Abstract
Immunotherapy in the context of treated HIV-1 infection aims to improve immune responses to achieve better control of the virus. To date, multifaceted immunotherapeutic approaches have been shown to reduce immune activation and increase CD4 T-lymphocyte counts, further to the effects of antiretroviral therapy alone, in addition to improving HIV-1-specific T-cell responses. While sterilizing cure of HIV-1 would involve elimination of all replication-competent virus, a functional cure in which the host has long-lasting control of viral replication may be more feasible. In this commentary, we discuss novel strategies aimed at targeting the latent viral reservoir with cure of HIV-1 infection being the ultimate goal, an achievement that would have considerable impact on worldwide HIV-1 infection.
Keywords: antiretroviral therapy, functional cure, HIV-1, immunotherapy, retroviral persistence, T-cell responses, therapeutic vaccines
Introduction
Combination antiretroviral therapy (cART) reduces morbidity and mortality in HIV-1-infected individuals;1 however, it cannot eradicate the virus and does not enable the immune system to control HIV-1 replication.2,3 Viral reservoirs persist and viral rebound is seen following treatment interruption.4-7 Immunotherapy in treated HIV-1 infection aims to diminish viral reservoirs by reversing T-cell dysfunction and modifying immune responses to mirror those found in long-term nonprogressors or HIV controllers.8,9 Ideally, immunotherapeutic approaches targeting the vulnerable T-cell population would induce and maintain HIV-1-specific responses, increase polyfunctionality, enhance thymic function, reduce levels of immune activation and reverse T-cell anergy and exhaustion. Novel approaches may further benefit clinical outcomes by providing more sustained viral control, preventing reservoir re-seeding, reducing risk of both HIV-1-related and inflammation-related morbidity and mortality,10 and most optimistically even eradicating the reservoir.7 In this context, the rational design of interventions must take into consideration the sequence, timing, dosage and kinetics of immunotherapeutic agents, in order to optimize the desired immune response. A major target in HIV-1 eradication is to reactivate latent integrated provirus in cART-treated patients,7 however reports suggest that current latency reversal agents alone are not sufficient to expunge the HIV-1 reservoir.8 The induction of robust virus-specific cell-mediated responses that will eliminate reactivated provirus is thought to be key.11 The same immunological principles may apply in the absence of eradication where functional cure is achieved by maintenance of viral control,9 hence the renewed interest in therapeutic vaccines and immunotherapies to be used in conjunction with candidate latency reversal agents.
Early Initiation of cART Facilitates Efficacious Immunotherapeutic Intervention
Studies seeking to optimize the timing and duration of cART have highlighted the advantages of early intervention with antiretroviral drugs. Initiation of cART in early HIV-1 infection results in lower residual viral reservoirs compared to levels observed in individuals treated during chronic HIV-1 infection.2 Moreover, early treatment with cART restrains the level of immune activation and facilitates improved polyfunctionality of HIV-1-specific T-cell responses.12 Early treatment also prevents the latent viral reservoir from becoming dominated by HIV-1 variants that have mutated to escape cytotoxic T lymphocytes (CTL); this has implications for both therapeutic vaccine design and HIV cure strategies.13 Furthermore, earlier initiation of cART and higher nadir CD4 T-cell counts have been associated with the preservation of functional recall T-cell responses.14 In a study investigating interleukin (IL)-2 administration and immunization with the therapeutic HIV-1 vaccine Remune™, functional responses were not boosted in cART-treated HIV-1-infected patients who had low pre-cART CD4 T-cell counts.15 This lack of immune recovery was postulated to be due to protracted immunosuppression and/or clonal dysfunction,15 and differed from previous Remune™ studies which demonstrated proliferative responses in patients that had both higher nadir and pre-immunotherapy baseline CD4 T-cell counts.16 For this reason, patients enrolled in our recent Phase I immunotherapy trial (ClinicalTrials.gov Identifier: NCT01130376) were carefully considered in terms of CD4 T-cell counts and duration of suppressed viraemia (Fig. 1).17 On an individual patient basis, those demonstrating the greatest benefit from immunotherapy (with improved clinical and immunological parameters) received cART at earlier stages following infection, which concurs with a number of reports.18,19 There is an emerging consensus that early initiation of cART is an essential aspect of long-term control of HIV-1 and likely to be critical in future latency reversal approaches.19
Figure 1.
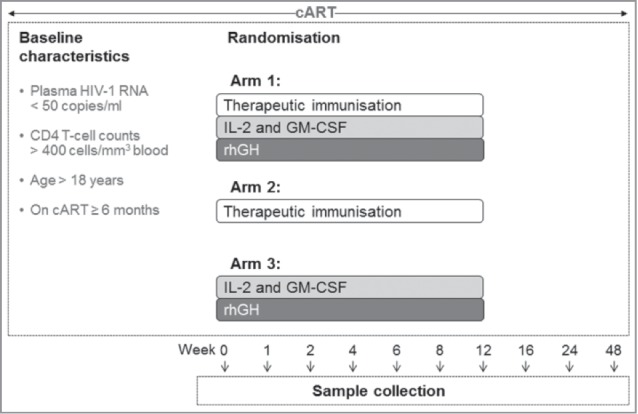
Immunotherapy trial treatment schedule overview: A multifaceted approach. In our recent study,17 patients were eligible to participate if they were over 18 y of age, chronically infected with HIV-1, on a stable cART regimen for ≥6 months, with CD4 T-cell counts >400 cells/mm3 blood, and plasma HIV-1 RNA <50 copies/ml. Subjects could not be receiving or have received immunomodulatory drugs or immunisation. Patients who met the eligibility criteria were randomized into one of 3 arms of the trial: Arm 1) to receive the GTU®-MultiHIV DNA Clade B vaccine (FIT Biotech Plc, Tampere, Finland) at baseline, followed by administration of IL-2 (Aldesleukin, Proleukin; Novartis, Camberley, UK) and GM-CSF (Sargramostim, Leukine™; Berlex, Seattle, WA) for 5 d during week 1, and rhGH (Somatropin, Saizen™; Merck-Serono International, Geneva, Switzerland) for 5 d during week 2, with vaccine further administered at weeks 6 and 12; Arm 2) to receive vaccine alone; or Arm 3) to receive cytokines and rhGH alone, all at the aforementioned time points. Vaccine was administered at 1mg/ml as 10 intradermal injections (5 100µl injections per arm); IL-2 given twice daily, 5 × 106 Units, administered by subcutaneous injection, 8h apart; GM-CSF, 150µg, was administered subcutaneously once daily, 4h from the IL-2; and rhGH self-administered subcutaneously daily at 4mg/day. Blood was drawn at 2 screen visits, at baseline (week 0) and at weeks 1, 2, 4, 6, 8, 12, 24 and 48.
Essential Role of Therapeutic Vaccines to Provide Antigenic Stimulus
To date no therapeutic vaccine has been licensed for use in the treatment of HIV-1 infection. However, immunization in conjunction with cART may lead to a reversal of anergy by selective induction and activation of specific memory T-cell responses against a number of viral proteins.3,20 By presenting viral proteins in novel ways, with or without specific adjuvants, or by presenting both immunodominant and subdominant epitopes, specific beneficial memory responses may be induced or augmented,13 and T-cell anergy reversed. That said, even administered in the context of cART, therapeutic immunization is likely to have little efficacy if used without other concomitant immunotherapies due to the immunocompromised status of chronically HIV-1-infected patients.20 In a study we recently conducted, we hypothesized that HIV-1-specific responses would be enhanced by the administration of cytokines and recombinant human growth hormone (rhGH) in chronic treated HIV-1 infection.17 Timing and delivery route of immunization were carefully considered in order to achieve the most robust antigen-specific responses. In this Phase I clinical trial, the FIT Biotech GTU® Multi-HIVB DNA vaccine was administered via 10 intradermal injections resulting in modest immunogenicity, whereas another therapeutic DNA vaccine study reported markedly enhanced HIV-1-specific CD8 T-cell responses following exfoliation of the skin and topical administration of the immunogen with a patch.21 In vivo electroporation delivery of a DNA vaccine was found to significantly improve cellular immune responses in terms of rate, magnitude, duration and breadth to a number of antigens,22 which concurred with results from animal models demonstrating improved responses with electroporation.23 The GTU® Multi-HIVB vaccine used in untreated HIV-1+ individuals induced antigen-specific responses, with beneficial effects on plasma HIV-1 RNA and CD4 T-cell recovery, particularly following intramuscular administration.20,24 Given the renewed interest in therapeutic vaccines as a component of latency reversal strategies, it is of utmost importance to determine the optimal timing and route of administration, in addition to the choice of immunogen.
Immunomodulatory Biologic Agents and Timing of their Administration
While previous immunotherapy studies have had limited efficacy to date, the lessons we have learned from them regarding the agents utilized and timing of their administration must be used to inform the rational design of future clinical trials.3,9 Immunotherapy with cytokines in conjunction with cART has been explored as a way of purging the viral reservoir, and targeting immune dysfunction.25 We have recently shown that administration of IL-2 following immunization as part of an immunotherapeutic program that included granulocyte-macrophage colony-stimulating factor (GM-CSF) and rhGH was safe and well-tolerated. In this recent study, immunization was used to prime specific cellular responses within cART-treated-HIV-1+ individuals followed by the administration of a regime of cytokines/rhGH along with further vaccine boosts designed to augment and sustain memory T-cell responses to HIV-1.3 Although the long-term clinical benefit of IL-2 administration in HIV-1+ subjects has been challenged, IL-2 therapy has been shown to increase CD4 T-cell counts,26 reduce the pool of resting CD4 T cells harbouring replication-competent virus,27 and induce HIV-1-specific T-cell responses.28 In our trials utilizing IL-2 immunotherapy (bi-daily subcutaneous administration of 5 × 106 Units), we demonstrated greater clinical and immunological improvements associated with this cytokine.15,17 The potential of this cytokine to augment virus-specific T-cell responses along with therapeutic immunization and antiretroviral drugs warrants further investigation. In our studies, we have shown IL-2 to be safe to administer with side effects limited by pre-administration prophylaxis.15,17,28 This lack of toxicity was also reported for daily low-dose IL-2 therapy in HIV-1 infection.29 Timing of IL-2 administration must be considered with regard to any planned immunization schedule. In animal models, relevant responses were preserved and maintained when IL-2 was administered during the antigen-specific T-cell contraction phase of the immune response.30,31 In contrast, IL-2 given pre-immunization in HIV-1+ cART-treated patients failed to increase specific T-cell proliferation.32,33 In 2 case studies, IL-2 administered following tetanus immunization in HIV-1-infected persons was found to better sustain tetanus-specific T-cell responses than IL-2 given together with vaccine or before immunization.34 Therefore IL-2 is likely to be more beneficial for augmenting vaccine-induced responses when given following immunization. In the context of HIV-1 infection, GM-CSF has been reported to increase CD4 T-cell counts and reduce plasma HIV-1 RNA.35 GM-CSF activates antigen-presenting cells, stimulates macrophage differentiation and proliferation,36,37 and may target the viral reservoir in these cells. In addition, IL-2 and GM-CSF have shown efficacy as adjuvants for DNA- or peptide-based vaccines.38 The use of rhGH to treat HIV-1-associated wasting revealed demonstrable improvements in a number of factors, including quality of life.39 Immunological benefits of rhGH include: reversal of thymic involution;40-43 increase in total and naive CD4 T-cell counts;40,41,43,44 restoration of T-cell responses against HIV-1;44-46 and reduction in expression of activation and apoptosis markers.41 Pharmacological and low doses of rhGH have been found to be effective and confer clinical benefit when administered for 12 weeks or more,40–46 which should be taken into account for future strategies.
The novel combination immunotherapy approach was well-tolerated, and subjects who received immunization, IL-2, GM-CSF and rhGH showed the most marked changes at the final study time point compared to baseline.17 This combination of cytokines, subsequent hormone administration and immunization resulted in a significant increase in CD4 T-cell counts, improved CD4/CD8 ratios, enhanced functional T-cell responses to Gag and a reversal of unfavorable (activated/exhausted) immunophenotypes. In a previous study in which IL-2 was administered during weeks 0, 4 and 8, HIV-1-specific lymphoproliferative responses were not detected until week 24.28 These responses were principally directed against Gag p24, and associated with transient 'blips' in plasma HIV-1 RNA levels.28 Such 'blips' may provide a form of autoimmunization, which could facilitate greater immune control of the induced latent reservoir. Similarly we reported a delayed Gag-specific response at week 48,17 although the last dose of vaccine was given at week 12, and these responses were not associated with transient viral 'blips'. Transient 'blips' in viraemia during immunotherapeutic intervention or latency reversal approaches may not necessarily lead to de novo infection, since this is thought to be prevented by cART.47 However, the pharmacokinetic profiles of the drugs administered appear to be insufficient to enable complete tissue penetration, such that a program of cART intensification should be considered in this setting, for example including CCR5 antagonists.18,48 Administration of IL-2 and GM-CSF in cART-treated patients with inadequate immune reconstitution despite virological suppression was found to result in HIV-1-specific proliferative responses and IL-2 production.49 Changes observed in our recent trial were most striking for subjects who received all interventions, suggesting a combinatorial outcome of these agents with distinct biological effects. While there was an initial cytokine-induced immune activation, which was short-lived, overall there was a reduction in activation state for individuals who received IL-2 and GM-CSF compared to subjects who did not receive these cytokines. This was accompanied by decreased surface expression of the negative regulator, programmed cell death 1 (PD-1), on both CD4 and CD8 T cells at week 48 compared to baseline for all treatment groups regardless of randomization, likely due to prolonged cART. The percentage of HIV-1-specific CD4+IFN-γ+PD-1+ T cells is significantly lower in cART-treated patients compared to untreated viraemic subjects,50 and PD-1 has been proposed as a marker to identify incomplete immune reconstitution.51 In the lymphocytic choriomeningitis virus (LCMV) mouse model, blockade of IL-10 during therapeutic DNA immunization or blockade of PD-1 during vaccinia vector vaccination enhanced control of LCMV, suggesting concurrent blockade of both may be sufficient to restore exhausted cellular immune responses.52,53 Combining IL-2 administration and PD-1 ligand 1 (PD-L1) blockade in the mouse model of LCMV reduced viral load and increased virus-specific CD8 T-cell responses.54 These studies support the rationale for a multifarious approach aimed at reversal of HIV-1 latency and subsequent cure.
Proviral DNA and Quantification of Replication-Competent Virus: Prospective Biomarkers to Measure Reversal of Latency
Across a number of our studies we have shown reductions in proviral HIV-1 DNA, be it with early cART, rhGH therapy or combined vaccine/cytokine/hormone immunotherapy.2,17,45 In our recent study, on an individual patient basis, subjects who showed the most improved HIV-1-specific responses at week 48 also showed a reduction of proviral DNA, which might be due to the low levels of proviral DNA at baseline, as these individuals started cART in the first month following diagnosis. To date there is no universally agreed method to accurately quantify latent infection, i.e. measurement of the proportion of integrated HIV-1 DNA capable of infectious virion production.
Long-lived resting memory CD4 T cells along with other tissue reservoirs that include the brain, lymphoid tissue (lymph node and gut), bone marrow, and genital tract, may each contribute to viral rebound and are difficult to sample clinically. In some of these tissue reservoirs drug concentrations may be suboptimal.55 Understanding the generation and maintenance of latent proviruses and latently infected T cells is central to HIV-1 cure strategies, however the mechanisms involved are complex56 and beyond the scope of this commentary. Currently, at least 2 steps are thought to be essential: HIV-1 induction and expression from latently infected cells; and virus-specific CTL recognition and killing of infected cells. Studies evaluating the extent of latency and potential for its reversal have been varied.57 The vast majority of viruses in the latent reservoir of patients treated during chronic HIV-1 infection comprise CTL escape mutations, which are rare in the reservoirs of subjects treated during acute infection.13 Recent work shows that only 12% of proviruses are non-mutated and replication competent.58 However, measurement of HIV-1 latency reversal ex vivo using a different approach revealed that only 1.5% of proviruses in resting CD4 T cells were able to produce virions following CD3/CD28 activation. Considering the high proportion of defective provirus in the latent reservoir (88–98.5%), an agreed method to accurately measure potential latency reversal by various candidate agents is urgently needed.59,60 The reported failures of various first generation latency reversal agents on their own resulted in modifying such approaches. To date, a number of potential latency reversal agents have failed to upregulate HIV-1 gene expression in resting CD4+ T cells from treated patients, with the exception of the protein kinase C agonist, bryostatin-1.47,59 The next generation of candidate agents will hopefully yield more promising results. Ongoing and planned trials of these interventions are incorporating the strategies we have reviewed here, including early cART, therapeutic immunization, cytokines and cART intensification (Table 1). A number of clinical trials are optimizing dosing, sequence and timing, in addition to utilizing multifaceted approaches. Combinations of drugs with pharmacokinetic profiles that achieve comprehensive tissue distribution are needed to target latency, as we fill gaps in our knowledge regarding in vivo latency mechanisms. As mentioned, the methods to measure latency reversal are varied. This is particularly troubling because the emerging consensus is that existing assays either overestimate or underestimate the size of the latent HIV-1 reservoir.47 The ultimate measure of success for latency reversal strategies will be structured treatment cessation, though this approach must be used with great caution.5,6
Table 1.
Immunotherapeutic interventions and their potential for HIV-1 latency reversal: A combined approach to induce cure of HIV-1 infection
| Intervention | Immunological benefits | HIV-1 reservoir and latency reversal |
|---|---|---|
| None | Infected CD4+ lymphocytes and cells of the monocytic lineage; dysfunctional APC; dysregulated NK cells; excessive immune activation; immunosuppression; T-cell anergy and unresponsiveness to HIV-1 | Establishment of the latent reservoir |
| cART during chronic HIV-1 infection | Increased numbers of CD4 T cells (including naïve); functional improvement in T-cell responses to recall antigens; partial normalization of activation, exhaustion, and regulatory function; some normalization of NK cell and APC function; incomplete reconstitution of fully functional HIV-1-specific CD4 and CD8 T-cell responses | Persistence of latent reservoir not impacted by either cART or immune reconstitution; latent reservoir dominated by CTL escape mutations |
| cART during acute HIV-1 infection | Increased numbers of CD4 T cells (including naïve); improved polyfunctionality of HIV-1-specific responses; enhanced immune reconstitution and better response to immunisation/immunotherapy | Reduced size of the latent viral reservoir comparable to that observed in LTNP; latent viruses carry fewer CTL escape mutations |
| cART intensification (e.g. CCR5 antagonists) | Favourably adjusts HIV-1 associated T-cell activation and differentiation profiles; prevention of de novo infection of susceptible cells from viral replication or reactivation | Potential to prevent reservoir re-seeding |
| HIV-1 immunogens | Provision of unpathogenic antigenic stimulation; induced/boosted anti-HIV-1 functional responses (new and memory) | Potential to deplete viral reservoirs (or at least reduce these to levels observed in LTNP or elite controllers) |
| Cytokines (e.g., IL-2) | Increased numbers of CD4 T cells; improved T-cell growth, survival, differentiation/maturation; reversal of T-cell anergy; regulation and maintenance of T cells along distinct differentiation pathways; increased frequency and function of T effectors and Tregs, particularly HIV-1-specific CD4 HTL and CD8 CTL | Lower numbers of HIV-1-infected latent CD4 T cells; potential to purge virus through transient viral ‘blips’ |
| Cytokines (e.g. GM-CSF) | Reversal of anergy; increased T effector cells; increased frequency of HIV-1-specific HTL and CTL; enhanced APC and NK cell function | Potential to purge viral reservoirs in cells of the monocytic lineage |
| Hormones (e.g., rhGH) | Increased thymic activity; increased pool of naïve T cells; decreased systemic hyperactivation; restored differentiation/maturation, prevention of apoptosis, and promotion of proliferation; increased NK cell function | Possible impact on integrated provirus |
| Latency reversal agents (e.g. protein kinase C agonists) | HIV-1 induction and expression from latently infected cells; proteins/peptides processed and presented to immune cells | Potential to reactivate the latent viral reservoir in infected resting CD4+ cells; however stimulation of HIV-1-specific CTL prior to reactivating latent HIV-1 is thought to be crucial; successful strategies might include HIV-1 immunogens and low-dose IL-2 |
| Immune modulatory drugs (e.g., PD-1/PD-L1 blockade) | Restoration of proliferative and effector function of CD4 and CD8 T cells, respectively; restoration of HIV-1-specific CD8 T-cell effector function due to reversal of exhaustion | Impact on HIV-1 reservoir to be determined |
APC, antigen-presenting cell; cART, combination antiretroviral therapy; CTL, cytotoxic T lymphocyte; GM-CSF, granulocyte macrophage colony stimulating factor; HDAC, histone deacetylase; HTL, helper T lymphocyte; IL-2, interleukin-2; LTNP, long-term nonprogressor; NK, natural killer cell; PD-1, programmed cell death-1; PD-L1, PD-1 ligand 1; rhGH, recombinant human growth hormone; T-reg, regulatory T cell.
Conclusions and Perspectives
Irrespective of the mechanisms responsible for the establishment of HIV-1 latency, it represents a major hurdle for HIV-1 clearance. A number of agents and strategies have been proposed to activate latent HIV-1 in order to deplete the reservoir by a combination of viral cytopathic effects and modulated cell-mediated immunity ultimately resulting in control of HIV-1 replication. Such control is observed in long-term nonprogressors or HIV controllers and forms the foundation for functional cure. Larger studies employing various biological agents and accurate quantification of latency reversal are needed to fully elucidate the clinical benefit of these therapies. Approaches aimed at purging virus by stimulating HIV-1-specific T cells in the absence of potentially detrimental global T-cell activation, need not abandon strategies employing low-dose IL-2 immunotherapy in addition to treatment with other agents described herein. Whether at lower physiological or higher pharmacological doses, current candidate agents have been associated with improved thymic function, enhanced T-cell functionality, lessened immune activation, and reduced proviral DNA; again lending weight to the idea of synergistic approach. Longer-term follow-up would assess whether or not these improvements are maintained. Current endeavors seek to establish consensus on combined therapies, their optimal timing and dosing, given the antigen loss post-cART. There has been an optimistic shift toward the development of curative strategies, in which the focus of prospective immunotherapy will not only be to induce and maintain increased T-cell functionality, improve immunophenotypes and confer clinical benefit, but also to target the latent reservoir. While eradication of the reservoir is the aim of a sterilizing cure, containment of the reservoir is essential to achieve a functional cure of HIV-1 infection. Successful immunological approaches may require different step-wise novel interventions, grouping of existing and new generation immunotherapeutic and latency reversal agents, and larger patient cohorts, alongside the development of accurate and consensus measurement of latency reversal, to result in curative intervention. Further detailed investigations are required to identify immunological, virological and clinical biomarkers for accurate assessment of end-point efficacy in the approaches described.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Dr. Gareth A D Hardy, Dr. Jocelyn S Downey and Dr. Adriano Boasso for helpful discussion and critical comments.
Funding
The authors were funded by grants from the MRC (Grant number G0501957), Westminster Medical School Research Trust and St Stephen's AIDS Trust.
References
- 1.Sidibe M, Zuniga JM, Montaner J. Leveraging HIV treatment to end AIDS, stop new HIV infections, and avoid the cost of inaction. Clin Infect Dis 2014; 59 Suppl 1:S3-6; PMID:24926030; http://dx.doi.org/ 10.1093/cid/ciu321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pires A, Hardy G, Gazzard B, Gotch F, Imami N. Initiation of antiretroviral therapy during recent HIV-1 infection results in lower residual viral reservoirs. J Acquir Immune Defic Syndr 2004; 36:783-90; PMID:15213561; http://dx.doi.org/ 10.1097/00126334-200407010-00004 [DOI] [PubMed] [Google Scholar]
- 3.Imami N, Westrop S, Cranage A, Burton C, Gotch F. Combined use of cytokines, hormones and therapeutic vaccines during effective antiretroviral therapy. Future HIV Therapy 2007; 1:171-79; http://dx.doi.org/ 10.2217/17469600.1.2.171 [DOI] [Google Scholar]
- 4.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 2003; 9:727-8; PMID:12754504; http://dx.doi.org/ 10.1038/nm880 [DOI] [PubMed] [Google Scholar]
- 5.Burton CT, Nelson MR, Hay P, Gazzard BG, Gotch FM, Imami N. Immunological and virological consequences of patient-directed antiretroviral therapy interruption during chronic HIV-1 infection. Clin Exp Immunol 2005; 142:354-61; PMID:16232224; http://dx.doi.org/ 10.1111/j.1365-2249.2005.02918.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, Babiker A, Burman W, Clumeck N, Cohen CJ, et al.. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006; 355:2283-96; PMID:17135583; http://dx.doi.org/ 10.1056/NEJMoa062360 [DOI] [PubMed] [Google Scholar]
- 7.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science 2009; 323:1304-7; PMID:19265012; http://dx.doi.org/ 10.1126/science.1165706 [DOI] [PubMed] [Google Scholar]
- 8.Blankson JN. Effector mechanisms in HIV-1 infected elite controllers: highly active immune responses? Antiviral Res 2010; 85:295-302; PMID:19733595; http://dx.doi.org/ 10.1016/j.antiviral.2009.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imami N, Westrop SJ, Grageda N, Herasimtschuk AA. Long-Term Non-Progression and Broad HIV-1-Specific Proliferative T-Cell Responses. Front Immunol 2013; 4:58; PMID:23459797; http://dx.doi.org/ 10.3389/fimmu.2013.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633-45; PMID:24138880; http://dx.doi.org/ 10.1016/j.immuni.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shan L, Deng K, Durand C, Rabi A, Blankson J, Siliciano RF. Elimination of the latent reservoir for HIV-1 requires induction of cytolytic T lymphocyte responses 19th Conference on Retroviruses and Opportunistic Infections (CROI). Seattle, Washington, USA, 2012. [Google Scholar]
- 12.Cellerai C, Harari A, Stauss H, Yerly S, Geretti AM, Carroll A, Yee T, Ainsworth J, Williams I, Sweeney J, et al.. Early and prolonged antiretroviral therapy is associated with an HIV-1-specific T-cell profile comparable to that of long-term non-progressors. PLoS One 2011; 6:e18164; PMID:21483676; http://dx.doi.org/ 10.1371/journal.pone.0018164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng K, Pertea M, Rongvaux A, Wang L, Durand CM, Ghiaur G, Lai J, McHugh HL, Hao H, Zhang H, et al.. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature 2015; 517:381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lange CG, Valdez H, Medvik K, Asaad R, Lederman MM. CD4+ T-lymphocyte nadir and the effect of highly active antiretroviral therapy on phenotypic and functional immune restoration in HIV-1 infection. Clin Immunol 2002; 102:154-61; PMID:11846457; http://dx.doi.org/ 10.1006/clim.2001.5164 [DOI] [PubMed] [Google Scholar]
- 15.Hardy GA, Imami N, Nelson MR, Sullivan AK, Moss R, Aasa-Chapman MM, Gazzard B, Gotch FM. A phase I, randomized study of combined IL-2 and therapeutic immunisation with antiretroviral therapy. J Immune Based Ther Vaccines 2007; 5:6; PMID:17428345; http://dx.doi.org/ 10.1186/1476-8518-5-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robbins GK, Addo MM, Troung H, Rathod A, Habeeb K, Davis B, Heller H, Basgoz N, Walker BD, Rosenberg ES. Augmentation of HIV-1-specific T helper cell responses in chronic HIV-1 infection by therapeutic immunization. AIDS 2003; 17:1121-6; PMID:12819512; http://dx.doi.org/ 10.1097/00002030-200305230-00002 [DOI] [PubMed] [Google Scholar]
- 17.Herasimtschuk A, Downey J, Nelson M, Moyle G, Mandalia S, Sikut R, Adojaan M, Stanescu I, Gotch F, Imami N. Therapeutic immunisation plus cytokine and hormone therapy improves CD4 T-cell counts, restores anti-HIV-1 responses and reduces immune activation in treated chronic HIV-1 infection. Vaccine 2014; 32:7005-13; PMID:25454870; http://dx.doi.org/ 10.1016/j.vaccine.2014.09.072 [DOI] [PubMed] [Google Scholar]
- 18.Ananworanich J, Schuetz A, Vandergeeten C, Sereti I, de Souza M, Rerknimitr R, Dewar R, Marovich M, van Griensven F, Sekaly R, et al.. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One 2012; 7:e33948; PMID:22479485; http://dx.doi.org/ 10.1371/journal.pone.0033948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ananworanich J, Dube K, Chomont N. How does the timing of antiretroviral therapy initiation in acute infection affect HIV reservoirs? Curr Opin HIV AIDS 2015; 10:18-28; PMID:25415421; http://dx.doi.org/ 10.1097/COH.0000000000000122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ensoli B, Cafaro A, Monini P, Marcotullio S, Ensoli F. Challenges in HIV Vaccine Research for Treatment and Prevention. Front Immunol 2014; 5:417; PMID:25250026; http://dx.doi.org/ 10.3389/fimmu.2014.00417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gudmundsdotter L, Wahren B, Haller BK, Boberg A, Edback U, Bernasconi D, Butto S, Gaines H, Imami N, Gotch F, et al.. Amplified antigen-specific immune responses in HIV-1 infected individuals in a double blind DNA immunization and therapy interruption trial. Vaccine 2011; 29:5558-66; PMID:21300092; http://dx.doi.org/ 10.1016/j.vaccine.2011.01.064 [DOI] [PubMed] [Google Scholar]
- 22.Vasan S, Hurley A, Schlesinger SJ, Hannaman D, Gardiner DF, Dugin DP, Boente-Carrera M, Vittorino R, Caskey M, Andersen J, et al.. In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers. PLoS One 2011; 6:e19252; PMID:21603651; http://dx.doi.org/ 10.1371/journal.pone.0019252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallengard D, Haller BK, Maltais AK, Gelius E, Nihlmark K, Wahren B, Brave A. Comparison of plasmid vaccine immunization schedules using intradermal in vivo electroporation. Clin Vaccine Immunol 2011; 18:1577-81; PMID:21752954; http://dx.doi.org/ 10.1128/CVI.05045-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vardas E, Stanescu I, Leinonen M, Ellefsen K, Pantaleo G, Valtavaara M, Ustav M, Reijonen K. Indicators of therapeutic effect in FIT-06, a Phase II trial of a DNA vaccine, GTU((R))-Multi-HIVB, in untreated HIV-1 infected subjects. Vaccine 2012; 30:4046-54; PMID:22549090; http://dx.doi.org/ 10.1016/j.vaccine.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 25.Catalfamo M, Le Saout C, Lane HC. The role of cytokines in the pathogenesis and treatment of HIV infection. Cytokine Growth Factor Rev 2012; 23:207-14; PMID:22738931; http://dx.doi.org/ 10.1016/j.cytogfr.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abrams D, Levy Y, Losso MH, Babiker A, Collins G, Cooper DA, Darbyshire J, Emery S, Fox L, Gordin F, et al.. Interleukin-2 therapy in patients with HIV infection. N Engl J Med 2009; 361:1548-59; PMID:19828532; http://dx.doi.org/ 10.1056/NEJMoa0903175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chun TW, Engel D, Mizell SB, Hallahan CW, Fischette M, Park S, Davey RT Jr., Dybul M, Kovacs JA, Metcalf JA, et al.. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med 1999; 5:651-5; PMID:10371503; http://dx.doi.org/ 10.1038/9498 [DOI] [PubMed] [Google Scholar]
- 28.Sullivan AK, Hardy GA, Nelson MR, Gotch F, Gazzard BG, Imami N. Interleukin-2-associated viral breakthroughs induce HIV-1-specific CD4 T cell responses in patients on fully suppressive highly active antiretroviral therapy. AIDS 2003; 17:628-9; PMID:12598786; http://dx.doi.org/ 10.1097/00002030-200303070-00020 [DOI] [PubMed] [Google Scholar]
- 29.Jacobson EL, Pilaro F, Smith KA. Rational interleukin 2 therapy for HIV positive individuals: daily low doses enhance immune function without toxicity. Proc Natl Acad Sci U S A 1996; 93:10405-10; PMID:8816813; http://dx.doi.org/ 10.1073/pnas.93.19.10405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med 2003; 9:540-7; PMID:12692546; http://dx.doi.org/ 10.1038/nm866 [DOI] [PubMed] [Google Scholar]
- 31.Nacsa J, Edghill-Smith Y, Tsai WP, Venzon D, Tryniszewska E, Hryniewicz A, Moniuszko M, Kinter A, Smith KA, Franchini G. Contrasting effects of low-dose IL-2 on vaccine-boosted simian immunodeficiency virus (SIV)-specific CD4+ and CD8+ T cells in macaques chronically infected with SIVmac251. J Immunol 2005; 174:1913-21; PMID:15699118; http://dx.doi.org/ 10.4049/jimmunol.174.4.1913 [DOI] [PubMed] [Google Scholar]
- 32.Levy Y, Durier C, Krzysiek R, Rabian C, Capitant C, Lascaux AS, Michon C, Oksenhendler E, Weiss L, Gastaut JA, et al.. Effects of interleukin-2 therapy combined with highly active antiretroviral therapy on immune restoration in HIV-1 infection: a randomized controlled trial. AIDS 2003; 17:343-51; PMID:12556688; http://dx.doi.org/ 10.1097/00002030-200302140-00008 [DOI] [PubMed] [Google Scholar]
- 33.Valdez H, Mitsuyasu R, Landay A, Sevin AD, Chan ES, Spritzler J, Kalams SA, Pollard RB, Fahey J, Fox L, et al.. Interleukin-2 Increases CD4+ lymphocyte numbers but does not enhance responses to immunization: results of A5046s. J Infect Dis 2003; 187:320-5; PMID:12552459; http://dx.doi.org/ 10.1086/346056 [DOI] [PubMed] [Google Scholar]
- 34.Hardy GA, Imami N, Sullivan AK, Nelson MR, Gazzard B, Gotch FM. Tetanus vaccination with IL-2 during highly active antiretroviral therapy induces sustained and pronounced specific CD4 T-cell responses. AIDS 2004; 18:2199-202; PMID:15577655; http://dx.doi.org/ 10.1097/00002030-200411050-00014 [DOI] [PubMed] [Google Scholar]
- 35.Brown PA, Angel JB. Granulocyte-macrophage colony-stimulating factor as an immune-based therapy in HIV infection. J Immune Based Ther Vaccines 2005; 3:3; PMID:15904525; http://dx.doi.org/ 10.1186/1476-8518-3-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Disis ML, Bernhard H, Shiota FM, Hand SL, Gralow JR, Huseby ES, Gillis S, Cheever MA. Granulocyte-macrophage colony-stimulating factor: an effective adjuvant for protein and peptide-based vaccines. Blood 1996; 88:202-10; PMID:8704175 [PubMed] [Google Scholar]
- 37.Waller EK. The role of sargramostim (rhGM-CSF) as immunotherapy. Oncologist 2007; 12 Suppl 2:22-6; PMID:18039636 [DOI] [PubMed] [Google Scholar]
- 38.Toubaji A, Hill S, Terabe M, Qian J, Floyd T, Simpson RM, Berzofsky JA, Khleif SN. The combination of GM-CSF and IL-2 as local adjuvant shows synergy in enhancing peptide vaccines and provides long term tumor protection. Vaccine 2007; 25:5882-91; PMID:17602804; http://dx.doi.org/ 10.1016/j.vaccine.2007.05.040 [DOI] [PubMed] [Google Scholar]
- 39.Moyle GJ, Daar ES, Gertner JM, Kotler DP, Melchior JC, O'Brien F, Svanberg E, Serono Study T. Growth hormone improves lean body mass, physical performance, and quality of life in subjects with HIV-associated weight loss or wasting on highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2004; 35:367-75; PMID:15097153; http://dx.doi.org/ 10.1097/00126334-200404010-00006 [DOI] [PubMed] [Google Scholar]
- 40.Napolitano LA, Lo JC, Gotway MB, Mulligan K, Barbour JD, Schmidt D, Grant RM, Halvorsen RA, Schambelan M, McCune JM. Increased thymic mass and circulating naive CD4 T cells in HIV-1-infected adults treated with growth hormone. AIDS 2002; 16:1103-11; PMID:12004268; http://dx.doi.org/ 10.1097/00002030-200205240-00003 [DOI] [PubMed] [Google Scholar]
- 41.Napolitano LA, Schmidt D, Gotway MB, Ameli N, Filbert EL, Ng MM, Clor JL, Epling L, Sinclair E, Baum PD, et al.. Growth hormone enhances thymic function in HIV-1-infected adults. J Clin Invest 2008; 118:1085-98; PMID:18292808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansen BR, Kolte L, Haugaard SB, Dirksen C, Jensen FK, Ryder LP, Sorensen AL, Flyvbjerg A, Nielsen SD, Andersen O. Improved thymic index, density and output in HIV-infected patients following low-dose growth hormone therapy: a placebo controlled study. AIDS 2009; 23:2123-31; PMID:19625946; http://dx.doi.org/ 10.1097/QAD.0b013e3283303307 [DOI] [PubMed] [Google Scholar]
- 43.Smith K, Zheng L, Bosch R, Margolis DM, Tenorio A, Napolitano L, Saag M, Connick E, Gross B, Francis I, et al.. Treatment with recombinant growth hormone is associated with modest improvement in CD4 lymphocyte reconstitution in HIV-infected persons on antiretroviral therapy: results of ACTG A5174. AIDS Res Hum Retroviruses 2010; 26:425-32; PMID:20415638; http://dx.doi.org/ 10.1089/aid.2009.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pires A, Pido-Lopez J, Moyle G, Gazzard B, Gotch F, Imami N. Enhanced T-cell maturation, differentiation and function in HIV-1-infected individuals after growth hormone and highly active antiretroviral therapy. Antivir Ther 2004; 9:67-75; PMID:15040538 [PubMed] [Google Scholar]
- 45.Herasimtschuk AA, Westrop SJ, Moyle GJ, Downey JS, Imami N. Effects of recombinant human growth hormone on HIV-1-specific T-cell responses, thymic output and proviral DNA in patients on HAART: 48-week follow-up. J Immune Based Ther Vaccines 2008; 6:7; PMID:18976455; http://dx.doi.org/ 10.1186/1476-8518-6-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herasimtschuk AA, Hansen BR, Langkilde A, Moyle GJ, Andersen O, Imami N. Low-dose growth hormone for 40 weeks induces HIV-1-specific T cell responses in patients on effective combination anti-retroviral therapy. Clin Exp Immunol 2013; 173:444-53; PMID:23701177; http://dx.doi.org/ 10.1111/cei.12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blankson JN, Siliciano JD, Siliciano RF. Finding a cure for human immunodeficiency virus-1 infection. Infect Dis Clin North Am 2014; 28:633-50; PMID:25277513; http://dx.doi.org/ 10.1016/j.idc.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westrop SJ, Moyle G, Jackson A, Nelson M, Mandalia S, Imami N. CCR5 antagonism impacts vaccination response and immune profile in HIV-1 infection. Mol Med 2012; 18:1240-8; PMID:22875102; http://dx.doi.org/ 10.2119/molmed.2012.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pires A, Nelson M, Pozniak AL, Fisher M, Gazzard B, Gotch F, Imami N. Mycobacterial immune reconstitution inflammatory syndrome in HIV-1 infection after antiretroviral therapy is associated with deregulated specific T-cell responses: beneficial effect of IL-2 and GM-CSF immunotherapy. J Immune Based Ther Vaccines 2005; 3:7; PMID:16181494; http://dx.doi.org/ 10.1186/1476-8518-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kassu A, Marcus RA, D'Souza MB, Kelly-McKnight EA, Golden-Mason L, Akkina R, Fontenot AP, Wilson CC, Palmer BE. Regulation of virus-specific CD4+ T cell function by multiple costimulatory receptors during chronic HIV infection. J Immunol 2010; 185:3007-18; PMID:20656923; http://dx.doi.org/ 10.4049/jimmunol.1000156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grabmeier-Pfistershammer K, Steinberger P, Rieger A, Leitner J, Kohrgruber N. Identification of PD-1 as a unique marker for failing immune reconstitution in HIV-1-infected patients on treatment. J Acquir Immune Defic Syndr 2011; 56:118-24; PMID:20980914; http://dx.doi.org/ 10.1097/QAI.0b013e3181fbab9f [DOI] [PubMed] [Google Scholar]
- 52.Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J Exp Med 2008; 205:533-41; PMID:18332180; http://dx.doi.org/ 10.1084/jem.20071948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med 2008; 205:543-55; PMID:18332181; http://dx.doi.org/ 10.1084/jem.20071949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.West EE, Jin HT, Rasheed AU, Penaloza-Macmaster P, Ha SJ, Tan WG, Youngblood B, Freeman GJ, Smith KA, Ahmed R. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest 2013; 123:2604-15; PMID:23676462; http://dx.doi.org/ 10.1172/JCI67008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fletcher CV, Staskus K, Wietgrefe SW, Rothenberger M, Reilly C, Chipman JG, Beilman GJ, Khoruts A, Thorkelson A, Schmidt TE, et al.. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A 2014; 111:2307-12; PMID:24469825; http://dx.doi.org/ 10.1073/pnas.1318249111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mbonye U, Karn J. Transcriptional control of HIV latency: cellular signaling pathways, epigenetics, happenstance and the hope for a cure. Virology 2014; 454-455:328-39; PMID:24565118; http://dx.doi.org/ 10.1016/j.virol.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spina CA, Anderson J, Archin NM, Bosque A, Chan J, Famiglietti M, Greene WC, Kashuba A, Lewin SR, Margolis DM, et al.. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog 2013; 9:e1003834; PMID:24385908; http://dx.doi.org/ 10.1371/journal.ppat.1003834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, Lai J, Blankson JN, Siliciano JD, Siliciano RF. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013; 155:540-51; PMID:24243014; http://dx.doi.org/ 10.1016/j.cell.2013.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med 2014; 20:425-9; PMID:24658076; http://dx.doi.org/ 10.1038/nm.3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cillo AR, Sobolewski MD, Bosch RJ, Fyne E, Piatak M Jr., Coffin JM, Mellors JW. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A 2014; 111:7078-83; PMID:24706775; http://dx.doi.org/ 10.1073/pnas.1402873111 [DOI] [PMC free article] [PubMed] [Google Scholar]


