Scholl TO. Association of elevated free fatty acids during late pregnancy with preterm delivery. Obstet Gynecol 2008;112:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA. Placental metabolism and transport of lipid. Fed Proc 1986;45:2519–2523. [PubMed] [Google Scholar]
- De Jong AJ, Kloppenburg M, Toes RE, Ioan-Facsinay A. Fatty acids, lipid mediators, and T-cell function. Front Immunol 2014;5:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmastro-Greenwood M, Freeman BA, Wendell SG. Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids. Annu Rev Physiol 2014;76:79–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton S. Control of mitochondrial beta-oxidation flux. Prog Lipid Res 2002;41:197–239. [DOI] [PubMed] [Google Scholar]
- Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, Yagi N, Ohto U, Kimoto M, Miyake K, et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab 2012;15:518–533. [DOI] [PubMed] [Google Scholar]
- Field CJ, Angel A, Clandinin MT. Relationship of diet to the fatty acid composition of human adipose tissue structural and stored lipids. Am J Clin Nutr 1985;42:1206–1220. [DOI] [PubMed] [Google Scholar]
- Forman BM, Chen J, Evans R. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA 1997;94:4312–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Sánchez A, Koletzko B, Larqué E. Current understanding of placental fatty acid transport. Curr Opin Clin Nutr Metab Care 2012;15:265–272. [DOI] [PubMed] [Google Scholar]
- Haggarty P, Page K, Abramovich DR, Ashton J, Brown D. Long-chain polyunsaturated fatty acid transport across the perfused human placenta. Placenta 1997;18:635–642. [DOI] [PubMed] [Google Scholar]
- Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, Kirwan JP. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 2009;58:337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera E. Implications of dietary fatty acids during pregnancy on placental, fetal and postnatal development--a review. Placenta 2002;23(Suppl A):S9–S19. [DOI] [PubMed] [Google Scholar]
- Hwang D, Rhee SH. Receptor-mediated signaling pathways: potential targets of modulation by dietary fatty acids. Am J Clin Nutr 1999;70:545–556. [DOI] [PubMed] [Google Scholar]
- Innis SM. Metabolic programming of long-term outcomes due to fatty acid nutrition in early life. Matern Child Nutr 2011;7(Suppl 2):112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkhoff RK. Impact of maternal fuels and nutritional state on fetal growth. Diabetes 1991;40(Suppl 2):61–65. [DOI] [PubMed] [Google Scholar]
- Kien CL, Bunn JY, Stevens R, Bain J, Ikayeva O, Crain K, Koves TR, Muoio DM. Dietary intake of palmitate and oleate has broad impact on systemic and tissue lipid profiles in humans. Am J Clin Nutr 2014;99:436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF. Purification, characterization and in vitro. Differentiation of cytotrophoblasts from human term placenta. Endocrinology 1986;118:1567–1582. [DOI] [PubMed] [Google Scholar]
- Lakin V, Haggarty P, Abramovich DR, Ashton J, Moffat CF, McNeill G, Danielian PJ, Grubb D. Dietary intake and tissue concentration of fatty acids in omnivore, vegetarian and diabetic pregnancy. Prostaglandins Leukot Essent Fatty Acids 1998;59:209–220. [DOI] [PubMed] [Google Scholar]
- Larqué E, Gil-Sánchez A, Prieto-Sánchez MT, Koletzko B. Omega 3 fatty acids, gestation and pregnancy outcomes. Br J Nutr 2012;107(Suppl 2):S77–S84. [DOI] [PubMed] [Google Scholar]
- Lee JY, Hwang DH. The modulation of inflammatory gene expression by lipids: mediation through Toll-like receptors. Mol Cells 2006;21:174–185. [PubMed] [Google Scholar]
- Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem 2001;276:16683–16689. [DOI] [PubMed] [Google Scholar]
- McKeegan PJ, Sturmey RG. The role of fatty acids in oocyte and early embryo development. Reprod Fertil Dev 2011;24:59–67. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997;388:394–397. [DOI] [PubMed] [Google Scholar]
- Pathmaperuma AN, Maña P, Cheung SN, Kugathas K, Josiah A, Koina ME, Broomfield A, Delghingaro-Augusto V, Ellwood DA, Dahlstrom JE, et al. Fatty acids alter glycerolipid metabolism and induce lipid droplet formation, syncytialisation and cytokine production in human trophoblasts with minimal glucose effect or interaction. Placenta 2010;31:230–239. [DOI] [PubMed] [Google Scholar]
- Phelps RL, Metzger BE, Freinkel N. Carbohydrate metabolism in pregnancy. XVII. Diurnal profiles of plasma glucose, insulin, free fatty acids, triglycerides, cholesterol, and individual amino acids in late normal pregnancy. Am J Obstet Gynecol 1981;140:730–736. [PubMed] [Google Scholar]
- Pietsch J, Batra A, Stroh T, Fedke I, Glauben R, Okur B, Zeitz M, Siegmund B. Toll-like receptor expression and response to specific stimulation in adipocytes and preadipocytes: on the role of fat in inflammation. Ann N Y Acad Sci 2006;1072:407–409. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCR mice: mutations in Tlr4 gene. Science 1998;282:2085–2088. [DOI] [PubMed] [Google Scholar]
- Potter JM, Nestel PJ. The hyperlipidemia of pregnancy in normal and complicated pregnancies. Am J Obstet Gynecol 1979;133:165–170. [DOI] [PubMed] [Google Scholar]
- Schaeffler A, Gross P, Buettner R, Bollheimer C, Buechler C, Neumeier M, Kopp A, Schoelmerich J, Falk W. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology 2009;126:233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling JD, Machkovech HM, He L, Sidhu R, Fujiwara H, Weber K, Ory DS, Schaffer JE. Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. J Biol Chem 2013;288:2923–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessler AM, Ntambi JM. Polyunsaturated fatty acid regulation of gene expression. J Nutr 1998;128:923–926. [DOI] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006;116:3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med 1999;189:1777–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan E, Homko CJ, Whittaker PG, Reece EA, Chen X, Boden G. Free fatty acids and insulin resistance during pregnancy. J Clin Endocrinol Metab 1998;83:2338–2342. [DOI] [PubMed] [Google Scholar]
- Skeaff CM, Hodson L, McKenzie JE. Dietary-induced changes in fatty acid composition of human plasma, platelet, and erythrocyte lipids follow a similar time course. J Nutr 2006;136:565–569. [DOI] [PubMed] [Google Scholar]
- Szabo AJ, De Lellis R, Grimaldi RD. Triglyceride synthesis by the human placenta. I. Incorporation of labelled palmitate into placental triglycerides. Obstet Gynecol 1973;115:257–262. [DOI] [PubMed] [Google Scholar]
- Villa PM, Laivuori H, Kajantie E, Kaaja R. Free fatty acid profiles in preeclampsia. Prostaglandins Leukot Essent Fatty Acids 2009;81:17–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.