Abstract
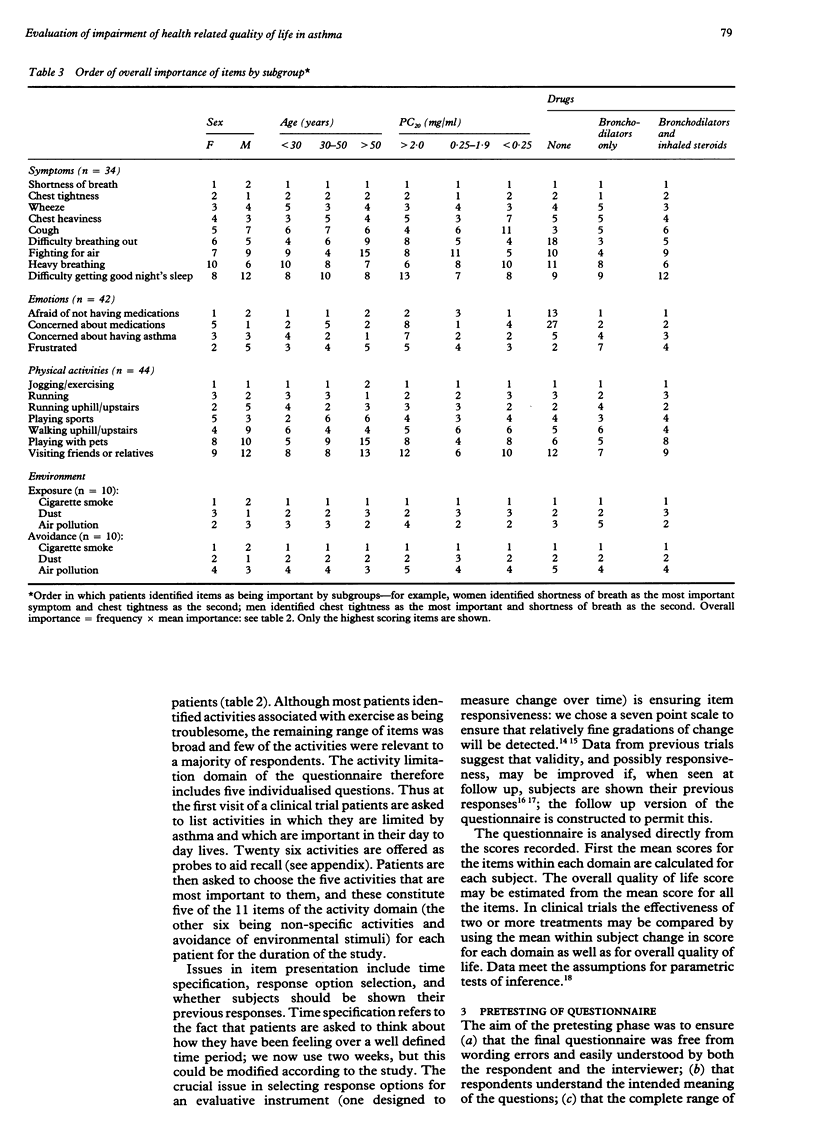
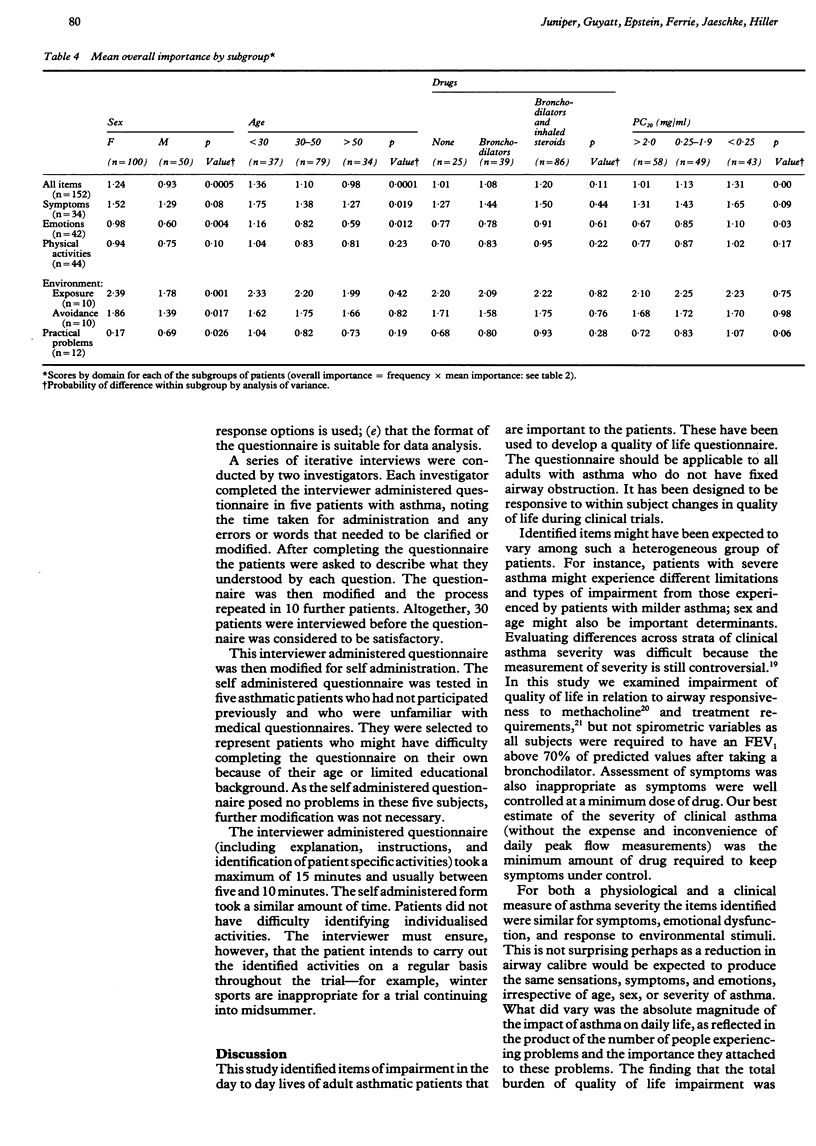
BACKGROUND: In the past only physiological and clinical outcomes have been used to assess the effect of asthma interventions and the effect of the intervention on the lives of the patients has not been determined. The objective of this study was to assess health related impairment of quality of life in adult asthmatic patients and to develop a questionnaire for measuring quality of life in clinical trials in asthma. METHODS: Impairment of quality of life in adults with asthma was evaluated from structured interviews in which patients were asked to identify the parts of their daily lives affected by asthma. On the basis of these results, an asthma quality of life questionnaire was developed in an interviewer and self administered form and tested for comprehension and acceptability. A total of 150 adults with asthma and with a wide range of airway hyperresponsiveness were enrolled from previous clinical trials, local asthma clinics, and notices in the media. RESULTS: Areas of quality of life impairment included symptoms classically associated with asthma, responses to environmental stimuli, the need to avoid these stimuli, limitation of activities, and emotional dysfunction. Areas of impairment were similar across strata of airway hyperresponsiveness, age, and treatment requirements and between sexes, thus allowing a single questionnaire suitable for all adults with asthma to be developed. The questionnaire contains 32 items and takes 5-10 minutes to administer; in the pretesting it was shown to be acceptable to a wide range of patients. CONCLUSIONS: The questionnaire includes areas of quality of life impairment that are important to adult asthmatic patients. It has been designed to be responsive to within subject change and therefore may be used as a measure of outcome in clinical trials in asthma.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergner M., Bobbitt R. A., Carter W. B., Gilson B. S. The Sickness Impact Profile: development and final revision of a health status measure. Med Care. 1981 Aug;19(8):787–805. doi: 10.1097/00005650-198108000-00001. [DOI] [PubMed] [Google Scholar]
- Greenough A., Wood S., Morley C. J., Davis J. A. Pancuronium prevents pneumothoraces in ventilated premature babies who actively expire against positive pressure inflation. Lancet. 1984 Jan 7;1(8367):1–3. doi: 10.1016/s0140-6736(84)90177-6. [DOI] [PubMed] [Google Scholar]
- Guyatt G. H., Berman L. B., Townsend M., Taylor D. W. Should study subjects see their previous responses? J Chronic Dis. 1985;38(12):1003–1007. doi: 10.1016/0021-9681(85)90098-0. [DOI] [PubMed] [Google Scholar]
- Guyatt G. H., Bombardier C., Tugwell P. X. Measuring disease-specific quality of life in clinical trials. CMAJ. 1986 Apr 15;134(8):889–895. [PMC free article] [PubMed] [Google Scholar]
- Guyatt G. H., Nogradi S., Halcrow S., Singer J., Sullivan M. J., Fallen E. L. Development and testing of a new measure of health status for clinical trials in heart failure. J Gen Intern Med. 1989 Mar-Apr;4(2):101–107. doi: 10.1007/BF02602348. [DOI] [PubMed] [Google Scholar]
- Guyatt G. H., Townsend M., Berman L. B., Pugsley S. O. Quality of life in patients with chronic airflow limitation. Br J Dis Chest. 1987 Jan;81(1):45–54. doi: 10.1016/0007-0971(87)90107-0. [DOI] [PubMed] [Google Scholar]
- Guyatt G. H., Townsend M., Keller J. L., Singer J. Should study subjects see their previous responses: data from a randomized control trial. J Clin Epidemiol. 1989;42(9):913–920. doi: 10.1016/0895-4356(89)90105-4. [DOI] [PubMed] [Google Scholar]
- Guyatt G., Mitchell A., Irvine E. J., Singer J., Williams N., Goodacre R., Tompkins C. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989 Mar;96(3):804–810. [PubMed] [Google Scholar]
- HUME K. M., JONES E. R. Bronchodilators and corticosteroids in asthma. Forced expiratory volume as an aid to diagnosis and treatment. Lancet. 1960 Dec 17;2(7164):1319–1322. doi: 10.1016/s0140-6736(60)92518-6. [DOI] [PubMed] [Google Scholar]
- Hargreave F. E., Ryan G., Thomson N. C., O'Byrne P. M., Latimer K., Juniper E. F., Dolovich J. Bronchial responsiveness to histamine or methacholine in asthma: measurement and clinical significance. J Allergy Clin Immunol. 1981 Nov;68(5):347–355. doi: 10.1016/0091-6749(81)90132-9. [DOI] [PubMed] [Google Scholar]
- Jaeschke R., Singer J., Guyatt G. H. A comparison of seven-point and visual analogue scales. Data from a randomized trial. Control Clin Trials. 1990 Feb;11(1):43–51. doi: 10.1016/0197-2456(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Josephs L. K., Gregg I., Mullee M. A., Holgate S. T. Nonspecific bronchial reactivity and its relationship to the clinical expression of asthma. A longitudinal study. Am Rev Respir Dis. 1989 Aug;140(2):350–357. doi: 10.1164/ajrccm/140.2.350. [DOI] [PubMed] [Google Scholar]
- Juniper E. F., Daniel E. E., Roberts R. S., Kline P. A., Hargreave F. E., Newhouse M. T. Improvement in airway responsiveness and asthma severity during pregnancy. A prospective study. Am Rev Respir Dis. 1989 Oct;140(4):924–931. doi: 10.1164/ajrccm/140.4.924. [DOI] [PubMed] [Google Scholar]
- Juniper E. F., Daniel E. E., Roberts R. S., Kline P. A., Hargreave F. E., Newhouse M. T. Improvement in airway responsiveness and asthma severity during pregnancy. A prospective study. Am Rev Respir Dis. 1989 Oct;140(4):924–931. doi: 10.1164/ajrccm/140.4.924. [DOI] [PubMed] [Google Scholar]
- Juniper E. F., Frith P. A., Dunnett C., Cockcroft D. W., Hargreave F. E. Reproducibility and comparison of responses to inhaled histamine and methacholine. Thorax. 1978 Dec;33(6):705–710. doi: 10.1136/thx.33.6.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juniper E. F., Frith P. A., Hargreave F. E. Airway responsiveness to histamine and methacholine: relationship to minimum treatment to control symptoms of asthma. Thorax. 1981 Aug;36(8):575–579. doi: 10.1136/thx.36.8.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juniper E. F., Guyatt G. H. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin Exp Allergy. 1991 Jan;21(1):77–83. doi: 10.1111/j.1365-2222.1991.tb00807.x. [DOI] [PubMed] [Google Scholar]
- Juniper E. F., Kline P. A., Vanzieleghem M. A., Ramsdale E. H., O'Byrne P. M., Hargreave F. E. Effect of long-term treatment with an inhaled corticosteroid (budesonide) on airway hyperresponsiveness and clinical asthma in nonsteroid-dependent asthmatics. Am Rev Respir Dis. 1990 Oct;142(4):832–836. doi: 10.1164/ajrccm/142.4.832. [DOI] [PubMed] [Google Scholar]
- Kinsman R. A., Luparello T., O'Banion K., Spector S. Multidimensional analysis of the subjective symptomatology of asthma. Psychosom Med. 1973 May-Jun;35(3):250–267. doi: 10.1097/00006842-197305000-00008. [DOI] [PubMed] [Google Scholar]
- Kirshner B., Guyatt G. A methodological framework for assessing health indices. J Chronic Dis. 1985;38(1):27–36. doi: 10.1016/0021-9681(85)90005-0. [DOI] [PubMed] [Google Scholar]
- Levine M. N., Guyatt G. H., Gent M., De Pauw S., Goodyear M. D., Hryniuk W. M., Arnold A., Findlay B., Skillings J. R., Bramwell V. H. Quality of life in stage II breast cancer: an instrument for clinical trials. J Clin Oncol. 1988 Dec;6(12):1798–1810. doi: 10.1200/JCO.1988.6.12.1798. [DOI] [PubMed] [Google Scholar]
- Stewart A. L., Hays R. D., Ware J. E., Jr The MOS short-form general health survey. Reliability and validity in a patient population. Med Care. 1988 Jul;26(7):724–735. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]



