INTRODUCTION
Although aging is the leading risk factor for Alzheimer disease, it is estimated that at least 200,000 people under age 65 have what is commonly known as young-onset dementia.1 Individuals with young-onset dementia can differ dramatically from those with older onset in the types of symptoms they express. Even those with the same clinical diagnosis (eg, dementia of the Alzheimer type [DAT]) can present with different neuro-cognitive profiles of impairment.2–4 Individuals with young-onset dementia and their families have different needs, concerns, and access to resources than older adults who develop dementia.5 This fact is related not only to the type of symptoms expressed but also to the time of life when the illness strikes. Individuals with young-onset dementia are likely to be healthier than individuals with late-onset dementia and less likely to have coexisting illnesses, such as cardiovascular disease, diabetes, or hearing loss and other sensory changes, making them good candidates for targeted therapies. Unfortunately, most psychosocial models of dementia care and intervention focus on a clinical diagnosis, usually dementia or Alzheimer disease dementia, without paying attention to the specific presenting symptoms. This approach may place individuals into programs or services solely based on a dementia diagnosis, regardless of whether or not a person is cognitively suited or age-appropriate for the intervention. One exception is the Tailored Activity Program.6 This program trains occupational therapists to assess persons with dementia who exhibit behavioral symptoms for preserved capabilities that are then used to customize activities. The family and environment are included and considered critical to the intervention's success. Similarly, the CARE-D model identifies and builds on strengths based on a person's neurocognitive/behavioral profile and incorporates the family and environmental capacities; however, skills of both occupational therapists and speech-language pathologists are engaged to tailor appropriate interventions.
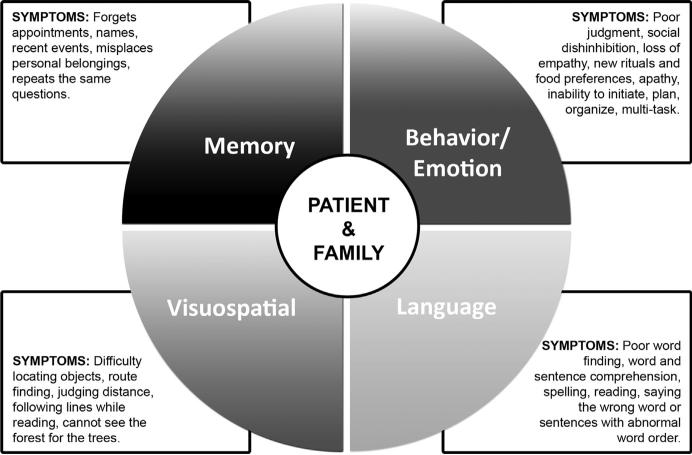
This article describes the conceptual design and implementation of CARE-D. The model is built on a framework provided by the neuropsychological characterization of cognitive and behavioral strengths and weaknesses in the early stages of illness and on a comprehensive psychosocial assessment (Fig. 1).
Fig. 1.
The Care Pathway Model for Dementia (CARE-D).
The model rests on the theory that psychosocial and rehabilitative interventions should address individual symptoms and distinctive neuropsychological profiles to improve quality of life and daily functioning for both the diagnosed individual and the family.3,7,8 Although the model is targeted at mild and moderate stages of a dementia where there may be only one major area of difficulty, it can still be used in later stages when there are more cognitive and behavioral limitations and where the goal is to identify the most disruptive symptom needing intervention.
CARE-D was developed in a multidisciplinary outpatient clinical setting of behavioral neurologists, neuropsychologists, neuropsychiatrists, and social workers and relies on input from different members of the team. CARE-D has 3 components: (1) determination of a patient's neurocognitive profile; (2) psychosocial assessment; and (3) development of specific recommendations and strategies based on a patient's strengths and weaknesses within the context of the social network and environment.
Although any cognitive, behavioral, and motor symptoms can mark the earliest stages of a dementia, this article focus on 4 profiles that have well-established diagnostic criteria and good evidence of their symptoms, course, and biological features: progressive memory, language, visuospatial, and executive/behavioral/comportmental dysfunction. The clinical syndromes that embody these major domains of impairment are DAT, a memory dementia; primary progressive aphasia (PPA), a language dementia; posterior cortical atrophy (PCA), a visuospatial dementia; and behavioral variant frontotemporal dementia (bvFTD), a behavioral-social dementia.3,9–14 Changes in mood, motor function, and sleep patterns also can be present in any of these profiles and need to be addressed in treatment planning but are discussed only briefly.
DETERMINATION OF A PATIENT's NEUROCOGNITIVE PROFILE
The neurocognitive evaluation is completed by a neuropsychologist who conducts a detailed review of attention, language, memory, executive functions, visuospatial skills, and reasoning. A behavioral neurologist or neuropsychiatrist can often identify major areas of weakness with a briefer examination but may not include some of the process analysis that is derived from the neuropsychological evaluation. Findings from these examinations aid in identifying a patient's strengths and weaknesses so that the appropriate dementia care pathway can be implemented.
Memory Profile
Perhaps the most common early symptom of neurodegenerative dementia is amnesia, or loss of episodic, short-term (retentive) memory not due to inattentiveness or lack of motivation. Retentive memory impairment can be caused by damage to the hippo-campus and surrounding components of the temporolimbic system that are responsible for the acquisition and retention of new information.15 Alzheimer neuropathology, for reasons not well understood,16 often targets this region early in the course of illness and typically starts by causing a progressive memory loss; thus, an amnestic dementia profile also is referred to as DAT. The neuropsychological evaluation demonstrates deficits on tests of learning and delayed recall of word lists, stories, and designs and differentiates among distinct stages of memory function, for example, between not being able to retrieve information and not being able to recognize it from choices.
Language Profile
Progressive impairment in language functioning due to neurodegenerative brain disease, also known as PPA, typically presents with trouble thinking of words in conversation, understanding the meaning of words, problems writing, and mistakes in grammar when speaking and/or writing.17 The aphasia is caused by dysfunction of the perisylvian region in the language dominant (usually left) hemisphere of the brain. Neuropsychologists and/or speech-language pathologists assess language functioning by having the individual complete tests that assess the ability to name objects, repeat words, point to objects that are named, read, write, execute commands, and quickly list words related to a cue, such as a specific category (eg, animals) or a specific letter (eg, the letter “F”). They also test an individual's ability to understand the meaning of single words and grammatically simple and complex sentences.
Visuospatial Profile
Visuospatial and other types of visual perceptual changes occur when the brain loses its ability to “make sense” of visual information in the environment. These changes are not due to problems with visual acuity, or “eyesight,” but, instead, individuals with this disorder have difficulty scanning the environment in an informative manner and paying attention to salient information in the periphery or even in the central field of vision. This syndrome, also referred to as PCA,13 is associated with degeneration in the occipitoparietal and temporoparietal areas of the brain,18,19 regions implicated in the interpretation of the nature of visual stimuli and their locations. Neuropsychologists assess visuospatial and perceptual abilities by asking an individual to search for shapes or letters on a page containing many target and nontarget stimuli, draw and copy geometric designs, complete visual puzzles, or estimate angles.
Behavior/Comportment Profile
Changes in behavior and personality are noticeable when an individual acts or behaves uncharacteristically. For example, the once kind and polite individual becomes rude and makes inappropriate comments, violating social norms. Conversely, someone who was always outgoing and gregarious becomes placid and lacks initiative. Sometimes abnormal behaviors are an exaggeration of preexisting personality traits. Research diagnostic criteria have been proposed and validated for this syndrome, which is referred to as bvFTD.12,20 Abnormal behaviors and personality changes arise from frontal network dysfunction. This network regulates emotional reactivity, judgment, and social conduct. Early symptoms in daily life include impaired judgment, perseverating on certain topics, apathy, social disinhibition, impulsivity, and changes in usual emotional reactions (eg, loss of empathy and sympathy). The key to making a diagnosis is not only whether or not a symptom is present but also if it represents a change from a prior customary state.
Additional Psychiatric and Neurologic Symptoms Considered for the Care Pathway Model for Dementia
Dementia can be accompanied by motor symptoms in several types of disease, including motor neuron disease (amyotrophic lateral sclerosis), corticobasal syndrome, progressive supranuclear palsy, and cortical Lewy body disease (LBD). Vascular cognitive impairment caused by cerebrovascular disease can also be associated with motor symptoms. These symptoms reflect that the disease is affecting systems involved with motor coordination, speed, and planning of movement and complex sequences of actions. If motor symptoms exist, care recommendations can include physical and occupational therapy.
Depression and other affective symptoms are known to be present in individuals diagnosed with DAT, PPA,21 and other dementia syndromes22 and can have a negative impact on activities of daily living, progression of disease, and quality of life.23 Affective symptoms can have a significant impact on the implementation of strategies and interventions and need to be considered when making recommendations to patients and caregivers. Mood and other psychiatric symptoms can be assessed by interviewing patients or their caregivers and having them respond to self-report questionnaires, such as the Geriatric Depression Scale24 or the brief questionnaire form of the Neuropsychiatric Inventory25
Some patients may also have disorders of sleep, particularly if the underlying cause is LBD.26 Sleep disorders can often lead to worsening of symptoms during waking hours and are also disruptive to caregivers who may be awakened during the night or, conversely, may have difficulty if a patient falls asleep often during the day. Sleep disorders should be evaluated because treatment may improve quality of life.
THE PSYCHOSOCIAL ASSESSMENT
After a neurocognitive assessment, patients are referred to social workers for a psychosocial assessment, which is an evaluation of a patient and family's mental, physical, and emotional health. Usually, a psychosocial assessment is obtained in a conversational interview with the patient and family to assess how individuals and their families are coping with the illness; their familial and other historical relationships; their emotional, social, and financial resources; and overall strengths and challenges. The psychosocial assessment allows for a holistic understanding of how patients and families are living with dementia symptoms, what resources are needed, and their capacity to reorganize themselves around the changes that the illness brings (and will continue to bring) to their lives.
The psychosocial assessment engages the family in a trusting working relationship to learn how a patient and family view the “problem” in their words. How well do the individual and family understand the symptoms and the connection between symptoms and the diagnosis? How are the cognitive symptoms specifically affecting the person's life and what effects do these changes have on the family? How do memory loss, aphasia, visuospatial deficits, or behavioral changes affect social relationships, safety, personal interests, and ability to participate in meaningful activities? How do the predominant symptoms specifically interfere with the ability to perform routine daily activities and carry out normal day-to-day responsibilities? How do the symptoms affect their mood? What symptoms are identified by the person with dementia and their family as most concerning?
Personal, familial, socioeconomic, community, and cultural influences on the patient and family are also addressed. For example, how is the family as a whole managing and coping? What is most challenging and what financial, emotional, and social resources do they have available over the course of the illness? An understanding of the family's past relational functioning influences how they experience current changes.
The psychological, social, family, and financial issues that affect individuals with young-onset dementia are very different from those that affect individuals with late-onset dementia. Not only are the patients younger but also their families are younger. Job-related tasks cannot be performed when patients find it difficult to carry on conversations or control inconsiderate behaviors or when they are increasingly forgetful. Employers and coworkers do not understand why an individual is having difficulty. Individuals are at risk of receiving poor performance evaluations, bringing a loss of self-esteem and a feeling of diminished productivity, and may be terminated from their positions before the illness is understood or diagnosed. Therefore, persons with young-onset dementia who are often still working have to stop working in the prime of their careers when they are saving for retirement and supporting a young family. Because of the loss of employment, a spouse or partner may need to seek additional work to meet the family's financial needs, or other family members, such as siblings and sometimes even parents, step in to offer financial support. The loss of income also has an impact on the affordability of future long-term care. Many have not saved enough yet and may also have young children they are supporting. Living with young-onset dementia is a defining experience for the entire family. It can have negative and positive ramifications and care must be taken to both understand how everyone is coping and recognize that each person in the family is experiencing the illness in his or her own way.
INTERVENTION STRATEGIES
Outcomes from the neurocognitive and psychosocial assessments are used to identify the most appropriate care pathway for a patient's specific symptoms. Interventions in CARE-D take a holistic approach to address functional impairments that focus on increasing an individual's participation in meaningful, productive activities throughout the course of the disease while maintaining dignity at all times. Cognitive retraining and introduction to compensatory strategies are targeted in the early stages; environmental modifications and compensatory strategies are emphasized in the later stages. Ongoing family/caregiver education and training to promote generalization and implementation of strategies is essential throughout all stages. Home safety and driving evaluations are necessary components of each care pathway. For each care pathway, it is also important to assess an individual's hobbies and interests, motivation, and awareness of deficits as well as the level of caregiver involvement and support.
Memory Care Pathway
The goal of the memory care pathway is to compensate for the inability to retain information and enable an individual to maintain independence through use of external memory aids, memory devices, and environmental modifications. Strategies also aim to reduce frustration and stress using techniques that not only maximize maintaining a routine, structure, and consistency but also provide safety in areas where forgetfulness could result in financial or personal jeopardy. If spontaneous retrieval is impaired but recognition memory is relatively preserved, strategies could use cues/triggers to aid recall. Specific interventions are provided in Table 1. Speech-language pathologists and occupational therapists are best suited to identify appropriate practical strategies for each family.
Table 1.
Four care pathways for dementia: common neuropsychological profiles, deficits in daily living activities, and strategies and interventions
| Common Neurocognitive Findings | Common Symptoms in Daily Living Reflecting Primary Deficit | Common Strategies/Interventions |
|---|---|---|
| Memory Dementia Care Pathway | ||
| Poor orientation to time and place Acquisition deficits: scores are low on tests of learning words, stories, and designs, and, despite repeated trials, cannot increase the amount of information recalled immediately after presentation Retention and retrieval deficits: after a delay even as brief as 3 min, cannot recall the information initially learned Performance does not improve with multiple-choice recognition and the patient may also “recognize” information that was not previously presented (ie, make false-positive identifications) |
Mixes up appointments; takes medications at the wrong times or completely forgets them; does not know the day, date, and, when more advanced, time of day Despite repeated information/instructions, cannot retain information; may ask the same question over and over again within a short interval of time Information evaporates and patients may argue that they were never told the information Cannot use cues to remember; seeing a familiar landmark does not remind the patient of location or direction to move in |
Spaced retrieval training (ie, gradually increasing recall intervals to promote learning)37,38 Errorless learning: learning that occurs in a facilitated environment that eliminates errors39 Vanishing cues hierarchy: the systematic reduction of cue information across learning trials40 Use of memory wallets/books to promote recall of orientation facts, important names, past and recent events, and other functional facts, such as safety precautions41 External or electronic memory aids, such as schedule boards42,43 Environmental modifications to improve participation in activities of daily living and safety (eg, lighting, flooring, color schemes, wall hangings, furniture, and noise/sound) Ongoing training of caregivers/family members on the use of positive communication strategies and the importance of avoiding questions that rely on short-term memory also contributes to a reduction in difficult behaviors44 Safety devices, such as GPS personal locator devices, home monitor device, adaptive telephones, identification bracelets |
| Language Dementia Care Pathway | ||
| Low scores on tests of object naming; makes errors (may say “microscope” instead of “stethoscope”) May not understand single words even when they are common (eg, asking “What is salt?”) or sentences that are grammatically complex (eg, “Who is the boy that John kicked?”) Low scores on tests of reading comprehension and writing |
Gropes for words so that speech is interrupted by long pauses for word-finding Cannot carry out instructions; may misunderstand what is being said, despite normal hearing, and misinterpret messages, which can lead to anger and even paranoia Cannot understand written communications (newspapers, books); cannot write normally; makes errors in spelling and/or grammar |
Self-cueing strategies (eg, semantic circumlocution) Home exercise program targeting the rehearsal of personally relevant words Oral reading tasks, including strategies to increase motor sequencing for multisyllabic words (eg, syllabic segmentation, Melodic Intonation Therapy)45 Personal picture description tasks Script rehearsal for telephone conversations and in social contexts Communication tools to supplement spoken language as the disease progresses include use of a communication wallet, communication boards/book, and possibly an augmentative and alternate communication device29,46 Strategies to help an individual compensate for dyslexia and dysgraphia, including technology that provides auditory and visual cues to promote reading comprehension; for dysgraphia, speech recognition apps and word prediction features can help facilitate functional writing tasks |
| Visuospatial Dementia Care Pathway | ||
| Low scores on tests requiring spatial perception, such as deciding if 2 lines are at the same angle as a model Difficulty finding specific targets on a sheet containing many different types of letters or shapes; search strategy is unsystematic and patient may miss many targets despite normal visual acuity Difficulty drawing a clock or copying a simple geometric figure; difficulty putting blocks together to make a visual pattern Facial discrimination problems and inability to decide if 2 faces seen from different perspectives are the same person or not |
Misjudging distance and relationship of body to external space, which can result in car accidents or scrapes when trying to park or pass between fences Difficulty searching for household items; items may be in plain view and, especially if surrounded by other items (as an item in a drawer or in a refrigerator), are “invisible” to the patient Inability to place objects on a table surface; difficulty assembling kitchen utensils; difficulty orienting clothing to one's body or working knobs and accessories in the car Misrecognizing people; not able to easily recognize facial differences Larger letters can be more difficult to recognize due to “simultanagnosia” |
Environmental modifications: increasing organization around the home, decreasing clutter, using proper lighting, controlling glare, and increasing contrast Use of technology for compensation: talking watches/alarm clocks, a large numbered phone, a reading machine, a magnifier with a light feature, and a variety of voice-activated enabled smartphones Reduce the size of print for individuals with simultanagnosia |
| Behavior Dementia Care Pathway | ||
| Has difficulty reasoning and performing cognitive shifting tasks Loses knowledge about what is socially acceptable in different settings Cannot inhibit automatic responses May incorporate stimuli irrelevant to the task at hand because they are accessible, known as stimulus-bound behavior |
Makes poor decisions, which may jeopardize financial or personal safety or the safety of others; thinking is rigid; cannot come up with or understand alternative solutions to problems Engages in embarrassing behaviors (eg, talking loud or laughing during a funeral service); makes insulting remarks; sloppy eating habits May unintentionally engage in criminal behavior (eg, “shoplifting”); touches objects; may dial the phone just because it is there |
Communication tips or visual cue strategies to best respond to behavioral changes, such as agitation disinhibition, poor judgment, etc.47 Emotional support and respite care for the primary caregiver Safety devices, such as GPS personal locator devices, home monitor device, adaptive telephones, identification bracelets |
Language Care Pathway
Strategies for the language care pathway aim to support communication with the affected individual by offering education and communication tips for family members and augmentative communication methods so that a person with dementia can maintain independence in daily activities. A combination of impairment-directed interventions that are focused on enhancing a particular area of deficiency (eg, improving motor sequencing for patients with apraxia of speech) and activity/participation-based interventions that are focused on the patient participating in a particular action or activity in daily life (eg, training a patient to use an augmentative and alternate communication device to help participate in a conversation)27 are often appropriate, with frequent adjustments to the recommended strategies to meet an individual's changing communication needs as the disease progresses. Interventions should focus on personally relevant stimuli to generalize to functional contexts as much as possible (see Table 1 for example strategies).28–30 A speech-language pathologist is the most relevant referral for this pathway.
Visuospatial Care Pathway
For those individuals experiencing perceptual or visuospatial deficits, the goal of the visuospatial care pathway is to modify the home environment to accommodate these changes and incorporate the use of technology to improve safety and independence. Table 1 provides examples of environmental and technological modifications that are often used by occupational therapists. In addition to referrals to an occupational therapist, specialized services for individuals with low vision may be useful, even though the main obstacle is not visual acuity.
Behavior Care Pathway
The goal of the behavior care pathway is to maximize safety for a person with dementia who lacks judgment and decision-making ability and to minimize the stress of family members involved in the care by replacing confrontation with alternative responses to behavior changes (see Table 1 for example strategies).30 Many of the strategies for this pathway require implementation by family members rather than the affected individual who often lacks the initiative to follow instructions. Psychiatrists and social workers are most commonly consulted but speech-language pathologists and occupational therapists may contribute by providing recommendations for home safety and assessing the amount of supervision needed at home and in the community.
PROCEDURES OF THE CARE PATHWAY PROGRAM
The CARE-D program grew out of a seed grant obtained in 2010 to pilot test the role of a Care Pathway Resource Coordinator to connect individual clinic patients and families with services and maintain communication among all involved. The experience from this initial trial resulted in reconfiguring the model not to create a bottleneck for services but to implement a clinic procedure in which all clinicians participate. After the clinical evaluation, typically neurologic and neuropsychological examinations, a patient is discussed at weekly clinic management rounds attended by speech and occupational therapists and social workers. A list of needed professionals is generated and the patient is referred directly to the one(s) identified as most central in the care pathway. All patients and families are also referred to social work. Continuity of care is maintained through medical record communication and feedback at the weekly meetings about how the care plan is proceeding and if any changes are needed. The ability for the clinicians and care providers to communicate efficiently with one another is a sine qua non of this program.
Costs are reimbursable by Medicare Part B and other insurance providers as part of a person's care plan. Speech and occupational therapies are provided both at home and in the outpatient clinical setting based on the specific needs of the individual and family.
The interdisciplinary nature of the CARE-D model, what is believed to be its success, requires increased attention be paid to the clinical training and education received in dementia not only within schools of medicine but also within schools of speech, occupational therapies, social work, and other allied health professions who come into contact with persons with dementia and have the opportunity to contribute to their optimal level of functioning throughout the course of the illness. Recognizing the need of these professions for specialized dementia education, the Northwestern clinical team is committed to continuing education and training of those with whom we work to provide optimal care and treatment. For example, in collaboration with the Association for Frontotemporal Degeneration and the National Aphasia Association, a 3-part webinar series on PPA and other clinical dementia syndromes for speech-language pathologists was designed and implemented36 and is accessible free of charge (http://www.brain.northwestern.edu/about/events/webinar.html). Another helpful source is the International PPA Connection Web site (http://www.ppaconnection.org), which provides resources and support for clinicians, researchers, patients, and family members.
DISCUSSION
CARE-D builds a tailored care plan based on data from an individual's psychosocial and neuropsychological assessments. The psychosocial context is an essential component and consideration is given to patients’ and families’ living situations, social support systems, life stages, financial resources, and understanding of their preexisting coping strategies and historical relationships. This ensures that the recommendations are realistic for families, from both psychological and practical perspectives. For an individual who has dementia, the interventions focus on the person's abilities and strengths. The introduction of tailored interventions and activities may allow family and friends to focus more on their relationship with the person as a spouse/partner, adult child, sibling, or friend rather than their role as a caregiver. The ultimate goal is to enhance quality of life for all as they significantly restructure their lives to cope with an individual's progressive disability.
The CARE-D model relies on a skilled interdisciplinary team to be successful. The evaluation by a neuropsychologist to establish the neurocognitive profile and symptoms as well as remaining strengths is vital to establishing the interventions. The CARE-D model has been developed in close collaboration with speech and occupational therapists knowledgeable about dementia and the neurodegenerative nature of the illness, who focus on compensatory strategies and remaining abilities. Thus, persons with dementia and their families are provided with helpful methods to manage the changes and contribute to a better quality of life. Understanding the strengths of the family, their relationships, and the context in which they are able to cope with and implement the proposed recommendations informs the team of what challenges the family is facing and what psychosocial support is needed to help sustain the family over time.
Finally, for this model to have wider replication in other clinic and primary care settings, research is needed to quantify and identify measureable outcomes. There is the potential for this model to have an impact on the larger health care system. By making recommendations that target symptom-specific profiles, disease stage, and life stage and focus on remaining strengths, this approach to care may help reduce unnecessary health care costs associated with improper/inadequate care, reduce hospitalizations, and delay long-term care placement. The model recognizes the complexity of dementia syndromes and the unique needs of each person with dementia and the families. This is particularly important because dementia is being diagnosed at earlier stages than in the past, at a time when cognitive impairments may be isolated to 1 or 2 cognitive and/or behavioral domains and may remain that way for several years prior to more generalized impairment.
Table 2.
Memory dementia care pathway vignette
| How Memory Loss is Affecting Betty's Daily Life | Betty's Identified Strengths | Corresponding Recommendations for Betty |
|---|---|---|
| Misplacing items | Visuospatial function intact | Implemented a system at home where the most important items (cell phone, purse, keys, etc.) are left in one well-marked spot in the house. A home occupational therapy consultation was ordered to focus on memory and organizational strategies to reduce clutter and to create systems for better organization of personal belongings, paperwork, and bathroom and kitchen items for improved activities of daily living performance. |
| Forgetting/missing appointments | Able to read | Advised Betty to use a large calendar on which she could write many details about her appointments including time, location, topic, and phone number. Set up a reminder system for her family to check her calendar on a daily basis. Implemented use of a dry-erase board next to the calendar to list times of daily activities and other important reminders. Betty crossed off each day of the calendar as it passed, using a clock next to the calendar that displayed the time and date. |
| Withdrawing from activities, including reading and socializing with friends | Only minor problems with language functioning | Modified activities, which do not rely on memory but fit Betty's interests, including water aerobics and using a computer with a visual memory aid displaying a picture and short description for each step for checking and sending e-mails. Referral made to speech-language therapy at home to help formulate visual memory aids. Family trained to assist Betty with writing down 2 stimulating activities per day on her dry-erase board. Audio and visual reminders set up by family on her phone to increase initiation of the selected activities. |
Table 3.
Language dementia care pathway vignette
| How the Aphasia is Affecting Margaret's Daily Life | Margaret's Identified Strengths | Corresponding Recommendations for Margaret |
|---|---|---|
| Margaret is less active socially and finds it hard to stay busy when her partner is at work. She often watches TV and wishes she had more to do. | She is independent in most of her personal activities of daily living. | New modified community activities were suggested, including volunteer work that does not rely heavily on communication with others, such as walking dogs for a local shelter. |
| Margaret has word finding pauses and hesitations and increased difficulty with auditory comprehension at the conversation level. Spontaneous speech is especially difficult when speaking over the telephone. | Margaret has preserved memory for recent events; no behavioral changes have been reported. | Create a communication friendly environment by reducing distractions, using one-on-one conversations and speaking in simple sentences to facilitate auditory comprehension. Communication enhancement strategies (eg, family asking more “yes-no” questions and Margaret using gestures to help explain what she is trying to say). Speech therapy focused on creating home program targeting the rehearsal of personally relevant words, including names, important locations, and other important words she uses during daily conversations. Telephone scripts were designed and rehearsed to promote word retrieval and fluency during telephone conversations. |
| Her partner and children are less likely to converse with her for fear of creating frustrating situations when she cannot find a word. | She has preserved insight into her condition and resulting changes. | Speech-language pathologist trained Margaret and her family to create a system for when to help fill in the words for her during conversation. Also trained family on appropriate verbal cues to increase Margaret's use of semantic circumlocution, so she can self-cue or communicate her message more easily to others. A communication wallet was also created, which contains lists of words by category that Margaret frequently uses during conversations with family and friends. Margaret was trained to use the wallet when unable to retrieve the word. Education on the disease and support was provided for her young sons and her partner. Directed family to the Association for Frontotemporal Dementia Web site for kids and teens http://www.aftdkidsandteens.org. |
| Errors with spelling | She is interested in writing e-mails to friends and family. | Used augmentative technology, such as the Google application or Dragon software on Margaret's smartphone to assist her with spelling. With this application, she could speak the word and the device used speech recognition software to spell out the word for her. |
Table 4.
Visuospatial dementia care pathway vignette
| How the Visuospatial Perceptual Deficits are Affecting Steve's Daily Life | Steve's Identified Strengths | Corresponding Recommendations for Steve |
|---|---|---|
| Steve enjoyed an active social life but lately has withdrawn from socializing with others due to impaired driving ability. | He maintains an interest in socializing with friends and he has no problems with verbal communication. | New modified community activities that do not rely on Steve's visual perception, such as attending art- and music-based activities and programs in the community accompanied by a companion so he does not need to drive. |
| Steve is having difficulty writing and using a calendar. | He has only minor problems with short-term memory and no other cognitive impairments at this time. | Referral to an occupational therapist to increase use of compensatory strategies for schedule management, including selection of visually simple calendar, use of highlighting and color coding, and use of block lettering with space in between each letter. A large display clock with the time and date was recommended. |
| Due to his visual changes, there are safety risks for activities within his home including cooking, ironing, and driving. | Steve is independent in all his personal activities of daily living. | Introduced environmental changes, including the use of bright lights, decluttering, and removing throw rugs to decrease falls. In the kitchen Steve was encouraged to use labels to help him find objects and organize the refrigerator so that he could find objects based on location (eg, “second shelf”) if he could not “see” them. The occupational therapist also reviewed safety issues at home with Steve's wife. |
| He walks more slowly and hesitates when on sidewalks with curbs and while using stairs. | Steve is physically strong and has good balance. | Referral to a local low vision support program for additional services to accommodate visual changes. |
Table 5.
Behavior dementia care pathway vignette
| How the Behavioral Deficits are Affecting Sam's Daily Life | Sam's Identified Strengths | Corresponding Recommendations for Sam |
|---|---|---|
| Fixating on topics for long periods of time | Enjoys wood working | Communication strategies were provided for Sam's wife; for example, using distraction when Sam fixates on topics. During the appointment, Sam's wife and a therapist role-played this strategy using the example of refocusing Sam's attention to an enjoyable activity, such as woodworking. Written cues in a memory wallet were also introduced to decrease perseveration on topics and to increase initiation of meaningful questions during conversations with family members. |
| Taking an extended time dressing and getting ready in the morning due to changes in initiation | Can follow simple written instructions | Step-by-step instructions with images to help Sam get ready in the morning were designed to assist Sam with brushing his teeth and hair. A referral was made to speech-language therapy in the home to help formulate these visual memory aids. |
| Problems with motivation and making decisions | Performs well with structured routine and participates in some household work | A daily schedule of activities was created so that Sam was not faced with decisions and was offered structure. Activities were written on a dry-erase schedule board to increase recall of past/upcoming activities. Activities focused on his interests in a modified and simplified way. For example, instead of using the woodworking equipment, Sam spent time sanding various pieces of wood for a project that his friend later helped him put together. |
| Makes inappropriate comments to strangers that are embarrassing to his wife | Sam's wife was counseled on strategies to help her cope with Sam's disinhibition. These included disclosing the diagnosis to close friends and family to increase their awareness and, as much as possible, avoiding public situations where Sam's behavior could be particularly troubling to her. Sam's wife was also given a card that she could show to strangers briefly explaining Sam's diagnosis and asking for their patience and understanding. Sam was referred to a behavioral home health program and a psychiatrist to assist with managing behavior changes. Sam's wife was referred to a frontotemporal dementia caregiver support group to help her cope with Sam's changes as well as help guide her with long-term planning. |
|
| Frequent irritability | The social worker explored and identified triggers for Sam's irritability and counseled family on interventions that may help to avoid these symptoms, such as playing calming music and remaining nonconfrontational. |
KEY POINTS.
Individuals with young-onset dementia can differ dramatically in the types of symptoms they express; therefore, a one-size-fits-all model of care for dementia is inadequate for this population.
The Care Pathway Model for Dementia (CARE-D) prescribes tailored care based on results from psychosocial and neuropsychological assessments.
Interventions focus on a person's abilities and strengths and are adapted over time as needs and abilities change.
The psychosocial context is an essential component. Consideration should be given to the living situation, social supports, life stage, financial resources, and individual's and family's preexisting coping strategies.
The goal is to enhance quality of life by maximizing independence and safety, identifying helpful modifications to activities and the environment, and providing emotional support for individuals with young-onset dementia and their families.
Case Vignettes: Care Pathway Model for Dementia In Action.
Memory dementia care pathway
Betty (age 62) was given a clinical diagnosis of DAT 2 years earlier. Bill, to whom Betty had been married 35 years, continued to work full time in the family business. Their 3 daughters lived nearby and tried to spend a lot of time with her but had a difficult time knowing what might be most helpful for their mother and were torn between helping their mother and caring for their own school-age children. Betty worked as a grade school teacher until it became too difficult to manage her lesson plans. She enjoyed learning new skills through adult learning classes in her community. She attended Catholic Mass every Sunday. Neuropsychological examination was completed and episodic memory was the primary problem, with other domains either normal or less impaired than memory. For example, on the Repeatable Battery for the Assessment of Neuropsychological Status Update,31 she was able to learn 8 of 10 words on the list learning subtest but after a brief delay could recall only 2 and could not recognize all the words on multiple choice. In contrast, she had no difficulty copying a complex figure, her digit span was 8 forward and 6 backward, and she was able to achieve a normal number of categories on the Wisconsin Card Sorting Test (WCST).32 Practical interventions were aimed at providing supports for impaired short-term memory.
Psychosocial assessment established that Betty was frustrated with being forced to give up driving. Her increased dependency on her family was straining her relationships with her husband and her daughters as she attempted to hold on to as much independence as possible. Reciprocally, her daughters did not know how much to assist their mother and what they could allow her to do safely. Helping Betty maintain her independence by focusing on her remaining strengths and identifying helpful strategies to compensate for changes helped reduce her frustration and stress. Psychosocial interventions of counseling and education helped the family know when to step in to help, when to step back and support Betty's autonomy, and how to balance their mother's needs with those of their children and identify and address their own emotional needs for self-care. Table 2 delineates the symptoms, strengths, and recommendations for this pathway.
Language dementia care pathway
Margaret (age 52) was diagnosed with PPA 3 years earlier. She obtained a PhD in education and, after a career of teaching at the university level, she became dean at a local college. Margaret was forced to stop working when communication problems began to interfere with her ability to deliver her classroom lectures and interact with her colleagues. She also had difficulty finishing a book that she started writing a few years earlier. Margaret, with her partner of 23 years, had 2 sons, ages 10 and 12. Her interests included reading biographies and traveling. Margaret's partner worked full time.
Neuropsychological testing found language the primary problem. Although Margaret could understand single words, she had difficulty when presented with complex grammatical constructions and multistep commands. She also had significant difficulty naming objects. On the Boston Naming Test,33 she scored 35 of a possible 60. Her errors indicated that she recognized the objects but had difficulty retrieving the words without a phonemic cue. Finally, word-finding difficulty was also evident in spontaneous speech. When trying to tell the “Picnic” story from the Western Aphasia Battery-Revised,34 she had frequent hesitations before nouns and often provided circumlocution for words she could not retrieve.
The psychosocial assessment brought to light a close supportive relationship between Margaret and her partner who adjusted his work life as a journalist to accommodate her increasing needs and to focus on the care of their 2 children. Finances were a major concern. An application was submitted for Social Security Disability Insurance and they consulted with an elder law attorney for estate planning. Margaret's awareness of her declining abilities contributed to her depressed mood, and her partner, although supportive, expressed much grief at the losses they were enduring as a family. Disclosing the diagnosis to their young sons was aided by consulting the Association for Frontotemporal Degeneration's specialized resources (www.aftdkidsandteens.org). The goal of CARE-D intervention was to support Margaret's communication and enhance mood by offering education and communication strategies for her family. The program also provided methods for Margaret to maintain independence in daily activities and engage in meaningful activities. Table 3 identifies symptoms, strengths, and CARE-D recommendations for Margaret.
Visuospatial dementia care pathway
Steve (age 57) was diagnosed 2 months earlier with visuospatial dysfunction (PCA). Steve lived with his spouse of 25 years in their home. His spouse worked a demanding full-time job at the peak of her career. They had 1 adopted daughter, who was married with a newborn child. Neuropsychological testing found visual perception as the primary problem. Visual target search was erratic and he missed many items on both sides of the page. Constructions were severely impaired and he was unable to complete the Trail Making Tests due to difficulty visually locating the appropriate next item, even though he had not lost his sequencing ability. In contrast, despite difficulty initially acquiring a list of words, he was able to retain the amount he initially learned after a delay. In daily life, the words seemed to “jump off the page” while reading, he had difficulty accurately reaching for objects, and driving became harrowing due to his problems with depth perception and getting lost. Steve also began having difficulty navigating his home as the illness began to eventually affect gait and balance.
An in-depth psychosocial evaluation revealed that Steve and his wife had experienced a troubled relationship on and off throughout their marriage. Steve's wife resented his increasing dependence on her, because this interfered with her demanding workload. Steve called his wife several times during the day because he had more trouble navigating his world independently. Steve's wife admitted that she feared that her goals of achieving a higher-level position, in the work that she loved, would be compromised by the illness because Steve required so much of her attention. Fortunately, Steve and his wife had a long-term care insurance policy that allowed her to hire care at home while she worked. This helped relieve some of the stress she experienced, and she sought counseling and participated in a support group to help manage her feelings of anger and grief. Occupational therapy recommendations for changes in Steve's home environment helped accommodate visuospatial deficits and improve his safety and independence. Table 4 identifies Steve's symptoms, strengths, and CARE-D recommendations.
Behavior dementia care pathway
Sam (age 61) was diagnosed with bvFTD 4 years earlier. Ruth, who is Sam's wife of 23 years, worked full time as a journalist. Her daughter from her previous marriage lived nearby. Sam had 2 adult children from a previous marriage; however, they were estranged. Sam was a law professor for 30 years, until he was forced to retire due to his symptoms. Sam used to be active in his community through a civic club and politics but had not been involved in these activities for the past 10 years. Neuropsychological testing identified that reasoning and comportment were Sam's primary areas of difficulty. Although Sam's scores on tests of episodic memory and even on the executive attention test, Trail Making Test, Part B, were normal, on the WCST he had difficulty with mental flexibility and switching from one category to another. Ruth identified significant changes on the Frontal Systems Behavioral Scale,35 including disinhibition, which was not part of his personality in the past. In addition, he also had signs of increasing executive dysfunction and apathy in daily life. Practical interventions were based on addressing obstreperous behaviors and maintaining a regular schedule.
Psychosocial assessment revealed that Sam's increased apathy over several years and misdiagnosis of his illness had led his wife to consider divorce. She expressed being overwhelmed and confused regarding how to respond to her husband's lack of emotion for her, in addition to the grief over the loss of their close relationship and dreams for the future. Interventions focused on maximizing Sam's safety and helping Ruth learn communication strategies while minimizing the daily stress she experienced. Table 5 delineates the symptoms, strengths, and recommendations.
Abbreviations
- bvFTD
Behavioral variant frontotemporal dementia
- CARE-D
Care Pathway Model for Dementia
- DAT
Dementia of the Alzheimer type
- LBD
Cortical Lewy body disease
- PCA
Posterior cortical atrophy
- PPA
Primary progressive aphasia
- WCST
Wisconsin Card Sorting Test
Footnotes
Disclosures: B. Khayum is the president and a speech pathologist of MemoryCare Corporation. The other authors have no disclosures to report.
REFERENCES
- 1.Alzheimer's Association . Early-onset dementia: a national challenge, a future crisis. Alzheimer's Association; Washington, DC: 2006. [Google Scholar]
- 2.McKhann GM, Albert MS, Grossman M, et al. Clinical and pathological diagnosis of frontotemporal dementia. Arch Neurol. 2001;58:1803–9. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 3.Weintraub S, Mesulam MM. Four neuropsychological profiles in dementia. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Elsevier; Amsterdam: 1993. pp. 253–81. [Google Scholar]
- 4.Price BH, Gurvit H, Weintraub S, et al. Neuropsychological patterns and language deficits in 20 consecutive cases of autopsy-confirmed Alzheimer's disease. Arch Neurol. 1993;50:931–7. doi: 10.1001/archneur.1993.00540090038008. [DOI] [PubMed] [Google Scholar]
- 5.Freyne A, Kidd N, Coen R, et al. Burden in carers of dementia patients: higher levels in carers of younger sufferers. Int J Geriatr Psychiatry. 1999;14:784–8. [PubMed] [Google Scholar]
- 6.Gitlin LN, Winter L, Vause Earland T, et al. The Tailored Activity Program to reduce behavioral symptoms in individuals with dementia: feasibility, acceptability, and replication potential. Gerontologist. 2009;49:428–39. doi: 10.1093/geront/gnp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weintraub S, Morhardt D. Treatment, education, and resources for non-alzheimer dementia: one size does not fit all. Alzheimer's Care Today. 2005;6:201–14. [Google Scholar]
- 8.Weintraub S. Neuropsychological assessment of dementia: a large-scale neuro-anatomical network approach. In: Dickerson BC, Atri A, editors. Dementia: comprehensive principles and practice. Oxford University Press; New York: 2014. pp. 487–507. [Google Scholar]
- 9.Weintraub S, Mesulam MM. From neuronal networks to dementia: four clinical profiles. In: Foret F, Christen Y, Boller F, editors. La Demence: Pourquoi? Foundation Nationale de Gerontologie; Paris: 1996. pp. 75–97. [Google Scholar]
- 10.Weintraub S, Rubin NP, Mesulam MM. Primary progressive aphasia. Longitudinal course, neuropsychological profile, and language features. Arch Neurol. 1990;47:1329–35. doi: 10.1001/archneur.1990.00530120075013. [DOI] [PubMed] [Google Scholar]
- 11.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rascovsky K, Hodges JR, Kipps CM, et al. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): current limitations and future directions. Alzheimer Dis Assoc Disord. 2007;21:S14–8. doi: 10.1097/WAD.0b013e31815c3445. [DOI] [PubMed] [Google Scholar]
- 13.Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch Neurol. 1988;45:789–93. doi: 10.1001/archneur.1988.00520310107024. [DOI] [PubMed] [Google Scholar]
- 14.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2004;82:171–7. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Mesulam MM. Neuroplasticity failure in Alzheimer's disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–9. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 17.Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49:425–32. [PubMed] [Google Scholar]
- 18.Mendez MF, Perryman KM. Neuropsychiatric features of frontotemporal dementia: evaluation of consensus criteria and review. J Neuropsychiatry Clin Neurosci. 2002;14:424–9. doi: 10.1176/jnp.14.4.424. [DOI] [PubMed] [Google Scholar]
- 19.Renner JA, Burns JM, Hou CE, et al. Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology. 2004;63:1175–80. doi: 10.1212/01.wnl.0000140290.80962.bf. [DOI] [PubMed] [Google Scholar]
- 20.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medina J, Weintraub S. Depression in primary progressive aphasia. J Geriatr Psychiatry Neurol. 2007;20:153–60. doi: 10.1177/0891988707303603. [DOI] [PubMed] [Google Scholar]
- 22.Ballard C, Patel A, Oyebode F, et al. Cognitive decline in patients with Alzheimer's disease, vascular dementia and senile dementia of Lewy body type. Age Ageing. 1996;25:209–13. doi: 10.1093/ageing/25.3.209. [DOI] [PubMed] [Google Scholar]
- 23.Lyketsos CG, Steele C, Baker L, et al. Major and minor depression in Alzheimer's disease: prevalence and impact. J Neuropsychiatry Clin Neurosci. 1997;9:556–61. doi: 10.1176/jnp.9.4.556. [DOI] [PubMed] [Google Scholar]
- 24.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 25.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–9. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 26.Ferman TJ, Boeve BF, Smith GE, et al. REM sleep behavior disorder and dementia: cognitive differences when compared with AD. Neurology. 1999;52:951–7. doi: 10.1212/wnl.52.5.951. [DOI] [PubMed] [Google Scholar]
- 27.Croot K, Nickels L, Laurence F, et al. Impairment- and activity/participation-directed interventions in progressive language impairment: clinical and theoretical issues. Aphasiology. 2009;23:125–60. [Google Scholar]
- 28.Khayum B, Wieneke C, Rogalski E, et al. Thinking outside the stroke: treating Primary Progressive Aphasia (PPA). Perspect Gerontol. 2012;17(2):37–49. doi: 10.1044/gero17.2.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor C, Kingma RM, Croot K, et al. Speech pathology services for primary progressive aphasia: exploring an emerging area of practice. Aphasiology. 2009;23:161–74. [Google Scholar]
- 30.Kortte KB, Rogalski EJ. Behavioural interventions for enhancing life participation in behavioural variant frontotemporal dementia and primary progressive aphasia. Int Rev Psychiatry. 2013;25:237–45. doi: 10.3109/09540261.2012.751017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Randolph C. Repeatable battery for the assessment of neuropsychological status update. Pearson; San Antonio (TX): 2012. [DOI] [PubMed] [Google Scholar]
- 32.Heaton RK. Wisconsin card sorting test (WCST) manual revised and expanded. Psychological Assessment Resources; Odessa (FL): 1993. [Google Scholar]
- 33.Goodglass H, Kaplan E, Weintraub S. Boston naming test. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- 34.Kertesz A. Western aphasia battery - revised (WAB-R) Pro-Ed; Austin (TX): 2006. [Google Scholar]
- 35.Grace J, Mallory PF. Frontal systems behavioral scale professional manual. Psychological Assessment Resources Inc; Lutz, FL: 2001. [Google Scholar]
- 36.Ganzfried E, Morhardt D, Denny S. The SLP & Primary Progressive Aphasia (PPA): developing an education coalition. American Speech and Hearing Association Annual Convention; Chicago: 2013. [Google Scholar]
- 37.Camp CJ. Spaced retrieval: a model for dissemination of a cognitive intervention for persons with dementia. In: Attix DK, Welsh-Bohmer KA, editors. Geriatric neuropsychology: assessment and intervention. The Guilford Press; New York: 2006. pp. 275–92. [Google Scholar]
- 38.Hopper T, Bourgeois M, Pimentel J, et al. An evidence-based systematic review on cognitive interventions for individuals with dementia. Am J Speech Lang Pathol. 2013;22:126–45. doi: 10.1044/1058-0360(2012/11-0137). [DOI] [PubMed] [Google Scholar]
- 39.Clare L, Jones RS. Errorless learning in the rehabilitation of memory impairment: a critical review. Neuropsychol Rev. 2008;18:1–23. doi: 10.1007/s11065-008-9051-4. [DOI] [PubMed] [Google Scholar]
- 40.Glisky EL, Schacter DL, Tulving E. Learning and retention of computer-related vocabulary in memory-impaired patients: method of vanishing cues. J Clin Exp Neuropsychol. 1986;8:292–312. doi: 10.1080/01688638608401320. [DOI] [PubMed] [Google Scholar]
- 41.Bourgeois MS, Camp C, Rose M, et al. A comparison of training strategies to enhance use of external aids by persons with dementia. J Commun Disord. 2003;36:361–78. doi: 10.1016/s0021-9924(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 42.Oriani M, Moniz-Cook E, Binetti G, et al. An electronic memory aid to support prospective memory in patients in the early stages of Alzheimer's disease: a pilot study. Aging Ment Health. 2003;7:22–7. doi: 10.1080/1360786021000045863. [DOI] [PubMed] [Google Scholar]
- 43.Lancioni GE, Singh NN, O'Reilly MF, et al. Technology-aided verbal instructions to help persons with mild or moderate Alzheimer's disease perform daily activities. Res Dev Disabil. 2010;31:1240–50. doi: 10.1016/j.ridd.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 44.Egan M, Berube D, Racine G, et al. Methods to enhance verbal communication between individuals with Alzheimer's disease and their formal and informal caregivers: a systematic review. Int J Alzheimers Dis. 2010:2010. doi: 10.4061/2010/906818. http://dx.doi. org/10.4061/2010/906818. [DOI] [PMC free article] [PubMed]
- 45.Norton A, Zipse L, Marchina S, et al. Melodic Intonation Therapy. Annals of the New York Academy of Sciences. 2009;1169:431–6. doi: 10.1111/j.1749-6632.2009.04859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beukelman DR, Fager S, Ball L, et al. AAC for adults with acquired neurological conditions: a review. Augment Altern Commun. 2007;23:230–42. doi: 10.1080/07434610701553668. [DOI] [PubMed] [Google Scholar]
- 47.Logsdon RG, McCurry SM, Teri L. Evidence-based psychological treatments for disruptive behaviors in individuals with dementia. Psychol Aging. 2007;22:28–36. doi: 10.1037/0882-7974.22.1.28. [DOI] [PubMed] [Google Scholar]