Abstract
Background
A number of studies have linked neuropsychiatric symptoms to increase risk of dementia.
Objective
To determine if risk of conversion to mild cognitive impairment or dementia among healthy controls varied as a function of their pattern of neuropsychiatric symptoms.
Method
We studied individuals in the National Alzheimer Coordinating Center dataset collected from 34 Alzheimer Disease Centers between 2005 and 2013. The analysis included 4,517 volunteers who were ≥ 60 years old, cognitively normal, and had complete Neuropsychiatric Inventory data at their baseline visit, and had at least one follow-up. We used latent class analysis to identify 4 classes based on patterns of NPI symptoms. We used a cox proportional hazards model to determine if time to MCI or dementia varied by baseline latent class membership.
Results
We identified 4 latent classes of neuropsychiatric symptoms: irritable, depressed, complex (depression, apathy, irritability and nighttime behaviors) and asymptomatic. 873 participants converted to MCI or dementia. Hazard ratios for conversion by class were 1.76 (95% CI: 1.34, 2.33) for the irritable class, 3.20 (95% CI: 2.24, 4.58) for the complex class, and 1.90 (95% CI: 1.49, 2.43) for the depressed class, with the asymptomatic class as the reference.
Conclusions
Membership in all 3 symptomatic classes was associated with greater risk of conversion to MCI or dementia; the complex class had the greatest risk. Different patterns of neuropsychiatric symptoms may represent different underlying neuropathological pathways to dementia. Further work imaging and pathology research is necessary to determine if this is the case.
Search Terms: Alzheimer, dementia, neuropsychiatric symptoms, depression, latent class analysis
Introduction
The burden of Alzheimer’s disease (AD) is compounded by neuropsychiatric symptoms (NPS) which occur in almost all patients [1, 2], and are usually persistent [3], NPS are associated with accelerated cognitive and functional decline [4, 5], worse quality of life [6, 7], earlier institutionalization [8–10], and accelerated mortality [10, 11]. Depression, apathy, anxiety, agitation and irritability are the most commonly observed NPS [2, 12].
High rates of NPS (50–85%) are also reported in mild cognitive impairment (MCI) [2, 13–15]. and are a major predictor of dementia.[16, 17] Specific NPS linked to dementia incidence include depression [17–19], apathy [19–21], and nighttime behaviors [22–24]. Rates of NPS are generally lower among non-cognitively impaired individuals [13, 25], but depression in particular has been associated with dementia incidence [24, 26].
Mild behavioral impairment (MBI) is defined as behavioral change lasting six months or longer, in the absence of cognitive and functional impairment (excluding a diagnosis of dementia or MCI or a psychiatric diagnosis) [27]. One prospective outpatient cohort study found that MBI, in the absence of cognitive impairment or a psychiatric diagnosis, was associated with significant risk of dementia (68% to frontotemporal dementia) [27]. This study described the rates of specific NPS in the sample, but did not address the issue of which specific NPS or patterns of NPS are associated with cognitive decline and comprise the diagnosis of MBI.
As NPS are a major predictor of cognitive impairment and dementia, they may be helpful for identifying individuals to be targeted for dementia prevention trials [28]. Additionally, the NPS themselves may represent an important target for prevention development, if they are shown to be associated with pathology that subsequently results in dementia, but signal such pathology prior to the onset of any cognitive decline. To develop hypotheses regarding neurobiology of NPS, it is important to determine specifically which NPS, or patterns of NPS, are associated with dementia. Our aim was to assess whether there are specific patterns of NPS in cognitively normal individuals that are associated with conversion to MCI or dementia.
Latent class analyses of NPS in dementia have been published [29, 30], and may be useful for identifying groups with different underlying neuropathology. In prevalent AD cases, three classes were modeled: asymptomatic, predominantly affective, and one with psychotic symptoms [30]. A later analysis yielded similar results [29]. In the present study, latent class analysis was applied to elucidate classes among cognitively unimpaired volunteers, and hazard of conversion to MCI or dementia was modeled as a function of class membership. This approach is preferable to looking at each symptom separately, as it avoids multiple comparisons, and facilitates the identification of combinations or interactions among NPS that confer risk. We hypothesized that the majority would be members of an asymptomatic class, but that at least one cluster of symptomatic individuals would be identified. We hypothesized that hazard of conversion to MCI or dementia would vary as a function of latent class membership, and would be lowest in the asymptomatic class.
Materials and Methods
National Alzheimer Coordinating Center
The sample were volunteers classified as cognitively unimpaired at their first visits to 34 past and present Alzheimer disease centers (ADCs) [31]. Data were collected between September 2005 and August 2013. All ADCs were overseen by local IRBs and written informed consent was obtained. Volunteers were evaluated (in ADCs or at home) annually by trained clinicians. A full description of NACC methods and the Uniform Data Set (UDS) (including demographics, medical history, family history, behavioral and functional assessments, and a neuropsychological battery), is published elsewhere [32]. Race/ethnicity were based on subjects’ report [32–34].
2.2 Measures
The Neuropsychiatric Inventory-Questionnaire (NPI-Q) was administered to informants by trained and certified clinicians or health professionals; the administration instructions explicitly state that it should not be filled out by the participant themselves [34]. NPI-Q is a simplified clinical measure of dementia-related behavioral disturbances in 12 domains: agitation, delusions, hallucinations, depression, euphoria, aberrant motor behavior, apathy, irritability, disinhibition, anxiety, sleep, and eating [35]. Presence of each in the past month representing a change from baseline was measured as a dichotomous variable. The Mini-Mental Status Exam [36] and the 15-item Geriatric Depression Scale (GDS), a screening measure for depression in older adults were also administered [37].
Cognitive Impairment and Dementia Diagnoses
The majority of diagnoses were made via consensus conference, with the remainder made by a single physician, using all available data [34, 38]. Mild cognitive impairment (MCI) diagnoses were made using modified Petersen criteria [39, 40], Alzheimer disease (AD) diagnoses were made using NINCSD/ADRDA criteria [41]. Lewy body dementia (LBD) diagnoses were made using consortium criteria as described in McKeith et al, [42] vascular dementia diagnoses were made using NINDS/AIREN criteria, [43] and frontotemporal dementia (FTD) using criteria as described in Neary, et al [44].
Statistical Methods
Conversion was defined as incident MCI or dementia. Baseline comparisons between converters and non-converters used t-tests or chi-square tests. All tests were two-sided, assumed unequal variances, and used Satterthwaite’s approximation for degrees of freedom [45].
Our latent class analyses (LCA) used dichotomous ratings on each of the 12 NPI-Q domains. Domains were categorized as 0 (0) or 1 (>0). LCA posits the existence of underlying groups (classes) of individuals. LCA uses patterns of responses on categorical variables to estimate two sets of parameters: latent class probabilities and conditional probabilities. Latent class probabilities are the prevalences of each class. Conditional probabilities are rates of each observed variable given membership in each latent class. Using these estimates, it is possible to calculate each individual’s probability of membership in each latent class, given their symptom pattern, and their modal (mostly likely) class membership. We used bootstrapped likelihood ratio tests and BIC to choose the optimal number of classes [46].
LCA assumes that after conditioning on latent class membership, observed variables are independent [47]; the latent classes should explain the associations among the observed variables. We tested this assumption using standardized bivariate residuals [48]. Model fit was assessed by comparing observed and model-based counts of individuals for each symptom pattern.
We fit Cox proportional hazards models with latent classes as predictors and adjusting for baseline age, MMSE, sex, race, education, and current antidepressant use. Time to conversion was measured in years. We used an adaptation of Vermunt’s three-step method to correct for biases related to uncertainty in class membership, instead of fitting a joint latent class survival model. This was done to prevent survival status from affecting the estimation of the latent classes. [49, 50].
Our survival model allows for inclusion of right-censored individuals (those who have not failed), but inferences from these models assume that censorship is unrelated to failure [51]. In our case, this implies an assumption that individuals who have converted are as likely to attend follow-up visits as non-converters. This assumption could be violated both ways: impaired individuals might have more difficulty attending their next visit, but they might also be more motivated to be evaluated. This is a known limitation of the NACC [52], and many other longitudinal datasets [53]. We compared proportion of “administrative censoring” by class, as such censoring is generally considered to be non-informative. Individuals whose last visit was less than 24 months prior to the data freeze were considered administratively censored. We also compared lengths of follow-up by modal class assignment.
To compare the utility of latent class membership and NPI-Q total scores for prediction of conversion to mci or dementia, we used the R package risksetROC v.1.0.4 [54] to estimate ROC curves from the censored survival data and C index [55], and the R package boot v 1.5 [56] to construct confidence intervals for the C-indices. Because the data are censored survival data, it is not appropriate to calculate metrics such as sensitivity and specificity because these metrics assume all participants are observed for the same amount of time. There is currently no software package that we are aware of that accommodate both the uncertainty of class membership and the censored nature of the survival data. As such, it is likely that estimates of the C-indices for modal class membership are biased downward by at least 5% [55], and should be interpreted with caution. All analyses were done using Stata v11.0 [57], MPLUS v7.1 [58], or R v3.1.2 [59].
Results
Our data request included all visits for all 7,424 volunteers who were cognitively normal at their first visit. We excluded 574 with missing NPI-Q, 1,812 without follow-up, and 521 under age 60. Of the 4,517 remaining, 3,644 (81%) were normal at last follow-up, 635 converted to MCI, 111 converted to MCI and then to dementia, and 127 individuals converted directly to dementia. Table 1 shows demographics, baseline assessments, and follow-up time by conversion status. At visits 1 through 8, the sample sizes were 4517, 4517, 3306, 3015, 2331, 1256, 682, and 168, respectively. Individuals without follow-up were younger (72.8 (8.0) vs. 74.6 (8.0)), and had higher GDS scores (1.57 (2.2) vs. 1.24 (1.9)). In comparing baseline characteristics between those with short (2–3 visits) to longer (4+ visits) follow-up, we observed that those with shorter follow-up had higher GDS scores (1.34 (2.0) vs. 1.17 (1.9)) and were more likely to be non-white (78.6% vs. 83.9%). Converters were older at baseline, and more likely to be male and white. Converters had slightly less education, lower MMSE, but higher NPI-Q scores. Depression was the most commonly endorsed NPI-Q symptom (13%), followed by irritability (11%), nighttime behaviors (9%), anxiety (8%), agitation (5%), eating behaviors (5%), and apathy (5%). All other domains had prevalences of 2% or less. Of the 4,096 possible unique patterns of the 12 dichotomous NPI-Q domain ratings, 218 were represented in our analytic sample.
Table 1.
Volunteer Characteristics at Baseline
| Non-Converters (N=3,644) | Converters (N=873) | t or χ2 Statistica | |
|---|---|---|---|
| Age (yrs) | 73.79 (7.78)b | 77.97 (7.95) | −14.01, df=1301.28, p<0.001 |
| Female | 2,429 (67%)c | 525 (60%) | 13.23, df=1, p<0.001 |
| Education (yrs) | 15.61 (3.03) | 15.26 (3.26) | 2.92, df=1249.18, p=0.004 |
| Race | |||
| White | 2950 (81%) | 742 (85%) | 8.52, df=2 p=0.014 |
| African American | 592 (16%) | 116 (13%) | |
| Other | 102 (3%) | 15 (2%) | |
| MMSE | 28.93 (1.41) | 28.46 (1.66) | 7.48, df=1150.15, p<0.001 |
| NPI-Q | 0.67 (1.53) | 1.26 (2.61) | −6.43, df=1018.60, p<0.001 |
| GDS | 1.13 (1.79) | 1.68 (2.27) | −6.68, df=1134.30, p<0.001 |
| Depression (last two years) | 567 (16%) | 186 (21%) | 16.79, df=1, p<0.001 |
| Depression (past) | 576 (16%) | 103 (12%) | 8.88, df=1, p=0.003 |
| Current Use of Antidepressant | 481 (13%) | 144 (16%) | 6.41, df=1, p=0.011 |
| Number of Visits | 4.16 (1.87) | 4.67 (1.73) | −7.68, df=1402.73, p<0.001 |
| Follow-up (yrs) | 3.63 (2.00) | 4.21 (1.81) | −8.33, df=1421.02, p<0.001 |
Abbreviations: MMSE – Mini-Mental Status Examination; NPI-Q - Neuropsychiatric Inventory-Q; GDS – Geriatric Depression Scale (15-item)
χ2 test statistics are presented for categorical variables, t test statistics for continuous variables.
Continuous variables are described with mean (SD)
Categorical variables are described with number (%).
We fit LCA models to the 12 dichotomous domains with 1–5 classes. Using bootstrapped likelihood ratio tests [46], a two class model fit significantly better than a one class model (−2LL difference: 2384.44, df=13, p<0.001), a three class model fit significantly better than a two class model (−2LL difference: 171.206, df=13, p<0.001), a four class model fit significantly better than a three class model (−2LL difference: 98.40, df=13, p<0.001), but a five class model did not fit significantly better than a four class model (−2LL difference: 20.34, p=0.09). The four class model also had the lowest BIC. As such, a four class model was chosen. We fit an analogous model including individuals without follow-up and the class structure did not differ.
The 264 standardized bivariate residuals showed no associations between domains with the possible exception of delusions and hallucinations. Given our low rate of hallucinations, this did not justify altering the model. Standardized residuals comparing observed and expected counts of individuals for each pattern of domains showed excellent fit.
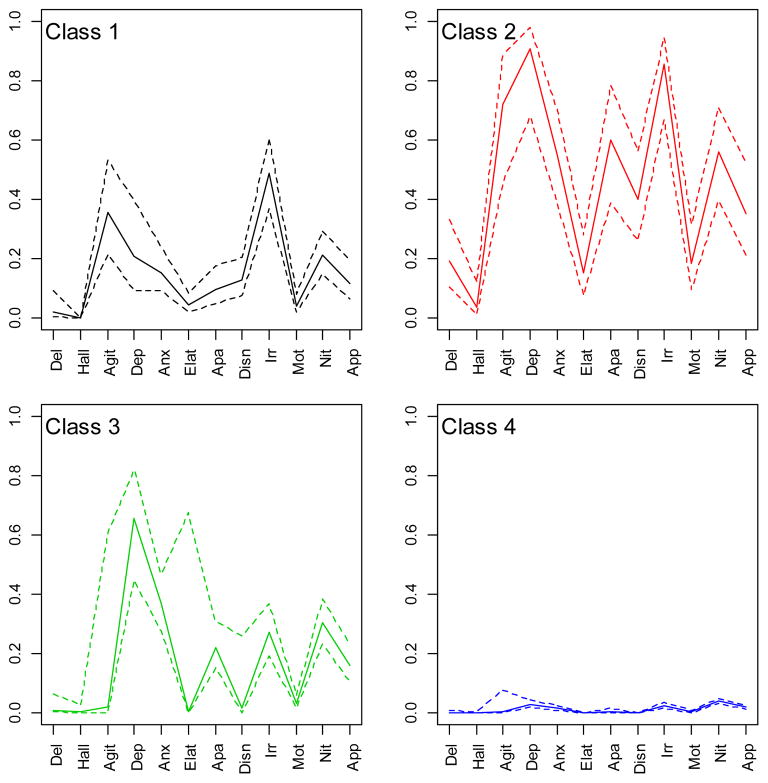
Figure 1 shows estimated conditional probabilities for each NPI-Q domain, by class. Class 1 would be expected to include 10% of the sample, and had high probabilities of agitation (0.36) and irritability (0.49). Class 2 would include only 2% of the sample, and had high probabilities of almost all of the domains. Class 3 would include 11% of the sample, and had a high probability of depression. The majority (78%) of the sample would be in class 4, which had very low probabilities on all domains. Results from an LCA with 6,850 subjects (including those <60 and without follow-up) were quite similar. We refer to classes 1 and 3 as “irritable” and “depressed”, as those were the domains with the highest conditional probabilities.
Figure 1. LCA Profile Plot.
These plots show conditional probability estimates (y-axis) for each NPI-Q domain (x-axis) for each class. Solid lines are point estimates, dotted lines denote 95% confidence intervals.
A list of modal class assignment by every possible pattern of NPI-Q dichotomous responses would be intractable, so in order to demonstrate the makeup of the classes, a calculator for modal class assignment as a function of pattern of NPI domains can be found at www.hopkinsmedicine.org/psychiatry/bayview/research/Leoutsakos.html. The majority of individuals assigned to class 4 had no symptoms. Individuals with just one symptom were also assigned to class 4 unless that symptom were apathy (class 3) or agitation, elation, or disinhibition (class 1). Among individuals with 2 to 4 symptoms, those whose patterns included agitation or irritability were almost always assigned to class 1; those assigned to class 3 almost always had either depression or apathy. Those with 5 or more symptoms were typically assigned to class 2, particularly if they had symptoms characteristic of both classes 1 and 3 (e.g., both irritability and apathy). Individuals with psychosis were typically assigned to class 2.
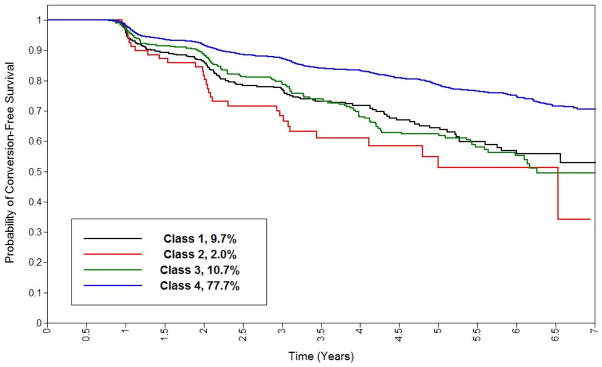
All three non-reference classes were statistically significantly associated with greater hazard of conversion to MCI or dementia (table 2), with class 2 (complex) showing the greatest hazard. An analogous model that only considered dementia conversions showed similar patterns of associations, as did one which adjusted, for all of the tests in the full cognitive battery. We tested the proportional hazards assumption by regressing scaled Schoenfeld residuals on time, [60] and found no evidence of violation. Examination of Cox-Snell residuals [61] showed good fit. Rates of administrative censoring as a function of all censoring across classes were 40%, 37%, 31% and 31% for classes 1 through 4, respectively. Mean follow-up was 3.57, 3.60, 3.66, and 3.77 years, for classes 1 through 4, respectively.
Table 2.
Cox Proportional Hazards Model for MCI or Dementia
| Hazard Ratio (95% Confidence Interval) | p-value | |
|---|---|---|
| Class 1 | 1.69 (1.28–2.22) | <0.001 |
| Class 2 | 3.09 (2.16–4.4) | <0.001 |
| Class 3 | 1.81 (1.41–2.31) | <0.001 |
| Class 4 | Reference Class | |
| Age (yrs) | 1.06 (1.05–1.07) | <0.001 |
| Female | 0.83 (0.73–0.95) | 0.007 |
| Education (yrs) | 0.99 (0.99–1) | 0.048 |
| White | 1.01 (0.84–1.22) | 0.889 |
| MMSE | 0.99 (0.99–0.99) | <0.001 |
| Antidepressant use | 1.47 (1.23–1.75) | <0.001 |
Abbreviations: MMSE – Mini Mental Status Exam
In assessing predictive utility, we found that modal class membership had a bias-corrected C-index for prediction of progression to MCI or dementia for up to 6 years of 0.653 (0.637, 0.670), while total NPI-Q score had a C-index of 0.646 (0.638, 0.660).
Table 3 depicts conversions to MCI and clinical dementia type by modal class. Class 2 had the highest rate of MCI conversion (35%), and over half were amnestic, multi-domain MCI. The other classes had fewer MCI conversions and higher proportions of amnestic, single-domain MCI. The majority of dementia conversions were to probable or possible AD for each of the latent classes. There was only 1 conversion to FTD.
Table 3.
Conversion Type by Modal Class Assignment
| Class 1 | Class 2 | Class 3 | Class 4 | |
|---|---|---|---|---|
| Irritable (N=289)1 | Complex (N=75) | Depressed (N=394) | Asymptomatic (N=3759) | |
| Any Conversion | 82 (28%)2 | 30 (40%) | 107 (27%) | 654 (17%) |
| Any MCI | 62 (21%) | 26 (35%) | 97 (25%) | 569 (15%) |
| Amnestic Single | 30 (48%)3 | 8 (31%) | 33 (34%) | 251 (44%) |
| Amnestic Multi | 16 (26%) | 14 (54%) | 36 (37%) | 185 (33%) |
| Non-Amnestic Single | 14 (23%) | 3 (12%) | 19 (20%) | 102 (18%) |
| Non-Amnestic Multi | 2 (3%) | 1 (4%) | 9 (9%) | 31 (5%) |
| Any Dementia4 | 29 (10%) | 9 (12%) | 29 (7%) | 171 (5%) |
| AD | 20 (69%)5 | 7 (78%) | 21 (72%) | 129 (75%) |
| VaD | 2 (7%) | 1 (11%) | 2 (7%) | 7 (4%) |
| Mixed AD/VaD | 1 (3%) | 0 (0%) | 3 (10%) | 15 (9%) |
| FTD | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) |
| DLB | 0 (0%) | 1 (11%) | 1 (3%) | 2 (1%) |
| Other/Unknown | 6 (21%) | 0 (0%) | 2 (7%) | 17 (10%) |
All counts based on modal (most likely) class assignment, not true class assignment, which is unknown.
For the any conversion, any MCI, and any dementia rows, the denominator for calculating percentages is the total count of individuals in that class.
For MCI subtypes, the denominator for calculating percentages is the total count of individuals in that class with MCI.
Individuals may be counted in this table twice if they converted first to MCI, and then to dementia.
For dementia subtypes, the denominator for calculating percentages is the total count of individuals in that class with dementia.
4. Discussion
Given the relationships between NPS, cognitive decline, and dementia, we hypothesized that one or more latent classes of cognitively normal NACC volunteers would be associated with conversion to MCI or dementia. We estimated 4 classes; a large asymptomatic class, an “irritable” class, a “depressed” class, and a small “complex” class with high probabilities of several NPS. There was a clear gradient of conversion risk as a function of class membership, with the complex class having the greatest risk, followed by the depressed and irritable classes.
Our findings are consistent with the bulk of the literature relating major depression or depressive symptoms to risk of cognitive decline. To our knowledge, this is the first demonstration of variable risk as a function of different latent classes estimated from patterns of NPS among cognitively normal individuals. Though formal depression diagnoses were not made, only a minority in each class (Irritable: 7%, Complex 9%: Depressed: 17%, Asymptomatic: 2%) would have screened positive on the GDS (e.g., score>5). We note that the NPI-Q depression domain focuses on depressed mood, feelings of worthlessness and suicidality and does not include vegetative symptoms, social withdrawal or psychomotor retardation that are aspects of the DSM-V diagnosis of major depressive disorder. We suggest that latent class analysis produced more nuanced groupings (for example, depression alone was not enough to get into class 3, another symptom was needed), and allowed identification of individuals at increased risk of conversion even among those with very mild NPS. Individually, every NPI-Q domain except elation was associated with statistically significantly greater conversion risk. Delusions and hallucinations, though rare, had the highest hazard ratios of 4.5 and 3.4, respectively. Looking solely at counts of symptoms, each additional positive NPI-Q domain increased risk. Beyond this however, our LCA allowed for the identification of patterns with different conversion risks. For example, individuals with depression, agitation, nighttime behaviors, and apathy would be placed in class 2, with the highest risk, whereas individuals with depression, agitation, nighttime behaviors, and motor symptoms would be placed in class 3, with lower risk. Although the c-index for classification using latent class membership was only very slightly better than for total NPI-Q score, we would argue that identification of patterns or interactions between NPS related to risk is important, because it allows for the identification of differences in the underlying pathology, whereas individual symptoms or counts of symptoms may not.
In the previous cohort study relating MBI to dementia incidence, the majority of non-cognitively impaired subjects ultimately developed clinically diagnosed frontotemporal dementia.[62] By contrast, the majority of dementia conversions in this sample were to clinical AD. This is likely a reflection of the sample: healthy ADC controls may be more likely to develop AD (due to family history) than either the general population or a specialty clinic. A specialty clinic might also be more likely to see patients with more severe NPS, and less common types of dementia. Delusions, disinhibition and anxiety were much more frequent in the previous cohort study than in the NACC sample.
Strengths of our analyses include the large sample, which allowed for precise estimation and the ability to include rare NPI domains and to identify relatively rare subtypes. An additional strength is the use of latent class analysis to model conversion risk based on NPS patterns. Weaknesses include potential selection biases; the sample were highly educated volunteers. As such, rates of NPS, latent class prevalences, and rates of conversion may be biased. We note that prevalences of each NPI-Q domain are quite similar to those found among cognitively normal participants in the Mayo Clinic Study of Aging, a population-based cohort study (N=1,587)[63]. Further, hazard rates of conversion by domain were also similar, with the possible exception of hallucinations and delusions, which were extremely rare in the Mayo Study and in NACC. In our analyses, Caucasian race was not associated with less risk in the survival model; this is inconsistent with reports showing minorities to be at greater risk for dementia [64–66]. In looking at the relationship between class membership and race, when we assigned individuals to their modal class, we saw that class 2, had a higher percentage of non-whites (75%) than the other classes (87%, 86%, and 81%, respectively, for classes 1,3, and 4). As class 2 was associated with the greatest risk of conversion, it may be that the reason we did not see an effect of race on conversion risk after controlling for class membership was because the effect of race was at least partially mediated by class membership. Though diagnostic criteria were uniform across ADCs, there may be some variation in diagnostic practices[38]. In a study at a single ADRC, the authors found inter-rater reliability of the consensus diagnoses of 0.78[67], which is comparable other longitudinal aging studies[68]. We are not aware of site-to-site interrater reliability studies, but such differences are likely to bias estimates toward, rather than away from the null hypothesis of no association between NPS classes and conversion risk. We would argue that these methodological concerns are offset by the large and geographically widespread sample available from NACC. We are not aware of any other dataset in which it would have been possible to perform our analysis including all 12 NPI-Q domains.
We used dichotomous NPI-Q domain ratings made by each participant’s knowledgeable informant; they are subject to recall bias as well as cultural biases, and only refer to the month prior (as per the instrument instructions) [62]. Though ratings are made by an informant and not through direction observation by a clinician, NPI-Q ratings have been shown to have good correspondence to NPI ratings [35], and factor structure of the domains has been shown to be consistent between NACC and ADNI [69]. Dichotomization may have resulted in loss of information, but given the rarity of severe NPS in healthy controls, including the actual ratings (0–3) would likely not have added enough information to offset the tripling of parameters this would have entailed. We note that individuals with intermittent or treated NPS might not be identified, and antidepressant use was a clear predictor of conversion. To our knowledge, the NPI-Q has not been validated in cognitively normal subjects, and may not be sensitive to all neuropsychiatric changes in this population. The questionnaire is completed by a proxy; this is necessary when the participant has dementia, but in the case of a cognitively normal individual, a proxy may be not be as good an informant as the participant themselves. Some of the domain questions are very clearly intended for use in a dementia population. For example, the anxiety question specifically refers to anxiety related to separation from a caregiver, though it does go on to ask if they have any other signs of nervousness. While the NPI-Q is likely not the ideal instrument to measure subtle NPS among cognitively normal individuals, we are no aware of another dataset with a better instrument which would cover the full range of NPS. Again, these issues would likely bias our results toward, rather than away from the null.
Although we limited our analyses to individuals classified as healthy controls with no cognitive impairment, and adjusted our survival models for MMSE, it is possible that some volunteers were already experiencing cognitive decline at baseline. We repeated our cox proportional hazards models with additional adjustment for logical memory, digit span forward and backward, category fluency, trail making, digit symbol substitution, and Boston naming, and found no substantial alteration in inference. 1,164 of the volunteers (26%) reported some subjective memory decline at baseline. We repeated our survival model with these individuals excluded and again found no substantial alteration in inference.
We fit the LCA using volunteers’ baseline NPI-Q, thus including the most person-years at risk. This did not account for possible shifts between latent classes over time. We also fit an LCA for individuals’ second visits, found a similar class structure, and 78% remained in the same class. Individuals in class 2 at both visits had the highest rate (62%) rate of conversion, but shifts from other classes into class 2 were also ominous: rates of conversion were 58% (class 1), 57% (class 3), and 42% (class 4). As some did change modal class membership over time, we do not suggest that the latent classes represent static groups of individuals. Rather, we suggest that the classes represent groups at a given time point who differ in their patterns of NPS, and who may be closer or further away from developing dementia in a way that relates to their pattern of NPS at that time point.
As NPS are not uncommon among healthy controls, and predict conversion, NPS may play an important role in prevention and treatment. They may afford an earlier view of emerging dementia pathology, and since they indicate increased dementia risk, they could be used to enrich future prevention trials. The medications used to treat the major psychiatric disorders (e.g., depression, psychosis) are not very effective at treating these symptoms in AD [70] and have not been fully investigated in MCI. Safe and effective treatments for NPS are needed and would have a substantial impact on the burden of AD to the patient and caregivers, as well as societal costs.
The neurobiological mechanisms underlying NPS and subsequent cognitive decline, particularly in the pre-clinical phase of dementia, are poorly understood. It is unclear whether NPS are part of a causal pathway for dementia, or represent a prodrome. The available data suggest that more complex neurobiological models are needed. For example, a longitudinal clinical-pathologic cohort study observed that “distress” (psychological distress, neuroticism and depressive symptoms) was associated with cognitive decline even when controlling for AD and cerebrovascular pathology [71]. Thus, a neurobiological model to explain the role of NPS in cognitive decline must include other mechanisms. Molecular imaging studies are ongoing to test such a neurobiological model in mild cognitive impairment and normal aging, based on observations of degeneration of monoamine systems that precede beta amyloid deposition in transgenic mouse models of amyloid pathology [72] and will be applied to evaluate the classes identified in the present study. In fact, as NPS precede many neurodegenerative diseases, the approach described in the present study may have implications for similar investigations in other neurodegenerative diseases, including Parkinson’s and Huntington’s disease.
Figure 2. Kaplan-Meier Plot for Conversion to Cognitive Impairment or Dementia By Latent Class.
This Kaplan-Meier plot shows survival (y axis) as a function of time (x-axis) for each latent class.
Acknowledgments
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIAfunded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Steven Ferris, PhD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016570 (PI David Teplow, PhD), P50 AG005131 (PI Douglas Galasko, MD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P50 AG005136 (PI Thomas Montine, MD, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), and P50 AG005681 (PI John Morris, MD).
Ms. Forrester was supported by NIH Institutional NRSA T32AG027668; Dr. Smith is funded by AG038893, MH086881
Footnotes
Author Contributions:
Dr. Leoutsakos designed the study, conducted data analysis, interpreted analytic results, and prepared the manuscript. She had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Ms. Forrester assisted with data analysis, assisted in the preparation of the manuscript, and reviewed and approved the manuscript.
Dr. Lyketsos designed the study and reviewed and approved the manuscript.
Dr. Smith designed the study, assisted with the preparation of the manuscript, and reviewed and approved the manuscript.
Conflicts of Interest
Dr. Leoutsakos has received NIH funding and is an unpaid statistical consultant for Lilly.
Ms. Forrester has no conflicts of interest and no financial relationships to report.
Dr. Lyketsos has received grant support (research or CME) from NIMH, NIA, Associated Jewish Federation of Baltimore, Weinberg Foundation, Forest, Glaxo-Smith-Kline, Eisai, Pfizer, Astra-Zeneca, Lilly, Ortho-McNeil, Bristol-Myers, Novartis, National Football League, Elan, and Functional Neuromodulation. He serves as a consultant for Astra-Zeneca, Glaxo-Smith Kline, Eisai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lundbeck, Merz, Lilly, Pfizer, Genentech, Elan, NFL Players Association, NFL Benefits Office, Avanir, Zinfandel, BMS, Abvie, Janssen, and Orion. He has received honoraria or travel support from Pfizer, Forest, Glaxo-Smith Kline, and Health Monitor.
Dr. Smith has received NIH funding.
References
- 1.Steinberg M, Shao H, Zandi P, Lyketsos CG, Welsh-Bohmer KA, Norton MC, Breitner JC, Steffens DC, Tschanz JT, Cache County I. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23:170–177. doi: 10.1002/gps.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg M, Tschanz JT, Corcoran C, Steffens DC, Norton MC, Lyketsos CG, Breitner JC. The persistence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2004;19:19–26. doi: 10.1002/gps.1025. [DOI] [PubMed] [Google Scholar]
- 4.Rabins PV, Schwartz S, Black BS, Corcoran C, Fauth E, Mielke M, Christensen J, Lyketsos C, Tschanz J. Predictors of progression to severe Alzheimer’s disease in an incidence sample. Alzheimers Dement. 2013;9:204–207. doi: 10.1016/j.jalz.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer K, Lupo F, Perri R, Salamone G, Fadda L, Caltagirone C, Musicco M, Cravello L. Predicting disease progression in Alzheimer’s disease: the role of neuropsychiatric syndromes on functional and cognitive decline. J Alzheimers Dis. 2011;24:35–45. doi: 10.3233/JAD-2010-101836. [DOI] [PubMed] [Google Scholar]
- 6.Buckley T, Fauth EB, Morrison A, Tschanz J, Rabins PV, Piercy KW, Norton M, Lyketsos CG. Predictors of quality of life ratings for persons with dementia simultaneously reported by patients and their caregivers: the Cache County (Utah) Study. Int Psychogeriatr. 2012;24:1094–1102. doi: 10.1017/S1041610212000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black BS, Johnston D, Morrison A, Rabins PV, Lyketsos CG, Samus QM. Quality of life of community-residing persons with dementia based on self-rated and caregiver-rated measures. Qual Life Res. 2012;21:1379–1389. doi: 10.1007/s11136-011-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodaty H, Connors MH, Xu J, Woodward M, Ames D group Ps. Predictors of institutionalization in dementia: a three year longitudinal study. J Alzheimers Dis. 2014;40:221–226. doi: 10.3233/JAD-131850. [DOI] [PubMed] [Google Scholar]
- 9.Afram B, Stephan A, Verbeek H, Bleijlevens MH, Suhonen R, Sutcliffe C, Raamat K, Cabrera E, Soto ME, Hallberg IR, Meyer G, Hamers JP, RightTimePlaceCare C. Reasons for institutionalization of people with dementia: informal caregiver reports from 8 European countries. J Am Med Dir Assoc. 2014;15:108–116. doi: 10.1016/j.jamda.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Okura T, Plassman BL, Steffens DC, Llewellyn DJ, Potter GG, Langa KM. Neuropsychiatric symptoms and the risk of institutionalization and death: the aging, demographics, and memory study. J Am Geriatr Soc. 2011;59:473–481. doi: 10.1111/j.1532-5415.2011.03314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganguli M, Dodge HH, Shen C, Pandav RS, DeKosky ST. Alzheimer disease and mortality: a 15-year epidemiological study. Arch Neurol. 2005;62:779–784. doi: 10.1001/archneur.62.5.779. [DOI] [PubMed] [Google Scholar]
- 12.Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157:708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- 13.Peters ME, Rosenberg PB, Steinberg M, Tschanz JT, Norton MC, Welsh-Bohmer KA, Hayden KM, Breitner JC, Lyketsos CG, Cache County I. Prevalence of neuropsychiatric symptoms in CIND and its subtypes: the Cache County Study. Am J Geriatr Psychiatry. 2012;20:416–424. doi: 10.1097/JGP.0b013e318211057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karttunen K, Karppi P, Hiltunen A, Vanhanen M, Valimaki T, Martikainen J, Valtonen H, Sivenius J, Soininen H, Hartikainen S, Suhonen J, Pirttila T group As. Neuropsychiatric symptoms and quality of life in patients with very mild and mild Alzheimer’s disease. Int J Geriatr Psychiatry. 2011;26:473–482. doi: 10.1002/gps.2550. [DOI] [PubMed] [Google Scholar]
- 15.Monastero R, Mangialasche F, Camarda C, Ercolani S, Camarda R. A systematic review of neuropsychiatric symptoms in mild cognitive impairment. J Alzheimers Dis. 2009;18:11–30. doi: 10.3233/JAD-2009-1120. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg PB, Mielke MM, Appleby BS, Oh ES, Geda YE, Lyketsos CG. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry. 2013;21:685–695. doi: 10.1016/j.jagp.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters ME, Rosenberg PB, Steinberg M, Norton MC, Welsh-Bohmer KA, Hayden KM, Breitner J, Tschanz JT, Lyketsos CG, Cache County I. Neuropsychiatric symptoms as risk factors for progression from CIND to dementia: the Cache County Study. Am J Geriatr Psychiatry. 2013;21:1116–1124. doi: 10.1016/j.jagp.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsang RS, Sachdev PS, Reppermund S, Kochan NA, Kang K, Crawford J, Wen W, Draper B, Trollor JN, Slavin MJ, Mather KA, Assareh A, Seeher KM, Brodaty H. Sydney Memory and Ageing Study: an epidemiological cohort study of brain ageing and dementia. Int Rev Psychiatry. 2013;25:711–725. doi: 10.3109/09540261.2013.860890. [DOI] [PubMed] [Google Scholar]
- 19.Zeki Al, Hazzouri A, Vittinghoff E, Byers A, Covinsky K, Blazer D, Diem S, Ensrud KE, Yaffe K. Long-term Cumulative Depressive Symptom Burden and Risk of Cognitive Decline and Dementia Among Very Old Women. J Gerontol A Biol Sci Med Sci. 2013 doi: 10.1093/gerona/glt139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richard E, Schmand B, Eikelenboom P, Yang SC, Ligthart SA, Moll van Charante EP, van Gool WA Alzheimer’s Disease Neuroimaging I. Symptoms of apathy are associated with progression from mild cognitive impairment to Alzheimer’s disease in non-depressed subjects. Dement Geriatr Cogn Disord. 2012;33:204–209. doi: 10.1159/000338239. [DOI] [PubMed] [Google Scholar]
- 21.Palmer K, Di Iulio F, Varsi AE, Gianni W, Sancesario G, Caltagirone C, Spalletta G. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J Alzheimers Dis. 2010;20:175–183. doi: 10.3233/JAD-2010-1352. [DOI] [PubMed] [Google Scholar]
- 22.Hahn EA, Wang HX, Andel R, Fratiglioni L. A Change in Sleep Pattern May Predict Alzheimer Disease. Am J Geriatr Psychiatry. 2013 doi: 10.1016/j.jagp.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Sterniczuk R, Theou O, Rusak B, Rockwood K. Sleep disturbance is associated with incident dementia and mortality. Curr Alzheimer Res. 2013;10:767–775. doi: 10.2174/15672050113109990134. [DOI] [PubMed] [Google Scholar]
- 24.Osorio RS, Pirraglia E, Aguera-Ortiz LF, During EH, Sacks H, Ayappa I, Walsleben J, Mooney A, Hussain A, Glodzik L, Frangione B, Martinez-Martin P, de Leon MJ. Greater risk of Alzheimer’s disease in older adults with insomnia. J Am Geriatr Soc. 2011;59:559–562. doi: 10.1111/j.1532-5415.2010.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geda YE, Roberts RO, Knopman DS, Petersen RC, Christianson TJ, Pankratz VS, Smith GE, Boeve BF, Ivnik RJ, Tangalos EG, Rocca WA. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry. 2008;65:1193–1198. doi: 10.1001/archpsyc.65.10.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brodaty H, Heffernan M, Draper B, Reppermund S, Kochan NA, Slavin MJ, Trollor JN, Sachdev PS. Neuropsychiatric symptoms in older people with and without cognitive impairment. J Alzheimers Dis. 2012;31:411–420. doi: 10.3233/JAD-2012-120169. [DOI] [PubMed] [Google Scholar]
- 27.Taragano FE, Allegri RF, Krupitzki H, Sarasola DR, Serrano CM, Lon L, Lyketsos CG. Mild behavioral impairment and risk of dementia: a prospective cohort study of 358 patients. J Clin Psychiatry. 2009;70:584–592. doi: 10.4088/jcp.08m04181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leoutsakos JM, Bartlett AL, Forrester SN, Lyketsos CG. Simulating effects of biomarker enrichment on Alzheimer’s prevention trials: Conceptual framework and example. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2013.05.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moran M, Walsh C, Lynch A, Coen RF, Coakley D, Lawlor BA. Syndromes of behavioural and psychological symptoms in mild Alzheimer’s disease. Int J Geriatr Psychiatry. 2004;19:359–364. doi: 10.1002/gps.1091. [DOI] [PubMed] [Google Scholar]
- 30.Lyketsos CG, Sheppard JM, Steinberg M, Tschanz JA, Norton MC, Steffens DC, Breitner JC. Neuropsychiatric disturbance in Alzheimer’s disease clusters into three groups: the Cache County study. Int J Geriatr Psychiatry. 2001;16:1043–1053. doi: 10.1002/gps.448. [DOI] [PubMed] [Google Scholar]
- 31.Beekly DL, Ramos EM, van Belle G, Deitrich W, Clark AD, Jacka ME, Kukull WA Centers NI-AsD. The National Alzheimer’s Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18:270–277. [PubMed] [Google Scholar]
- 32.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 34.Morris JC National Alzheimer’s Coordinating Center. Aging NIo. ADC Clinical Task Force, NACC, University of Washington; 2008. [Google Scholar]
- 35.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 36.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40:812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 37.Sheikh JI, Yesavage JA. Clinical Gerontology: A Guide to Assessment and Intervention. The Haworth Press; New Yok: 1986. [Google Scholar]
- 38.Steenland K, Macneil J, Bartell S, Lah J. Analyses of diagnostic patterns at 30 Alzheimer’s disease centers in the US. Neuroepidemiology. 2010;35:19–27. doi: 10.1159/000302844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 40.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 41.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 42.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 43.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 44.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 45.Satterthwaite FE. An Approximate Distribution of Estimates of Variance Components. Biometrics Bulletin. 1946;2:110–114. [PubMed] [Google Scholar]
- 46.Nylund K, Bellmore A, Nishina A, Graham S. Subtypes, severity, and structural stability of peer victimization: what does latent class analysis say? Child Dev. 2007;78:1706–1722. doi: 10.1111/j.1467-8624.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- 47.Bandeen-Roche K, Miglioretti DL, Zeger SL, Rathouz PJ. Latent Variable Regression for Multiple Discrete Outcomes. Journal of the American Statistical Association. 1997;92:1375–1286. [Google Scholar]
- 48.Hagenaars JA, McCutcheon AL, editors. Latent Structure Analysis. Cambridge University Press; 2002. [Google Scholar]
- 49.Vermunt JK. Latent Class Modeling with Covariates: Two Improved Three-Step Approaches. Political Analysis. 2010;18:450–469. [Google Scholar]
- 50.Asparouhov TMB. [Accessed June 11];Auxiliary Variables in MIxture Modeling: 3-Step Approaches using Mplus. Statmodel.com.
- 51.Siannis F, Copas J, Lu G. Sensitivity analysis for informative censoring in parametric survival models. Biostatistics. 2005;6:77–91. doi: 10.1093/biostatistics/kxh019. [DOI] [PubMed] [Google Scholar]
- 52.Center AsDR. NACC Checklist for Authors. National Institute on Aging; [Accessed June 11]. https://http://www.alz.washington.edu/WEB/checklist.html. [Google Scholar]
- 53.Wu Y, Furnary AP, Grunkemeier GL. Using the National Death Index to validate the noninformative censoring assumption of survival estimation. Ann Thorac Surg. 2008;85:1256–1260. doi: 10.1016/j.athoracsur.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 54.Heagerty P, Saha-Chaudhuri P. Package ‘risksetROC’: Riskset ROC curve estimation from censored survival data. Saha-Chaudhri; [Accessed June 11]. http://cran.r-project.org/web/packages/risksetROC/risksetROC.pdf. [Google Scholar]
- 55.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 56.Canty A, Ripley B. Package “Boot”: Bootstrap Functions. 2012. [Google Scholar]
- 57.StataCorp. Stata Statistical Software: Release 13. StataCorp LP; 2013. [Google Scholar]
- 58.Múthen LMBO. Múthen & Múthen; Los Angeles, CA: 1998–2012. [Google Scholar]
- 59.Team RC. R. Foundation for Statistical Computing. Vienna, Austria: 2014. [Google Scholar]
- 60.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 61.Baltazar-Aban IP, Edsel A. Properties of Hazard-Based Residuals and Implications in Model Diagnostics. Journal of the American Statistical Association. 1995:90. [Google Scholar]
- 62.de Medeiros K, Robert P, Gauthier S, Stella F, Politis A, Leoutsakos J, Taragano F, Kremer J, Brugnolo A, Porsteinsson AP, Geda YE, Brodaty H, Gazdag G, Cummings J, Lyketsos C. The Neuropsychiatric Inventory-Clinician rating scale (NPI-C): reliability and validity of a revised assessment of neuropsychiatric symptoms in dementia. Int Psychogeriatr. 2010;22:984–994. doi: 10.1017/S1041610210000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Geda YE, Roberts RO, Mielke MM, Knopman DS, Christianson TJ, Pankratz VS, Boeve BF, Sochor O, Tangalos EG, Petersen RC, Rocca WA. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry. 2014;171:572–581. doi: 10.1176/appi.ajp.2014.13060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Katz MJ, Lipton RB, Hall CB, Zimmerman ME, Sanders AE, Verghese J, Dickson DW, Derby CA. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord. 2012;26:335–343. doi: 10.1097/WAD.0b013e31823dbcfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Demirovic J, Prineas R, Loewenstein D, Bean J, Duara R, Sevush S, Szapocznik J. Prevalence of dementia in three ethnic groups: the South Florida program on aging and health. Ann Epidemiol. 2003;13:472–478. doi: 10.1016/s1047-2797(02)00437-4. [DOI] [PubMed] [Google Scholar]
- 66.Froehlich TE, Bogardus ST, Jr, Inouye SK. Dementia and race: are there differences between African Americans and Caucasians? J Am Geriatr Soc. 2001;49:477–484. doi: 10.1046/j.1532-5415.2001.49096.x. [DOI] [PubMed] [Google Scholar]
- 67.Duara R, Loewenstein DA, Greig M, Acevedo A, Potter E, Appel J, Raj A, Schinka J, Schofield E, Barker W, Wu Y, Potter H. Reliability and validity of an algorithm for the diagnosis of normal cognition, mild cognitive impairment, and dementia: implications for multicenter research studies. Am J Geriatr Psychiatry. 2010;18:363–370. doi: 10.1097/jgp.0b013e3181c534a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trzepacz PT, Saykin A, Yu P, Bhamditipati P, Sun J, Dennehy EB, Willis B, Cummings JL Alzheimer’s Disease Neuroimaging I. Subscale validation of the neuropsychiatric inventory questionnaire: comparison of Alzheimer’s disease neuroimaging initiative and national Alzheimer’s coordinating center cohorts. Am J Geriatr Psychiatry. 2013;21:607–622. doi: 10.1016/j.jagp.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gauthier S, Cummings J, Ballard C, Brodaty H, Grossberg G, Robert P, Lyketsos C. Management of behavioral problems in Alzheimer’s disease. Int Psychogeriatr. 2010;22:346–372. doi: 10.1017/S1041610209991505. [DOI] [PubMed] [Google Scholar]
- 71.Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology. 2003;61:1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- 72.Liu Y, Yoo MJ, Savonenko A, Stirling W, Price DL, Borchelt DR, Mamounas L, Lyons WE, Blue ME, Lee MK. Amyloid pathology is associated with progressive monoaminergic neurodegeneration in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2008;28:13805–13814. doi: 10.1523/JNEUROSCI.4218-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]